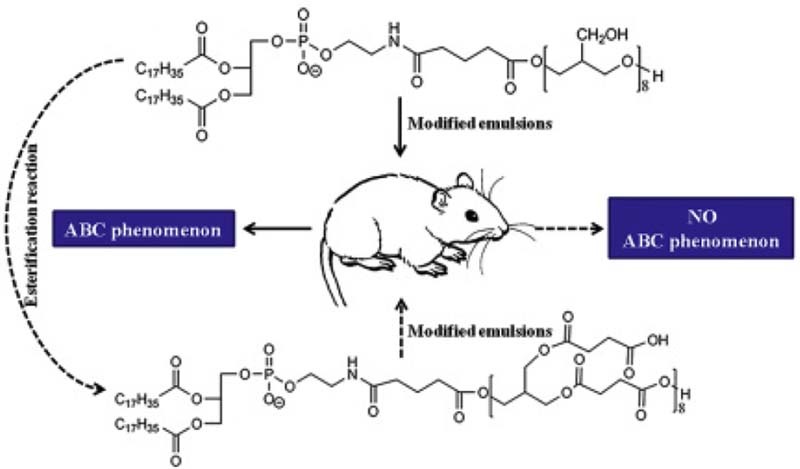
Graphical Abstract
The first dose of the 1,2-distea-royl-sn-glycero-3-phosphoethanolamine-n-polygly-cerine-610 modified nanoemulsions can induce the ABC phenomenon of the second dose of the PEGylated nanoemulsions. We synthesized polycarboxyl compound via the esterification reaction of 1,2-distea-royl-sn-glycero-3-phosphoethan-olamine-n-polyglycerine-610 and succinic anhydride. The polycarboxyl structure in the new modified compound we synthesized can circumvent the ABC phenomenon of the second dose of the PEGylated nanoemulsions.
Keywords: ABC phenomenon, Nanoemulsion, Liver accumulation, Kupffer cells, Polyglycerin, Polycarboxyl
Abstract
For investigating the accelerated blood clearance (ABC) phenomenon of polyglycerin modified nanoemulsions upon cross administration with polyethylene glycol (PEG) covered nanoemulsion, we used the 1,2-distea-royl-sn-glycero-3-phosphoethanolamine-n-polyglycerine-610 and the 1,2-distearoyl-n-glycero-3-phosphoethanolamine-n-[me-thoxy(polyethylene glycol)-2000] as modify materials, the dialkylcarbocyanines as fluorescence indicator. Exhausted macrophages rat model was established and new material containing polycarboxyl structure was synthesized. The microplate reader and the in vivo optical imaging system were applied to measure the concentration of nanoemulsions in tissues. The results show that the first dose of polyglycerin modified nanoemulsion can induce the ABC phenomenon of the second dose of PEGylated nanoemulsion. With the increase in the amount of the surface polyglycerin, the extent of the ABC phenomenon decreases. Liver accumulation has positive relationship with the ABC phenomenon. Furthermore, kupffer cells in liver can get more immune information from polyhydroxy structure than polycarboxyl group in the modify compound. The results of our work imply that the polycarboxyl structure has advantages to eliminate the ABC phenomenon.
1. Introduction
The use of PEGylated nanocarriers is a milestone breakthrough in the field of drug delivery due to its important application for increasing the serum stability and blood circulation time [[1], [2], [3]]. However, an unexpected pharmacokinetic issue, the so-called accelerated blood clearance (ABC) phenomenon, has been revealed afterwards [[4], [5], [6], [7], [8], [9], [10]]. In this phenomenon, an intravenous injection of PEGylated nanocarriers causes a second dose of PEGylated nanocarriers, which are injected a few days later, to be accumulated in liver resulting in the lost of long-circulating character of the PEGylated nanocarriers. Therefore, the ABC phenomenon can reduce the therapeutic efficacy of the encapsulated drugs, alter tissue distribution pattern of the drugs and cause some other adverse effects. For these reasons, how to eliminate the ABC phenomenon has garnered much interest and become a research focus.
Over the past decades, several excellent alternative materials have been reported, such as hemoglobin [[11], [12], [13]], biomimetic red blood cell membrane [[14], [15]], sixth generation of lysine dendrimer [16], poly(amino acid)-poly(hydroxyethyl-l-asparagine) [17], poly(N-vinyl-2-pyrrolidone) [[18], [19]], cleavable PEG-cholesterol derivatives [20], poly(sarcosine)60-block-(l-Leu-Aib)6 [21], poly(carboxybetaine) [[22], [23], [24]], and et. al [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. These materials can decrease even eliminate the ABC phenomenon. As reported, polyglycerol is a more hydrophilic polymer than PEG, and can reduce the uptake by the mononuclear phagocyte system [35]. In addition, 1,2-distea-royl-sn-glycero-3-phosphoethanolamine-n-polyglycerine-760 (PG-760) eliminates the ABC phenomenon of liposomes and ensures the effective delivery doxorubicin to the target site upon repeated administration [33]. Moreover, repeated injection of the pDNA–lipoplex modified with PG-760 cannot induce the ABC phenomenon and can accumulate in tumor efficiently [34]. Although PG760 can eliminate the ABC phenomenon in a repeated injection regimen, but more work needs to be done in the field of studying the ABC phenomenon associated with nanocarriers coated with other kind of polyglycerine.
In this work, the ABC phenomenon of the 1,2-distea-royl-sn-glycero-3-phosphoethanolamine-n-polyglycerine-610 (PG610-DSPE) has been studied. We find that the nanoemulsions modified with the PG610-DSPE can induce the ABC phenomenon of the second dose of PEGylated nanoemulsions (PE), and the nanoemulsions modified with lower density of PG-610 can induce stronger ABC phenomenon. Through the in vivo tracing experiment and exhausted macrophages rat model, as expected, we confirm that the liver accumulation level of the first dose has positive relationship with the intensity of the ABC phenomenon. The kupffer cells (KCs) in liver not only play a “cleaner” role in the final stage, but also as an external information “getter” or “transporter” in the early steps of the ABC phenomenon. This result is quite agreed with the conclusion proposed by Dams group [4], Ishida group [[36], [37], [38]] and our group [39]. In addition, the blocking out polyhydroxy structure experiment reveals that the KCs in liver can get more immune information from the polyhydroxy structure than polycarboxyl group in the modify compound. The KCs deliver the information to other part of the immune system and finally increases the blood clearance ability. We propose that the modifier which contains polycarboxyl structure has chance to eliminate the ABC phenomenon. In addition, preclinical studies of new materials modified nanocarriers should include the all-sided cross injection regimen with PEGylated nanocarriers, because the long circulation carriers such as PEGylated nanoemulsions which can amplify some weak signal of immunology changes can be a good ABC phenomenon detector.
2. Materials and methods
2.1. Materials and animals
1,2-distea-royl-sn-glycero-3-phosphoethanolamine-n-polyglycerine-610 (PG610-DSPE, NOF corporation, Japan); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[me-thoxy(polyethylene glycol)-2000] (mPEG2000-DSPE, Genzyme, USA); Egg phosphatidylcholine (E80, Lipoid GmbH, Germany); Hydrogenated soy phosphatidylcholine (HSPC, Avanti polar lipid, USA); Cholesterol (CH, National medicines corporation, China); Medium-chain triglycerides (MCTs, Beiya Medicated Oil, China); Dialkylcarbocyanines (DiR, AAT Bioquest, USA); Triethylamine (TEA, Tianjin Bodi chemistry, China); Dichloromethane (DCM, Zhengxin high-tech research institute, China); Succinic acid anhydride (SAA, Zhengxin high-tech research institute, China); N,N-Dimethylamino-2-pyridine (DMAP, Tianjin Bodi chemistry, China); Alendronate sodium (AD, Beijing HWRK chem, China); Dimethyldioctadecylammonium bromide (DDAB, Sigma-aldrich, China); Sigma 1 KDa MWCO cellulose ester membrane (Sigma-aldrich, China); Sephadex G-50 (Sigma-aldrich, China); Male Wistar rats (180–200 g, the experimental animal center of Shenyang Pharmaceutical University, China). All animal care and experiments were carried out according to the guidelines of the animal welfare committee of Shenyang Pharmaceutical University.
2.2. The structural transformation of the PG610-DSPE
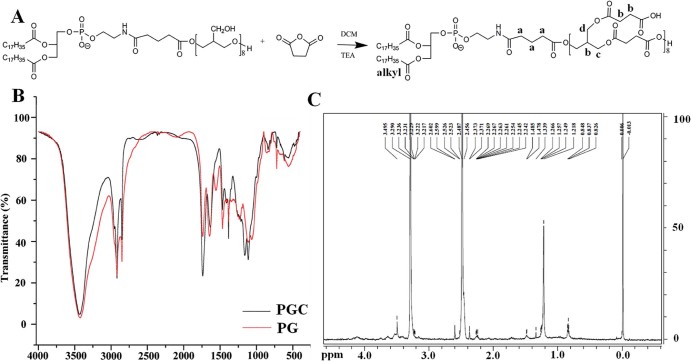
The PGC was synthesized using PG610-DSPE and SAA with the DMAP / TEA as catalyst (Fig. 1A). Briefly, PG610-DSPE (30 mg, mM), TEA (10 µl, mM) and DMAP (6.7 mg, mM) were dissolved in 4 ml DCM which contained SAA (54.7 mg) and stirred for 30 min in ice water bath. Then the reaction temperature was elevated to room temperature. After the reaction processed for 24 h under nitrogen, the mixture was diluted and fully dialyzed against water using a cellulose ester membrane of 1 KDa MWCO. The retentate was then lyophilized to yield the final product, which was analyzed by 1H NMR (Bruker 600-MHz) using DMSO as the solvent and FT-IR (Bruker IFS 55) using KBr as the reference.
Fig. 1.
PGC synthesized by esterification reaction of PG610-DSPE and SAA with TEA as catalyst and DCM as organic solvent. The characterization of the PGC was measured by FT-IR and 1H NMR. (A) The structural formula PGC. (B) FT-IR spectra of PGC and PG. (C) 1H NMR spectrum of PGC.
2.3. The preparation of the nanoemulsions
The oil phase containing DiR, MCTs, E80 and PG610-DSPE / mPEG2000-DSPE / PGC was dissolved at 55 °C. Sterile water was quickly injected into this oil phase with stirring for 10 min at 55 °C in a water bath to obtain the primary emulsions. The final emulsions were obtained by using a laboratory ultrasonic cell pulverizer (JY92-II, Ningbo Scientz Biotechnology, Zhejiang, China) at 200 W for 2 min and at 400 W for 6 min, respectively. The final emulsions were extruded through polycarbonate membranes with a pore size of 0.22 µm respectively, and were made isotonic with 50% (m/v) glucose (Shandong Yuwang industry, China). The nanoemulsions modified with 1 mol%, 3 mol% or 9 mol% PG610-DSPE were named as 1%GE, 3%GE 9%GE respectively. The nanoemulsion modified with 9 mol% mPEG2000-DSPE was named as 9%PE. The nanoemulsion modified with 1 mol% PGC was named as 1%HE. The nanoemulsions, 1%GE, 3%GE 9%GE, 9%PE and 1% HE, were used as detection nanocarriers for containing DiR in the formulations. The empty nanoemulsions for the first injection in repeated injection regime were prepared using the similar formulations without DiR. These empty nanoemulsions were named as 1%GE-B, 3%GE-B, 9%GE-B, 9%PE-B and 1%HE-B. The particle size distribution and Zeta potential were determined by dynamic light scattering using a Nicomp 380 HPL submicron particle analyzer (Particle Sizing Systems, Santa Barbara, CA, USA) and the results are shown in the Table 1.
Table 1.
The characteristics of nanoemulsions.
| Type | Composition | Mean diameter (nm) | C.V. | Zeta Potential (mV) |
|---|---|---|---|---|
| 1%GE | DiR/MCT/E80/ PG610-DSPE | 161.1 ± 4.2 | 0.318 ± 0.007 | −12.50 ± 1.77 |
| (3.10/77.50/19.04/0.36, w/w) | ||||
| 1%GE-B | MCT/E80/ PG610-DSPE | 158.4 ± 3.7 | 0.339 ± 0.013 | −18.50 ± 3.41 |
| (77.50/19.04/0.36, w/w) | ||||
| 3%GE | DiR/MCT/E80/ PG610-DSPE | 152.5 ± 3.2 | 0.388 ± 0.011 | −25.31 ± 5.22 |
| (3.10/77.5/18.32/1.08, w/w) | ||||
| 3%GE-B | MCT/E80/ PG610-DSPE | 155.7 ± 2.9 | 0.305 ± 0.009 | −29.01 ± 3.69 |
| (77.5/18.32/1.08, w/w) | ||||
| 9%GE | DiR/MCT/ E80/ PG610-DSPE | 151.3 ± 6.6 | 0.374 ± 0.017 | −28.32 ± 1.09 |
| (2.95/77.64/16.38/3.03, w/w) | ||||
| 9%GE-B | MCT/ E80/ PG610-DSPE | 153.2 ± 3.0 | 0.320 ± 0.006 | −27.83 ± 3.17 |
| (77.64/16.38/3.03, w/w) | ||||
| 9%PE | DiR/MCT/E80/mPEG2000-DSPE | 138.9 ± 2.1 | 0.428 ± 0.003 | −30.20 ± 1.68 |
| (3.21/72.89/17.05/6.85, w/w) | ||||
| 9%PE-B | MCT/E80/mPEG2000-DSPE | 135.7 ± 4.5 | 0.315 ± 0.006 | −29.77 ± 3.43 |
| (72.89/17.05/6.85, w/w) | ||||
| 1%HE | DiR/MCT/E80/PGC | 179.8 ± 3.9 | 0.404 ± 0.023 | −35.79 ± 4.722 |
| (3.10/77.50/18.83/0.57, w/w) | ||||
| 1%HE-B | MCT/E80/PGC | 168.9 ± 3.5 | 0.379 ± 0.018 | −33.09 ± 5.174 |
| (77.50/18.83/0.57, w/w) | ||||
| AD-L | CH/HSPC/DDAB | 503.8 ± 7.6 | 0.424 ± 0.013 | 69.40 ± 3.12 |
| (50.00/16.66/33.34, w/w) |
2.4. The preparation of the alendronate liposome
Alendronate liposome (AD-L) was prepared using passive loading method with a combination of the extrusion method. Briefly, the lipid mixture (Table 1) was dissolved in 5 ml ethanol in 250 ml round-bottom flask. Added micro glass beads into the flask and then used RE52CS spin steaming instrument (Shanghai yarong biochemical instrument plant, China) to evaporate it at 60 °C to near dryness. The resulting lipid film was hydrated with 5 ml AD solution at 60 °C for 20 min under fast stirring. After hydration, the dispersion was extruded through 0.8 µm and then 0.6 µm polycarbonate films three times by LIPEX extrusion apparatus (Northen lipid, Canada). For removing non-encapsulated drug, the liposome was passed through a Sephadex G-50 column eluted with 5% glucose. The mean diameter and Zeta potential of AD-L were determined by Nicomp 380 HPL submicron particle analyzer. The formulation, size and Zeta potential of AD-L are shown in the Table 1.
2.5. The determination of the encapsulation efficiency
The AD-L preparation was taken and the unentrapped AD was removed by Sephadex G-50 chromatography to determine the encapsulation efficiency (%EE). The %EE was assessed by high performance liquid chromatography-evaporative light scattering detection (HPLC-ELSD, Chromatographic column: Kramasil C8 column; Mobile phase: n-butyl amine : acetonitrile (9 : 1, v/v); Drift tube temperature: 50 °C; Air carrier gas pressure: 3.0 bar) using a standard curve method. Briefly, the 0.1 ml sample was loaded onto a Sephadex G-50 minicolumn (~20 mm) and then eluted them using 5% glucose. We calculated the concentration of AD which was entrappped into the liposome (Clip). Another 0.1 ml sample was dissolved by demulsifier containing 90% (v/v) isopropyl alcohol and 1.0 M HCl. We calculated the total concentration of AD (Ctot). The %EE was calculated using this equation: EE% = (Clip / Ctot) × 100%.
2.6. The pharmacokinetic and the ABC phenomenon studies of the nanoemulsions
In order to study the pharmacokinetic of nanoemulsions, the 1%GE, 3%GE, 9%GE and 9%PE prepared at a dose of 5 µmol phospholipid/kg were injected via tail vein injection into rats respectively. At selected post-injection time points, 0.083, 0.25, 0.5, 1, 2, 4 h, blood was sampled through marginal veins of the eye. The liver and spleen were sacrificed after withdrawing the last blood sample at 4 h and rinsed with saline. Then these tissues were stored at −20 °C. When studied the ABC phenomenon, 5 µmol phospholipid/kg of the first injections, 1%GE-B, 3%GE-B, 9%GE-B and 9%PE-B, were injected intravenously via the femoral vein respectively. Control group received 5 % (m/v) glucose solution. 7 d later, the second injection, 9%PE, was administered intravenously via the femoral vein at a dose of 5 µmol phospholipid/kg. Similarly, blood was sampled through marginal veins of the eye at time points, 0.083, 0.25, 0.5, 1, 2, and 4 h. The liver and spleen were sacrificed after withdrawing the last blood sample at 4 h, rinsed with saline and stored at −20 °C.
2.7. The in vivo tracking of the nanoemulsions
Rats were divided into four groups and these rats were administered with 1%GE, 3%GE, 9%GE and 9%PE at the dose of 5 µmol phospholipid/kg by tail vein injection respectively and anesthetized with pentobarbital. At 15, 30, 60, 240, and 480 min after the injections, the sedated animals were then imaged using the in vivo optical imaging system and near-infrared fluorescence (NIRF) probe (DiR, 0.65 mg/kg). The detection wavelengths were 750 nm (excitation) and 790 nm (emission).
2.8. The effect of macrophage depletion
For the first injections, rats were injected with the 1%GE-B, 3%GE-B, 9%GE-B (5 µmol phospholipid/kg) respectively via tail vein injection. 5 d after the first injection of the non-labeled nanemulsions, each group received the AD-L (i.v., AD 3 mg/kg), and then two days later, the rats were intravenously administered 9%PE (5 µmol phospholipid/kg), which was injected intravenously via the femoral vein. Control group was pretreated with 5 % (m/v) glucose solution. Blood samples were collected at multiple time points after the 9%PE injection (0.083, 0.25, 0.5, 1, 2, and 4 h) and the rats in each treatment group were euthanized at 4 h. Liver and spleen were excised, rinsed with saline, and stored at −20 °C.
2.9. The effect of changing hydrophilic group in modified phospholipids
Rats were intravenously administered the 1%HE (5 µmol phospholipid/kg) in order to study the pharmacokinetic of nanoemulsions. After the injection, at selected post-injection time points (0.083, 0.25, 0.5, 1, 2, and 4 h), blood was sampled through marginal veins of the eye. The liver and spleen were sacrificed, rinsed with saline and stored at −20 °C. When studied the ABC phenomenon, the first injection, 1%HE-B, at the doses of 5 µmol phospholipid/kg, was injected intravenously via the femoral vein. Control group received 5 % (m/v) glucose solution. After 7 d, 9%PE (5 µmol phospholipid/kg), was administered via the femoral vein. As described above, at post-injection time points, 0.083, 0.25, 0.5, 1, 2, and 4 h, blood was sampled through marginal veins of the eye. The liver and spleen were sacrificed, rinsed with saline, and stored at −20 °C.
2.10. The method for detecting DiR concentration in the tissue samples
The plasma samples and tissue samples were treated as follows: 100 µl of the plasma samples were mixed with ethylalcohol (900 µl); 200 µl homogenates (equivalent to 0.1 g tissue) were mixed with ethylalcohol (800 µl); the entire mixture was vortexed for 5 min and centrifuged at 10 000 rpm for 10 min; the supernatant (600 µl) was centrifuged at 10 000 rpm for 10 min again; the final supernatant (200 µl) was added into 96 plates and analysised by a microplate reader (Bio-Rad Laboratories, Hertfordshire, UK). The detection wavelengths were 750 nm (excitation) and 790 nm (emission).
2.11. Statistical analysis
The statistical analysis was performed using unpaired Student's t-test with SPSS 16.0 (IBM, Armonk, NY, USA) software for statistically testing whether the central tendencies of two groups are different from each other on the basis of samples of the two groups. A two-sided P value, P < 0.05 was considered significant. The fluorescence intensity was calculated using the carestream MI SE (Carestream, USA). The data are presented as the means ± standard deviation.
3. Results and discussion
3.1. Synthesis and characterization of the PGC
As shown in the Fig. 1A, PGC was synthesized via the esterification reaction of PG610-DSPE and SAA. Comparing the red and black lines in the Fig. 1B (FT-IR spectra), we can find that a new absorption peak at 1739 /cm (C = O) appears. Moreover, the absorption peaks at 1162.3 /cm and 1142.3 /cm (C-O-C) become stronger. These results reveal that new ester bond has formed. The absorption peak at 3426 /cm(-OH) becomes shaper and the absorption peaks at 1559.8 /cm and 1236.8 /cm (hydrogen bond) disappear, indicating that the hydroxyl number decreases and the combined water in polyhydroxyl structures disappears. The structure of PGC is also confirmed by 1H NMR (DMSO, dppm): 0.83 (t, 6H, alkyl), 1.00~1.20 (s, 52H, alkyl), 1.30 (m, 4H, alkyl), 1.40 ~ 1.50 (m, 4H, alkyl), 2.1~2.3 (s, 10H, H-a, alkyl), 2.3~2.7 (m, 44H, H-b), 3.10~3.4 (t, 28H, H-c), 3.55 (d, 14H, H-d) (Fig. 1C). Therefore, we can confirm that the polyhydroxyl structures have been blocked by carboxyl and the PGC has been synthesized successfully.
3.2. The characterization of nanoemulsions
The average sizes of the GE, PE, PL, HE and AD-L are about 155 nm, 140 nm, 120 nm, 175 nm and 500 nm respectively. Moreover, the size distributions of all the formulations stay in a low level for the coefficient of variation (C.V.) ranging from 0.318 to 0.428. Except the AD-L, all nanoemulsions are negatively charged for E80 molecule, mPEG2000-DSPE, PG610-DSPE and PGC at physiological pH. The AD-L is positively charged for DDAB at physiological pH. From the Zeta potential results, modifying agents have coated on the surface of nanoemulsions successfully (Table 1). In addition, the EE% of the AD-L measured by Sephadex G-50-HPLC-ELSD method is 1.3%.
3.3. The pharmacokinetics of GE
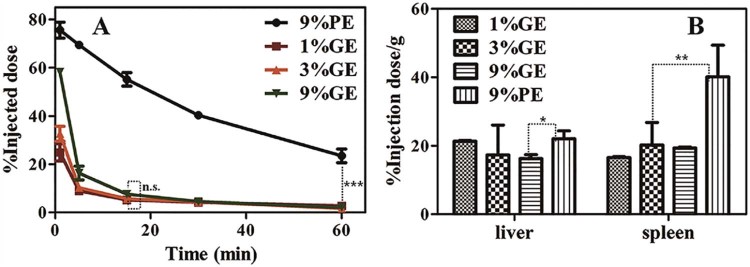
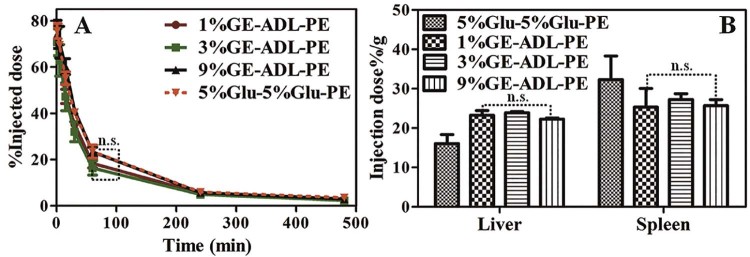
As reported, increasing the PG760-derived lipid concentration from 5 to 15 mol% does not significantly affect the pharmacokinetic profile and organ distribution of PG760-coated liposomes [33]. In this study, we treated rats with the 1%GE, 3%GE and 9%GE at the dose of 5 µmol phospholipid/kg, and the pharmacokinetic parameters and organ distribution were evaluated. As shown in the Fig. 2A, increasing the PG-derived lipid concentration from 1 mol% to 9 mol%, the pharmacokinetic profiles do not have significant changes. Table 2 revealed that, the AUC(0–60 min) values for the 1%GE, 3%GE and 9%GE are comparable (1224.44 ± 24.65, 1746.27 ± 22.83 and 2201.15 ± 27.37 µg/ml min respectively) (P > 0.05). %GE (MRT(0–∞), 10.93 ± 1.23 min) is not significantly shorter than that of the 3%GE(MRT(0–∞), 11.83 ± 0.96 min) (Table 2). The circulation time cannot be further prolonged when the modified density to 9 mol% (MRT(0–∞), 12.47 ± 1.54 min) is increased. Compared with the 1%GE, the 9%PE prolongs the circulation significantly (***P < 0.005). The AUC(0–60 min) value of the 9%PE is 5723.06 ± 85.14 µg/ml/min. The Fig. 2B reveals that the liver and spleen accumulations of PE are larger than those of the GE groups.
Fig. 2.
The pharmacokinetic profiles and organ distributions of intravenous injected nanoemulsions (1%GE, 3%GE, 9%GE and 9%PE) at the dose of 5 µmol phospholipid/kg in rats, n = 3. (A) Plasma concentration of the PE/GE. (B) Tissue concentration of the PE/GE. Each value represents the mean ± SD of three animals. n.s. P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.005.
Table 2.
Pharmacokinetic parameters of formulations.
| Group | AUC(0–60 min)a (µg/ml/min) | MRT(0–60)b (min) | |
|---|---|---|---|
| 1st dose | 1%GE | 1224.44 ± 24.65 | 10.93 ± 1.23 |
| 3%GE | 1746.27 ± 22.83 | 11.83 ± 0.96 | |
| 9%GE | 2201.15 ± 27.37 | 12.47 ± 1.54 | |
| 9%PE | 5723.06 ± 85.14 | 25.30 ± 2.83 | |
| 1%HE | 1107.47 ± 21.28 | 10.13 ± 0.78 | |
| 2nd dose (9%PE) | Pretreated with 1%GE-B | 1236.65 ± 27.23 | 19.45 ± 1.54 |
| Pretreated with 3%GE-B | 2081.18 ± 28.44 | 22.48 ± 2.12 | |
| Pretreated with 9%GE-B | 2712.66 ± 22.56 | 23.49 ± 2.76 | |
| Pretreated with 9%PE-B | 204.15 ± 9.38 | 9.92 ± 0.73 | |
| Pretreated with 1%HE-B | 5593.62 ± 84.23 | 24.94 ± 2.31 | |
| 3rd dose (9%PE) | Pretreated with 1%GE-B then AD treatment | 5023.59 ± 86.75 | 22.01 ± 2.28 |
| Pretreated with 3%GE-B then AD treatment | 5423.86 ± 78.48 | 23.98 ± 2.54 | |
| Pretreated with 9%GE-B then AD treatment | 5323.08 ± 82.83 | 23.25 ± 3.05 | |
| Pretreated with 5%Glu-B then AD treatment | 5642.75 ± 85.37 | 24.68 ± 2.83 | |
AREA under curve from time 0 to 60 min.
The mean retention time from time 0 to 60 min.
3.4. The ABC phenomenon of the GE
For evaluating the effect of PG610-DSPE on the induction of the ABC phenomenon in rats, rats were treated with the 1%GE-B, 3%GE-B, 9% GE-B and 9%PE-B at 5 µmol phospholipid/kg as the first dose respectively. 7 d later, the rats were injected with the 9%PE (5 µmol phospholipid/kg). The result of Fig. 3A showed that, the plasma concentrations of the second dose of the 9%PE decrease. Compared with control group which pretreated with 5% glucose, rats pretreated with the 1%GE-B induce the rapidly clearance of the 9%PE (AUC(0–60 min) values, 5723.06 ± 85.14 and 1236.65 ± 27.23 µg/ml/min, respectively, **P < 0.01, Table 2). The decreasing extends from strong to weak are 1%GE > 3%GE > 9%GE (MRT(0–60) (min), 19.45 ± 1.54, 22.48 ± 2.12, 23.49 ± 2.76 min respectively). This suggests that the ABC phenomenon is induced by the nanoemulsion coated with PG610-DSPE. This is the first report that the GE induces the ABC phenomenon of the second dose of the 9%PE. As shown in the Table 2, pharmacokinetic parameters reveal that rats pretreated with the 9%PE-B can induce stronger ABC phenomenon than the 1%GE-B (AUC(0–60 min) values, 204.15 ± 9.38 and 1236.65 ± 27.23 µg/ml/min, respectively). The parameters between the control group and the group pretreated with the 1%GE-B are compared. The differences in parameters between these two groups are significant (**P < 0.01) (Table 2). The Fig. 2B shows that the liver and spleen accumulations are increased due to the induction of the ABC phenomenon. As we all know, PEG endows the nanoparticles “stealth”. Some researchers think that the reason why the PEGylated nanoemulsion induces the ABC phenomenon is that the long lifetime of nanoparticles trigger the immune system [40]. However, from the results of our work, the ABC phenomenon is also induced by the nanocarriers in short circulation.
Fig. 3.
The effect of the first dose of none labeled nanoemulsions (5 µmol phospholipid/kg), the 1%GE-B, the 3%GE-B, the 9%GE-B and the 9%PE-B, to the second dose of the 9%PE (5 µmol phospholipid/kg), n = 3. (A) Plasma concentration of the second dose of the 9%PE. (B) Tissue concentration of the second dose of the 9%PE. Each value represents the mean ± SD of three animals. **P < 0.01, ***P < 0.005.
3.5. The in vivo tracking of the nanoemulsions
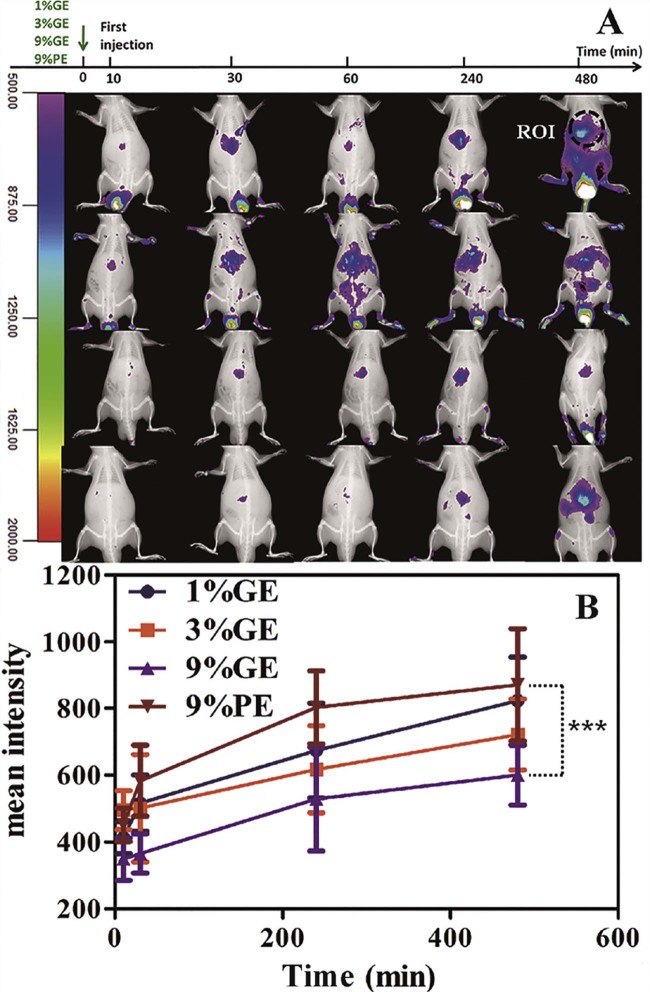
In order to find the reason why the GE can induce the ABC phenomenon of the second dose of the PE, we studied the tissue distributions of nanocarriers by the optical in vivo imaging method. As shown in the Fig. 4A, the nanoemulsions spread over the whole body. The tissue distributions of the 1%GE, 3%GE, 9%GE and 9%PE are quite different. Compared with 9%PE, nanoemulsions modified with the PG610-DSPE trend to accumulate into the joint, and are easily metabolized and excrete from the urinary system. That is the reason why the circulation time of the GE is shorter than the PE and why the liver accumulation of the GE is not higher than the PE at the time of 4 h after the first injection. However, 9%PE which has “stealth” feature prefer to accumulate in the liver region. The liver accumulations from more to less are 9%PE > 1% GE > 3% GE > 9% GE and that are also approved by the Region-of-interest (ROI) analyses (Fig. 4B). We conclude that liver accumulation due to mononuclear phagocyte system plays an important role in the induction of the ABC phenomenon by the GE to the second dose of the 9%PE.
Fig. 4.
The in vivo tracking of the intravenous injected nanoemulsions at the dose of 5 µmol phospholipid/kg (1%GE, 3%GE, 9%GE and 9%PE) in rats, n = 3. (A) The in vivo imaging performed at 10, 30, 240 and 480 min after the injection of the 1%GE, 3%GE, 9%GE and 9%PE respectively. (B) Time profiles of region-of-interest (ROI) analyses. Each value represents the mean ± SD of three animals. ***P < 0.005.
3.6. The effect of macrophage depletion
The mechanism of the ABC phenomenon described as follows: the first dose of PEGylated nanocarriers which acts as TI-2 antigens [[38], [41], [42], [43]] contacts with the B-cells located in the splenic marginal zone [43], to secrete the anti-PEG IgM; the anti-PEG IgM binds to the second dose of the PEGylated liposome, activating complement and increasing uptake of opsonized nanoparticles by resident liver macrophages [[44], [45], [46], [47], [48], [49]]. Laverman group reported that when macrophages are depleted before the first and second injections, the second injection of PEGylated liposomes has a normal long circulation time. This reveals that macrophages are involved in the production of the ABC phenomenon [5]. In addition, it is also clear that the splenectomized rat fails to completely reverse the ABC phenomenon [36]. Ishida group speculated that macrophages, including the KCs, acquire the ability to recognize and aggressively take up PEGylated liposomes [[37], [38]]. Furthermore, Ishida group also proposed that, the first dose PEGylated liposomes do not increase the intrinsic phagocytic activity of the KCs and the additive effect of activated liver macrophages and complement activation may exist in the ABC phenomenon [50]. In this study, we also find that the liver communication has positive relationship with the ABC phenomenon from in vivo tracing experiment. We confirm that, the KCs in liver induce the ABC phenomenon at the initial phase.
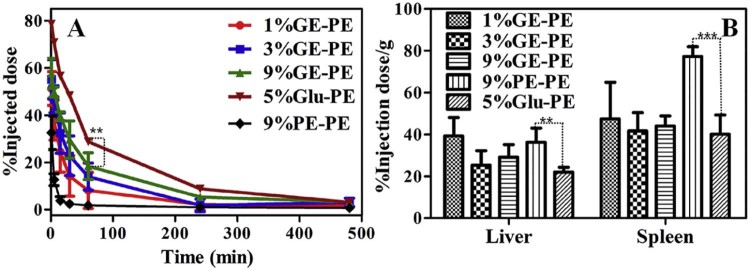
For studying the effect of the mononuclear phagocyte system in liver in the ABC phenomenon, the depletion of the KCs were determined following the first administration of various GE. Firstly, rats in different groups were given the 1%GE-B, 3%GE-B and 9%GE-B respectively. 5 d later, each group was assigned to the AD-L treatments of AD 3 mg/kg. Then two days later, each group was administrated with the 9%PE (5 µmol phospholipid/kg). For the control group, the first two injections were the 5%Glu and the third injection was the 9%PE. The result shows that, for the AD-L treatments, the first injection of the GE only slightly influence the plasma concentration and the tissue distribution of the second dose of the 9%PE (Fig. 5). Pharmacokinetic parameters were also determined. As shown in the Table 2, the AUC(0–60 min) values of the second dose of the 9%PE are 5023.59 ± 86.75, 5423.86 ± 78.48 and 5323.08 ± 82.83 respectively. The differences in parameters for the control group and the group pretrated with the 1%GE-B are not significant (P > 0.05). This suggests that the KCs can play an important role in the induction phase of the ABC phenomenon.
Fig. 5.
The effect of the macrophage depletion in the ABC phenomenon induced by GE. rats in different groups were given the 1%GE-B, 3%GE-B and 9%GE-B respectively. 5 d later, each group was assigned to the AD-L treatments of AD 3 mg/kg. Then 2 d later, each group was administrated with the 9%PE (5 µmol phospholipid/kg). For the control group, the first two injections were the 5%Glu and the third injection was the 9%PE, n = 3. (A) Plasma concentration of the second dose of the 9%PE. (B) Tissue concentration of the second dose of the 9%PE. Each value represents the mean ± SD of three animals. n.s. P > 0.05.
3.7. The effect of changing hydrophilic group in modified phospholipids
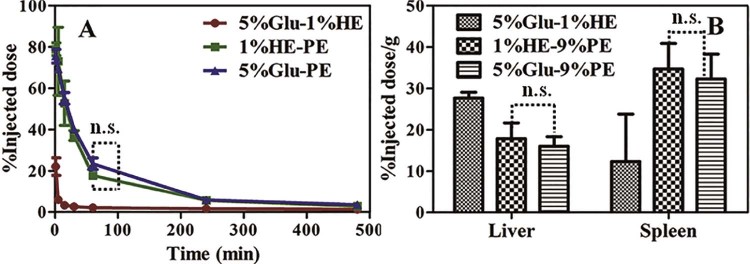
In order to find out the immune signals that the KCs receives from the GE, we replaced the polyhydroxy structure with polycarboxylic group and named this new compound as PGC. Interestingly, nanoemulsions modified with the PGC cannot decrease the plasma concentration of the second dose of the 9%PE and only slightly increase the tissue accumulation (Fig. 6). From pharmacokinetic parameters, the MRT(0–60) value of the second dose of the 9%PE is 24.94 ± 2.31 min. It has no significant difference with the single dose of the 9%PE (MRT(0–60), 25.30 ± 2.83 min) (P > 0.05). When we block out the polyhydroxy structure with the SAA, the ABC phenomenon is eliminated. The result reveals that the KCs in liver can get more immune information from the polyhydroxy structure than the polycarboxyl group in the modify compound. Sperling and co-workers reported that the hydroxyl groups modified on the surfaces of nanocarriers strongly activate the complement system. In addition, with the increase in amount of the surface -OH, the amount of the deposition of C3b on these surfaces increases [[51], [52]]. As reported, nanocarriers mediated complement activating occurs predominantly via the classical pathway and the alternative pathway [53]. The activated complement can cause leakage of liposome by the formation of a membrane attack complex and results in nanocarriers' rapid clearance by macrophages and hypersensitivity reactions [53]. In addition, terminating group that has been used to functionalize nanocarriers can effects on complement activation pathway. As reported, the PEGylated lipid polymeric nanoparticles functionalized with -COOH activate the alternative pathway, but not the classical pathway [54]. Interestingly, the activation of complement which needs the assistance of anti-PEG IgM belongs to the classical pathway and the major cause of the induction of the accelerated blood clearance of PEGylated liposome is the anti-PEG IgM-mediated complement activation [[4], [50]]. Hence, the KCs may need signal from complement which generated from classical pathway and polycarboxyl structure has advantages to eliminate the ABC phenomenon.
Fig. 6.
The effect of changing the hydrophilic group in modified phospholipids in the ABC phenomenon induced by the GE. Rats in different groups were given the 1%HE-B. 7 d later, each group was assigned to the 9%PE (5 µmol phospholipid/kg). For the control group, the first injection was the 5%Glu. n = 3. (A) The Plasma concentration of the second dose of the 9%PE. (B) Tissue concentration of the second dose of the 9%PE. Each value represents the mean ± SD of three animals. n.s. P > 0.05.
In our work, we find that the first injection of the GE can induce the ABC phenomenon of the second dose of the PE. This is the first report to show the influence of the second dose of the PE by the first dose of the GE. However, the first injection of the PEGylated liposome cannot induce the ABC phenomenon of the second administration of the polyglycerin coated liposome [33]. These results revealed that the long circulation carriers such as PEGylated nanoemulsions, which can amplify some weak signal of immunology changes, can be a good ABC phenomenon detector. Moreover, the all-sided cross injection regimen with the PEGylated nanocarriers should be done in a preclinical study of new materials modified nanocarriers.
4. Conclusion
In this study, we confirm that the first dose of the GE can induce the ABC phenomenon of the second dose of the PE. One of the mechanisms is that the KCs in liver can obtain the immune signal from the polyhydroxy structure and then increase the body clearance ability to the second dose of the PE. Our results revealed that the polycarboxyl structure in modified compound can circumvent the ABC phenomenon. The classical pathway activation is important for the ABC phenomenon. In addition, preclinical study of new nanocarriers materials should do the all-sided cross injection regimen with PEGylated nanocarriers. In the future, we plan to study the interaction between the inmmune system and the compound containing polycarboxyl for better understanding the ABC phenomenon.
Conflict of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos. 81072602, 81373334).
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Abuchowski A., McCoy J.R., Palczuk N.C. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 2.Gref R., Lück M., Quellec P. Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 3.Bose R.J., Soo-Hong L., Hansoo P. Biofunctionalized nanoparticles: an emerging drug delivery platform for various disease treatments. Drug Discov Today. 2016;21:1303–1312. doi: 10.1016/j.drudis.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Dams E.T., Laverman P., Oyen W.J. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 5.Laverman P., Carstens M.G., Boerman O.C. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther. 2001;298:607–612. [PubMed] [Google Scholar]
- 6.Ishida T., Maeda R., Ichihara M. The accelerated clearance on repeated injection of pegylated liposomes in rats: laboratory and histopathological study. Cell Mol Biol Lett. 2002;7:286. [PubMed] [Google Scholar]
- 7.Ishida T., Maeda R., Ichihara M. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J Control Release. 2003;88:35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y., Wang C., Wang L. A frustrating problem: accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in rats. Eur J Pharm Biopharm. 2012;81:506–513. doi: 10.1016/j.ejpb.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y., Wang L., Yan M. Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int J Nanomedicine. 2012;7:2891–2900. doi: 10.2147/IJN.S30943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla S., Dorand R.D., Myers J.T. Multiple administrations of viral nanoparticles alter in vivo behavior – insights from intravital microscopy. ACS Biomater Sci Eng. 2016;2:829–837. doi: 10.1021/acsbiomaterials.6b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai H., Masada Y., Horinouchi H. Physiological capacity of the reticuloendothelial system for the degradation of hemoglobin vsicles (artificial oxygen carriers) after massive intravenous doses by daily repeated infusions for 14 days. J Pharmacol Exp Ther. 2004;311:874–884. doi: 10.1124/jpet.104.073049. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi K., Urata Y., Anraku M. Hemoglobin vesicles, polyethylene glycol (PEG) ylated liposomes developed as a red blood cell substitute, do not induce the accelerated blood clearance phenomenon in mice. Drug Metab Dispos. 2009;37:2197–2203. doi: 10.1124/dmd.109.028852. [DOI] [PubMed] [Google Scholar]
- 13.Taguchi K., Maruyama T., Iwao Y. Pharmacokinetics of single and repeated injection of hemoglobin-vesicles in hemorrhagic shock rat model. J Control Release. 2009;136:232–239. doi: 10.1016/j.jconrel.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Naeem S., Kiew L.V., Yong C.L. Drug delivery and innovative pharmaceutical development in mimicking the red blood cell membrane. Rev Chem Eng. 2015;31:491–508. [Google Scholar]
- 15.Rao L., Bu L.L., Xu J.H. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11:6225–6236. doi: 10.1002/smll.201502388. [DOI] [PubMed] [Google Scholar]
- 16.Okuda T., Kawakami S., Akimoto N. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J Control Release. 2006;116:330–336. doi: 10.1016/j.jconrel.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Romberg B., Oussoren C., Snel C.J. Pharmacokinetics of poly (hydroxyethyl-l-asparagine)-coated liposomes is superior over that of PEG-coated liposomes at low lipid dose and upon repeated administration. BBA Biomembr. 2007;1768:737–743. doi: 10.1016/j.bbamem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara T., Maeda T., Sakamoto H. Evasion of the accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers. Biomacromolecules. 2010;11:2700–2706. doi: 10.1021/bm100754e. [DOI] [PubMed] [Google Scholar]
- 19.Kierstead P.H., Okochi H., Venditto V.J. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J Control Release. 2015;213:1–9. doi: 10.1016/j.jconrel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H., Wang K.Q., Deng Y.H. Effects of cleavable PEG-cholesterol derivatives on the accelerated blood clearance of PEGylated liposomes. Biomaterials. 2010;31:4757–4763. doi: 10.1016/j.biomaterials.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Hara E., Ueda M., Kim C.J. Suppressive immune response of poly–(sarcosine) chains in peptide–nanosheets in contrast to polymeric micelles. J Pept Sci. 2014;20:570–577. doi: 10.1002/psc.2655. [DOI] [PubMed] [Google Scholar]
- 22.Yang W., Liu S.J., Bai T. Poly (carboxybetaine) nanomaterials enable long circulation and prevent polymer-specific antibody production. Nano Today. 2014;9:10–16. [Google Scholar]
- 23.Li Y., Liu R., Yang J. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials. 2015;41:1–14. doi: 10.1016/j.biomaterials.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Zhang X. Zwitterionic poly (carboxybetaine) modified liposomes enhancing tumor therapy without accelerated blood clearance phenomenon. J Control Release. 2015;213:e125. doi: 10.1016/j.jconrel.2015.05.211. [DOI] [PubMed] [Google Scholar]
- 25.Lowe S., O'Brien-Simpson N.M., Connal L.A. Antibiofouling polymer interfaces: poly (ethylene glycol) and other promising candidates. Polymer Chem UK. 2015;6:198–212. [Google Scholar]
- 26.Judge A., McClintock K., Phelps J.R. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara T., Takeda M., Sakamoto H. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res Dordr. 2009;26:2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z.S., Xie C., Liu Q. The effect of hydrophilic chain length and iRGD on drug delivery from poly (ε-caprolactone)-poly (N-vinylpyrrolidone) nanoparticles. Biomaterials. 2011;32:9525–9535. doi: 10.1016/j.biomaterials.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 29.Chen D.Q., Liu W.H., Shen Y. Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomedicine. 2011;6:2053–2061. doi: 10.2147/IJN.S24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H., Yang C.L., Hu Z.P. Ethylene glycol oligomer modified-sodium alginate for efficiently improving the drug loading and the tumor therapeutic effect. J Mater Chem B. 2013;1:5933–5941. doi: 10.1039/c3tb20968g. [DOI] [PubMed] [Google Scholar]
- 31.Matsui H., Ueda M., Hara I. Precise control of nanoparticle surface by host–guest chemistry for delivery to tumor. RSC Adv. 2015;5:35346–35351. [Google Scholar]
- 32.Zhang Q., Deng C.F., Fu Y. Repeated administration of hyaluronic acid coated liposomes with improved pharmacokinetics and reduced immune response. Mol Pharm. 2016;13:1800–1808. doi: 10.1021/acs.molpharmaceut.5b00952. [DOI] [PubMed] [Google Scholar]
- 33.Abu Lila A.S., Nawata K., Shimizu T. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. Int J Pharm. 2013;456:235–242. doi: 10.1016/j.ijpharm.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 34.Abu Lila A.S., Uehara Y., Ishida T. Application of polyglycerol coating to plasmid DNA lipoplex for the evasion of the accelerated blood clearance phenomenon in nucleic acid delivery. J Pharm Sci. 2014;103:557–566. doi: 10.1002/jps.23823. [DOI] [PubMed] [Google Scholar]
- 35.Maruyama K., Okuizumi S., Ishida O. Phosphatidyl polyglycerols prolong liposome circulation in vivo. Int J Pharm. 1994;111:103–107. [Google Scholar]
- 36.Ishida T., Ichihara M., Wang X.Y. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T., Syuntaro K., Hiroshi K. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Ichihara M., Shimizu T., Imoto A. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2010;3:1–11. doi: 10.3390/pharmaceutics3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Wang C.L., Jiao J. Tolerance-like innate immunity and spleen injury: a novel discovery via the weekly administrations and consecutive injections of PEGylated emulsions. Int J Nanomedicine. 2014;9:3645. doi: 10.2147/IJN.S66318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara E., Ueda M., Makino A. Factors influencing in vivo disposition of polymeric micelles on multiple administrations. ACS Med Chem Lett. 2014;5:873–877. doi: 10.1021/ml500112u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semple S.C., Harasym T.O., Clow K.A. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acid. J Pharmacol Exp Ther. 2005;312:1020–1026. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 42.Koide H., Asai T., Hatanaka K. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392:218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T., Ishida T., Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218:725–732. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- 44.Ishida T., Ichikawa T., Ichihara M. Effect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in mice. J Control Release. 2004;95:403–412. doi: 10.1016/j.jconrel.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Ishida T., Atobe K., Wang X.Y. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Ishida T., Ichihara M., Wang X.Y. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Ishida T., Wang X.Y., Shimizu T. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Kaminskas L.M., McLeod V.M., Porter C.J.H. Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance. J Pharm Sci. 2011;100:5069–5077. doi: 10.1002/jps.22682. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto Y., Shimizu T., Abu Lila A.S. Relationship between the concentration of anti-polyethylene glycol (PEG) immunoglobulin M (IgM) and the intensity of the accelerated blood clearance (ABC) phenomenon against PEGylated liposomes in mice. Biol Pharm Bull. 2015;38:417–424. doi: 10.1248/bpb.b14-00653. [DOI] [PubMed] [Google Scholar]
- 50.Ishida T., Kashima S., Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Sperling C., Schweiss R.B., Streller U. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials. 2005;26:6547–6557. doi: 10.1016/j.biomaterials.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 52.Arima Y., Toda M., Iwata M. Complement activation on surfaces modified with ethylene glycol units. Biomaterials. 2008;29:551–560. doi: 10.1016/j.biomaterials.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Jiskoot W., van Schie R.M.F., Carstens M.G. Immunological risk of injectable drug delivery systems. Pharm Res Dordr. 2009;26:1303–1314. doi: 10.1007/s11095-009-9855-9. [DOI] [PubMed] [Google Scholar]
- 54.Salvador-Morales C., Zhang L.F., Langer R. Immunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groups. Biomaterials. 2009;30:2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]