Graphical Abstract
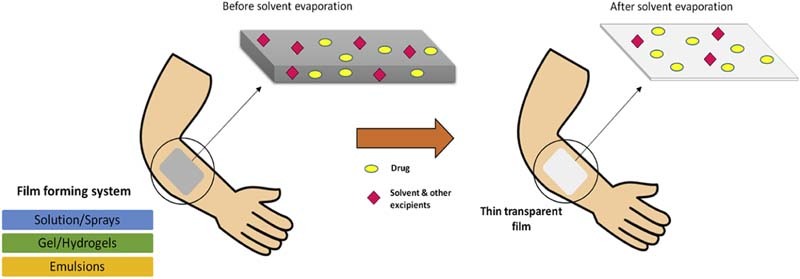
After application of the film forming system to the skin, the composition of the film forming system changes significantly due to the loss of the volatile components of the vehicle which results in formation of residual transparent film on the skin surface.
Keywords: Film forming polymers, Topical drug delivery, Gelling agents
Abstract
Skin is considered as an important route of administration of drugs for both local and systemic effects. The effectiveness of topical therapy depends on the physicochemical properties of the drug and adherence of the patient to the treatment regimen as well as the system's ability to adhere to skin during the therapy so as to promote drug penetration through the skin barrier. Conventional formulations for topical and dermatological administration of drugs have certain limitations like poor adherence to skin, poor permeability and compromised patient compliance. For the treatment of diseases of body tissues and wounds, the drug has to be maintained at the site of treatment for an effective period of time. Topical film forming systems are such developing drug delivery systems meant for topical application to the skin, which adhere to the body, forming a thin transparent film and provide delivery of the active ingredients to the body tissue. These are intended for skin application as emollient or protective and for local action or transdermal penetration of medicament for systemic action. The transparency is an appreciable feature of this polymeric system which greatly influences the patient acceptance. In the current discussion, the film forming systems are described as a promising choice for topical and transdermal drug delivery. Further the various types of film forming systems (sprays/solutions, gels and emulsions) along with their evaluation parameters have also been reviewed.
1. Introduction
The skin is the most readily accessible organ of the body and acts as a barrier against the micro and macromolecules of the environment because of its low permeability to such substances [1]. The skin of an average adult body has approximately 2 m2 surface area and it receives about one-third of the total blood circulating throughout the body [2]. Percutaneous absorption of drug through skin mainly occurs via stratum corneum. Stratum corneum is made up of dead, keratinized epidermal cells having thickness of 10 µm and acts as a barrier for permeation of drugs. Therefore transport of drug molecules across the skin is difficult [3].
The goal of drug administration through skin is for topical treatment of skin diseases or for transdermal absorption of drugs in the systemic circulation. The topical route offers a large and varied surface in addition to the ease of application via self-administration and provides an alternative to oral delivery of drugs as well as hypodermic injection [4]. The rate and extent of drug absorption through skin depends on the skin physiology and physicochemical properties of drugs as well as the delivery system. The current dosage forms, i.e. patches, ointments, creams, etc., are associated with several limitations. Patches have various disadvantages, most commonly skin irritation [5], because of their occlusive properties causing obstruction of sweat ducts, which in turn prevents loss of water vapor from skin surface, difficulty in applying on the curved surfaces, pain while peeling off and poor aesthetic appeal. Semisolid preparations like creams and ointments overcome some of these drawbacks but have other limitations. These do not ensure persistent contact with the skin surface and can be easily wiped off by patient's clothes [6]. Hence repeated application is required in case of chronic diseases like athlete's foot, ringworm and candidiasis [7]. Also these leave a sticky and greasy feel after application leading to poor patient compliance [8], [9]. Therefore there is a need for development of a dosage form which permits less frequent dosing by maintaining a close contact with the skin for prolonged time period thereby improving the patient compliance.
Film forming system (FFS) is a novel approach which can be used as an alternative to conventional topical and transdermal formulations. It is defined as non-solid dosage form that produces a film in situ, i.e. after application on the skin or any other body surface. These systems contain the drug and film forming excipients in a vehicle which, upon contact with the skin, leaves behind a film of excipients along with the drug upon solvent evaporation. The formed film can either be a solid polymeric material that acts as matrix for sustained release of drug to the skin or a residual liquid film which is rapidly absorbed in the stratum corneum [10].
2. Mechanism of film formation and permeation
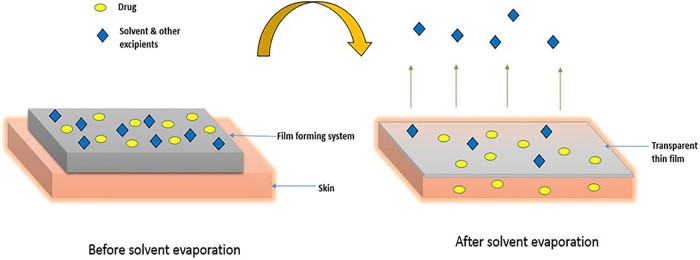
Film forming system is applied directly to the skin and it forms a thin, transparent film in situ upon solvent evaporation as shown in Fig. 1.
Fig. 1.
Mechanism of film formation.
After application of the formulation to the skin, the composition of the film forming system changes significantly due to the loss of the volatile components of the vehicle which results in formation of residual film on the skin surface. In this process the concentration of drug increases, reaching saturation level and with the possibility of reaching supersaturation level on the skin surface. Supersaturation results in the enhanced drug flux through the skin by increasing the thermodynamic activity of the formulation without affecting the skin's barrier, thereby reducing the side effects or irritation [10], [11].
The concept of supersaturation can be explained by the modified form of Fick's law of diffusion. Fick's law of diffusion is given by Eq. (2.1):
| (2.1) |
where
J = rate of drug permeation per unit area of skin per unit time (flux)
D = diffusion coefficient of drug
Cv = concentration of drug
h = thickness of barrier to diffusion
From this equation, it is clear that the rate of drug permeation across the skin is proportional to the concentration of the drug. However this is true when all the drug is dissolved in the vehicle.
Equation (2.2) describes the modified form of Fick's law of diffusion:
| (2.2) |
where
α = thermodynamic activity of drug within formulation
γ = thermodynamic activity of drug within membrane
According to this equation, the flux of the drug is directly proportional to the thermodynamic activity of the system, which is related to saturation. However increasing the supersaturation increases thermodynamic instability [12].
FFS creates supersaturated systems immediately after application to the skin, overcoming the problem of instability. Thus it improves the drug permeation through skin compared to other transdermal dosage forms.
The delivery efficiency of the film forming solutions for ethinylestradiol was investigated. The permeation of ethinylestradiol from the film forming solution prepared with enhancer or without enhancer was compared to the permeation from the commercially available patch (EVRA®) through human epidermis in vitro. The film forming formulations showed a higher permeation than the commercial patch. Without enhancer the formulation transported more than double the ethinylestradiol than the marketed patch. With enhancer, the formulation delivered about seven times as much ethinylestradiol as that of the marketed patch. Thus these systems prove to be useful in enhancing the drug permeation [13].
3. Comparison of topical drug delivery systems
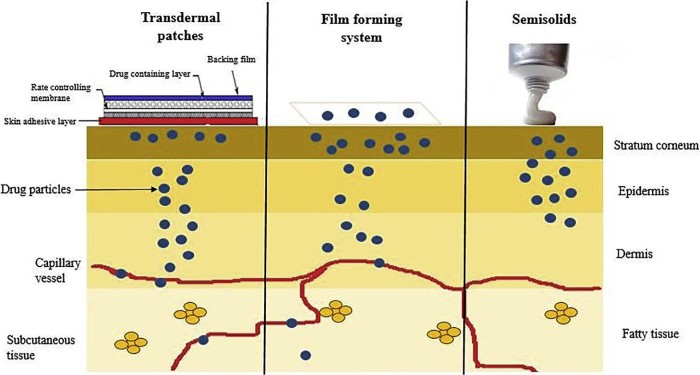
FFS form an intermediate between the transdermal patches and semisolid dosage forms. Thus exhibiting the advantages of both systems. Table 1 summarizes the superiority of film forming systems over patches and ointments. Fig. 2 depicts the drug permeation pattern of all the three systems. In case of transdermal patches the drug is stored in a reservoir from which the drug release occurs slowly and the drug is absorbed into the capillaries from where it is transported to systemic circulation or it is formulated as a topical patch so as to penetrate the skin to reach the target tissue for localized action. Drugs incorporated into semisolids show their activity on the skin surface or penetrate into skin layers to reach the site of action but systemic delivery of drugs is limited due to various factors. Film forming systems can function as both semisolids and patches and can provide topical as well as transdermal delivery as desired.
Table 1.
Comparison of topical drug delivery systems.
| Patches | Film forming system | Semisolids | |
|---|---|---|---|
| Visual appearance | Highly visible | Almost invisible | Visible |
| Skin feel | Non-sticky, non-greasy | Non-sticky, non-greasy | Sometimes sticky, greasy |
| Administration | Convenient | Convenient | Sometimes messy |
| Dose adjustment | Low | High | High |
| Dosing frequency | 1–7 d | 1–2 d | 1 d or less |
| Sustained release | Yes | Yes | No |
| Occlusive properties | Yes | No | No |
| Wipe off resistance | Yes | Yes | No |
| Residual remains | Possible | No | No |
Fig. 2.
Release profile of the topical and transdermal drug delivery systems.
4. Applications of film forming systems
Initially film forming systems were predominantly used in the field of surgery or wound care. Film forming solutions or gels have been used as tissue glues for the closing of operative wounds. The film formers used for this purpose may be natural like fibrin or synthetic like cyanoacrylates. These wound care preparations can be without drugs or with antimicrobial agents to prevent infections in the wounds [14]. The film forming wound care products are listed in Table 2 [13]. It can also be used for non-medical uses, such as the delivery of active ingredients contained in beauty products like silicone film forming technologies used to prepare cosmetic creams and ointments [15]. It can also be used as transparent peel off mask technologies for skin hydration treatment, acne problems, etc. [16]. The film forming technology also has potential application as a substrate for various barrier membranes used in the industry. Barrier membranes are widely used to protect workers from detergents, acids, bases and other hazardous chemicals, infra-red heat, UV exposure etc., e.g. hydrophilic and hydrophobic creams and ointments, UV protecting creams [17]. Film forming polymers are sprayed on the soil which forms a membrane film thus increasing the integrity of soil and elevating the soil temperature, which is useful in crop protection [18].
Table 2.
Film forming wound care products (reproduced from Ref. [14]).
| Trade names | Manufacturer | Film forming polymer |
|---|---|---|
| Dermabond® | Ethicon GmbH, Germany | Octylcyanoacrylate |
| EPIGLU® Gewebekleber | Meyer-Haake GmbH, Germany | Ethylcyanoacrylate, Poly(methylmethacrylate) |
| Flint® Sprühverband | Togal, Germany | Poly(butylmethacrylate, methylmethacrylate) |
| BandAid® Sprühpflaster | Ethicon GmbH, Germany | Cellulose acetate butanoate |
| Opsite® Spray | Smith & Nephew GmbH, Austria | Poly(methylacrylate) |
5. Properties of film forming system
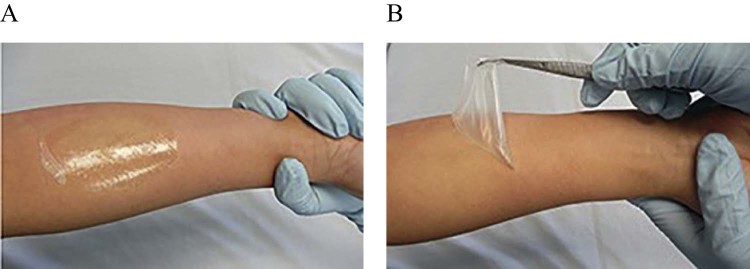
The film forming preparation can be applied to the site regardless of shape and area, and can be retained for a long time as compared to conventional semi-solid preparations. Fig. 3(A) shows that FFS forms an almost completely transparent fast drying film on application. Fig. 3(B) shows that after drying, a non-tacky, flexible and easily peelable film is formed. There is an excellent adhesion of the formed film to the skin, hence wipe off resistance. Therefore the risk of transfer of active ingredients to other people or clothes is reduced.
Fig. 3.
Appearance of film forming system: (A) Formation of transparent film on application; (B) Non-tacky, flexible, easily peelable film after drying.
6. Film forming formulations
6.1. Sprays/solutions
Film forming solutions and sprays is an attractive approach in transdermal dosage form. In this the polymeric solution is applied to the skin as a liquid or sprayed on the skin and forms an almost transparent film by solvent evaporation [19].
The film forming sprays/solutions are made up of four main components – drug, solvent systems i.e. volatile and non-volatile vehicles, polymers and penetration enhancers. The non-volatile component present in the solvent system prevents the drug from precipitating in solution when the volatile solvent component evaporates. The non-volatile component is chosen such that it itself partitions rapidly into the stratum corneum and also aids in partitioning of the drug into the stratum corneum, as well as increases drug diffusivity by disrupting the ordered intercellular lipids and enhance permeation. This type of delivery system creates an invisible depot of drug in the stratum corneum from which the drug can be slowly absorbed into the systemic circulation. Thus a sustained and enhanced permeation of drug across the skin can be achieved following once a day application [20], [21].
The formulation preparation involves addition of the polymer to the vehicle and stirring of the solution overnight to ensure complete dissolution of the polymer. Once a clear polymeric solution is obtained other optional excipients such as cross linker or plasticizer are added. After addition of all excipients the solution is stirred for 24 h [22]. For the physical stability of the API, the polymers are chosen such that they function as anti-nucleating agents and crystallization inhibitors which prevent crystallization of drug even after solvent evaporation, e.g. polyvinyl pyrrolidone, polyethylene glycol, hydroxyl propyl methyl cellulose.
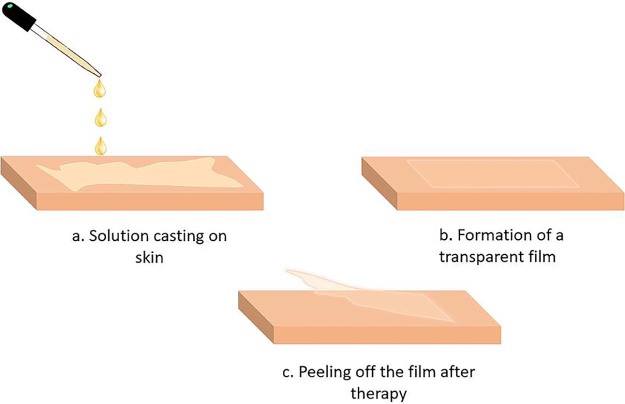
Film forming solutions can be applied with an applicator to the skin and allowed to dry. Film forming spray is manufactured as a metered dose pump dispenser to provide fixed amount of drug and it is sprayed on the topical site to form a film. These systems form a stable fast drying, non-irritating invisible film from which the drug is available for transdermal therapy [23]. Following administration, the film can be peeled off once the desired results are obtained or for the termination of therapy as shown in Fig. 4.
Fig. 4.
Application of film forming solution on skin.
Misra et al. prepared a liquid film forming solution using a mixture of polyvinyl pyrrolidone and polyvinyl alcohol in isopropanol as film forming polymeric solutions for the biphasic delivery of testosterone [24]. Ammar et al. developed a film forming polymeric solution of ketorolac using Eudragit and polyvinyl pyrrolidone in ethanol as film forming agents [22]. The mechanical properties and appearance of the prepared formulations were evaluated.
Gohel and Nagori developed a fluconazole spray containing ethyl cellulose and Eudragit RS 100 as film formers [25]. Yu et al. developed transdermal film-forming spray containing estradiol and optimized the formulation using different polymers and plasticizers for efficient penetration of estradiol for longer duration of time as compared to gel and patch [26].
6.2. Gels
Gels are defined as semisolid dosage form containing both solid and liquid components. The liquid component may be hydrophobic or hydrophilic in nature, immobilized in a three dimensional network of the interconnected solid components [27]. Hydrogels are the aqueous gels containing hydrophilic polymers that form three dimensional network in water [28].
The development of transdermal formulations is being focused on employing several polymers as film forming agents along gelling agents. Various gelling agents are listed in Table 3. The administration of film forming gel involves applying a dose on the arms, shoulders, internal parts of the thighs or abdomen to form a thin bioadhesive film on the skin [32]. The drug substance is dissolved in film forming vehicle and is thus incorporated in the film formed on skin. The film can function as an external reservoir or limit the supply of drug substance to the skin thereby controlling the release of drug [33].
Table 3.
Gelling agents.
| Gelling agent | Properties |
|---|---|
| Gellan gum |
|
| Carbomer (Carbopol®) Grades – ETD 2020, 171, 910, 934, 934P, 940, 1342 NF, 1971P [29] |
|
| Carboxymethyl cellulose |
|
| HPMC (E series, F series, K4M, K15M, K100M) |
|
| Hydroxy ethyl cellulose |
|
| Poloxamer (polyethylene–polypropylene glycol co-polymer) Grades – 124, 182, 188, 407 [30] |
|
| Sepineo P 600 (acrylamide/sodium acryloyldimethyltaurate copolymer) |
|
Complete skin contact over the entire application is essential; therefore, the formulation requires high flexibility to adapt to the movement of the skin, high substantivity, strong adhesion to the skin for constant delivery and absorption of drug. Hence, along with gelling agents, film forming agents, plasticizers, preservatives etc. are used in the formulation. Compared to other forms, these systems offer easier use and application, appropriate consistency and adhesiveness, good flexibility and elasticity and ease of manufacturing [34].
Vij and Saudagar developed a film forming gel for prolonged delivery of terbinafine hydrochloride. The polymers used were Eudragit and hydroxypropyl cellulose in combination to provide a matrix film which would allow the release of terbinafine for a prolonged time. The formulations were prepared using 32 full factorial design [33]. Li et al. developed a film-forming gel formulation for sustained release of rotigotine with hydroxypropyl cellulose and Carbomer 934. To optimize this formulation, the response surface analysis technique was applied [35].
Film forming hydrogels are majorly used in wound healing. The formulation applied to the wounded site provides a film that is resistant to physiological stress caused by the movement of skin.
Liu et al. developed the formulation of sustained release transparent film-forming hydrogels of tolterodine and studied the effects on stratum corneum with the help of response surface methodology technique [36]. An et al. developed a polyvinyl alcohol based soft hydrogel of testosterone for transdermal delivery. The formulation was in semisolid phase inside the tubes, but formed thin films within 2–3 min when applied to the skin [37].
6.3. Emulsions
Emulsions are semisolid or liquid preparations having the ability to solubilize both lipophilic and hydrophilic drugs. Pharmaceutical emulsions consist of mixtures of aqueous phase and oily phases stabilized by suitable emulsifying agents [38]. These can be oil-in-water (O/W) emulsions (oil phase is dispersed in the water phase) or water-in-oil (W/O) emulsions (water phase dispersed in an oily continuous phase). The type of emulsion formed depends mainly on the type of emulsifiers, which is characterized by the hydrophilic–lipophilic balance (HLB). The HLB is a scale from 1 to 20 and the higher the HLB, the more hydrophilic is the surface active agent. An emulsifying agent is a substance which stabilizes the emulsion. There are different types of emulsifying agents including surfactants, polymers, proteins (gelatin) and finely divided solid particles (bentonite).
Film forming emulsions, in addition to the oil phase and the aqueous phase, contain film forming polymer. The volatile components present in the emulsions evaporate leading to the changes in the tissue, allowing absorption of the drug [39]. The advantage of film forming emulsions over semisolid formulations is that, it allows treatment of larger areas of affected skin with an extended contact time and adequate substantivity, thus allowing sustained dermal therapy of chronic diseases [40].
The delivery of the drug through skin depends on the nature of the API and the type of emulsion. The dermal delivery of the lipophilic sunscreen agent ethylhexyl methoxycinnamate was higher from the W/O emulsion than from the O/W emulsion most probably because of the occlusive effect of the oily vehicle. But other studies have shown a discrepancy. It was observed that the skin permeation of lipophilic parabens was enhanced from O/W emulsions compared with the W/O emulsion. This was explained by a higher affinity of the parabens for the vehicle than for the stratum corneum in the case of the w/o emulsion [38].
Lunter et al. developed film forming emulsions for sustained dermal delivery of nonivamide containing Eudragit NE and RS 30D as film formers [41]. In another study by Lunter et al., the in vitro skin permeation and penetration of nonivamide from the prepared film-forming emulsions was studied. It was found that the rate of permeation of the active substance is determined by diffusion through the polymeric matrix in which the droplets were embedded. Thereby, constant permeation rates and efficient API concentrations in the skin could be maintained for a period of 12 h [40].
7. Components of film forming systems
7.1. Drug
For transdermal application of film forming systems, the drugs need to have suitable properties which are independent of the dosage form. Generally the drugs which are applicable to these systems are highly potent which permeate the skin rapidly, which cause no skin irritation and which are relatively stable to the enzymes present in the epidermis. Other properties of the drug like partition coefficient dictate the pathway a drug will follow through the skin. Second, the molecular weight of drug is an important factor in drug permeation as small molecules cross human skin than large molecules. The ideal properties of the drug suitable for transdermal drug delivery system are listed in Table 4.
Table 4.
Ideal properties of drug for transdermal delivery.
| Parameter | Properties |
|---|---|
| Dose | <10 mg/day |
| Half-life | 10 h or less |
| Molecular weight | <500 Dalton |
| Partition coefficient Log P (octanol/water) |
Between 1 and 3 |
| Skin reaction | Non irritating and non-sensitizing |
| Oral bioavailability | Low |
7.2. Polymers
Polymers are the foundation of the FFS and a variety of polymers are available for the preparation of these systems. In order to achieve the desired film properties, these polymers can be used alone or in combination with other film forming polymers [42]. These polymers should form a clear flexible film at skin temperature. The list of polymers along with their molecular weight and properties are mentioned in Table 5.
Table 5.
Film forming polymers.
| Polymer | Properties |
|---|---|
| Hydroxypropyl Methylcellulose (HPMC) HPMC (E4M, E15, E50M K4M) [43] |
|
| Ethyl cellulose (EC) |
|
| Hydroxypropyl cellulose |
|
| Polyvinyl pyrrolidine (PVP) (PVP K30, PVP VA64) |
|
| Polyvinyl alcohol (PVA) |
|
| Chitosan | |
| Eudragit (polymethacrylates copolymer) Eudragit RS 100, RL 100, NE, RS 30D, S 100 |
|
| Silicones Polydimethylsiloxane (PDMS) |
|
| Acrylates copolymer Avalure® AC 118, AC 120 |
|
7.3. Solvents
The solvents form an important component in film formation. The solvent used in film forming systems help in solubilizing the drugs as well as have an impact on drug permeation. Commonly used solvents for topical and transdermal use [53] are listed in Table 6. As these solvents are widely used, the safety of these has been established on long term use.
Table 6.
Solvents used in topical systems.
| Category | Examples |
|---|---|
| Glycols | Propylene glycols, polyethylene glycols |
| Alcohols | Ethanol, butanol, isopropanol, benzyl alcohol, lanolin alcohols, fatty alcohols |
| Other solvents | Ethyl acetate, oleic acid, isopropyl myristate |
7.4. Plasticizers
Plasticizers are used in the film forming systems to impart flexibility to the film and improve the tensile strength of the film formed. The plasticizer used should be compatible with the polymers used and should have low skin permeability. Commonly used plasticizers are glycerine, polyethylene glycol, sorbitol, dibutyl phthalate, propylene glycol, triethyl citrate etc. [54].
8. Evaluation of film forming system
8.1. Film formation
The films are formed in a Petri dish or on an excised pig ear skin. Film-formation is evaluated and rated as complete and uniform, incomplete or non-uniform, with or without precipitation of the film-forming polymer. The cosmetic aspects of the film are given in terms of transparency or opaque, sticky or dry, peelable or non-peelable [55].
8.2. Film flexibility
Film flexibility is evaluated on the basis of cracking and skin fixation and this is determined by stretching the skin in 2–3 directions. The film is rated flexible if there is no cracking or skin fixation and non-flexible if there is cracking and skin fixation.
8.3. Drying time
For the evaluation of the drying time the formulation is applied to the inner sides of the forearm of a volunteer. After a fixed time period a glass slide is placed on the film without pressure. If no liquid is visible on the glass slide after removal, the film is considered dry [56]. If remains of the liquid are visible on the glass slide the experiment is repeated with an increase in drying time. A good FFS should have a minimum drying time to avoid long waiting time for the patient.
8.4. Stickiness
The stickiness of the film formed is determined by pressing cotton wool on the dry film with low pressure. Depending on the quantity of cotton fibres that are retained by the film, the stickiness is rated high if there is dense accumulation of fibers on the film, medium if there is a thin fiber layer on the film and low if there is an occasional or no adherence of fibers. This evaluation parameter is essential, as the formulation should be non-sticky to avoid adherence to the patients' clothes [33].
8.5. Mechanical properties
The polymeric films are produced by solvent evaporation on a Teflon plate. The dry films are cut with the help of a scalpel. Film thickness is measured with a digital micrometer. The mechanical properties of the films are determined with a tensile tester.
The tensile strength (σ) is calculated as:
where Fmax (N) is the maximum force and A (m2) is the cross-sectional area [19].
8.6. Determination of the water vapor permeability
The water vapor permeability is defined as the quantity of water transmitted through a unit area of film in unit time. These water vapor permeation data are important in determining the permeation characteristics of the film as they have influence on skin properties like hydration of stratum corneum, blood flow, and skin temperature [57]. Films are produced with a solvent evaporation technique on a Teflon plate and dried for 72 h at room temperature. Circular samples are cut from the dry film sheets. For the sample preparation glass vials with an opening are filled with distilled water, covered with the circular film samples and a silicone ring, and sealed tightly with an aluminum vial cap. The weight of the vial is determined and then placed into a desiccator creating an atmosphere of 58% relative humidity or low relative humidity (approximately 0%). They are kept at a determined temperature for 72 h and weighed after predetermined intervals. From the weight loss of the vials W (g) the water vapor permeability is calculated as the amount of water that permeates through the film in relation to the surface area A (cm2) and the time t (h) [19]:
8.7. Swab studies
Swab test can be performed to evaluate the residence time of film forming system. For adhesion testing, glass was used as a polar, hydrophilic substrate. Glass was chosen as test surface because films adhering strongly to it would also show strong adherence to skin because both materials display a polar surface structure [41].
Dry swab test: This test indicates the behavior of FFS on the skin in dry condition. Dry swab test can be carried out on a glass plate. The glass plate is marked with 6 squares of 1 × 1 cm2. Developed formulation is applied in this area. Dry cotton swabs of the same volume are taken. Swabbing on the applied film is carried out at 0 min, 30 min, 2 h, 4 h, 6 h and 8 h and checked for drug content after extraction of drug from the swab.
Wet swab test: This test depicts the behavior of FFS when it comes in contact with water or sweat. The procedure for the wet swab test is the same as dry swab test except the swab taken is soaked in water before and then the formulations are swabbed with this wet swab.
8.8. Film topography
Atomic force microscopy (AFM) provides information about the topographic and mechanical properties of the polymeric films and helps to match the mechanical properties of the formed films to those of skin. It generates a nanoscale image of the film's homogeneity and roughness and requires no special treatment prior to the measurement [58].
8.9. Film homogeneity
Raman spectroscopy provides information about the chemical composition of the polymeric films. The chemical maps obtained from Raman spectra provide a measure of chemical homogeneity of films. Techniques based on Raman scattering can also be used to track the permeation of topically applied compounds through the skin [58].
8.10. In vitro diffusion study
The in vitro diffusion studies are used to predict the permeation characteristics of drug in vivo. Franz diffusion cell is used to determine the release profile of the drug from the film forming system. The cell is made up of two compartments, the donor and the receiver compartment between which the diffusion membrane is attached (egg membrane or cellophane). The donor compartment is exposed to the atmosphere and the receptor compartment contains the diffusion medium. The sampling arm in the receptor compartment allows for sampling. Predetermined quantity of the drug containing film forming formulation is placed on the donor compartment. Samples are collected and analyzed by suitable spectroscopic method for drug release [33].
8.11. Ex vivo permeation study
The ex vivo permeation studies are performed to study the effects of skin barrier on the developed film forming system. Franz diffusion cell/Keshary–Chien diffusion cell can be used for permeation study. Rat's skin is mounted between the two compartments, stratum corneum facing the donor compartment and dermis facing the receptor compartment. The formulation is applied to the skin surface which forms a film after drying. The receptor compartment contains phosphate buffered saline (pH 7.4) maintained at 37 ± 0.5 °C. Aliquots are collected at specific time intervals and analyzed by suitable spectroscopic method [59].
8.12. Skin penetration studies
The formulation is applied evenly on the skin using a pipette or a spatula. After fixed time intervals (e.g. 15 min, 1 h, 3 h, 6 h, 8 h, etc.) post application, the remaining formulation is removed. The film is wiped off with the help of cotton pads and the amount of drug present in the cotton pads is calculated, which is equivalent to the amount of drug remaining in the film. Therefore the amount of drug penetrated can be calculated by subtracting the remaining amount from the total amount of drug present in the formulation [60].
9. Commercialized film forming products
A number of companies have tried to develop film forming systems as a drug delivery platform and have marketed their products successfully. The companies with their products based on film forming technology are listed in Table 7.
Table 7.
Commercialized film forming system.
| Product | Drug | Company | Formulation type |
|---|---|---|---|
| Lamisil Once® [61] | Terbinafine hydrochloride | Novartis Consumer Health, Australasia, Pty Ltd | Film forming Solution |
| Axiron® [62] | Testosterone | Lilly USA, LLC | Film forming spray |
| Medspray® the Patch-in-a-Can® [63] | Terbinafine hydrochloride | MedPharm Ltd, UK | Film forming spray |
| Liqui-Patch technology [64] | Testosterone hydrocortisone | Epinamics GmbH, Germany | Film forming spray |
| Durapeel Technology [65] | Ropivacane | Crescita Therapeutics, Inc | Film forming gel |
| PharmaDur®Technology [66] | Hydroquinone | Polytherapeutics, Inc | Film forming emulsion-gel |
10. Conclusion and future prospects
The film forming system presents a novel platform to deliver drugs to the skin both topical and transdermal. These film forming systems are simple and offer advantages of transparency, non-greasy, lower skin irritation, wipe off resistance, longer retention, greater increased dosage flexibility, improved patient compliance and aesthetic appearance Although considerable work has been done on these systems, not much data are available on its delivery efficiency. Hence the marketed products available are less. Additional research is necessary to prove the relevance of film forming system as transdermal dosage form, but the obtained results are encouraging for further development of this novel topical drug delivering technology.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors acknowledge Vivekanand Education Society's College of Pharmacy, Chembur, Mumbai, for their support and encouragement.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Renata C.V., Tatiele K., Silvia G.S. Drug transport across skin. In: Muro S., editor. Drug delivery across physiological barriers. Taylor and Francis Group LLC; 2016. pp. 132–134. [Google Scholar]
- 2.Sharma N., Agarwal G., Rana A. A review: transdermal drug delivery system a tool for novel drug delivery system. Int J Drug Dev Res. 2011;3:70–84. [Google Scholar]
- 3.Michaels A.S., Chandrasekaran S.K., Shaw J.E. Drug permeation through human skin: theory and in vitro experimental measurement. AIChE J. 1975;21(5):985–996. [Google Scholar]
- 4.Prausnitz M.R., Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhiman S., Singh G.T., Rehni A.K. Transdermal patches: a recent approach to new drug delivery system. Int J Pharm Pharm Sci. 2011;3(5):26–34. [Google Scholar]
- 6.Advanced Film-Forming Agent for Transdermal Drug Delivery. http://marketplace.yet2.com/app/insight/techofweek/28842?Sid=350 Available from: [Accessed 12 December 2016]
- 7.Fungal Infections of the Skin. http://www.webmd.com/skin-problems-and-treatments/guide/fungal-infections-skin#1 Available from: [Accessed 24 December 2016]
- 8.Devaux S., Castela A., Archier E. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(3):61–67. doi: 10.1111/j.1468-3083.2012.04525.x. [DOI] [PubMed] [Google Scholar]
- 9.Tan X., Feldman S.R., Chang J. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9(10):1263–1271. doi: 10.1517/17425247.2012.711756. [DOI] [PubMed] [Google Scholar]
- 10.Mcauley W.J., Caserta F., Hoboken N.J. Film-forming and heated systems. In: Donnelly R.F., Singh T.R.R., editors. Novel delivery systems for transdermal and intradermal drug delivery. John Wiley & Sons; United States: 2015. pp. 97–107. [Google Scholar]
- 11.Frederiksen K., Guy R.H., Petersson K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin Drug Deliv. 2015;13(3):349–360. doi: 10.1517/17425247.2016.1124412. [DOI] [PubMed] [Google Scholar]
- 12.Brown M.B., Jones S.A., Limited M. 2006. Topical film-forming monophasic formulations. Patent CA2622624C. [Google Scholar]
- 13.Zurdo Schroeder I. 2007. Film forming polymeric solutions as drug delivery systems for the skin. Saarland University, Saarbrücken, Germany. [Accessed 21 December 2016] [DOI] [PubMed] [Google Scholar]
- 14.Bajaj H., Kumar T., Singh V. Film forming gels: a review. Res J Pharm Biol Chem Sci. 2016;7(4):2085–2091. [Google Scholar]
- 15.Klykken P., Servinski M., Thomas X. 2009. Silicone film-forming technologies for health care applications. 2–8. [Google Scholar]
- 16.Tech nature Peel-off masks,second skin effect. http://www.tech-nature.com/ Available from: [Accessed 21 November 2016]
- 17.Kurpiewska J., Liwkowicz J. The composition of waterproof barrier creams' ingredients and their barrier properties. CHEMIK. 2012;66(9):991–996. [Google Scholar]
- 18.Reddy P.P. vol. 95. Springer India; 2013. Disguising the leaf surface; pp. 91–95. (Recent advances in crop protection). ISBN: 9788132207238. [Google Scholar]
- 19.Zurdo Schroeder I., Franke P., Schaefer U.F. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur J Pharm Biopharm. 2007;65(1):111–121. doi: 10.1016/j.ejpb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Algin Y.E., Inal Ö. Transdermal spray in hormone delivery. Trop J Pharm Res. 2014;13(3):469–474. [Google Scholar]
- 21.Lu W., Luo H., Wu Y. Preparation and characterization of a metered dose transdermal spray for testosterone. Acta Pharm Sin B. 2013;3(6):392–399. [Google Scholar]
- 22.Ammar H.O., Ghorab M., Mahmoud A.A. Rapid pain relief using transdermal film forming polymeric solution of ketorolac. Pharm Dev Technol. 2011;18(5):1005–1016. doi: 10.3109/10837450.2011.627867. [DOI] [PubMed] [Google Scholar]
- 23.Lulla A., Malhotra G., Raut P. 2000. Topical spray compositions. Patent US6962691. [Google Scholar]
- 24.Misra A., Pal R., Majumdar S. Biphasic testosterone delivery profile observed with two different transdermal formulations. Pharm Res. 1997;14(9):1264–1268. doi: 10.1023/a:1012179529090. [DOI] [PubMed] [Google Scholar]
- 25.Gohel M.C., Nagori S.A. Fabrication of modified transport fluconazole transdermal spray containing ethyl cellulose and Eudragit® RS100 as film Formers. AAPS Pharm Sci Tech. 2009;10(2):684–691. doi: 10.1208/s12249-009-9256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Z., Liang Y., Liang W. Development and in vitro evaluation of estradiol transdermal film-forming spray. Acta Pharm Sin. 2013;48(5):746–751. [PubMed] [Google Scholar]
- 27.Rehman K., Zulfakar M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm. 2013;40(4):433–440. doi: 10.3109/03639045.2013.828219. [DOI] [PubMed] [Google Scholar]
- 28.Nerkar T.S., Gujarathi N.A., Rane B.R. In-situ gel: novel approach in sustained and controlled drug delivery system. Pharma Sci Monitor An Int J Pharm Sci. 2013;4(4):1–18. [Google Scholar]
- 29.Carbopol® and Pemulen™ products for topical applications; Comparison Overview of Carbopol®. https://www.lubrizol.com/en/Life-Sciences/Products/Carbopol-Polymer-Products/Products-for-Topical-Applications Available from: [Accessed 24 January 2017]
- 30.Niazi S.K. 3rd ed. vol. 3. Informa Healthcare; USA: 2004. p. 156. (Handbook of pharmaceutical manufacturing formulations). [Google Scholar]
- 31.SEPPIC Emulsifier, stabilizing agent, thickening agent. 2011. http://www.seppic.com/human-health/emulsifier-thickening-stabilizing-sepineo-p600-@/view-461-seproduit.html SEPINEO P600 – human health; Available from: [Accessed 24 January 2017]
- 32.Guo R., Du X., Zhang R. Bioadhesive film formed from a novel organic–inorganic hybrid gel for transdermal drug delivery system. Eur J Pharm Biopharm. 2011;79(3):574–583. doi: 10.1016/j.ejpb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Vij N.N., Saudagar R.B. Formulation, development and evaluation of film-forming gel for prolonged dermal delivery of terbinafine hydrochloride. Int J Pharm Sci Res. 2014;5(9):537–554. [Google Scholar]
- 34.Kim D.W., Kim K.S., Seo Y.G. Novel sodium fusidate-loaded film-forming hydrogel with easy application and excellent wound healing. Int J Pharm. 2015;495(1):67–74. doi: 10.1016/j.ijpharm.2015.08.082. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Zhang R., Liang R. Preparation and characterization of sustained-release rotigotine film-forming gel. Int J Pharm. 2014;460(1–2):273–279. doi: 10.1016/j.ijpharm.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Liu X., Fu L., Dai W. Design of transparent film-forming hydrogels of tolterodine and their effects on stratum corneum. Int J Pharm. 2014;471(1–2):322–331. doi: 10.1016/j.ijpharm.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 37.An N.M., Kim D.D., Shin Y.H. Development of a novel soft Hydrogel for the Transdermal delivery of testosterone. Drug Dev Ind Pharm. 2003;29(1):99–105. doi: 10.1081/ddc-120016688. [DOI] [PubMed] [Google Scholar]
- 38.Otto A., du Plessis J., Wiechers J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int J Cosmet Sci. 2009;31(1):1–19. doi: 10.1111/j.1468-2494.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 39.Nielloud F., Marti-Mestres G. Marcel Dekker, Inc.; New York: 2000. Pharmaceutical emulsions and suspensions. [Google Scholar]
- 40.Lunter D., Daniels R. In vitro skin permeation and penetration of nonivamide from novel film-forming emulsions. Skin Pharmacol Physiol. 2013;26(3):139–146. doi: 10.1159/000348464. [DOI] [PubMed] [Google Scholar]
- 41.Lunter D.J., Daniels R. New film forming emulsions containing Eudragit® NE and/or RS 30D for sustained dermal delivery of nonivamide. Eur J Pharm Biopharm. 2012;82(2):291–298. doi: 10.1016/j.ejpb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Karki S., Kim H., Na S.J. Thin films as an emerging platform for drug delivery. Asian J Pharm Sci. 2016;11(5):559–574. [Google Scholar]
- 43.Chandak A.R., Verma P.R.P. Development and evaluation of HPMC based matrices for transdermal patches of tramadol. Clin Res Regul Aff. 2008;25(1):13–30. [Google Scholar]
- 44.Vijaya R., Pratheeba C., Anuzvi A. Study of the hydroxy propyl methyl cellulose (hpmc) combinations in the development of transdermal film for amitriptyline HCl and their in vitro characterization. Int J Pharm Chem Biol Sci. 2015;5(3):548–556. [Google Scholar]
- 45.Patel D.P., Setty C.M., Mistry G.N. Development and evaluation of ethyl cellulose-based transdermal films of furosemide for improved in vitro skin permeation. AAPS Pharm Sci Tech. 2009;10(2):437–442. doi: 10.1208/s12249-009-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klucel™ hydroxypropylcellulose physical and chemical properties. www.ashland.com Available from: [Accessed 21 March 2017]
- 47.Bühler V. Berlin: Springer-Verlag Berlin and Heidelberg GmbH & Co. K; 2004. Polyvinylpyrrolidone excipients for pharmaceuticals povidone, crospovidone, and copovidone. [Google Scholar]
- 48.Kwon J.S., Kim D.Y., Seo H.W. Preparation of erythromycin-loaded poly(vinylalcohol) film and investigation of its feasibility as a transdermal delivery carrier. Tissue Eng Regen Med. 2014;11(3):211–216. [Google Scholar]
- 49.Sun Y., Cui F., Shi K. The effect of chitosan molecular weight on the characteristics of spray-dried methotrexate-loaded chitosan microspheres for nasal administration. Drug Dev Ind Pharm. 2009;35(3):379–386. doi: 10.1080/03639040802395185. [DOI] [PubMed] [Google Scholar]
- 50.Can A., Erdal M., Güngör S. Optimization and characterization of chitosan films for transdermal delivery of ondansetron. Molecules. 2013;18(5):5455–5471. doi: 10.3390/molecules18055455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joshi M. Role of eudragit in targeted drug delivery. Int J Curr Pharm Res. 2013;5(2):58–62. [Google Scholar]
- 52.Klykken P., Servinski M., Thomas X. Silicone film-forming technologies for health care applications. Dow Corning, 1–8.
- 53.Williams A.C., Walters K.A. Chemical penetration enhancement: possibilities and problems. In: Walters K.A., Roberts M.S., editors. Dermatologic, cosmeceutic, and cosmetic development: therapeutic and novel approaches. Informa Healthcare; New York: 2007. p. 502. [Google Scholar]
- 54.Güngör S., Erdal S.M., Özsoy Y. Plasticizers in transdermal drug delivery systems. In: Luqman M., editor. Recent advances in plasticizers. Intech; 2012. pp. 92–99.http://cdn.intechopen.com/pdfs/32871 Available from: [Google Scholar]
- 55.Frederiksen K., Guy R.H., Petersson K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur J Pharm Biopharm. 2015;91:9–15. doi: 10.1016/j.ejpb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Indrė S., Vitalis B. Effect of film-forming polymers on release of naftifine hydrochloride from nail lacquers. Int J Polym Sci. 2017;2017:7. doi: 10.1155/2017/1476270. [DOI] [Google Scholar]
- 57.Bharkatiya M., Nema R.M., Bhatnagar M. Development and characterization of transdermal patches of metoprolol tartrate. Asian J Pharm Clin Res. 2010;3(2):130–134. [Google Scholar]
- 58.Garvie-Cook H., Frederiksen K., Petersson K. Characterization of topical film-forming systems using atomic force microscopy and Raman microspectroscopy. Mol Pharm. 2015;12(3):751–757. doi: 10.1021/mp500582j. [DOI] [PubMed] [Google Scholar]
- 59.De A., Chakraborty S., Mukherjee A. Formulation & optimization of the transdermal film of 5-FU with in-vitro and ex-vivo study using ethyl cellulose and two grades of hydroxy propyl methyl cellulose. Pharm Sin. 2013;4(4):103–111. [Google Scholar]
- 60.Garvie-Cook H., Frederiksen K., Petersson K. Biophysical elucidation of the mechanism of enhanced drug release and topical delivery from polymeric film-forming systems. J Control Release. 2015;212:103–112. doi: 10.1016/j.jconrel.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Products – Lamisil Once®. Lamisil Once, Single dose treatment for athelete's foot. http://lamisil.com.au/product-oncefilm.html Available from: [Accessed 25 November 2016]
- 62.AXIRON (testosterone) topical solution. 2016. http://www.axiron.com/ Available from: [Accessed 25 November 2016]
- 63.MedSpray® Patch-in-a-Can® – MedPharm – topical pharmaceutical development services – dermal formulation – transdermal drug delivery – drug delivery service online. 2013. http://www.medpharm.co.uk/delivery-solutions/medspray-patch-in-a-can-2/ Available from: [Accessed 25 November 2016]
- 64.Kehr W. Platform technology. 2016. http://www.epinamics.com/platform-technology/ Epinamics GmbH, Germany; Available from: [Accessed 6 January 2017]
- 65.DuraPeel Crescita Therapeutics Inc Self-occluding, film-forming cream/gel formulations that provide extended release delivery to the site of application. 2016. http://www.crescitatherapeutics.com/technology/durapeel/ Available from: [Accessed 25 November 2016]
- 66.Polytherapeutics Transdermal drug delivery. 2010. http://www.polytherapeutics.com/node/5 Available from: [Accessed 25 November 2016]