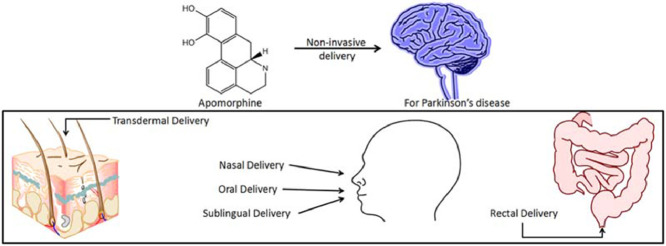
Graphical abstract
Keywords: Apomorphine, Drug delivery, Parkinson's disease, Alternative apomorphine therapy, Non-invasive delivery, Excipients
Abbreviations: PD, Parkinson's disease; SEDDS, Self-emulsifying drug delivery system; L-dopa, Levodopa; PAA-cys-2MNA, poly(acrylic acid)-cysteine-2-mercaptonicotinic acid
Abstract
Parkinson's disease (PD) is a chronic debilitating disease affecting approximately 1% of the population over the age of 60. The severity of PD is correlated to the degree of dopaminergic neuronal loss. Apomorphine has a similar chemical structure as the neurotransmitter dopamine and has been used for the treatment of advanced PD patients. In PD patients, apomorphine is normally administered subcutaneously with frequent injections because of the compound's extensive hepatic first-pass metabolism. There is, hence, a large unmet need for alternative administrative routes for apomorphine to improve patient compliance. The present review focuses on the research and development of alternative delivery of apomorphine, aiming to highlight the potential of non-invasive apomorphine therapy in PD, such as sublingual delivery and transdermal delivery.
1. Introduction
The earliest reported synthesis of apomorphine was described by Arppe in 1845 and later by Matthiesen and Wright in 1869, which involved reacting morphine with hydrochloric acid or sulfuric acid, respectively, to obtain apomorphine [1]. Apomorphine found its early use in veterinary therapeutics to treat issues associated with farmyard animal behavior. By 1874, it was known that apomorphine had effects on the central nervous system along with emetic effects [1], [2]. In human medical use, the compound was recommended as an emetic, sedative, as well as a treatment for narcotic and alcohol addiction [3]. Weil in 1884 suggested apomorphine for its potential use in treating Parkinson's disease (PD) [4], [5]. Schwab and coworkers and Cotzias confirmed Weil's suggestion, as they found a significant decrease in tremors and rigidity associated with PD in humans [6], [7], [8], [9]. In today's medical treatment, subcutaneous apomorphine is used for the treatment of advanced PD in patients undergoing motor disabilities that do not respond to other PD treatments. There is a large unmet need for alternative apomorphine delivery systems, as this treatment is provided by multiple subcutaneous injections daily. This review covers the importance of apomorphine in PD management and emphasizes the challenges with apomorphine therapy for PD patients. The present review also aims at highlighting some key research developments in the area of non-invasive delivery of apomorphine.
2. Physicochemical properties of apomorphine
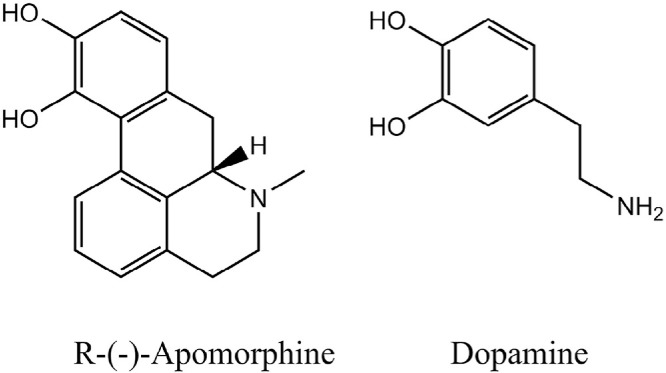
Apomorphine (6-methyl-6αβ-noraporphine- 10,11-diol) belongs to the class of β-phenylethylamines sharing structural similarities to the neurotransmitter, dopamine, both having a catechol moiety (Fig. 1). Apomorphine is a chiral molecule (MW: 267.32 Da). The R-form is a dopamine agonist, while the S-form of the molecule may possess anti-dopaminergic activity [10], [11].
Fig. 1.
Chemical structures of R-(-)-apomorphine and dopamine.
Apomorphine is water-soluble with an intermediate lipophilicity represented by a log P of 2.0 [12]. The compound has two pKa values, at 7.0 and 8.9 [13], and it is generally available as the hydrochloride salt. The hydrophilic character of apomorphine allows it to be solubilized and therefore, formulated as an aqueous solution. Moreover, it is readily mixed in tissue fluids and can be absorbed into the systemic circulation and subsequently cross the lipophilic blood-brain barrier [14].
3. Therapeutic uses of apomorphine
Apomorphine has been used in the treatment of several ailments. Table 1 illustrates the various apomorphine products currently available on the market. In veterinary practices, apomorphine is utilized as an emetic agent, which induces vomiting as a part of managing poisoning in dogs and other animals. Apomorphine acts on the dopamine receptors in the ‘chemoreceptor trigger zone’ in the area postrema of the medulla oblongata as well as the receptor cerebrospinal fluid side [2].
Table 1.
Currently marketed apomorphine formulations.
| Product name | Company | Dosage form and route of administration | Indication |
|---|---|---|---|
| Apometic ® [15] | Forum Animal Health | Solution for subcutaneous injection | Emesis for veterinary practice |
| Uprima®, Ixense®, Spontane ®, TAK 251 [16] | Pentech Pharmaceuticals Inc. (originator and developer); Takeda Pharmaceutical Company Ltd. (developer) | Sublingual tablet | Erectile dysfunction |
| APO-go, Apokinon, Apokyn, Apomine, Britaject, KW-6500, Li Ke Ji, MOVAPO ® [17] | Britannia Pharmaceuticals Ltd. (originator and developer); Kyowa Hakko Kirin Co. Ltd. and US WorldMeds (developer) | Solution for subcutaneous injection | Parkinson's disease |
One of the prescribed uses of apomorphine is its application in male erectile dysfunction. Apomorphine has been demonstrated to be an effective erectogenic agent by stimulating the postsynaptic dopamine receptors in the hypothalamus [18], [19]. A sublingual apomorphine formulation has been reported to have 18–19 min as an onset time of erection requiring very low (<1.5 ng/dl) apomorphine concentrations at the site of action [19]. Apomorphine therapy in improving erectile response can be considered as a valuable alternative to other treatments for erectile dysfunction.
The predominant therapeutic use of apomorphine is its use in PD. PD is a debilitating disease which is characterized by chronic neurodegeneration of the striatal region of the brain causing a deficiency of neurotransmitter dopamine [20], [21]. Replacing the loss of dopamine by using dopamine agonist is the choice of treatment for many PD patients. Apomorphine, a potent dopamine agonist, is commonly used as rescue therapy in subcutaneous formulations in advanced stage PD patients. Apomorphine and its role in PD are discussed in detail in the following sections.
Apomorphine has demonstrated its therapeutic effects in treating Alzheimer's disease. Alzheimer's disease is characterized by a loss of memory and cognitive functions, which is attributed to hyperphosphorylated tau protein and amyloid β-protein [22]. Apomorphine increases amyloid β-protein degradation and protects neuronal cells from oxidative stress. Therefore, it restores memory function and improves pathology traits of Alzheimer's disease assessed in a mice model [23]. More such studies in the future might establish apomorphine as a novel therapeutic agent in treating Alzheimer's disease.
4. Parkinson's disease and its treatment
PD is one of the most common chronic neurodegenerative diseases affecting about 1% of the population over the age of 60 [20]. As aforementioned, the dopamine-secreting neurons in the nigrostriatal region of brain undergoes a progressive degeneration leading to a depletion of dopamine in patients with PD [21]. The low levels of dopamine in the striatum have several implications on motor abilities, such as rest tremor, bradykinesia (slowness of movement), rigidity, postural instability, and falls. The non-motor complications involving the non-dopaminergic brain regions, such as neuropsychiatric and autonomic disturbances, cognitive impairment, etc. generally surface as the disease progresses [20].
PD is yet incurable; however, various symptomatic therapies are available to improve the quality of life as well as longevity for the PD patients. The severity of PD correlates to the degree of dopaminergic neuronal loss. In order to understand the need for apomorphine in the treatment of PD, one must understand the usage of levodopa (L-dopa) in managing PD. L-dopa, a precursor of dopamine, is the most efficacious oral drug available for alleviating symptoms of early stages of PD. L-dopa (dihydroxyphenylalanine) is also an endogenous intermediate in the synthesis of catecholamine neurotransmitters. In contrast to dopamine, L-dopa can be absorbed across the blood-brain barrier and converted to dopamine in the striatum [24].
In early stages of PD, it has been shown that L-dopa eases several symptoms, such as freezing, somnolence, edema, and hallucinations [25]. However, long-term therapy with L-dopa is limited due to a decrease in its efficacy. The prolonged use of L-dopa also causes appearance of adverse effects of dopaminergic motor functions especially dyskinesia (impairment in voluntary movements) [26], [27] and wearing off (‘on–off’ phenomenon) [28]. The “on–off” phenomenon in PD refers to a switch between mobility and immobility, which occurs as a worsening of motor function or, much less commonly, as sudden and unpredictable motor fluctuations as the disease progresses. As a result, dopamine receptor agonists are used alone or in combination with L-dopa to delay the onset of motor complications. Dopamine agonists can also be effective in early PD, especially in cases where the disease occurs in “younger” patients, where dyskinesia is a greater risk [29], [30]. Dopamine receptor agonists stimulate the post- and presynaptic dopaminergic receptors [31].
4.1. Apomorphine therapy
More than 50% of Parkinson's disease patients develop ‘on–off phenomenon’ with a prolonged L-dopa use of greater than 5 years, which warrants the use of dopamine agonists such as apomorphine [32], [33]. Apomorphine, a mixed D1 and D2 dopamine agonist, predominantly finds its use as a rescue medicine to treat the ‘off’ period in L-dopa therapy. The D1 potency by apomorphine is exhibited by the catechol moiety [34], while the bulky aromatic part of the molecule may contribute to the affinity toward D2 receptors [35]. The potency of apomorphine and L-dopa has been shown to be comparable to each other [36], [37], [38]. Unlike oral L-dopa therapy, apomorphine is administered subcutaneous in the abdomen region as an intermittent injection or by continuous infusion using an infusion pump [39]. The avoidance of dyskinesia is a major advantage of apomorphine treatment over L-dopa therapy. However, apomorphine administration has several disadvantages such as nausea and vomiting; thus, requiring anti-emetics, which are often co-dosed (such as, domperidone) [40]. Additionally, subcutaneous apomorphine therapy may give rise to problems with patient compliance associated with needle phobia or local pain due to irritation and inflammation followed by a formation of subcutaneous nodules [41], [42], [43].
4.2. Challenges with apomorphine therapy
Although apomorphine has medical applications, its inherent instability poses a complication in clinical practice. Oxidation of apomorphine is one of the pharmaceutical challenges when it is formulated as an aqueous solution. Apomorphine spontaneously undergoes oxidative decomposition in aqueous solution to yield a bluish-green color in the presence of light and air [44], [45]. The catechol group of the apomorphine molecule is highly susceptible to oxidation leading to the formation of a quinone [44], [46], [47]. The decomposition of apomorphine is dependent on its concentration as well as the pH and temperature of the solution [44], [48]. The chemical half-life of apomorphine is reported to be 39 min under conditions similar to that of plasma (at 37 °C and pH 7.4) [48]. The additions of antioxidants, chelating agents, or alteration of the formulation pH are some of the approaches reported in the literature to prevent the autoxidation of the molecule [44], [49], [50].
Another major challenge associated with apomorphine is the deactivation upon metabolism of apomorphine after administration. The in vivo conversion from the R-form of apomorphine, which is pharmacologically active, to the S-form lowers the pharmacological activity of the molecule [51]. Additionally, apomorphine is metabolized via numerous enzymatic pathways predominantly in the liver, but also in the brain and tissue fluids [52]. Some of the metabolic pathways include sulfation, glucuronidation, and catechol-O-methyltransferase [51], [53], [54], [55], [56]. Moreover, a large pharmacokinetic variability is reported between PD patients after subcutaneous administration of apomorphine [53]. This inter-subject variability may be caused by the interactions of the drug and its various metabolites with plasma and tissue, which complicates the clinical dose setting [44].
The clinical utility of apomorphine upon oral administration is highly limited due to hepatic first-pass metabolism [57]. The oral absorption of apomorphine in rats with surgical portacaval venous anastomosis or shunting was reported to be similar to the absorption after subcutaneous application. In contrast, the sham operated rats with intact portal-hepatic venous circulation had undetectable tissue concentration of apomorphine [52]. This suggested that apomorphine was well absorbed when given orally, but underwent an extensive metabolism in the liver. The metabolic constraints can account for a poor oral bioavailability of less than 4% in PD patients [57].
Due to a high extent of apomorphine metabolism, a plasma half-life of about 32 min is reported after subcutaneous administration [48], [58]. This short plasma half-life necessitates a higher frequency of injections to maintain a concentration of apomorphine in the blood and subsequently, in the brain. A continuous subcutaneous infusion may be used if apomorphine subcutaneous injections exceed 7–9 times daily [40]. As mentioned previously, this causes inconvenience to patients with PD and often leads to patient non-compliance. Additionally, self-injection might prove difficult for patient in late stage PD during ‘off’ periods due to impairment of motor functions [59].
5. Drug delivery approaches for apomorphine
There is a high medical need for improving apomorphine bioavailability via various approaches and/or administrative routes to improve patient convenience and compliance. The sections below highlight the developments in non-invasive delivery of apomorphine, which are setting a pathway for the future of apomorphine therapy in PD.
5.1. Chemical modification: Prodrug approach
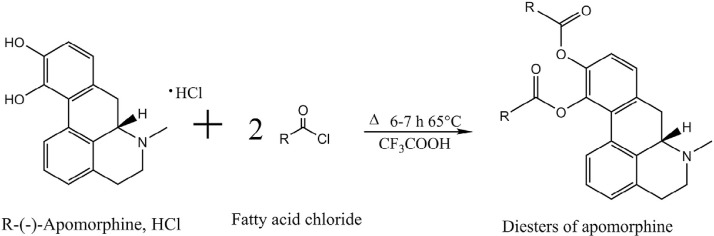
Synthesizing prodrugs can be a method to improve physicochemical, biopharmaceutical and/or pharmacokinetic properties of an active compound. Prodrugs are derivatives of drug compounds which undergo enzymatic or chemical biotransformation to yield the pharmacologically active parent drug in vivo, which can exert its therapeutic action. One prominent site for derivatization for apomorphine is the catechol moiety in the molecule to synthesis diesters of apomorphine. Fig. 2 illustrates the general apomorphine esterification reaction yielding apomorphine diesters. Early literature on derivatization of apomorphine molecule was reported by Borgman et al., where diacetyl, dipropionyl, diisobutyryl, dipivaloyl, and dibenzoyl esters of apomorphine were synthesized [60], [61]. Stereotyped gnawing behavior and unilateral rotation were investigated upon intraperitoneal administration to rats. The duration of action of the diesters was significantly increased compared to that of free apomorphine. Apart from altering the pharmacokinetics, the advantage of esterification on the catechol moiety is that it inhibits oxidation of hydroxyl groups [62], thereby enabling the development of more chemically stable formulations.
Fig. 2.
General scheme of esterification of R-(-)-apomorphine to yield apomorphine diesters.
Although the first reported apomorphine esters were administered via an invasive intraperitoneal route, there are several studies of apomorphine esters which explored non-invasive routes of administration, such as the transdermal and oral route. Liu et al. investigated the transdermal delivery of two diesters of apomorphine: diacetyl and diisobutyryl apomorphine [12]. The diester prodrugs were more lipophilic than apomorphine and were determined as substrates to the esterases present in esterase medium, nude mouse skin homogenate, and human plasma. Apomorphine and its diester prodrugs formulated in lipid emulsions were investigated for their permeation across the skin of nude mouse (area of 0.785 cm2) using Franz diffusion cell. Diacetyl and diisobutyryl apomorphine yielded 11 and 3 folds higher fluxes, respectively, when compared to apomorphine. The results indicated a promising transdermal delivery of apomorphine by incorporating bioreversible lipophilic prodrugs in lipid-based carrier.
Apomorphine diester prodrugs have also been investigated for their oral delivery. Borkar and colleagues investigated the possibility of developing oral apomorphine formulations by synthesizing lipophilic derivatives of the molecule and incorporating them into lipid formulations [63]. The objective of utilizing lipophilic derivatives in lipid carrier was to stimulate the lymphatic drug transport, which will be further discussed in Section 5.2.1. Lipidifying apomorphine via prodrug was successfully demonstrated by obtaining highly lipophilic diesters: dilauroyl and dipalmitoyl apomorphine, which were 6.5 and 8.5 times more lipophilic (based on the logarithm of partition coefficient (log P) value), respectively, than their parent drug. This high lipophilicity of the diesters allowed them to be dissolved in lipid vehicles required to obtain lipid-based formulations. Additionally, the apomorphine diesters exhibited differences in their enzymatic degradation when incubated in biorelevant medium containing pancreatic extract. About 28% dipalmitoyl diester remained intact, while only 4% dilauroyl apomorphine was left undegraded after 5 min of incubation. It was suggested that the longer alkyl chain of dipalmitoyl apomorphine (C16) might have exhibited higher steric hindrance during enzymatic cleavage of the ester bond than dilauroyl apomorphine (C12). The need for enzymatic hydrolysis of the apomorphine diesters before initiation of the therapeutic action was also demonstrated by the presence of a lag time (about 30 min) in a 6-hydroxydopamine-induced rotational rat model [64].
Another study used R-(–)-11-O-valeryl-N-n-propylnoraporphine HCl as an oral apomorphine derivative to evaluate its motor effects on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated marmosets [65]. The fast onset and prolonged duration of the therapeutic effects meant a reversal of motor deficits and improvement of dyskinesia in the PD marmoset model. These studies demonstrated that the various parameters in the apomorphine prodrug synthesis, such as chain length, branching and different types of substituents can be modified to alter the physicochemical and biopharmaceutical properties for the drug molecule.
5.2. Formulation and non-invasive drug delivery strategies
Various delivery strategies have been investigated to administer apomorphine using an approach which utilizes alternative routes besides the subcutaneous route. This section focuses on the non-invasive apomorphine delivery approaches investigated in previous studies.
5.2.1. Oral delivery
Oral delivery of apomorphine suffers from a very poor oral bioavailability (less than 4%) because of extensive hepatic first-pass metabolism [57]. Early in vivo investigations of apomorphine administered orally revealed that there was relief in PD symptoms at high doses of apomorphine (up to 500 mg), although azotemia was observed [8]. Tsai and coworkers investigated the possibility of developing an oral delivery of apomorphine by incorporating it into solid lipid nanoparticles [66]. Glyceryl monostearate and polyethylene glycol monostearate were individually used as emulsifiers in the lipid nanoparticles composed of a lipid phase (tripalmitin and hydrogenated soybean phosphatidylcholine) and an aqueous phase (Pluronic® F68 and L-ascorbic acid). The relative oral bioavailability in rats increased to 25%–28% after administration of apomorphine in solid lipid nanoparticles, while it was a mere 2% for the apomorphine aqueous solution (control). An improved therapeutic efficacy was demonstrated by a 5–6 fold increase in rotation scores in 6-hydroxydopamine-lesioned rats from the groups receiving the solid lipid nanoparticle formulations compared to the control group.
One way to avoid the hepatic first-pass metabolism upon oral administration is to stimulate drug transport via the lymphatics [67], [68], [69]. Generally, compounds with a log P value > 5 and a triglyceride solubility >50 mg/g have a larger potential to be transported lymphatically after oral administration, but exceptions also exist [69], [70]. Apomorphine has a log P value of 2.0 [12], so in order to have any potential for lymphatic transport the lipophilicity of the molecule needs to be increased. Borkar and coworkers lipidified apomorphine to synthesize prodrugs and incorporated these prodrugs in self-emulsifying drug delivery systems (SEDDS) [63]. Various SEDDS formulation compositions were used with excipients like triglycerides (medium chain, soybean oil, or castor oil), Kolliphor® RH40 (surfactant), MaisineTM 35–1 (co-surfactant) and ethanol (co-solvent). The incorporation of the diester prodrugs in SEDDS kept the prodrugs as intact molecules preventing their enzymatic hydrolysis in the presence of pancreatic lipase and other esterases when incubated in simulated intestinal fluids. This was further carried out to assess the prodrug affinity toward chylomicrons, which are the predominant class of lipoproteins mediating the intestinal lymphatic drug transport [71]. Even though the diester prodrugs had a low affinity toward chylomicrons, a pharmacokinetic and pharmacodynamic study was conducted in order to assess the drug absorption process in a dynamic in vivo system. Various lipid-based formulations such as, oil-in-water, water-in-oil emulsions, SEDDS, pure co-surfactant and pure oil loaded with apomorphine prodrug were assessed in rats [72]. Borkar and coworkers reported that apomorphine loaded in soybean oil had a long tmax, which was possibly caused by an increase in gastric emptying time and a slower triglyceride digestion, subsequently a slower drug release. Although the relative bioavailabilites from the lipid-based vehicles investigated were in the low end, a pharmacodynamic study was carried out in 6-hydroxydopamine-lesioned rats to evaluate if the obtained plasma concentrations could lead to a therapeutic effect in the brain [64]. SEDDS formulations gave a sustained behavior response for 6 h while, oil-in-water emulsion loaded with apomorphine diester prodrug yielded a response which lasted for about 2.5 h after administration. It was demonstrated that a sustained oral delivery of apomorphine prodrug using lipid-based formulations was feasible and thereby, could hold a potential in reducing or eliminating the frequent subcutaneous injections.
5.2.2. Sublingual delivery
Sublingual apomorphine delivery is beneficial for several reasons; the predominant reason being the avoidance of hepatic first-pass metabolism and an ease in administration with rapid onset of action. Lees et al. conducted a study with sublingual apomorphine in nine patients with idiopathic PD with 12 years as an average duration of the disease [73]. The sublingual apomorphine yielded a comparable therapeutic efficacy when compared to the efficacy observed after subcutaneous apomorphine administration. There was a latency observed in the pharmacological effect after sublingual dose, which was suggested to be induced by the time required for tablet dissolution, which was 33 min on average [73]. Similarly, an onset time of 30 min was reported by Durif et al. after sublingual administration of a apomorphine tablet compared to 14 min after subcutaneous injection in a patient study including 8 patients [74]. The therapeutic effects after sublingual administration were also noted to be sustained for a longer period of time relative to the subcutaneous administration.
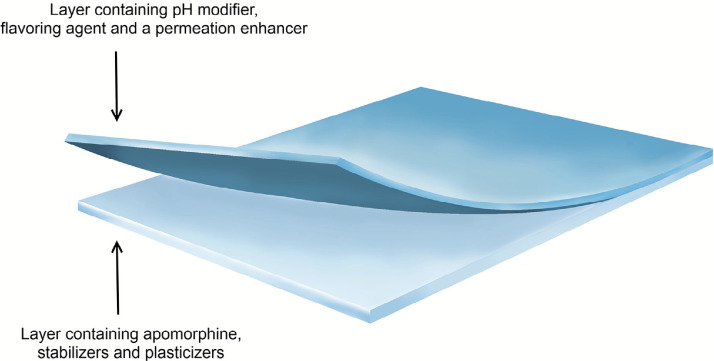
A formulation currently being investigated and developed is apomorphine sublingual film strip formulation (APL-130277) [75], [76]. It is a dissolvable thin-film strip formulated with apomorphine as a laminated bilayer to prevent mucosal irritation (Fig. 3). The first layer (light blue layer in Fig. 3) is a cellulose ether-based film containing apomorphine, stabilizers and plasticizers, while the second layer (dark blue layer in Fig. 3) is composed of a similar cellulose film base consisting of pH modifier, flavoring agent and a permeation enhancer [77].
Fig. 3.
A graphical representation of APL-130277 bilayer sublingual strip formulation. Modified and reprinted with permission from Cynapsus Therapeutics, Inc. © Cynapsus Therapeutics, Inc., Toronto, Ontario, Canada.
In a study performed with 19 patients, 15 patients responded and had a quick conversion from ‘off’' state to ‘on’ state within 15–30 min. Sixty percent of the responders had a response duration which lasted for at least 60 min [75]. A study conducted in Syrian golden hamsters revealed no irritation of the buccal mucosa after three times daily applications of APL-130277 for 28 consecutive days [77]. A phase III clinical trial is currently in the pipeline to investigate the efficacy, safety and tolerability of APL-130277 (ClinicalTrials.gov, Identifier: NCT02469090). APL-130277 could provide for a therapeutically effective and patient compliant way to administer apomorphine during ‘off’ episodes in PD patients.
5.2.3. Nasal or inhalation delivery
Drug delivery through the nasal cavity have been extensively explored for its intrinsic advantages, such as ease of application, circumvention of the hepatic first-pass metabolism and degradation in the gastrointestinal tract enabling a reduction in drug dose compared to the oral dose. The considerably larger absorption area in the nasal cavity, due to the presence microvilli along with rather extensive vascularization, often results in quick drug absorption and a fast onset of action upon nasal administration [78].
One of the early investigations for administering apomorphine via the nasal route was conducted by Kapoor et al [79]. The onset of motor response was on average reported to occur 8.9 min after administration, lasting for an average of 44 min. Sam et al. studied apomorphine pharmacokinetics after nasal and subcutaneous administration [58]. The nasal absorption half-life was reported to be 8.6 ± 2.6 min. This suggested that apomorphine was absorbed rapidly, which was similar to subcutaneous administration where the absorption half-life was 5.8 ± 1.9 min. Therefore, nasal administration seems to be a promising route of administration as a ‘rescue’ therapy of apomorphine.
The research stemming out from the initial investigations on nasal absorption of apomorphine focused on increasing the residence time of the drug loaded formulation on the nasal mucosa. For instance, Ugwoke and colleagues prepared formulations of freeze-dried powder containing apomorphine using excipients such as, carboxymethylcellulose, degradable starch microspheres and lactose [80]. An in vitro drug release study was conducted in phosphate buffer (pH 6.0) and an in vivo pharmacokinetic study was performed, where the drug containing powder was insufflated in the nasal cavity of New Zealand white rabbits. The in vitro drug release and in vivo absorption profile of the carboxymethylcellulose formulation demonstrated a sustained release and absorption of apomorphine, where a 15% (w/w) drug loaded formulation had 50% of Cmax maintained for 70 min compared to degradable starch microspheres and lactose formulations, which were 35 min or less. In another study, Ugwoke et al. reported further sustained release of apomorphine by incorporation of mucoadhesive polymers (Carbopol 971P and polycarbophil) into the powder formulations containing apomorphine [81]. Apomorphine was detected in the plasma of the rabbits for as long as 6–8 h after administration of the mucoadhesive powder formulations. The nasal drug clearance using radiolabeling was assessed, where the mucoadhesive polymers in combination with apomorphine increased their residence time within the nasal cavity enabling a sustained drug release and absorption [82]. Ugwoke et al. also tried to combine excipients for immediate drug release (lactose powder) with sustained release formulation (Carbopol matrix) to increase the initial plasma drug concentration, which was, however, an unsuccessful attempt [83].
Recently, the use of thiomers as a mucoadhesive biomaterial in apomorphine formulation has been explored as an effort to prolong the residence time of the formulation in the nasal cavity [84]. Various poly(acrylic acid) thiomers were synthesized. These thiomers were pre-activated to yield poly(acrylic acid)-cysteine-2-mercaptonicotinic acid (PAA-cys-2MNA) with varying degrees of pre-activation, which protected the thiomer from oxidation and thus, retained its mucoadhesive properties. High degree of pre-activation of PAA-cys-2MNA, as an excipient in aqueous solution of apomorphine, yielded a higher absolute bioavailability of about 27% when compared to the oral and intranasally administered apomorphine solution without the thiomer. Moreover, these thiomers have been reported to be non-toxic toward Caco-2 cells [85] thereby, potentially possessing low risk of local toxicity on the nasal mucosa.
In a phase II, placebo-controlled, double blind clinical study, Grosset and coworkers investigated the therapeutic efficacy of inhaled dry powder of apomorphine (VR040) via an inhalation device, i.e. pulmonary delivery [86]. Apomorphine was absorbed very quickly (2–7 min) leading to a rapid transformation (mean 10 min) to the ‘on’ state from ‘off’ state. The incidences of adverse effects were not different between VR040 and the placebo group, suggesting no compromise in safety. The quick onset of action and the ease of administration make nasal apomorphine delivery a viable option used for ‘rescue’ therapy.
5.2.4. Transdermal delivery
Transdermal drug delivery possesses similar benefits as nasal delivery, which favors apomorphine absorption. Liu et al. investigated the potential of apomorphine transdermal delivery by loading apomorphine free base, its hydrochloride salt, and two of its diesters (diacetyl and diisobutyryl apomorphine) into lipid emulsions [12]. Prior to emulsification, the compounds were dissolved in the lipid phase consisting of 12% (w/v) mineral oil and 0.3% (w/v) MyverolTM, while the aqueous phase contained water and 2.5% (w/v) Pluronic® F68. As an exception, apomorphine HCl was dissolved in the aqueous phase. The flux across nude mouse skin (area of 0.785 cm2) of diisobutyryl apomorphine was determined and found to be lower than that of diacetyl apomorphine when the prodrug was incorporated into lipid emulsions. It was suggested that the higher log P value of diisobutyryl apomorphine retained the compound in the oil phase retarding its release from the formulation. This characteristic may potentially be utilized to obtain a prolonged release and absorption of the apomorphine diester. However, the unpleasant oily or sticky feeling on the skin from the lipid vehicle might limit the pharmaceutical applicability.
Peira et al. studied absorption via the transdermal route using apomorphine loaded microemulsions [87]. The formulations were composed of apomorphine HCl, octanoic acid, 1,2 propanediol, sodium hexanoate, sodium glycocholate (or taurocholate) dissolved in water (pH 6.0), Epikuron 200 as surfactant and an oil phase of isopropylmyristate–decanol. In addition to the formulation vehicle, ion pairs of apomorphine–octanoic acid were prepared to increase lipophilicity of the drug and to enhance the transdermal permeation across hairless mouse skin with a diffusible area of 1.7 cm2. As a follow up study, Priano et al. conducted an in vivo study using such apomorphine microemulsions on twenty-one idiopathic PD patients, who exhibited long term L-dopa syndrome or a lack of complete reduction of ‘off’ period [88]. The apomorphine microemulsion was applied as a 1 mm thick layer creating a reservoir on to 100 cm2 skin area over the chest surrounded by 1 mm thick biocompatible foam tapes and enclosed by a polyester-based and an occlusive membrane. Apomorphine microemulsions applied with oral administration of L-dopa demonstrated a rapid increase in apomorphine plasma concentration to obtain therapeutic levels and a prolonged absorption with a tmax of 5.1 h compared to tmax of 20 min after subcutaneous administration.
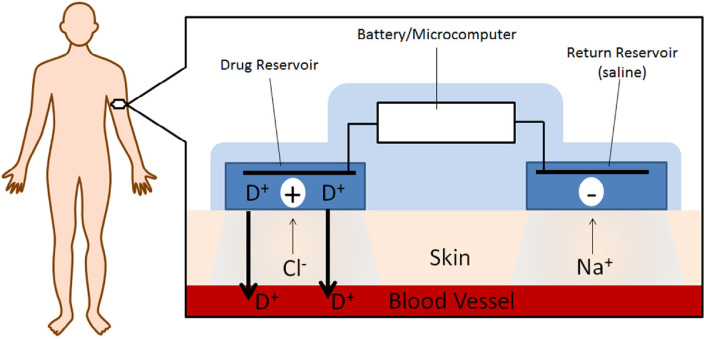
One of the novel routes for controlled delivery of apomorphine is transdermal iontophoresis. The salient feature and a major advantage of transdermal iontophoresis is that it possesses the potential to deliver apomorphine as per the need, not only by measuring patient's pharmacokinetics, but by the possibility of measuring their pharmacodynamic output. Fig. 4 illustrates a representation of a typical iontophoretic patch. Li and coworkers evaluated a iontophoretic patch designed with a anodal compartment filled with R-apomorphine solution (sodium chloride, ascorbic acid and citrate buffer, pH 5.0) and the cathodal compartment filled with a phosphate buffered saline solution (pH 7.4) [90]. The delivery of apomorphine can be regulated by the current density in the device. In this study, the current density was 250 µA/cm2 with a skin contact patch area of 20 cm2. This study aimed at investigating the in vivo effects of the transdermal iontophoretic patch with and without a surfactant pre-treatment between cohorts of advanced PD patients. The patients treated with pre-treatment showed a significantly higher bioavailability (13%) and steady state flux (98.3 ± 12.1 nmol/cm2h) from the non-pre-treated group with 10% bioavailability and 75.3 ± 6.6 nmol/cm2h steady state input rate. Similarly, Li et al. explored different surfactant formulation pre-treatment to enhance the apomorphine transdermal iontophoretic delivery across skin area of 0.64 cm2 with an 500 µA/cm2 current density [91]. In this study, Li and colleagues prepared various compositions of surfactant formulations consisting of laureth-3 oxyethylene ether, laureth-7 oxyethylene ether, and cholesterol sulphate and demonstrated a 2-fold increase in the steady state flux to 181.5 ± 22.6 nmol/cm2h relative to the group without any surfactant pre-treatment. These studies indicated a safe alternative delivery methodology yielding only minor and temporary local skin irritation [90], [91]. This patient customized apomorphine delivery system with iontophoresis could hold a great potential for optimizing the drug dose of apomorphine.
Fig. 4.
A graphical representation of an iontophoretic patch. Modified from Green, 1996 [89].
5.2.5. Rectal delivery
In a study conducted in three patients, apomorphine was administered with a rectal enema solution at the beginning of the ‘off’ period [92]. The observed clinical efficacy in the patients was comparable to that after subcutaneous or intranasal administration. Thereby, the administration of rectal enema showed its potential for a quick relief from ‘off’ state symptoms. Huges et al. studied rectal apomorphine administration using suppositories [93]. Five out of eleven patients had a full reversal from the ‘off’ state and had a mean latency of response of 32 min. The plasma levels of apomorphine after rectal administration were sustained, but became lower than that after sublingual and subcutaneous administration, and exhibited higher inter-patient variability. The slower onset of action might prove this route applicable as an adjunct to subcutaneous administration, for patients requiring frequent subcutaneous injections, or patients having disabilities at night. Van Laar and colleagues performed a comparative study with a rectal apomorphine solution, a gelatin and a Witepsol®-H15 suppository [94]. The group receiving Witepsol® suppository exhibited a longer tmax (127.5 ± 7.5 min) and a longer duration of action than the group receiving the rectal solution (tmax 16.0 ± 2.5 min and duration of action 50.0 ± 13.1 min). A high inter-patient variability in the bioavailabilities was noted, the group of patients receiving gelatin suppository demonstrated a bioavailability of 40.2 ± 22.9%, which was higher than the group receiving Witepsol® (18.9 ± 8.1%). Witepsol® suppository base can prove to be useful in achieving sustained release of apomorphine; however, due to the inconvenience of rectal administration, it would probably only be the administration route chosen when alternative routes have adverse events making them non-feasible.
6. Efforts by pharmaceutical industry on development of apomorphine delivery
As mentioned in the sections above, a number of formulations containing apomorphine have already been introduced to the market, such as the solution for subcutaneous injection and tablet for sublingual administration for PD and erectile dysfunction, respectively. While these formulations cover a medical need, the discussions above have clarified some of the disadvantages dealing with poor bioavailability and pharmacokinetics in particular with the non-invasive PD treatment with apomorphine. Several companies, including Vectura Group PLC, Amarin Pharma Inc., and Britannia Pharmaceuticals Ltd. have worked or are still working on drug delivery solutions that may potentially overcome some of these disadvantages.
The different delivery attempts of apomorphine for the use in PD including non-invasive sublingual, intranasal, and pulmonary deliveries have continued into clinical trials, but stopped before commercialization for various reasons. Many of the companies that have been active in the field no longer mention apomorphine on their homepages and all seem closed, but one program by Cynapsus Therapeutics Inc. that has a sublingual version of apomorphine in clinical phase III, as discussed in Section 5.2.2. While this is naturally a disappointment to the patients suffering from PD, that no alternative delivery options beyond subcutaneous injection seem to be available within the near future, there is still reason to believe that all stones have not been turned yet. However, non-invasive drug delivery of apomorphine is certainly a hard challenge to solve from a drug delivery perspective.
7. Conclusion
The challenges with non-invasive apomorphine therapy range from drug stabilities to absorption barriers. The chemical stability of apomorphine could be improved by addition of antioxidants and chelating agents, or prodrug strategy, whereas the enzymatic stability of apomorphine should be considered in connection with the alternative formulation choice or delivery route. Combining prodrugs principle with lipid-based formulations via the oral route could be a promising way to tweak the pharmacokinetics of apomorphine. Additionally, using polymers to increase residence time in the nasal cavity or employing lipid-based formulations or iontophoresis for transdermal application could be a way to achieve prolonged or measured systemic exposure to apomorphine. Sublingual delivery of apomorphine could be a better approach for avoiding hepatic first-pass metabolism and enabling rapid onset of action. Hence, currently, it seems to be one of the most promising non-invasive routes for delivery of apomorphine to PD patients, also from the perspective of pharmaceutical industry.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgment
We would like to acknowledge The Lundbeck Foundation for the financial support (R108-A10772).
References
- 1.Taba P., Lees A., Stern G. Erich Harnack (1852–1915) and a short history of apomorphine. Eur Neurol. 2013;69(6):321–324. doi: 10.1159/000346762. [DOI] [PubMed] [Google Scholar]
- 2.Harding R.K., Hugenholtz H., Kucharczyk J. Central mechanisms for apomorphine-induced emesis in the dog. Eur J Pharmacol. 1987;144(1):61–65. doi: 10.1016/0014-2999(87)90009-4. [DOI] [PubMed] [Google Scholar]
- 3.Raymond M.J. The treatment of addiction by aversion conditioning with apomorphine. Behav Res Ther. 1963;1(2–4):287–291. [Google Scholar]
- 4.Tyne H.L., Parsons J., Sinnott A. A 10 year retrospective audit of long-term apomorphine use in Parkinson's disease. J Neurol. 2004;251(11):1370–1374. doi: 10.1007/s00415-004-0547-4. [DOI] [PubMed] [Google Scholar]
- 5.Weil E. De l'apomorphine dans certain troubles nerveux. Lyon Med. 1884;48:411–419. [Google Scholar]
- 6.Haq I., Lewitt P., Fernandez H. Apomorphine therapy in Parkinson's disease: a review. Expert Opin Pharmacother. 2007;8(16):2799–2809. doi: 10.1517/14656566.8.16.2799. [DOI] [PubMed] [Google Scholar]
- 7.Cotzias G.C., Papavasiliou P.S., Fehling C. Similarities between neurologic effects of L-Dopa and of apomorphine. N Engl J Med. 1970;282(1):31–33. doi: 10.1056/NEJM197001012820107. [DOI] [PubMed] [Google Scholar]
- 8.Cotzias G.C., Papavasiliou P.S., Tolosa E.S. Treatment of Parkinson's disease with aporphines. Possible role of growth hormone. N Engl J Med. 1976;294(11):567–572. doi: 10.1056/NEJM197603112941101. [DOI] [PubMed] [Google Scholar]
- 9.Schwab R.S., Amador L.V., Lettvin J.Y. Apomorphine in Parkinson's disease. Trans Am Neurol Assoc. 1951;56:251–253. [PubMed] [Google Scholar]
- 10.Campbell A., Baldessarini R.J., Teicher M.H. S(+)Apomorphines. Selective inhibition of excitatory effects of dopamine injected into the limbic system of the rat. Neuropharmacology. 1985;24(5):391–399. doi: 10.1016/0028-3908(85)90023-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhang A., Zhang Y., Branfman A.R. Advances in development of dopaminergic aporphinoids. J Med Chem. 2007;50(2):171–181. doi: 10.1021/jm060959i. [DOI] [PubMed] [Google Scholar]
- 12.Liu K.-S., Sung K.C., Al-Suwayeh S.A. Enhancement of transdermal apomorphine delivery with a diester prodrug strategy. Eur J Pharm Biopharm. 2011;78(3):422–431. doi: 10.1016/j.ejpb.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong J., Barlow R.B. The ionization of phenolic amines, including apomorphine, dopamine and catecholamines and an assessment of zwitterion constants. Br J Pharmacol. 1976;57(4):501–516. doi: 10.1111/j.1476-5381.1976.tb10377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neef C., van Laar T. Pharmacokinetic-pharmacodynamic relationships of apomorphine in patients with Parkinson's disease. Clin Pharmacokinet. 1999;37(3):257–271. doi: 10.2165/00003088-199937030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Forum Animal Health-Apometic; 2014. Available from: http://www.forumanimalhealth.co.uk/small_animal_products_apometic.asp. [Accessed 20 June 2017].
- 16.Adis Insight – Apomorphine - Takeda; 2013. Available from: http://adisinsight.springer.com/drugs/800010470. [Accessed 20 June 2017].
- 17.Adis Insight – Apomorphine subcutaneous – Britannia Pharmaceuticals; 2017. Available from: http://adisinsight.springer.com/drugs/800020307. [Accessed 20 June 2017].
- 18.Heaton J.P.W., Morales A., Adams M.A. Recovery of erectile function by the oral administration of apomorphine. Urology. 1995;45(2):200–206. doi: 10.1016/0090-4295(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 19.Altwein J.E., Keuler F.U. Oral treatment of erectile dysfunction with apomorphine SL. Urol Int. 2001;67(4):257–263. doi: 10.1159/000051001. [DOI] [PubMed] [Google Scholar]
- 20.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. J Am Med Assoc. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 21.Fahn S., Libsch L.R., Cutler R.W. Monoamines in the human neostriatum: topographic distribution in normals and in Parkinson's disease and their role in akinesia, rigidity, chorea, and tremor. J Neurol Sci. 1971;14(4):427–455. doi: 10.1016/0022-510x(71)90178-x. [DOI] [PubMed] [Google Scholar]
- 22.Perl D.P. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 2010;77(1):32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himeno E., Ohyagi Y., Ma L. Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation. Ann Neurol. 2011;69(2):248–256. doi: 10.1002/ana.22319. [DOI] [PubMed] [Google Scholar]
- 24.Arai R., Karasawa N., Geffard M. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett. 1995;195(3):195–198. doi: 10.1016/0304-3940(95)11817-g. [DOI] [PubMed] [Google Scholar]
- 25.Tarsy D. Treatment of Parkinson disease: a 64-year-old man with motor complications of advanced Parkinson disease. J Am Med Assoc. 2012;307(21):2305–2314. doi: 10.1001/jama.2012.4829. [DOI] [PubMed] [Google Scholar]
- 26.Rascol O., Brooks D.J., Korczyn A.D. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342(20):1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 27.Holloway R.G., Shoulson I., Fahn S. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004;61(7):1044–1053. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]
- 28.Chase T.N., Mouradian M.M., Engber T.M. Motor response complications and the function of striatal efferent systems. Neurology. 1993;43(12 Suppl. 6):S23–S27. [PubMed] [Google Scholar]
- 29.Wickremaratchi M.M., Ben-Shlomo Y., Morris H.R. The effect of onset age on the clinical features of Parkinson's disease. Eur J Neurol. 2009;16(4):450–456. doi: 10.1111/j.1468-1331.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 30.Fox S.H., Katzenschlager R., Lim S.Y. The movement disorder society evidence-based medicine review update: treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl. 3):S2–S41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 31.Hisahara S., Shimohama S. Dopamine receptors and Parkinson's disease. Int J Med Chem. 2011;2011 doi: 10.1155/2011/403039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsden C.D., Parkes J.D., Quinn N. Fluctuations and disability in Parkinson's disease: clinical aspects. In: Marsden C.D., Fahn S., editors. Movement Disorders. Butterworth Scientific; London: 1982. [Google Scholar]
- 33.Deleu D., Hanssens Y., Northway M. Subcutaneous apomorphine. Drugs Aging. 2004;21(11):687–709. doi: 10.2165/00002512-200421110-00001. [DOI] [PubMed] [Google Scholar]
- 34.Bonner L.A., Laban U., Chemel B.R. Mapping the catechol binding site in dopamine D(1) receptors: synthesis and evaluation of two parallel series of bicyclic dopamine analogues. ChemMedChem. 2011;6(6):1024–1040. doi: 10.1002/cmdc.201100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan H., DuRand C.J., Teeter M.M. Structural determinants of pharmacological specificity between D1 and D2 dopamine receptors. Mol Pharmacol. 2006;69(1):185–194. doi: 10.1124/mol.105.017244. [DOI] [PubMed] [Google Scholar]
- 36.Hughes A.J., Lees A.J., Stern G.M. Apomorphine test to predict dopaminergic responsiveness in Parkinsonian syndromes. Lancet. 1990;336(8706):32–34. doi: 10.1016/0140-6736(90)91531-e. [DOI] [PubMed] [Google Scholar]
- 37.Rossi P., Colosimo C., Moro E. Acute challenge with apomorphine and levodopa in Parkinsonism. Eur Neurol. 2000;43(2):95–101. doi: 10.1159/000008142. [DOI] [PubMed] [Google Scholar]
- 38.Lees A.J. Dopamine agonists in Parkinson's disease: a look at apomorphine. Fundam Clin Pharmacol. 1993;7(3–4):121–128. doi: 10.1111/j.1472-8206.1993.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 39.Katzenschlager R. Apomorphine in the treatment of Parkinson's disease. Eur Neurol Rev. 2009;4(1):28. [Google Scholar]
- 40.Zaleska B., Domzal T. Apomorphine in treatment of Parkinson's disease with fluctuations. Neurol Neurochir Pol. 1999;33(6):1297–1303. [PubMed] [Google Scholar]
- 41.Pietz K., Hagell P., Odin P. Subcutaneous apomorphine in late stage Parkinson's disease: a long term follow up. J Neurol Neurosurg Psychiatry. 1998;65(5):709–716. doi: 10.1136/jnnp.65.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albanese A. Acute challenge with apomorphine and levodopa in Parkinsonism. Focus Parkinsons Dis. 2001;13(3):60–63. doi: 10.1159/000008142. [DOI] [PubMed] [Google Scholar]
- 43.Deffond D., Durif F., Tournilhac M. Apomorphine in treatment of Parkinson's disease: comparison between subcutaneous and sublingual routes. J Neurol Neurosurg Psychiatry. 1993;56(1):101. doi: 10.1136/jnnp.56.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrido J.M.P.J., Delerue-Matos C., Borges F. New insights into the oxidation pathways of apomorphine. J Chem Soc Perkin Trans II. 2002;10:1713–1717. [Google Scholar]
- 45.Burkman A.M. Some kinetic and thermodynamic characteristics of apomorphine degradation. J Pharm Sci. 1965;54(2):325–326. doi: 10.1002/jps.2600540242. [DOI] [PubMed] [Google Scholar]
- 46.Ng Ying Kin N.M., Lal S., Thavundayil J.X. Stability of apomorphine hydrochloride in aqueous sodium bisulphite solutions. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(7):1461–1468. doi: 10.1016/s0278-5846(01)00188-9. [DOI] [PubMed] [Google Scholar]
- 47.Cheng H.Y., Strope E., Adams R.N. Electrochemical studies of the oxidation pathways of apomorphine. Anal Chem. 1979;51(13):2243–2246. [Google Scholar]
- 48.Sam E., Augustinjns P., Verbeke N. Stability of apomorphine in plasma and its determination by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;658:311–317. doi: 10.1016/0378-4347(94)00239-8. [DOI] [PubMed] [Google Scholar]
- 49.Wilcox R.E., Humphrey D.W., Riffee W.H. Stability of apomorphine in solutions containing ascorbic acid and bisulfite and effects of antioxidants on apomorphine-induced cage climbing and hypothermia in mice. J Pharm Sci. 1980;69(8):974–976. doi: 10.1002/jps.2600690830. [DOI] [PubMed] [Google Scholar]
- 50.Priston M.J., Sewell G.J. The analysis of apomorphine formulations for ambulatory infusions. Pharm Pharmacol Commun. 1995;1(2):91–94. [Google Scholar]
- 51.van der Geest R., van Laar T., Kruger P.P. Pharmacokinetics, enantiomer interconversion, and metabolism of R-apomorphine in patients with idiopathic Parkinson's disease. Clin Neuropharmacol. 1998;21(3):159–168. [PubMed] [Google Scholar]
- 52.Cambell A., Kula N., Jeppsson B. Oral bioavailability of apomorphine in the rat with a portacaval venous anastomosis. Eur J Pharmacol. 1980;67:139–142. doi: 10.1016/0014-2999(80)90022-9. [DOI] [PubMed] [Google Scholar]
- 53.LeWitt P.A. Subcutaneously administered apomorphine: pharmacokinetics and metabolism. Neurology. 2004;62(6 Suppl. 4):S8–S11. doi: 10.1212/wnl.62.6_suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 54.Missala K., Lal S., Sourkes T.L. O-methylation of apomorphine and the metabolic prolongation of apomorphine-induced stereotyped behaviour. Eur J Pharmacol. 1973;22(1):54–58. doi: 10.1016/0014-2999(73)90183-0. [DOI] [PubMed] [Google Scholar]
- 55.McKenzie G.M., White H.L. Evidence for the methylation of apomorphine by catechol-O-methyl-transferase in vivo and in vitro. Biochem Pharmacol. 1973;22(18):2329–2336. doi: 10.1016/0006-2952(73)90014-2. [DOI] [PubMed] [Google Scholar]
- 56.Thomas N.L., Coughtrie M.W.H. Sulfation of apomorphine by human sulfotransferases: evidence of a major role for the polymorphic phenol sulfotransferase, SULT1A1. Xenobiotica. 2003;33(11):1139–1148. doi: 10.1080/00498250310001609192. [DOI] [PubMed] [Google Scholar]
- 57.Gancher S., Nutt J., Woodward W. Absorption of apomorphine by various route in Parkinsonism. Mov Disord. 1991;6(3):212–216. doi: 10.1002/mds.870060304. [DOI] [PubMed] [Google Scholar]
- 58.Sam E., Jeanjean A.P., Maloteaux J.M. Apomorphine pharmacokinetics in parkinsonism after intranasal and subcutaneous application. Eur J Drug Metab Pharmacokinet. 1995;20(1):27–33. doi: 10.1007/BF03192285. [DOI] [PubMed] [Google Scholar]
- 59.Lees A.J. The on-off phenomenon. J Neurol Neurosurg Psychiatr. 1989;52(Suppl.):29–37. doi: 10.1136/jnnp.52.suppl.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borgman R.J., Baldessarini R.J., Walton K.G. Diester derivatives as apomorphine prodrugs. J Med Chem. 1976;19(5):717–719. doi: 10.1021/jm00227a026. [DOI] [PubMed] [Google Scholar]
- 61.Borgman R.J., McPhillips J.J., Stitzel R.E. Synthesis and pharmacology of centrally acting dopamine derivatives and analogs in relation to Parkinson's disease. J Med Chem. 1973;16(6):630–633. doi: 10.1021/jm00264a011. [DOI] [PubMed] [Google Scholar]
- 62.Liu K.S., Wen C.J., Yen T.C. Combined strategies of apomorphine diester prodrugs and nanostructured lipid carriers for efficient brain targeting. Nanotechnology. 2012;23(9):1–14. doi: 10.1088/0957-4484/23/9/095103. [DOI] [PubMed] [Google Scholar]
- 63.Borkar N., Li B., Holm R. Lipophilic prodrugs of apomorphine I: preparation, characterisation, and in vitro enzymatic hydrolysis in biorelevant media. Eur J Pharm Biopharm. 2015;89:216–223. doi: 10.1016/j.ejpb.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Borkar N., Andersson D.R., Yang M. Efficacy of oral lipid-based formulations of apomorphine and its diester in a Parkinson's disease rat model. J Pharm Pharmacol. 2017;69(9):1110–1115. doi: 10.1111/jphp.12758. [DOI] [PubMed] [Google Scholar]
- 65.Lincoln L., Fisher R., Jackson M.J. Oral r-(-)-11-o-valeryl-n-n-propylnoraporphine reverses motor deficits in MPTP-treated marmosets. Mov Disord. 2016;31(9):1381–1388. doi: 10.1002/mds.26626. [DOI] [PubMed] [Google Scholar]
- 66.Tsai M.J., Huang Y.B., Wu P.C. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. 2011;100(2):547–557. doi: 10.1002/jps.22285. [DOI] [PubMed] [Google Scholar]
- 67.Blomhoff R., Helgerud P., Dueland S. Lymphatic absorption and transport of retinol and vitamin D-3 from rat intestine evidence for different pathways. Biochim Biophys Acta. 1984;772(2):109–116. doi: 10.1016/0005-2736(84)90033-6. [DOI] [PubMed] [Google Scholar]
- 68.Sylvén C., Borgström B. Intestinal absorption and lymphatic transport of cholesterol in the rat: influence of the fatty acid chain length of the carrier triglyceride. J Lipid Res. 1969;10(4):351–355. [PubMed] [Google Scholar]
- 69.Holm R., Hoest J. Successful in silico predicting of intestinal lymphatic transfer. Int J Pharm. 2004;272(1):189–193. doi: 10.1016/j.ijpharm.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Trevaskis N.L., Charman W.N., Porter C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60(6):702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borkar N., Chen Z., Saaby L. Apomorphine and its esters: differences in Caco-2 cell permeability and chylomicron affinity. Int J Pharm. 2016;509(1–2):499–506. doi: 10.1016/j.ijpharm.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Borkar N., Holm R., Yang M. In vivo evaluation of lipid-based formulations for oral delivery of apomorphine and its diester prodrugs. Int J Pharm. 2016;513(1–2):211–217. doi: 10.1016/j.ijpharm.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 73.Lees A.J., Montastruc J.L., Turjanski N. Sublingual apomorphine and Parkinson's disease. J Neurol Neurosurg Psychiatr. 1989;52(12):1440. doi: 10.1136/jnnp.52.12.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Durif F., Serre F., Deffond D. Efficacy of sublingual apomorphine in idiopathic Parkinson's disease. Eur J Pharmacol. 1990;183(2):528–529. [Google Scholar]
- 75.Hauser R.A., Olanow C.W., Dzyngel B. Sublingual apomorphine (APL-130277) for the acute conversion of OFF to ON in Parkinson's disease. Mov Disord. 2016;31(9):1366–1372. doi: 10.1002/mds.26697. [DOI] [PubMed] [Google Scholar]
- 76.Dubow J., Dzyngel B., Bilbault T. Baseline disease severity not predictive of sublingual apomorphine (APL-130277) dose needed to convert a PD patient from the off to on state. Parkinsonism Relat Disord. 2016;22:e21–e22. [Google Scholar]
- 77.Bilbault T., Taylor S., Walker R. Buccal mucosal irritation studies of sublingual apomorphine film (APL-130277) in Syrian golden hamsters. Ther Deliv. 2016;7(9):611–618. doi: 10.4155/tde-2016-0043. [DOI] [PubMed] [Google Scholar]
- 78.Jadhav K., Gambhire M., Shaikh I. Nasal drug delivery system-factors affecting and applications. Curr Drug Ther. 2007;2(1):27–38. [Google Scholar]
- 79.Kapoor R., Turjanski N., Frankel J. Intranasal apomorphine: a new treatment in Parkinson's disease. J Neurol Neurosurg Psychiatr. 1990;53(11):1015. doi: 10.1136/jnnp.53.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ugwoke M.I., Kaufmann G., Verbeke N. Intranasal bioavailability of apomorphine from carboxymethylcellulose-based drug delivery systems. Int J Pharm. 2000;202(1–2):125–131. doi: 10.1016/s0378-5173(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 81.Ugwoke M.I., Exaud S., Van Den Mooter G. Bioavailability of apomorphine following intranasal administration of mucoadhesive drug delivery systems in rabbits. Eur J Pharm Sci. 1999;9(2):213–219. doi: 10.1016/s0928-0987(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 82.Ugwoke M.I., Agu R.U., Vanbilloen H. Scintigraphic evaluation in rabbits of nasal drug delivery systems based on carbopol 971p® and carboxymethylcellulose. J Control Release. 2000;68(2):207–214. doi: 10.1016/s0168-3659(00)00258-3. [DOI] [PubMed] [Google Scholar]
- 83.Ugwoke M.I., Sam E., Van Den Mooter G. Nasal mucoadhesive delivery systems of the anti-Parkinsonian drug, apomorphine: influence of drug-loading on in vitro and in vivo release in rabbits. Int J Pharm. 1999;181(1):125–138. doi: 10.1016/s0378-5173(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 84.Netsomboon K., Partenhauser A., Rohrer J. Preactivated thiomers for intranasal delivery of apomorphine: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;109:35–42. doi: 10.1016/j.ejpb.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 85.Iqbal J., Shahnaz G., Dunnhaupt S. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials. 2012;33(5):1528–1535. doi: 10.1016/j.biomaterials.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grosset K.A., Malek N., Morgan F. Inhaled dry powder apomorphine (VR040) for ‘off’ periods in Parkinson's disease: an in-clinic double-blind dose ranging study. Acta Neurol Scand. 2013;128(3):166–171. doi: 10.1111/ane.12107. [DOI] [PubMed] [Google Scholar]
- 87.Peira E., Scolari P., Gasco M.R. Transdermal permeation of apomorphine through hairless mouse skin from microemulsions. Int J Pharm. 2001;226(1–2):47–51. doi: 10.1016/s0378-5173(01)00759-1. [DOI] [PubMed] [Google Scholar]
- 88.Priano L., Albani G., Brioschi A. Transdermal apomorphine permeation from microemulsions: a new treatment in Parkinson's disease. Mov Disord. 2004;19(8):937–942. doi: 10.1002/mds.20054. [DOI] [PubMed] [Google Scholar]
- 89.Green P.G. Iontophoretic delivery of peptide drugs. J Control Release. 1996;41(1):33–48. [Google Scholar]
- 90.Li G.L., de Vries J.J., van Steeg T.J. Transdermal iontophoretic delivery of apomorphine in patients improved by surfactant formulation pretreatment. J Control Release. 2005;101(1–3):199–208. doi: 10.1016/j.jconrel.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Li G.L., Danhof M., Frederik P.M. Pretreatment with a water-based surfactant formulation affects transdermal iontophoretic delivery of R-Apomorphine in vitro. Pharm Res. 2003;20(4):653–659. doi: 10.1023/a:1023211219118. [DOI] [PubMed] [Google Scholar]
- 92.van Laar T., Jansen E.N., Essink A.W. Rectal apomorphine: a new treatment modality in Parkinson's disease. J Neurol Neurosurg Psychiatr. 1992;55(8):737. doi: 10.1136/jnnp.55.8.737-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hughes A.J., Bishop S., Lees A.J. Rectal apomorphine in Parkinson's disease. Lancet. 1991;337(8733):118. doi: 10.1016/0140-6736(91)90780-s. [DOI] [PubMed] [Google Scholar]
- 94.van Laar T., Jansen E.N.H., Neef C. Pharmacokinetics and clinical efficacy of rectal apomorphine in patients with Parkinson's disease: a study of five different suppositories. Mov Disord. 1995;10(4):433–439. doi: 10.1002/mds.870100405. [DOI] [PubMed] [Google Scholar]