Graphical Abstract
Three dimensional hydrogel containing titrated extract of Centella asiatica was prepared following Trojan et al. (2005) with slight medication. The hydrogel was cultured with periodontal ligament fibroblasts which are triggered with TNF-α, then the senescence marker of fibroblasts was measured. Migration of fibroblasts using in vitro wound healing assay was also evaluated.
Keywords: 3D TECA, Cellular senescence, SA-β-gal, TNF-α, Fibroblast migration
Abstract
This study was designed to investigate the effect of 3D TECA hydrogel on the inflammatory-induced senescence marker, and to assess the influence of the gel on the periodontal ligament fibroblasts (PDLFs) migration in wound healing in vitro. PDLFs were cultured with 20 ng/ml TNF-α to induce inflammation in the presence and absence of 50 µM 3D TECA gel for 14 d. The gel effect on the senescence maker secretory associated-β-galactosidase (SA-β-gal) activity was measured by a histochemical staining. Chromatin condensation and DNA synthesis of the cells were assessed by 4′,6-diamidino-2-phenylindole and 5-ethynyl-2′-deoxyuridine fluorescent staining respectively. For evaluating fibroblasts migration, scratch wound healing assay and Pro-Plus Imaging software were used. The activity of senescence marker, SA-β-gal, was positive in the samples with TNF-α-induced inflammation. SA-β-gal percentage is suppressed (>65%, P < 0.05) in the treated cells with TECA gel as compared to the non-treated cells. Chromatin foci were obvious in the non-treated samples. DNA synthesis was markedly recognized by the fluorescent staining in the treated compared to non-treated cultures. Scratch wound test indicated that the cells migration rate was significantly higher (14.9 µm2/h, P < 0.05) in the treated versus (11 µm2/h) for control PDLFs. The new formula of 3D TECA suppresses the inflammatory-mediated cellular senescence and enhanced fibroblasts proliferation and migration. Therefore, 3D TECA may be used as an adjunct to accelerate repair and healing of periodontal tissues.
1. Introduction
Periodontal disease is an inflammatory condition which results in the destruction of tooth supporting tissues (alveolar bone, periodontal ligament and cementum) and triggered by the bacterial infection from dental biofilm [1]. Chronic periodontitis affects the quality of life negatively as it may eventually lead to the loss of teeth involved [2], [3]. Treatment of the affected teeth requires not only combating the bacterial infection, but also restoring the lost structure supporting the tooth.
Cellular senescence is a state of irreversible cell growth arrest (irreversible cell cycle arrest) which occurs due to aging [4] or due to other stimuli such as ionizing radiation, chemical exposure, or bacterial infection and inflammation [5], [6], [7], [8]. Inflammation can be provoked by a variety of inducers such as tumor necrosis factor-α (TNFα), interleukin-1β, IL-6, reactive oxygen species, ultraviolet irradiation or bacterial lipopolysaccharides [9], [10], [11].
The typical senescence phenotype consists of an enlarged or swollen cell, and an increases in the activity of senescence biomarker called “senescence-associated β galactosidase” (SA-β-gal). In the senescent cells, the accumulation of membrane-impermeant proteins increases the osmotic pressure within the cell [12]. Senescent cells are unable to release their proteins to be part of the connective stroma. Protein accumulation in a cell creates inflows of water and/or ions across the water-permeable plasma membrane, and results in cell distention and swelling [13], [14], [15], [16].
During senescence, the cells are not able to proliferate so they release mediators that escalate and spread the inflammation. A specific feature of senescent cells is condensed heterochromatic regions, known as senescence-associated heterochromatic foci (SAHF) [17], [18]. SAHF plays a role in limiting proliferation-promoting genes 19]. Disturbance of SAHF formation causes mutagenic changes 20], which assumes that SAHF provides a barrier to malignant transformation and contributes to the tumor suppressive function of senescence. SAHF may also prolong the viability of the senescent cells by limiting the DNA damage signaling 21]. However, during chronic inflammation, apoptosis takes place due to the extensive DNA damage [8].
Senescent cells undergo apoptosis and then clearance by phagocytosis [22], [23], [24]. In a case of chronic periodontal infection, it is evident that the inflamed senescent supporting structure cells are cleared and definite replacement of the lost structure is not materialized. This is very obviously manifested by the gingival recession, pocketing, and loss of attachment [25], [26].
Migration of the healing cells to the wound or inflamed site is crucial to achieve accelerated healing with minimal loss or changes in the tissues architecture [27], [28]. During periodontal healing, the proliferation and remodeling stages are essential phases in proper wound repair and regeneration. Periodontal ligament fibroblasts play an important role in these phases. PDLFs are recruited to damaged site to reinstitute tissue repair and matrix remodeling [29], [30], [31].
2D gel environment is not the real representation of the physiological cell environment as cell movement; cell signaling and the exposure to the extracellular matrix (ECM) are limited in 2D environment [32]. 3D gel environment is therefore, considered to be a more reflective picture of the morphological and physiological characters of the in vivo environment that facilitate cell–cell and cell–ECM interactions which ultimately influence gene expression and cellular behavior [33], [34]. Growth of cells in 3D is steadier than that in 2D environment which require the cells to undergo trypsinization in order to provide sufficient nutrients for normal cell growth [35], [36]. In vitro 3D system can provide analogous ECM properties and simulate the mechanical environment of the in vivo cell microenvironment. 3D scaffolds can be made from natural or synthetic materials and can be engineered to allow cells to migrate and grow within the network of the scaffold [37], [38], [39]. A hydrogel is a biologically compatible (biocompatible) polymer network with high water content and physical properties that closely mimic the natural extracellular matrix (ECM) and the living cells can be cultured either in or on a hydrogel [38]. Biodegradable polymers are mainly used where the transient existence of materials is required and they find applications as sutures, scaffolds for tissue regeneration, tissue adhesives, hemostats, and transient barriers for tissue adhesion, as well as drug delivery systems [39].
Centella asiatica herb has been used for hundreds of years as a traditional medicine of many Asian countries for healing of skin cuts and ulcers. These properties are ascribed to the active ingredients, asiatic acid, asiaticoside, and madecassic acid. In a previous study, titrated extract of C. asiatica (TECA) was used to improve connective tissue formation, epithelization, and angiogenesis when applied locally on wounds [40].
Although many adjunctive remedies are currently available for the non-surgical or surgical treatment of periodontal disease, almost all of them are made by a complicated manufacturing process, require training and skill for handling, and are mostly not cost-effective [41]. Although several gel preparations are used for gum treatment, the statistics for these conventional gum treatments is entirely disappointing [42], [43], [44], [45]. In this study, the effect of 3D TECA hydrogel on the cellular senescence of the periodontal fibroblasts has been tested by assessing the senescence marker. The effect of gel on the cells migration during healing was evaluated by in vitro scratch wound healing assay.
2. Materials and methods
2.1. 3D gel preparation
In this study, silanized hydroxyl propyl methyl cellulose gel (Si-HPMC) was prepared. The natural water-soluble cellulose ethers HPMC 2%–4% w/w (Methocels E4M, Colorcon, Kent, UK) was grafted with 3-glycidoxypropyltrimethoxysilane (3-GPTMS) (Sigma-Aldrich, Germany). Then, the silanized Si-HPMC was dissolved in NaOH (pH 12.9). The basic Si-HPMC solution was made more viscous by adding 0.26M HEPES, buffer (Sigma-Aldrich, Germany) and 10% (v/v) of culture medium (Fibroblast medium, Sigma-Aldrich, Germany) supplemented with 10% FCS, 1% penicillin/streptomycin and 1% L-glutamine. The pH of hydrogel was adjusted with a Mettler Toledo pH meter (Mettler Toledo, Greifensee, Switzerland), and ranged from 7 to 8 [46]. The final composition of the hydrogel includes 50 µM titrated extract of C. asiatica (TECA). The percentages of the bioactive components of TECA were previously tested and customized for enhancing the proliferation of PDLFs in our previous study (unpublished data).
2.2. Cell culture
Human periodontal ligament fibroblast (HPDLF) was obtained from the ScienCell Research Laboratories, (Carlsbad, CA, USA). HPDLF cells were cultured in a fibroblast medium containing antibiotics (10% Fetal bovine serum (FBS), 1% of penicillin/streptomycin and 1% of Gentamycin). The Fetal bovine serum was deactivated by immersing the container in water bath at 50 °C for 5 min. The cell culture was incubated in 5% CO2 at 37 °C and 95% air and the medium was changed every three days. Seven days later, each confluent culture dish was trypsinized and split onto 5 new 10 cm-dishes in 10% DMEM. The growing cells were kept in vials with 10% dimethyl sulfoxide/20% DMEM and stored in a liquid nitrogen tank. The cell cultures were examined by using phase contrast microscopy (Olympus, Tokyo, Japan).
Cell cultures after 25 passages were also prepared to check the naturally expressed senescence marker in aged cells. Three groups of samples were made and analyzed; two of them were treated with TNF-α. The third group of samples used was serially sub-cultured cells or naturally senescent cells.
2.3. Senescence-associated β-galactosidase (SA-β-gal) activity
PDLFs were cultured with 20 ng/ml TNF-α [47], [48] for 14 days in a 3D gel medium in the presence or absence of 50 µM TECA. The SA-β-gal-positive cell ratios were determined using a senescence detection kit, according to manufacturer's protocol (BioVision, CA, USA). In brief, sparse cultures at a density of 4×103 cells were washed once with 1 ml of 1X PBS. Then, the cells were fixed with the kit Fixative Solution for 10 min at room temperature. Staining of the samples was done by adding 470 µl of kit Staining Solution, 5 µl of Staining Supplement 25 µl of 20 mg/ml X-gal in DMF for each plate well. The samples were washed twice with 1 ml of 1X PBS. Then, another 0.5 ml of the kit Staining Solution Mix was added to each well. The plates were incubated overnight at 37 °C. Observation of the cells was done under a phase contrast microscope for development of blue color (200× total magnification).
2.4. DNA replication assay, EdU and DAPI analysis
Analysis of DNA replication or cell proliferation was achieved by cells treatment with 10 mM 5-ethynyl-2′-deoxyuridine (EdU) (ThermoFisher) followed by incubation of the samples for 24 h, then fixation with 3.7% formaldehyde in 0.1 M PBS for 10 min. Permeabilization was done by a 0.5% Triton® X-100 for 10 min. All steps of the assay were performed in the dark. The Click-iT® reaction cocktail was prepared following the protocol from Click-iT EdU Alexa Fluor 488 Imaging Kit (ThermoFisher). 0.5 ml of Click-iT® reaction cocktail was added to each well containing a coverslip followed by plate incubation for 30 min at room temperature. The reaction cocktail was then removed and each well was washed with 3% BSA in PBS for 2 min. Before microscopic observation, samples were washed with ddH2O and stained with 2.5 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, MO, USA) in PBS for 20 min. Afterwards, the samples were carefully placed face down on glass slides and mounted with Vectashield antifade (Vector Laboratories, CA, USA). The samples were observed on a Nikon Eclipse 80i fluorescence microscope.
2.5. Cell migration/scratch wound healing assay
6-well Costar® culture plates (Corning Inc., NY, USA) were layered with 2 ml of the 3D hydrogel with or without TECA. Each well was seeded with 500,000 cells in 10% FCS/DMEM and placed at 37 °C and 5% CO2 incubator for 48 h. Wounds were induced by scratching the samples with a sterile pipette tip to leave a scratch of approximately 1 mm width. Suspended cells were removed by aspiration. Digital images were captured with an inverted microscope (Nikon Eclipse Ti-U, Nikon, Japan) and QImaging Retiga 2000R CCD digital camera (QImaging, BC, Canada). The selected fields were captured every 2 min during 24 h using phase microscopy. The distance between the selected cells and wound edge was measured. Migration rate was calculated as cell migration distance/time (µm2/h). The digitized images were analyzed using Image-J software and Pro-Plus Imaging software [49], [50].
2.6. Statistical analysis
Data presented in this study are means ± standard error of the mean. Kruskal–Wallis test was run followed by a pairwise Wilcoxon rank sum test. For scratch wound assay, statistical significance was obtained by Student's t-test. Variances with a value of P < 0.05 were considered significant.
3. Results and discussion
3.1. Detection of senescence-associated β-galactosidase (SA-β-gal) activity
Cellular senescence was confirmed by measurements of SA-β-gal positive cells. The activities of SA-ß-gal activity, chromatin condensation (DAPI), and detecting DNA proliferation marker were analyzed 14 days after treatment with TECA gel (Fig. 1). Late passage cell samples were used as a control.
Fig. 1.
Senescence marker histochemical blue staining (A1, B1 & C1), fluorescent staining by DAPI for SAHF (A2, B2 & C2) and by EdU for DNA synthesis (A3, B3 & C3).
Detection of biomarker SA-β-gal activity permits the identification of senescent cells in culture and mammalian tissues [51], [52], [53], [54]. In this study, senescence marker SA-β-gal percentage (Fig. 1; A1 & B1) is suppressed (>65%, P < 0.05) in the treated cells (~32% positive cells) as compared to the non-treated cells (~81% positive cells) (Fig. 2 ). The histochemical analysis has showed that inflammatory senescence was induced in the fibroblasts cultured with TNF-α only. Detection of senescence was manifested by the blue staining of the enlarged senescent cells in the non-treated samples compared to the test samples treated with TECA. The samples which consisted of late passages cells were also exhibited with senescence marker stain. Treatment of the cells with TECA encountered the TNF-α mediated inflammation and subsequently limited the senescence process in these test samples (Fig. 1; A1). This result is consistent with previous studies that revealed blockade of TNF-α would be beneficial in suppressing inflammation and senescence [55], [56], [57]. The results also indicate that TECA hydrogel was having a remarkable action in sustaining the cell vitality and protecting the cells from senescence. Cellular senescence is characterized by permanent cell cycle arrest [58], [59], [60]. Senescent cells secrete bioactive peptides [61], [62], generate and release ROS [60], [63] which provoke further inflammatory reaction within the affected tissues [64]. Senescent cells generate DNA damage response and induce a bystander effect, spreading senescence toward their neighbors in vitro and, possibly, in vivo [65], [66]. Thus, senescent cells may contribute to loss of tissue homeostasis.
Fig. 2.
Senescence marker SA-β-gal percentages.
Previous studies on osteogenic activity of fibroblast reported that osteocalcin production is impaired in senescent periodontal ligament fibroblasts which exhibit short telomere and loss of the production potential of bone-associated proteins during periodontal disease [67]. The presence of the senescent cells in the tissues, by itself, has a damaging effect as senescent cells release pro-inflammatory cytokines which, in turn, provoke the inflammation and increase the intensity of destruction and apoptosis [68], [69], [70], [71], [72], [73], [74], [75], [76], [77]. By suppression of inflammatory senescence, the new formulated TECA hydrogel may be able to maintain tissues homeostasis, reduce the DNA damage and limit the periodontal tissue loss.
3.2. Detection of SAHF
SAHF were observed by the blue fluorescence DAPI stain. The heterochromatic condensations were evident in the non-treated samples (Fig. 1; B2) compared to the test samples. The non-treated cells presented with SAHF (similar to that in aging cells sample) are probably undergoing cell growth arrest and no more proliferation as SAHF plays a role in sequestering proliferation-promoting genes during inflammation [18], [19], [78]. Condensation of chromatin was not materialized in the treated samples with TECA because these samples were almost free of inflammatory senescence (Fig. 1; A2). The late passage cells were clearly showing the chromatin foci as one of the features of senescence (Fig. 1; C2) [79]. The results are consistent with previous study [72] which concluded that pro-inflammatory cytokines released in chronic inflammation conditions are similar to that released due to aging. Indeed, heterochromatin foci (which are formed due to inflammation) are associated with repression of E2F target genes and have been shown to be a hallmark of senescent cells, thus, these foci were thought to harbor epigenetic modifications that govern maintenance of senescence [19], [68], [80].
3.3. DNA replication assay (EdU)
Proliferation assay was performed by EdU (5-ethynyl-2′-deoxyuridine) fluorescence staining. EdU provided in the kit is a nucleoside analog of thymidine and is incorporated into cell DNA during active DNA synthesis [81]. The EdU contains the alkyne which can be reacted with an azide-containing detection reagent, to form a stable, triazole ring. In our study, the treated samples were shown to be proliferating and actively synthesizing their DNA (Fig. 1; A3). However, the non-treated as well as the late passage samples (Fig. 1; B3 & C3) showed no signs of clear green fluorescence stain, probably because these samples were not proliferating, nor replicating their DNA and undergoing irreversible growth arrest [82], [83].
These results indicated that the cells treated with TECA gel were able to survive the inflammation and produced a new DNA (or their DNA is replicating) which was stained by the fluorescent stain EdU. Previous studies found that collagen synthesis was suppressed in fibroblast during chronic inflammation [84], [85]. In our study, TECA hydrogel protected the cells which were consequently proliferating and positive to the DNA synthesis marker EdU.
Regarding TECA, it elicits a strong and consistent change in the levels of gene expression in fibroblast culture [86], [87], induces cell-cycle progression and collagen type-1 synthesis in human dermal fibroblasts [88], [89]. Of 1053 genes analyzed, 82 were found to have statistically recognizable changes in expression [86]; considering the direction of this change along with the role of these genes play in cellular function provides an insight into the biological activity of TECA.
Non-TECA hydrogel treated samples and also samples of the late passages senescent cells (control) showed very low percentages of cells positive for EdU. DNA synthesis is an indication of cell growth or cell cycle progression and proliferation [90], [91], [92], [93] where DNA must be duplicated precisely once before cell division occurs [94], [95].
3.4. Cell migration/scratch wound healing assay
The effect of TECA hydrogel on the cell migration during inflammatory senescence was assessed by scratch wound healing assay (Fig. 3 ). The in vitro scratch assay is an easy, low-cost and well-developed method to measure cell migration in cell culture [96]. The basic steps involve creating a “scratch” in a cell monolayer [37], [97], [98], capturing the images at the beginning and at regular intervals during cell migration to close the scratch, and comparing the images to quantify the migration rate of the cells [37], [99], [100]. Stimulated by the availability of empty space (scratch), the cells at the edges of the newly created gap proliferate and move toward the center of the scratch area [101], [102].
Fig. 3.
Cell migration assay at 0 h and after 24 h, closure of the scratch was remarkable in the TECA treated samples. Normally cultured fibroblasts were used as a control.
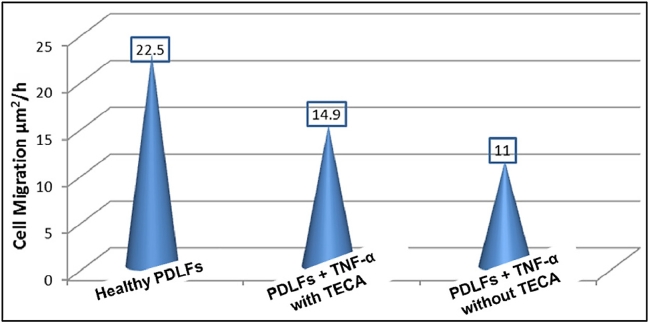
In this study, scratch wound assay showed that the cells migration rate (Fig. 3, Fig. 4) was higher (14.9 µm2/h, P < 0.05) in the TECA treated samples versus (11 µm2/h) for non-treated PDLFs samples. Healthy PDLFs without TNF-α or TECA treatment were used as a control.
Fig. 4.
Cell migration rates. Non-treated cells migration was remarkably lower than the normal healthy samples.
Application of the 3D TECA gel was significantly enhancing the migration of cells to the scratch field. The explanation for the higher migration rate in the treated sample would be due to the suppression of senescence activity and limiting the condensation of chromatin foci. As the treated cells samples were almost free of inflammatory senescence and SAHF, they were able to move faster than the enlarged senescent cells [103], [104], [105]. The reduced ability to migrate may be related to alterations in the cytoskeleton during cellular senescence [106]. Cellular migration requires actin which is an important component of the cytoskeleton. However, in senescent fibroblasts, actin is down-regulated and instead, vimentin is produced [106]. This migration discrepancy has negative implications during wound healing since cells are stimulated to migrate into the wound, proliferate and build a new extracellular matrix. Senescent cells secrete pro-inflammatory cytokines, chemokines, and proteases which provoke the inflammation and degrade the matrix [107]; hence, wound repair would be further impaired [108].
4. Conclusion
In the current study, inflammatory senescence was induced in PDLF cultured with TNF-α. Elimination of the senescence was possible with the use of TECA gel as samples treated with TECA gel showed a reduced percentage of cells with senescence-associated-β-galactosidase (SA-β-gal) staining and suppressed SAHF formation.
Senescence (irreversible cell growth arrest) of periodontal cells occurs during chronic inflammation of these cells which means that aging factor is not required for the periodontal fibroblast to be senescent cells. The formation of senescent cells during chronic periodontitis would not be beneficial as the apoptotic senescent cells (as in the case of periodontitis) are not replaced by new cells; consequently, this leads to a very evident loss of tooth supporting tissues. As the formula of 3D TECA has suppressed the inflammatory mediated cellular senescence, preserved cell proliferation and enhanced the migration of fibroblasts, it may be used as an adjunct to accelerate tissues repair and healing of periodontal tissues.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the support from the Ministry of Science, Technology and Innovation for providing the grant number (MOSTI SF01.01.09) and the Ministry of Higher Education for providing the Research Acculturation Grant Scheme (RAGS 119/2013) and UiTM DANA (RIF 751/2012).
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Socransky S.S., Haffajee A.D. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 2.Meusel D.R., Ramacciato J.C., Motta R.H. Impact of the severity of chronic periodontal disease on quality of life. J Oral Sci. 2015;57(2):87–94. doi: 10.2334/josnusd.57.87. [DOI] [PubMed] [Google Scholar]
- 3.Araujo A.C., Gusmao E.S., Batista J.E. Impact of periodontal disease on quality of life. Quintessence Int (Berl) 2010;41(6):e111–e118. Berlin, Germany: 1985. [PubMed] [Google Scholar]
- 4.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 5.Cmielova J., Havelek R., Soukup T. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int J Radiat Biol. 2012;88(5):393–404. doi: 10.3109/09553002.2012.666001. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q.M., Liu J., Merrett J.B. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347(Pt 2):543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiyoshima T., Enoki N., Kobayashi I. Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblasts. Int J Mol Med. 2012;30(5):1007–1012. doi: 10.3892/ijmm.2012.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng R., Choudhury D., Liu C. Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Discov. 2015;1:15046. doi: 10.1038/cddiscovery.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao S., Ogtata Y., Shimizu E. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol Cell Biochem. 2002;238(1–2):11–18. doi: 10.1023/a:1019927616000. [DOI] [PubMed] [Google Scholar]
- 10.Chang J., Wang Z., Tang E. Inhibition of osteoblast functions by IKK/NF-κB in osteoporosis. Nat Med. 2009;15(6):682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arabaci T., Cicek Y., Canakci V. Immunohistochemical and stereologic analysis of NF-kappaB activation in chronic periodontitis. Eur J Dent. 2010;4(4):454–461. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann E.K., Dunham P.B. Membrane mechanisms and intracellular signalling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- 13.Hamill O.P., Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81(2):685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 14.Blazkova H., Krejcikova K., Moudry P. Bacterial intoxication evokes cellular senescence with persistent DNA damage and cytokine signalling. J Cell Mol Med. 2010;14(1–2):357–367. doi: 10.1111/j.1582-4934.2009.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson E.C., Manning B., Zhang H. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol. 2013;203(6):929–942. doi: 10.1083/jcb.201306073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takauji Y., Wada T., Takeda A. Restriction of protein synthesis abolishes senescence features at cellular and organismal levels. Sci Rep. 2016;6:18722. doi: 10.1038/srep18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R., Adams P.D. Heterochromatin and its relationship to cell senescence and cancer therapy. Cell Cycle. 2007;6(7):784–789. doi: 10.4161/cc.6.7.4079. [DOI] [PubMed] [Google Scholar]
- 18.Kosar M., Bartkova J., Hubackova S. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a) Cell Cycle. 2011;10(3):457–468. doi: 10.4161/cc.10.3.14707. [DOI] [PubMed] [Google Scholar]
- 19.Narita M., Nunez S., Heard E. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 20.Narita M., Narita M., Krizhanovsky V. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126(3):503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 21.Di Micco R., Sulli G., Dobreva M. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol. 2011;13(3):292–302. doi: 10.1038/ncb2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horikawa I., Fujita K., Jenkins L.M. Autophagic degradation of the inhibitory p53 isoform Delta133p53alpha as a regulatory mechanism for p53-mediated senescence. Nat Commun. 2014;5:4706. doi: 10.1038/ncomms5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demirovic D., Nizard C., Rattan S.I. Basal level of autophagy is increased in aging human skin fibroblasts in vitro, but not in old skin. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0126546. e0126546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B.R., Zhang L.C., Permatasari F. ALA-PDT elicits oxidative damage and apoptosis in UVB-induced premature senescence of human skin fibroblasts. Photodiagnosis Photodyn Ther. 2016;14:47–56. doi: 10.1016/j.pdpdt.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Lamster I.B., Asadourian L., Del Carmen T. The aging mouth: differentiating normal aging from disease. Periodontol 2000. 2016;72(1):96–107. doi: 10.1111/prd.12131. [DOI] [PubMed] [Google Scholar]
- 26.Ozturk V.O., Belibasakis G.N., Emingil G. Impact of aging on TREM-1 responses in the periodontium: a cross-sectional study in an elderly population. BMC Infect Dis. 2016;16(1):429. doi: 10.1186/s12879-016-1778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anitua E., Troya M., Orive G. Plasma rich in growth factors promote gingival tissue regeneration by stimulating fibroblast proliferation and migration and by blocking transforming growth factor-beta1-induced myodifferentiation. J Periodontol. 2012;83(8):1028–1037. doi: 10.1902/jop.2011.110505. [DOI] [PubMed] [Google Scholar]
- 28.Semlali A., Chakir J., Goulet J.P. Whole cigarette smoke promotes human gingival epithelial cell apoptosis and inhibits cell repair processes. J Periodontal Res. 2011;46(5):533–541. doi: 10.1111/j.1600-0765.2011.01370.x. [DOI] [PubMed] [Google Scholar]
- 29.Cho J.W., Kim S.A., Lee K.S. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. 2012;29(1):32–36. doi: 10.3892/ijmm.2011.803. [DOI] [PubMed] [Google Scholar]
- 30.Caceres M., Hidalgo R., Sanz A. Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol. 2008;79(4):714–720. doi: 10.1902/jop.2008.070395. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y., Zhu L., Liu L. Interleukin-17A stimulates migration of periodontal ligament fibroblasts via p38 MAPK/NF-kappaB-dependent MMP-1 expression. J Cell Physiol. 2014;229(3):292–299. doi: 10.1002/jcp.24444. [DOI] [PubMed] [Google Scholar]
- 32.Gurski L.A., Jha A.K., Zhang C. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30(30):6076–6085. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J., Cuddihy M.J., Kotov N.A. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14(1):61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 34.Edmondson R., Broglie J.J., Adcock A.F. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun T., Jackson S., Haycock J.W. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol. 2006;122(3):372–381. doi: 10.1016/j.jbiotec.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Justice B.A., Badr N.A., Felder R.A. 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today. 2009;14(1–2):102–107. doi: 10.1016/j.drudis.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Liang C.C., Park A.Y., Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 38.Langer R., Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 39.Nair L.S., Laurencin C.T. Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv Biochem Eng Biotechnol. 2006;102:47–90. doi: 10.1007/b137240. [DOI] [PubMed] [Google Scholar]
- 40.Maquart F.X., Chastang F., Simeon A. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol. 1999;9(4):289–296. [PubMed] [Google Scholar]
- 41.Ripamonti U., Petit J.C. Bone morphogenetic proteins, cementogenesis, myoblastic stem cells and the induction of periodontal tissue regeneration. Cytokine Growth Factor Rev. 2009;20(5–6):489–499. doi: 10.1016/j.cytogfr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Slots J., Jorgensen M.G. Efficient antimicrobial treatment in periodontal maintenance care. J Am Dent Assoc. 2000;131(9):1293–1304. doi: 10.14219/jada.archive.2000.0383. [DOI] [PubMed] [Google Scholar]
- 43.Kotsovilis S., Markou N., Pepelassi E. The adjunctive use of platelet-rich plasma in the therapy of periodontal intraosseous defects: a systematic review. J Periodontal Res. 2010;45(3):428–443. doi: 10.1111/j.1600-0765.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 44.Del Fabbro M., Bortolin M., Taschieri S. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J Periodontol. 2011;82(8):1100–1111. doi: 10.1902/jop.2010.100605. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi M., Haapasalo M., Imazato S. Dentistry in the 21st century: challenges of a globalising world. Int Dent J. 2014;64(6):333–342. doi: 10.1111/idj.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trojani C., Weiss P., Michiels J.F. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials. 2005;26(27):5509–5517. doi: 10.1016/j.biomaterials.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Yucel-Lindberg T., Olsson T., Kawakami T. Signal pathways involved in the regulation of prostaglandin E synthase-1 in human gingival fibroblasts. Cell Signal. 2006;18(12):2131–2142. doi: 10.1016/j.cellsig.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Bage T., Lindberg J., Lundeberg J. Signal pathways JNK and NF-kappaB, identified by global gene expression profiling, are involved in regulation of TNFalpha-induced mPGES-1 and COX-2 expression in gingival fibroblasts. BMC Genomics. 2010;11:241. doi: 10.1186/1471-2164-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomic-Canic M., Mamber S.W., Stojadinovic O. Streptolysin O enhances keratinocyte migration and proliferation and promotes skin organ culture wound healing in vitro. Wound Repair Regen. 2007;15(1):71–79. doi: 10.1111/j.1524-475X.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 50.Walter M.N., Wright K.T., Fuller H.R. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316(7):1271–1281. doi: 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 51.Debacq-Chainiaux F., Erusalimsky J.D., Campisi J. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4(12):1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 52.Itahana K., Campisi J., Dimri G.P. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 53.Itahana K., Itahana Y., Dimri G.P. Colorimetric detection of senescence-associated beta galactosidase. Methods Mol Biol. 2013;965:143–156. doi: 10.1007/978-1-62703-239-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gary R.K., Kindell S.M. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal Biochem. 2005;343(2):329–334. doi: 10.1016/j.ab.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J., Gao Y., Wang H. Human endothelial senescence can be induced by TNF-α. Chin Phys Lett. 2002;47(2):119–123. [Google Scholar]
- 56.Huvers F.C., Popa C., Netea M.G. Improved insulin sensitivity by anti-TNFalpha antibody treatment in patients with rheumatic diseases. Ann Rheum Dis. 2007;66(4):558–559. doi: 10.1136/ard.2006.062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu F., Wu S., Ren H. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13(3):254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 58.Dalle Pezze P., Nelson G., Otten E.G. Dynamic modelling of pathways to cellular senescence reveals strategies for targeted interventions. PLoS Comput Biol. 2014;10(8) doi: 10.1371/journal.pcbi.1003728. e1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Victorelli S., Passos J.F. Telomeres and cell senescence – size matters not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maria J., Ingrid Z. Effects of bioactive compounds on senescence and components of senescence associated secretory phenotypes in vitro. Food Funct. 2017;8(7):2394–2418. doi: 10.1039/c7fo00161d. [DOI] [PubMed] [Google Scholar]
- 61.Coppe J.P., Patil C.K., Rodier F. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuilman T., Peeper D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 63.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 64.Youn H.J., Kim K.B., Han H.S. 23-Hydroxytormentic acid protects human dermal fibroblasts by attenuating UVA-induced oxidative stress. Photodermatol Photoimmunol Photomed. 2017;33(2):92–100. doi: 10.1111/phpp.12294. [DOI] [PubMed] [Google Scholar]
- 65.Nelson G., Wordsworth J., Wang C. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11(2):345–349. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acosta J.C., Banito A., Wuestefeld T. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawa Y., Phillips A., Hollard J. Impairment of osteocalcin production in senescent periodontal ligament fibroblasts. Tissue Cell. 2000;32(2):198–204. doi: 10.1054/tice.2000.0104. [DOI] [PubMed] [Google Scholar]
- 68.Ren J.L., Pan J.S., Lu Y.P. Inflammatory signaling and cellular senescence. Cell Signal. 2009;21(3):378–383. doi: 10.1016/j.cellsig.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elliott M.R., Ravichandran K.S. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189(7):1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davalos A.R., Coppe J.P., Campisi J. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29(2):273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coppe J.P., Desprez P.Y., Krtolica A. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freund A., Orjalo A.V., Desprez P.Y. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tchkonia T., Zhu Y., van Deursen J. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar M., Seeger W., Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2014;51(3):323–333. doi: 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y., Armstrong J.L., Tchkonia T. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 76.Hsueh Y., Chen H.L., Chang T.C. Pro-inflammatory cytokines induce mesenchymal stem cell secretory phenotype by increasing autophagosomes and lysosomes. FASEB J. 2016;30(1 Suppl.):1062.2. [Google Scholar]
- 77.Oishi Y., Manabe I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis. 2016;2:16018. doi: 10.1038/npjamd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salminen A., Kauppinen A., Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell Signal. 2012;24(4):835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Adams P.D. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene. 2007;397(1–2):84–93. doi: 10.1016/j.gene.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lasry A., Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36(4):217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 81.Salic A., Mitchison T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105(7):2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Q., Ames B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA. 1994;91(10):4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8(7):512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 84.Riquet F.B., Lai W.F., Birkhead J.R. Suppression of type I collagen gene expression by prostaglandins in fibroblasts is mediated at the transcriptional level. Mol Med. 2000;6(8):705–719. [PMC free article] [PubMed] [Google Scholar]
- 85.Dohi T., Miyake K., Aoki M. Tissue inhibitor of metalloproteinase-2 suppresses collagen synthesis in cultured keloid fibroblasts. Plast Reconstr Surg Glob Open. 2015;3(9):e520. doi: 10.1097/GOX.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coldren C.D., Hashim P., Ali J.M. Gene expression changes in the human fibroblast induced by Centella asiatica triterpenoids. Planta Med. 2003;69(8):725–732. doi: 10.1055/s-2003-42791. [DOI] [PubMed] [Google Scholar]
- 87.James J.T., Dubery I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules. 2009;14(10):3922–3941. doi: 10.3390/molecules14103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu L., Ying K., Wei S. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int J Dermatol. 2004;43(11):801–807. doi: 10.1111/j.1365-4632.2004.02047.x. [DOI] [PubMed] [Google Scholar]
- 89.Lee J., Jung E., Kim Y. Asiaticoside induces human collagen I synthesis through TGF beta receptor I kinase (TbetaRI kinase)-independent SMAD signaling. Planta Med. 2006;72(4):324–328. doi: 10.1055/s-2005-916227. [DOI] [PubMed] [Google Scholar]
- 90.Heng N.H., Zahlten J., Cordes V. Effects of enamel matrix derivative and transforming growth factor-beta1 on connective tissue growth factor in human periodontal ligament fibroblasts. J Periodontol. 2015;86(4):569–577. doi: 10.1902/jop.2015.120448. [DOI] [PubMed] [Google Scholar]
- 91.Pandey R., Velasquez S., Durrani S. MicroRNA-1825 induces proliferation of adult cardiomyocytes and promotes cardiac regeneration post ischemic injury. Am J Transl Res. 2017;9(6):3120–3137. [PMC free article] [PubMed] [Google Scholar]
- 92.Mitarai H., Wada N., Hasegawa D. Transgelin mediates transforming growth factor-beta1-induced proliferation of human periodontal ligament cells. J Periodontal Res. 2017;52(6):984–993. doi: 10.1111/jre.12466. [DOI] [PubMed] [Google Scholar]
- 93.Sun X., Bizhanova A., Matheson T.D. Ki-67 contributes to normal cell cycle progression and inactive X heterochromatin in p21 checkpoint-proficient human cells. Mol Cell Biol. 2017 doi: 10.1128/MCB.00569-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nishitani H., Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7(6):523–534. doi: 10.1046/j.1365-2443.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 95.Nishitani H., Lygerou Z. DNA replication licensing. Front Biosci. 2004;9:2115–2132. doi: 10.2741/1315. [DOI] [PubMed] [Google Scholar]
- 96.Grada A., Otero-Vinas M., Prieto-Castrillo F. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol. 2017;137(2):e11–e16. doi: 10.1016/j.jid.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez L.G., Wu X., Guan J.L. Wound-healing assay. Methods Mol Biol. 2005;294:23–29. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- 98.Cai A.Q., Landman K.A., Hughes B.D. Multi-scale modeling of a wound-healing cell migration assay. J Theor Biol. 2007;245(3):576–594. doi: 10.1016/j.jtbi.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 99.Zhang H., Han Y., Tao J. Cellular repressor of E1A-stimulated genes regulates vascular endothelial cell migration by the ILK/AKT/mTOR/VEGF(165) signaling pathway. Exp Cell Res. 2011;317(20):2904–2913. doi: 10.1016/j.yexcr.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 100.Chieng-Yane P., Bocquet A., Letienne R. Protease-activated receptor-1 antagonist F 16618 reduces arterial restenosis by down-regulation of tumor necrosis factor alpha and matrix metalloproteinase 7 expression, migration, and proliferation of vascular smooth muscle cells. J Pharmacol Exp Ther. 2011;336(3):643–651. doi: 10.1124/jpet.110.175182. [DOI] [PubMed] [Google Scholar]
- 101.Block E.R., Matela A.R., Sundarraj N. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279(23):24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 102.Poujade M., Grasland-Mongrain E., Hertzog A. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA. 2007;104(41):15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sandeman S.R., Allen M.C., Liu C. Human keratocyte migration into collagen gels declines with in vitro ageing. Mech Ageing Dev. 2000;119(3):149–157. doi: 10.1016/s0047-6374(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 104.Reed M.J., Corsa A.C., Kudravi S.A. A deficit in collagenase activity contributes to impaired migration of aged microvascular endothelial cells. J Cell Biochem. 2000;77(1):116–126. [PubMed] [Google Scholar]
- 105.Ruiz-Torres A., Lozano R., Melon J. Age-dependent decline of in vitro migration (basal and stimulated by IGF-1 or insulin) of human vascular smooth muscle cells. J Gerontol A Biol Sci Med Sci. 2003;58(12):B1074–B1077. doi: 10.1093/gerona/58.12.b1074. [DOI] [PubMed] [Google Scholar]
- 106.Nishio K., Inoue A. Senescence-associated alterations of cytoskeleton: extraordinary production of vimentin that anchors cytoplasmic p53 in senescent human fibroblasts. Histochem Cell Biol. 2005;123(3):263–273. doi: 10.1007/s00418-005-0766-5. [DOI] [PubMed] [Google Scholar]
- 107.Rodier F., Coppe J.P., Patil C.K. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caceres M., Oyarzun A., Smith P.C. Defective wound-healing in aging gingival tissue. J Dent Res. 2014;93(7):691–697. doi: 10.1177/0022034514533126. [DOI] [PMC free article] [PubMed] [Google Scholar]