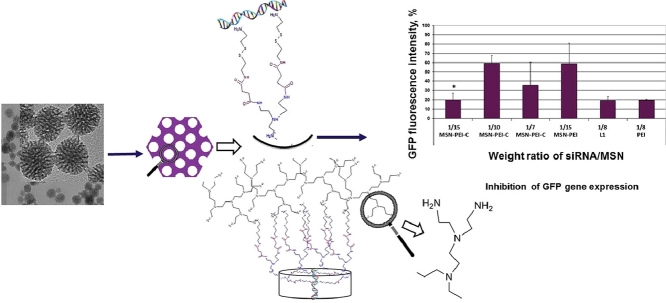
Abstract
Gene therapy using siRNA molecules is nowadays considered as a promising approach. For successful therapy, development of a stable and reliable vector for siRNA is crucial. Non-viral and non-organic vectors like mesoporous silica nanoparticles (MSN) are associated with lack of most viral vector drawbacks, such as toxicity, immunogenicity, but also generally a low nucleic acid carrying capacity. To overcome this hurdle, we here modified the pore walls of MSNs with surface-hyperbranching polymerized poly(ethyleneimine) (hbPEI), which provides an abundance of amino-groups for loading of a larger amount of siRNA molecules via electrostatic adsorption. After loading, the particles were covered with a second layer of pre-polymerized PEI to provide better protection of siRNA inside the pores, more effective cellular uptake and endosomal escape. To test the transfection efficiency of PEI covered siRNA/MSNs, MDA-MB 231 breast cancer cells stably expressing GFP were used. We demonstrate that PEI-coated siRNA/MSN complexes provide more effective delivery of siRNAs compared to unmodified MSNs. Thus, it can be concluded that appropriately surface-modified MSNs can be considered as prospective vectors for therapeutic siRNA delivery.
Keywords: Gene therapy, Nanocarriers, siRNA delivery, Mesoporous silica nanoparticles
Graphical abstract
Modified mesoporous silica nanoparticles (MSNs) where the pores are surface functionalized with polyethylene imine (PEI) and linkers containing intracellularly cleavable S—S bonds, and a particle surface covered with pre-polymerized PEI were evaluated for their transfection efficiency in MDA-MB 231 breast cancer cells stably expressing GFP. PEI-coated siRNA/MSN complexes provide significantly more effective delivery of siRNAs compared to unmodified MSNs.
1. Introduction
Nowadays new approaches for gene and drug delivery into cells are actively being developed. The most widespread vectors for gene delivery are viral vectors, which can provide effective cell transfection. An ideal virus-based vector must be able to infect cells like viruses do, but at the same time such infection must not lead to expression of viral genes, which are responsible for replication and toxicity. However, viral vectors have some drawbacks, which include insertional mutagenesis, toxicity and immunogenicity [1].
The most popular and promising alternate approach is design of non-viral vectors that are non-toxic, biodegradable, have high loading capacity, can protect the cargo from degradation during the whole route of the cargo/vector complex transit to the final destination, provide effective targeted delivery and intracellular or nuclear release of the cargo. They should not cause immune response, which allows repeated administration of the genetic constructs into the organism, and moreover, they are generally cheaper. Besides these, non-viral vectors might be easily modified with various molecular signals for overcoming barriers of gene delivery into the cells [2].
One novel and promising type of non-viral and non-organic vector is mesoporous silica nanoparticles (MSNs), which have an ordered porous structure. Porous silica particles have been known in their bulk form by materials scientists for over 40 years [3], [4], but started to be used outside of materials engineering and petrochemicals fields about 25 years ago [5] and have been proposed for drug delivery approximately 15 years ago [6], [7]. One of the major advantages of MSNs is that they are especially suitable for the delivery of hydrophobic substances, due to the fact that drugs to be delivered are packed into the pores after the particle synthesis and thus shielded by the hydrophilic silica matrix [8]. Moreover, the size of the particles and their surface can be flexibly and easily modified [9] in different manners for better interaction and protection of the cargo molecules. To provide stronger interaction of negatively charged cargo with the pore surface of MSN, it could be modified with cationic surface groups such as amines. For reaching a high abundance of amine groups on the surface, the hyperbranched polymer polyethylenimine (hbPEI) is particularly advantageous [10], [11]. In addition, PEI covering of loaded MSNs can provide capping of the pore entrances for better retention of the cargo by so-called “molecular gate-keeping” [12], [13] and internalization of loaded particles into cells by endocytosis [12], [14].
Therapy based on RNA interference (RNAi) provides treatment options in those cases when current drug technology fails [15]. RNAi is a process of gene expression silencing during different stages of genes processing. The advantage of small interfering RNAs (siRNA) is that siRNA is fully complementary to target sequences, which leads to silencing based on Argonaute 2–mediated degradation [16]. However, controlled siRNA delivery remains challenging for two main reasons: (1) existing delivery systems cannot be endowed with high loading efficiency, stability and slow release, hence long-term therapeutic effects would require repeated drug administration; (2) biological barriers are still remaining a challenging obstacle [17], [18]. Moreover, for potential in vivo use, the nanocarrier must retain the oligonucleotide molecules very tightly during circulation and cellular internalization, but slowly and sustainably dissociate them in the intracellular environment according to individual requirements. MSNs have the potential for meeting both challenges, due to established low toxicity and high uptake.
PEI is known to be an effective vector for gene delivery since it exhibits a unique “proton-sponge” effect to exit endosomal compartments. The interest nowadays is focused on applying of this unique property of PEI to nanocarriers, e.g. by conjugation of PEI molecules with silica nanoparticles [12]. This modification also provides positive charge on the surface of MSNs for loading of negatively charged nucleic acids. Several studies have been published on utilizing PEI of different molecular sizes and degrees of branching. The only disadvantage of PEI is cytotoxicity, though it is dependent on its molecular weight. Xia and coauthors demonstrated toxicity of MSN-PEI (25 kDa) complexes on macrophage (RAW 264.7) and bronchial epithelial (BEAS-2B) cell lines [19]. PEI of different molecular weights was tested, whereby the results confirmed the absence of toxicity of PEIs with lower molecular weight, such as 0.6, 1.2 and 1.8 kDa. In addition, this study also showed high nucleic acid binding capacity that was mostly independent of the PEI MW [19]. At the same time, Li with coauthors were the first ones in 2011 who succeeded to load siRNA not only on the surface of magnetic MSNs, but inside the pores [20]. To prepare a reliable vector for siRNA delivery, they treated the loaded particles with PEI to block the detachment of siRNA. Low toxicity of such constructions was proven although branched PEI with the weight 25 kDa was chosen for further experiments.
In one of our previous publications, MSN-PEI hybrid carrier coupled to redox-sensitive surface linkers that break under intracellular conditions was developed, thereby converting the surface charge to negative which drives the nucleic acids to release from the pores [21]. These MSNs were capped with PEG chains, which did not promote effective endosomal escape after cellular uptake. In the subsequent study, PEG was replaced with pre-polymerized PEI (in contrast to the surface-grafted PEI which was grown via surface-initiated polymerization), which lead to enhanced cellular uptake and endosomal escape [22]. Hence, the work described in the current manuscript is to be considered a follow-up of this study by Prabhakar et al. 2016 [22] devoted to transfection of MDA-MB 231 cell line with modified MSN particles loaded with cell-killing siRNA. The used AllStars Hs Cell Death Control siRNA mechanism of action involves five different target genes, which makes it relatively unspecific. Thus, in the current study, we decided to use GFP-expressing MDA-MB 231 cell line to transfect with MSN/anti-GFP siRNA complexes to confirm the silencing origin of the observed effect. Further, we compare the siRNA delivery efficacy with non-PEI-modified MSNs, to confirm the advantage of PEI as construct. MDA-MB cell lines, MDA-MB 231 in particular, are very important for breast cancer research [23], while GFP-MDA-MB cell lines are useful for optimization of siRNA transfection with non-viral vectors.
2. Materials and methods
2.1. Materials
Unloaded MSNs (in 96% ethanol, 3 mg/ml), with cystamine-PEI coated pores, were synthesized as described previously [21]. All the in vitro experiments were performed with the use of MDA-MB-231 cell culture constitutively expressing GFP gene. PEI 1300 Da (Lupasol G 20 WF) was used for MSN pores covering with hyperbranched PEI after siRNA loading. For transfection experiments, anti-GFP siRNA was used: sense 5′-CAA GCU GAC CCU GAA GUU Ctt-3′, antisense 5′-GAA CUU CAG GGU CAG CUU Gtt-3′. siRNA was purchased from Syntol JSC, Russia. Two non-viral vectors were used as controls: peptide-based vector L1, modified with CXCR4 receptor ligand developed in Laboratory of Prenatal Diagnostics of Inherited and Inborn Disorders in The Research Institute of Obstetrics, Gynaecology and Reproductology named after D. O. Ott, Saint Petersburg [24], [25] and polyethyleneimine (branched PEI 25 kDa; Sigma-Aldrich). Weight ratios used in transfection experiments were the following: siRNA/L1-1/8.42 and siRNA/PEI-1/1.1
2.2. Methods
2.2.1. Particles preparation
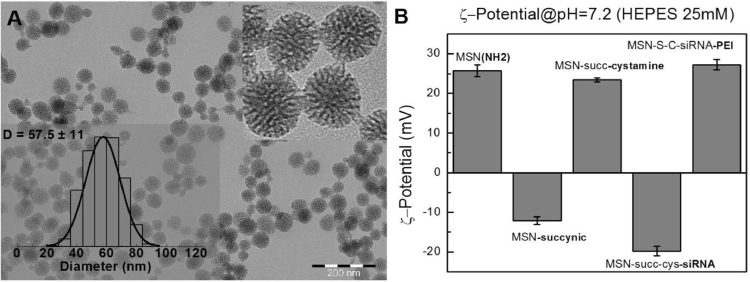
siRNA loading and PEI covering of loaded MSN was performed according to Prabhakar et al. [22]. Briefly, MSN-PEI hybrid carrier nanoparticles of roughly 60 nm in diameter (Fig. 1A) coupled to redox-sensitive surface linkers (cystamine molecules with an intracellularly cleavable S—S bond) were prepared through sequential steps involving first the succinylation of the MSN surface amines, followed by the linking of cystamine to the succinic acid residues by EDC coupling. After this, complexes of siRNA/MSN were formed in following weight ratios: 1/15, 1/10, 1/7 (200 nM of siRNA corresponding to 41.2 µg/ml, 27.4 µg/ml and 9.59 µg/ml MSNs). Specifically, Anti-GFP-siRNA was added to the prepared suspension of MSN-cystamine in MES buffer at these weight ratios. It is necessary to state that GFP is not a natural protein in MDA-MB-231 cells and is known to not to affect cellular physiology. Final solution was mixed for 1 h and centrifuged (5000 × g/10 min). For PEI covering the complexes were resuspended in ethanol. Then PEI (1300 Da) and N,N′-disuccinimidyl carbonate (DSC) were added under stirring to provide PEI surface polymerization. The solution was incubated at room temperature for 30 min, then centrifuged (5000 × g/10 min) for purification. The final solution was resuspended in HEPES buffer. The success of the different functionalization steps was assessed by zeta potential measurements, where the expected modification of the particle net surface charge can be observed after each step (Fig. 1B). MSN binding to siRNA was monitored using SybrGreen fluorescence quenching method. The particles were analyzed at following weight ratios (1/15, 1/10, 1/7).
Fig. 1.
Characterization of the MSN system used in this work. (A) Representative TEM images and size distribution of the starting MSN-NH2 particles. (B) Zeta potential measurements of the MSN-NH2 particles after synthesis and after the different sequential functionalization steps with succinic anhydride, cystamine, siRNA and PEI. Particles were dispersed in HEPES buffer (10 mM; pH 7.2) at a 0.1 mg/ml mass concentration for comparability of the measurements. It can be observed the expected modification of the particle surface charge after each step (negatively charged after succinylation and siRNA loading and positively charged for the MSN after synthesis, after cystamine conjugation and finally after PEI adsorption onto MSN-siRNA loaded particles.
2.2.2. Particles characterization
MSN samples were characterized by means of different techniques such as transmission electron microscopy (TEM), zeta potential (ζ-potential) and dynamic light scattering (DLS). Transmission electron microscopy (TEM) images were obtained using a JEM 1400-Plus (JEOL Ltd., Tokyo, Japan) operating at 80 kV with a tungsten filament and an 11-Mpx CCD camera. Preparation of the samples was performed onto a carbon coated copper TEM grids by drop casting. Different parts of the grid were observed and images were taken at different magnifications to ensure obtaining statistically significant results for MSNs size distribution. More than 1000 particles were counted using Image J software (Fig. 1A). Zeta potential (ζ-potential) measurements were performed using a Malvern ZetaSizer Nano ZS Instrument operating at a light source wavelength of 532 nm and a fixed scattering angle of 173° for detection. After synthesis and after the different functionalization steps, a volume of 0.8 ml of the MSNs (0.1 mg/ml) dispersed in HEPES (10 mM; pH 7.2) were placed into the specific cuvette, and the software was arranged with the specific parameters of refractive index and absorption coefficient of the MSN and viscosity of the solvent, data required to obtain the correct ζ-potential value for the samples (Fig. 1B). Finally, a slight increase in dynamic light scattering (DLS) values along with the maintenance of the polydispersity index indicates that MSN maintain their colloidal stability after the different functionalization steps (data not shown).
2.2.3. Loading efficiency
SybrGreen displacement was assayed following the decrease in emission fluorescence at 590 nm (485 nm excitation). After loading of MSN-based vectors with prelabeled siRNA solution with 1 × SybrGreen (AMRESCO), one part of the loaded particles solution was covered with hyperbranched PEI, while the rest were left uncovered. The concentration of siRNA was 0.2 µg per well. Two weight ratios of siRNA and MSN were tested: 1/7 and 1/10. Fluorescence measurements were performed by means of Wallac 1420D scanning multilabel counter (Thermo Fisher Scientific Oy, Vantaa, Finland). Displacement was calculated as (F − Ff)/(Fb − Ff), where Ff and Fb are the fluorescence intensities of SybrGreen in the absence and presence of siRNA, respectively.
2.2.4. Transfection experiments and estimation of transfection efficiency
Transfection experiments were performed in triplicate. Before transfection, cell culture medium was replaced with medium without FBS. siRNA-MSN complexes at three weight ratios established before (1/15, 1/10, 1/7) were added and incubated with MDA-MB-231 cells for 4 h. The final concentration of siRNA was 200 nM in each well, or 1.37 µg. The concentration of siRNA for transfection experiments was set based on existing protocols previously shown to provide a good readout [25], according to which the amounts of MSN were calculated (41.2, 27.4 and 9.59 µg/ml). After incubation in normal culture medium for the next 48 h, the media was removed from wells and 80 µl of lysis-buffer were added to each well with transfected cells. To provide efficient lysis of cells they were put into freezer (−70°) for at least 1 h. To measure the GFP fluorescence intensity the cells were centrifuged (2500 × g/5 min) and 50 µl of supernatant was taken to Wallac 1420 Multilabel Counter (emission 535 nm/excitation 485 nm). The GFP gene expression was normalized by the total protein concentration of the cell extracts, measured with Bradford reagent (Helicon, Russia). Fluorescence of MDA-MB-231 cells transfected with naked siRNA was used as a control with maximal fluorescence taken as 100%.
2.2.5. Hemolysis assay
Hemolysis assay was performed according to methodology described before by Evans et al. [26]. 5 ml of blood was obtained into tube with Na2EDTA from an anonymous human donor according to ethical requirements. Red blood cells (RBCs) were washed three times with NaCl and phosphate-buffered saline (PBS) (pH 7.4) and diluted up to 50 times with PBS pH 7.4. Stock solutions of MSN-PEI-Linker-PEI of different concentrations were prepared. For each assay we took 200 µl of RBC and 800 µl of particles stock solutions to reach the final concentration of MSN-PEI-Linker-PEI 41.2, 27.4 and 9.59 µg/ml. For positive control tubes we added 800 µl of 1, 25% Triton X-100 to 200 µl. For negative control tubes, we added 800 µl of PBS pH 7.4. Tubes were incubate at 37 °C for 1 h and 24 h. After incubation tubes were centrifuged for 5 min at 500 × g to pellet intact erythrocytes. Hemolysis was estimated in UV/Vis spectrum, from the 540 nm absorbance of hemoglobin released into the supernatant using NanoDrop™ 2000, the results were expressed as % hemolysis. Negative control which corresponds to 0% hemolysis was taken as the absorbance of the supernatant of erythrocyte suspensions in PBS pH 7.4. Absorbance of the supernatant of erythrocyte suspensions in presence of Triton X-100 was taken as 100% hemolysis.
2.2.6. Statistics
Statistically significant differences were analyzed by Student's t-test, using Instat (GraphPad Software Inc., USA). P < 0.05 was considered statistically significant.
3. Results and discussion
The present study is focused on evaluation of hyperbranched PEI covered MSN as vehicles for siRNA delivery into MDA-MB-231 breast cancer cells for GFP expression inhibition. The structure of MSN particles and surface functions may be variable and flexibly tuned for increasing their loading capacity [27]. Considering this, the interaction of oligonucleotides and surface-modified MSNs and their behavior inside pores were studied previously [21], [22]. As a result, it has been shown that short oligonucleotides (up to 20 bp) can be more effectively bound by functionalized pores in comparison with longer DNA molecules [28], which led to further studies of siRNA delivery by MSNs. Recently it has been shown that modification of MSN pores with amino groups is a promising approach to increase the amount of siRNA which could be loaded into MSNs [29].
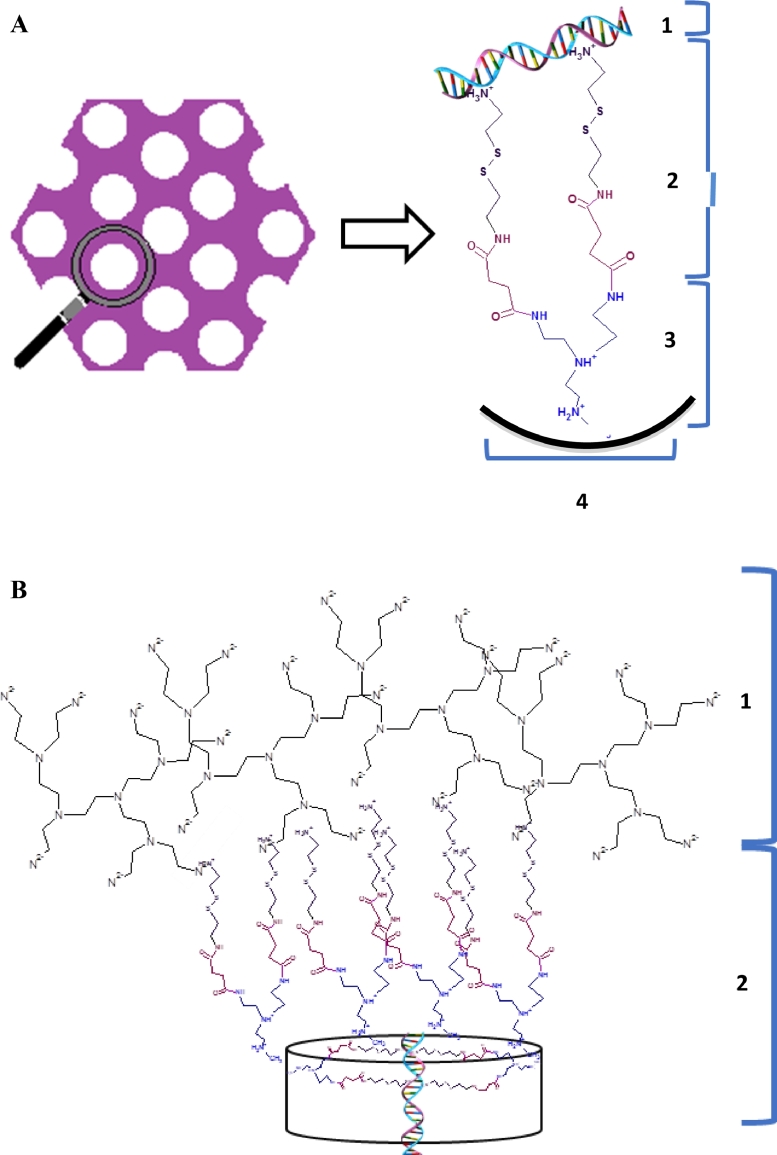
The most effective means to increase siRNA loading of MSN is to achieve low N/P ratio inside the functionalized pores, ranging from 0.7 to 1.0, by providing high density of amine-terminated organic linkers (one linker per 3.3–3.7 nm2) [21]. Here, cystamine was chosen as organic linker because it contains amino-groups and a disulfide bond, which could be easily disrupted inside cells (Fig. 2).
Fig. 2.
Schematic representation of (A) the construction of cleavable organic linkers on MSN pores surface through hyperbranched PEI with loaded NA: 1. NA, 2. Cystamine with amino-group and disulfide bond, 3. PEI, 4. MSN pore surface; (B) the NA loading, particle surface grafting of exterior PEI: 1. Hyperbranched PEI, 2. MSN-PEI-Linker.
To provide a better protection of siRNA inside pores, increased cellular uptake and endosomal escape, the MSN surface was covered with a coating of pre-polymerized PEI (1300 Da) after siRNA loading. It has been shown experimentally that low-molecular weight PEI polymers are not toxic for cells [30] and especially when used as a construct in silica hybrid materials, the cytotoxicity can be suppressed even for higher MW PEI [29]. Besides, the outside layer of PEI provides the “proton sponge” effect for endosomal escape of siRNA/MSN complexes [19].
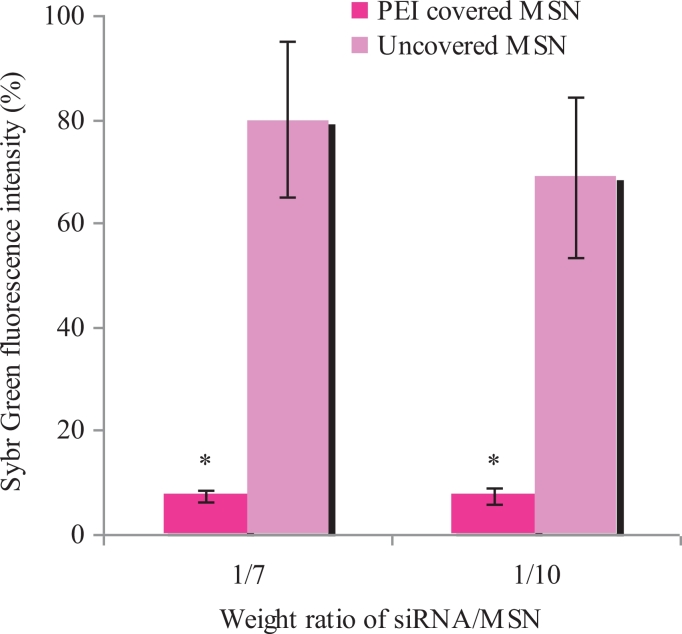
To estimate the efficiency of MSN/anti-GFP siRNA complexes formation, exclusion tests were performed. Intercalating dye SybrGreen releases out of DNA during complex formation, which causes fluorescence intensity decrease. Free DNA was used as a control with maximal fluorescence taken as 100%. Data obtained prove that the PEI coating prevents SybrGreen access to siRNA inside pores, which results in decreased fluorescence in comparison with uncovered particles (Fig. 3).
Fig. 3.
SybrGreen exclusion from complexes of siRNA and MSNs with and without PEI covering. *P < 0.05 when compared with uncovered MSN particles.
Two of the most important characteristics of vectors for intracellular delivery are toxicity and transfection efficiency. Previously, Prabhakar and co-authors showed MSN-PEI-Linker-PEI vectors to be not toxic for MDA-MB 231 cell culture [22] by WST-1 assay, which is based on the conversion of the tetrazolium salt WST-1 into a colored dye by mitochondrial dehydrogenase enzymes.
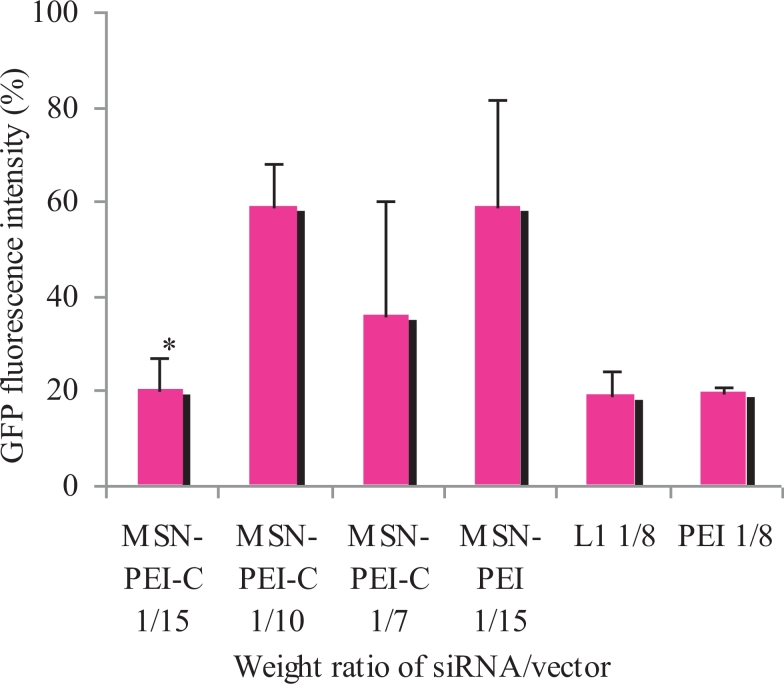
Transfection experiments were subsequently performed for evaluation of siRNA delivery efficiency by MSNs into cells. For these experiments, MDA-MB-231 cell culture was used because previously low toxicity of MSN/NA complexes was proved for this cell line [22]. Uncovered particles (without an outer coating of pre-polymerized PEI) with the weight rate of siRNA/MSN 1/15 were taken for comparison (MSN-PEI in Fig. 4). As control, we used branched PEI 25 kDa and previously described peptide vector L1, modified with ligand to CXCR4 receptor [24], [31]. As shown earlier, MDA-MB-231 cells overexpress CXCR4 receptors on their surface [32]. Transfection efficiency was estimated by the level of GFP expression silencing in the transfected cells. No differences in transfection efficacy in MDA-MB-231 cells between PEI-modified siRNA/MSN formed at low weight ratios and unmodified siRNA/MSN were found, whereas efficacy of siRNA/MSN formed at weight ratio 1/15 was significantly decreased after PEI modification (Fig. 4).
Fig. 4.
Relative GFP fluorescence intensity (%) after transfection of MDA-MB-231 cells with anti-GFP siRNA/MSN complexes at weight ratios 1/15, 1/10 and 1/7. Complexes of anti-GFP siRNA and vectors L1 and PEI were used as controls. The result of transfection with naked anti-GFP siRNA is taken as 100%. *P < 0.05 when compared with uncovered MSN particles.
Results obtained show that PEI-covered complexes with higher siRNA/MSN weight ratios provide more effective delivery of siRNAs (Table 1). Thus, our results confirm the assumption about the advantages of PEI-covered siRNA/MSN.
Table 1.
Relative GFP fluorescence intensity (%) in MDA-MB-231 cell culture after transfection with complexes of anti-GFP siRNA with MSNs of weight ratios 1/15, 1/10, 1/7, and complexes of anti-GFP siRNA with L1 and PEI as controls. The level of GFP intensity is expressed in percentage terms, where result of transfection with naked anti-GFP siRNA is taken as 100%. *P < 0.05 when comparing with uncovered particles.
| Name of vector and siRNA/MSN weight ratio | GFP intensity (%, mean ± SEM) |
|---|---|
| MSN-PEI-covered 1/15 | 19.96* ± 7.05 |
| MSN-PEI-covered 1/10 | 59.08 ± 8.68 |
| MSN-PEI-covered 1/7 | 35.82 ± 24.33 |
| MSN-PEI-uncovered 1/15 | 58.77 ± 22.5 |
| L1 1/8 | 19.28 ± 4.63 |
| PEI 1/8 | 19.53 ± 1.11 |
The hemolysis assay is required for all devices or materials which are introduced into the body via the blood stream to define hemolytic properties. This test measures the damage to red blood cells which can occur due to nanocarrier exposure, and compares it to positive and negative controls.
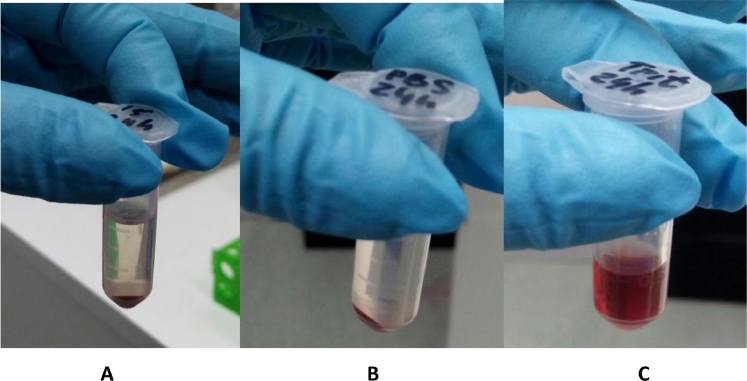
Three different concentrations of MSN-PEI-Linker-PEI were chosen for the assay in accordance with concentrations utilized for transfection efficiency test: 41.2 µg/ml, 27.4 µg/ml and 9.59 µg/ml. Before analyzing the absorbance level, the effect of delivery complexes can be detected visually (Fig. 5).
Fig. 5.
Example of visualization of RBC after incubation with MSN-PEI-Linker-PEI vectors: (A) 41.2 µg/ml, (B) negative control (PBS pH 7.4), (C) positive control (Triton X-100).
For measuring the absorbance level of supernatants of each assay mixture, a wavelength of 540 nm was chosen, and the percentage of hemolysis was counted according to a previously reported formula [26]. It can be seen that our particles do not exhibit any hemolytic activity in comparison with the positive control. Results obtained for both time points, 1 h and 24 h, show no significant difference between the negative control (0% of hemolysis) and the studied samples (Table 2).
Table 2.
Hemolytic activity of MSN-PEI-Linker-PEI particles of different concentrations after 1 h and 24 h of incubation. The hemolytic activity is expressed in percentage terms. * no significant difference.
| Name of vector | Concentration of MSN-PEI-Linker-PEI (µg/ml) | Hemolysis (%) |
Statistical difference | |
|---|---|---|---|---|
| 1 h | 24 h | |||
| MSN-PEI-Linker-PEI | 41.2 | 0.2–0.2 | −0.2 | NS* |
| MSN-PEI-Linker-PEI | 27.4 | −0.4 | −0.7 | NS* |
| MSN-PEI-Linker-PEI | 9.59 | 0 | −0.5 | NS* |
| PBS pH 7.4 | 100 | 0 | ||
| Triton X-100 | 100 | |||
Although the investigated siRNA delivery system must still be further optimized for clinical studies, we can already envision that these types of delivery systems will have a significant impact. There is a great demand for the developing of effective siRNA carriers that would exhibit high specificity, significant therapeutic effect, minor side effects and ease of synthesis. Moreover, the field of use for siRNA therapy is quite broad; the silencing mechanism is effective against many genetic and infectious disorders, such as cancer or hepatitis C. With the first gene therapy for a genetic disease approved by the FDA in late 2017 [33], based on a viral vector, we envision the development of gene delivery systems will continue to promote more effective gene therapies in the future.
4. Conclusion
During the past decades, vectors for gene therapy are being rapidly developed, but almost all of them have disadvantages, such as toxicity, short-term effect, low transfection efficiency, high price etc. Use of non-viral vectors may solve most of these problems, while non-organic non-viral vectors, like MSNs, are also much cheaper and robust. In the current study, we demonstrated that MSNs conjugated with cationic polymers can provide safety of the fragile NA cargo and higher transfection efficiency in comparison with pure MSNs. Thus, we can conclude that modified mesoporous silica nanoparticles can be considered as suitable vectors for delivery of siRNA into cells.
Conflicts of interest
The authors declare that there is no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported in part by Russian Science Foundation grant 17-15-01230 (biological characterization), Academy of Finland project nos. 284542, 384542 (JMR) and Jane and Aatos Erkko Foundation (EC). Anna Egorova is supported by President of Russian Federation scholarship (SP-2162.2015.4). Anna Slita was supported by the scholarship within Saint Petersburg State University bilateral exchange program for study abroad.
References
- 1.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 2.Egorova A.A., Kiselev A.V. Peptide modules for overcoming barriers of nucleic acids transport to cells. Curr Top Med Chem. 2016;16:330–342. doi: 10.2174/1568026615666150812120755. [DOI] [PubMed] [Google Scholar]
- 3.Beau R, Duchene J, Le Page M. Porous silica particles containing a crystallized phase and method, patent USPTO 3493341, 1970.
- 4.Mieville R.L. Measurement of microporosity in the presence of mesopores. J. Colloid Interface Sci. 1972;41:371–373. [Google Scholar]
- 5.Inagaki S, Fukushima Y, Okada A, Kato C, Kuroda K. Manufacture of layer-form silica-metal oxide porous intercalation compounds useful as adsorbents and catalysts. Toyota Central Research and Development Laboratories, Inc., Japan. assignee.: patent JP04238810A, 1992.
- 6.Muller U, Reck B, Roser J. Basf Aktiengesellschaft, Germany. Assignee. Mesoporous silica and its preparation for use as catalysts or supports for catalysts, drugs, enzymes or pigments patent: EP831059A1. 1998
- 7.Roggers R., Kanvinde S., Boonsith S., Oupicky D. The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. AAPS PharmSciTech. 2014;15:1163–1171. doi: 10.1208/s12249-014-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maleki A., Kettiger H., Schoubben A., Rosenholm J., Ambrogi V., Hamidi M. Mesoporous silica materials: from physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J Control Release. 2017 doi: 10.1016/j.jconrel.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Rosenholm J.M., Sahlgren C., Lindén M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—opportunities & challenges. Nanoscale. 2010;2:1870–1883. doi: 10.1039/c0nr00156b. [DOI] [PubMed] [Google Scholar]
- 10.Rosenholm J.M., Duchanoy A., Lindén M. Hyperbranching surface polymerization as a tool for preferential functionalization of the outer surface of mesoporous silica. Chem Mater. 2007;20:1126–1133. [Google Scholar]
- 11.Rosenholm J.M., Lindén M. Wet-chemical analysis of surface concentration of accessible groups on different amino-functionalized mesoporous SBA-15 silicas. Chem Mater. 2007;19:5023–5034. [Google Scholar]
- 12.Rosenholm J.M., Meinander A., Peuhu E. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano. 2008;3:197–206. doi: 10.1021/nn800781r. [DOI] [PubMed] [Google Scholar]
- 13.Niemelä E., Desai D., Nkizinkiko Y., Eriksson J.E., Rosenholm J.M. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur J Pharm Biopharm. 2015;96:11–21. doi: 10.1016/j.ejpb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Karaman D.S., Desai D., Senthilkumar R. Shape engineering vs organic modification of inorganic nanoparticles as a tool for enhancing cellular internalization. Nanoscale Res Lett. 2012;7:358. doi: 10.1186/1556-276X-7-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shubina A.N., Egorova A.A., Baranov V.S., Kiselev A.V. Recent advances in gene therapy of endometriosis. Recent Pat DNA Gene Seq. 2013;7:169–178. doi: 10.2174/18722156113079990021. [DOI] [PubMed] [Google Scholar]
- 16.Bobbin M.L., Rossi J.J. RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu Rev Pharmacol Toxicol. 2016;56:103–122. doi: 10.1146/annurev-pharmtox-010715-103633. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miele E., Spinelli G.P., Miele E. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int J Nanomed. 2012;7:3637–3657. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia T., Kovochich M., Liong M. Polyethyleneimine coating enhances the cellular uptake of mesoporous silicananoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Xie Q.R., Zhang J., Xiab W., Gu H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials. 2011;32.35:9546–9556. doi: 10.1016/j.biomaterials.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Niemelä M., Westermarck J., Rosenholm J.M. Mesoporous silica nanoparticles with redox-responsive surface linkers for charge-reversible loading and release of short oligonucleotides. Dalton Trans. 2014;43:4115–4126. doi: 10.1039/c3dt53071j. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar N., Zhang J., Desai D. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNa delivery. Int J Nanomed. 2016;11:6591. doi: 10.2147/IJN.S120611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacroix M., Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 24.Egorova A., Bogacheva M., Shubina A., Baranov V., Kiselev A. Development of a receptor-targeted gene delivery system using CXCR4 ligand-conjugated cross-linking peptides. J Gene Med. 2014;16:336–351. doi: 10.1002/jgm.2811. [DOI] [PubMed] [Google Scholar]
- 25.Egorova A., Shubina A., Sokolov D., Selkov S., Baranov V., Kiselev A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int J Pharm. 2016;515:431–440. doi: 10.1016/j.ijpharm.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 26.Evans B.C., Nelson C.E., Shann S.Y. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013;73:1–5. doi: 10.3791/50166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kecht J., Schlossbauer A., Bein T. Selective functionalization of the outer and inner surfaces in mesoporous silica nanoparticles. Chem Mater. 2008;20:7207–7214. [Google Scholar]
- 28.Lebold T., Schlossbauer A., Schneider K. Controlling the mobility of oligonucleotides in the nanochannels of mesoporous silica. Adv Funct Mater. 2012;22:106–112. [Google Scholar]
- 29.Desai D., Karaman D.S., Prabhakar N. Design considerations for mesoporous silica nanoparticulate systems in facilitating biomedical applications. Mesoporous Biomater. 2014;1:1. [Google Scholar]
- 30.Neu M., Fischer D., Kissel T. Recent advances in rational gene transfer vector design based on poly (ethylene imine) and its derivatives. J Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 31.Egorova A., Kiselev A., Hakli M. Chemokine-derived peptides as carriers for gene delivery to CXCR4 expressing cells. J Gene Med. 2009;11:772–781. doi: 10.1002/jgm.1366. [DOI] [PubMed] [Google Scholar]
- 32.Lee B.C., Lee T.H., Avraham S., Avraham H.K. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–338. [PubMed] [Google Scholar]
- 33.http://ir.sparktx.com/news-releases/news-release-details/fda-approves-spark-therapeutics-luxturnatm-voretigene-neparvovec [Accessed 22 December 2017].