Graphical Abstract
The present study was conducted to decipher the role of 0.5 ml Hesperetin conjugated gold nanoparticles (Au-mPEG(5000)-S-HP NPs) during diethylnitrosamine (DEN)-induced hepatocarcinogenesis in male Wistar albino rats and shows the better antioxidant that possesses anti-inflammatory, anti-proliferation and anticarcinogenic properties and may modulate signaling pathways. Here, we review the role of mast cell counts, tumor necrosis factor alpha (TNF-α), transcription factor nuclear factor-κB (NF-κB), levels of glycoconjugates, proliferating cell nuclear antigen (PCNA) and argyrophilic nucleolar organizing regions, are the master regulator of inflammation and proliferation, in the development of hepatocellular injury, liver fibrosis and HCC.
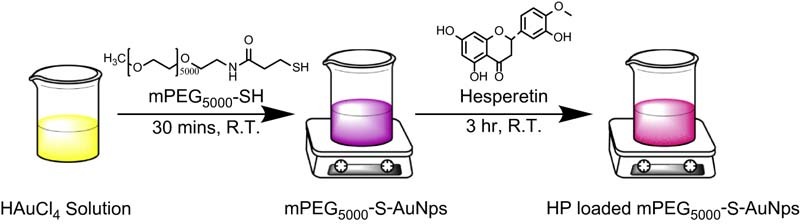
Schematic illustration for the chemical synthesis of mPEG(5000)-SH polymer stabilized gold nanoparticles and hesperetin (HP) loaded mPEG(5000)-SH stabilized gold nanoparticles.
Keywords: Hesperetin, Gold nanoparticles, Mast cells, Glycoconjugates, TNF-α, NF-κB, PCNA
Abstract
Liver cancer is the fifth most common cancer and one of the leading causes of death in the world, and second most common cause of death in men. Natural products emerge as the most enduring approaches in the development of anticancer targeting drug. Hesperetin (HP), one of the abundant flavonoids found naturally in citrus fruits, has received considerable attention in anti-cancer promotion and progression. The present study was conducted to decipher the role of 0.5 ml hesperetin conjugated gold nanoparticles (Au-mPEG(5000)-S-HP NPs) during diethylnitrosamine (DEN)-induced hepatocarcinogenesis in male Wistar albino rats and shows the better antioxidant that possesses anti-inflammatory, anti-proliferation and anticarcinogenic properties and may modulate signaling pathways. The confirmation of polymer functionalized gold nanoparticles and drug loaded polymer gold nanoparticles were characterized by HR-TEM with EDAX, and DLS with Zeta potential techniques. The drug encapsulation efficiency and release properties were carried out in PBS at pH 7.4 for Au- mPEG(5000)-S-HP and compared with the control pure hesperetin (HP). Here, we review the role of mast cell counts, tumor necrosis factor alpha (TNF-α), transcription factor nuclear factor-κB (NF-κB), levels of glycoconjugates, proliferating cell nuclear antigen (PCNA) and argyrophilic nucleolar organizing regions, are the master regulator of inflammation and proliferation, in the development of hepatocellular injury, liver fibrosis and HCC. DEN-administered animals showed increased mast cell counts, tumor necrosis factor alpha, transcription factor nuclear factor-κB, glycoconjugates, proliferating cell nuclear antigen, and argyrophilic nucleolar organizing regions. Whereas Au-mPEG(5000)-S-HP NPs supplementation considerably suppressed all the above abnormalities. These results suggest that the Au-mPEG(5000)-S-HP NPs exhibited the better potential anticancer activity by inhibiting cell inflammation and proliferation in DEN-induced hepatocellular carcinogenesis.
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequent form of primary liver cancer and it is the fifth most common cancer in the world and third most common cause of cancer mortality [1]. The incidence of HCC is rising across the globe, especially in the United States, with 71% increase over the last 25 y [2]. The incidence of liver cancer has been increasing in recent years in India and the major risk factors of HCC may be age, gender, hepatitis B and C, alcohol consumption, hormone exposure, haemochromatosis, vinyl chloride, arsenic poisoning, aflatoxin B1, obesity, diabetes, renal transplant patients, tobacco smoking and parasitic infections such as clonorchiasis and schistosomiasis which results in liver damage such as cirrhosis [3]. The main denominator in HCC of different etiology is the induction of oxidative stress by inflammatory cells, resulting in chronic hepatic injury and cell death, followed by oncogenic transformation of surviving hepatocytes and compensatory proliferation that leads to oncogenesis [4], [5]. Diethylnitrosamine (DEN) or N-nitrosodiethylamine (NDEA) is a powerful environmental carcinogen that has been used as an initiating agent for hepatocarcinogenic activities. N-nitroso compounds are considered to be a tragic health hazards to man, and these compounds were present in tobacco products, cheddar cheese, cured and fried meals, occupational settings, cosmetics, agricultural chemicals and pharmaceutical agents [6]. The pre-treatment and the post-treatment of cancer is still a big challenge in medicine and the chemoprevention serves as an attractive and alternative to prevent cancer [7]. The term cancer chemoprevention is the use of several natural, synthetic, and biological agents to reverse, inhibit, or delay carcinogenic progression to invasive cancer which has been identified as a novel approach against several types of cancers [8]. Considerable efforts were taken to search for naturally occurring compounds that can curtail several stages of carcinogenesis. Plant derived substances have recently gained importance, owing to their versatile applications such as quenching reactive oxygen species and protect critical cellular components like DNA, proteins, and lipid from oxidative damage [9]. It may also interfere with intracellular signaling pathways which as regulate cell proliferation, initiation of apoptosis, and response to oxidative damage [10]. Epidemiology and animal studies have suggested that a high intake of flavonoids may be linked to a reduced risk of cancer.
Hesperetin (5, 7, 3'-trihydroxy-4'methoxy flavanone), a Chinese traditional medicine, is a bioflavonoid occurring abundant in citrus fruits which occurs as hesperidin (its glycoside form) in nature and it has received considerable attention in cancer prevention [11]. It exhibits various pharmacological activities, such as anti-inflammatory, anti-hypertensive and anti-atherogenic effects [12], [13], [14]. Despite the challenging application of hesperetin (HP) in cancer therapy, but the clinical use of HP was restricted because of the poor water solubility. Therefore, many researchers are now focusing on improving its bioavailability through several approaches including innovative drug delivery systems [15], [16], [17]. In this condition we need an effective drug delivery system with the help of various biomaterials such as biodegradable nanoparticles (NPs). To enhance the efficacy and the solubility of the cancer therapeutic agent, the use of nanoparticle-based drug formulation is an important aspect of nanomedicine [18]. In particular, gold NPs possess biological activities like antioxidant, anti-inflammatory, anti-angiogenesis and anticancer properties and therefore it has been used for the delivery of drugs, proteins, peptides and oligonucleotides etc. [19]. The uses of biocompatible functionalized polymers emerged as an attractive candidate for the delivery of various therapeutic agents and also playing a dual role as reducer and stabilizer [20]. The major advantage of using a polymer as a stabilizing agent not only the enhancement of their long-term stability, adjustment of the solubility and amphiphilicity, but also their functionalization with polymers to achieve higher and tunable surface-density of shell/brush morphology and to tailor its properties, beyond promoting their compatibility and processibility of the nanoparticles by preventing particle agglomeration [21], [22]. Hence, it is clear that the binding ability of the AuNPs to the cell membrane and the functionalization of the m-PEG-thiol polymer on the AuNPs make it to serve as a good drug carrier and solubility.
In our previous study we have introduced a new method for effective drug delivery system to improve the drug efficacy, solubility and bioavailability with the help of nanomaterials by synthesizing gold (Au) NPs stabilized and reduced with polymer O-[2-(3-mercaptopropionylamino)ethyl]-O'-methyl polyethylene glycol (mPEG(5000)-SH). Further, it is capped with anticancer drug-HP for effective chemotherapy drug to treat DEN induced HCC in male Wistar albino rats. The effect of better anti-inflammatory effect and anti-proliferative effect of hesperetin conjugated gold nanoparticles (Au-mPEG(5000)-S-HP NPs) is not yet documented. Hence the present study was aimed to elucidate the better protective role of Au-mPEG(5000)-S-HP NPs on the expressions of cell inflammation and cell proliferation during diethylnitrosamine-induced hepatocarcinogenesis in male Wistar albino rats.
2. Materials and methods
2.1. Sources of chemicals
Tetrachloroauric acid (HAuCl4), O-[2-(3-mercaptopropionylamino) ethyl]-O'methylpolyethylene glycol-5000(PEG-SH), diethylnitrosamine and hesperetin were purchased from sigma chemical company (St. Louis, MO, USA) with the purity of >99 and 98%, respectively.
2.2. Animals
Male, Wistar strain albino rats weighing about 150–180 g were obtained from Tamil Nadu Veterinary and Animal Science University (TANUVAS), Madhavaram, Chennai, India. The animals were housed in cages under proper environmental conditions and were fed with a commercial pelleted diet (M/S Hindustan Foods Ltd, Bangalore, India). The animals had free access to water. All the experiments were designed and conducted according to the ethical norms approved by Institutional Animal Ethics Committee guidelines (IAEC No: 12/02/2012).
2.3. Experimental design
Experimental animals were divided into six groups of six rats each as follows. Group 1 (Control) normal control animals fed with standard diet and pure drinking for 16 weeks. Group 2 (DEN induced) animals were administered with DEN (0.01%) in drinking water for 16 weeks to induce liver cancer. Group 3 (Drug control) animals were treated with HP alone by intraperitoneally twice a week at a dose of 20 mg/kg b.wt (based on effective dose fixation studies) for 16 weeks to study the toxicity (if any) induced by HP. Group 4 (HP treatment) animals were treated with pure HP (20 mg/kg b.wt by intraperitoneally) twice a week for one week prior to the first dose of 0.01% DEN administration and continued for 15 weeks. Group 5 (Gold NPs encapsulated HP control) animals were treated with 0.5 ml of Au-mPEG(5000)-S-HP NPs (i.e. 1.5 mg/0.5 ml) intraperitonealy twice a week for 16 weeks to study the cytotoxicity (if any) induced by gold naoparticles. Group 6 (Gold NPs encapsulated HP treatment) animals received 0.01% DEN along with 0.5 ml of Au-mPEG(5000)-S-HP NPs (i.e.1.5 mg/0.5 ml) intraperitoneally for twice a week. Au-mPEG(5000)-S-HP NPs was started one week prior to the first dose of DEN administration and continued for 16 weeks. After the experimental period, the animals were fasted overnight and euthanized. The liver was excised immediately and rinsed with ice-cold saline. A portion of the liver was homogenized in 0.1 M Tris buffer, pH 7.4, and used for the further analysis.
2.4. Characterization of gold nanoparticles and in vitro drug release study
The samples for HR-TEM imaging was prepared by placing a drop of gold solution on a carbon coated copper grid and drying at room temperature. The dynamic light scattering (DLS) and Zeta potential of the samples were determined by Zetasier nano ZS (Malvern Instruments, UK). A He-Ne diode laser (633 nm) as the source was scattered at a fixed angle of 900 °C at room temperature. The experiments were performed in triplicate. The in vitro drug release from the gold nanoparticles loaded hesperetin and pure hesperetin was studied in phosphate buffered saline solution (PBS) at pH 7.4. Au-mPEG(5000)-S-HP micelles (4 mg/ml), dispersed in PBS solution, were sealed in dialysis bag (MW cut-off: 12–16 kDa), and incubated in the release medium (25 ml) at 37 °C under oscillation at 90 r/min. To measure the drug release content, samples (1 ml) were periodically removed and replaced with an equivalent volume of the phosphate buffer solution. The amount of pure hesperetin and gold nanoparticles loaded hesperetin was analyzed with a spectrophotometer at 289 nm in triplicate.
2.5. Biocompatibility study of pure hesperetin (HP) and gold nanoparticles loaded hesperetin
The biocompatibility study of pure HP and Au-mPEG(5000)-S-HP NPs was analyzed in rats by giving 20 mg/kg b.wt of pure HP and 0.5 ml of Au-mPEG(5000)-S-HP NPs were given by intraperitoneally for 21 d, the rats were scarified by cervical decapitation and the tissues of liver samples and kidney samples were investigated by histophathological examination.
2.6. Preparation of slides for mast cell staining, PAS staining, AgNOR staining and immunohistochemistry
Mast cells, glycoconjugates, argyrophilic nucleolar organizing region (AgNOR), and immunohistochemistry were performed on a lobe of the liver for groups 1, 3 and 5. For groups 2, 4 and 6 both tumorous and non-tumorous portions (i.e., the number of sections prepared for groups 2, 4, and 6 are 12 each, whereas for groups 1, 3 and 5 it was 6 each) of the liver will be fixed in 10% formalin and embedded in paraffin wax, and the sections were cut 5 µm thick and then stained.
2.7. Mast cell staining
Histochemical analysis of mast cell was carried out by the method mentioned in [23]. Briefly, the 5 µm thickness sections were dewaxed in xylene and rehydrated through decreasing concentrations of ethanol until distilled water. The sections were stained with toluidine blue for 2 min and washed with distilled water, then stained using light green SF for 30 s, washed using distilled water, dehydrated in increasing concentrations through alcohol series, and mounted using DPX. High power objective field (40×) was chosen for counting the total number of mast cells in ten different fields/slides. The number of ruptured cells and intact cells per field was noted. The ruptured cells were easily identified by their irregular shape and by their discharged granules.
2.8. Periodic acid Schiff's staining (PAS)
Liver sections were deparaffinized in xylene, using three changes of fresh xylene, 10 min each and further rehydrated gradually through descending grades of alcohol (95%, 90%, 70%, 50%, and 30%) for 5 min each. Finally the sections were hydrated with distilled water. Selected sections were placed in 2% periodic acid for 5 min then rinsed in distilled water. To these sections, Schiff's reagent was added and kept in dark for 20 min. The sections were then rinsed in distilled water for 10 min and counterstained with hematoxylin for 5 min and then washed in running tap water for 5 min or distilled water. Then dehydrated gradually in ascending grades of alcohol (70%, 75% and 100%) for 5 min each. Then the sections were mounted in DPX (mount) with cover slips and allowed to dry [24].
2.9. Argyrophilic nucleolar organizing regions (AgNORs) staining
AgNOR staining was performed according to the method used in Ref. [25]. Sections of 5 µm, obtained from each paraffin block were stained by the one-step silver colloid method. Briefly, the sections were dewaxed in xylene and rehydrated through decreasing concentrations of ethanol to distilled, deionized water. The AgNOR solution was freshly prepared by dissolving gelatin at a concentration of 2 g/dl in 1 g/dl aqueous formic acid. This solution was added to 50 g/dl aqueous silver nitrate solution (1:2, v/v). The final solution was then immediately poured on to the slides, which were left in the dark at room temperature for 45 min. The silver colloid was washed from the sections with distilled, deionized water and the sections were dehydrated through a graded series of ethanol to xylene. The AgNOR dots were easily identified as black points within the nuclei. The results were expressed in terms of number of AgNORs/nuclei in each group.
2.10. Immunohistochemical analysis of TNF-α and PCNA
Briefly, the tissue sections were deparaffinized in two changes of xylene at 60 °C for 20 min each and hydrated through a graded series of alcohol, the slides were incubated in citrate buffer (pH 6.0) for three cycles of five min each in a microwave oven for antigen retrieval. The sections were then allowed to cool at room temperature and then rinsed with 1× Tris buffered saline (TBS), and treated with 0.3% H2O2 in methanol for 10 min to block endogenous peroxidase activity. Non-specific binding was blocked with 3% BSA in room temperature for 1 h. The sections were then incubated with the following primary antibodies: TNF-α and PCNA (Spring Bioscience, USA) rabbit polyclonal antibodies at a dilution of 1:50 and 1:100 at 4 °C overnight. The slides were washed with TBS and then incubated with anti-rabbit HRP labeled secondary antibody (Genei, Bangalore, India) at a dilution of 1:500 for 1 h in room temperature. The peroxidase activity was visualized by treating the slides with 3, 3'-diaminobenzidine tetrahydrochloride (SRL, Mumbai, India); the slides were counterstained with Meyer's hematoxylin. Negative controls were incubated with TBS instead of primary antibodies.
2.11. Protein extraction and immunoblotting analysis of TNF-α, NFκB and PCNA
Liver tissues of control and experimental groups of rats were homogenized in 135 mM NaCl, 20 mM Tris, 2 mM EDTA, and 1 Mm PMSF (pH 7.4). The homogenate was centrifuged (15 min, 12,000 × g at 4 °C), and then protein content of the supernatant was determined. Aliquots of supernatant (30 µg total protein) were boiled for 5 min in sample buffer (0.2 M Tris–HCL buffer, 10% glycerol, 2% SDS, 0.02% β-mercaptoethanol). Equal amount of protein from each of the samples was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gel and transferred electrophoretically to nitrocellulose membrane (Amersham Biosciences, NJ). The membrane was blocked with 5% BSA in Tris-Tween buffered saline at room temperature for 1 h. The membrane was then incubated with TNF-α, NFκB and PCNA (Spring Bioscience, USA) rabbit polyclonal antibodies at a dilution of 1:1000 and 1:500 and β-actin (Sigma) mouse monoclonal antibody at a dilution of 1:2000 overnight at 4 °C. The membrane was then incubated with corresponding horseradish peroxidase conjugated secondary antibody for 1 h. Protein antibody complexes were detected by the addition of diaminobenzidine tetrahydrochloride (SRL, Mumbai) as a substrate. The membrane was photographed and quantitated with image analysis software.
2.12. Statistical analysis
Data were evaluated with SPSS/software version 10. Hypothesis testing methods included one-way analysis of variance (ANOVA) followed by least significance difference (LSD) test. Statistical significance was defined as P values less than 0.05. All the results were expressed as mean ± standard deviation.
3. Results and discussion
It is well known that flavonoids act as general cell growth inhibitors [26]. This biological capacity of flavonoids suggests their potential use in cancer chemotherapy. The usage of anticancer drugs is limited due to short plasma half-life and common side effects such as systemic toxicity due to high dosage and no specificity with respect to healthy cells and low bioavailability. Hesperetin (HP) has the similar problem of poor bioavailability and solubility in water and thus restricting its use in chemotherapy. Nanoparticles possess the ability to improve the solubility and permeabilize the cells more efficiently than microspheres due to their smaller size, which facilitates administration of large quantities of drugs to give better efficacy. Encapsulation of anticancer drugs in polymeric nanoparticles could potentially overcome both P-glycoprotein (P-gp) mediated drug efflux and first pass metabolism [27]. In our previous report [28] we concluded that the hesperetin conjugated PEGylated gold nanoparticles improves solubility and shows better chemopreventive against DEN-induced HCC in rats. In the present study, the hesperetin conjugated PEGylated gold nanoparticles improves the drug bioavailability and shows better antioxidant that possesses anti-inflammatory and anti-proliferation properties during diethylnitrosamine (DEN)-induced hepatocarcinogenesis in male Wistar albino rats.
3.1. Characterization of Au-mPEG(5000)-SH NPs and Au-mPEG(5000)-S-HP NPs
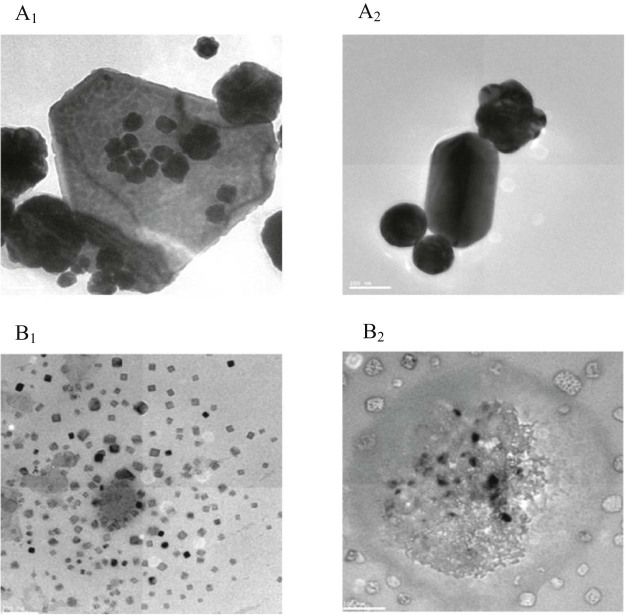
The high resolution transmission electron microscopy (HR-TEM) image of Au-mPEG(5000)-SH NPs and Au-mPEG(5000)-S-HP nanoparticles were shown in Fig. 1. From the images it is observed that the gold nanoparticles are spherical, triangular and pentagon in shape. Fig. A1 and A2 shows the average size of Au-mPEG(5000)-SH nanoparticles is 220 nm. The size of the gold nanoparticles which is embedded in the thiol functiolized poly ethylene glycol polymer with diameter 300 nm that is, they form a core shell type where the gold nanoparticle forms the core which is surrounded by the polymer coating thus stabilizing the nanometal and preventing from agglomeration and Fig. B1 and B2 shows the average size of Au-mPEG(5000)-S-HP NPs is 110–120 nm in size respectively. When compared with the control (Au-mPEG(5000)-SH), the size of the nanoparticles will be reduced due to the addition of the hesperetin. Generally flavonoid, it has the properties of reduction of gold ions [29]. Huang et al. reported that various polyols and terpenoids were responsible for the generation and stabilization of NPs and its play the major role in bio-reduction [30], [31]. The EDAX spectrum of Au-mPEG(5000)-SH and Au-mPEG(5000)-S-HP highlights the presence of gold in addition to the carbon and oxygen alone increases in drug loaded polymer AuNPs. This confirms that the drug HP is loaded on the polymer coated gold nanoparticles.
Fig. 1.
HR-TEM image of (A1) and (A2) shows the different shape and size of gold nanoparticles stabilized with polymer (mPEG(5000)-SH). Whereas the (B1) and (B2) shows the different shape and size of gold nanoparticles stabilized with polymer and encapsulated hesperetin drug (Au-mPEG(5000)-S-HP).
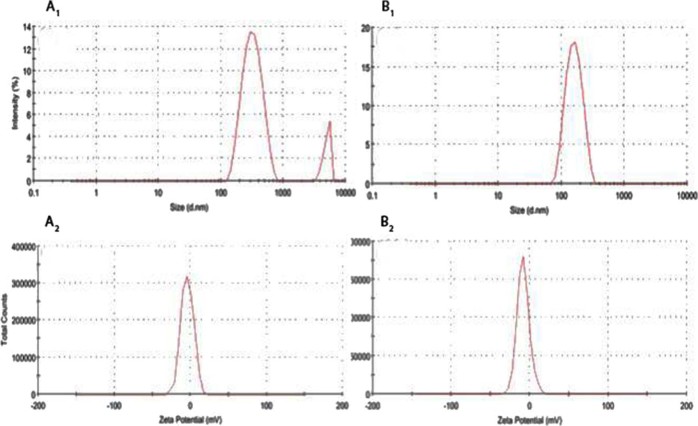
Fig. 2 shows the size of Au-mPEG(5000)-SH and Au-mPEG(5000)-S-HP nanoparticles were determined using DLS and Zeta potential. The Fig. 2 (A1) shows the size distribution graph of Au-mPEG(5000)-SH narrow area peaks shows the homogeneity of the NPs formed and the average diameter of Au-mPEG(5000)-SH NPs is 320 nm. Fig. 2 (B1) peak shows the nanoparticles formed and the average diameter of Au-mPEG(5000)-S-HP NPs is 150 nm. Likewise the Zeta potential distribution of Fig. 2 (A2) of Au-mPEG(5000)-SH NPs shows negative charge greater than −4.38 mV and the Fig. (B2) Au-mPEG(5000)-S-HP NPs shows negative charge greater than −8.42 mV.
Fig. 2.
Dynamic light scattering measurement (A1 & B1) and Zeta potential distribution (A2 & B2) graph of Au-mPEG(5000)-SH NPs and Au-mPEG(5000)-S-HP NPs.
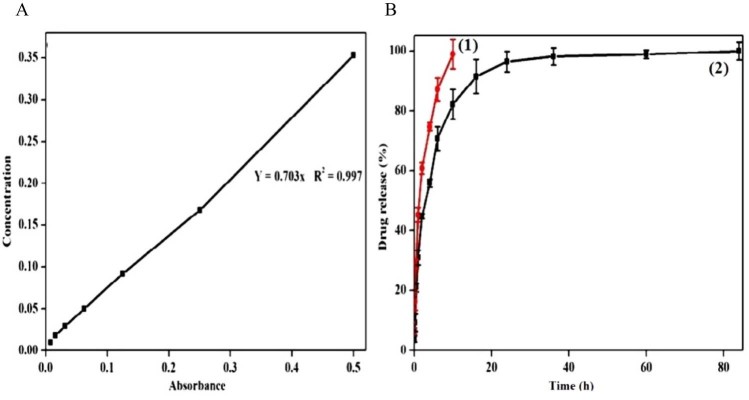
The in vitro drug release profile of Au-mPEG(5000)-S-HP NPs was illustrated in Fig. 3 as compared with standard graph Fig. 3(A) of hesperetin drug. Fig. B(1) showed that pure hesperetin (HP) was released to the extent of 99% within 8 h, the same as reported in Kuntal Maiti et al. [32]; when compared with the pure hesperetin drug. Fig. 3 B(1), shows the sudden release within 10 h whereas in Fig. 3 B(2) Au-mPEG(5000)-S-HP NPs can be sustained and persisted release for 72 h. The result produced by the hesperetin loaded AuNPs may be a combined effect of sustained release property. It shows that more than 80% of HP was released from AuNPs for 72 h suggested the potential of the nanoparticles as a sustained drug delivery system. Other antidiabetic drugs e.g. rosiglitazone loaded gelatin nanoparticles have similar sustained release behavior from drug loaded nanoparticles [33]. These results indicated that the Au-mPEG(5000)-S-HP NPs could be a good candidate for drug carriers.
Fig. 3.
In vitro release study of (A) hesperetin (HP) from B(1) pure hesperetin (HP) suspension and B(2) hesperetin loaded gold nanoparticles (Au-mPEG(5000)-S-HP NPs) at different time points. Values are mean ± SEM (n = 3).
3.2. Biocompatibility study of pure hesperetin (HP) and Au-mPEG(5000)-S-HP nanoparticles
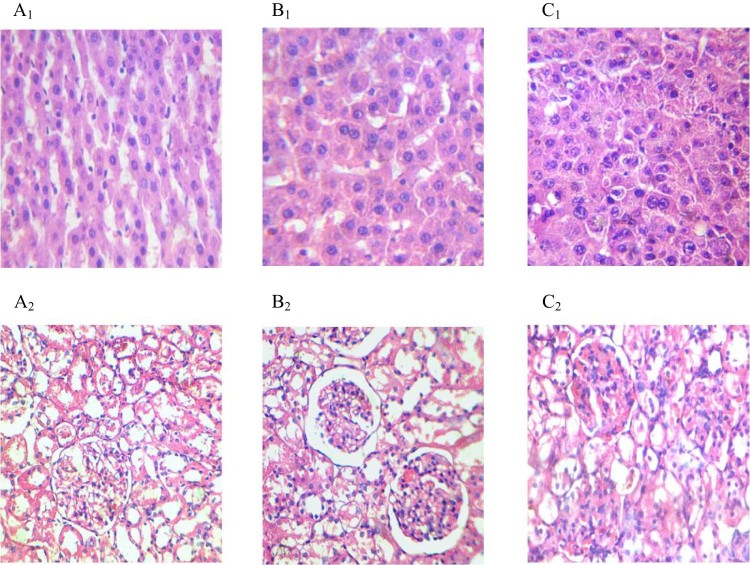
Fig. 4 shows the histopathological study of liver and kidney tissues for checking the biocompatibility of pure hesperetin (HP) and Au-mPEG(5000)-S-HP NPs. Fig. 4 shows no cellular architectural changes were observed in (A1) (A2) as control, (B1) (B2) as pure HP and (C1) (C2) as Au-mPEG(5000)-S-HP NPs of liver and kidney images. Both liver tissue and kidney tissue have similar architecture and these clearly concluded that no toxicity was observed in pure HP and Au-mPEG(5000)-S-HP NPs. These results prove that the polymer encapsulated gold nanoparticles should be a biocompatible and no toxicity was proved.
Fig. 4.
Histological alterations in the liver and kidney at 21 d after administration of pure HP and Au-mPEG(5000)-S-HP nanoparticles (H&E staining, 40×). Both (A1) and (A2) photomicrograph of liver and kidney section of control rat demonstrated normal cellular structure. Both (B1) and (B2) sections of liver and kidney of pure HP drug at 20 mg/kg b.wt show normal cellular architecture and both (C1) and (C2) section of liver and kidney of Au-mPEG(5000)-S-HP nanoparticles drug at 0.5 ml depicted normal cellular architecture.
3.3. Au-mPEG(5000)-S-HP NPs reduces mast cell density in DEN-induced rat hepatocarcinogenesis
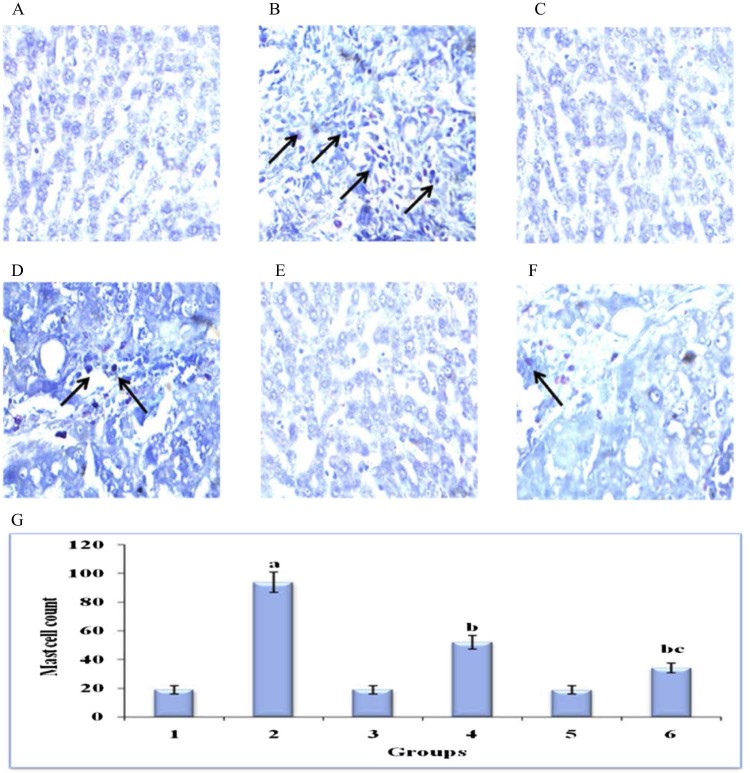
Fig. 5 displays the histochemical staining for mast cells by toluidine blue method in the liver of control and experimental groups of rats. DEN induced (group 2) animals showed significant increase in the number of mast cells (can be termed as mast cell density or MCD) when compared with (group 1) control animals. The treatment in Au-mPEG(5000)-S-HP NPs (group 6) animals showed significant decrease in mast cell density than the pure hesperetin (group 4) animals when compared with (group 2) animals. The hesperetin alone treated (group 3) animals and Au-mPEG(5000)-S-HP NPs treated (group 5) animals did not show any significant changes and proves that the gold nanoparticles should be biocompatible.
Fig. 5.
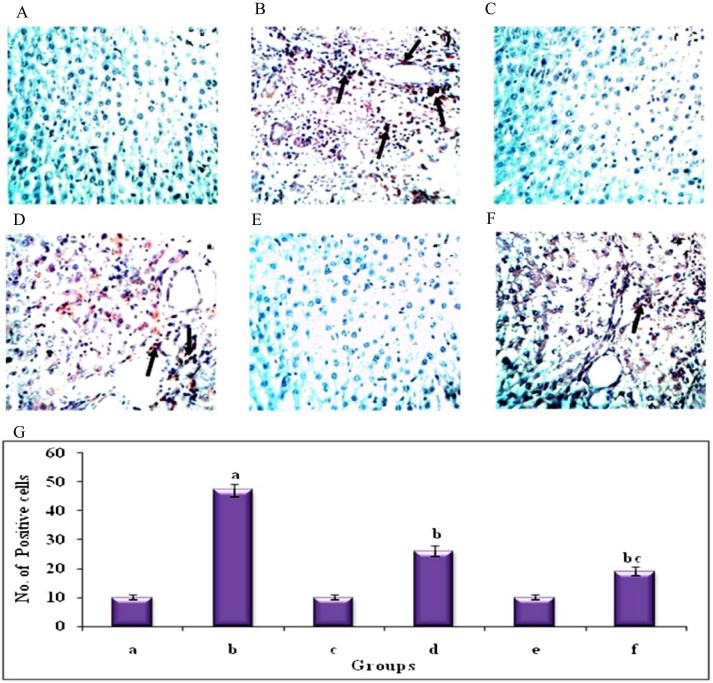
Histochemical analysis of mast cells by toluidine blue in the liver of control and experimental groups of animals (40×). Plate's a–f corresponds to the liver sections of groups 1–6, respectively, (A) Control group. (B) DEN-induced group. (C) Hesperetin alone treated group. (D) DEN + hesperetin treatment group. (E) Au-mPEG(5000)-S-HP NPs alone treated group. (F) DEN + Au-mPEG(5000)-S-HP NPs treated group. (G) Quantitative analysis of total mast cell count. Results are expressed as mean ± SD for six rats in each group. Statistical significance at P < 0.05 compared with agroup 1, bgroup 2 and cgroup 4. Arrow represents mast cells.
Inflammation is one of the important factors for tumor progression and one of hallmarks of cancer. Mast cells are derived from pluripotent hematopoietic stem cells in the bone marrow, which leave and circulate as immature cells only; upon reaching their destination they mature [34]. Hepatic mast cells consistently increase in number with progression of various liver diseases. Studies of cirrhosis, fibrosis, hepatitis and other cholangiopathies demonstrate that, histologically, hepatic mast cell counts generally increase as diseases progress implicating a significant role for mast cells in hepatic disorders [35]. Mast cells can secrete several proangiogenic factors, which can jump-start tumor angiogenesis switch [36] and they have been shown to accumulate in tissues undergoing angiogenesis during tumor growth [37]. Mast cell accumulation has been shown in liver cancer [38] suggesting a role for these cells in tumor immunology. In line with above findings, our results demonstrated an increased level of toluidine blue stained mast cells in the liver section of DEN induced tumor bearing animals, whereas Au-mPEG(5000)-S-HP nanoparticles treated animals shows significantly decreased levels of mast cells in the liver. This shows that the PEGylated gold nanoparticles loaded hesperetin is biocompatible and also shows the better anti-inflammatory effects than the pure hesperetin.
3.4. Anti-proliferative potential of Au-mPEG(5000)-S-HP NPs as revealed by decreased expression of inflammative markers TNF-α and NF-κB in the liver tissue of DEN-induced rats
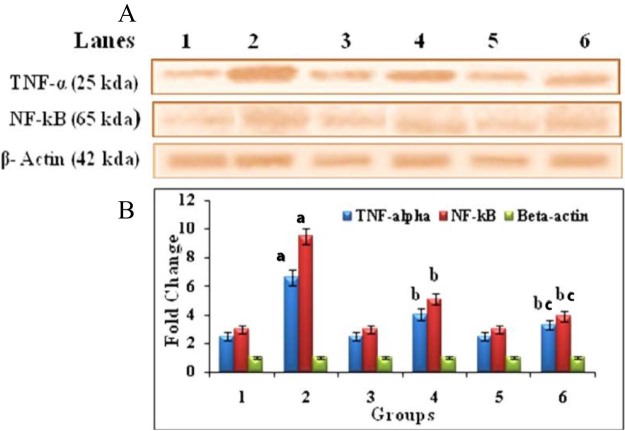
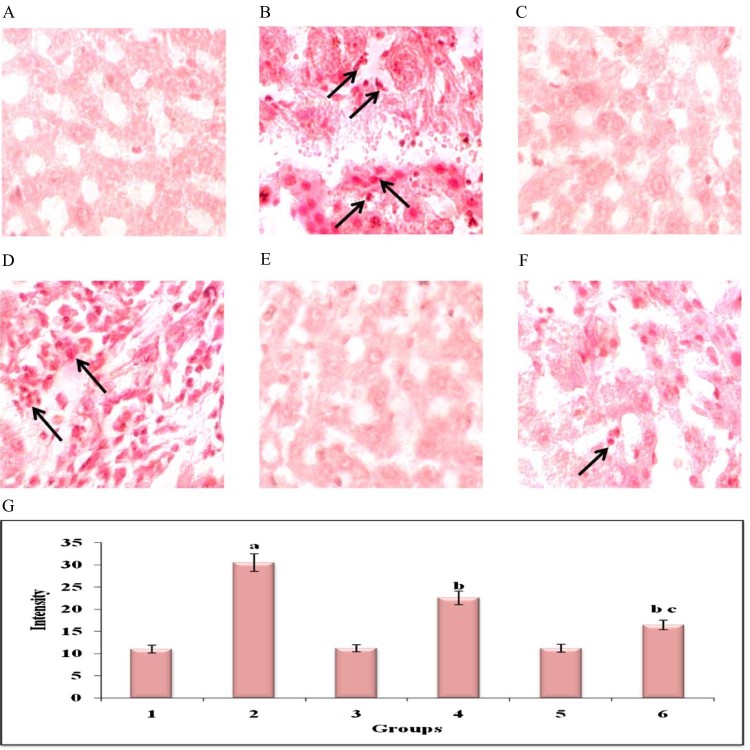
Fig. 6 shows the immunohistochemisty and quantitative analysis to confirm the protein expression levels of TNF-α in liver of control and experimental groups of animals. The expression of TNF-α was significantly (P < 0.05) high in DEN-induced (group 2) animals compared to normal control (group 1) animals. HP treated (group 4) animals showed significantly (P < 0.05) reduce the levels of TNF-α expression when compared with (group 2) animals. Whereas Au-mPEG(5000)-S-HP NPs treatment (group 6) animals caused a drastic (P < 0.05) reduction in the protein expression than the HP treated (group 4) animals when compared with DEN induced (group 2) animals. Fig. 7 reveals the immunoblotting and densitometric analysis to confirm the inflammation protein expression levels of TNF-α, NF-κB and β-actin in control and experimental groups of animals. The DEN-induced (group 2) tumor bearing animals showed there was an increase in significant (P < 0.05) expression levels of TNF-α and NF-κB. The treatment in HP (group 4) animals showed there was a significant (P < 0.05) decrease in the levels of these protein where as the Au-mPEG(5000)-S-HP NPs treated (group 6) animals showed there was much more significant (P < 0.05) decrease than the treatment with HP (group 4) animals when compared with (group 2) animals.
Fig. 6.
Immunohistochemical analysis of TNF-α in the liver of control and experimental groups of animals (40Χ). Plate's a–f corresponds to the liver sections of groups 1–6, respectively, (A) Control group. (B) DEN-induced group. (C) hesperetin alone treated group. (D) DEN + hesperetin treatment group. (E) Au-mPEG(5000)-S-HP NPs alone treated group. (F) DEN + Au-mPEG(5000)-S-HP NPs treated group. (G) Quantitative analysis of TNF-α expression. The number of stained cells/100 cells was counted across ten fields for each slide. Results are expressed as mean ± SD for six rats in each group. Statistical significance at P < 0.05 compared with agroup 1, bgroup 2 and cgroup 4. Arrow represents TNF-α positive stained cells.
Fig. 7.
Western blot analysis of TNF-α, NF-kB and β-Actin in the liver of control and experimental groups of animals. (A) Lane 1 – Control, Lane 2 – DEN induced group, Lane 3 – hesperetin alone treated group, Lane 4 – DEN + hesperetin treatment group, Lane 5 – Au-mPEG(5000)-S-HP NPs alone treated group, Lane 6 – DEN + Au-mPEG(5000)-S-HP NPs treated groups (B) Densitometric analysis of TNF-α, NF-kB and β-Actin in control and experimental groups of animals. Results are expressed as mean ± SD for six rats in each group. Statistical significance at P < 0.05 compared with agroup 1, bgroup 2 and cgroup 4.
TNF-α is a key regulator of the immune and inflammatory responses to cancer with important functions in pro-inflammatory cytokine, that induces hepatocyte apoptosis and necrosis through the activation of caspase-3, and thereby leads to liver cell DNA shift [39] and various inflammatory liver diseases [40]. Serum and hepatic TNF-α levels are increased in patients with acute and chronic viral hepatitis, alcoholic hepatitis, and NASH [41]. Existing evidence indicates that high levels of TNF-α can favor cell survival and tumor progression [42]. Their expression is mainly regulated by the transcription factor NF-kB, which is constitutively active in most tumors and is induced by carcinogens and chemotherapeutic agents. Nuclear factor-kappa B (NF-kB) is a ubiquitous transcription factor that is activated by various cytokines and mitogens, and is a key regulator in the inflammatory response infection [43]. The activation of NF-kB has been proven to play an important role in enhancing the expression of several inflammatory cytokine genes, including TNF-α, IL-6 and IL-8 [44]. TNF-α induced NF-kB had been extensively studied in line with anti-apoptotic activity in many cancers [45]. Under normal circumstances death factors such as TNF-α and Fas ligand (FasL) induce apoptosis and can cause tissue destruction [46]. But in tumor cells TNF-α binding to the TNF receptor potentially initiates both apoptosis and activates NF-kB, which suppresses apoptosis by induction of NF-kB responsive genes including inhibitor of apoptotic proteins [47]. In our present study, the immunohistochemical and western blotting analysis of DEN induced animal tissues showed up-regulation of TNF-α and NF-kB expression when compared with control rats. Whereas, Au-mPEG(5000)-S-HP nanoparticles treated animals, showed the down-regulated protein expression of TNF-α and NF-kB which clearly indicates that PEGylated gold nanoparticles loaded hesperetin act as an anti-inflammatory agent and suppresses the HCC induced by DEN than the pure hesperetin treatment.
3.5. Au-mPEG(5000)-S-HP NPs reduces the glycoconjugates level in DEN-induced rat hepatocarcinogenesis
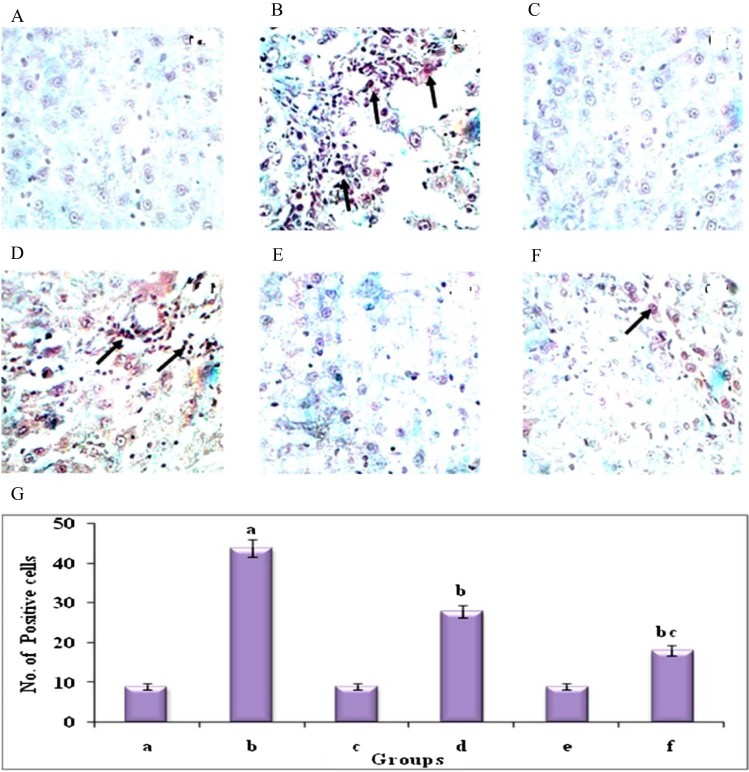
Fig. 8 shows the periodic Schiff's staining for glycoconjugates in the liver of control and experimental groups of animals. DEN induced (group 2) animals showed increased in the levels of glycoprotein and showed increased intensity of staining for glycoconjugates when compared with (group 1) control animals. The Au-mPEG(5000)-S-HP NPs treated (group 6) animals showed considerable decrease in the intensity of staining than in the treatment of HP (group 4) animals. This shows that the Au-mPEG(5000)-S-HP NPs should have reduced the cell proliferation.
Fig. 8.
Histochemical analysis of Glycoconjugates by PAS staining in the liver of control and experimental groups of animals (40×). Plate's a–f corresponds to the liver sections of groups 1–6, respectively, (A) Control group. (B) DEN-induced group. (C) Hesperetin alone treated group. (D) DEN + hesperetin treatment group. (E) Au-mPEG(5000)-S-HP NPs alone treated group. (F) DEN + Au-mPEG(5000)-S-HP NPs treated group. (G) Quantitative analysis of intensity of staining for glycoconjugates. Arrow depicts glycoconjugates expression.
Cell proliferation is thought to play an important role in several steps of the carcinogenic process. Increase in the level of glycoconjugates shows the nature of proliferation in various tumors. Glycoconjugates are necessary for the assembly of the oligosaccharide moieties of the glycoprotein chains and their levels have been found to be elevated in neoplastic conditions and can therefore be designated as non-specific markers of malignancy [48]. Its levels are high in tumor tissue due to increased lipid peroxidation resulting in lowered antioxidant status [49] and aberrant glycosylation [50]. Elevations of glycoprotein components serve as a classical marker and as an indicator in the progression of tumor growth. In line with the findings, our results showed similar changes with an increase in the amount of glycoprotein level in DEN induced liver tissues which was confirmed by histochemical analysis using PAS staining. Whereas the Au-mPEG(5000)-S-HP nanoparticles treated animals, shows arbitrary decrease in the amount of glycoprotein level when compared to DEN induced cancer bearing animals. More over the results showed there was a high decrease in the level of glycoprotein than the pure hesperetin treated animals which show the PEGylated gold nanoparticles loaded hesperetin should be a biocompatible and promising anti-proliferative nature during liver carcinogenesis.
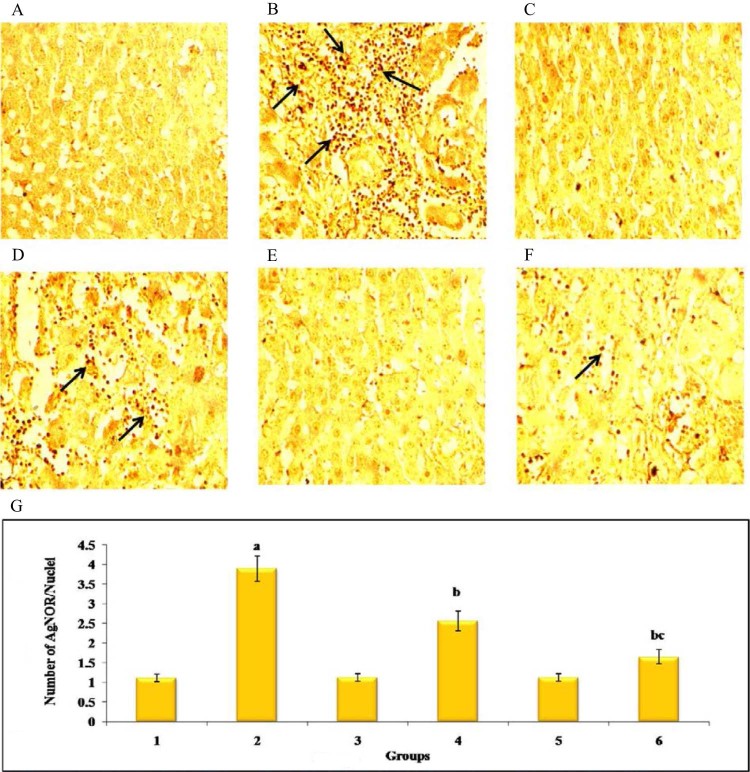
Fig. 9 shows immunohistochemical staining of PCNA in the liver of control and experimental group of animals. DEN-induced (group 2) showed a significant increase in the number of PCNA positive nuclei when compared with (group 1) normal control animals, while Au-mPEG(5000)-S-HP NPs treated (group 6) the number of PCNA positive nuclei in animals notably decreased more than in the HP treated (group 4) animals when compared with (group 2) animals. Fig. 10 represents the immunoblot analysis to confirm the protein expression levels of PCNA and β-actin, respectively, in the liver of control and experimental animals. DEN-induced (group 2) animals showed that there was a significant (P < 0.05) increase in expression levels of PCNA. The treatment in HP (group 4) animals showed there was a significant (P < 0.05) decrease in the levels of these protein, where as the Au-mPEG(5000)-S-HP NPs treated (group 6) animals showed there was much more significant (P < 0.05) decrease in the expression level than the treatment with HP (group 4) animals when compared with (group 2). It is clearly revealed that antiproliferative effect of gold nanoparticles loaded HP against DEN-induced hepatocellular carcinogenesis. Fig. 11 shows the levels of AgNORs in the liver of control and experimental animals. Tumor-induced (group 2) animals showed a significant increase in the number of the AgNORs/nuclei when compared with (group 1) normal control animals. Whereas in Au-mPEG(5000)-S-HP NPs treated (group 6) animals (slide F) showed more reduction in the levels of AgNORs than the pure hesperetin treatment (group 4) animals. This could be reason of inhibition or arrest of some degree of cell proliferation by PEGylated gold nanoparticles coated hesperetin may contribute more decrease in tumor formation. The (group 1) control animals (slide A), Hesperetin alone treated (group 3) animals (slide C) and Au-mPEG(5000)-S-HP NPs treated (group 5) animals (slide E) did not show any significant changes animals which show the PEGylated gold nanoparticles loaded hesperetin should be a biocompatible.
Fig. 9.
Immunohistochemical analysis of PCNA in the liver of control and experimental groups of animals (40×). Plate's a–f corresponds to the liver sections of groups 1–6, respectively, (A) Control group. (B) DEN-induced group. (C) hesperetin alone treated group. (D) DEN + hesperetin treatment group. (E) Au-mPEG(5000)-S-HP NPs alone treated group. (F) DEN + Au-mPEG(5000)-S-HP NPs treated group. (G) Quantitative analysis of PCNA expression. The number of stained cells/100 cells was counted across ten fields for each slide. Results are expressed as mean ± SD for six rats in each group. Statistical significance at P < 0.05 compared with agroup 1, bgroup 2, and cgroup 4. Arrow represents PCNA positive cells.
Fig. 10.
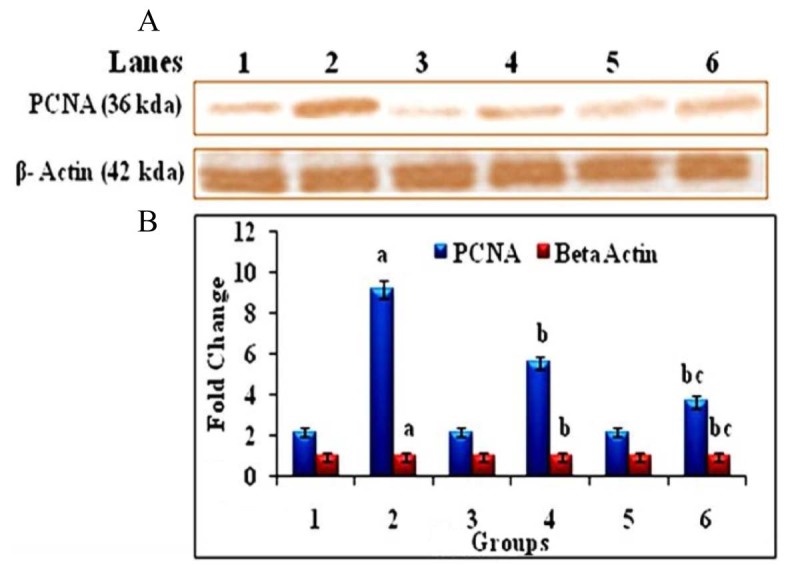
Western blot analysis of PCNA and β-Actin in the liver of control and experimental groups of animals. (A) Lane 1 – Control, Lane 2 – DEN induced group, Lane 3 – hesperetin alone treated group, Lane 4 – DEN + hesperetin treatment group, Lane 5 – Au-mPEG(5000)-S-HP NPs alone treated group, Lane 6 – DEN + Au-mPEG(5000)-S-HP NPs treated groups (B) Densitometric analysis of PCNA and β-Actin in control and experimental groups of animals. Results are expressed as mean ± SD for six rats in each group. Statistical significance at P < 0.05 compared with agroup 1, bgroup 2, and cgroup 4.
Fig. 11.
AgNOR analysis in liver of control and experimental group of animals (40×). Plate's A–F represents the liver sections of groups 1–6 of experimental animals, respectively. Plate G shows the bar graph representing the number of AgNORs/nuclei in 10 different fields in control and experimental groups. Results are expressed as mean ± SD for six rats in each group, P < 0.05 compared with agroup 1, bgroup 2 and cgroup 4. Arrow represents AgNOR positive cells.
Proliferative cell nuclear antigen (PCNA) is a nuclear protein related to cell proliferation. It is a 36 kDa nuclear protein and its expression in the nucleus is associated with the DNA synthesis phase of cell cycle, and serves as a biomarker for proliferation [51]. It is an accessory protein to DNA polymerase-γ and plays an important role in the activation of DNA replication. An increase in PCNA's expression indicates that the cells are highly proliferative [52]. Their high expression suggested that the ability of cell proliferation became stronger, and this was closely related to malignant cell proliferation and carcinogenesis [53]. Elevated expression of PCNA in the liver of DEN-administered animals is considered as a common index for hepatocyte proliferation at late G1- and early S-phase [54]. In the present study, PEGylated gold nanoparticles loaded hesperetin-mediated suppression of PCNA expression in immunohistochemical and immunoblotting analysis with pre-neoplastic rat liver may reflect its anti-proliferative potentials in vivo. Argyrophilic nucleolar organizer region (AgNOR) proteins is a group of proteins that are associated with the nucleolar organizer regions and are selectively stained by silver staining [55]. The amount of AgNOR proteins can be used as a marker for cell proliferation [56]. In the interphasic nuclei, AgNOR staining usually results in black dots granules located in the nucleoli. The intensity of staining depends on the transcriptional activity of the cells [57]. More recently, it was suggested that the amount of silver-stained proteins is directly proportional to cell proliferation activity [58]. In the present study, the amounts of AgNOR were significantly increased in DEN-induced liver cancer bearing animals, which indicates the hyper-proliferative activity of tumor cells [59]. Au-mPEG(5000)-S-HP nanoparticles treatment significantly reduced the amount of AgNORs, when compared with DEN-induced cancer bearing animals suggesting that PEGylated gold nanoparticle loaded hesperetin could be a potent inhibitor of cancer cell proliferation.
4. Conclusion
In conclusion, the results of present study conclusively demonstrate that the PEGylated gold nanoparticle loaded hesperetin attenuates hepatocellular carcinoma by inhibiting cell inflammation and cell proliferation. The expression of inflammatory markers (TNF-α, NF-kB) and proliferative marker (PCNA) was found to be down regulated in Au-mPEG(5000)-S-HP nanoparticles treated animals than in the treatment of pure hesperetin, which proves that the PEGylated gold nanoparticles loaded hesperetin is be a better anti-inflammatory and anti-proliferative agent during liver carcinogenesis. Therefore, the PEGylated gold nanoparticles encapsulated hesperetin drug possesses as a powerful tool in enhancing the better treatment of HCC, to minimize the side effects and reduce the dose of chemotherapy drug.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
The author K. Gokuladhas thanks Indian Council of Medical Research, New Delhi, India, for the financial support provided in the form of Senior Research Fellowship (SRF-3/2/2/156/2011/NCD-III).
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Ahmedin Jemal D.V.M., Rebecca Siegel M.P.H., Murray T. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H.B. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2007;127:27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G., Stroffolini T., Zagni I. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Fausto N. Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin Liver Dis. 1999;19:243–252. doi: 10.1055/s-2007-1007114. [DOI] [PubMed] [Google Scholar]
- 5.Maeda S., Kamata H., Luo J.L. IK K beta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121(7):977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan B.P., Meyer T.J., Stershic M.T. Acceleration of N-nitrosation reactions electrophiles. IARC Sci Publ. 1991;105:370–374. doi: 10.1002/chin.199210322. [DOI] [PubMed] [Google Scholar]
- 7.Kapadia G.J., Azuine M.A., Takayasu J. Inhibition of Epstein–Barr virus early antigen activation promoted by 12-O-tetradecanoylphorbol-13-acetate by the non-steroidal anti-inflammatory drugs. Cancer Lett. 2006;161:221–229. doi: 10.1016/s0304-3835(00)00616-9. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz K.A., Potter J.D. Vegetables, fruits and cancer, I. Epidemiology. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 9.Rafter J.J. Scientific basis of biomarkers and benefits of functional foods for reduction of disease risk: cancer. Br J Nutr. 2002;88(2):S219–S224. doi: 10.1079/BJN2002686. [DOI] [PubMed] [Google Scholar]
- 10.Loo G. Redox-sensitive mechanisms of phytochemical mediated inhibition of cancer cell proliferation. J Nutr Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Izquierdo A., Gil M.I., Ferreres F. In vitro availability of flavonoids and other phenolics in orange juice. J Agric Food Chem. 2001;49:1035–1041. doi: 10.1021/jf0000528. [DOI] [PubMed] [Google Scholar]
- 12.Galati E.M., Monforte M.T., Kirjavainen S. Biological effects of hesperidin, a citrus flavonoid. (Note I): antiinflammatory and analgesic activity. Farmaco. 1994;40(11):709–712. [PubMed] [Google Scholar]
- 13.Garg A., Garg S., Zaneveld L.J. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother Res. 2001;15(8):655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 14.Galati E.M., Trovato A., Kirjavainen S. Biological effects of hesperidin, a citrus flavonoid (Note III): antihypertensive and diuretic activity in rat. Farmaco. 1996;51(3):219–221. [PubMed] [Google Scholar]
- 15.Ficarra R., Tommasini S., Raneri D. Study of flavonoids/beta-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J Pharm Biomed Anal. 2002;29(6):1005–1014. doi: 10.1016/s0731-7085(02)00141-3. [DOI] [PubMed] [Google Scholar]
- 16.Maiti K., Mukherjee K., Murugan V. Exploring the effect of hesperetin-HSPC complex – a novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech. 2009;10(3):943. doi: 10.1208/s12249-009-9282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fathi M., Varshosaz J., Mohebbi M. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: preparation, characterization, and modeling. Food Bioprocess Technol. 2013;6(6):1464–1475. [Google Scholar]
- 18.Wang J., Zhou N., Zhu Z. Detection of flavonoids and assay for their antioxidant activity based on enlargement of gold nanoparticles. Anal Bioanal Chem. 2007;388:1199–1205. doi: 10.1007/s00216-007-1295-y. [DOI] [PubMed] [Google Scholar]
- 19.Paciotti G.F., Kingston D.G.I., Tamarkin L. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev Res. 2006;67:47–54. [Google Scholar]
- 20.Sardar R., Park J.-W., Shumaker-Parry J.S. Polymer-induced synthesis of stable gold and silver nanoparticles and subsequent ligand exchange in water. Langmuir. 2007;23:11883. doi: 10.1021/la702359g. [DOI] [PubMed] [Google Scholar]
- 21.Barnes K.A., Karim A., Douglas J.F. Suppression of dewetting in nanoparticle-filled polymer films. Macromolecules. 2000;33:4177. [Google Scholar]
- 22.Kim B.J., Bang J., Hawker C.J. Effect of areal chain density on the location of polymer-modified gold nanoparticles in a block copolymer template. Macromolecules. 2006;39:4108. [Google Scholar]
- 23.Ranieri G., Labriola A., Achille G. Microvessel density mast cells and thymidine phosphorylase expression in oral squamous carcinoma. Int J Oncol. 2002;21:1317–1323. [PubMed] [Google Scholar]
- 24.Meloan S.N., Valentine L.S., Puchtler H. On the structure of carminic acid and carmine. Histochem Cell Biol. 1971;27:87–95. doi: 10.1007/BF00284950. [DOI] [PubMed] [Google Scholar]
- 25.Aubele M., Biesterfeld S., Derenzini M. Guidelines of AgNOR quantitation. Committee on AgNOR quantitation within the European Society of Pathology. Zentralbl Pathol. 1994;140:107–108. [PubMed] [Google Scholar]
- 26.Singh R.P., Agarwal R. Natural flavonoids targeting deregulated cell cycle progression in cancer cells. Curr Drug Targets. 2006;7:345–354. doi: 10.2174/138945006776055004. [DOI] [PubMed] [Google Scholar]
- 27.Pandey R., Zahoor A., Sharma S. Nano-encapsulation of azole antifungals: potential applications to improve oral drug delivery. Int J Pharm. 2005;301:268–276. doi: 10.1016/j.ijpharm.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Gokuladhas K., Jayakumar S., Rajan B. Exploring the potential role of chemopreventive agent, hesperetin conjugated pegylated gold nanoparticles in diethylnitrosamine-induced hepatocellular carcinoma in male Wistar albino rats. Ind J Clin Biochem. 2015;31(2):171–184. doi: 10.1007/s12291-015-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y., Lin W., Huang J. Biosynthesis of gold nanoparticles by foliar broths: roles of biocompounds and other attributes of the extracts. Nanoscale Res Lett. 2005;5:1351–1359. doi: 10.1007/s11671-010-9652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J.L., Li Q.B., Sun D.H. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18:105104. [Google Scholar]
- 31.Shankar S.S., Rai A., Ankamwar B. Biological synthesis of triangular gold naoprisms. Nat Mater. 2004;3:482. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- 32.Maiti K., Mukherjee K., Murugan V. Exploring the effect of hesperetin–HSPC complex – a novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech. 2009;10:3. doi: 10.1208/s12249-009-9282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh V., Chaudhary A.K. Development and characterization of rosiglitazone loaded gelatin nanoparticles using two step desolvation method. Int J Pharm Sci Rev Res. 2010;5:100–103. [Google Scholar]
- 34.Ishizaka T., Mitsui H., Yanagida M. Development of human mast cells from their progenitors. Curr Opin Immunol. 1993;5:937–943. doi: 10.1016/0952-7915(93)90109-6. [DOI] [PubMed] [Google Scholar]
- 35.Stoyanova I.I. Relevance of mast cells and hepatic lobule innervation to liver injury. Rom J Gastroenterol. 2004;13:203–209. [PubMed] [Google Scholar]
- 36.Coussens L.M., Raymond W.W., Bergers G. Inflammatory mast cells up-regulated angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heissig B., Rafii S., Akiyama H. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;407:770–776. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grizzi F., Franceschini B., Chiriva-Internati M. Mast cells and human hepatocellular carcinoma. World J Gastroenterol. 2003;9:1469–1473. doi: 10.3748/wjg.v9.i7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B.S., Ma Y.J., Wang Y. Protective effect and mechanism of stronger neo-minophagen C against fulminant hepatic failure. World J Gastroenterol. 2007;13:462–466. doi: 10.3748/wjg.v13.i3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwabe R.F., Brenner D.A. Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:583–589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 41.Nelson D.R., Lim H.L., Marousis C.G. Activation of tumor necrosis factor-α system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 42.Mocellin S., Rossi C.R., Pilati P. Tumor necrosis factor, cancer and anticancer therpy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Roman J., Colell A., Blasco C. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HepG2 cells: effect on transcription factors AP-2 and NF-kappa B. Hepatology. 1999;30:1473–1480. doi: 10.1002/hep.510300623. [DOI] [PubMed] [Google Scholar]
- 44.Blackwell T.S., Christman J.W. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 45.Shishodia S., Aggarwal B.B. Nuclear factor-kappa B activation: a question of life or death. J Biochem Mol Biol. 2002;35(1):28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 46.Guicciardi M.E., Gores G.J. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54(7):1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucharczak J., Simmons M.J., Fan Y. To be, or not to be: NF-kappaB is the answer; role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22(56):89611–89682. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 48.Sen U., Guha S., Chowdhury J.R. Serum fucosyl transferase activity and serum fucose levels as diagnostic tools in malignancy. Acta Med Okayama. 1983;37:457–462. doi: 10.18926/AMO/32405. [DOI] [PubMed] [Google Scholar]
- 49.Scholz D., Horpacsy G., Mebel M. Late prognosis in acute post-transplant renal failure in 102 patients. Eur Urol. 1979;5:14–17. doi: 10.1159/000473052. [DOI] [PubMed] [Google Scholar]
- 50.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo (glyco) lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 51.Maga G., Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 52.Risio M. Cell proliferation in colorectal tumor progression: an immunohistochemical approach to intermediate biomarkers. J Cell Biochem. 1992;16:79–87. doi: 10.1002/jcb.240501115. [DOI] [PubMed] [Google Scholar]
- 53.Liu L.X., Jiang H.C., Zhu A.L. Cell cycle and growth regulators gene expression in liver cancer tissues and adjacent normal tissues. Zhonghua ShiyanWaikeXue Zazhi. 2001;18:123–126. [Google Scholar]
- 54.Qin L.X., Tang Z.Y. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howell W.M. Selective staining of nucleolus organizer regions (NORs) In: Busch H., Rothblum L., editors. The cell nucleus. Academic Press; New York: 1982. pp. 89–142. [Google Scholar]
- 56.Jayakumar S., Gokuladhas K., Rajan B. Carvacrol modulates instability of xenobiotic metabolising enzymes and down regulates the expressions of PCNA, MMP-2, and MMP-9 during diethylnitrosamine-induced hepatocarcinogenesis in rats. Mol Cell Biochem. 2014;395(1–2):65–76. doi: 10.1007/s11010-014-2112-5. [DOI] [PubMed] [Google Scholar]
- 57.Moreno F.J., Rodrigo R.M., Garcia-Herdugo G. AgNOR proteins and rDNA transcriptional activity in plant cells. J Histochem Cytochem. 1990;38:1879–1887. doi: 10.1177/38.12.1701461. [DOI] [PubMed] [Google Scholar]
- 58.Jagan S., Ramakrishnan G., Anandakumar P. Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol Cell Biochem. 2008;319:51–59. doi: 10.1007/s11010-008-9876-4. [DOI] [PubMed] [Google Scholar]
- 59.Srisesharam S., Ilaveni S., Indira A. Apoptosis associated inhibition of DEN-induced hepatocellular carcinogenesis by ellagic acid in experimental rats. Biomed Prev Nutr. 2012;2:1–8. [Google Scholar]