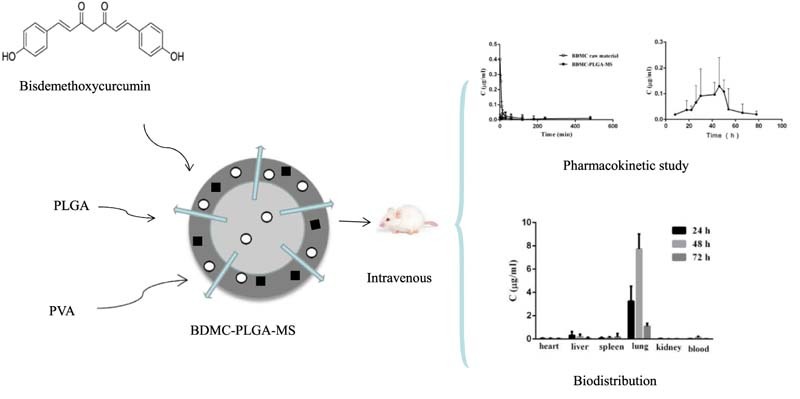
Graphical Abstract
Poly (lactic-co-glycolic acid) (PLGA)-based microspheres formulation of Bisdemethoxycurcum (BDMC) was prepared by emulsion-solvent evaporation method. The pharmacokinetics and tissue distribution in rats were conducted to evaluate BDMC-PLGA-MS preliminarily.
Keywords: Bisdemethoxycurcumin, Microsphere, Poly (lactic-co-glycolic acid), Solvent evaporation method, Pharmacokinetics
Abstract
The aim of the present study was to develop a novel long-acting Poly (lactic-co-glycolic acid) (PLGA)-based microspheres formulation of Bisdemethoxycurcum (BDMC) by emulsion-solvent evaporation method. Meanwhile, the effects of the volume ratio of the dispersed phase and continuous phase, the concentration of PLGA and PVA, the theoretical drug loading and stirring speed were investigated. The mean diameter of the microspheres was 8.5 µm and the size distribution was narrow. The encapsulation efficiency (EE) and drug loading efficiency (DLE) of BDME loaded PLGA microspheres (BDMC-PLGA-MS) was 94.18% and 8.14%, respectively. In an in vitro study of drug release, it can be concluded that the BDMC-PLGA-MS exhibited sustained and long-term release properties for 96 h. Stability studies suggested that the microspheres we prepared had a very good stability. Furthermore, the results of an in vivo study indicated that the BDMC-PLGA-MS had sustained release effect and was mainly distributed in the lung tissue, and less distribution in other tissues, which indicated that microspheres could be an effective parenteral carrier for the delivery of BDMC in lung cancer treatment.
1. Introduction
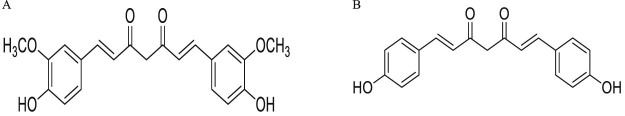
Bisdemethoxycurcumin (BDMC) is an α, β-unsaturated 1, 3-diketone that constitutes one of the three major components of the Curcuma longa [1]. Compared with curcumin, a β-unsaturated BDMC (Fig. 1) [2], consists of two phenyl and two hydroxyl groups which enhance its activities. And it is found more efficacious than curcumin and the increased potency is attributed to better stability in physiological media with a larger number of phenolic groups in BDMC [[3], [4]]. The potential prosperities of BDMC in anti-oxidant [[5], [6]], anti-inflammatory [[7], [8]], anti-diabetes [9], and in the treatment of inflection with tumors have been extensively studied in both intensive laboratories and clinical researches. Topical application of commercial grade BDMC has an equally potent inhibitory effect on the adhesion, invasion and migration of human ovarian cancer cells [10]. BDMC can downregulate vimentin and upregulate E-cadherin expression so as to inhibit the invasion and migration in highly metastatic human lung cancer cells [11]. The current research suggests that BDMC can potentially be used as a chemotherapeutic agent in human leukemic cancer [[12], [13]]. Besides it may have a therapeutic function in the restoration of WIF-1 expression in non-small cell lung cancer cell lines [14]. However, most cancer deaths are not caused by the growth of the primary tumor but result from its invasive spread to the secondary site. It is demonstrated that BDMC shows higher antimetastasis potency than curcumin by the differentially down-regulation of ECM degradation enzymes [15]. Furthermore, BDMC has the potential to inhibit multidrug resistance in cancer chemotherapy [16].
Fig. 1.
Chemical structures of (A) curcumin and (B) bisdemethoxycurcumin.
Despite great therapeutic potentials for BDMC utilization in a variety of diseases, its clinical development has been hindered due to its fast metabolism and poor water solubility. So suitable administration is needed to avoid repeated administration, and some alternative formulation approaches such as liposomes, nanospheres and microspheres need to be taken into account [17]. The modem microencapsulation of bioactive substances has been an important formulation strategy since its inception about 70 years ago, which focused on the encapsulation of larger molecules, e.g., peptides [18], proteins [19], and DNA/RNA [[20], [21]] for potential use as vaccines or as long-acting release. Microsphere systems have been used for drugs with a low solubility and a low permeability to improve the therapeutic response as well as to reduce side effects at the local site [[22], [23]]. Microsphere formulations are particularly well suited for the local treatment of diseases as they are injectable, and the drug encapsulates at high efficiency and the release rate is slow over a period of months [[24], [25]].
Biodegradable microparticles composed of Poly (lactic-co-glycolic acid) (PLGA) are well established drug delivery systems, possessing high potential to serve as carriers for drugs [26]. PLGA has been successfully used as a biodegradable polymer, because it undergoes hydrolysis in the body to produce the original monomer, lactic acid and glycolic acid. And it is a copolymer with demonstrated safety used in a host of Food and Drug Administration (FDA) approved therapeutic devices.
In this study, we prepared BDMC-PLGA-MS by emulsion solvent evaporation method. The particle size, morphological characteristics, encapsulation efficiency and drug release properties in vitro of BDMC-PLGA-MS were examined. The results suggested that it might be a good formulation. We also evaluated plasma pharmacokinetic and tissue distribution of BDMC-PLGA-MS in rats to obtain a better understanding of its pharmacological characteristics.
2. Materials and methods
2.1. Materials, reagents and animals
Bisdemethoxycurcumin (BDMC), standard substance (purity: ≥ 99%) was purchased from Shanghai Guigu Biological Technology Co. Ltd. BDMC raw material (purity: ≥ 99%) was prepared by Department of Clinical pharmacy, Anhui University of Traditional Chinese. Poly (lactic-co-glycolic acid) (PLGA) containing a 50:50 ratio of lactic and glycolic acids (50:50, IV = 0.46–0.55 dl/g, Mw = 50000) was purchased from Jinan Daigang Biomaterial Co., Ltd. Poly (vinyl alcohol) (PVA-124) was supplied by the Nanfang Chemical and Glasses Company. Sodium dodecyl sulfate (SDS) was purchased from Longxi Biomaterial Co., Ltd. Ethyl acetate, methylene chloride, and methanol (AR) were supplied by Shanghai Xinghua Biomaterial Co., Ltd. Acetonitrile was of high-performance liquid chromatography grade. All other reagents and solvents were of analytical grade, and were commercially available.
Sprague-Dawley rats (220 ± 20 g) were supplied by Laboratory Animal Center of Anhui Medical University (Hefei, Anhui, China permit number SCXK 2011-0002). It took at least one week to make rats adapt to the environment with standardized temperature 24 ± 2 °C and 50 ± 10% humidity. The rats were fed with standard laboratory chow and allowed access to water ad libitum before experiments begin. But they were fasted overnight with water available before experiments. All animal experiments were performed in accordance with the approval of the Animal Ethics Committee of Anhui Medical University.
2.2. Preparation of BDMC-PLGA-MS by emulsion-solvent evaporation method
BDMC-PLGA-MS was prepared using an emulsion-solvent evaporation method. Briefly, 21–35 mg peptide was an oil-in water emulsion solvent evaporation technique that was used for BDMC microsphere formulation. 100 mg of PLGA was dissolved in 0.8 ml of methylene chloride and 0.2 ml ethyl acetate, and 10 mg of BDMC was dispersed in this solution. This organic phase was added slowly to 100 ml of 1.5% (w/v) aqueous PVA solution and stirred at 800 rpm. for 4 h. The resulting microspheres were washed with double distilled deionized sterile water for 3 times and frozen dried for 24 h.
2.2.1. HPLC analysis of BDMC and method validation in vitro
The content of BDMC was determined by high performance liquid chromatography (HPLC). The HPLC is composed of binary LC-15C pumps (Shimadzu, Japan) and a SPD-15C UV detector (Shimadzu, Japan). Data processing was performed with LC-Solution software. The analytical column was an Inertsil/wondasilTM C18 column (150 mm × 4.6 mm, 5 µm) with the temperature at 30 °C. The mobile phase was acetonitrile and 4% acetic acid solution (48:52, v/v) and was delivered at a flow rate of 1.0 ml/min. The injection volume was 20 µl and detection of BDMC was carried out at 430 nm. The specificity, linearity, precision, recovery and repeatability experiments were performed to evaluate the HPLC method. The linearity test of the method was examined with standard solutions by injecting a series of concentrations, and the regression equations of BDMC was calculated in the form of y = ax + b, where y and x were peak area and concentration, respectively.
2.2.2. Measurement of particle size and surface morphology
Microsphere morphology and shape were characterized by scanning electron microscopy (SEM). The particle size was determined by laser diffraction (LD) using a Zetasizer (3000HS, USA). Microparticles were added to an aqueous solution of 2% CMC-Na. The suspension was sonicated for 30 s and was stirred during the measurement.
2.2.3. Thermal analysis
Differential scanning calorimetry (DSC) was used to determine the thermal properties of the polymer, drug and all formulations (BDMC, blank PLGA-MS, BDMC-PLGA-MS and the mechanical mixture of blank PLGA-MS and BDMC).
2.2.4. Determination of drug entrapment efficiency and drug loading
Drug efficiency (EE) was determined by measuring the total amount of drug in the collected samples. Approximately 1 mg of BDMC-PLGA-MS was dissolved in acetonitrile (20 µl). Then this solution was diluted in 1000 µl methanol and centrifuged for 10 min. The suspension was filtered (0.45 µm) and analyzed by HPLC. The EE and DLE were determined by the following equations:
2.2.5. Stability test of BDMC-PLGA-MS
In order to study the stability of microspheres, the suspension of BDMC-PLGA-MS were stored in high temperature (40 °C, 60 °C), high humidity and bright light environment. At intervals of specified time, BDMC-PLGA-MS dispersions were evaluated with respect to appearance, color and contents.
2.2.6. Release kinetics of BDMC-PLGA-MS
The drug release behavior from microspheres was evaluated using dialysis bag diffusion technique in phosphate buffered saline (PBS, pH 7.4) containing 0.5% SDS. Typically, 12.40 mg BDMC-PLGA-MS dispersion was transferred into a pre-swelled dialysis bag (21 mm, Mw: 8000–14400 Da). The sealed dialysis bag with 5 ml release medium was incubated in 100 ml release medium. The system was controlled at 37 °C, at a stirring rate at 100 rmp. At predetermined time intervals, 2 ml sample of the medium was withdrawn and replenished with the same amount of fresh medium. The samples were analyzed by HPLC method. All measurements were conducted in triplicate.
2.3. Pharmacokinetic study
2.3.1. Experimental conditions and method validation in vivo
The content of BDMC in plasma and tissue samples was detected through HPLC. The experimental conditions were the same as the method applied in the content of BDMC in the process of microsphere preparation. The method validation of HPLC in vivo was evaluated by the specificity, linearity, precision, recovery and stability experiments at different levels. The results were fitted to linear regression analysis using 1/x as weighting factor. The concentration of BDMC was directly calculated from the corresponding calibration curve.
2.3.2. Biological sample preparation
Plasma samples were prepared by spiking 100 µl samples, 20 µl internal standard solution (curcumin) and 300 µl methanol into a 1.5 ml centrifuge tube. And samples were mixed for 3 min, centrifuged for 10 min at 12000 rpm, the upper organic layer were transferred into a clean centrifuge tube and then was filtered through a 0.22 µm millipore film before injection for HPLC analysis. Tissues samples were grinded with deionized water at a ratio of 1:3 (w:w) and then processed as plasma samples.
2.3.3. Plasma pharmacokinetic study of BDMC-PLGA-MS
A plasma pharmacokinetic study was designed to evaluate the BDMC-PLGA-MS by comparing with BDMC raw material. In the plasma pharmacokinetic study, rats were randomly divided into two groups with five in each group, Group 1 was randomly selected and treated with BDMC raw material at a dose of 10 mg/kg intravenously, while a corresponding dose of BDMC-PLGA-MS was administered to Group 2. Blood samples (0.3 mL each) were collected into heparinized tubes at 0 (before dosing), 5, 10, 15, 30, 60, 120, 180, 240, 480 min after dosing, and plasma was separated immediately. The plasma samples were stored at −20 °C until assayed.
2.3.4. Plasma pharmacokinetic study of BDMC-PLGA-MS for a long time
Five rats were treated with BDMC-PLGA-MS at a dose of 10 mg/kg by tail-vein injection. Blood samples of about 0.3 ml were collected from the suborbital vein into heparinized centrifuge tubes, at 0 (before dosing), 8, 18, 22, 26, 30, 42, 46, 50, 54, 66, 78 h after dosing, and plasma was separated immediately. The plasma samples were stored at −20 °C until assayed.
2.3.5. Biodistribution of BDMC-PLGA-MS
The tissue distribution study was carried out on 18 rats which were randomly divided into 3 groups, with six in each. BDMC-PLGA-MS were given intravenously at a dose of 10 mg/kg via the caudal vein. After administration, the rats were sacrificed at 24, 48, 72 h, and tissues (heart, liver, spleen, lung, kidney) were collected at the same time. The tissue samples were rinsed in ice-cold normal saline, blotted dry with filter paper, and then stored at −20 °C until assayed.
2.4. Statistical analysis
The content of BDMC was detected through HPLC, and the concentration of BDMC was calculated according to corresponding calibration curves. The pharmacokinetic parameters including the area under curve (AUC), CL, t1/2 were calculated using WinNonlin software (Pharsight Co., Mountain View, CA). Data were expressed as mean ± stand deviation (SD).
3. Results and discussion
3.1. Method validation for BDMC analysis in vitro and in vivo
3.1.1. In vitro
To check the selectivity of the method, blank microspheres, blank microspheres spike with BDMC and BDMC-PLGA-MS were injected into HPLC system. No interference was observed. Linear calibration curve was obtained for BDMC in the range of 0.0104~10.4 µg/ml with r = 0.9998. The RSD of within-day and between-day precision were in the range of 0.66% ~ 2.72% and the RSD of stability was 1.71%. The recovery were in the range of 98.89~100.58% with RSD values of 1.33~2.62% which indicated that the HPLC method developed was suitable for determining the content of BDMC.
3.1.2. In vivo
Specificity was assessed by analyzing blank plasma and tissue homogenates samples, blank plasma and tissue homogenates samples spiked with BDMC, and rat plasma and tissue homogenates samples after i.v. administration of BDMC-PLGA-MS. There were no endogenous substance interfering with the BDMC and internal standard. The HPLC method had good linearity (r = 0.9985, r = 0.9980, r = 0.9980, r = 0.9990, r = 0.9990, r = 0.9985) and enabled the quantification of BDMC in plasma and heart, liver, spleen, lung, kidney samples within the range of 0.001~0.8 µg/ml, 0.002~1 µg/ml, 0.002~1 µg/ml, 0.002~1 µg/ml, 0.1~16 µg/ml and 0.002~1 µg/ml. Precision, recovery and stability were also conformed to the requirement of Guidance for Industry Bioanalytical Method Validation.
3.2. Characterization of BDMC-PLGA-MS
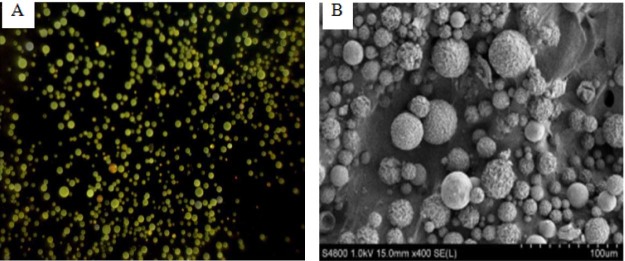
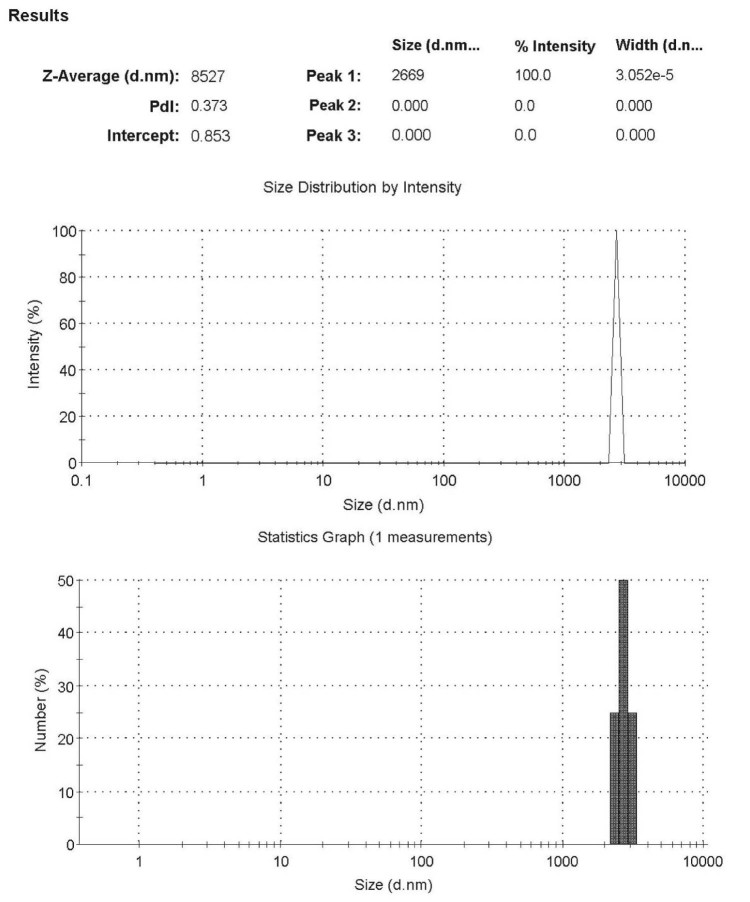
The morphology of the microparticles was determined by light microscopic (Fig. 2A) and SEM (Fig. 2B). Microparticles appeared flaxen spherical with a smooth surface. SEM analysis showed that the surface of the microspheres was spherical and smooth. And the total scale is 100 µm in SEM, so each scale is 10 µm. From the Fig. 2B, it can be seen that the particle size is about 8.5 µm. LD analysis also showed that the average particle size of the optimal prescription was 8.5 µm and the size distribution was narrow (Fig. 3). In our study, the EE and DLE of BDMC-PLGA-MS was 94% and 8.41%, respectively.
Fig. 2.
The morphology of the microparticles: (A) Light microscopy picture and (B) SEM photography.
Fig. 3.
The size distribution of BDMC-PLGA-MS.
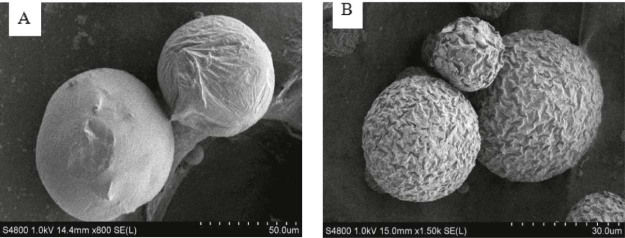
This study showed that the volume ratio of the dispersed phase and continuous phase, the concentration of PLGA and PVA, the theoretical drug loading and stirring speed were the key factors for the emulsion-solvent evaporation method. It is important to select suitable solvent as dispersed phase for emulsion-solvent evaporation method. In our experiments, the microspheres prepared by ethyl acetate showed a rough surface and irregular morphology, and there were fiber-like agglomerates in the system. It may be due to the partial miscibility of ethyl acetate in water. As the dispersed phase spread rapidly into the continuous phase, there was no enough time for PLGA solidifying. With the use of dichloromethane, the microspheres we got were brownish-red, and there was a small amount of drug crystals on the surface of the microsphere, so the burst release was obvious. Taking the solubility of the drug and boiling point of the volatile solvent into account, we chose the mixture of dichloromethane and ethyl acetate. The ratio of the mixture affected the particle size obviously. We found that too vigorous stirring would generate much foam, which led to the leakage of BDMC, so the encapsulation efficiency fell. We had also inspected the influence of the organic solvent evaporation time, and it almost had no effect on the particle size, EE and DLE. Drying method also influenced the characterization of BDMC-PLGA-MS. Normal temperature drying and freeze-drying were used in our study. The former showed particle crushing and poor redispersibility (Fig. 4A), and the latter showed a shrinkage surface, but the microspheres were complete and the redispersion was good (Fig. 4B). Appropriate lyoprotectant needs to be screened to protect microspheres in the freeze-drying process in further studies.
Fig. 4.
SEM-picture of BDMC-PLGA-MS: (A) It was dried at normal temperature and (B) dried by using a freeze-drying method.
3.3. DSC studies and stability studies
DSC analysis was used to obtain the thermograms of BDMC, PLGA-MS, BDMC-PLGA-MS and physical mixture of BDMC and PLGA-MS. DSC graphs of samples described above were presented in Fig. 5. The raw material BDMC and PLGA-MS revealed a sharp peak at 220 °C and 50 °C, respectively. The melting peaks both appeared when the physical mixture of them was analyzed by DSC. However, the samples of BDMC-PLGA-MS led to a new signal peak appearing near at 45 °C and there was no melting peak for BDMC. After high temperature, high humidity and bright light processing for 5 days BDMC-PLGA-MS were still yellow power and the contents were almost unchanged. Maintaining these conditions for another 5 days, results showed that the appearance and color were still unchanged and the contents were decreased slightly (Table 1).
Fig. 5.
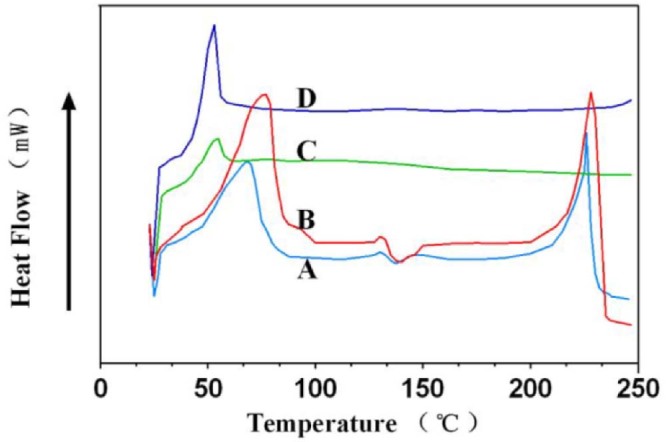
Differential scanning calorimetry of samples: (A: PLGA-MS; B: BDMC-PLGA-MS; C: BDMC; D: physical mixture of BDMC and PLGA-MS) .
Table 1.
Stability studies of BDMC-PLGA-MS.
| Factors | Time (d) | Appearance | Contents (%) |
|---|---|---|---|
| 0 | Yellow power | 98.56 | |
| High temperature 40 °C |
5 | Yellow power | 97.87 |
| 10 | Yellow power | 97.56 | |
| High temperature 60 °C |
5 | Yellow power | 96.24 |
| 10 | Yellow power | 95.59 | |
| High humidity 92.5% |
5 | Yellow power | 97.74 |
| 10 | Yellow power | 97.03 | |
| Bright light 4000Lx |
5 | Yellow power | 97.89 |
| 10 | Yellow power | 96.14 |
According to DSC studies and stability studies we could speculate that BDMC were dispersed homogeneously in microspheres and were formed into a new system instead of adhering to the surface of the microspheres. The microspheres we prepared could detect that being embedded by the polymer could protect the drug from the external environment.
3.4. Release kinetics
The release of BDMC from microspheres was investigated to evaluate its sustained ability. The release rates of BDMC from microspheres against time were plotted in Fig. 6. There was a very small initial burst with only about 3.63% of the total loaded amount at the beginning 26 h, followed by sustained release for up to 96 h. It could be concluded that the BDMC-PLGA-MS exhibited sustained and long-term release property in vitro. The possible mechanism for the release behavior was that there was little drug left on the surface and most of them were dispersed in PLGA.
Fig. 6.
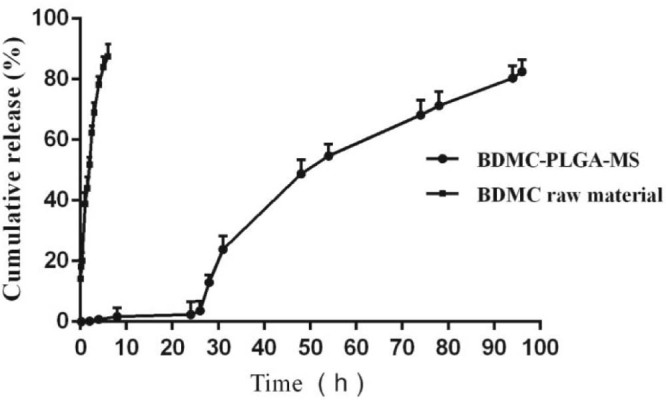
In vitro release profile of BDMC-PLGA-MS.
3.5. Pharmacokinetic study
Due to poor absorption and rapid metabolism, BDMC is rarely detected in plasma when administered orally [27]. Therefore, tail vein administration was performed in this study. Previous reports indicated there was no visible damage at the injected sites, no significant pathological changes in tissues and vessel wall after administering microspheres within the size range of 5–20 µm through intravenous injection. In addition, the organ weights did not change significantly [[28], [29]]. In this study, there were no abnormal performance, no death phenomenon, no adverse reaction and the activity was normal after rats were treated by intravenous injection. All these indicated that the microspheres we prepared were relatively safe in intravenous injection.
3.5.1. Plasma pharmacokinetic study
The pharmacokinetic parameters and the plasma concentration versus time curves of BDMC in BDMC raw material group and BDMC-PLGA-MS group were shown in Table 2 and Fig. 7. After 120 min, BDMC in raw material was undetectable in plasma from animals but still was detectable in BDMC-PLGA-MS group. These results showed that BDMC in BDMC raw material group metabolized rapidly in rats. BDMC could sustain release with low concentration in BDMC-PLGA-MS group. Probably because BDMC-PLGA-MS was a controlled release formulation and has a slow-release effect. On the basis of the above results, pharmacokinetic study of BDMC-PLGA-MS for a long time was investigated further. The plasma concentration versus time curve of BDMC in BDMC-PLGA-MS group for a long time is shown in Fig. 8. BDMC in BDMC-PLGA-MS was still detectable after 78 h.
Table 2.
Main pharmacokinetic parameters of BDMC.
| Compounds | PK parameters | BDMC raw material | BDMC-PLGA-MS |
|---|---|---|---|
| BDMC | V(c) (l/kg) | 328.505 ± 42.259 | 1033.607 ± 386.348 |
| T1/2 (min) | 66.056 ± 10.755 | 305.707 ± 195.006 | |
| CL(s) (ml/min) | 3.471 ± 0.261 | 2.656 ± 0.693 | |
| AUC (µg/min/ml) | 4.893 ± 0.29 | 3.309 ± 0.183 |
Fig. 7.
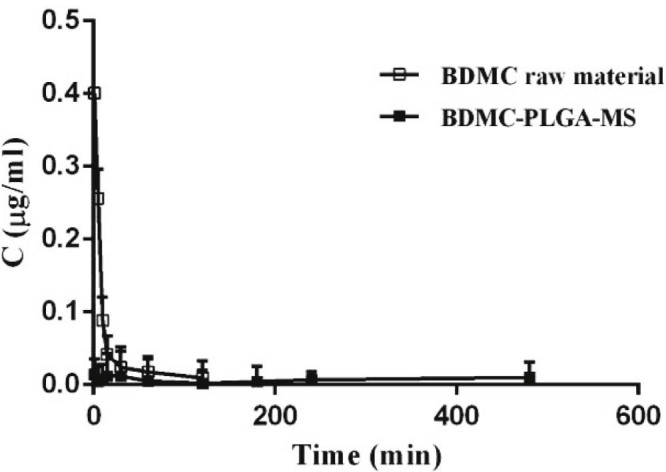
Mean plasma concentration-time course of BDMC in BDMC-PLGA-MS in rats, plasma. Data are expressed as mean ± SD (n = 5).
Fig. 8.
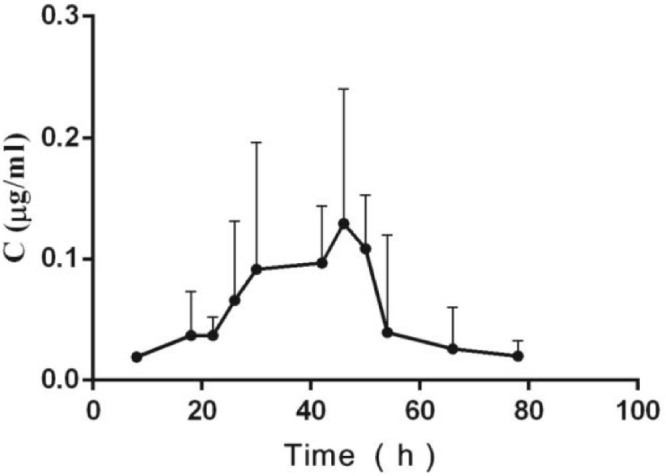
Mean plasma concentration-time course of BDMC in BDMC-PLGA-MS in rats, plasma. Data are expressed as mean ± SD (n = 5).
3.6. Tissues distribution study
Concentrations of BDMC in BDMC-PLGA-MS in heart, liver, spleen, lung, kidney tissues were also measured, as displayed in Fig. 9. For the BDMC-PLGA-MS, the highest BDMC concentrations were found in lung, followed by liver, spleen, heart, kidney and blood. The highest concentrations were achieved in lung at 48 h. It is reported that the particle size is an important parameter for drug targeting with microspheres, as the size helps in discharge of drug in controlled manner and uptake of drugs into the tissues. Particles with size between 7 and 30 µm could be uptaken by lung through mechanical filtration resistance [30]. The average particle size of BDMC-PLGA-MS we prepared was 8.5 µm, which indicated this microsphere may be able to target in lung. Tissues distribution study also proved this point preliminarily.
Fig. 9.

Tissue distribution of BDMC in rats, heart, liver, spleen, lung, kidney, and blood. Data are expressed as mean ± SD (n = 6).
4. Conclusion
Curcuminoids are safe natural yellow pigments used as food coloring agents and traditional drugs with a variety of biological functions such as antitumor and antioxidant activities [31]. Among the main three curcuminoids, BDMC may be more potent in pulmonary, hepatic and esophageal cancer [32]. However, in an aqueous environment, such as the human gastrointestinal tract, BDMC are only sparingly soluble. Clinical advancement of this promising molecule has been hindered by its poor water solubility, short biological half-life, and low bioavailability following oral administration [[33], [34]]. We developed BDMC-PLGA-MS to improve the treatment efficiency, and provided a new method for clinical application of BDMC. The preparation and in vitro release of BDMC-PLGA-MS were reported here for the first time. In the process of the organic solvent evaporated, the carrier material in the emulsion droplet concentrate gradually, then the viscosity of the emulsion droplets increased, the microspheres became solidified, and at last the drug was wrapped in the microspheres. Adding excessive water in the continuous phase increased the contact area of gas and liquid, adopting the freeze-drying method could further remove the organic solvent and ensure the safety of the formulation. The result of a preliminary plasma pharmacokinetic study and tissues distribution study showed that BDMC-PLGA-MS had sustained release effect and was mainly distributed in the lung tissue, less in other tissues. The highest concentrations of BDMC in BDMC-PLGA-MS about 48 h and 72 h in lung tissue are still high. Although the plasma elimination time of BDMC is relatively short, the microspheres was swelling and dissolution in vivo, which could make BDMC release continuously. The effect of BDMC in the lung tissue can get better to treat lung cancer, which is conductive for this formulation to play the role of anti-lung cancer drug. Further research should be carried out in the future.
Conflicts of interest
The authors report no conflicts of interest.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Yuan T., Zhang C., Qiu C. Chemical constituents from Curcuma longa L. and their inhibitory effects of nitric oxide production. Nat Prod Res. 2017;20:1–6. doi: 10.1080/14786419.2017.1354185. [DOI] [PubMed] [Google Scholar]
- 2.Sheu M.J., Lin H.Y., Yang Y.H. Demethoxycurcumin, a major active curcuminoid from Curcuma longa, suppresses balloon injury induced vascular smooth muscle cell migration and neointima formation: an in vitro and in vivo study. Mol Nutr Food Res. 2013;57:1586–1597. doi: 10.1002/mnfr.201200462. [DOI] [PubMed] [Google Scholar]
- 3.Luo C., Du Z., Wei X. Bisdemethoxycurcumin attenuates gastric adenocarcinoma growth by inducing mitochondrial dysfunction. Oncol Lett. 2015;9:270–274. doi: 10.3892/ol.2014.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hua Y., Dolence J., Ramanan S. Bisdemethoxycurcumin inhibits PDGF-induced vascular smooth muscle cell motility and proliferation. Mol Nutr Food Res. 2013;57:1611–1618. doi: 10.1002/mnfr.201200852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y.B., Gao J.L., Lee S.M. Bisdemethoxycurcumin protects endothelial cells against t-BHP-induced cell damage by regulating the phosphorylation level of ERK1/2 and Akt. Int J Mol Med. 2011;27:205–211. doi: 10.3892/ijmm.2010.578. [DOI] [PubMed] [Google Scholar]
- 6.Kalaycioglu Z., Gazioglu I., Erim F.B. Comparison of antioxidant, anticholinesterase, and antidiabetic activities of three curcuminoids isolated from Curcuma longa L. Nat Prod Res. 2017;13:1–4. doi: 10.1080/14786419.2017.1299727. [DOI] [PubMed] [Google Scholar]
- 7.Ramadan G., Al-Kahtani M.A., El-Sayed W.M. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation. 2011;34:291–301. doi: 10.1007/s10753-010-9278-0. [DOI] [PubMed] [Google Scholar]
- 8.Li Y.B., Zhong Z.F., Chen M.W. Bisdemethoxycurcumin Increases Sirt1 to Antagonize t-BHP-Induced Premature Senescence in WI38 Fibroblast Cells. Evid Based Complement Alternat Med. 2013;2013:851714. doi: 10.1155/2013/851714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponnusamy S., Zinjarde S., Bhargava S. Discovering Bisdemethoxycurcumin from Curcuma longa rhizome as a potent small molecule inhibitor of human pancreatic alpha-amylase, a target for type-2 diabetes. Food Chem. 2012;135:2638–2642. doi: 10.1016/j.foodchem.2012.06.110. [DOI] [PubMed] [Google Scholar]
- 10.Pei H., Yang Y., Cui L. Bisdemethoxycurcumin inhibits ovarian cancer via reducing oxidative stress mediated MMPs expressions. Sci Rep. 2016;6:28773. doi: 10.1038/srep28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J., Yang H., Zhou X. Bisdemethoxycurcumin suppresses migration and invasion of highly metastatic 95D lung cancer cells by regulating E-cadherin and vimentin expression, and inducing autophagy. Mol Med Rep. 2015;12:7603–7608. doi: 10.3892/mmr.2015.4356. [DOI] [PubMed] [Google Scholar]
- 12.Tima S., Ichikawa H., Ampasavate C. Inhibitory effect of turmeric curcuminoids on FLT3 expression and cell cycle arrest in the FLT3-overexpressing EoL-1 leukemic cell line. J Nat Prod. 2014;77:948–954. doi: 10.1021/np401028h. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y.L., Yang H.P., Gong L. Hypomethylation effects of curcumin, demethoxycurcumin and bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung cancer cell lines. Mol Med Rep. 2011;4:675–679. doi: 10.3892/mmr.2011.473. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y.L., Yang H.P., Zhou X.D. The hypomethylation agent bisdemethoxycurcumin acts on the WIF-1 promoter, inhibits the canonical wnt pathway and induces apoptosis in human non-small-cell lung cancer. Curr Cancer Drug Targets. 2011;11:1098–1110. doi: 10.2174/156800911798073041. [DOI] [PubMed] [Google Scholar]
- 15.Boonrao M., Yodkeeree S., Ampasavate C. The inhibitory effect of turmeric curcuminoids on matrix metalloproteinase-3 secretion in human invasive breast carcinoma cells. Arch Pharm Res. 2010;33:989–998. doi: 10.1007/s12272-010-0703-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang H.-J., Yang Z.-X., Dai X.-T. Bisdemethoxycurcumin sensitizes cisplatin-resistant lung cancer cells to chemotherapy by inhibition of CA916798 and PI3K/AKT signaling. Apoptosis. 2017;22:1157–1168. doi: 10.1007/s10495-017-1395-x. [DOI] [PubMed] [Google Scholar]
- 17.Lu X.F., Zhou Y., Zhang J. Pharmacokinetics and tissue distribution of larotaxel in rats: comparison of larotaxel solution with larotaxel-loaded folate receptor-targeting amphiphilic copolymer-modified liposomes. Xenobiotica. 2017;47:416–422. doi: 10.1080/00498254.2016.1195936. [DOI] [PubMed] [Google Scholar]
- 18.Ng S.M., Choi J.Y., Han H.S. Novel microencapsulation of potential drugs with low molecular weight and high hydrophilicity: hydrogen peroxide as a candidate compound. Int J Pharm. 2010;384:120–127. doi: 10.1016/j.ijpharm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Rawat S., Kohli N., Suri C.R. Molecular mechanism of improved structural integrity of protein in polymer based microsphere delivery system. Mol Pharm. 2012;9:2403–2414. doi: 10.1021/mp2004065. [DOI] [PubMed] [Google Scholar]
- 20.Ishii A., Hiruta Y., Heinemann D. Intracellular localization and delivery of plasmid DNA by biodegradable microsphere-mediated femtosecond laser optoporation. J Biophotonics. 2017 doi: 10.1002/jbio.201600323. [DOI] [PubMed] [Google Scholar]
- 21.Frede A., Neuhaus B., Knuschke T. Local delivery of siRNA-loaded calcium phosphate nanoparticles abates pulmonary inflammation. Nanomedicine (Lond) 2017 doi: 10.1016/j.nano.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohr A., Kristensen J., Stride E. Preparation of microspheres containing low solubility drug compound by electrohydrodynamic spraying. Int J Pharm. 2011;412:59–67. doi: 10.1016/j.ijpharm.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Han B., Wang H.T., Liu H.Y. Preparation of pingyangmycin PLGA microspheres and related in vitro/in vivo studies. Int J Pharm. 2010;398:130–136. doi: 10.1016/j.ijpharm.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Salis A., Porcu E.P., Gavini E. In situ forming biodegradable poly (epsilon-caprolactone) microsphere systems: a challenge for transarterial embolization therapy. In vitro and preliminary ex vivo studies. Expert Opin Drug Deliv. 2017;14:453–465. doi: 10.1080/17425247.2017.1295036. [DOI] [PubMed] [Google Scholar]
- 25.Liu S.S., Liu L.J., Xiao L.Y. Design of silk-vaterite microsphere systems as drug carriers with pH-responsive release behavior. J Mater Chem B Mater Biol Med. 2015;3:8314–8320. doi: 10.1039/C5TB01692D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mir M., Ahmed N., Rehman A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–231. doi: 10.1016/j.colsurfb.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 27.Metzler M., Pfeiffer E., Schulz S.I. Curcumin uptake and metabolism. Biofactors. 2013;39:14–20. doi: 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- 28.Hao Z., Qu B., Wang Y. Preparation and characterization of lung-targeting ceftiofur-loaded gelatin microspheres. Drug Dev Ind Pharm. 2011;37:1422–1428. doi: 10.3109/03639045.2011.584192. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Yang Z., Sun R. Preparation of lung-targeting, emodin-loaded polylactic acid microspheres and their properties. Int J Mol Sci. 2014;15:6241–6251. doi: 10.3390/ijms15046241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramaiah B., Nagaraja S.H., Kapanigowda U.G. High azithromycin concentration in lung by way of bovine serum albumin microspheres as targeted drug delivery: lung targeting efficiency in albino mice. Daru. 2016;24:14. doi: 10.1186/s40199-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jäger R., Lowery R.P., Calvanese A.V. Comparative absorption of curcumin formulations. Nutr J. 2014;13:11. doi: 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramezani M., Hatamipour M., Sahebkar A. Promising anti-tumor properties of bisdemethoxycurcumin: a naturally occurring curcumin analogue. J Cell Physiol. 2017 doi: 10.1002/jcp.25795. [DOI] [PubMed] [Google Scholar]
- 33.Mehanny M., Hathout R.M., Geneidi A.S. Studying the effect of physically-adsorbed coating polymers on the cytotoxic activity of optimized bisdemethoxycurcumin loaded-PLGA nanoparticles. J Biomed Mater Res A. 2017;105:1433–1445. doi: 10.1002/jbm.a.36028. [DOI] [PubMed] [Google Scholar]
- 34.Yeh C.C., Su Y.H., Lin Y.J. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des Devel Ther. 2015;9:2285–2300. doi: 10.2147/DDDT.S78277. [DOI] [PMC free article] [PubMed] [Google Scholar]