Abstracts
Diabetes is one of the most prevalent diseases in the world with high-mortality and complex complications including diabetic foot ulcer (DFU). It has been reported that the difficulties in repairing the wound related to DFU has much relationship with the wound infection, change of inflammatory responses, lack of extracellular matrix (ECM), and the failure of angiogenesis. Following the development of medical materials and pharmaceutical technology, nanofibers has been developed by electrospinning with huge porosity, excellent humidity absorption, a better oxygen exchange rate, and some antibacterial activities. That is to say, as a potential material, nanofibers must be a wonderful candidate for the DFU treatment with so many benefits. Careful selection of polymers from natural resource and synthetic resource can widen the nanofibrous application. Popular methods applied for the nanofibrous fabrication consist of uniaxial electrospinning and coaxial electrospinning. Furthermore, nanofibers loading chemical, biochemical active pharmaceutical ingredient (API) or even stem cells can be wonderful dosage forms for the treatment of DFU. This review summarizes the present techniques applied in the fabrication of nanofibrous dressing (ND) that utilizes a variety of materials and active agents to offer a better health care for the patients suffering from DFU.
Keywords: Nanofibers, Nanofibrous dressing, Diabetic foot ulcer, Uniaxial electrospinning, Coaxial electrospinning
Graphical abstract
1. Introduction
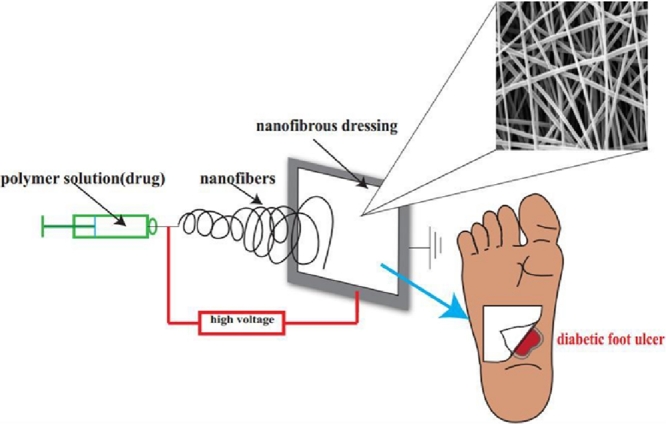
According to statistics, it is estimated that there were 366 million people worldwide with diabetes in 2013, and the number will be increased to almost 552 million by 2030 [1]. Diabetes is a kind of disease that relates to the dysfunction of glucose control, the destroyed management on the protein metabolism and lipid metabolism [2]. Following the deterioration of diabetes, a portion of diabetic patients will suffer from diabetic foot ulcer (DFU), one of the complications of diabetes, which possesses the characteristics of long term process of wound closure and has the tendency to hospitalization and even amputation in the future. Unfortunately, apart from the physical and mental impairment on diabetic patients, the cost for DFU treatment may be another heavy load. To solve this problem, we need to have a clear idea of the reason why it is so difficult to heal the wound and we need to figure out what has prolonged the healing process. Peripheral neuropathy, deformity, and macrovascular disease can be the main factors that cause the failure of DFU healing process [3]. Additionally, new findings can be the supplements to the factors [3], which including lacking resistance to infection, changing on microcirculation function, and damaging to growth factors (GF) expression and activity. Furthermore, another study is conducted to find out whether the peripheral arterial disease has a correlation with DFU, and the results indicate that there is some correlation between them, and DFU can be classified to two disease states according to whether there exists the peripheral arterial disease [4]. Other factors, which have significant contributions to DFU including low proliferative capacity of the fibroblasts, downregulation of receptors, and the absence of a suitable protein matrix in the dermis [5]. In brief, the mechanism of this issue is relatively complex, and there still has a big room for pharmaceutists to participate in DFU treatment. The methods of DFU treatment varies from individual symptoms and the disease stages. Several therapeutic approaches have been reported, glycemic regulation, such as adequate insulin administration, is essential. Debridement, skin graft and tissue replacement has relevantly high-efficiency of wound closure [3]. Other approaches such as vascular reconstruction, hyperbaric oxygen therapy, and granulocyte-colony stimulating factor are also options to treat DFU. In most cases, the wound dressing is of use to create a beneficial environment for the open wound to achieve a better and faster wound closure [6]. Unfortunately, conventional wound dressings like gauze possess limited basic function for their defective material properties. What is more, different phases of the diabetic wound healing have different pathology features so that multifunctional wound dressing with specific material is under the urgency. Currently, advanced dressing technology like nanofibrous dressing (ND) that can employ specific materials to fit specific need for DFU treatment is harvesting large interests and attention. ND is the collection of nanofibers ranging from nanometers to micrometers, as shown in Fig. 1, ND is easy to be removed [7]. The technique of nanofibers fabrication has a long history, and it was firstly reported since 1900 [8]. Nanofibers as a promising matrix have lots of advantages such as small diameter, narrow diameter distribution, and high-specific surface area. Various polymers have been studied and developed into fabricate nanofibers for textiles, electrical and optical component, sensors, and filtration devices [9], [10]. The products manufactured through this technology are very soft and highly flexible, lacking of sharp corners and vulnerable to turn to sheets, tubes, and coatings [11]. ND employed by pharmaceutics shows great benefits and an increased prevalence in the drug delivery system. It utilizes the excipients or accessories to deliver the therapeutic agents to the site with high-efficiency, and low-adverse effects. For the DFU treatment, it is necessary to put emphasis upon the vascularization, collagen accumulation and normal physiological functions to control the deterioration process and even cure the wound [12]. Kinds of active pharmaceutical agents like molecules and cells play the key roles in this process; however, the matrix loaded them can also be a fundamental stage for their functional realization. Basically, it has crucial activities of the absorption of exudation and the exchange of oxygen, water, and nutrient [13]. Additionally, nanofibers with the similar diameter to the extracellular matrix (ECM) have been proven to accelerate the process of cell adhesion and proliferation. 1D–3D nanofibers production also has been investigated in biomaterials field [14].
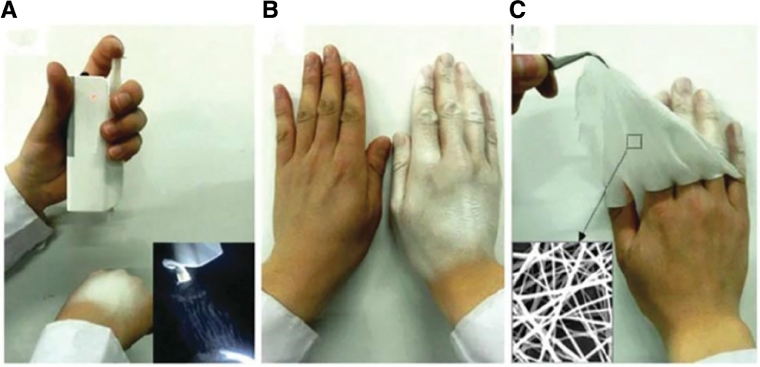
Fig. 1.
(A) PLA fibers electrospun by portable electrospinning device on hand; (B) the final appearance of a homogenous ND; (C) removing the ND with a tweezer from skin (Reproduced with permission from [7].). Copyright 2015, The Royal Society of Chemistry.
In this article, we try to discuss the common polymers used in the fabrication of ND, especially for the DFU treatment. According to the resources, polymers are classed into two categories, natural and synthetic polymers. Biological and physicochemical properties are also discussed. The next attention will be paid to the fabrication approaches including uniaxial and coaxial electrospinning. Attempt to introduce the device configuration and the process in the nanofibers formation has been conducted. In the meantime, underlying factors influencing nanofibers fabrication are listed in detail. An overview of the application of ND in treating DFU will be given in the end.
2. Polymers for nanofibers
There is no universal standard for the selection of the polymers applied for individual nanofibers, and the choice relies on the desirable function of the nanofibers. Different polymers with different molecules and sources will lead to notable differences on properties such as spinning solution viscosity, nanofibers morphology, mechanical strength, biocompatibility and physicochemical characteristics. A large number of polymers have been developed in the study of nanofibers utilized for DFU healing. They may be broadly classified into two categories: synthetic and natural polymers. In general, natural polymers have a better biocompatibility such as degradation and lower immune resistance, whereas the synthetic polymers possess an easier electrospinning with excellent mechanical strength such as flexibility and stiffness. To get the maximum benefits from those materials, taking the blending strategy is advisable. For example, chitosan and alginate with the addition of polyethylene oxide (PEO) or polyvinyl alcohol (PVA) has been fabricated into nanofibers [15]. Table 1 shows various polymers and corresponding parameters applied in the ND fabrication.
Table 1.
Synthetic polymers and natural polymers used in ND fabrication and the relevant parameters set in the studies.
| Polymer | Polymer resource | Solvent | Drug | Feed rate (μl/min) | Voltage supply (kV) | Collector distance (cm) | Reference |
|---|---|---|---|---|---|---|---|
| PLGA | Synthetic | Acetone | Amoxicillin | 300 | 14 | 12 | 15 |
| PLGA | Synthetic | Dichloromethane/DMF | Dopamine/bFGF | 600 | 12 | 15 | 16 |
| PCL | Synthetic | Dichloromethane /DMF | Curcumin | 13 | 8.5 | 16 | 19 |
| PVA | Synthetic | Formic acid | Recombinant spider silk protein | 50–167 | 80–100 | 18–22 | 28 |
| Chitosan/PLA | Synthetic/natural | Acetic acid | – | 2 | 15 | 10 | 32 |
| Alginate/PEO/triton x-100 | Synthetic/natural | Deionized water | – | 8.3 | 6–12 | 15 | 34 |
| Gelatin/hyaluronic acid | Natural | Distilled water and ethanol | – | 60 | 22 | 15 | 38 |
2.1. Synthetic polymers
Poly (lactic-co-glycolic acid) (PLGA) is a biodegradable and biocompatible polymer synthesized by two monomers: lactic acid and glycolic acid. Some articles [15], [16], [17], [18] have shown that PLGA is an applicable polymer for the nanofibrous fabrication with excellent mechanical strength and flexibility. The ratio of lactic acid to glycolic acid, molecular weight, crystallinity and the medium pH have some certain influences on the degradation rate of PLGA. The degradation rate can be slowed by the reduction of glycolic acid and the improvement of the molecular weight [16]. However, organic solvents are often used to dissolve PLGA for its water-insolubility that can cause cell toxicity preventing the DFU healing. Thus, modification on PLGA is favorable for its application in ND. Study presented by Zhou et al. [19] indicates that N-carboxyethyl PLGA synthesized by Michael addition reaction of acrylic acid has a better water solubility. Even though PLGA exhibits poor solubility in water, nanofibers with PLGA also has been successfully developed into hybrid scaffolds in the diabetic wound treatment [20]. In their study, the addition of chitosan and recombinant human platelet-derived GF increases the hydrophilic characteristics, and water contact angle of the nanofibers made of the two materials compared with the pure PLGA material proves it. The area of simulative diabetic wound dramatically decreases in the in vivo test. Polymers like PEO and PVA are recommended to apply for reducing the viscosity of PLGA solution due to its chain entanglements or promoting the conductivity of N-carboxyethyl PLGA aqueous solution [19], [21].
Poly (lactic acid) (PLA) is the thermoplastic aliphatic polyester derived from natural resources, and it has been widely used in pharmaceutics and medical medicine, for example, drug delivery system, sutures, and stents. PLA also possesses some activities of biocompatibility and mechanical strength, despite the approval from FDA, there still exists the fact that poor compatibility can be observed when it contacts the blood [22]. However, it does not mean that PLA is not worthy in the DFU treatment, because blending with other polymers like chitosan derivatives can modulate the blood compatibility [23]. Accordingly, as a platform, it can promote the cell attachment and proliferation [24].
Poly (ɛ-caprolactone) (PCL) is a biodegradable and biocompatible polymer with a functional ester bond that can be degraded into nontoxic fractions under the physiological environment. Even though PCL mimics the ECM, its capacity of cell attachment and proliferation has been limited for the hydrophobic properties [25]. As reported in the texture, high-porosity on the surface of the PCL nanofibers can overcome this drawback, co-utilization with other hydrophilic polymers such as gelatin, collagen, or chitosan is another good choice [21]. For instance, PCL with gelatin can be electrospun into nanofibers in two forms, alignment and random [14], [26], and these topologies do affect the fibroblasts behavior. The essential point in this research is that fibroblasts can produce more actin attaching the align nanofibers. In other words, the alignment of nanofibers with PCL and gelatin must has some specific relationship with DFU wound recovery. Further study about the alignment of PCL nanofibers with scanning electronic microscope (SEM) and differential scanning calorimetry (DSC) finds that a single aligned nanofiber consists of several nanofibrils and each nanofibril includes the crystalline and the amorphous region [27].
Polyvinyl alcohol (PVA) is aqueous soluble, nontoxic, and no carcinogenic with excellent mechanical properties, and chemical resistance. Besides, PVA has a fair flexibility and swelling capacity. One defection of PVA is that it turns to be unstable in aqueous medium, so in many researches, grafting, crosslinking, and copolymerization have been done with other polymers like polyvinyl pyrrolidone (PVP), and sterculia [28]. Blending PVA, pluronic, and polyethyleneimine (PEI) can obtain a better stable nanofibers employed in the antibacterial wound dressing [29]. Silver or AgNO3 incorporated in nanofibers made by PVA has the ability of anti-bacteria [15]. A research by Zhao et al. [30] developed an electrospinning membrane employing PVA blending with a protein named pnsr16 to cure Sprague Dawley rat skin burns, the formation of granulosa tissue and the contraction of wound in test group are better than the negative groups.
Poly (ethylene oxide) (PEO) has been widely used as an ideal hydrophilic carrier for active ingredients in nanoscales [31], [32], [33], [34]. For reasons like water interaction and steric exclusion, the introduction of PEO has little interference on plates and plasma protein [35], [36]. This could provide a relatively favorable environment for the DFU healing. The addition of PEO to other materials like cyclodextrin and chitosan with poor electrospinning properties has been reported to be useful in the long and bead free fibers fabrication [37], [38]. Meanwhile, for fragile biomaterials incorporated in the core shell of coaxial nanofibers, mixing with aqueous PEO solution also performed well to stable them [39]. In another investigation, it can be seen obviously that with the ratio of PEO increased, better morphology of nanofibers was found under the SEM images [40]. Molecular chain orientation and crystallinity of PEO were believed to have some relationship with its excellent performance, furthermore, other underlying factors like constrained annealing and solvents also contributed to it [41].
2.2. Natural polymers
Chitosan is a biodegradable polysaccharide which can be obtained through chitin deacetylation. It has the same component glycosaminoglycans as in the ECM. Apart from the biocompatibility, biodegradability, and hemostasis ability, the activity of anti-bacteria and anti-fungi is the special property of chitosan [42]. This advantage does add assistance to the acceleration of DFU recovery. However, chitosan with poor mechanical properties limits its application in the DFU treatment, and crosslinking with other natural polymers like gelatin and collagen can improve the mechanical properties [43]. The composite of chitosan and dextran synthesized by Michael addition reaction also has the ability to move away from DFU without cellular adhesion compared with the traditional wound dressing materials [44]. It is a time-saving and cost-saving approach to utilize chitosan in ND fabrication. However, there are indeed some difficulties to fabricate uniform ultrafine nanofibers as the strong molecular interaction of chitosan [45]. In their research, blending strategy with PVA shows an ideal way to ease the use of chitosan. On the other hand, chitosan has the effect of loosening the tight junctions between cells [46]. It may be unbeneficial for the DFU wound closure, but drugs incorporated in the ND aiming at the deeper tissue need it.
Alginate is also a biodegradable polysaccharide extracted from seaweed. Similar to chitosan, it also has the glycosaminoglycan which is the major component of ECM. The properties of bleeding and exudate absorption make alginate an excellent wound dressing material. Nevertheless, similar to most biopolymers, electrospinning processibility of aqueous alginate is still a challenge. To solve this problem [47], utilizing a cosolvent glycerol added to the aqueous solution can disrupt the H-bonds between inter and intra-molecule, consequently, new H-bonds establish between glycerol and sodium alginate. Rheological characterization shows that the viscosity of the sodium alginate increases to a good level and the flexibility of the ND exhibits the same effect. In most cases, researchers prefer to use alginate with PEO [48], [49], PEO in the suitable molecular such as 2000 kDa can provide the entanglement effect for the alginate to be electrospun into nanofibers [49].
Collagen is a critical component which can be found in the ECM with the structure of fibrill and three-dimensional networks. As the biochemical activity of collagen, it is reported to stimulate the migration of the fibroblasts and modulate the metabolism of the granulation tissue [50]. It is beneficial for the cell attachment and proliferation during the DFU recovery. However, it is better to use other polymers like PCL together due to the instability of collagen under the high-voltage in the nanofibers fabrication process. Additionally, similar to PLGA, collagen cannot be electrospun without the use of toxic organic solvents and zein from natural sources co-electrospun with collagen can ease the fabrication process. Gelatin is a high-biodegradable polymer denaturalized from collagens with the identical functional groups as collagen. It is believed that gelatin also cannot be electrospun to nanofibers with aqueous solution. However, a research by Li et al. [51] aiming at finding the potential factors that influencing the electrospinning processibility of aqueous gelatin indicates that controlling the spinning solution temperature at which the gelling behavior is suppressed can increase the electrospinning processibility to get a desirable ND.
3. Methods for nanofiber fabrication
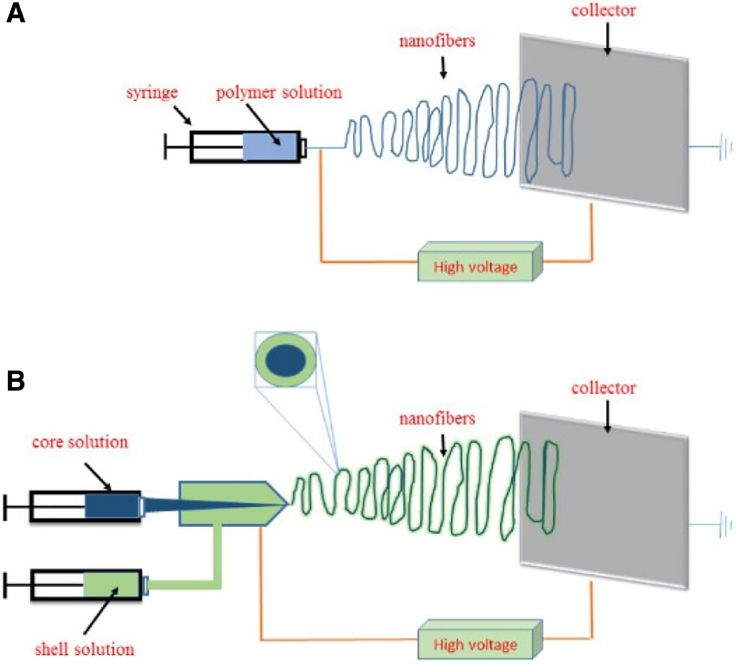
Currently, the setup for nanofibers fabrication is relatively simple consisting of several components, such as micro pump, syringe, spinneret, voltage supply, electrode metal collector and polymer solution [52]. Two cartons representing the typical facilities used for the fabrication of nanofibers are shown as in the below, Fig. 2A exhibits the apparatus used for uniaxial electrospinning and Fig. 2B the coaxial electrospinning. The material attributes and process parameters, for example, the drug to polymer ratio, the solution viscosity, and the solution feed rate are crucial to the final function of the nanofibrous products. Each way of drug incorporation using respective polymers and nozzles needs individual material attributes and process parameters for a desired drug release profile including immediate release and controlled release [53].
Fig. 2.
(A) Schematic apparatus used for the uniaxial electrospinning and (B) the coaxial electrospinning.
3.1. Uniaxial electrospinning
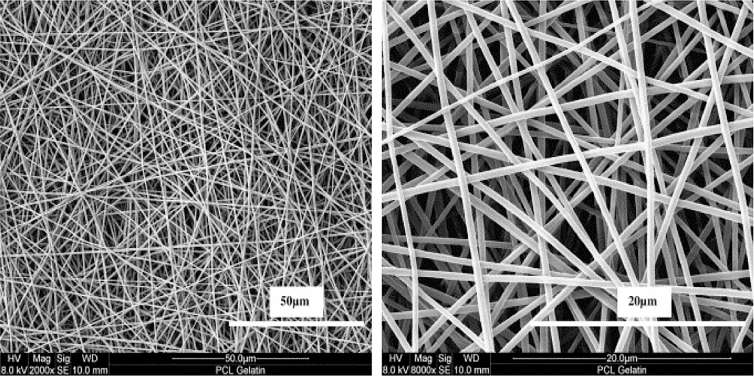
One of the most distinguished features of uniaxial electrospinning about the setup is the nozzle configuration. That is to say, it just has one nozzle layer contrast to the coaxial electrospinning setup with two concentric nozzle layers. The process of uniaxial electrospinning and the consideration on the parameters are relatively less than the coaxial electrospinning. Firstly, the polymer solution should be prepared with desired drug–polymer ratio to obtain an ideal viscosity or conductivity. Then, the solution can be extruded from the syringe tube with a suitable force on the syringe. The critical technique applied for this process is the Tylor cone which refers to a cone like extrusive solution instead of a traditional cylinder like solution under the voltage supply on the nozzle. The voltage is extremely high to even 27 kV [54]. As the reason why it becomes a cone like solution, a study [55] claims that the Tylor cone appears with the charge repulsion force exceeding the surface tension of the solution so that a desired nanofibers with extremely thin, elongated features can be observed. Following the solution going forward with the predefined rate, solvents will be volatilized or evaporated immediately and the nanofibers appears on the metal collector that is stationary as a foil or rotating as a column. In the end, a ND with thousands of nanofibers can be seen. Typical features of uniaxial nanofibers taken by Field-emission Electron Microscope shows in Fig. 3 [56].
Fig. 3.
Field-emission electron micrographs of uniaxial nanofibers made by PCL-gelatin at (A) 50 μm and (B) 20 μm. Reproduced with permission from [56]. Copyright 2007, Elsevier.
Actually, much attention has been paid to the approach of drug loading. As we know, the interaction between two substances can be chemical or physical. In the same way, the active ingredients also can be loaded chemically or physically in the polymer matrix. A nanofibrous drug delivery system contains therapeutic agents of ciprofloxacin conjugated to the polymer matrix of PLA with diameters ranging from 150 nm to 400 nm and pore size from 62 to 102 nm are developed, the release study shows that the release kinetic can be modulated through drug content changing [54]. This is an excellent example of the chemical link between drug and polymer matrix used in nanofibers. The other way to load drugs is physical incorporation, simple mixture between drug and polymer is an ideal choice. Zhang et al. [57] applied the electrospinning technology to fabricate the vancomycin ND that is collected on the aluminum foil. Consequently, the in vivo and in vitro studies indicate a satisfactory antibacterial property. Following the careful selection of the drug loading method, considerations of variables on the uniaxial nanofibrous properties come to the next. Firstly, it is necessary to pay attention to the various forces underlying the formation of nanofibers. The forces applied in the process come from gravity, pump driving, surface strength, and electrical field. It is also obvious that the pump driving and electrical field are the positive factors that can be helpful to the fibrous formation and elongation. The electrical field induced by voltage supply is the dominant factor for a stable and continuous fibrous formation [58], [59]. The primary features of nanofibers including fiber size distribution, morphology, porosity, and drug release profile are the crucial elements influencing the nanofibrous function. With respect to the fiber size and size distribution, the feed rate can be one of the main factors that affect them. The fibrous diameter ranging from nanometers to some millimeters is positively proportional to the solution deliver rate under a rational electrical field. However, the charge strength from the electrical field also has a huge influence on the fibrous diameter; the fibrous diameter will be thinner as the charge strength improves with a medium solution deliver rate. There is a mass balance underlying the combined action between the deliver rate of the jet solution to the nozzle and the rate at which the solution is forced to be elongated by the electric field [60]. Apart from the forces burdened on the solution, the characters of the solution itself also determine the nanofibrous diameter and the diameter distribution. Especially, the conductivity of it is just the thing; the solution with high-conductivity will lead to wide size distribution [61]. When it comes to nanofibrous morphology, the bead structure is the common phenomenon produced by inconsistent forces between the syringe pump and the electrical field. For example, the beaded structure can be observed with the deliver rate by the syringe pump extremely surpassing the rate at which the solution is forced to be elongated by the electric field. Additionally, the excessively poor conductivity of the solution and the relatively high-electrical field can be another unfavorable factor to form the homogenous nanofibers, and the charge prefers accumulating on the surface of the jet wire and migrate on the collector with short distance between nozzle and collector to form the bead structure. Porosity is another property that plays a key role in the drug release profile and exudation absorption. The porosity can be increased when the so called thermally induced phase separation grows up. It refers to a process that the non-solvent rich phase separates from the non-solvent poor phase. The site of the pore is located at the non-solvent poor phase [62]. Humidity is also another element that influences the forming of porosity in the principle of vapor-induced separation or breathe figures with non-water solvent evaporation. The drug release kinetics is not only influenced by the primary properties discussed above but also the interaction between the drug and the polymer matrix. In a study, caffeine is loaded into the polymer PCL and the drug crystallization induced by the interaction between them has caused the decreased release compared with the non-crystallization group [63]. Another potential factor can be the ionization state of incorporated drugs; high-ionic strength of the drug will lead the drug to locate to the surface of the fibers [53]. What is more, surface location behavior can also result in burst release in some cases.
3.2. Coaxial electrospinning
As previous discussion, the typical structure of coaxial electrospinning nozzle comprises two concentric and separate spinneret to form a core–shell architecture nanofibers. Two different jet solutions will be prepared and each is transported to the relevant spinneret layers forming the core or shell layer, respectively. The process of this technology is basically similar to the uniaxial electrospinning. The images of a coaxial nanofibers taken by transmission electron microscope (TEM) shown in Fig. 4 [64].
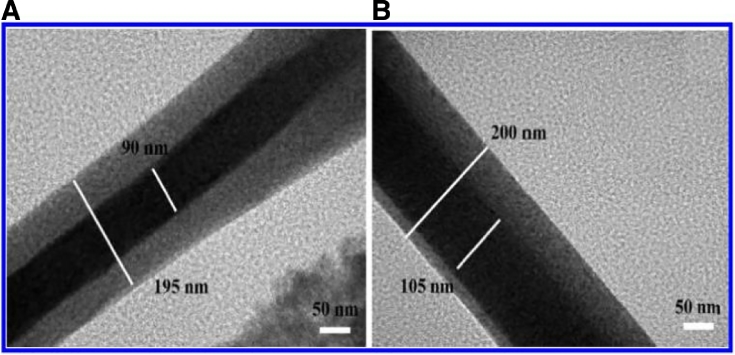
Fig. 4.
TEM images of coaxial nanofibers made by PEO and chitosan. (A) Concentric and (B) eccentric core and shell structures (Reproduced with permission from [64].). Copyright 2012, American Chemical Society.
No matter how the drug is loaded into the nanofibers, the fundamental principle of this way is equal to uniaxial electrospinning: physical and chemical linkage. Even though drugs applied in this method can be dissolved or suspended in the core or shell polymer solution, most nanofibers prefer putting the active ingredient in the inner layer to keep it from negative factors such as moisture and oxygen. A study [59] has fabricated nanofibers containing fish oil and zine in the core layer, the shell layer made by PVP is the protectant. A further investigation on the oxidative stability compared with uniaxial nanofibers has shown that coaxial method was an efficient encapsulation way to protect the active agent fish oil against oxidation. Another benefit is controlling the release characters of it as immediate release model or sustained release model. Han et al. [65] develop a stimuli-responsive ND with coaxial electrospinning technology, out of the core layer which comprises encapsulated active molecules is the smart shell layer. It consists of the stimuli-responsive polymers, and once the shell layer recognizes the stimulating signals, the depolymerization of the polymers occurs with the active molecules released from the core layer. The further study on the triggered release shows there is no release action without the stimulating signals. However, once the signal is provided, the encapsulated active agents are released swiftly. Additionally, no matter whether there exists drug or not in the nanofibers, one layer of the two can be a functional structure that assists the other [66]. For instance, the electrospinnable polymer solution used in one layer can be fabricated as a spinning aid for the inelectrospinnable in the other layer. In the same way, having a clear idea of the effect of variables can be beneficial to obtain a desirable coaxial nanofibers. In fact, the variables discussed here are partially similar to the uniaxial. For example, the effect of jet rate, electrical field strength, solution conductivity and concentration on the diameter, diameter distribution, and morphology is parallel. However, the integrity with the interaction between the two jet parts can be one of the greatest challenges to form a homogeneous nanofibers. Sometimes, the two layers will separate from each other and the integrity disappears. To some extent [66], the interfacial strength plays a key role in the affinity of the two layers. So it is recommended to dissolve or suspend the polymer with similar or even the same solvent and prepare a harmony concentration to obtain a relatively low-interfacial strength. The interfacial strength refers to the compatibility among the ingredients including the active agents, polymers, and solvents. The secondary bonds such as electrostatic and hydrophobic interactions have the improving effect on compatibilities of the components used for nanofibrous fabrication [59].
4. Characterization of nanofibers
Various analytical instruments with different mechanisms have applied for the characterization of the nanofibers. Optical microscope is a favorable choice for the primary examination on the production with high-resolution. First impression with optical microscope to observe the outline of the product is necessary, that is to say, beads and non-fibers can be checked. Another common measure technique is the SEM. To have a better knowledge of the drug distribution, DSC usually provides a method with the peak shift and the endothermic or exothermic fluctuation [19]. Another important characterization item of nanofibers is water contact angles, as for the cell proliferation during the DFU occlusion needs hydrophilic matric to support it. When drugs are incorporated in the nanofibers, their existence, morphology, especially crystallinity, and distribution can also be described through Fourier transform infrared spectroscopy (FTIR) [16], and X-ray photoelectron spectroscope [17]. The final functional test model of the nanofibers includes cell model and animal model. The cell model is relatively simple and inexpensive. In the cell model, cells such as human dermal fibroblasts or mouse fibroblasts (L929) are cultured with the nanofibers for several days, then the cytotoxicity, cell attachment, and cell proliferation are investigated through a cell counting machine. The animal model utilizes rabbit or mouse to directly evaluate the wound healing efficiency of ND on DFU. After the anesthetization, a specific number of simulated DFU wound with designed dimension are created on the local skin of the animals under the sterile conditions. To supply a better wound bed for the functional exhibition of the ND, the epidermis, dermis, and perichondrium should be removed. The data about the closure effect collected from them can be analyzed with the statistical approach.
5. Application of ND for DUF
5.1. Benefits of ND for DFU wound healing
Prior to discussing the nanofibrous application, it is necessary to have a deep insight into the process of DFU wound healing. Normal skin comprises epidermis, dermis, and the subcutaneous tissue from the out layer to the deep layer. Keratinocytes located in the epidermis and fibroblasts embedded in the dermis, they are both incorporated with the three-dimensional matrix consisting of fibers such as collagen, fibrinogen and elastin. ECM is just the three-dimemsional matrix. Obviously, vein and lymph distribute among them offering required nutrition and immune activity. One of the most important roles of the skin is providing a natural barrier preventing the harmful damage like mechanical impacts and microbial infection. Considering the DFU wound with chronic recovery, it needs more realization about the mechanism of wound healing. Furthermore, reforming on the wound dressing consistent with the mechanism is imperative. As we know, once the DFU injury develops into the dermis or even the subcutaneous tissue, many procedures may occur in the wound recovery. In the inflammatory phase, there are some specific biological changes as the fluid leaks from blood or lymph, especially with the aggregation of platelets and the next clots by it. Keratinocytes may release GF or cytokines under the stimulating signals from platelets. Then, immune cells like macrophages migrate to the wound bed to fight against the germs and fungus, simultaneously, new blood and lymph vessels are reconstructed. The next phase is proliferation. Re-epithelialization occurs flowing the proliferation of keratinocytes and myofibroblasts that are critical for the generation of granulation tissue and ECM. In the final maturation phase, the rearrangement of collagen and the form of elastin enhance the normal properties of the renewal skin. Unfortunately, studies have indicated that severe disorders of the healing phase usually happen in DFU patients [67]. Obviously, the healing of the DFU wound is so complex that the applied wound dressing with high-performance capacities is evitable. Firstly, hemostasis of the wound dressing must be helpful for the clot formation [18], ND with marvelous specific surface area just fits this requirement, and the exudate absorption capacity is also excellent. The oxygen exchange ability is also needed for the blood vessel renewal and cell proliferation, in addition, abundant oxygen has the effect of decreasing the risk to infection [6]. As discussed previously, ND is the perfect candidate for its huge porosity to oxygen penetration. Secondly, providing a suitable stage for the renewal cells like fibroblasts and keratinocytes to adhere and spread may sharply increase the DFU wound occlusion, fortunately, ND has been proven to enhance the cells growth as a good stage [8]. Effect of ND for diabetic wound closure shows in Fig. 5 as below [68]. Another capability of substitutability of antimicrobial agents is also profitable for the performance of the wound dressing, using chitosan is advisable for its activity of inhibiting the growth of fungi and bacteria [69]. In addition, superior active pharmaceutical ingredient (API) entrapment of wound dressing can ease the modification of the release kinetic, for example, the sustained release and the controlled release, as in the coaxial electrospinning chapter, nanofibers with a controlled out layer may realize these release profile.
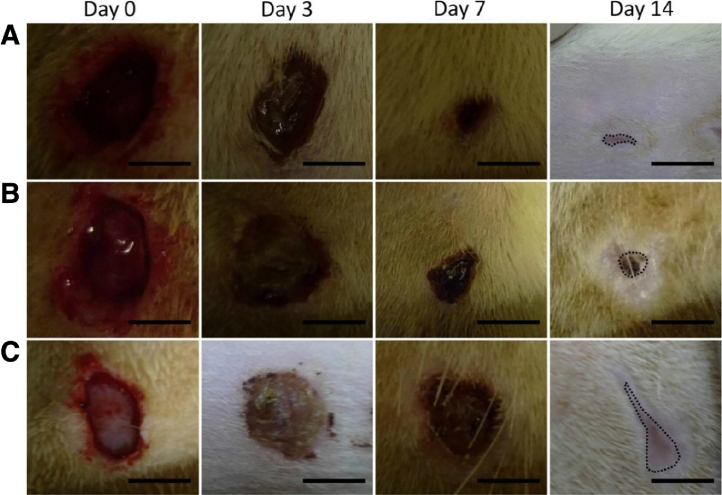
Fig. 5.
Photographs of the wound closure of diabetic rats on days 0, 3, 7, 14, (A) collagen/PLGA with glucophage-loaded group, (B) pure collagen/PLGA group, (C) gauze sponge group (Reproduced with permission from [68].). Copyright 2015, Elsevier.
5.2. Active agents in the nanofibrous dressing for diabetic foot ulcer
5.2.1. Small molecules
Wound infection is a common progressive spreading symptom. With the dysfunction of glucose metabolism in DFU patients, immunological response is disrupted so that the DFU wound becomes more and more susceptible to infection [70]. In most conditions, infection deterioration may lead to amputation. Compared with the wound in non-diabetic Yorkshire pigs, the diabetic Yorkshire pigs show wound healing delay and secondary infection by endogenous bacteria [71]. Corynebacterium spp is the dominant bacterial genus associated with DFU [72]. Other bacterial communities including Streptococcus, Serratia, Staphylococcus and Enterococcus spp are also responsible for the DFU infection [71], [73]. In 2004, IDSA (the Infectious Diseases Society of America) has pointed out that the infection should be identified by the biological findings instead of the clinical criteria avoiding antibiotic abuse [74]. Once the severity of the infection and the types of the bacterial are identified, corresponding antibiotics can be administrated in the ND forms for DFU treatment assisting the systemic administration. For example, fluoroquinolones such as ciprofloxacin, levofloxacin and clindamycin are recommended in non-severe or severe phase of DFU infection [75]. Several antibiotics have been successfully developed into ND forms with proper synthetic or natural polymers. Gentamycin as an anti-Staphylococcus is entrapped in the Eudragit RS/RL 100 to fabricate ND [76]. SEM micrograph indicate that smooth surface without bead and excellent size distribution presents the ND feasibility of gentamycin. Further thermal analysis with DSC and TGA validates the increased thermal stability of gentamycin loaded in the polymers. In vivo study demonstrates that the fabricated ND has the bioactivity of inhibiting the bacterial growth. Another practical antibiotic incorporated in ND for DFU is tetracycline hydrochloride (TCH) which possesses broad spectrum [77]. To obtain a sustained release profile, attempt of emulsion electrospinning has been made. With respect to the emulsion, TCH dissolved in water as water phase and diblock polymer PEG-PLA dissolved in chloroform as oil phase. API with fluorescent substance incorporated in the core of the nanofibers can be seen from the image taken by confocal microscope (CM). Under this bilayer structure, the bioactivity of the TCH and the sustained release character has been observed in the antibacterial test. Additionally, there are also many other antibiotics under the way to be developed into ND. Ciprofloxacin is an efficient antibiotics with the potential to be loaded in nanofibers, and it has been successfully applied in the cure of S. Aureus infection [78].
In the inflammatory phase of the DFU recovery, side effects induced by free radical, cell debris, and cytotoxic enzyme can disturb and prolong the recovery span [79]. As discussed above, a series of signal cascades and large number of cells including platelets, neutrophils, keratinocytes and macrophages participate in the DUF recovery. Despite of the unclear mechanism of anti-inflammatory agents, some chemical entities have been proven to be efficient as an API used in the ND for DFU. Bixin, a pigment extracted form Bixa Orellana L. Seed, shows a great bioactivity of anti-inflammatory, anti-oxidant, and healing properties [80]. In a study [79], under the uniaxial electrospinning technology, bixin with PCL is fabricated into an ideal ND. Apart from the routinely physical detection, cell proliferation assay and in vivo study indicates that the Bixin-loaded PCL nanofibers decrease the time of wound closure more than virgin PCL nanofibers. Another natural compound curcumin derived from turmeric has lots of biological activities, for example, anti-tumor, anti-oxidant, and anti-inflammatory has been proven [81], [82], [83]. Allowing for the poor solubility and low-bioavailability of curcumin, researchers select the PCL as a matrix for the sustained release ND fabrication. To elevate the anti-inflammatory effect, IL-6 (interleukin-6), a representative member of inflammatory factor released from monocyte-macrophages, is quantified. In the anti-inflammatory experiment, the inflammatory-induced J774A1 cells with curcumin-PCL nanofibers express low IL-6. Consequent diabetic mouse wound closure examination exhibits decreased time for diabetic wound closure to be done. Another anti-inflammatory agent is quercetin. It is a flavonoid that has been shown anti-oxidative and anti-inflammatory activities [84], [85]. Additional cell adhesion, angiogenesis also has been reported [86].Incorporated quercetin by zein following the uniaxial electrospinning technique shows great benefits for neuropathy in diabetic wound. Researchers [87] prepare the quercetin spinning solutions in a gradient concentration to determine the best quercetin zein ratio, a set of fabrication parameters like 16 kV voltage supply, 0.2 ml/h feed rate, 15 cm collection distance are investigated. Diabetic rats are divided into several groups according to the gradient quercetin concentration. Morphology analysis demonstrates that the increased ratio of quercetin to zein has no significant influence on the diameters. Good API distribution and potential hydrogen bonding between drug and vehicle are detected by FTIR and XRD (X-ray diffraction). The detection of quercetin release is conducted in the acetate buffer and shows an increased rate from low to high-concentration of API. A further investigation on the motor function of the sciatic nerve reveals the favorable effect of quercetin for neuropathy in diabetic wound. Additionally, quercetin is also treated as an anti-carcinogenic chemical [88], no matter how broad application it possesses, the fundamental mechanism for DFU treatment may be relevant to its potent antioxidant and ant-inflammatory capacities [89], [90].
Metformin is a common first-line clinical drug for hyperglycemia, developed with PLGA under the uniaxial electrospinning technology metformin suggests a massive potential in the application of DFU treatment [91], [92]. Metformin is a hydrophilic agent, in order to get the aim of prolonged release character, hydrophobic and biodegradable PLGA is the very candidate to be tested. Simultaneously, the addition of metformin to PLGA solution decreases the viscosity of the solution and increases the feasibility of nanofibers fabrication. The release profile analysis indicates that first outburst of metformin may be relevant to the API located on the surface of nanofibers, the sustained release following the slowly degradation of PLGA [91]. Study has confirmed that metformin not only regulates the hepatic glucose output, but also improves the insulin sensitivity [93], [94]. In fact, metformin released from nanofibers just acts as a topical drug delivery system with limited absorption to systemic body. The explanations for the effectiveness of the metformin on wound include enhanced endothelial precursor cells circulating, improved angiogenesis, and balanced collagen from the inhibiting MMP (matrix metalloproteinases) [68], [95].
As discussed in the DFU recovery phase, normal vessel reconstruction and refunction are critical for the wound closure. Angiogenesis is one strategy in dealing with this issue, relevant biological agents intervened in practical are hypoxia-inducible factor 1α (HIF-α), neuropeptide Y (NPY), and angiopoietin-1 [96], [97], [98]. In the field of ND for DFU treatment, dimethyloxalylglycine (DMOG)-embedded PCL ND has proved to be feasible for the diabetic wound treatment [99]. With the bioactivity of inhibiting oxygen-dependent degradation of HIF-α, DMOG exhibits the angiogenesis function in wound repairment [100]. In the study present by Zhang et al. [99], DMOG and PCL are dissolved in the dichloromethane (DM) and dimethylformamide (DMF), the final ratio of DMOG to PCL in nanofibers is controlled in 0.1 mmol/g. The electric potential and collector distance are about 8 kV and 9 cm respectively. After the uniaxial electrospinning, the ND is cut into equal dimension of square. Organic solvent evaporation is completed by vacuum chamber and air dryer. Fiber diameter and surface roughness are evaluated by SEM and CM, respectively. The diameter is about 300 nm and the surface roughness 2.13 μm; moreover, there is no significant difference between virgin PCL and drug-loaded PCL. Homogeneous API distribution in PCL also has been demonstrated by fluorescent probe molecule Rhodamine 6 G. Compared with the controls or the virgin PCL groups, DMOG-loaded PCL ND shows accelerated wound closure. Increased CD-31 (cluster-differentiation 31) positive cells observed in the immunostained analysis indicates that angiogenesis is significant statistically. Another promising “drug” applied in ND for the DFU recovery is the silicate-based bioceramics (NAGEL, Ca7Si2P2O16), the original intention of NAGEL application is to be an alternative to metallic materials for its higher biocompatibility [101]. Currently, NAGEL has been intensively used in the tissue engineering to promote cell attachment, growth and new tissue formation [102].With respect to wound healing effect, relevant report also can be seen [12], in view of modulating the degradation time of the polymers applied in nanofibers, PCL blending with gelatin is prepared to vehicle solution. Coaxial electrospinning technology is utilized with NAGEL embedded in the PCL/gelatin tube. A gradient ratio of NAGEL to PCL/gelatin is designed to elevate various physical and biological index, other parameters such as feed rate, voltage supply, and collector distance are set to be 0.025 ml/min, 10 kV, and 12 cm. The crosslink for the primary nanofibers is completed by glutaraldehyde/ethyl alcohol disposition. The proliferation test of the HUVECS (human umbilical vein endothelial cells) seeded on the ND with different NAGEL concentration reveals NAGEL's no apoptosis. But the cell adhesion analysis shows that drug-loaded nanofibers is more effective than the pure nanofibers. Direct quantification and assessment of the number of neovascular in the wound bed is conducted by the digital camera photos, images taken from it confirm that NAGEL does have the capacity to improve the vessel especially the capillary formation compared with the control and virgin group. The higher level of CD31 is also a specific proof.
5.2.2. Macromolecules
Potential macromolecules for DFU healing mainly refer to cytokine or GF. It consists of diverse groups of polypeptides and most groups have their own different family members [103]. Platelet-derived growth factor (PDGF) [104], basic fibroblast growth factors (BFGF), vascular endothelial growth factors (VEGF) and epidermal growth factors (EGF) are relevant GF [105], [106]. PDGF was firstly identified as a disulfide-linked dimer of two peptides [107]; it is beneficial for the growth of specific cells [108], the formation of blood vessel, and the early hematopoiesis. GF deficiency in DFU wound bed is one of the most inducers for the prolonged wound healing [109], recombinant human PDGF (RHPDGF-BB) has been successfully developed to ND for the initial phase of diabetic wound treatment [110], PLGA as a biodegradable polymer is selected to load the active RHPDGF-BB, 17 kV voltage power and 12 cm collector distance are set for the fabrication attributes. The concentration of the two materials is 0.28 g/ml and 0.005 g/ml respectively in the solvent hexafluoroisopropyl alcohol. SEM images of the final ND show that the drug-loaded PLGA has a thinner fibers than the virgin PLGA. Even though the tensile strength of the drug-loaded ND is larger than the pure, the result of the elongation at break is just the opposite. Contact angles of water indicate that the addition of RHPDGF-BB is helpful for the increased hydrophilicity of the PLGA. Consequent wound repair and histology test also demonstrate that RHPDGF-BB possesses the features of accelerating the diabetic wound healing and enhancing the density of stratum corneum. These results suggest a substantial potential of PDGF for the DFU treatment. Other GF like recombinant human EGF (RHEGF) has been used for the DFU with the form of intralesional administration, the clinical profile of this method exhibits enormous benefits [111], in the same way, RHEGF may be a proper candidate loaded in ND for the DFU recovery.
5.2.3. Stem cells
Considering the full-thickness wound, just as the deterioration of DFU, conventional strategies discussed above may not be efficient [112]. As an advanced wound treatment modality, stem cells therapy has become more and more prevalent in dealing with the complicated wound [113]. Common stem cells are usually divided into embryonic and adult stem cells. Various resources from bone marrow, peripheral blood, and hair follicles contain the adult stem cells. With the ability of prolonging self-renewal and differentiation into targeted tissues like skin, stem cells has been broadly investigated for the chronic skin wound treatment [114], [115], [116], [117]. As for the mechanism of the adult stem cells applied for the skin wound recovery, two aspects are reported, one is repopulating the lost or injured tissue with the differentiated cells and the other is recruiting inflammatory cells or tissue progenitor cells through signal molecule release [118]. In order to obtain the selection of endothelial precursor cells (EPCs) enrichment and the efficiency of EPCs delivery. Mononuclear cells derived from murine bone marrow are seeded into the nanofibers made of PCL/gelatin [119]. Regular physical parameters of nanofibers are determined by SEM and TEM; mononuclear cells are extracted from the femurs of the mice, and primary cellular suspension is centrifuged and washed to obtain the pure mononuclear cells. After seeded in the PCL/gelatin matrix, the cell starts to differentiate to EPCs incubated in specific conditions, and the characterization of EPCs shows that PCL/gelatin matrix is a suitable stage for EPCs growth. Further EPCs adhesion, colony formation, and proliferation are investigated, and the results indicate the positive effect of PCL/gelatin matrix for EPCs. In vivo test on diabetic mice wound reveals that the EPCs-loaded PCL/gelatin not only accelerates the wound closure but also improves the quality. Despite of unspecific application for DFU, other cells like mesenchymal stem cells [120], [121] and epidermal stem cells [122], [123], [124] are broadly utilized for full-thickness wound treatment seeded in proper nanofibrous matrix.
6. Further perspectives
DFU imposes risks of amputation and economic lost on diabetic patients. Wound dressing is necessary in protecting and accelerating the wound closure. ND is an advanced technique with characters of thin diameters ranging from nanometers to micrometers, consequent capacities of oxygen penetration and ECM reconstruction are obtained. Furthermore, utilization of various polymers from natural and synthetic sources renders ND a better selection for diabetic wound closure compared with conventional dressing types. Additionally, bioactive small molecules, macromolecules, and cells can be loaded in the nanofibers to optimize the DFU recovery process. Proper selection of polymers and process parameters will fabricate desirable ND for DFU treatment. However, the rheological behavior that influences the physicochemical properties of nanofibers and the mathematical model for it in pharmaceutical cycle still has a big room for researchers to study and establish.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (No. 81600353) and the Career Development Program for Young Teachers in Shenyang Pharmaceutical University.
Reference
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. Idf diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Majd SA, Khorasgani MR, Moshtaghian SJ, Talebi A, Khezri M. Application of chitosan/pva nano fiber as a potential wound dressing for streptozotocin-induced diabetic rats. Int J Biol Macromol. 2016;92:1162–1168. doi: 10.1016/j.ijbiomac.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Veves A, Falanga V, Armstrong DG, Sabolinski ML, . The Apligraf Diabetic Foot Ulcer Study Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 4.Prompers L, Schaper N, Apelqvist J. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. Eurodiale Study Diabetol. 2008;51(5):747–755. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study Group The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers – results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–1705. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 6.Hanna JR, Giacopelli JA. A review of wound healing and wound dressing products. J Foot Ankle Surge. 1997;36(1):2–79. doi: 10.1016/s1067-2516(97)80003-8. Official Publication Of The American College of Foot and Ankle Surgeons. [DOI] [PubMed] [Google Scholar]
- 7.Xu SC, Qin CC, Yu M. A battery-operated portable handheld electrospinning apparatus. Nanoscale. 2015;7(29):12351–12355. doi: 10.1039/c5nr02922h. [DOI] [PubMed] [Google Scholar]
- 8.Babitha S, Rachita L, Karthikeyan K. Electrospun protein nanofibers in healthcare: a review. Int J Pharm. 2017;523(1):52–90. doi: 10.1016/j.ijpharm.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Zeng H, Cui J, Cao B. Electrochemical deposition of ZNO nanowire arrays: organization, doping, and properties. Sci Adv Mater. 2010;2(3):336–358. [Google Scholar]
- 10.Zhang C, Miao X, Chabak K D, Li X. A review of iii-v planar nanowire arrays: selective lateral vls epitaxy and 3d transistors. J Phys D Appl Phys. 2017;50(39) [Google Scholar]
- 11.Blakney AK, Ball C, Krogstad E A, Woodrow K A. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013;100:S9–S16. doi: 10.1016/j.antiviral.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Lv F, Wang J, Xu P. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017;60:128–143. doi: 10.1016/j.actbio.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Kim HN, Hong Y, Kim MS, Kim SM, Suh K-Y. Effect of orientation and density of nanotopography in dermal wound healing. Biomaterials. 2012;33(34):8782–8792. doi: 10.1016/j.biomaterials.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Chen SX, Liu B, Carlson MA. Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017;12(11):1335–1352. doi: 10.2217/nnm-2017-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma R, Singh H, Joshi M. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carr Syst. 2014;31(3):187–217. doi: 10.1615/critrevtherdrugcarriersyst.2014008193. [DOI] [PubMed] [Google Scholar]
- 16.Sofokleous P, Stride E, Edirisinghe M. Preparation, characterization, and release of amoxicillin from electrospun fibrous wound dressing patches. Pharm Res. 2013;30(7):1926–1938. doi: 10.1007/s11095-013-1035-2. [DOI] [PubMed] [Google Scholar]
- 17.Sun XM, Cheng LY, Zhao JW. Bfgf-grafted electrospun fibrous scaffolds via poly(dopamine) for skin wound healing. J Mater Chem B. 2014;2(23):3636–3645. doi: 10.1039/c3tb21814g. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Mater Sci Eng C Mater Biol Appl. 2017;76:1413–1423. doi: 10.1016/j.msec.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YS, Yang DZ, Chen XM. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9(1):349–354. doi: 10.1021/bm7009015. [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Chao YK, Chang SH. Nanofibrous rhpdgf-eluting plga-collagen hybrid scaffolds enhance healing of diabetic wounds. RSC Adv. 2016;6(8):6276–6284. [Google Scholar]
- 21.Wang J, Windbergs M. Functional electrospun fibers for the treatment of human skin wounds. Eur J Pharm Biopharm. 2017;119:283–299. doi: 10.1016/j.ejpb.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Lv J, Yin X, Zeng Q. Preparation of carboxymethyl chitosan nanofibers through electrospinning the ball-milled nanopowders with poly (lactic acid) and the blood compatibility of the electrospun NCMC/PLA mats. J Polym Res. 2017;24(4) [Google Scholar]
- 23.Chen Y, Lin J, Wan Y. Preparation and blood compatibility of electrospun PLA/curcumin composite membranes. Fibers Polym. 2012;13(10):1254–1258. [Google Scholar]
- 24.Jang SI, Mok JY, Jeon IH. Effect of electrospun non-woven mats of dibutyryl chitin/poly(lactic acid) blends on wound healing in hairless mice. Molecules. 2012;17(3):2992–3007. doi: 10.3390/molecules17032992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bui HT, Chung OH, Dela Cruz J, Park JS. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res. 2014;22(12):1288–1296. [Google Scholar]
- 26.Fee T, Surianarayanan S, Downs C, Zhou Y, Berry J. Nanofiber alignment regulates NIH3T3 cell orientation and cytoskeletal gene expression on electrospun pcl plus gelatin nanofibers. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Zhao H, Turng LS, Li Q. Crystalline morphology of electrospun poly(epsilon-caprolactone) (PCL) nanofibers. Ind Eng Chem Res. 2013;52(13):4939–4949. [Google Scholar]
- 28.Jannesari M, Varshosaz J, Morshed M, Zamani M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomed. 2011;6:11. doi: 10.2147/IJN.S17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Aassar MR, El Fawal GF, El-Deeb NM, Hassan HS, Mo XM. Electrospun polyvinyl alcohol/ pluronic F127 blended nanofibers containing titanium dioxide for antibacterial wound dressing. Appl Biochem Biotechnol. 2016;178(8):1488–1502. doi: 10.1007/s12010-015-1962-y. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L, Chen DL, Yao QH, Li M. Studies on the use of recombinant spider silk protein/polyvinyl alcohol electrospinning membrane as wound dressing. Int. J. Nanomed. 2017;12:8103–8114. doi: 10.2147/IJN.S47256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon KW, Kang SW. Highly permeable and selective CO2 separation membrane to utilize 5-hydroxyisophthalic acid in poly(ethylene oxide) matrix. Chem Eng J. 2018;334:1749–1753. [Google Scholar]
- 32.Pencheva V, Margaritova E, Borinarova M. A novel approach for fabricating nanocomposite materials by embedding stabilized core-shell micelles into polysaccharide cryogel matrix. Carbohydr Polym. 2018;183:165–172. doi: 10.1016/j.carbpol.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Kuang G, Zhang Z, Liu S. Biphasic drug release from electrospun polyblend nanofibers for optimized local cancer treatment. Biomater Sci. 2018;6(2):324–331. doi: 10.1039/c7bm01018d. [DOI] [PubMed] [Google Scholar]
- 34.Asim S, Wasim M, Sabir A. The effect of nanocrystalline cellulose/gum arabic conjugates in crosslinked membrane for antibacterial, chlorine resistance and boron removal performance. J Hazard Mater. 2018;343:68–77. doi: 10.1016/j.jhazmat.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Tan J, Mcclung WG, Brash JL. Nonfouling biomaterials based on polyethylene oxide-containing amphiphilic triblock copolymers as surface modifying additives: Protein adsorption on peo-copolymer/polyurethane blends. J. Biomed. Mater. Res. Part A. 2008;85A(4):873–880. doi: 10.1002/jbm.a.31554. [DOI] [PubMed] [Google Scholar]
- 36.Yoo HJ, Kim HD. Properties of crosslinked blends of pellethene and multiblock polyurethane containing poly(ethylene oxide) for biomaterials. J. Appl. Polym. Sci. 2004;91(4):2348–2357. [Google Scholar]
- 37.Celebioglu A, Uyar T. Cyclodextrin short-nanofibers using sacrificial electrospun polymeric matrix for VOC removal. J Incl Phenom Macrocycl Chem. 2018;90(1-2):135–141. [Google Scholar]
- 38.Dresvyanina E, Yudenko A, Lebedeva I. Comparison of electrospinning and wet-spinning methods for the production of chitosan-based composite fibers. Mater Tehnol. 2018;52(1):39–42. [Google Scholar]
- 39.Gong T, Liu T, Zhang L. Design redox-sensitive drug-loaded nanofibers for bone reconstruction. ACS Biomater. Sci. Eng. 2018;4(1):240–247. doi: 10.1021/acsbiomaterials.7b00827. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Soin N, Prashanthi K. Emulsion electrospinning of polytetrafluoroethylene (PTFE) nanofibrous membranes for high-performance triboelectric nanogenerators. ACS Appl Mater Interfaces. 2018;10(6):5880–5891. doi: 10.1021/acsami.7b18442. [DOI] [PubMed] [Google Scholar]
- 41.Song Z, Chiang SW, Chu X. Effects of solvent on structures and properties of electrospun poly(ethylene oxide) nanofibers. J Appl Polym Sci. 2018;135(5) [Google Scholar]
- 42.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2013;11(8):866. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu P, Kumar UN, Manjubala I. Wound healing materials - a perspective for skin tissue engineering. Curr Sci. 2017;112(12):2392–2404. [Google Scholar]
- 44.Tang Y, Cai X, Xiang Y. Cross-linked antifouling polysaccharide hydrogel coating as extracellular matrix mimics for wound healing. J Mater Chem B. 2017;5(16):2989–2999. doi: 10.1039/c6tb03222b. [DOI] [PubMed] [Google Scholar]
- 45.Zhang YY, Huang XB, Duan B. Preparation of electrospun chitosan/poly(vinyl alcohol) membranes. Colloid Polym Sci. 2007;285(8):855–863. [Google Scholar]
- 46.Jean M, Smaoui F, Lavertu M. Chitosan-plasmid nanoparticle formulations for IM and SC delivery of recombinant FGF-2 and PDGF-BB or generation of antibodies. Gene Ther. 2009;16(9):1097–1110. doi: 10.1038/gt.2009.60. [DOI] [PubMed] [Google Scholar]
- 47.Nie HR, He A H, Zheng JF. Effects of chain conformation and entanglement on the electrospinning of pure alginate. Biomacromolecules. 2008;9(5):1362–1365. doi: 10.1021/bm701349j. [DOI] [PubMed] [Google Scholar]
- 48.Kaassis AYA, Young N, Sano N. Pulsatile drug release from electrospun poly(ethylene oxide)-sodium alginate blend nanofibres. J Mater Chem B. 2014;2(10):1400–1407. doi: 10.1039/c3tb21605e. [DOI] [PubMed] [Google Scholar]
- 49.Saquing CD, Tang C, Monian B. Alginate-polyethylene oxide blend nanofibers and the role of the carrier polymer in electrospinning. Ind Eng Chem Res. 2013;52(26):8692–8704. [Google Scholar]
- 50.Veves A, Sheehan P, Pham HT, Promogran Diabetic Foot Ulcer S. A randomized, controlled trial of promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137(7):822–827. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 51.Li JX, He A H, Zheng J F, Han C C. Gelatin and gelatin-hyaluronic acid nanofibrous membranes produced by electrospinning of their aqueous solutions. Biomacromolecules. 2006;7(7):2243–2247. doi: 10.1021/bm0603342. [DOI] [PubMed] [Google Scholar]
- 52.Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomed. 2013;8:2997–3017. doi: 10.2147/IJN.S43575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chou SF, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Control Release. 2015;220:584–591. doi: 10.1016/j.jconrel.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parwe SP, Chaudhari PN, Mohite KK. Synthesis of ciprofloxacin-conjugated poly(l-lactic acid) polymer for nanofiber fabrication and antibacterial evaluation. Int. J. Nanomed. 2014;9:1463–1477. doi: 10.2147/IJN.S54971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release. 2016;240:77–92. doi: 10.1016/j.jconrel.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chong EJ, Phan TT, Lim IJ. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3(3):321–330. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Yan J W, Yin ZW. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014;9:3027–3036. doi: 10.2147/IJN.S63991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao ZC, Chen SC, Ahmad Z. Essential oil bioactive fibrous membranes prepared via coaxial electrospinning. J. Food Sci. 2017;82(6):1412–1422. doi: 10.1111/1750-3841.13723. [DOI] [PubMed] [Google Scholar]
- 59.Yang H, Wen P, Feng K. Encapsulation of fish oil in a coaxial electrospun nanofibrous mat and its properties. RSC Adv. 2017;7(24):14939–14946. [Google Scholar]
- 60.Weng L, Xie JW. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr Pharm Des. 2015;21(15):1944–1959. doi: 10.2174/1381612821666150302151959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayati I, Bailey AI, Tadros TF. Investigations into the mechanisms of electrohydrodynamic spraying of liquids: I. Effect of electric field and the environment on pendant drops and factors affecting the formation of stable jets and atomization. J Colloid Interface Sci. 1987;117(1):205–221. [Google Scholar]
- 62.Megelski S, Stephens JS, Chase DB, Rabolt JF. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules. 2002;35(22):8456–8466. [Google Scholar]
- 63.Seif S, Franzen L, Windbergs M. Overcoming drug crystallization in electrospun fibers–elucidating key parameters and developing strategies for drug delivery. Int. J. Pharm. 2015;478(1):390–397. doi: 10.1016/j.ijpharm.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 64.Pakravan M, Heuzey M-C, Ajji A. Core–shell structured peo-chitosan nanofibers by coaxial electrospinning. Biomacromolecules. 2012;13(2):412–421. doi: 10.1021/bm201444v. [DOI] [PubMed] [Google Scholar]
- 65.Han D, Yu X, Chai Q, Ayres N, Steckl AJ. Stimuli-responsive self-immolative polymer nanofiber membranes formed by coaxial electrospinning. ACS Appl Mater Interfaces. 2017;9(13):11858–11865. doi: 10.1021/acsami.6b16501. [DOI] [PubMed] [Google Scholar]
- 66.Zhu J, Huang W, Zhang Q. Aqueous-based coaxial electrospinning of genetically engineered silk elastin core-shell nanofibers. Materials. 2016;9(4) doi: 10.3390/ma9040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goova MT, Li J, Kislinger T. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159(2):513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee CH, Chang SH, Chen WJ. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/plga scaffold membranes. J Colloid Interface Sci. 2015;439:88–97. doi: 10.1016/j.jcis.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 69.Desai K, Kit K, Li J, Zivanovic S. Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules. 2008;9(3):1000–1006. doi: 10.1021/bm701017z. [DOI] [PubMed] [Google Scholar]
- 70.Mathew SM, Suchithra TV. Zymogram profiling of myeloperoxidase in association with increased risk of infection susceptibility in diabetic foot ulcer. Int J Diabetes Dev Ctries. 2017;37(4):459–463. [Google Scholar]
- 71.Hirsch T, Spielmann M, Zuhaili B. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 2008;8 doi: 10.1186/1471-2482-8-5. 5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dowd SE, Wolcott RD, Sun Y. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shettigar K, Bhat DV, Satyamoorthy K, Murali TS. Severity of drug resistance and co-existence of enterococcus faecalis in diabetic foot ulcer infections. Folia Microbiol (Praha) 2017;63(1):115–122. doi: 10.1007/s12223-017-0547-2. [DOI] [PubMed] [Google Scholar]
- 74.Massara M, De Caridi G, Serra R. The role of procalcitonin as a marker of diabetic foot ulcer infection. Int Wound J. 2017;14(1):31–34. doi: 10.1111/iwj.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waspadji S. Antibiotic choices in the infected diabetic foot/ulcer. Acta Med Indones. 2005;37(2):94–101. [PubMed] [Google Scholar]
- 76.Dwivedi C, Pandey I, Pandey H. In vivo diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J Biomed Mater Res Part A. 2017 doi: 10.1002/jbm.a.36268. [DOI] [PubMed] [Google Scholar]
- 77.Xu XL, Zhong W, Zhou SF, Trajtman A, Alfa M. Electrospun PEG-PLA nanofibrous membrane for sustained release of hydrophilic antibiotics. J Appl Polym Sci. 2010;118(1):588–595. [Google Scholar]
- 78.Gnanadhas DP, Elango M, Ben Thomas M, Gopalan J, Chakravortty D. Remotely triggered micro-shock wave responsive drug delivery system for resolving diabetic wound infection and controlling blood sugar levels. RSC Adv. 2015;5(17):13234–13238. [Google Scholar]
- 79.Pinzon-Garcia AD, Cassini-Vieira P, Ribeiro CC. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J Biomed Mater Res Part B Appl Biomater. 2017;105(7):1938–1949. doi: 10.1002/jbm.b.33724. [DOI] [PubMed] [Google Scholar]
- 80.Piva RM, Batista Rodrigues Johann AC, Costa CK. Bixin action in the healing process of rats mouth wounds. Curr. Pharm. Biotechnol. 2013;14(9):785–791. doi: 10.2174/1389201014666131227111026. [DOI] [PubMed] [Google Scholar]
- 81.Franco-Robles E, Campos-Cervantes A, Murillo-Ortiz BO. Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl Physiol Nutr Metabol. 2014;39(2):211–218. doi: 10.1139/apnm-2013-0133. [DOI] [PubMed] [Google Scholar]
- 82.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149(7):3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4 doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demir F, Guzel A, Kati C. A combination of methylprednisolone and quercetin is effective for the treatment of cardiac contusion following blunt chest trauma in rats. Braz J Med Biol Res. 2014;47(9):766–772. doi: 10.1590/1414-431X20144021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Tang ZG, Yang JQ. Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Onco Targets Ther. 2017;10:4023–4028. doi: 10.2147/OTT.S136821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lan H, Hong W, Fan P. Quercetin inhibits cell migration and invasion in human osteosarcoma cells. Cell Physiol Biochem. 2017;43(2):553–567. doi: 10.1159/000480528. [DOI] [PubMed] [Google Scholar]
- 87.Thipkaew C, Wattanathorn J, Muchimapura S. Electrospun nanofibers loaded with quercetin promote the recovery of focal entrapment neuropathy in a rat model of streptozotocin-induced diabetes. Biomed Res Int. 2017 doi: 10.1155/2017/2017493. 2017493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nwaeburu CC, Bauer N, Zhao Z. Up-regulation of microrna Let-7c by quercetin inhibits pancreatic cancer progression by activation of numbl. Oncotarget. 2016;7(36):58367–58380. doi: 10.18632/oncotarget.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mckay TB, Karamichos D. Quercetin and the ocular surface: what we know and where we are going. Exp Biol Med. 2017;242(6):565–572. doi: 10.1177/1535370216685187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoon JS, Chae MK, Jang SY, Lee SY, Lee EJ. Antifibrotic effects of quercetin in primary orbital fibroblasts and orbital fat tissue cultures of graves' orbitopathy. Invest Ophthalmol Vis Sci. 2012;53(9):5921–5929. doi: 10.1167/iovs.12-9646. [DOI] [PubMed] [Google Scholar]
- 91.Lee CH, Hsieh MJ, Chang SH. Enhancement of diabetic wound repair using biodegradable nanofibrous metformin-eluting membranes: In vitro and in vivo. ACS Appl Mater Interfaces. 2014;6(6):3979–3986. doi: 10.1021/am405329g. [DOI] [PubMed] [Google Scholar]
- 92.Zhao P, Sui BD, Liu N. Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell. 2017;16(5):1083–1093. doi: 10.1111/acel.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin NR, Bose S, Wang JH. Flos lonicera combined with metformin ameliorates hepatosteatosis and glucose intolerance in association with gut microbiota modulation. Front Microbiol. 2017:8. doi: 10.3389/fmicb.2017.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gutierrez-Lara EJ, Navarrete-Vazquez G, Sanchez-Lopez A, Centurion D. Pharmacological evaluation of metformin and N-benzylbiguanide, a novel analogue of metformin, on the vasopressor responses to adrenergic system stimulation in pithed rats with fructose-induced insulin resistance. Eur J Pharmacol. 2017;814:313–323. doi: 10.1016/j.ejphar.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 95.Han X, Tao Y, Deng Y. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol Med Rep. 2017;16(6):8691–8698. doi: 10.3892/mmr.2017.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ekstrand AJ, Cao RH, Bjorndahl M. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA. 2003;100(10):6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho CH, Sung HK, Kim KT. Comp-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA. 2006;103(13):4946–4951. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Marti GP, Wei X. Age-dependent impairment of HIF-1 alpha expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217(2):319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Q, Oh JH, Park CH. Effects of dimethyloxalylglycine-embedded poly(ε-caprolactone) fiber meshes on wound healing in diabetic rats. ACS Appl Mater Interfaces. 2017;9(9):7950–7963. doi: 10.1021/acsami.6b15815. [DOI] [PubMed] [Google Scholar]
- 100.Zhu T, Park HC, Son KM, Yang HC. Effects of dimethyloxalylglycine on wound healing of palatal mucosa in a rat model. BMC Oral Health. 2015;15 doi: 10.1186/s12903-015-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Aza PN, De Aza AH, De Aza S. Crystalline bioceramic materials. Bol Soc Esp Ceram Vidrio. 2005;44(3):135–145. [Google Scholar]
- 102.Yunos DM, Bretcanu O, Boccaccini A R. Polymer-bioceramic composites for tissue engineering scaffolds. J Mater Sci. 2008;43(13):4433–4442. [Google Scholar]
- 103.Carpenter G, Cohen S. Epidermal growth factor. J Biolog Chem. 1990;265(14):7709–7712. [PubMed] [Google Scholar]
- 104.Das S, Majid M, Baker AB. Syndecan-4 enhances pdgf-bb activity in diabetic wound healing. Acta Biomater. 2016;42:56–65. doi: 10.1016/j.actbio.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Chu Y, Yu D, Wang P. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18(5):499–505. doi: 10.1111/j.1524-475X.2010.00612.x. [DOI] [PubMed] [Google Scholar]
- 106.Dumantepe M, Fazliogullari O, Seren M, Uyar I, Basar F. Efficacy of intralesional recombinant human epidermal growth factor in chronic diabetic foot ulcers. Growth Factors. 2015;33(2):128–132. doi: 10.3109/08977194.2015.1031898. [DOI] [PubMed] [Google Scholar]
- 107.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heldin CH. Structural and functional studies on platelet-derived growth factor. EMBOJ. 1992;11(12):4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia Herrera AL, Febles Sanabria R D J, Acosta Cabadilla L D L A, Moliner Cartaya M. Curative metatarsal bone surgery combined with intralesional administration of recombinant human epidermal growth factor in diabetic neuropathic ulceration of the forefoot: a prospective, open, uncontrolled, nonrandomized, observational study. Curr Ther Res Clin Exp. 2017;85:2–7. doi: 10.1016/j.curtheres.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee CH, Liu KS, Chang SH. Promoting diabetic wound therapy using biodegradable rhPDGF-loaded nanofibrous membranes consort-compliant article. Medicine. 2015;94(47):8. doi: 10.1097/MD.0000000000001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yera-Alos IB, Alonso-Carbonell L, Valenzuela-Silva CM. Active post-marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacol Toxicol. 2013;14 doi: 10.1186/2050-6511-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Datta S, Rameshbabu AP, Bankoti K. Oleoyl-chitosan-based nanofiber mats impregnated with amniotic membrane derived stem cells for accelerated full -thickness excisional wound healing. ACS Biomater Sci Eng. 2017;3(8):1738–1749. doi: 10.1021/acsbiomaterials.7b00189. [DOI] [PubMed] [Google Scholar]
- 113.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25(1):73–78. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 114.Kidd S, Spaeth E, Dembinski JL. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324(5935):1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 116.Sasaki M, Abe R, Fujita Y. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 117.Chen L, Tredget E E, Liu C, Wu Y. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4(9) doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18(11):921–933. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 119.Kanitkar M, Jaiswal A, Deshpande R, Bellare J, Kale VP. Enhanced growth of endothelial precursor cells on PCG-matrix facilitates accelerated, fibrosis-free, wound healing: a diabetic mouse model. PLoS One. 2013;8(7):15. doi: 10.1371/journal.pone.0069960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shou K, Huang Y, Qi B. Induction of mesenchymal stem cell differentiation in the absence of soluble inducer for cutaneous wound regeneration by a chitin nanofiber-based hydrogel. J Tissue Eng Regen Med. 2018;12(2):E867–E880. doi: 10.1002/term.2400. [DOI] [PubMed] [Google Scholar]
- 121.Ma K, Liao S, He L. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Part A. 2011;17(9–10):1413–1424. doi: 10.1089/ten.TEA.2010.0373. [DOI] [PubMed] [Google Scholar]
- 122.Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97(3):173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21(7):1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 124.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12(3):170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]