Abstract
Polyurethane foam dressings for dermal wounds were formulated with natural polyols in order to improve the foam characteristics and the release of 2 active agents, silver and asiaticoside (AS) as an antimicrobial agent and an herbal wound healing agent, respectively. The foam was instantly formed by interaction of polyols and diisocyanate. Hydroxypropyl methylcellulose, chitosan and sodium alginate were individually mixed with the main polyols, polypropylene glycol, in the formulation while the active components were impregnated into the obtained foam dressing sheets. Although the type and amount of the natural polyols slightly affected the pore size, water sorption-desorption profile and compression strength of the obtained foam sheets, a prominent effect was found in the release of both active components. Among natural polyols formulations, foam sheets with alginate showed the highest silver and AS release. Non-cytotoxicity of these foam sheets to human fibroblast cells was confirmed. Antimicrobial testing on four bacteria strains showed that 1 mg/cm2 silver in formulations with 6% of natural polyols and without natural polyols had sufficient content of the silver release with comparable inhibition zone and significantly larger zone than other formulations. In pig study, the foam dressing with 6% alginate, 1 mg/cm2 silver and 5% AS could improve wound healing in both the percentage of the wound closure and histological parameters of the dermal wound without any dermatologic reactions. In conclusion, this innovative foam dressing had potential to be a good candidate for wound treatment.
Keywords: Polyurethane foam dressing, Natural polyols, Silver nanoparticles, Asiaticoside, Wound healing
Graphical abstract
The developed foam dressing consisted of three parts; natural polyols, silver nanoparticles and asiaticoside. Adding the natural polyols in polyurethane foam preparation was to increase hydrophilic properties and their characteristics were evaluated. The silver and asiaticoside were then impregnated and their releasing profiles were determined. The animal studies were performed to evaluate the efficacy and safety of the prepared foam dressings.
1. Introduction
The primary objectives of wound care are rapid wound closure, minimal complications and less chance of hypertrophic scar formation. Dermal wound or partial thickness wound has pathology in both epidermis and dermis layers, and is characterized by blebs, pink-red color, moist and pain. Because of available cells, this wound type can be self-healed under appropriate treatment especially with wound dressing that possesses tissue regeneration and healing properties. There are many factors to be considered to develop advanced wound dressing. Ideally, wound dressing should provide the effectiveness in exudate absorption, retaining hydration, prevention of micro-organism contamination and avoiding dressing related trauma [1].
Due to thicker than other wound dressings, the foam dressing provides a protective cushioning effect over the wound. Not only prevent exudates pooling and periwound maceration, it also keeps hydration so that the appropriate moisture environment will promote the epithelialization and healing through the encouragement of the migration of cells [1], [2]. Polyurethane (PU) foam was generally synthesized by the reaction of polyols with isocyanate [3]. The open cells and linked porosity in foam structure were caused by two reactions; gelling and blowing. The carbon dioxide gas from the blowing reaction expanded the foam while simultaneously the gelling reaction would produce the urethane linkage which increased the foam strength. Concerning the water sorption and moisture protection, hydrophilic natural polymers such as cellulose and polysaccharides were for the first time to be incorporated in foam formulation.
Apart from possess water-favorable property, hydroxypropyl methylcellulose (HPMC, H), chitosan (CLMW, C) and sodium alginate (Alg, A) are investigated due to low cost, commercial availability, and excellent biocompatibility. To date, carboxymethylcellulose (CMC) has been incorporated in various commercial wound products such as IntraSite™ Gel, GranuGel™, and Aquacel® Ag, but not HPMC. Only investigation on HPMC based hydrogel were reported that over 95% wound closure and collagen deposition in a few weeks after application of ofloxacin-loaded HPMC hydrogels [4]. In rat study, HPMC/chitosan gel loaded with simvastatin showed significant increase in wound closure [5]. Chitosan has markedly wound healing properties due to its antimicrobial effect, hemostasis stimulation, and acceleration of tissue regeneration [6], [7], [8]. Numerous studies have reported on chitosan containing formulations for wound healing [5], [6], [9]. Sodium alginate, an anionic polysaccharide has outstanding in hydrophilic property. The alginate is commonly prepared as alginate hydrogel dressing which turns to gel after water sorption such as Algisite® M and Kaltostat™ [10]. The alginate dressing preserved a moist environment around the wound to promote wound healing including the histological results of facilitation on wound healing from neomycin sulfate-loaded alginate hydrogel dressing compared to the commercial product [11]. Thus the incorporation of these hydrophilic polymers in PU foam was expected to intensely improve foam dressing properties.
To reduce systemic antibiotic resistance, silver (Ag) in dressings locally improve wound treatment especially in chronic and infected prone wounds. This metal has 4 possible bacteriostatic/bactericidal mechanisms [12], [13], binding with bacterial cell wall leading to cell membrane disruption, binding microbial DNA effect thus inhibiting cell division, generating reactive oxygen species and free radicals and blocking the mitochondrial respiratory function by inhibiting respiratory chain enzymes. This agent is a valuable candidate for wound management with low incidence resistance [12], [14]. Moreover, numerous evidences have proved the efficacy of silver dressings in both in vitro and clinical trials and the removal of the bioburden in burn and open wound has been indicated [15], [16], [17].
Asiaticoside (AS), an active herbal compound from Centella asiatica, has been confirmed its potency in wound healing [18], [19], [20]. The solution containing AS increased the hydroxyproline content, tensile strength, collagen content and epithelialization resulting in facilitating the healing of diabetic wounds [21]. In an in vivo animal burn model, AS showed positive effects on the proliferation and cell growth of wound healing by stimulating the collagen synthesis and reducing the wound's oxidative stress [22], [23]. Faster and better maturation of collagen was reported in the extract-treated wounds whereas Smad 3 and 4 signaling in collagen synthesis was induced [24], [25]. Moreover, the Centella asiatica extracts were listed in the Indian Pharmacopoeia as wound healing agent [26].
Owing to similar skin structure to the human such as the process of reepithelialization, vascularization and percentage amount of collagen and elastic fiber in the extracellular matrix [27], [28], pig model has generally been used to investigate the in vivo effectiveness of wound treatment. The dermis thickness of pig is about 1400–2000 µm compared to human which is about 2000–3000 µm while the rat and mouse skin are thinner (1000–2000 and 200–600 µm, respectively) [29]. It also has sparse hair (10–20 hairs/cm2) rather than fur which differs from rodent and rabbit (289 and 658 hairs/cm2, respectively) [30]. There is only one sebaceous gland per hair follicle in pigs and humans whereas two opposite glands per each hair follicle are in rodents [31].
Thus the aim of this study was to investigate the influence of three natural polyols on the physical and mechanical behavior of PU foam dressings especially the effectiveness in exudate absorption and retaining hydration. The content of silver and AS, their releasing profiles and antimicrobial activity were also determined in Part I of this investigation to select the most appropriate formulation. Then, the optimized dressing would then be tested for the wound healing efficacy and safety in animal models in Part II study.
2. Materials and methods
2.1. Materials
The materials used for the study comprised polypropylene glycol (PPG, MW 3000), silicone copolymer surfactant (Dabco DC5810), amine catalyst (Dabco 33-LV), tin catalyst (T9), and toluene diisocyanate (TDI) that were obtained from the Air Products and Chemicals Company Limited (Pennsylvania, USA). Methylene chloride came from Fisher Scientific (New Hampshire, USA). Other hydrophilic polyols used included hydroxypropyl methylcellulose (HPMC, Methocel® E5, Colorcon Asia Pacific Pte. Ltd., Singapore), chitosan (CLMW, Sigma-Aldrich Chemical Company, Missouri, USA), and sodium alginate (Alg, Acros Organics, Geel, Belgium). Silver nanoparticles were bought from the Guangzhou Hongwu Material Technology Company Limited (Guangzhou, China; Batch No. HW-P160819). Asiaticoside powder (95% purity) was bought from the Xian Lyphar Biotech Company Limited (Shaanxi, China; Batch No. LYPH150927). All other chemicals were AR grades and used as received.
2.2. Preparation and evaluation of foam dressing properties (Part i)
2.2.1. Preparation and characterization of PU foam dressing
2.2.1.1. Preparation
PU foam was produced by the reaction of polyols with diisocyanate [3]. Briefly, polypropylene glycol (PPG) 100 g and 4%−6% of a hydrophilic natural polyols; HPMC (H4, H6), CLMW (C4, C6) or Alg (A4, A6), 2.0 g of deionized water, 1.5 g of silicone copolymer surfactant, 3.0 g of methylene chloride, and 0.2 g of amine catalyst were mixed and vigorously stirred by an impeller at 500 rpm for 2 min before being cooled to 20 °C. Tin catalyst was added to the mixture, stirred at 1900 rpm for one min followed by adding 31.71 g of cooled toluene diisocyanate (TDI) and stirring for a few seconds. The loaf of foam was immediately created after the mixture was poured in a mold at 25–30 °C and then cured for 72 h. Foam sheets were sliced by a foam cutting machine (Foam vertical cutting machine model IS-M, Albrecht Bäumer GmbH and Company KG, Freudenberg, Germany) to obtain a thickness of 6.0 ± 0.5 mm. The blank foam sheet without natural polyols (Bl) was also prepared.
2.2.1.2. Characterization
Microscopic appearance and pore size: Prior to the investigation, samples were cut with a blade and attached on the stub then coated with gold. Scanning electron micrographs of samples were taken at 10 kV, 15x magnification (JSM-7610F; JEOL, Tokyo, Japan), and the pore sizes from the top view were calculated [32] from approximately 300 pores using the Image J program (NIH, USA).
Density: The foams were measured in width, length and thickness using a Vernier caliper (Mitutoyo digimatic caliper series 500, Tokyo, Japan) in mm. Then, the samples were weighed and recorded in grams. The density was calculated and reported as g/cm3 unit [33].
Water absorption and %weight loss study: Foam sheets were dried in a desiccator for 24 h before the initial weighing (Wi) then held in stainless steel mesh tea ball apparatus prior to submerging in 120 ml of distilled water. After equilibrated at 37 °C for 48 h, the samples were then taken out of the water, suspended for 10 s for free drainage, absorbed excess water with paper and reweighed again to get Ww. Subsequently, the samples were kept in an incubator at 50% ± 5%RH, 37 ± 1 °C for 48 h, the samples were reweighed. Weight loss was the weight that was lost from the absorption and dehydration process and was calculated from the difference in weight between Wi and Wd. Five samples per formulation were evaluated. The percentages of the absorption and weight loss could be calculated from absorption %= {(Ww – Wi)/Wi} × 100 and weight loss% = {(Wi – Wd)/Wi} × 100 [34].
Foam degradation test: The study was applied from the enzyme degradation test [35]. The dry sample was weighed (W0) and then completely suspended in 7 ml of phosphate-buffered saline pH 7.4 with or without lysozyme (1.6 µg/ml) in the 6-well plate and incubated at 37 °C. The solution was refreshed daily. After 48 h, the sample was removed, washed with distilled water, and dried at 40 °C for 48 h. The dry weight was measured (W1). The percentage of degradation = (W0 – W1)/W0} × 100%.
Compression test: The study was applied from ASTM D3574 [36] using a Universal Testing Machine (UTM) (Shimadzu, model EZ-S 500 N, Osaka, Japan). The initial thickness of the sample was determined prior to placing between the horizontal plates of a compression device. The samples were compressed to 75% of their original thickness with a speed of 2 mm/min after that the samples were removed. The compressive strength values were reported at 50% of the strain. Five specimens per sample were tested. The value was reported as a mean value of those observed.
2.2.2. Preparation and determination of active compounds contents and their releasing profiles
2.2.2.1. Preparation
Active ingredients were loaded into the foam sheets of 10 × 10 cm2 by an absorption process. Silver nanoparticles of 0.4, 0.6, 0.8 and 1.0 mg/cm2 (0.4–1.0Ag) were homogeneously dispersed in deionized water of not more than optimal water absorption volume of the foam whereas asiaticoside powder was also incorporated at 5% in selected dispersions. After absorption, the foam sheets were oven dried at 40 °C for 48 h.
2.2.2.2. Determination of active compounds contents and their releasing profiles
Silver content
This experiment was adapted from Kulthong et al. [37]. The sample was added with 1 ml of 50% (v/v) of nitric acid and boiled in a water bath (Model B 22, Memmert GmBH + Company KG, Schwabach, Germany) at 70 °C for 2 h. Then, 0.5 ml of the acid solution was pipetted to 2 ml of distilled water. The solution was centrifuged at 3000 rpm for 5 min. The 1 ml supernatant was pipetted into 4 ml of purified water. The amount of silver ion was determined by a flame atomic absorption spectrophotometer (AAS, Varian model AA280FS, California, USA) at the wavelength and lamp current of 328.1 nm and 3 mA, respectively. The flame type was air/acetylene with air flow and acetylene flow of 13.20 and 1.8 l/min, respectively. The standard concentration was prepared from a standard silver solution (Merck KGaA, Damstadt, Germany).
Asiaticoside (AS) content
The cubed foam was suspended in 5 ml of methanol. The suspension was shaken in a water bath at 30 °C for one h. The process was repeated in new medium. Both volume of medium was then filtered through a 0.45 µm membrane filter prior to injection. The AS content was determined following the method by Hengsawas et al. [38] using HPLC with a UV detector at 220 nm (Shimadzu model LC-20AB, Shimadzu Scientific Instruments, Kyoto, Japan, detector model SPD-20A) and HALO-5® (C18) column (250 mm × 4.6 mm), 5 μm (Advance Materials Technology, USA). The injection volume was 20 µl at a flow rate of 1 ml/min. The mobile phase was water-acetonitrile with linear gradient conditions of water 70%, 0%, 70% and 70% of water in pump A, and 30%, 100%, 30%, 30% acetonitrile in pump B at the time intervals of 0, 12, 15 and 30 min, respectively.
Releasing profiles of active compounds
A release study was applied using the static Franz diffusion cells method [39]. Purified water and PBS pH 7.4 with 10% of methanol were used as receptor media for the content determination of silver and AS, respectively. The receptor compartment of each medium was maintained at 37 °C and magnetically stirred. A round shape dressing sample was placed between the donor and the receptor compartment. At various time intervals, the solutions of appropriate volume were sampled from the receptors for determination of the amount of silver and AS released, respectively and replaced with the same volume of fresh solution to maintain the fluid level. The 4 ml of silver sampling solution was mixed with 1 ml of 25% (v/v) nitric acid to dissolve the silver nanoparticles. The contents of silver and AS were quantified by using AAS and HPLC as aforementioned, respectively.
2.2.3. Antibacterial test
An agar diffusion method was performed in this study with some modifications [40] using four bacterial strains commonly found in trauma wounds; Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6633), Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) [41]. The bacteria were grown in a culture slant for overnight incubation. The bacterial culture was suspended in broth, and the turbidity of the bacterial suspension was adjusted to the 0.5 McFarland standards (1.5 × 108 cfu/ml). The broth of 100 µl was used to streak on Muller-Hinton agar plates in three directions to form a confluent lawn. The sample of the ascertained size was aseptically applied to the center of each lawn. The plates were left in the incubator at 37 °C for 24 h. The diameter of the clear zone surrounding the test dressing was measured for the zone of growth inhibition using a Vernier caliper and recorded in millimeters (mm). All zones of the inhibition (ZOI) were reported as a mean and standard deviation from three independent experiments.
2.2.4. Cytotoxicity test
The human fibroblasts (ATCC® CRL-2522™) were cultured in complete growth medium which composed of 10% of fetal bovine serum, 1% of antibiotic-antimycotic agent which contained amphotericin B, penicillin and streptomycin and Dulbecco's Modified Eagle's Medium (DMEM) which contained a saline solution, amino acids, 25 mM of D-glucose, and 1 mM of sodium pyruvate. The cell culture grew in a 37 °C and 5% humidified CO2 incubator.
Study of the viability of the cells in the presence of foam samples was modified from Burd et al. [42] whereby the fibroblasts were planted in a complete growth medium on 24 well plates at a density of 5 × 103/well. The plate was incubated in 5% of CO2 at 37 °C for 72 h to obtain 70% of the cells’ confluences. The complete growth medium was added into each well for 300 µl. At that time, the 1 × 1 cm2 dressing samples soaked with 200 µl of phosphate buffer solution were added to the culture well. The control was 500 µl of complete growth media solution. After 24 h of incubation, the dressing and medium were removed. The cell viability was determined by an MTT assay. The absorbance was measured at 560 nm in triplicate (100 µl in three wells of 96 well plates), using a microplate reader (Perkin Elmer Victor3™ Model 1420–050, Massachusetts, USA). The number of viable cells is correlated with a mitochondrial activity which reflected by the conversion of the tetrazolium salt MTT into formazan crystal by the mitochondrial dehydrogenase enzyme. The percentage of the cell viability was calculated from the following; cell viability% = (Absorbance of surviving fibroblast of samples / Absorbance of surviving fibroblast of control) × 100.
2.3. Efficacy and safety in the animal model
2.3.1. Skin irritation test of selected foam dressing on rabbits
This experiment was approved by the Institutional Animal Care and Use Committee of Thailand Institute of Scientific and Technological Research (TISTR) (Protocol No.TS-59,001) according to the OECD Guidelines (2015) [43]. Three rabbits were housed individually within approximately 20 ± 3 °C and 50% ± 10% RH, for adaptation to minimize stress and physiologic alteration before the experiment. Conventional laboratory diets with drinking water were provided ad libitum. Twenty-four hours before the test, the fur on the dorsal area was removed and avoided abrading the skin. The 4.0 cm2 of the tested dressing was soaked with 0.5 ml of normal saline solution before being applied on the skin. Then, it was covered with a sterile gauze patch and held in place with adhesive tape. The skin area, which was applied with the dressing, was called the study group. On the other side, the control group was applied with a gauze patch and adhesive dressing. The experiment was left for 4 h then removed and gently cleaned with cotton balls soaked with normal saline solution. The redness and swelling response grading were between 0– 4 points where 0 was defined as no serious skin reaction and 4 was a serious reaction. After dressing removal, the evaluations were recorded at 1, 24, 48 and 72 h, respectively. If there was any skin reaction, a confirmation test on two more animals would be taken into consideration.
2.3.2. Efficacy and safety of prepared foam sheets on pigs
Five domestic farm pigs with an average weight of 20–25 kg were allocated for the study after authorization by Faculty of Veterinary Science-Animal Care and Use Committee (FVS-ACUC), Mahidol University (Protocol No. MUVS-2016-09-34). The experiment followed the guide for the care and use of laboratory animals (NRC 2011) and the guide for the care and use of agricultural animals in research and teaching (FASS 2010). After seven acclimatization days, the animals were made to fast 12 h before surgery. All animals were intramuscularly injected with tiletamine-zolazepam to induce anesthesia and maintained by administering isoflurane in 100% oxygen. In each pig, the deep partial thickness of the excision wounds (area about 225 mm2) were created along the markings using toothed forceps, a surgical blade and pointed scissors. The total number of experimental wounds was 50, with 10 per animal. All wounds were cleaned with a sterile normal saline solution following the program for each group. These created wounds were randomly assigned into five groups of treatments as follows: Group I (comparative group I) was treated with a commercial PU foam dressing, Group II (comparative group II) was treated with a commercial silver coated PU foam dressing, Group III-V (study group I-III) was treated with PU foam dressing containing at least 2 components following; 1) natural polyols, 2) silver and/or 3) asiaticoside. These 3 study groups would be selected from releasing profiles. All of the wounds were applied to the dressing, then covered with sterile gauze, and changed every one-two days. During the observation of the wound healing, the wound area was periodically recorded and calculated (in cm2) at 0, 4, 7, 14 and 21 d A digital camera (SONY, model DSC TX9, Sony Company Limited, Japan) was used to collect the wound appearance with 10 cm above the wound. During this study, the animals were routinely checked for food and water consumption and mentation.
2.3.2.1. Histological evaluation
Punch biopsies were taken at two time points: 7 and 14 d post-wounding at the wound's edge. The tissue was taken and fixed in 10% of buffered formalin solution. Each specimen was embedded in a paraffin block and stained with hematoxylineosin and Masson's trichrome method. The tissue from the normal skin would be collected in order to compare with the tissue from the wounds. After that, the tissue was examined histologically under a light microscope (Nikon Eclipse E200, Nikon Instruments, Tokyo, Japan). The histologic examinations were modified from the studies of Abramov et al. and Karayannopoulou et al. [44], [45] and detected per high power field (HPF) at a magnification of 400. For the comparison to normal skin, the epithelium cell layer, amounts of the inflammatory cells and fibroblasts were counted and scored using 4 scales: 0 = normal, 1 = mild increase, 2 = moderate increase and 3 = marked increase. The new capillaries formation was assigned an angiogenesis score of 0 = < 3 new vessels, 1 = 3–10 new vessels, 2 = 11–30 new vessels and 3 = ≥ 31 new vessels detected per HPF.
2.4. Statistical analysis
The mean and standard deviation of the groups were calculated for each data set. The differences in all quantitative data; such as compression test, drug contents, the zone of inhibition, wound area, the day of epithelialization and histological score between group were compared using one-way ANOVA, and the releasing profiles were compared using repeated measured ANOVA. The comparison of histological score between day 7 and 14 within group was perform using paired t-test. The statistical significance was considered less than 0.05. All statistical data analyses were performed using SPSS 22.0 (SPSS Company Limited, Bangkok, Thailand). The qualitative data; such as dermatologic effect were reported as descriptive information.
3. Results and discussion
3.1. Preparation and evaluation of foam dressing properties (Part i)
3.1.1. Preparation and characterization of PU foam dressing
3.1.1.1. Preparation
The obtained foam dressings were white, soft, flexible and also immediately recovered after compression. Foams with chitosan had a little yellowish color.
3.1.1.2. Characterization
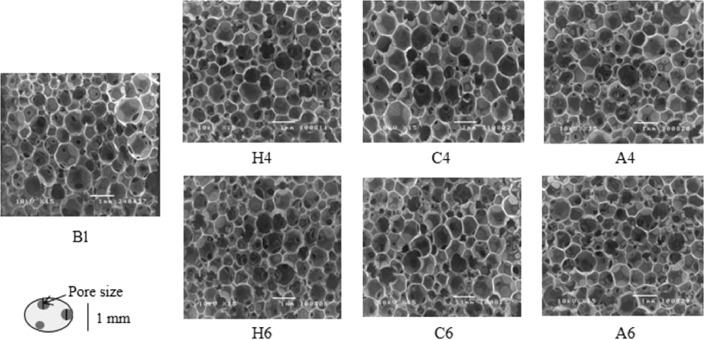
Microscopic appearance and pore size: The morphology observed by SEM showed several round-shaped and interconnected pores within the foam (Fig. 1). The average pore sizes from all groups were similar to commercial wound dressings [32]. Bl foam seemed to provide little larger pore size compare to natural polyols formulations (P > 0.05). The average pore size of natural polyols foams was in a range of 228–262 µm with no significant differences (P > 0.05). The porosity slightly decreased with increasing the polymer solution concentration. This might cause by the opportunity of natural polyols could react with diisocyanate more than foam without natural polyols [46]. The polymerization might compact the foam structure. Another reason was viscosity in the formulation. The natural polyols powder which was added in suspension could increase viscous and hinder gas creation [47], [48].
Fig. 1.
The SEM photographs of the obtained foams with 4% and 6% of HPMC (H4 and H6), CLMW (C4 and C6) and Alg (A4 and A6) compared with foam without natural polyols (Bl).
Density: The calculated density of Bl was less than foam with natural polyols (Table 1). The concentration of natural polyols might increase foam density, however, there were no significant results (P > 0.05). The density of foam might be an inverse relationship with pore size. The porosity of foam was expressed by P% = (1 - Dfoam/Dpolymer) × 100, where Dfoam and Dpolymer were density of foam and actual density of the polymer, respectively [49]. From this equation, it could be inferred that the density of foam would increase when the porosity decreased.
Table 1.
The physical properties and integrity of foam dressing.
| Mean pore size (× 10−3 mm) | Density (g/cm3) | Water absorption 48 h (%) | Weight loss 48 h (%) | Enzyme degrade 48 h (%) | PBS degrade 48 h (%) | Mechanical strength (× 10−3 MPa) | |
|---|---|---|---|---|---|---|---|
| B1 | 317.65 ± 264.76 | 41.09 ± 1.09 | 1104.29 ± 142.13 | 0.15 ± 0.34 | 0.72 ± 0.82 | 0.49 ± 0.90 | 4.60 ± 1.05 |
| H4 | 243.79 ± 238.99 | 44.54 ± 0.94 | 1353.05 ± 490.75 | 1.68 ± 0.41 | 1.28 ± 0.91 | 1.22 ± 1.43 | 5.41 ± 0.65 |
| H6 | 228.91 ± 226.36 | 44.81 ± 3.07 | 1515.25 ± 320.39 | 2.51 ± 1.94 | 2.19 ± 1.11 | 1.50 ± 1.00 | 5.69 ± 0.79 |
| C4 | 262.37 ± 238.31 | 41.25 ± 1.63 | 1231.88 ± 373.05 | 0.06 ± 0.34 | 0.49 ± 0.67 | 0.29 ± 0.87 | 5.08 ± 1.05 |
| C6 | 238.93 ± 225.15 | 41.20 ± 1.46 | 1253.47 ± 255.97 | 0.07 ± 0.11 | 0.58 ± 1.46 | 0.43 ± 0.64 | 5.18 ± 1.14 |
| A4 | 239.43 ± 267.74 | 41.94 ± 1.95 | 1301.83 ± 358.97 | 2.86 ± 0.68 | 2.61 ± 1.03 | 2.16 ± 1.57 | 4.99 ± 0.66 |
| A6 | 236.35 ± 238.52 | 42.28 ± 3.57 | 1512.69 ± 597.99 | 3.70 ± 0.45 | 4.34 ± 0.79 | 4.37 ± 1.05 | 5.29 ± 0.29 |
Water absorption and %weight loss study: The natural polyols could facilitate water absorption compared to foam without natural polyols (Table 1). Their percent water absorption at 48 h results were higher than Bl group but no significant differences were noted (P > 0.05). The polyol groups which were left from polymerization would react to water via hydrogen bonding. According to their structures, these three natural polymers had some different substituted groups; OH and OCH3 group from HPMC, a COO-Na+ group from Alg and NH2 group from CLMW. The foam with HPMC seemed to have water sorption higher than Alg foam. However, it has been reported that the tablets containing Alg could swell more rapidly than those of HPMC [50]. The obtained foam with Alg might consist of some unreacted Alg which could swell and dissolve easily. Thus it could not sustain much water within their network structure [51].
The foam containing Alg could absorb water more than CLMW foam in both two concentrations. The water sorption ability of foam dressings containing natural polyols might be explained from previous studies. The number of water molecules absorbed per repeating unit in the amorphous phase can be ranked as follows: alginate > chitosan [52]. The water sorption first occurred on polymer sites. The chitosan could interact with two water molecules per repeating unit at NH2 group while four molecules are bound per repeating unit at COONa group in Alg. HPMC foam also presented good water sorption property comparable to Alg foams. This might be explained by functional groups contained in polymers. The degree of substitution and viscosity grade are also involved in polymer hydration and drug release. Although cellulose generally could bind 2 water molecules per glucopyranosyl unit, while the HPMC E5 could bind 6 water molecules [53], [54]. The hydration of HPMC also presented the net exothermic value more than the other cellulose [53]. The stronger binding with water of functional groups might lead to increase absorption capacity.
Another factor related to water absorption is porosity. Small pore size could prevent the water leak out during handling. Thus larger average pore size of Bl foam could retain less water than foam with natural polyols. Among natural polyols groups, the H6 had the smallest pore size while C4 showed the largest. At 48 h, the water sorption of HPMC was the highest while the lowest was from CLMW.
The percent weight loss of natural polyols foams was higher than foam without natural polyols. The hydrophilic groups in natural polymers could interact with water and then solubilized. The polarity of organic compounds could be ranked: acid > alcohol > amine groups. This might reflect to their percent weight loss that Alg > HPMC > CLMW foams. The hydrolysis effect of Alg foam seemed to be higher than that of other foams because Alg contained a salt form of carboxyl groups (COONa) which presented the strongest hydrophilicity. The hydroxyl groups in HPMC could also interact with water molecules but showed lower percent compared to Alg. While CLMW had percent weight loss comparable to Bl possibly caused by the pKa value of 6.5 of CLMW. The solubility of chitosan depends on the protonation of free amino group that it could soluble in acidic solution and also hardly solubilize in deionized water which had pH nearly 7.0.
Foam degradation test: The percentage of the weight loss of various foam dressings in the solution of lysozyme which generally found in wounds and in the phosphate buffer pH 7.4 compared at 48 h are shown in Table 1. It could be seen that adding natural polyols seemed to increase the weight loss of the foam dressing% especially in formulation with Alg except in formulations with CLMW. Moreover, increasing the amount of polyols would increase the weight loss due to polymer solubilization. In addition, there was no significant difference in the weight loss in the lysozyme and buffer solutions (P > 0.05) indicating that lysozyme had no effect on the dressing integrity. Minute residue could be found in the wound due to the degradation of the natural polyols, especially from A6 formulation.
Compression test: Adding natural polyols seemed to increase the strength. The HPMC formulation had a similar strength to A6. At the same compressive distance, there were no significant differences between the groups (P = 0.593). The compressive strength was associated with pore size and density [55]. The higher amount of powder in the mixture such in formulation with 4% and 6% of natural polyols would lead to higher viscosity and also increase foam density and also increase the mechanical strength of the final foam. The compressive strength could explain the response of the foam dressing while it experienced a compressive load. The force would decrease the pore sizes.
3.1.2. Determination of the active compounds contents and their releasing profiles
3.1.2.1. Active compound contents
Silver content
The silver contents after preparation were in a range of 92.50%—94.50% of theoretical amount. Foam with Alg seemed to have the highest silver content while foam with chitosan showed the lowest (94.37% ± 8.61% and 92.50% ± 9.40%, respectively). However, there were no significant differences between the silver amounts among 4 formulations (P > 0.05). Some silver losses in all groups was due to some residue was found in container after absorption process. The agglomeration might occurred in the concentrated silver suspension which hardly solubilized and easily fallen in the bottom before the content determination.
Asiaticoside (AS) content
The AS content was 94.0%—96.0% after impregnation and no statistical differences between groups (95.47% ± 8.81%, 95.29% ± 8.35%, 95.21% ± 10.64% and 94.42% ± 10.90% for foam dressing without natural polyols impregnated with silver and AS (Bl-1Ag-AS), foam dressing with 6% HPMC impregnated with silver and AS (H6-1Ag-AS), foam dressing with 6% CLMW impregnated with silver and AS (C6-1Ag-AS) and foam dressing with 6% Alg impregnated with silver and AS (A6-1Ag-AS), respectively, P > 0.05). Apart from the residue after foam absorption, AS might degrade from drying process [56]. Heat condition resulted in a decrease of total triterpene glycosides.
3.1.2.2. Releasing of the active compounds
Silver releasing profiles
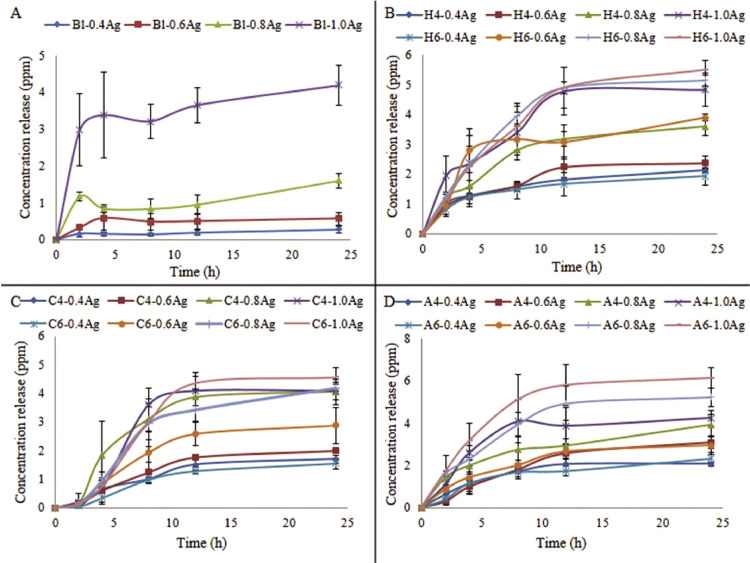
The releasing profiles of Bl, HPMC (H4 and H6), CLMW (C4 and C6) and Alg (A4 and A6) are shown in Fig. 2. Concentrations of both silver and natural polyols affected the silver releasing profiles. Higher amount of Ag in dressing provided the higher amount of Ag release. The burst release of silver after the water contact was due to some deposition on the surface. The releasing from pore and the agglomeration of small silver nanoparticles when releasing from foam dressing would prolong the release. The sized of released silver nanoparticles was shifted to a slightly larger population [57]. The silver releasing mechanism involved two phases; the initial silver dissolution and then agglomeration that the latter reduced the surface area leading to decrease the amount and also prolong the rate of releasing profiles. In addition, the silver nanoparticles might show low releasing profiles in deionized water. The property of a vehicle affected the active compound releasing. A lower pH of a vehicle would provide the silver dissolution more than neutral pH [58], [59]. Although low amount of silver releasing profiles, there was a concept of silver containing in suprasorbent wound dressing. In order to avoid periwound maceration, the dressing should absorb excess exudate. In this step, some bacteria in exudate were absorbed and killed by silver inside the dressing [60].
Fig. 2.
Silver releasing profiles of (A) foam without natural polyols (Bl), (B) foams with 4% and 6% of HPMC (H4 and H6), (C) CLMW (C4 and C6) and (D) Alg (A4 and A6) impregnated with different amounts of silver in ppm.
The higher concentration of polyols provided higher releasing profiles. The 6% of natural polyols could facilitate silver release more than 4% In the HPMC group, H6-1.0Ag > H4-1.0Ag > > H6-0.4Ag > H4-0.4Ag. In the CLMW group, C6-1.0Ag > C4-1.0Ag > > C6-0.4Ag > C4-0.4Ag. In the Alg group, A6-1.0Ag > A4-1.0Ag > > A6-0.4Ag > A4-0.4Ag. Among groups containing 1.0 mg/cm2, A6 gave the highest release, and higher than C4 and C6 (P = 0.013 and 0.021, respectively). The releasing profiles of silver from the Bl group were quite instant compared to the natural polyols group due to larger pore size which allowed water and drug to pass through easily. Moreover, the Bl foam had not hydrophilic functional groups thus there was no swelling effect to hinder permeation. Among three polyols, COONa from Alg foam was the strongest hydrophilic group. The erosion effect of Alg foam could increase the silver releasing profiles which could be confirmed by weight loss%. The OH group also presented water-favorable property. The releasing profiles of HPMC foam were comparable to Alg foam. The CLMW foam seemed to provide low releasing profiles. This might cause by the polarity of amine group which was weaker than COONa and OH group. From this study, 6% of the natural polyols and 1 mg/cm2 of the silver concentration was selected for the next experiments.
Asiaticoside releasing profiles
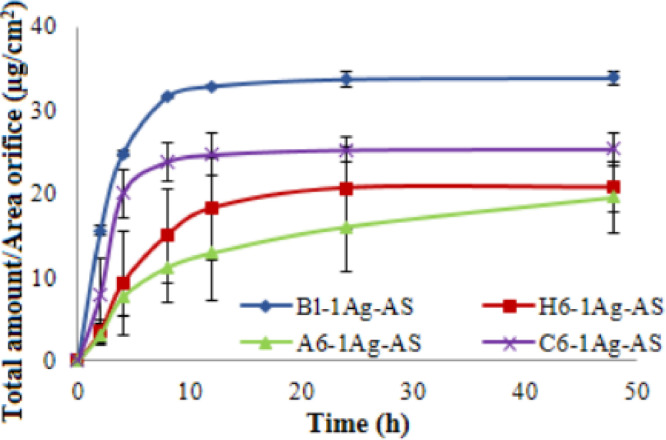
It was clearly shown that the Bl-1Ag-AS rapidly released the highest amount of AS, and the release was constant after 8 h (P < 0.05 ), followed by A6-1Ag-AS while C6-1Ag-AS was the lowest (Fig. 3). Similar to the silver release, AS from Bl-1Ag-AS was quite rapidly released then become gradually decrease. The releasing profiles of natural polyols foams were similar to foam without natural polyols. Similar to silver release profiles, AS on surface could burst release following by diffusion of drug through pores and channels. The larger porosity from foam without natural polyols provided faster rate of AS releasing profiles from foam with natural polyols. In addition, the latter foams could absorb water and swell more than the former foam that swelling effect and gelling behavior of polymer might retard the drug releasing in the hydration process [61], [62]. The erosion effect could be found in hydration confirmed by percent weight loss [63] that higher weight loss%from foam with Alg related to increase the release of the drug. Although formulations with H6-1Ag-AS and C6-1Ag-AS had a comparable low release, the latter foam gradually released the AS even after 48 h while the former reached the plateau after 24 h. The less swelling and solubility of chitosan in buffer solution could delay the releasing profiles. Moreover, the positive charge of amine group rich in CLMW might attract the AS which present partial negative charge and lead to prolonging release. In wound environment, the occlusive effect from the dressing involved the entrapment of water result in the rise in temperature and increase hydration of the wound site. This would increase the rate and amount of drug releasing.
Fig. 3.
Asiaticoside releasing profiles of foam without natural polyols (Bl-1Ag-AS), foams with 6% of HPMC (H6-1Ag-AS), CLMW (C6-1Ag-AS) and Alg (A6-1Ag-AS) impregnated with 1 mg/cm2 silver and 5% asiaticoside.
3.1.3. Antibacterial test
The results revealed that Bl, H6, C6, A6 and Bl-AS did not show clear zone inhibition while Bl-1Ag-AS, H6-1Ag-AS, C6-1Ag-AS and A6-1Ag-AS exhibited a large clear inhibition zone, which were statistically non-significant (P > 0.05) in every type of tested bacteria (Table 2). The MIC of the silver nanoparticles to P. aeruginosa, S. aureus, E. coli and B. subtilis were in a range of 0.4–3.1 ppm [64] while the foam dressing released approximately 4–5 ppm. These clear zones were statistically larger than other tested formulations except the Bl foam which was pipetted with Ag solution in 1 mg/cm2 (Bl + Ag solution), which showed a moderate clear zone area. Large surface area of the silver nanoparticles would have more contact area with the bacteria, thus a higher efficiency in bacteria inhibition. Moreover, the large pore size of the prepared foams facilitated the release of silver. The Bl + Ag solution had a smaller clear zone and was significantly different compared to foam dressing impregnated with silver and asiaticoside (P < 0.05). This was possibly due to the silver solution was obstructed within the middle area while pipetting onto the foam sheet.
Table 2.
Comparison of the inhibition zone of the prepared foam dressings on various bacteria.
| Formulations | Inhibition zone (mean ± SD, n = 3) |
|||
|---|---|---|---|---|
| S. aureus (mm) | B. subtilis (mm) | E. coli (mm) | P. aeruginosa (mm) | |
| Bl-1Ag-AS | 31.93 ± 3.46 | 25.52 ± 3.35 | 31.57 ± 4.76 | 28.67 ± 2.64 |
| H6-1Ag-AS | 31.53 ± 2.90 | 27.11 ± 3.29 | 30.23 ± 4.98 | 29.38 ± 2.62 |
| C6-1Ag-AS | 31.76 ± 4.40 | 29.41 ± 3.33 | 34.71 ± 5.32 | 30.17 ± 4.01 |
| A6-1Ag-AS | 31.34 ± 3.37 | 27.71 ± 4.84 | 34.02 ± 4.73 | 29.95 ± 4.02 |
| Bl + Ag solution (1 mg/cm2) | 16.13 ± 1.16 | 16.68 ± 1.59 | 18.56 ± 1.24 | 12.24 ± 1.06 |
| Bl | NZ | NZ | NZ | NZ |
| H6 | NZ | NZ | NZ | NZ |
| C6 | NZ | NZ | NZ | NZ |
| A6 | NZ | NZ | NZ | NZ |
| Bl-AS | NZ | NZ | NZ | NZ |
NZ = no zone of inhibition
3.1.4. Cytotoxicity test
The percent cell viability of Bl group was slightly less than PBS group (101.16% ± 7.07% and 107.29% ± 9.71%, respectively) (Table 3). No foam added in PBS group while other groups contained foam in well plate. The added foam in each well plate might interfere the cell growth. Although TDI in foam formulation was noted to be cytotoxic [65], [66], however, TDI could rapidly vaporize under room temperature [67]. In addition, there were reports that the PU foams did not cause any cytotoxicity [68], [69]. The natural polyols used in this study were biocompatibility [5], [8], [70] and presented comparable results implying that these polymers did not cause cytotoxicity. Similar to another study, AS containing wound dressing could increase the viability of the cultured cells [56]. The foam with 5% AS (Bl-AS) showed high percent cell viability (113.34% ± 9.97%). The AS concentration was varied from 1–1000 µM. Concentration at 1000 µM could decrease cell viability [19], [25]. From the releasing profiles, AS was just in the range of 15–35 µg.
Table 3.
Percentage of cell viability of various foam dressings.
| Group | PBS | Bl | H6 | C6 | A6 |
|---|---|---|---|---|---|
| Cell Viability (%) | 107.29 ± 9.71 | 101.16 ± 7.07 | 104.41 ± 8.18 | 102.35 ± 5.42 | 100.01 ± 5.77 |
| Group | Bl-5AS | Bl-1Ag-AS | H6-1Ag-AS | C6-1Ag-5AS | A6-1Ag-AS |
| Cell Viability (%) | 113.34 ± 9.97 | 100.22 ± 8.37 | 100.56 ± 10.84 | 101.02 ± 9.12 | 100.88 ± 10.19 |
The silver concentrations used in commercial wound dressings were ranged from 0.08–1.50 mg/cm2 [71], [72] while the silver in the prepared foam formulation was 1.0 mg/cm2. From the test, the result of foam with silver and AS could be concluded that combination of both ingredients did not affect cell proliferation. Moreover, the cytotoxicity from in vitro experiment might not confirm the in vivo toxicity. A wound dressing with nanocrystalline silver caused cytotoxicity in cell cultures but the effect could not be observed in mice [73].
From the studies of Part I, the A6-1Ag-AS was chosen for the studies in Part II due to the appropriate absorption-desorption properties, stability and non-cytotoxicity. It could provide the high silver and asiaticoside releasing profiles. The Bl-1Ag-AS was chosen to compare the efficacy of wound healing in the porcine model.
3.2. Efficacy and safety in the animal model (Part II)
3.2.1. Skin irritation test of selected foam dressing on rabbits
There was no redness and swelling on the tested area determined at time 1, 24, 48 and 72 h (Table 4). They were also no dermatologic effects after dressing the application compared to the control side. The total evaluation score was zero in A6-1Ag-AS group and control group. This result was first evidence in which the developed PU foam dressing with Alg and silver nanoparticles plus AS (A6-1Ag-AS) was safe to apply on the skin.
Table 4.
The redness and swelling score in the study and control group over 72 h.
| Group | 1 h |
24 h |
48 h |
72 h |
||||
|---|---|---|---|---|---|---|---|---|
| Redness | Swelling | Redness | Swelling | Redness | Swelling | Redness | Swelling | |
| Rabbit 1 – A6-1Ag-AS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rabbit 1 - Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rabbit 2 - A6-1Ag-AS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rabbit 2 - Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rabbit 3 - A6-1Ag-AS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rabbit 3 - Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
3.2.2. Efficacy and safety of prepared foam sheets on pigs
The average initial wound areas were 2.02 ± 0.05, 2.04 ± 0.17, 2.05 ± 0.15, 2.03 ± 0.23 2.02 ± 0.18 cm2 in the comparative group I-II, study group I-III which included Bl-1Ag-AS, A6-1Ag-AS and A6-1Ag groups, respectively with no significant difference (P = 0.995). The wounds were randomly assigned to be treated with five types of dressings. All wounds were deep partial thickness wounds, which had pathology in the dermis layer.
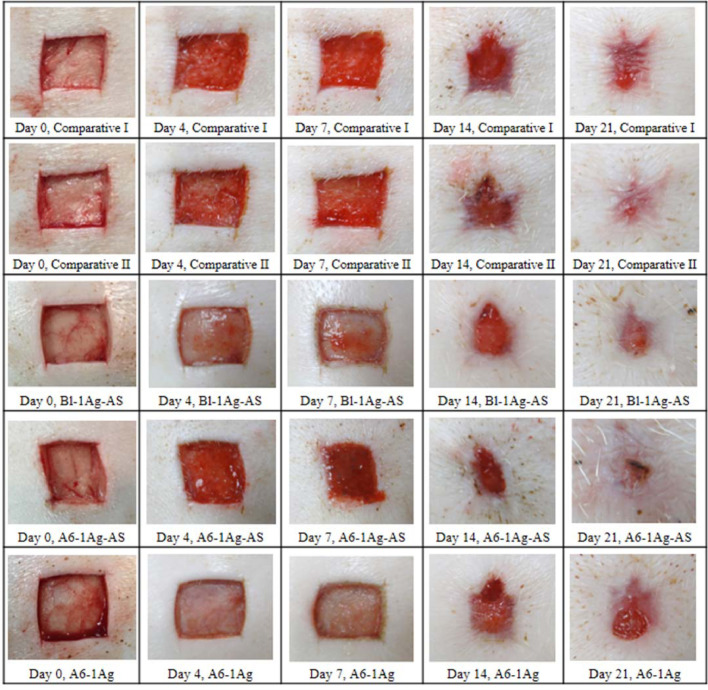
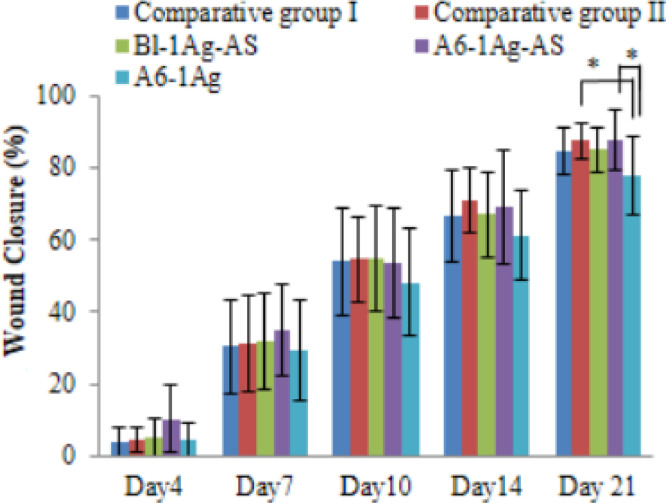
Fig. 4 shows the appearance of the wounds which received different treatments on 0, 4, 7, 14 and 21 d These photographs revealed a significant acceleration of the time of wound closure observed in comparative group II and study group II over study group III. At day 7, the granulation tissue was fully grown in study group II which demonstrated the red connective tissue compare to the white and dry wound appearance in study group III. The healthy red color in the new generative tissues over the wound bed could refer to blood supplies that were forming to deliver nutrients to the tissues. The cells in this area formed the extracellular matrix. The white and dry wound bed appearance demonstrated to show the slower rate of wound healing. At day 14 and 21, all groups except study group III show almost completely granulated tissues and epithelialization especially in comparative group II and study group II. It confirmed with the results in Fig. 6; the wound in these two groups were significant healed faster than the wound in study group III. The percentage of the wound closure of comparative groups and study groups are shown in Fig. 5. The average percentage of epithelialization was 84.59 ± 8.09 at day 21. Because the deep partial thickness wound was deep, the healing time might take longer than superficial partial thickness wounds. There were no significant differences between the group at day 4, 7 and 14. However, at the 21st d after the creation of the wound, the percentage mean of the wound's closure was significantly faster in the study group II and comparative group II than in study group III (P = 0.04 in both pairs). Study group I showed a smaller closure in size than study group III, but there was no statistical significance. It might have been caused by the AS in Bl-1Ag-AS and A6-1Ag-AS groups that could promote the healing process. Lee et al. [19] reported that this compound could stimulate the migration of epithelial cells. Moreover, the comparative groups I and II had a smaller pore size than the study groups. This might retain more hydration than the study groups. The moist wound could heal faster than a dry wound because the epithelial cells could migrate easily [74]. Moreover, comparative group II had an alginate content, which was the hydrophilic polymer; this might facilitate moisture at the wound bed and could detect the difference between comparative group II and study group III.
Fig. 4.
Wound appearance at day 0, 4, 7, 14 and 21 after treatment of commercial dressings (comparative I and II) compared to foam without natural polyols impregnated with silver and asiaticoside (Bl-1Ag-AS), foam with 6% Alg impregnated with silver and asiaticoside (A6-1Ag-AS) and foam with 6% Alg impregnated with silver (A6-1Ag). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
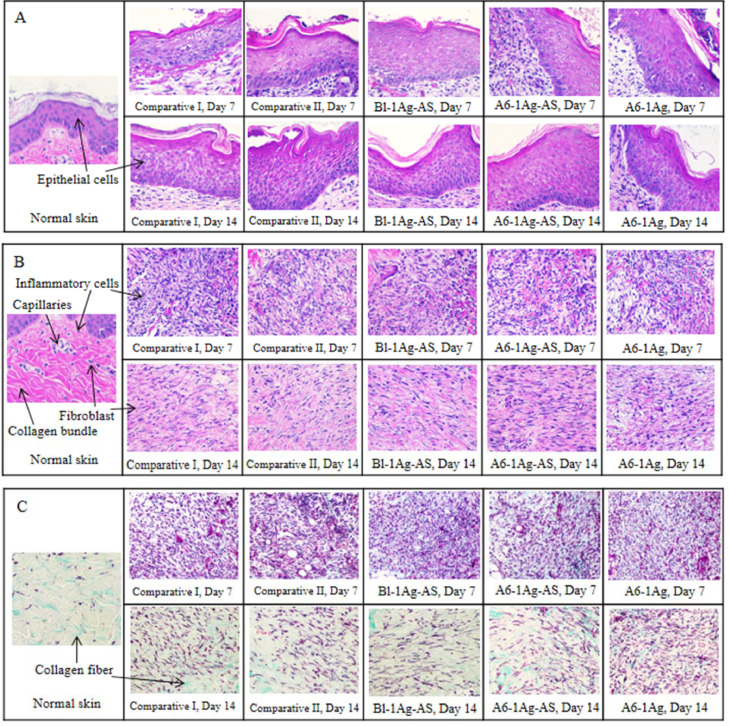
The histologic cross-section of normal skin (left) and the wounds (right) in (A) epithelial cells layer, (B) extracellular matrix (ECM) and (C) Masson's trichrome stained of extracellular matrix on day 7 and 14 from treatment of commercial dressings (comparative I and II) and PU foam dressings (Bl-1Ag-AS, A6-1Ag-AS and A6-1Ag).
Fig. 5.
Percentage of wound closures of commercial dressings (comparative I and II) compared to PU foam dressings (Bl-1Ag-AS, A6-1Ag-AS and A6-1Ag) for 21 d (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The positive effects of AS on wound healing, especially in reepithelialization and reparation were found in the histological analysis of the wounds, which were evaluated in four parameters: the epithelial cell layer, number of the inflammatory cells, number of fibroblasts and number of capillaries. Comparing the wound lesions between treatment groups, the epithelial cell layers, extracellular matrix and the Masson's trichrome stained of extracellular matrix were shown in Fig. 6. The normal skin was taken from an area, which had not been wounded including the epithelial cells, collagen bundles, fibroblasts and some of the inflammatory cells and new capillaries as shown on left side of Fig. 6A, 6B and 6C. The three skin layers were epidermis, which is the outer layer, the dermis which is the second thick layer and hypodermis, which mainly consists of adipose tissue. The histologic evaluations on day 7 and 14 including epithelium cell layer, amounts of the inflammatory cells and fibroblasts were reported in Table 5.
Table 5.
The histologic evaluation of the wounds.
| Parameters | Histological grading score (Day 7/ Day 14) |
||||
|---|---|---|---|---|---|
| Comparative I | Comparative II | Bl-1Ag-AS | A6-1Ag-AS | A6-1Ag | |
| Epithelium cell layer | 1.10 ± 0.99 / 2.28 ± 0.83 | 1.03 ± 0.80 / 2.24 ± 0.85 | 1.48 ± 0.73 / 2.13 ± 0.85 | 1.20 ± 0.90 / 2.65 ± 0.48* | 1.46 ± 0.88 / 2.44 ± 0.67 |
| Amount of inflammatory cell | 1.94 ± 0.74 / 0.91 ± 0.35 | 2.12 ± 0.63 / 0.88 ± 0.40 | 2.00 ± 0.78 / 0.86 ± 0.34 | 2.14 ± 0.67 / 0.88 ± 0.40 | 2.20 ± 0.57 / 0.80 ± 0.51 |
| Amount of fibroblast | 0.86 ± 0.57 / 1.85 ± 0.55 | 0.82 ± 0.72 / 1.92 ± 0.44 | 0.76 ± 0.69 / 2.10 ± 0.46 | 1.12 ± 0.75 / 2.14 ± 0.57* | 0.92 ± 0.70 / 1.78 ± 0.65 |
| Amount of new capillary | 1.28 ± 0.61 / 0.81 ± 0.54 | 1.26 ± 0.60 / 0.80 ± 0.40 | 1.28 ± 0.50 / 0.86 ± 0.42 | 1.38 ± 0.57 / 0.90 ± 0.52 | 1.27 ± 0.57 / 0.82 ± 0.52 |
Significance consider, P 〈 0.05, one way ANOVA test, The epithelial cell layer score of Bl-1Ag-AS < A6-1Ag-AS at 14 days, P = 0.01; The amount of fibroblast score of A6-1Ag-AS 〉 A6-1Ag at 14 d, P = 0.04.
The reepithelialization occurred for 7 d (Fig. 6A). There were some epithelial cells from the neighboring epidermis that began to replicate and migrate into the wound bed. In all groups, the average epithelial cell layer notably increased at day 14 compared to day 7 (P < 0.05). This might have been caused by the epithelial cell growth covering the wound bed, which would prevent dehydration and protect the wound externally. At day 14, the study group I had an epithelial cell layer score significantly less than study group II (P < 0.05). The alginate in study group II might be a reason to keep hydration and facilitate the proliferation of the epithelial cell. In addition, AS might be involved in this process. Cheng et al. [18] reported that this compound could activate intestinal epithelium cell growth.
Lots of inflammatory cells were observed on day 7 and dramatically decreased in day 14 (P < 0.05) (Fig. 6B). The inflammatory phase normally occurred within the first week after injury. Macrophage and neutrophil chemotaxis would remove debris cells and bacteria. After that, the extracellular matrix was produced in the proliferative phase in which the inflammatory cells would have less importance. Although there was some evidence about the Centella asiatica extract reducing inflammation [75], [76], there was no significant difference in the inflammatory cells score between the groups at each point of time (P > 0.05). This might be because these wounds were deep partial thickness wounds, and the inflammation might be greater than a superficial partial thickness wound. Moreover, itching of the wound might occur during the healing process, so the animal might scratch and let inflammatory cells be released in all the wounds.
At day 7, all the wounds had some granulation tissue. The collagen which was exhibited in pink fiber mixed with the fibroblasts showed as purple satellite-shaped cells. The amount of the fibroblasts increased in day 14 compared to day 7, especially in comparative group II and study group II (P = 0.02 in both pairs). At day 14, there were more fibroblasts found in study group II than those in study group III (P < 0.05). The collagen fiber became denser, which was the signs of regeneration of the dermis. This result confirmed the findings of previous studies [21], [77] in which AS activates fibroblast proliferation. The increasing of the fibroblasts led to an increase of the collagen fibers and wound's strength. The collagen fiber should be confirmed by the photos from the histologic stained with Masson's trichrome in which the collagen is stained in blue (Fig. 6C). The wound tissue from study group III had loose collagen fiber in both points of time when compared to other groups.
The new capillaries were found in day 14 less than day 7 (Fig. 6B). As a result of the nearly completed healing, and the nutrients and oxygen were a lesser necessity. Even though some data showed the AS activated angiogenesis [18], [78], there was no difference in these study groups. Also, other factors might be involved in the wound healing; such as animal genetics, food and water consumption, and self-traumatized site from the animal.
There was a dermatologic effect in comparative group I. There were some rashes on the skin surface around the wound. This may have been caused by the adhesive layer that recovered after discontinuing the dressing. Therefore, there was no dermatologic effect found in the study groups.
This study was performed in deep surgical wounds which were clean wounds. However, they could be infected due to animal behavior. The pigs usually scratched the wounds on the wall and the floor. The microbe might infect easily. The silver in PU dressing prevented the infection which might occur. Without infection, the wound could heal continuously without dermatologic reaction. Combination of silver and AS in PU foam would present satisfied results. The percentages of the wound's closure and histological data supported that the PU foam dressing with alginate and silver plus AS (A6-1Ag-AS) could accelerate wound healing through the migration of the epithelial cells and the proliferation of the fibroblast in a deep partial thickness wound of a porcine model.
4. Conclusion
The PU foam sheets could be successfully prepared with the addition of natural polyols. The investigated natural polyols especially alginate and hydroxypropyl methylcellulose improved the foam characteristics with the increase in the absorption property and compressive strength. All formulations confirmed comparable antimicrobial effect in the disk diffusion test and showed non-cytotoxicity. The foam dressing with A6-1.0Ag of silver showed the highest silver releasing results and subsequently presented satisfactory releasing of AS. This formulation (A6-1Ag-AS) could improve wound healing in both the percentages of wound closure and histological parameters in porcine model with deep partial thickness wound. The wound applied with A6-1Ag-AS presented the epithelial cells and fibroblasts notably proliferated and repaired the lesion. This also demonstrated a gentle skin effect on a rabbit model. Formulation of A6-1Ag-AS could be an alternative dressing for wound treatment. The efficacy and safety in clinical studies was planned to further investigated.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This research was supported by the Agricultural Research Development Agency (ARDA) and by the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship. We also thanked Assistant Professor Tawewan Tansatit for histological knowledge assistance. The skin irritation test was supported by the Industrial Metrology and Testing Service Center, Thailand Institute of Scientific and Technological Research (TISTR).
References
- 1.Vowden K, Vowden P. Wound dressings: principles and practice. Surgery )Oxford) 2014;32(9):462–467. [Google Scholar]
- 2.Jones V, Grey JE, Harding KG. Wound dressings. BMJ. 2006;332(7544):777–780. doi: 10.1136/bmj.332.7544.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szycher M. 2nd ed. CRC Press; FL, USA: 2013. Szycher's handbook of polyurethanes. [Google Scholar]
- 4.Agubata CO, Okereke C, Nzekwe IT, Onoja RI, Obitte NC. Development and evaluation of wound healing hydrogels based on a quinolone, hydroxypropyl methylcellulose and biodegradable microfibres. Eur J Pharm Sci. 2016;89:1–10. doi: 10.1016/j.ejps.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Varshosaz J, Taymouri S, Minaiyan M, Rastegarnasab F, Baradaran A. Development and in vitro/in vivo evaluation of HPMC/chitosan gel containing simvastatin loaded self-assembled nanomicelles as a potent wound healing agent. Drug Dev Ind Pharm. 2018;44(2):276–288. doi: 10.1080/03639045.2017.1391832. [DOI] [PubMed] [Google Scholar]
- 6.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15(1):74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 7.Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Wang C, Li C. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Advances. 2018;8(14):7533–7549. doi: 10.1039/c7ra13510f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devi N, Dutta J. Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int J Biol Macromol. 2017;104:1897–1904. doi: 10.1016/j.ijbiomac.2017.02.080. [DOI] [PubMed] [Google Scholar]
- 10.Limova M. Evaluation of two calcium alginate dressings in the management of venous ulcers. Ostomy Wound Manag. 2003;49(9):26–33. [PubMed] [Google Scholar]
- 11.Choi JS, Kim DW, Kim DS. Novel neomycin sulfate-loaded hydrogel dressing with enhanced physical dressing properties and wound-curing effect. Drug Deliv. 2016;23(8):2806–2812. doi: 10.3109/10717544.2015.1089958. [DOI] [PubMed] [Google Scholar]
- 12.Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res B. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 2012;112(5):841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 15.Opasanon S, Muangman P, Namviriyachote N. Clinical effectiveness of alginate silver dressing in outpatient management of partial-thickness burns. Int Wound J. 2010;7(6):467–471. doi: 10.1111/j.1742-481X.2010.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosti G, Magliaro A, Mattaliano V, Picerni P, Angelotti N. Comparative study of two antimicrobial dressings in infected leg ulcers: a pilot study. J Wound Care. 2015;24(3):121–122. doi: 10.12968/jowc.2015.24.3.121. 124-7. [DOI] [PubMed] [Google Scholar]
- 17.Ding X, Shi L, Liu C, Sun B. A randomized comparison study of Aquacel Ag and alginate silver as skin graft donor site dressings. Burns. 2013;39(8):1547–1550. doi: 10.1016/j.burns.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CL, Guo JS, Luk J, Koo MW. The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci. 2004;74(18):2237–2249. doi: 10.1016/j.lfs.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Kim HL, Lee MH. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine. 2012;19(13):1223–1227. doi: 10.1016/j.phymed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Paolino D, Cosco D, Cilurzo F. Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles. J Control Release. 2012;162(1):143–151. doi: 10.1016/j.jconrel.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 21.Shukla A, Rasik AM, Jain GK, Shankar R, Kulshrestha DK, Dhawan BN. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol. 1999;65(1):1–11. doi: 10.1016/s0378-8741(98)00141-x. [DOI] [PubMed] [Google Scholar]
- 22.Hou Q, Li M, Lu YH, Liu DH, Li CC. Burn wound healing properties of asiaticoside and madecassoside. Exp Ther Med. 2016;12(3):1269–1274. doi: 10.3892/etm.2016.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suguna L, Sivakumar P, Chandrakasan G. Effects of Centella asiatica extract on dermal wound healing in rats. Indian J Exp Biol. 1996;34(12):1208–1211. [PubMed] [Google Scholar]
- 24.Lee J, Jung E, Kim Y. Asiaticoside induces human collagen I synthesis through TGFbeta receptor I kinase )TbetaRI kinase)-independent Smad signaling. Planta Med. 2006;72(4):324–328. doi: 10.1055/s-2005-916227. [DOI] [PubMed] [Google Scholar]
- 25.Wu F, Bian D, Xia Y. Identification of major active ingredients responsible for burn wound healing of Centella asiatica herbs. Evid Based Complement Altern Med. 2012;2012:1–13. doi: 10.1155/2012/848093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine. 2000;7(5):427–448. doi: 10.1016/s0944-7113(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 27.Branski LK, Mittermayr R, Herndon DN. A porcine model of full-thickness burn, excision and skin autografting. Burns. 2008;34(8):1119–1127. doi: 10.1016/j.burns.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56(1):127–138. doi: 10.1093/ilar/ilv016. [DOI] [PubMed] [Google Scholar]
- 29.Wei JCJ, Edwards GA, Martin DJ, Huang H, Crichton ML, Kendall MAF. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: from mice, rats, rabbits, pigs to humans. Sci Rep. 2017;7(1):15885. doi: 10.1038/s41598-017-15830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes P. In: W Montagna RD, editor. Vol. 9. Pergamon: Oxford; 1969. pp. 419–432. (Advances in the biology of skin hair growth). editor. [Google Scholar]
- 31.Rittié L. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal. 2016;10(2):103–120. doi: 10.1007/s12079-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SM, Park IK, Kim YS. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater Res. 2016;20(1):15. doi: 10.1186/s40824-016-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SM, Park IK, Kim YS. Superior absorption and retention properties of foam-film silver dressing versus other commercially available silver dressing. Biomater Res. 2016;20:22. doi: 10.1186/s40824-016-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangsadthakun C, Kanokpanont S, Sanchavanakit N, Banaprasert T, Damrongsakkul S. Properties of collagen/chitosan scaffolds for skin tissue engineering. J Metals Mater Min. 2006;16:8. [Google Scholar]
- 36.ASTM Standard D3574-11 ``Standard test methods for flexible cellular materials- Slab, Bonded, and molded urethane foams”. ASTM Int. 2014 (Internet) [Google Scholar]
- 37.Kulthong K, Srisung S, Boonpavanitchakul K, Kangwansupamonkon W, Maniratanachote R. Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part Fibre Toxicol. 2010;7(1):8. doi: 10.1186/1743-8977-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soraya H. Faculty of Pharmaceutical Sciences: Chulalongkorn University; 2004. Formulation, evaluation and scale-up production of Centella asiatica extract film coated tables. [Google Scholar]
- 39.Ng SF, Rouse JJ, Sanderson FD, Meidan V, Eccleston GM. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS Pharm Sci Tech. 2010;11(3):1432–1441. doi: 10.1208/s12249-010-9522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallant-Behm CL, Yin HQ, Liu S. Comparison of in vitro disc diffusion and time kill-kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Regen. 2005;13(4):412–421. doi: 10.1111/j.1067-1927.2005.130409.x. [DOI] [PubMed] [Google Scholar]
- 41.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burd A, Kwok CH, Hung SC. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen. 2007;15(1):94–104. doi: 10.1111/j.1524-475X.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 43.OECD Guideline No.404 for the testing of chemicals, acute dermal irritation/corrosion [Internet]. Organization for Economic Co-operation and Develiopment. 2002 (cited 24 February 2018). Available from: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg404.pdf.
- 44.Abramov Y, Golden B, Sullivan M. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007;15(1):80–86. doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 45.Karayannopoulou M, Tsioli V, Loukopoulos P. Evaluation of the effectiveness of an ointment based on Alkannins/Shikonins on second intention wound healing in the dog. Can J Vet Res. 2011;75(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- 46.Bagdi MK, Molnár K, Sajó I, Pukánszky B. Specific interactions, structure and properties in segmented polyurethane elastomers. eXPRESS Polym Lett. 2011;5(5):10. [Google Scholar]
- 47.Oh CM, Heng PWS, Chan LW. A study on the impact of hydroxypropyl methylcellulose on the viscosity of PEG melt suspensions using surface plots and principal component analysis. AAPS PharmSciTech. 2015;16(2):466–477. doi: 10.1208/s12249-014-0204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X, Wang H, Zhang J, Zheng Z, Du G. Lightweight biobased polyurethane nanocomposite foams reinforced with pineapple leaf nanofibers )PLNFs) J Renew Mater. 2018;6(1):68–74. [Google Scholar]
- 49.Ryan AJ, Gleeson JP, Matsiko A, Thompson EM, O'Brien FJ. Effect of different hydroxyapatite incorporation methods on the structural and biological properties of porous collagen scaffolds for bone repair. J Anat. 2015;227(6):732–745. doi: 10.1111/joa.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis S, Subramanian G, Pandey S, Udupa N. Design, evaluation and pharmacokinetic study of mucoadhesize buccal tablets of nicotine for smoking cessation. Indian J Pharm Sci. 2006;68(6):829–831. [Google Scholar]
- 51.Dai M, Zheng X, Xu X. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J Biomed Biotechnol. 2009:1–9. doi: 10.1155/2009/595126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Despond S, Espuche E, Cartier N, Domard A. Hydration mechanism of polysaccharides: a comparative study. J Polym Sci B Polym Phys. 2005;43(1):48–58. [Google Scholar]
- 53.Joshi HN, Wilson TD. Calorimetric studies of dissolution of hydroxypropyl methylcellulose E5 )HPMC E5) in water. J Pharm Sci. 1993;82(10):1033–1038. [PubMed] [Google Scholar]
- 54.Fringant C, Desbrieres J, Milas M, Rinaudo M, Joly C, Escoubes M. Characterisation of sorbed water molecules on neutral and ionic polysaccharides. Int J Biol Macromol. 1996;18(4):281–286. doi: 10.1016/0141-8130(95)01087-4. [DOI] [PubMed] [Google Scholar]
- 55.Hadi A, Emadi R, Baghshahi S, Naghavi SH. Different pore size alumina foams and study of their mechanical properties. Ceramics-Silikaty. 2015;59(1):6–9. [Google Scholar]
- 56.Orawan S, Uracha R, Pitt S. In vitro biological evaluation of electrospun cellulose acetate fiber mats containing asiaticoside or curcumin. J Biomed Mater Res A. 2010;94A(4):1216–1225. doi: 10.1002/jbm.a.32797. [DOI] [PubMed] [Google Scholar]
- 57.Holbrook RD, Rykaczewski K, Staymates ME. Dynamics of silver nanoparticle release from wound dressings revealed via in situ nanoscale imaging. J Mater Sci Mater Med. 2014;25(11):2481–2489. doi: 10.1007/s10856-014-5265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hristovski KD, Westerhoff PK, Posner JD. Octanol-water distribution of engineered nanomaterials. J Environ Sci Health A. 2011;46(6):636–647. doi: 10.1080/10934529.2011.562859. [DOI] [PubMed] [Google Scholar]
- 59.Peretyazhko TS, Zhang Q, Colvin VL. Size-controlled dissolution of silver nanoparticles at neutral and acidic pH conditions: kinetics and size changes. Environ Sci Technol. 2014;48(20):11954–11961. doi: 10.1021/es5023202. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Hu H. Application of superabsorbent spacer fabrics as exuding wound dressing. Polymers. 2018;10(2):210. doi: 10.3390/polym10020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varma MVS, Kaushal AM, Garg A, Garg S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am J Drug Deliv. 2004;2(1):43–57. [Google Scholar]
- 62.Prabu D, Majdalawieh AF, Abu-Yousef IA. Preparation and characterization of gatifloxacin-loaded sodium alginate hydrogel membranes supplemented with hydroxypropyl methylcellulose and hydroxypropyl cellulose polymers for wound dressing. Int J Pharm Investig. 2016;6(2):86–95. doi: 10.4103/2230-973X.177810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maver T, Hribernik S, Mohan T, Smrke DM, Maver U, Stana-Kleinschek K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015;5(95):77873–77884. [Google Scholar]
- 64.Martinez-Gutierrez F, Olive PL, Banuelos A. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine. 2010;6(5):681–688. doi: 10.1016/j.nano.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Pons F, Fischer A, Frossard N, Lugnier A. Effect of toluene diisocyanate and its corresponding amines on viability and growth of human lung fibroblasts in culture. Cell Biol Toxicol. 1999;15(5):333–340. doi: 10.1023/a:1007671903406. [DOI] [PubMed] [Google Scholar]
- 66.Lange RW, Lantz RC, Stolz DB. Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol Sci. 1999;50(1):64–71. doi: 10.1093/toxsci/50.1.64. [DOI] [PubMed] [Google Scholar]
- 67.Vangronsveld E, Berckmans S, Spence M. Toluene diisocyanate emission to air and migration to a surface from a flexible polyurethane foam. Ann Occup Hyg. 2013;57(5):650–661. doi: 10.1093/annhyg/mes105. [DOI] [PubMed] [Google Scholar]
- 68.Minnen B, Leeuwen MBM, Stegenga B. Short-term in vitro and in vivo biocompatibility of a biodegradable polyurethane foam based on 1,4-butanediisocyanate. J Mater Sci Mater Med. 2005;16(3):221–227. doi: 10.1007/s10856-005-6683-2. [DOI] [PubMed] [Google Scholar]
- 69.Arévalo-Alquichire S, Ramírez C, Andrade L. Polyurethanes from modified castor oil and chitosan: synthesis, characterization, in vitro degradation, and cytotoxicity. J Elastom Plast. 2017;50(5):419–434. [Google Scholar]
- 70.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas S, McCubbin P. A comparison of the antimicrobial effects of four silver-containing dressings on three organisms. J Wound Care. 2003;12(3):101–107. doi: 10.12968/jowc.2003.12.3.26477. [DOI] [PubMed] [Google Scholar]
- 72.Lansdown AB, Williams A, Chandler S, Benfield S. Silver absorption and antibacterial efficacy of silver dressings. J Wound Care. 2005;14(4):155–160. doi: 10.12968/jowc.2005.14.4.26762. [DOI] [PubMed] [Google Scholar]
- 73.Supp AP, Neely AN, Supp DM, Warden GD, Boyce ST. Evaluation of cytotoxicity and antimicrobial activity of Acticoat burn dressing for management of microbial contamination in cultured skin substitutes grafted to athymic mice. J Burn Care Rehabil. 2005;26(3):238–246. [PubMed] [Google Scholar]
- 74.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 75.Park JH, Choi JY, Son DJ. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int J Mol Sci. 2017;18(4):738. doi: 10.3390/ijms18040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li HZ, Wan JY, Zhang L, Zhou QX, Luo FL, Zhang Z. Inhibitiory action of asiaiticoside on collagen-induced arthritis in mice. Acta Pharm Sin. 2007;42(7):698–703. [PubMed] [Google Scholar]
- 77.Muangman P, Pundee C, Opasanon S, Muangman S. A prospective, randomized trial of silver containing hydrofiber dressing versus 1% silver sulfadiazine for the treatment of partial thickness burns. Int Wound J. 2010;7(4):271–276. doi: 10.1111/j.1742-481X.2010.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimura Y, Sumiyoshi M, Samukawa K, Satake N, Sakanaka M. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur J Pharmacol. 2008;584(2–3):415–423. doi: 10.1016/j.ejphar.2008.02.036. [DOI] [PubMed] [Google Scholar]