Graphical Abstract
Keywords: Pectin, Zein, Emulsions, High-pressure homogenization, Stability
Abstract
The aim of this study was to investigate the effect of high-pressure homogenization on the droplet size and physical stability of different formulations of pectin–zein stabilized rice bran oil emulsions. The obtained emulsions, both before and after passing through high-pressure homogenizer, were subjected to stability test under environmental stress conditions, that is, temperature cycling at 4 °C/40 °C for 6 cycles and centrifugal test at 3000 rpm for 10 min. Applying high-pressure homogenization after mechanical homogenization caused only a small additional decrease in emulsion droplet size. The droplet size of emulsions was influenced by the type of pectin used; emulsions using high methoxy pectin (HMP) were smaller than that using low methoxy pectin (LMP). This is due to a greater emulsifying property of HMP than LMP. The emulsions stabilized by HMP–zein showed good physical stability with lower percent creaming index than those using LMP, both before and after passing through high-pressure homogenizer. The stability of emulsions after passing through high-pressure homogenizer was slightly higher when using higher zein concentration, resulting from stronger pectin–zein complexes that could rearrange and adsorb onto the emulsion droplets.
1. Introduction
Emulsions are a heterogeneous system of two immiscible liquids (generally oil and water), where one of the phases (dispersed phase) is distributed in the other one as a continuous phase [1]. Most emulsions are not thermodynamically stable and tend to break down during storage through a number of mechanisms, that is, creaming, flocculation, Ostwald ripening, coalescence and phase inversion. Still, emulsion stability is one of the most important parameters in managing the shelf-life of food and pharmaceutical products [2].
Combination of protein and polysaccharide has been commonly used as emulsifier in a wide range of applications such as food, cosmetic and pharmaceutical industries, due to its advantages such as fast adsorption, steric repulsion and viscosity enhancement [3]. The complexes can be formed, when protein and polysaccharide are combined, commonly through electrostatic interaction. Thus, there is an increasing interest in combining proteins and polysaccharides to form electrostatic complexes to stabilize emulsions. Many studies have revealed the advantages of combining protein and polysaccharide, by formation of protein–polysaccharide complexes, to emulsify and stabilize emulsions under appropriate conditions, leading to increased emulsion stability, for example, β-lactoglobulin and carrageenan [4], β-lactoglobulin and gum arabic [5], whey protein isolate and pectin [6], bovine serum albumin and pectin [7], etc.
Pectin is progressively gaining acceptance as an emulsifier in food and pharmaceutical industries. Previous studies have shown that pectin alone at low concentration is not a good emulsifying agent for stabilizing emulsion. This may be due to its low adsorption ability, which is not sufficient to provide effective steric stabilization over a long-term period and against harsh conditions [8], [9]. Pectin is also applied in the formation of interfacial polymeric complexes, particularly for the production of emulsified oils, e.g., soy bean oil and corn oils [10]. Pectin could form interfacial complexes with many proteins, for example, β-lactoglobulin [10], whey protein isolate [6], bovine serum albumin [7], and zein [11], [12]. In our previous report, the use of complexes between pectin and zein to stabilize o/w emulsions at different pHs was preliminary investigated [13]. However, the effects of other extrinsic factors as well as formulation factors have not yet been investigated.
Different methods can be used to produce emulsions: mechanical (or rotor-stator), high-pressure, ultrasound, and membrane systems. The mechanical system includes colloid mill, with a common characteristic of complex geometry; droplet size of emulsions produced by this system are of several microns. High-pressure homogenization can reduce the droplet size to less than 1 µm and can improve shelf-life of the emulsions by reducing creaming rate. In the high-pressure homogenizer, harsh processing conditions such as high pressure, shear stress and temperature can lead to a deterioration of protein and/or polysaccharide that used as emulsifiers. To the best of our knowledge, there is a lack of information to show the influence of high-pressure homogenization on stability of pectin–zein stabilized emulsions. Therefore, the objective of this study was to assess the effect of high-pressure homogenization on the stability, under environmental stress conditions, of rice bran oil emulsions in the presence of pectin and zein.
2. Materials and methods
2.1. Materials
Refined rice bran oil was purchased from Thai Edible Oil Co., Ltd. (Thailand). Commercial zein (Zein F4000, lot number F4000262-L) having a maximum molecular weight of 35 kDa, mostly 19 kDa and 22 kDa, was purchased from Freeman Industries (USA). High methoxy pectin with molecular weight of 200 kDa and degree of esterification (DE) of 70% (lot number 00501087) and low methoxy pectin with molecular weight of 70 kDa and DE of 38% (lot number 00412072) were a gift from Herbstreith & Fox KG (Germany) and referred to as HMP and LMP, respectively. All other chemicals were of analytical or pharmaceutical grade and used as supplied without further purification. Deionized water was used throughout the study.
2.2. Preparation of oil-in-water (o/w) emulsions
Stock solution of zein (10% w/w) was prepared by dispersing zein powder (10 g) in aqueous alcohol solution (consisting of 80% v/v ethanol), adjusting to 100 g. Stock solution of pectin (4% w/w LMP or HMP) was prepared by dissolving pectin powder in distilled water. Both zein and pectin solutions were stirred at ambient temperature (25 °C) for at least 2 h to ensure complete hydration and then centrifuged (model Universal 320R, Hettich, Germany) at 8500 rpm for 30 min to remove any insoluble particles.
The premix emulsions were prepared by mixing 20% w/w rice bran oil with 0.5–1.5% w/w pectin (HMP or LMP) at pH 4.0 using mechanical homogenizer (model Polytron, Kinematika, Switzerland) at a speed of 12,000 rpm for 3 min [13]. Zein solution (0–0.1% w/w) was slowly added to primary emulsions to obtain secondary emulsions. The obtained emulsions were homogenized at a speed of 12,000 rpm for 3 min and then passed through a high-pressure homogenizer (model NV-200-D, Nanomizer, Japan) at 100 MPa for 3 times. Schematic diagram of emulsion preparation using mechanical and high-pressure homogenizers is shown in Fig. 1. To preserve the emulsions, 0.1% w/w methyl-propyl paraben, an antimicrobial agent, was added. The emulsions were then stored at ambient temperature (25 °C) for 24 h before further analysis.
Fig. 1.
Schematic diagram of emulsion preparation using mechanical and high-pressure homogenizers.
In a preliminary study, the effects of homogenization pressure (50–150 MPa) and number of pass (3–100 cycles) through the high-pressure homogenizer on the droplet size of emulsion were studied. The pectin concentration used was 1.5% w/w or lower due to the viscosity limit of high-pressure homogenizer.
2.3. Measurement of droplet size
Emulsion droplet size was measured by a laser diffraction size analyzer (model LDSA-SPR3500A, Nikkiso Co., Ltd., Tokyo, Japan). The emulsion was dispersed or diluted in 5 mM acetate buffer (pH 4) with gentle stirring. The median droplet size was measured under continuous stirring condition. The measurements were done on at least three batches of emulsions.
2.4. Zeta potential measurement
The zeta potential of the obtained emulsions was measured by using Zetasizer (model 3000HS, Malvern, UK). The emulsion was dispersed or diluted in 5 mM acetate buffer (pH 4) with gentle stirring at a volume ratio of 1:100 before measurement. The average and standard deviation of the measurement of three batches of emulsions were reported.
2.5. Optical microscopy
A drop of emulsions was placed on a glass slide and then covered with a cover slip. The morphology of droplet of the emulsions was investigated by light microscope (model BX51, Olympus, USA).
2.6. Stability of o/w emulsions
All emulsions were transferred into glass vials and then stored under environmental stress conditions, i.e., temperature cycling at 4 °C/40 °C (6 cycles), and centrifugation test at 3000 rpm for 10 min. After test, a number of emulsions separated into optically opaque “cream layer” at the top and a transparent (or turbid) “serum layer” at the bottom. The total height of the emulsions (HE) and the height of the serum layer (HS) were measured. The extent of creaming was characterized by creaming index using the following equation:
| (1) |
Measurements were carried out on three samples and reported as the mean and standard deviation.
2.7. Statistical analysis
Data were analyzed using SPSS version 11.5 for Windows (SPSS Inc., USA). The results were represented as mean ± standard deviation (SD). Analysis of variance (ANOVA) was used to determine difference among the groups, and pairs were compared using either the Scheffé or Games-Howell test [14]. The statistical significance was set at P < 0.05.
3. Results and discussion
3.1. Preparation of pectin–zein stabilized emulsions
The o/w emulsions could be prepared by homogenizing pectin with rice bran oil and then adding zein solution at pH 4. In the previous studies those using only mechanical homogenizer, it is found that the pectin–zein complexes demonstrated better oil/water interfacial tension lowering properties than pectin alone [12] and thus improved the stability of emulsions by decreasing droplet size and increasing viscosity of the emulsion system [13]. In order to form emulsion with smaller droplet size, more mechanical energy was applied. In the homogenization process, an increase in mechanical energy is recognized through the increase of homogenization pressures or homogenization cycles. The increase of the pressure level permits to reduce the droplet size and thus to improve the stability of the obtained emulsions. In a high-pressure homogenizer, the oil and water mixture is subjected to intense turbulent and shear flow fields. Turbulence leads to the break-up of the dispersed phase into small droplets [15]. In this study, the effect of high-pressure homogenization conditions on droplet size of emulsions was preliminarily investigated. Different homogenization pressures (50–150 MPa) and homogenization cycles (3–100 cycles) in high-pressure homogenization process were investigated in order to adjust the suitable condition for the preparation of emulsions. HMP was used as emulsifier in a preliminary experiment.
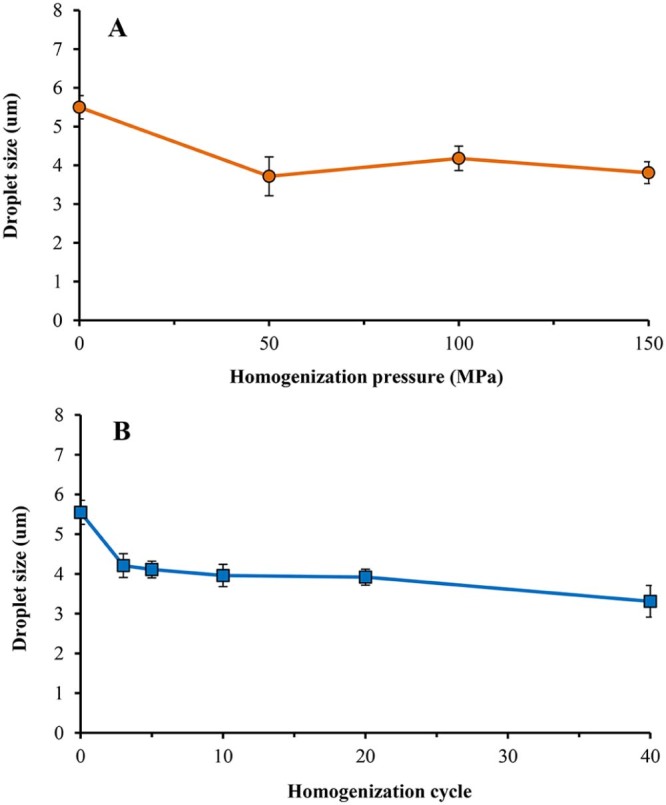
Fig. 2A shows droplet size of o/w emulsions stabilized by HMP, using various homogenization pressures. The average size of droplets generated by mechanical homogenizer (without high-pressure homogenization process) were about 5–6 µm. High-pressure homogenization with the pressure ranged from 50 to 150 MPa produced the emulsions with smaller droplet size. Generally, high-pressure homogenizer is capable of producing stable submicron emulsions by breaking down the oil droplets to the submicron scale with a narrow size distribution. However, in this study, the droplet size of 3–4 µm was achieved. The increased homogenization pressure did not significantly affect the emulsion droplet size (P > 0.05). The results are in agreement with the previous report [15], i.e., the pressure level does not markedly influence droplet size. Moreover, in this study, the droplet size of emulsions after passing through high-pressure homogenizer was in micrometer range. This is likely due to high concentration (20% w/w) of rice bran oil used in the formulations. The high concentration of oil content may cause greater viscosity, resulting in larger size of oil droplets. Floury and colleagues also found that the emulsions containing higher concentration of oil (50% w/w) demonstrate larger droplet size (about 10–20 µm) when using homogenization pressure of 20–150 MPa, compared to those containing 10% w/w oil (average droplet size of about 0.5–1 µm) [15]. Therefore, the homogenization pressure of 100 MPa was chosen for further investigation.
Fig. 2.
Droplet sizes of o/w emulsions containing 20% w/w rice bran oil, stabilized by HMP; effect of (A) homogenization pressure and (B) homogenization cycle.
Fig. 2B shows the droplet size of o/w emulsions stabilized by HMP, using various homogenization cycles. It appears that when the emulsions were homogenized for three cycles, the droplet size decreased significantly from 5.5 to 4 µm (P < 0.05). This would be expected because the increased number of pass allowed the increase in the energy input for emulsification. However, the droplet size insignificantly decrease (P > 0.05) when the homogenization was more than three cycles (Fig. 2B). Besides, at 100 cycles, emulsions were separated to two phases (data not shown). It is possible that a long-term high-pressure homogenization broke pectin chain up into shorter chain, leading to a higher coalescence rate after the homogenizing valve. Thus, the homogenization cycle for passing through the high-pressure homogenizer was set at three cycles for all formulations.
Various concentrations of pectin (both LMP and HMP) were used as an emulsifier, i.e., 0.5, 1.0 and 1.5% w/w. Low concentration of pectin (0.5% w/w) was not sufficient to prepare stable emulsions. The pectin concentration of greater than 2% w/w tended to obstruct the machine because of its high viscosity. Therefore, the concentration of pectin used in this study was fixed at 1.5% w/w for both LMP and HMP.
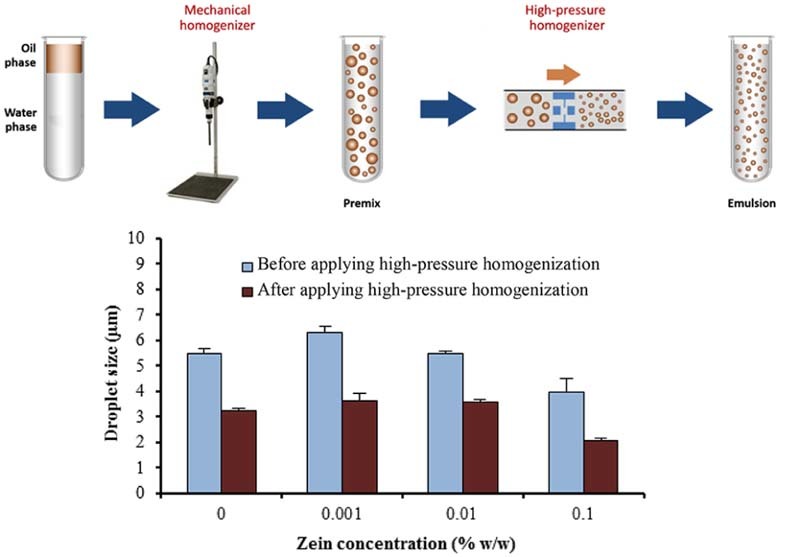
Fig. 3 shows microscopic images of o/w emulsions containing 1.5% w/w LMP and various concentrations of zein, before and after passing through high-pressure homogenizer. Freshly prepared emulsions were milky white in color and all of emulsions showed spherical droplets. The droplet size of emulsions containing 1.5% w/w LMP slightly decreased (P < 0.05) after passing through the high-pressure homogenizer but insignificantly decreased (P > 0.05) when the concentration of zein was increased (Fig. 4A). The size distribution of emulsion droplets also decreased slightly (Fig. 3). It is likely that the high-pressure homogenizer could reduce droplet size of the internal phase with forcing macro-emulsion through narrow gaps by imposing high-pressure [15]. Similar results have been reported by Pouliot et al. [16] that, although high-pressure homogenizer is capable of fabricating fine emulsions, it only accomplishes a small decrease in the average size of oil droplets after passing through the high-pressure homogenizer (microfluidizer). However, they observed a greater effect on size distribution, compared with classical homogenization.
Fig. 3.
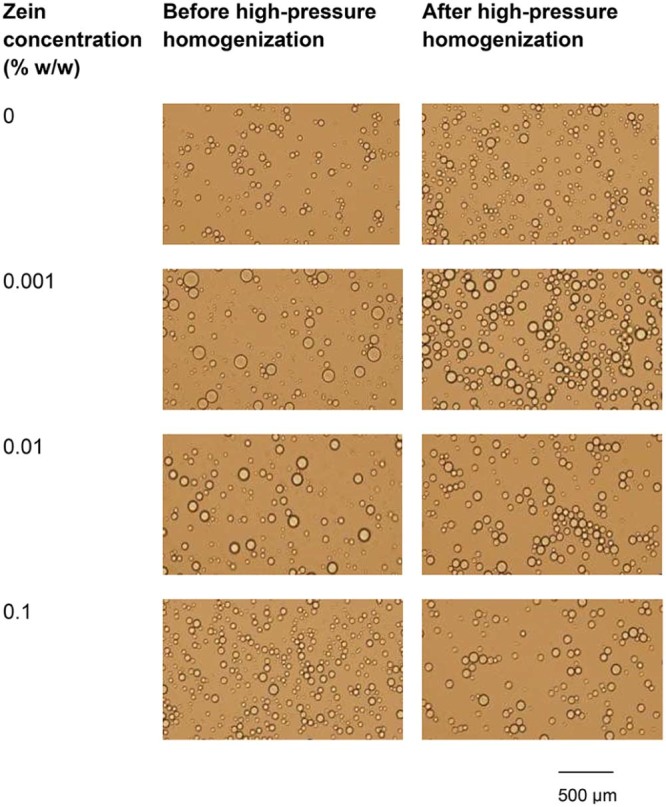
Microscopic images of o/w emulsions containing 20% w/w rice bran oil and 1.5% w/w LMP and various concentrations of zein, before and after passing through high-pressure homogenizer.
Fig. 4.
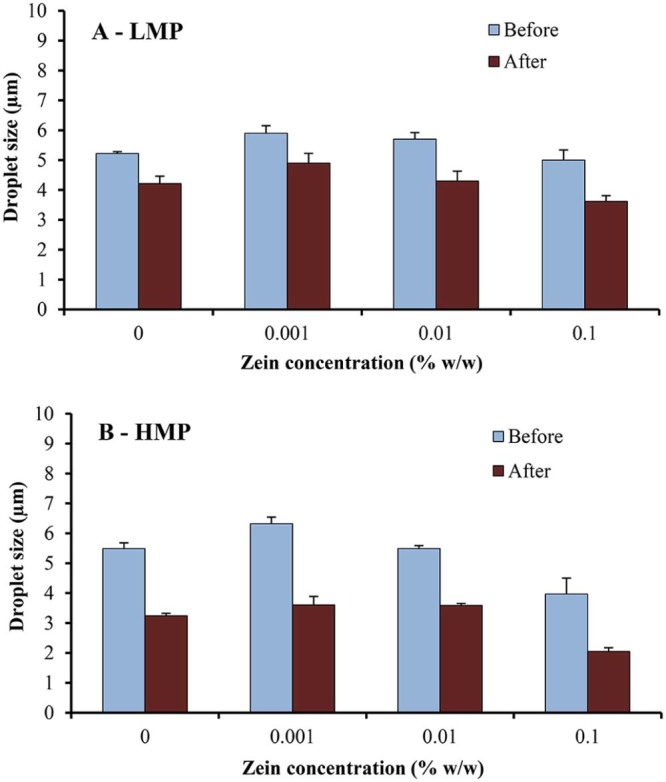
Droplet size of o/w emulsions containing 20% w/w rice bran oil, using (A) LMP or (B) HMP and various concentrations of zein, before and after passing through high-pressure homogenizer.
The droplet size of emulsions containing 1.5% w/w HMP obviously decreased (P < 0.05) after passing through the high-pressure homogenizer (Fig. 4B). This could be confirmed by the microscopic images of emulsions containing 1.5% w/w HMP and various concentrations of zein (Fig. 5). McClements [17] reported that emulsions subjected to secondary homogenization usually have a smaller droplet size than those subjected only to primary homogenization. Other authors [18], [19] studied the effect of rotor-stator homogenization and rotor-stator homogenization combined with high-pressure homogenization (microfluidization); they found that high-pressure homogenization significantly reduced emulsion droplet size. It is also observed that the size distribution of emulsions containing 1.5% w/w HMP decreased after passing through the high-pressure homogenizer, which is probably due to the fact that the emulsion droplets pass through a very narrow gap with extremely high velocity, as discussed above.
Fig. 5.
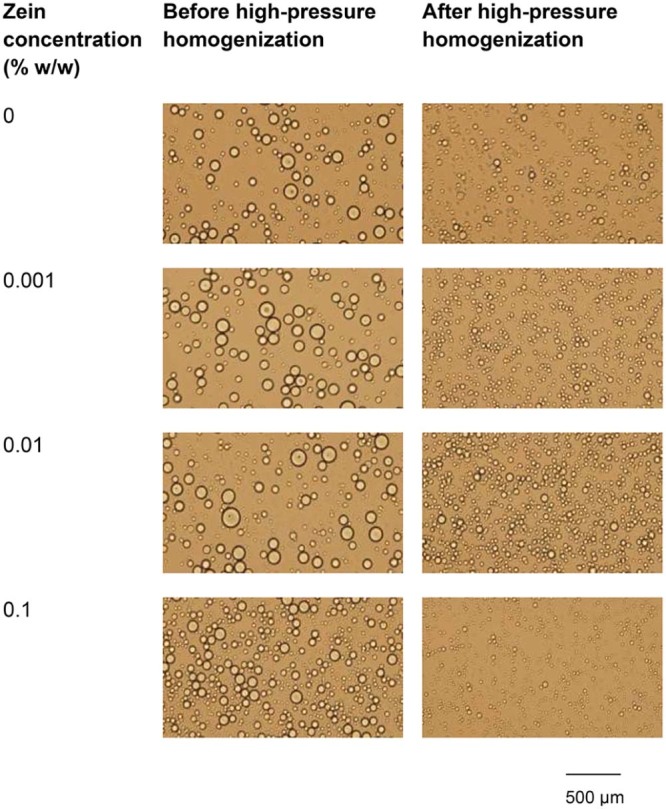
Microscopic images of o/w emulsions containing 20% w/w rice bran oil and 1.5% w/w HMP and various concentrations of zein, before and after passing through high-pressure homogenizer.
It is also evident that the droplet size of emulsion using HMP was smaller than that using LMP, after passing through high-pressure homogenizer. This may be due to a greater emulsifying property of HMP, resulting from more hydrophobic group in the structure compared to LMP [20]. In addition, the droplet size decreased when the zein concentration was increased. It is likely that zein in formulation may be able to improve emulsifying properties of o/w emulsions stabilized by HMP.
Table 1 shows zeta potential of o/w emulsions using LMP or HMP and various concentrations of zein, before and after passing through high-pressure homogenizer. The zeta potential of the emulsions before and after passing through high-pressure homogenization was not significantly different. It is likely that the surface charge of emulsions was still the same after passing through the high-pressure homogenizer. However, the zeta potential of emulsions using HMP was less negative than those using LMP, resulting from the higher amount of free carboxyl group in HMP [21].
Table 1.
Zeta potential of o/w emulsions using (A) LMP or (B) HMP and various concentrations of zein, before and after passing through high-pressure homogenizer (n = 3).
| Zein (% w/w) | Zeta potential (mV) ± S.D. | |||
|---|---|---|---|---|
| LMP | HMP | |||
| Before | After | Before | After | |
| 0 | −37.90 ± 0.61 | −36.90 ± 0.96 | −22.90 ± 2.26 | −23.67 ± 0.71 |
| 0.001 | −38.57 ± 0.55 | −38.47 ± 0.68 | −24.00 ± 0.36 | −24.20 ± 0.53 |
| 0.01 | −37.47 ± 1.21 | −37.13 ± 0.40 | −23.00 ± 1.35 | −23.73 ± 1.10 |
| 0.1 | −38.00 ± 0.20 | −37.37 ± 0.91 | −23.27 ± 0.75 | −23.97 ±0.74 |
3.2. Stability of pectin–zein stabilized emulsions prepared by high-pressure homogenizer
The physical stability of o/w emulsions was also investigated in this study and could be reflected by creaming index. After stability testing under stress conditions (i.e., centrifugation test, temperature cycling test), the emulsions underwent visible phase separation, with a white cream layer on top of a clear serum layer. Fig. 6 shows percent creaming index of o/w emulsions using LMP or HMP and various concentrations of zein, before and after passing through high-pressure homogenizer, determined by centrifugation test at 3000 rpm for 10 min. The percent creaming index of emulsions after temperature cycling test (4 °C/40 °C) for 6 cycles is given in Fig. 7.
Fig. 6.
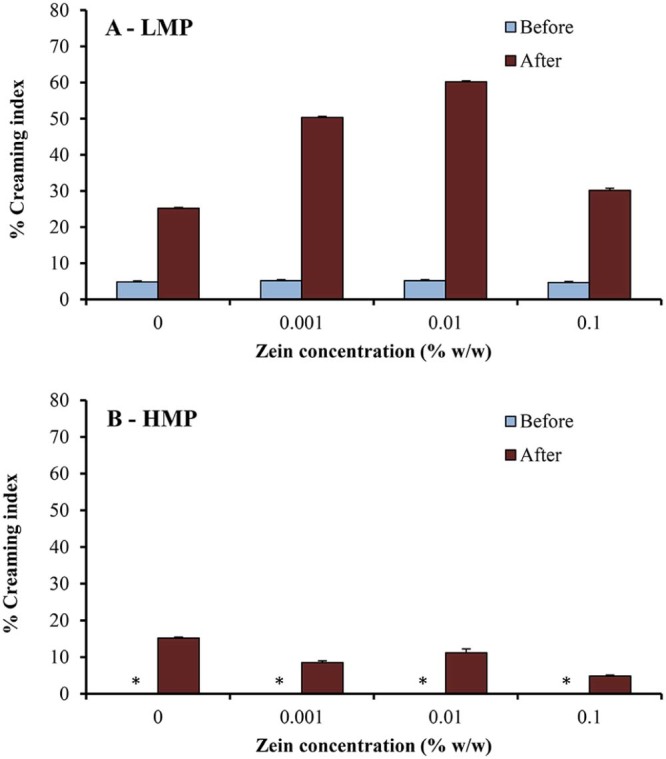
Percent creaming index of o/w emulsions using (A) LMP or (B) HMP and various concentrations of zein, before and after passing through high-pressure homogenizer, determined by centrifugation test at 3000 rpm for 10 min. * creaming index = 0, indicating stable emulsions after stability testing.
Fig. 7.
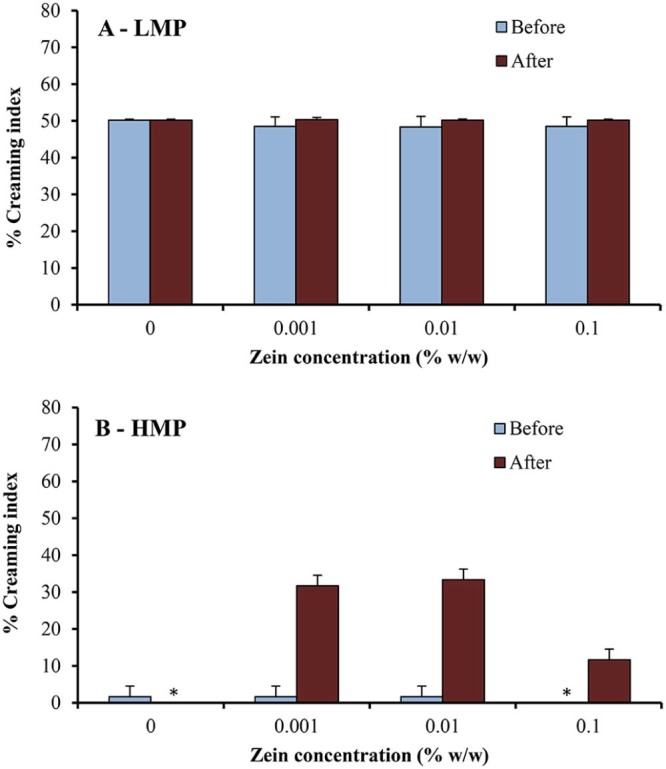
Percent creaming index of o/w emulsions using (A) LMP or (B) HMP and various concentrations of zein, before and after passing through high-pressure homogenizer, after temperature cycling test (4 °C/40 °C) for 6 cycles. * creaming index = 0, indicating stable emulsions after stability testing.
The emulsions prepared with pectin and various concentrations of zein were milky white in color. The results of this study revealed that emulsions stabilized by HMP–zein showed good physical stability, with no sign of phase separation, before passing through high-pressure homogenizer; their stability was better (lower percent creaming index) than those using LMP (Fig. 6, Fig. 7). The percent creaming index of the emulsions using LMP was about 5% after centrifugal test (Fig. 6A), indicating instability of emulsions. As expected, the emulsions using LMP were unstable after temperature cycling test (Fig. 7A) and they separated to two phases, resulting in a low stability (with 50% creaming index). This is probably due to the low emulsifying property and sensitivity to temperature of LMP [21].
To study the effect of high-pressure homogenization, the physical stability under stress conditions was also performed. It is found that a significant lower emulsion stability (with a higher % creaming index) of both pectin types after passing through the high-pressure homogenizer (Fig. 6, Fig. 7). This may be due to the fact that high-pressure homogenizer might destroy the long chain molecules and then reduce the emulsifying property of pectin [22]. Floury et al. [15] reported the effect of high-pressure homogenization on the change in emulsion droplet size and also the properties of the stabilizer molecules. They hypothesized an unfolding or partial denaturation of the globular proteins caused by treatments at high pressure. A similar mechanism may be suggested to explain the high-pressure effect on the o/w emulsions stabilized by pectin–zein complexes; that is, the high-pressure homogenization could cause the unfolding or partial denaturation of zein, leading to a network formation or rearrangement [23].
Similar to the emulsions prepared by only mechanical homogenizer (before passing through high-pressure homogenizer), the emulsions with HMP were more stable than those with LMP. It is likely due to more hydrophobic group in the HMP structure, resulting in a greater emulsifying property [20]. Moreover, when the HMP forms polyelectrolyte complex with zein, it can cause a more reduction in the surface tension at the oil–water interface than LMP [12]. In addition, the percent creaming index of the emulsions before passing through high-pressure homogenizer was not influenced by zein concentration. The results also demonstrated that the stability of emulsions after passing through high-pressure homogenizer was slightly higher when using high zein concentration, compared to that using low zein concentration. The higher zein concentration may result in the strong pectin–zein complexes, which could rearrange and adsorb onto the emulsion droplets [13], [14]. This resulted in a better protection of emulsion droplets by pectin–zein complexes [3].
4. Conclusion
The droplet size of emulsions prepared by using mechanical homogenizer and high-pressure homogenizer together decreased only slightly, which is probably due to the high oil content in the formulations. The emulsions stabilized by HMP–zein were smaller in size and showed good physical stability with lower percent creaming index than those using LMP, both before and after passing through high-pressure homogenizer. Zein concentration did not influence the percent creaming index of the emulsions before passing through high-pressure homogenizer but that of emulsions after passing through high-pressure homogenizer. Therefore, when aiming for a greater stability, the conditions would be (1) the use of high-pressure homogenizer for the emulsions containing only HMP, or (2) without high-pressure homogenizer for the emulsions containing HMP and zein (0.1% w/w).
Acknowledgments
This work was financially supported by the Research and Development Institute, Silpakorn University. Thanks to Herbstreith & Fox KG (Germany) who kindly donated pectin samples.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Dickinson E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems: review. Food Hydrocolloids. 2003;17:25–39. [Google Scholar]
- 2.Abdolmaleki K., Mohammadifar M.A., Mohammadi R. The effect of pH and salt on the stability and physicochemical properties of oil-in-water emulsions prepared with gum tragacanth. Carbohydr Polym. 2016;140:342–348. doi: 10.1016/j.carbpol.2015.12.081. [DOI] [PubMed] [Google Scholar]
- 3.Evans M., Ratcliffe I., Williams P.A. Emulsion stabilization using polysaccharide-protein complexes. Curr Opin Colloid Interface Sci. 2013;18:272–282. [Google Scholar]
- 4.Gu Y.S., Decker E.A., McClements D.J. Influence of pH and carrageenan type on properties of β-lactoglubulin stabilized oil-in-water emulsions. Food Hydrocolloids. 2004;19:83–91. doi: 10.1021/jf0352834. [DOI] [PubMed] [Google Scholar]
- 5.Bouyer E., Mekhloufi G., Le Potier I. Stabilization mechanism of oil-in-water emulsions by β-lactoglobulin and gum arabic. J Colloid Interface Sci. 2011;354:467–477. doi: 10.1016/j.jcis.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Lutz R., Aserina A., Wicker L. Double emulsions stabilized by a charged complex of modified pectin and whey protein isolate. Colloids Surf B Biointerfaces. 2009;72:121–127. doi: 10.1016/j.colsurfb.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Fang Y., Al-Assaf S. Complexation of bovine serum albumin and sugar beet pectin: stabilizing oil-in-water emulsions. J Colloid Interface Sci. 2012;388:103–111. doi: 10.1016/j.jcis.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Mirhosseini H., Tan C., Aghlara A. Influence of pectin and CMC on physical stability, turbidity loss rate, cloudiness and flavor release of orange beverage emulsion during storage. Carbohydr Polym. 2008;73:83–91. [Google Scholar]
- 9.Nakauma M., Funami T., Noda S. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocolloids. 2008;22:1254–1267. [Google Scholar]
- 10.Güzey D., McClements D.J. Impact of electrostatic interactions on formation and stability of emulsions containing oil droplets coated by β-lactoglobulin–pectin complexes. J Agri Food Chem. 2007;55:475–485. doi: 10.1021/jf062342f. [DOI] [PubMed] [Google Scholar]
- 11.Juttulapa M., Sriamornsak P. Effect of zein concentration on the formation of pectin-zein complexes. Adv Mater Res. 2012;506:319–322. [Google Scholar]
- 12.Piriyaprasarth S., Juttulapa M., Sriamornsak P. Formation and characterization of polyelectrolyte complexes containing pectin and zein. Walailuk J Sci Technol. 2016 in press. [Google Scholar]
- 13.Juttulapa M., Piriyaprasarth S., Sriamornsak P. Effect of pH on stability of oil-in-water emulsions stabilized by pectin–zein complexes. Adv Mater Res. 2013;747:127–130. [Google Scholar]
- 14.Sungthongjeen S., Sriamornsak P., Pitaksuteepong T. Effect of degree of esterification of pectin and calcium amount on drug release from pectin-based matrix tablets. AAPS PharmSciTech. 2004;5(1):50–57. doi: 10.1208/pt050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floury J., Desrumaux A., Lardieres J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov Food Sci Emerg Technol. 2000;1:127–134. [Google Scholar]
- 16.Paquin P. Technological properties of high pressure homogenizers: the effect of fat globules, milk proteins, and polysaccharides. Int Dairy J. 1999;9:329–335. [Google Scholar]
- 17.McClements D.J. Biopolymers in food emulsions. In: Kassapis S., Norton I.T., Ubbink J.B., editors. Modern biopolymer science: bridging the divide between fundamental treatise and industrial application. Elsevier Science; New York: 2009. pp. 129–166. [Google Scholar]
- 18.Ma W., Tang C., Yin S. Effect of homogenization conditions on properties of gelatin–olive oil composite films. J Food Eng. 2012;113(1):136–142. [Google Scholar]
- 19.Bonilla J., Atarés M., Vargas M. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocolloids. 2012;26(1):9–16. [Google Scholar]
- 20.Burapapadh K., Kumpugdee-Vollrath M., Chantasart D. Fabrication of pectin-based nanoemulsions loaded with itraconazole for pharmaceutical application. Carbohydr Polym. 2010;82:384–393. [Google Scholar]
- 21.Sriamornsak P. Application of pectin in oral drug delivery. Expert Opin Drug Deliv. 2011;8(8):1009–1023. doi: 10.1517/17425247.2011.584867. [DOI] [PubMed] [Google Scholar]
- 22.Anton N., Benoit J.P., Saulnier P. Design and production of nanoparticles formulated from nanoemulsion templates – a review. J Control Rel. 2008;128:185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Innocente N., Biasutti M., Venir E. Effect of high-pressure homogenization on droplet size distribution and rheological properties of ice cream mixes. J Dairy Sci. 2009;92(5):1864–1875. doi: 10.3168/jds.2008-1797. [DOI] [PubMed] [Google Scholar]