Abstract
Honokiol (HK) usage is greatly restricted by its poor aqueous solubility and limited oral bioavailability. We synthesized and characterized a novel phosphate prodrug of honokiol (HKP) for in vitro and in vivo use. HKP greatly enhanced the aqueous solubility of HK (127.54 ± 15.53 mg/ml) and the stability in buffer solution was sufficient for intravenous administration. The enzymatic hydrolysis of HKP to HK was extremely rapid in vitro (T1/2 = 8.9 ± 2.11 s). Pharmacokinetics studies demonstrated that after intravenous administration of HKP (32 mg/kg), HKP was converted rapidly to HK with a time to reach the maximum plasma concentration of ∼5 min. The prodrug HKP achieved an improved T1/2 (7.97 ± 1.30 h) and terminal volume of distribution (26.02 ± 6.04 ml/kg) compared with direct injection of the equimolar parent drug (0.66 ± 0.01 h) and (2.90 ± 0.342 ml/kg), respectively. Furthermore, oral administration of HKP showed rapid and improved absorption compared with the parent drug. HKP was confirmed to maintain the bioactivity of the parent drug for ameliorating ischemia-reperfusion injury by decreasing brain infarction and improving neurologic function. Taken together, HKP is a potentially useful aqueous-soluble prodrug with improved pharmacokinetic properties which may merit further development as a potential drug candidate.
Keywords: Phosphate prodrug, Honokiol, Pharmacokinetics, Focal cerebral ischemia-reperfusion
Graphical abstract
A phosphate prodrug of honokiol (HKP) enhanced the aqueous solubility of honokiol. HKP was converted to honokiol in vivo in rats after intravenous administration with improved pharmacokinetic properties.
1. Introduction
Honokiol (HK) is one of the major bioactive phytochemical agents isolated from Magnolia officinalis, a Chinese herb used for the treatment of stroke, headache, and ischemic heart disease [1], [2]. HK has been reported to possess anti-inflammatory [3], antioxidant [4], antidepressant [5], anticancer [6] and neuroprotective effects [7]. However, HK use is greatly restricted by its poor aqueous solubility and limited oral bioavailability in vivo [8]. Hence, several studies have shown that most of the diverse pharmacologic effects mentioned above are after intraperitoneal or intravenous administration rather than oral administration to achieve rapid onset [7], [9], [10].
Novel pharmaceutical approaches have been used recently to improve the hydrophilicity and oral absorption of HK, such as liposomes. However, liposomal formulations are cleared from the blood rapidly because plasma proteins attach to the phospholipid membrane of liposomes, thereby triggering the mononuclear phagocytic system to recognize and phagocytose the liposomes [11]. In addition, a HK microemulsion has been studied in recent years [12], [13]. However, these microemulsion preparations include a large amount of surfactant and co-surfactant, which may cause hemolysis or histopathologic alterations of tissue [14], [15] Some studies have aimed to improve the aqueous solubility and bioavailability of HK via preparation of nanoparticles and nano-micellar formulations [8], [16]. The present work describes a novel approach to counter both challenges by designing a prodrug of HK.
Prodrug technology has become a popular way to optimize the aqueous solubility, stability, drug release, and pharmacokinetics of drug molecules. Typically, prodrugs are designed by adding phosphate, ester, or carbamate groups to the parent drug [17], [18], [19]. Prodrugs are activated via enzymatic hydrolysis by ubiquitous esterases (carboxylesterases, butyrylcholinesterases, acetylcholinesterases, arylesterases) and alkaline phosphatase (which is present throughout the human body) [20], [21].
Among them, phosphate esters are probably the prodrug strategy used most extensively for improving aqueous solubility, not only for oral administration but also for drugs intended for intravenous administration [22]. Phosphate prodrugs release the active parent drug molecule rapidly by endogenous phosphatases, such as alkaline phosphatase, which is particularly abundant in hepatic, skeletal, and renal tissue [23].
There are many examples of successful application of phosphate prodrugs for parenteral administration. Dexamethasone phosphate is a form of dexamethasone based on the sodium phosphate salt. Dexamethasone phosphate increases the aqueous solubility 500-fold (50 mg/ml) when compared with the parent drug (0.01 mg/ml). Fosfluconazole is the phosphate prodrug of fluconazole, and is highly soluble (> 300 mg per ml) compared with fluconazole [24]. Propofol phosphate is an aqueous-soluble prodrug of the widely used intravenous anesthetic propofol. Propofol phosphate has a > 3000-fold increased aqueous solubility (460 mg/ml) when compared with propofol [25]. These phosphate prodrugs have significantly improved aqueous solubilities and, consequently, enhanced absorption, bioavailability, and patient acceptance compared with their parent drugs.
The promising results mentioned above prompted us to evaluate the possibility of improving HK solubility using a phosphate prodrug of HK. The latter has two hydroxyl groups, which provide sites for intervention using phosphate prodrugs whereby HK properties can be manipulated. More importantly, HK has important physiologic activities, such as protection from focal cerebral ischemia-reperfusion injury in rat brains [9], [13], [26]. Hence, whether HK prodrugs that retain these activities deserve attention and merit research.
The main purposes of the present study were to: synthesize and characterize a phosphate prodrug of HK (HKP) to enhance the hydrophilicity of HK; investigate HKP pharmacokinetics in rats; examine if HKP can ameliorate cerebral infarction and protect the brain against neural injury in rats subjected to focal cerebral ischemia-reperfusion injury [2].
2. Materials and methods
2.1. Materials
HKP was obtained from Chengdu Yishan Biotechnology (Chengdu, China) at > 85% yield and 97% purity. HK (Fig. 1) and alkaline phosphatase were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Phosphorus oxychloride and other reagents were obtained from Chengdu Kelong Chemical Company (Chengdu, China).
Fig. 1.

Chemical structure of HKP.
2.2. Synthesis and characterization of HKP
The reaction route for HKP is shown in Scheme 1. Phosphorus oxychloride was allowed to react with HK overnight at 4 °C. After disappearance of the reactant, the reaction was quenched with iced water to obtain honokiol bisphosphate with higher purity. Then, honokiol sodium tetraphosphate was salted out with sodium hydroxide in organic solvent. 1H NMR (400 MHz, D2O) δ 7.56 (dd, J = 8.4, 6.0 Hz, 2H), 7.52–7.44 (m, 2H), 7.33–7.22 (m, 2H), 6.18 (dddt, J = 27.8, 16.9, 10.1, 6.7 Hz, 2H), 5.28–5.11 (m, 4H), 3.58 (d, J = 6.6 Hz, 2H), 3.47 (d, J = 6.8 Hz, 2H). 13C NMR (100 MHz, D2O) δ 149.09, 149.02, 146.70, 146.63, 138.07, 136.93, 136.75, 133.88, 132.64, 132.58, 131.57, 131.51, 131.34, 130.85, 128.72, 128.42, 120.66, 119.71, 115.88, 115.63, 38.73, 34.00. The splitting of four carbon signals was observed in 13C NMR spectra. The HRMS (ESI, m/z) was calculated for C18H16Na4O8P2(M + H+) as 514.9984, and found to be 515.0018.
Scheme 1.
Synthesis of the phosphate prodrug of honokiol.
2.3. High-performance liquid chromatography (HPLC)
HPLC was carried out on a Waters 2695 apparatus coupled to a Waters 2996 photodiode array detector, assisted by Waters Empower 2 software (Waters, Milford, MA, USA). A Kromasil 100-5C18 column (5 µm, 250 mm × 4.6 mm) was used for HPLC (the mobile phase was 80% acetonitrile at a flow rate of 1 ml/min), with the detection absorbance set at 294 nm.
2.4. Comparison of the solubility of HKP and HK
The aqueous solubility of HKP and HK was determined at room temperature in ultrapure water. HK (10 mg) and excess amounts of HKP were added to 1 ml of water in a 1.5-ml polypropylene centrifuge microtube, respectively. Mixtures were stirred for 1 h, filtered (0.22 µm; Millipore, Billerica, MA, USA) and analyzed by HPLC.
2.5. Stability of HKP in physiologic (0.9%) saline
HKP was dissolved in 0.9% NaCl solution formulated as 2 mg/ml solution, then diluted to 500 µM with 0.9% NaCl solution, and aliquoted after sterilization via filtration. HKP saline solution was incubated at 37 °C and sampled at 4, 8, 12, and 24 h as well as 3, 6, 20 and 52 d using HPLC to determine the amount of HK released in HKP saline solution (which reflects the stability of HKP in saline).
2.6. Hydrolysis of HKP by alkaline phosphatase
To determine whether alkaline phosphatase activated the HKP prodrug, HKP was dissolved in alkaline phosphatase solution at pH 7.4. After sterilization by filtration, the HKP saline solution was incubated at 37 °C. Samples were taken at 2, 5, 10, 15 and 30 s as well as 1, 10 and 30 min, and the amount of HK released from HKP determined by HPLC.
2.7. Hydrolysis of HKP in human plasma
Blood donors were healthy individuals and provided written informed consent. Freshly donated whole blood was centrifuged for 5 min at 2000 ×g to remove cells and platelets, and supernatant plasma was collected. Subsequently, HKP was incubated with plasma (final concentration = 500 µM) at 0, 0.5, 2, 4, 8, 12 and 24 h. The plasma sample (200 µl) was mixed vigorously with 1 ml precooled acetonitrile for 5 min in a 1.5-ml Eppendorf tube, and then the mixture was centrifuged at 6000 ×g for 10 min. The clear supernatant was analyzed using HPLC by measuring the concentration of HK, which was released from HKP.
2.8. Pharmacokinetics study
Rats were divided into four groups: HKP intravenous treatment group (HKP-IV group, 32 mg/kg), HK intravenous treatment group (HK-IV group, 20 mg/kg), HKP oral treatment group (HKP-PO group, 64 mg/kg), and HK oral treatment group (HK-PO group, 40 mg/kg). Rats in the HKP-IV group and the HK-IV group or the HKP-PO group and the HK-PO group were intravenously injected or orally administered with equimolar amounts of HK.
For intravenous injection, HKP was dissolved in saline to obtain a concentration of 8 mg/ml of HKP solution (pH = 7.0–7.4) and the injection volume was 4 ml/kg; HK was dissolved in 5% dimethyl sulfoxide (DMSO) + 10% Solutol + 85% deionized water to give a concentration of 4 mg/ml of HK solution, and the injection volume was 5 ml/kg.
For oral administration, HKP was dissolved in deionized water to obtain a HKP solution of concentration 8 mg/ml, and the gavage volume was 8 ml/kg; HK was dissolved in 5% DMSO + 10% Solutol + 85% deionized water to obtain a HK solution of concentration 4 mg/ml, and the gavage volume was 10 ml/kg.
Blood samples (∼0.20 ml) were collected through a cannulated tube at 0, 5, 15, 30 min as well as 1, 2, 4, 6, 8, and 24 h and placed into tubes containing heparin sodium. Samples were centrifuged to separate plasma (6000 ×g, 6 min, 2–8 °C; plasma samples were stored at − 80 °C before analyses). Each plasma sample (15 µl) was collected in a 1.5-ml centrifuge tube and vortex-mixed with 300 µl of warfarin (200 ng/ml; internal standard solution) and then centrifuged at 17 000 ×g for 5 min. Finally, an aliquot of 200 µl of the supernatant was analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
2.9. Establishment of the middle cerebral artery occlusion (MCAO) model
Rats were anesthetized with halothane (2.5%–3%) and subjected to MCAO as described previously, with minor modifications of the method of Longa et al. [27]. Briefly, a 2-cm incision was made with ophthalmic scissors along the midline, and the muscle tissue of the right neck was separated and retracted. The right common carotid artery (CCA), external carotid artery, (ECA) and internal carotid artery (ICA) were exposed in turn. Then, the ECA was ligated, and the ICA was clamped temporarily. After threading at the proximal and distal CCA, respectively, the distal end was fastened, whereas the proximal end was tied loosely. An incision was made between the two lines on the CCA. A 4-0 monofilament nylon suture was inserted past the incision site of the ECA into the ICA for ∼18 mm. Suture insertion was halted if slight resistance was encountered. At this moment, the blood flow of the right MCA was blocked by the suture. Ischemic rats were placed into an environment at 37 °C for 2 h; then the suture was removed carefully to allow MCA reperfusion.
Seventy-nine rats were assigned randomly to four groups, and given intravenous injections of the following drugs: vehicle group, treated with 0.9% saline; positive control group, treated with 10 mg/kg butylphthalide (NBP); optimal-force group, treated with 100 µg/kg HKP; heavy-force group, treated with 5 mg/kg HKP. Rats were treated with HKP immediately for the first dose after modeling and then the second dose was given 6–7 h after modeling. Then, HKP was administered twice daily for the next 2 consecutive days, whereas the vehicle group and positive control group were treated with saline and NBP, respectively, according to the HKP administration method.
2.10. Measurement of infarct volume
Rats were sacrificed by exsanguination of the abdominal aorta under anesthesia after the end of treatments. The bony compartment of the skull was removed carefully to expose the whole brain; the latter was removed, weighed immediately, and placed in a freezer at − 20 °C until it was frozen completely. Frozen brain tissue was cut into slices of thickness 2 mm, posterior to the olfactory bulb, to yield six slices. Slices were placed separately in a six-well plate with 5% 2,3,5-triphenyl tetrazolium chloride (Sigma-Aldrich) at 37 °C in the dark for 15–20 min. Brain slices were removed before immersion in 4% formaldehyde solution protected from light. Non-infarcted areas appeared red after staining, whereas infarcted areas remained white. Each brain slice was placed on a filter paper in sequence, photographed with a digital camera, and white tissue was removed carefully and weighed. The infarction size was calculated as the percentage of the weight of infarcted tissue to the total weight of the brain.
2.11. Determination of the neurological severity score (NSS)
NSS evaluations were made according to the method of Chen and colleagues [28] on 1–3 d after MCAO by an investigator blinded to experimental grouping. The NSS is a composite of motor, sensory, reflex, and balance tests. The NSS is a useful parameter for evaluation of the neuronal effects of drugs in a model of closed head injury in rats [26]. A set of NSS (Supplementary Table 1) was graded on a scale of 0 to 18, where grade 0 represents normal neural function and grade 18 represents the maximal deficit. The higher the score, the more severe the brain injury.
2.12. Statistical analyses
Statistical analyses were undertaken using Prism (GraphPad Software, La Jolla, CA, USA). Results are the mean ± SD. Differences between the mean values of control and treatment groups were calculated using the Student's t-test.
3. Results and discussion
3.1. Solubility
Hydroxy phosphates-based prodrugs are a promising strategy for improving the aqueous solubility of a parent drug. HK is a poorly soluble compound with a solubility < 0.01 mg/ml, whereas HK-loaded micelles have a solubility of only 1.46 mg/ml [29].
Surprisingly, the HKP prodrug enhanced the aqueous solubility of HK (0.0023 ± 0.0003 mg/ml) immensely. This compound had a solubility of 127.54 ± 15.53 mg/ml (Table 1) and was 55,000-fold higher than HK. In general, a water-insoluble compound is defined as those that dissolve to the extent a solubility < 0.01/100 g of water-insoluble, and freely soluble compounds is defined as those that dissolve to the extent of 1 g or more per 100 g of water. Thus, HK was defined as a poorly soluble compound whose solubility limitations were overcome by using a prodrug.
Table 1.
The aqueous solubility of HKP in buffer solution, half-lives of HKP in buffer solutions and half-life of HKP in alkaline phosphatase solution and, all at pH 7.4 (mean ± SD.; n = at least 3).
| Compound | Solubility (mg/ml), r.t.a | Chemical stability, T1/2; | Enzymatic hydrolysis, T1/2; |
|---|---|---|---|
| HKP | 127.54 ± 15.53 | Stableb | 8.90 ± 2.11 s |
| HK | 0.0023 ± 0.0003 | –c | –c |
Room temperature.
No degradation was observed after three weeks.
Not determined.
3.2. Stability in buffer solutions
The stability of the prodrug was determined in saline solution at 37 °C. The assay showed that the HKP prodrug was highly stable, and there was no detection of the parent drug (HK) during 24-h incubation in saline, thereby suggesting that spontaneous hydrolysis of these compounds did not occur (Table 1). Even after 3 d of incubation in saline, the concentration of the HKP prodrug and its parent drug remained steady. Thus, the phosphate ester prodrug exhibited good chemical stability. Hence, not only was HKP solubility sufficient for intravenous administration, its stability could ensure the safety and efficacy of the drug if used clinically [30], [31]. The results mentioned above also suggest that HK released from the HKP prodrug in plasma in vitro in the subsequent experiment was not due to poor stability but instead the result of hydrolysis of HKP by alkaline phosphatase.
3.3. Hydrolysis of HKP in alkaline phosphatase solution and plasma
The enzymatic hydrolysis of HKP was determined in alkaline phosphatase solution at pH 7.4 and 37 °C. The half-life (T1/2) was determined to be 8.90 ± 2.11 s (Table 1, Fig 2A). HK was released completely by hydrolysis of HKP within ∼20 s. A hydrolysis study revealed satisfactory hydrolysis of the prodrug in alkaline phosphatase solution, which is promising for future drug development. However, the alkaline phosphatase used in this experiment was derived from bovine intestinal mucosa. Due to species differences, we should investigate further using human serum to ascertain if HKP can be hydrolyzed to release HK. However, serum has very low dephosphorylase activity for these substrates compared with intestine and liver homogenates [32]. Thus, it is unsurprising that in our assay, HKP release of 500 µM resulted in a concentration of only 32 µM of HK after 48 h, and the hydrolysis rate of HKP in serum was much slower than HKP in the enzyme reaction system (Fig. 2B). This decreased hydrolysis may have been due to the influence of complex components in serum, such as plasma proteins.
Fig. 2.
Alkaline phosphatase can hydrolyze HKP in vitro. The HK concentration was determined using HPLC. (A) 100 µM of HKP was hydrolyzed by alkaline phosphatase at pH 7.4 and 37 °C, with a half-life of 8.90 ± 2.11 s. (B), HKP (500 µM) was hydrolyzed in human plasma at 37 °C, and samples were evaluated at different time points.
Studies have demonstrated that prodrugs that show low protein binding could increase the availability of prodrugs for hydrolysis in plasma, and that high plasma protein binding of a prodrug elicits an extremely slow hydrolysis rate [33], [34]. These findings strongly suggest accelerated hydrolysis of phosphate prodrugs in vivo due to the additional phosphatase enzymes in tissues. Thus, the pharmacokinetics of HKP prodrugs should be investigated further.
3.4. Pharmacokinetics of HKP and HK in rats
After intravenous or oral administration of a single dose of HKP and HK to healthy rats, HK was detected rapidly in plasma samples. The pharmacokinetic parameters of HK in plasma samples of the four groups are listed in Table 2. The time to reach the maximum plasma concentration (Tmax) in the HKP-IV group occurred at 0.08 h, which was identical to that of the parent drug. This result indicated that the active parent drug (HK) was released rapidly from the phosphate prodrug (HKP) by the abundant phosphatases in vivo, which substantiated the feasibility of the phosphate prodrug method.
Table 2.
The non-compartmental pharmacokinetic parameters of HK in rat plasma samples of four groups after oral or intravenous administration.
| Variable | HKP-IV | HK-IV2 | HKP-PO | HK-PO |
|---|---|---|---|---|
| 32 mg/kg | 0 mg/kg | 64 mg/kg | 40 mg/kg | |
| T1/2 (h) | 7.97 ± 1.30 | 0.66 ± 0.01 | 2.27 ± 1.28 | 6.68 ± 6.97 |
| Tmax (h) | 0.08 | 0.08 | 0.25 | 1.00 |
| Cmax (ng/ml) | 32 554.80 ± 3331.71 | 12 256.90 ± 1761.85 | 312.25 ± 63.03 | 34.72 ± 19.68 |
| AUC0-t (h ng/ml) | 14 151.45 ± 987.73 | 6600.83 ± 838.38 | 356.66 ± 111.49 | 157.80 ± 59.33 |
| AUC0-∞ (h ng/ml) | 14 289.34 ± 967.70 | 6603.81 ± 838.69 | 379.09 ± 119.08 | 287.59 ± 99.01 |
| Vz (ml/kg) | 26.02 ± 6.04 | 2.90 ± 0.34 | ||
| CL (ml/h/kg) | 2.25 ± 0.15 | 3.06 ± 0.42 | ||
| MRT0-t (h) | 0.96 ± 0.13 | 0.50 ± 0.01 | 1.40 ± 0.02 | 3.27 ± 0.38 |
| MRT0-∞ (h) | 1.30 ± 0.14 | 0.50 ± 0.01 | 2.01 ± 0.45 | 10.19 ± 10.18 |
These results also showed improved pharmacokinetic properties using the prodrug design. The plasma concentration measured at 0.08 h for HKP-IV and HK-IV was 32 554.80 ± 3331.71 ng/ml and 12 256.90 ± 1761.85 ng/ml, respectively (Table 2). Both profiles exhibited a rapid distribution phase followed by a slower elimination phase (Fig. 3). However, the T1/2 after intravenous administration of HKP (T1/2 = 7.97 ± 1.30 h) was prolonged remarkably compared with those after intravenous administration of HK (T1/2 = 0.66 ± 0.01 h), which was due (at least in part) to the prodrug HKP diffusing into tissues with relatively less phosphatase and thus releasing the parent drug (HK) relatively slowly. This diffusion could also explain why the HK concentration in plasma declined apparently in the first hour after administration but, in the subsequent 23 h, the HK concentration decreased more slowly and smoothly. Compared with the HK-IV group, the HKP-IV group showed approximate doubling of the HK concentration in the area under the plasma concentration–time curve (AUC0-∞ = 14 289.34 ± 967.70 h ng/ml). The most important result was the terminal volume of distribution (Vz) of HKP, which showed a rate that was 9-fold higher (26.02 ± 6.04 ml/kg) than that of the parent drug (2.90 ± 0.342 ml/kg). As one of the most important pharmacokinetic properties of a drug candidate, the Vz is a major determinant of the T1/2 and dosing frequency of a drug [35]. Based on its improved pharmacokinetic properties, the prodrug HKP could merit further development as a drug candidate.
Fig. 3.
The HK concentration was determined by LC-MS/MS after intravenous and oral administration with equimolar amounts of HKP and HK in rat plasma in vivo at several time points. (A) Mean plasma concentration–time curves of HK after intravenous administration (HKP-32 mg/kg, HK-20 mg/kg) and (B) Mean plasma concentration–time curves of HK after oral administration (HKP-64 mg/kg, 40 mg/kg). Each point represents the mean ± SD (n = 3).
Rapid generation of the parent drug via intestinal alkaline phosphatase leading to precipitation of the parent drug may explain the failure of many oral phosphate prodrugs to demonstrate improved absorption compared with their soluble parent drugs [36]. Interestingly, the pharmacokinetics of these prodrugs after oral administration were improved. The maximum plasma concentration (Cmax; 312.25 ± 63.03 ng/ml) of the HKP-PO group was about 10-fold greater than that of the HK-PO group, suggesting that HKP had greater absorption than the parent drug (HK) (Table 2). Typically, phosphate prodrugs administered via the oral route are hydrolyzed rapidly by alkaline phosphatases in the gut epithelia to their parent drugs during absorption, with only minimal concentrations of prodrugs reaching the blood circulation [22]. Therefore, significant acceleration of the oral absorption rate in vivo using phosphate prodrugs is difficult. However, our study revealed that oral HKP phosphate had a short Tmax (∼15 min) compared with that of the HK-PO group (1 h), thereby reflecting rapid absorption compared with the parent drug. This increased absorption was (at least in part) because of increased permeability of the parent drug molecule due to its prodrug structure.
It has been reported that nanomicellar formulations of HK result in significant increase in oral bioavailability, and Cmax has been shown to be increased significantly (4.06 fold) compared with the free drug [8]. In our study, the Cmax and AUC of HK were increased dramatically in the HKP-PO group (10-fold), and the phosphate prodrug system seemed to have better absorption of HK compared with that of HK nanomicellar formulations. Thus, HKP had greater absorption, and its use in practical applications could be meaningful. Taken together, HKP was converted readily to HK in vivo in rats for administration via intravenous or oral routes with improved pharmacokinetic properties. Hence, whether HK prodrugs retain these pharmacologic activities deserves further research.
3.5. HKP ameliorated ischemia-reperfusion injury in MCAO rats
Studies have shown that HK has protective roles during ischemia-reperfusion injuries in rat brain through disruption of PSD95-nNOS interactions because it inhibits neutrophil infiltration, production of reactive oxygen species, and the cell cycle [7], [9], [13]. Lin and colleagues documented that HK can pass through the blood–brain barrier, induce the death of neuroblastoma cells [37], and that ∼10% of HK in plasma can cross the blood–cerebrospinal fluid barrier into cerebrospinal fluid [10]. HKP was confirmed to greatly optimize the pharmacokinetic parameters of its parent drug (HK) in our study. Therefore, it was necessary to confirm if HK can help to ameliorate ischemia-reperfusion injury after structural transformation. The Tmax of the HKP-IV group showed that the active parent drug (HK) was released from the phosphate prodrug (HKP) in vivo within 5 min. Therefore, intravenous injection of HKP could be regarded as HK administration. Thus, we thought it unnecessary to add an experimental group that was administered HK. Also, the poor solubility of HK makes its intravenous administration difficult. As for the positive drug NBP, several studies have shown that NBP aids amelioration of ischemia-reperfusion injury, and NBP is approved in China for the treatment of ischemic stroke [38], [39], [40]. Further, NBP and HKP can be dissolved in physiologic (0.9%) saline, but HK cannot. Thus, we did not create an HK experimental group but choose NBP as our positive drug to compare the differences between HKP and NBP groups in this experiment.
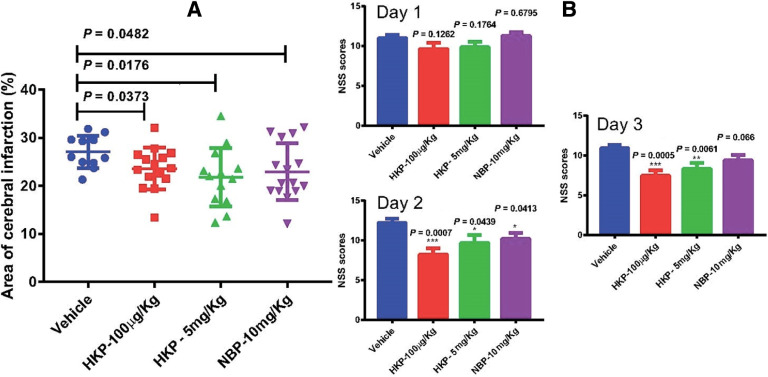
Following 2 h of blood-flow interruption and 15 min of reperfusion, the brain tissue in rats underwent a certain degree of edema and injury. Rats treated with HKP (100 µg/kg), HKP (5 mg/kg) or NBP (10 mg/kg) showed a significant decrease in infarct volume 72 h after reperfusion compared with the vehicle group (P = 0.0373, 0.0176 and 0.0482, respectively) (Fig. 4). The efficacy observed in the HKP-5 mg/kg group was comparable with that of the NBP group, but the HKP-5 mg/kg group showed slight superiority in reducing the brain infarct volume of rats compared with the NBP group. NSS evaluations were made 1–3 d after MCAO. The NSS for the vehicle group, HKP-100 µg/kg group, HKP-5 mg/kg group and NBP-10 mg/kg group 1–3 d after MCAO are shown in Fig. 4. There were no significant differences between the vehicle control group (11 ± 0.39) and HKP-100 µg/kg group (9.63 ± 0.75, P = 0.1262), HKP-5 mg/kg group (9.84 ± 0.71, P = 0.1764) or NBP-10 mg/kg group (11.26 ± 0.48, P = 0.6795) on day-1, respectively. There was a downward trend in the NSS of the HKP-100 µg/kg group and HKP-5 mg/kg group compared with the vehicle group. After 2 d of intravenous drug administration, the NSS of animals in the HKP-100 µg/kg group (P = 0.0007), HKP-5 mg/kg group (P = 0.0439), and NBP-10 mg/kg group (P = 0.0413) was 8.21 ± 0.83, 9.69 ± 1.02, and 10.18 ± 0.74, respectively, significantly lower than that of the model control group (12.21 ± 0.54). Significant functional improvement was observed in the HKP-100 µg/kg group (P = 0.0005), HKP-5 mg/kg group (P = 0.0061) 3 d after MCAO except for the NBP-5 mg/kg group (P = 0.066), with a score of 7.47 ± 0.71, 8.31 ± 0.75, and 9.41 ± 0.64, respectively, compared with the vehicle control group (10.93 ± 0.40). These NSS results demonstrated that the effect of lower doses of HKP is better than higher, and this phenomenon may be due to the inverted U-shaped dose-effect. HKP and NBP had roles in improving the NSS of model rats compared with the vehicle group. Furthermore, the neuroprotective effects of HKP on rats was stronger than that of the NBP group.
Fig. 4.
HKP attenuated focal cerebral ischemia-reperfusion injury. (A) Quantification of infarct volumes 72 h after focal ischemia. (B) Quantification of the neurologic severity score (NSS) 24, 48, and 72 h after focal ischemia. Bars represent mean ± SEM (n = 15).
In conclusion, our results confirmed that HKP could decrease brain infarction and improve the neurologic function of rats after cerebral ischemia-reperfusion injury, which was consistent with the results of two studies [1,2].
Stroke is a leading cause of long-term disability, and outcomes are directly related to timely intervention. Several studies and stroke guidelines have reported that the optimal treatment time-window of stroke is within 4.5 h and cannot exceed 24 h [41], [42], [43], which require the rapid onset of drugs. Hence, HKP could be used to treat stroke via intravenous injection owing to its excellent aqueous solubility to achieve rapid onset. These results suggest that HKP might be a potent neuroprotective agent against focal cerebral ischemia-reperfusion injury.
4. Conclusions
Our design of a phosphate prodrug focused on masking the hydroxy groups of HK. As the phosphate prodrug of HK, HKP enhanced the aqueous solubility of HK greatly. HKP was converted readily to HK in vivo in rats administrated via intravenous and oral routes with improved pharmacokinetic properties and retained its ability to aid amelioration of ischemia-reperfusion injury after structural transformation. Taken together, HKP is a potentially useful aqueous-soluble phosphate prodrug with improved pharmacokinetic properties that could merit further development as a drug candidate.
Acknowledgments
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by the Scientific Research Fund of the National Natural Science Foundation of China (81201668), Chengdu Science and Technology Bureau (2015-HM01-00506-SF, 2018-YF05-00454-SN), Scientific Research Fund of the Sichuan Provincial Education Department (17CZ0011, 17ZA0109), and the Scientific Research Fund of Chengdu Medical College (CYCG15-01). We thank the staff at Shanghai Medicilon for pharmacokinetic studies of HKP (www.medicilon.com/). We thank Arshad Makhdum, Ph.D., from Liwen Bianji (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2018.11.004.
Contributor Information
Bing Guang, Email: guangb@yahoo.com.
Tai Yang, Email: taiyang@cmc.edu.cn.
Appendix. Supplementary materials
References
- 1.Watanabe K., Watanabe H., Goto Y., Yamaguchi M., Yamamoto N., Hagino K. Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: Central depressant effects. Planta Med. 1983;49(2):103–108. doi: 10.1055/s-2007-969825. [DOI] [PubMed] [Google Scholar]
- 2.Liou K.T., Lin S.M., Huang S.S., Chih C.L., Tsai S.K. Honokiol ameliorates cerebral infarction from ischemia-reperfusion injury in rats. Planta Med. 2003;69(2):130–134. doi: 10.1055/s-2003-37707. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y.R., Chen H.H., Ko C.H., Chan M.H. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. 2007;81(13):1071–1078. doi: 10.1016/j.lfs.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Dikalov S., Losik T., Arbiser J.L. Honokiol is a potent scavenger of superoxide and peroxyl radicals. Biochem Pharmacol. 2008;76(5):589–596. doi: 10.1016/j.bcp.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Q., Yi L.T., Pan Y. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):715–725. doi: 10.1016/j.pnpbp.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Bai X., Cerimele F., Ushio-Fukai M. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278(37):35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 7.Wang H., Liao Z., Sun X. Intravenous administration of honokiol provides neuroprotection and improves functional recovery after traumatic brain injury through cell cycle inhibition. Neuropharmacology. 2014;86:9–21. doi: 10.1016/j.neuropharm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Godugu C., Doddapaneni R., Singh M. Honokiol nanomicellar formulation produced increased oral bioavailability and anticancer effects in triple negative breast cancer (TNBC) Colloids Surf B: Biointerfaces. 2017;153:208–219. doi: 10.1016/j.colsurfb.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou K.T., Shen Y.C., Chen C.F., Tsao C.M., Tsai S.K. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992(2):159–166. doi: 10.1016/j.brainres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Duan X., Yang G. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS One. 2011;6(4):e18490. doi: 10.1371/journal.pone.0018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X.H., Cai L.L., Zhang X.Y. Improved solubility and pharmacokinetics of PEGylated liposomal honokiol and human plasma protein binding ability of honokiol. Int J Pharm. 2011;410(1–2):169–174. doi: 10.1016/j.ijpharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Qin X., Yin J., Zhang W., Li J., Wen J., Chen S. Acute and subchronic toxicities in dogs and genotoxicity of honokiol microemulsion. Regul Toxicol Pharmacol. 2018;95:362–370. doi: 10.1016/j.yrtph.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Hu Z., Bian X., Liu X. Honokiol protects brain against ischemia-reperfusion injury in rats through disrupting PSD95-nNOS interaction. Brain Res. 2013;1491:204–212. doi: 10.1016/j.brainres.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 14.He C.X., He Z.G., Gao J.Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin Drug Deliv. 2010;7(4):445–460. doi: 10.1517/17425241003596337. [DOI] [PubMed] [Google Scholar]
- 15.von Corswant C., Thoren P., Engstrom S. Triglyceride-based microemulsion for intravenous administration of sparingly soluble substances. J Pharm Sci. 1998;87(2):200–208. doi: 10.1021/js970258w. [DOI] [PubMed] [Google Scholar]
- 16.Fang F., Gong C., Qian Z. Honokiol nanoparticles in thermosensitive hydrogel: Therapeutic effects on malignant pleural effusion. ACS Nano. 2009;3(12):4080–4088. doi: 10.1021/nn900785b. [DOI] [PubMed] [Google Scholar]
- 17.Jornada D.H., dos Santos Fernandes G.F., Chiba D.E., de Melo T.R., dos Santos J.L., Chung M.C. The prodrug approach: A successful tool for improving drug solubility. Molecules. 2015;21(1):42. doi: 10.3390/molecules21010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Li J., Li Z., Tang X., Zhang Z. Pharmacokinetics of a ternary conjugate based pH-responsive 10-HCPT prodrug nano-micelle delivery system. Asian J Pharm Sci. 2017;12(6):542–549. doi: 10.1016/j.ajps.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo C., Sun J., Sun B.J., He Z.G. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol Sci. 2014;35(11):556–566. doi: 10.1016/j.tips.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Zawilska J.B., Wojcieszak J., Olejniczak A.B. Prodrugs: A challenge for the drug development. Pharmacol Rep. 2013;65(1):1–14. doi: 10.1016/s1734-1140(13)70959-9. [DOI] [PubMed] [Google Scholar]
- 21.Song X., Sun Y., Zhao C., He Z. The effect of cephalexin in influencing the pharmacokinetics of a novel drug-5′-valyl-cytarabine hydrochloride. Asian J Pharm Sci. 2017;12(2):143–148. doi: 10.1016/j.ajps.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huttunen K.M., Raunio H., Rautio J. Prodrugs – from serendipity to rational design. Pharmacol Rev. 2011;63(3):750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 23.Sharma U., Pal D., Prasad R. Alkaline phosphatase: An overview. Indian J Clin Biochem. 2014;29(3):269–278. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoyama T., Ogata K., Shimizu M. Pharmacokinetics of fluconazole and fosfluconazole after intraperitoneal administration to peritoneal dialysis rats. Drug Metab Pharmacokinet. 2005;20(6):485–490. doi: 10.2133/dmpk.20.485. [DOI] [PubMed] [Google Scholar]
- 25.Lang B.C., Yang J., Wang Y. An improved design of water-soluble propofol prodrugs characterized by rapid onset of action. Anesth Analg. 2014;118(4):745–754. doi: 10.1213/ANE.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y., Li M., Su N. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol Med Rep. 2016;13(2):1353–1360. doi: 10.3892/mmr.2015.4660. [DOI] [PubMed] [Google Scholar]
- 27.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Li Y., Wang L. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 29.Song Z.M., Sun J.J., Deng P.Z. Oligochitosan-pluronic 127 conjugate for delivery of honokiol. Artif Cells Nanomed Biotechnol. 2018:1–11. doi: 10.1080/21691401.2018.1434785. [DOI] [PubMed] [Google Scholar]
- 30.Liang Y., Narayanasamy J., Schinazi R.F., Chu C.K. Phosphoramidate and phosphate prodrugs of (-)-beta-D-(2R,4R)-dioxolane-thymine: synthesis, anti-HIV activity and stability studies. Bioorg Med Chem. 2006;14(7):2178–2189. doi: 10.1016/j.bmc.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Kumpulainen H., Jarvinen T., Mannila A. Synthesis, in vitro and in vivo characterization of novel ethyl dioxy phosphate prodrug of propofol. Eur J Pharm Sci. 2008;34(2–3):110–117. doi: 10.1016/j.ejps.2008.02.121. [DOI] [PubMed] [Google Scholar]
- 32.Lo W.Y., Balasubramanian A., Helsby N.A. Hydrolysis of dinitrobenzamide phosphate prodrugs: The role of alkaline phosphatase. Drug Metabol Drug Interact. 2009;24(1):1–16. doi: 10.1515/dmdi.2009.24.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Rasheed A., Kumar C.K., Mishra A. Synthesis, hydrolysis studies and phamacodynamic profiles of amide prodrugs of dexibuprofen with amino acids. J Enzyme Inhib Med Chem. 2011;26(5):688–695. doi: 10.3109/14756366.2010.548327. [DOI] [PubMed] [Google Scholar]
- 34.Shameem M., Imai T., Yoshigae Y., Sparreboom A., Otagiri M. Stereoselective hydrolysis of O-isovaleryl propranolol and its influence on the clearance of propranolol after oral administration. J Pharm Sci. 1994;83(12):1754–1757. doi: 10.1002/jps.2600831221. [DOI] [PubMed] [Google Scholar]
- 35.Smith D.A., Beaumont K., Maurer T.S., Di L. Volume of distribution in drug design. J Med Chem. 2015;58(15):5691–5698. doi: 10.1021/acs.jmedchem.5b00201. [DOI] [PubMed] [Google Scholar]
- 36.Heimbach T., Oh D.M., Li L.Y., Rodriguez-Hornedo N., Garcia G., Fleisher D. Enzyme-mediated precipitation of parent drugs from their phosphate prodrugs. Int J Pharm. 2003;261(1–2):81–92. doi: 10.1016/s0378-5173(03)00287-4. [DOI] [PubMed] [Google Scholar]
- 37.Lin J.W., Chen J.T., Hong C.Y. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro Oncol. 2012;14(3):302–314. doi: 10.1093/neuonc/nor217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang P., Guo Z.F., Xu Y.M., Li Y.S., Song J.G. N-Butylphthalide (NBP) ameliorated cerebral ischemia reperfusion-induced brain injury via HGF-regulated TLR4/NF-kappaB signaling pathway. Biomed Pharmacother. 2016;83:658–666. doi: 10.1016/j.biopha.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 39.Wang S., Ma F., Huang L. Dl-3-n-butylphthalide (NBP): A promising therapeutic agent for ischemic stroke. CNS Neurol Disord Drug Targets. 2018;17(5):338–347. doi: 10.2174/1871527317666180612125843. [DOI] [PubMed] [Google Scholar]
- 40.Qin C., Zhou P., Wang L. Dl-3-N-butylphthalide attenuates ischemic reperfusion injury by improving the function of cerebral artery and circulation. J Cereb Blood Flow Metab. 2018:1–11. doi: 10.1177/0271678X18776833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers W.J., Rabinstein A.A., Ackerson T. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 42.Furie K.L., Jayaraman M.V. 2018 Guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49(3):509–510. doi: 10.1161/STROKEAHA.118.020176. [DOI] [PubMed] [Google Scholar]
- 43.Pena I.D., Borlongan C., Shen G., Davis W. Strategies to extend thrombolytic time window for ischemic stroke treatment: An unmet clinical need. J Stroke. 2017;19(1):50–60. doi: 10.5853/jos.2016.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.