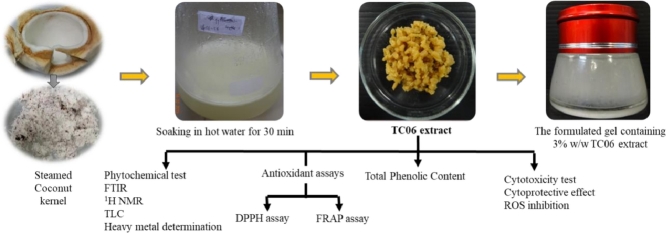
Abstract
The biological activity of coconut (Cocos nucifera L.) extracts from its kernels and various parts was reported by many previous studies, it is therefore believable that the extracts of its kernels might show some activities in topical formulations. Among several kernel extracts, the TC06 extract prepared by soaking the steamed coconut kernels in hot water showed the highest total phenolic content (6.98 ± 0.30 mg GAE/g extract) and the strongest antioxidant activity as determined using FRAP and DPPH methods with a reducing power value of 4.12 ± 0.16 mg AAE/g of extract and an SC50 value of 2.38 ± 0.14 mg/ml, respectively. In addition, this extract did not display any cytotoxic effects in the concentration range of 50–3200 µg/ml. Meanwhile, it revealed cytoprotective effects against t-BHP-induced cytotoxicity in HaCaT cells at concentrations higher than 400 µg/ml. The results of phytochemical investigations including a chemical color test, TLC, 1H NMR and FTIR suggested that the TC06 extract was mainly composed of flavonoids and terpenoids. Furthermore, the concentrations of heavy metals including As, Cd, Hg, and Pb in the TC06 extract were below permissible limits. According to the solubility, the TC06 extract was incorporated into gels using Carbopol Ultrez 21 as a gelling agent. The formulated gel containing 3% (w/w) TC06 extract was stable at 4 °C and 25 °C with 75% RH throughout the storage period. It was found that the Carbopol Ultrez 21-based hydroalcoholic gel containing an aqueous extract of coconut kernels exhibited antioxidant activities in the two assays and showed a sufficient consistency, a pleasing color, and a non-oily perception during the period of observation.
Keywords: Coconut kernel, Extract, Gel, Antioxidant activity, Phytochemical screening
Graphical abstract
Ethanolic and aqueous extracts of coconut kernels were composed of phenolic compounds exhibiting antioxidant activities as determined using DPPH and FRAP methods. An aqueous extract (TC06) was selected for the topical preparation of a hydroalcoholic gel due to its nontoxicity, water solubility, and chemical constituents. The hydroalcoholic gel composed of TC06 extract, Carbopol Ultrez 21, ethanol, and triethanolamine appeared to be a suitable formulation due to its physicochemical characteristics and stability.
1. Introduction
The coconut tree (Cocos nucifera L.) is a palm tree related to the family Arecaceae, which is widespread throughout tropical countries, especially Thailand. Many studies have unveiled that coconut kernels play a significant role in human health due to the fact that they contain essential fatty acids and amino acids, dietary fibers, and biologically active phytochemicals [1], [2], [3]. Furthermore, recent studies have demonstrated that coconut kernel extracts exhibit anti-inflammatory, antibacterial, antiviral, antiprotozoal and anti-hyperglycemic effects [3], [4], [5]. Generally, most antioxidants from plants, such as phenolic compounds, ascorbic acid, and omega 3, are safer than those from chemical synthesis. The combination of different natural antioxidants exhibited a synergistic effect to protect the human skin against oxidative stresses [6]. Coconut kernel extracts composed of various antioxidants should therefore be better than pure compounds isolated from those extracts. Currently, it is well-known that gel formulations are designed to deliver polar active ingredients efficiently, and to be less sticky, less oily, and easily washable [7]. However, there have been few studies on the antioxidant and cytoprotective activities of coconut kernel extracts. In the current study we prepared coconut kernel extracts under various conditions and then subjected them to preliminary phytochemical evaluation, measurement of polyphenolic contents using a Folin–Ciocalteu assay, analysis of heavy metal contents using an inductively coupled plasma-mass spectrometer (ICP-MS), determination of antioxidant capacities using ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) methods, and evaluation of cytotoxicity and cytoprotective effects in HaCaT cells using an MTT assay. Ultimately, the selected coconut kernel extract which exhibited the best properties among all extracts was incorporated into an appropriate gel formulation and investigated for its stability and antioxidant activities.
2. Materials and methods
2.1. Standards and reagents
Glacial acetic acid and ferric chloride were obtained from QRëC (Selangor, Malaysia). Ethanol, hydrochloric acid, and sodium hydroxide were purchased from Labscan (Dublin, Ireland). Dimethyl sulfoxide (DMSO), dimethyl sulfoxide-d6, ellagic acid, gallic acid, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), tert‑butyl hydroperoxide (t-BHP), 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA), and 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) were bought from the Sigma-Aldrich Chemical Corporation (Missouri, USA). l-ascorbic acid was obtained from Chem-Supply Pty Ltd (Gillman, South Australia). Folin–Ciocalteu was purchased from Loba Chemie (Mumbai, India). For TLC analysis, silica gel 60 F254 aluminium sheets and reference standards with at least 99% purity including lauric acid, monolaurin, dilaurin and trilaurin were purchased from Merck KGaA (Darmstadt, Germany) and Nu-Chek Prep, Inc. (Minnesota, USA), respectively. For cell testing, penicillin-streptomycin, Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), and 0.25% trypsin-EDTA solution were purchased from Life Technologies Inc. (California, USA). Hank's balanced salts, phosphate-buffered saline (PBS), and amphotericin B were obtained from Capricorn Scientific (Ebsdorfergrund, Germany). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was acquired from Bio Basic Inc. (Ontario, Canada). For gel formulations, Carbopol Ultrez 21 and Carbopol 940 were bought from P.C. Drug center (Bangkok, Thailand). Poloxamer 407 was purchased from BASF (Ludwigshafen, Germany). Triethnolamine 99% was bought from Ajax Finechem (New South Wales, Australia). Potassium bromide was purchased from Thermo Fisher Scientific (Massachusetts, USA). Finally, ICP multi-element standard solution XIII bought from Agilent Technologies, Santa Clara, USA, ultrapure water generated by GenPure equipment (TKA Wasseraufbereitungssysteme GmbH, Germany), and nitric acid bought from Merck, Darmstat, Germany were used to determine heavy metals.
2.2. Plant materials
Tall coconut fruits (C. nucifera var. typical) known in Thailand as “Maphrao Kang” were collected from 7 to 10-year-old palms in local areas of Nakhon Pathom, Thailand in January, 2016. The key morphological characteristics from the Coconut Varieties in Thailand by Narong Chomchalow [8] were used to identify this plant and a voucher specimen was kept at the Faculty of Pharmacy, Silpakorn University. The coconut kernels were separated from their shells, cleaned with deionized water, blended with a stainless steel blender, and then kept at − 20 °C.
2.3. Preparation of coconut kernel extracts
Nine coconut kernel extracts (TC01–TC09) were prepared from powdered coconut kernel using different kinds of kernels (fresh, steamed, and dried kernels) and extraction conditions (maceration with 50% (v/v) or 95% (v/v) ethanol for 24 h and soaking in hot water (100 °C) for 30 min) as shown in Table 1. Powdered coconut kernel (1 kg) was separately transferred into 2000 ml of each solvent and then extracted under several conditions as described in Table 1. After extraction, each of these mixtures was filtered using a piece of muslin. The filtrate was left overnight at 4 °C and then the aqueous phase (lower layer) was separated and the solvent was evaporated using a rotary evaporator at 45 °C under a reduced pressure. Finally, the residual water in all extracts was removed using a freeze dryer, Model 6112974 (Labconco, Kansas City, MO, USA). All extracts were kept in the dark at 4 °C until use.
Table 1.
Code of extracts showing various kinds of kernels and extraction conditions.
| Code of extracts | Kernel types |
Extraction conditions |
||||
|---|---|---|---|---|---|---|
| Fresh kernel | Steamed kernel | Dried kernel | Maceration with 95% (v/v) ethanol for 24 h | Maceration with 50% v/v ethanol for 24 h | Soaking in hot water (100 °C) for 30 min | |
| TC01 | √ | √ | ||||
| TC02 | √ | √ | ||||
| TC03 | √ | √ | ||||
| TC04 | √ | √ | ||||
| TC05 | √ | √ | ||||
| TC06 | √ | √ | ||||
| TC07 | √ | √ | ||||
| TC08 | √ | √ | ||||
| TC09 | √ | √ | ||||
2.4. Phytochemical tests
Twenty milligrams of each extract was dissolved in 20 ml of aqueous ethanol solution until the solution was clear. The redissolved extracts were evaluated to determine the presence of alkaloids, carbohydrates, flavonoids, tannins, steroids, and terpenoids according to standard methods [9]. The resulting solution was observed for color change and/or precipitate formation to indicate a positive test result. All tests were performed in duplicate.
2.4.1. Detection of alkaloids
One milliliter each of Wagner, Mayer, and Dragendorff reagents were separately added into 2 ml of the extract solution. A reddish brown precipitate (Wagner), cream colored precipitate (Mayer), and orange/reddish-brown precipitate (Dragendorff) indicated the presence of alkaloids.
2.4.2. Detection of carbohydrates
The identification of carbohydrates was performed using a Molisch's test. Three drops of α-naphthol were placed into 2 ml of the extract solution and 1.5 ml of concentrated sulfuric acid was then steadily added from the sides of a test tube. A violet ring at the interface between the acid and extract layers indicated the presence of carbohydrates.
2.4.3. Detection of flavonoids
2.4.3.1. Ferric chloride test
One milliliter of 5% (w/v) ferric chloride solution was added to 2 ml of the extract solution. Formation of a bluish color revealed the existence of the phenolic nuclei.
2.4.3.2. Sodium hydroxide test
One milliliter of 10% (w/v) sodium hydroxide solution was added to 2 ml of extract solution. The intense yellow color disappeared after adding 1 ml of diluted hydrochloric acid indicated the presence of xanthone groups related to flavonoids.
2.4.3.3. Shinoda test
Some magnesium ribbons and concentrated hydrochloric acid were placed into 2 ml of the extract solution. The appearance of orange to red color indicated the existence of flavonoids.
2.4.4. Detection of tannins
2.4.4.1. Ferric chloride test
One milliliter of 5% (w/v) ferric chloride solution was added to 2 ml of the extract solution. Formation of a blackish blue color indicated the existence of tannins.
2.4.4.2. Gelatin test
One milliliter of 1% (w/v) gelatin solution containing sodium chloride (10%, w/v) was added to 2 ml of the extract solution. The white cloudy precipitates which formed revealed the presence of tannins.
2.4.5. Detection of terpenoids and steroids
The Liebermann–Burchard test was used for the detection of steroids and terpenoids. Ten milligrams of the extract was dissolved in 5 drops of acetic anhydride and then 5 drops of concentrated sulfuric acid were carefully added. After mixing and waiting 5 min the reddish-brown color and green color indicated the presence of terpenoids and steroids respectively.
2.5. Thin layer chromatography (TLC) analysis
TLC using the method with external standards [10] was used for the qualitative analysis of chemical constituents in all extracts. Nine extracts, four polar standards (ascorbic acid, ellagic acid, gallic acid, and quercetin), and nonpolar standards (lauric acid, monolaurin, dilaurin, and trilaurin) were separately dissolved in an appropriate solvent to obtain a solution having a concentration of about 1 mg/ml. Each 5 µl of extract and standard solution was individually spotted onto silica gel 60 F254 TLC plates (250 µm thickness). The plates were separately developed in dichloromethane:methanol (7:3, v/v) and hexane:dichloromethane:ethyl acetate (8:1:1, v/v/v) for the detection of polar and nonpolar substances respectively. The mobile phases were modified from our previous method [11]. After drying, the TLC plates were examined under UV light at 366 and 254 nm (Camag UV cabinet, USA), exposed to iodine vapor, and then sprayed with a freshly prepared solution of p-anisaldehyde in sulfuric acid. The Rf values of the interesting spots were calculated.
2.6. Compound characterization
Fourier-transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance spectroscopy (1H NMR) were used for the characterization of the phytochemical constituents of each extract. The sample extract (5 mg) was triturated with dried, purified potassium bromide (100 mg) and then compressed into a pellet using a hydraulic press. The FTIR spectrum of the extract was recorded within the wavenumber range from 4000 to 400 cm−1 in an FTIR spectrometer (Thermo Electron Scientific Instruments Corporation, Madison, WI, USA).
The 1H NMR spectrum was recorded at 400 MHz on a Bruker AVANCE 400 NMR spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). A sample extract (5 mg) was dissolved in dimethyl sulfoxide-d6 (4 ml) with tetramethylsilane (TMS) as an internal standard and then placed in a 5 mm NMR tube prior to 1H NMR analysis.
2.7. Measurement of polyphenolic contents
The total phenolic contents of all extracts were evaluated using the modified Folin–Ciocalteu assay [12]. Thirty microliters of 10 mg/ml extract solution was combined with 150 µl of Folin–Ciocalteu reagent and then 2 ml of distilled water and 1 ml of 7% w/v sodium carbonate solution were added to the mixture. After mixing and standing for 30 min at room temperature in the dark, the values of absorbance were determined using a UV–visible spectrophotometer, Model U-2990 (Hitachi, Japan) at 765 nm wavelength. The total amount of phenolics was calculated as milligrams of gallic acid equivalent (GAE) from the calibration curve of a gallic acid standard solution using concentrations of 50, 100, 150, 250, and 500 µg/ml and presented as mg GAE/g extract. The data were reported as the average of triplicate analyses.
2.8. Antioxidant activity measurements
2.8.1. DPPH assay
The DPPH assay was performed with slight modifications according to a previous work [12]. The DPPH radical can obtain a hydrogen radical or electron from antioxidants and turns into a stable diamagnetic molecule, causing a color change of a DPPH solution from purple to yellow. A decrease in absorbance of DPPH radicals is measured at a wavelength of 515 nm.
One hundred microliters of individual sample extracts in a methanolic solution at concentrations of 1.25, 2.5, 5, 7.5, 10 mg/ml were added to 100 µl of a 0.5 mmol/l DPPH in methanol, and then the solutions were mixed thoroughly. After incubation for 30 min at room temperature in the dark, the decrease in absorbance of each solution was read at 517 nm using a microplate reader (Fusion universal microplate analyzer, Model A153601, Packard BioScience Company, USA) against a blank (50%, v/v, aqueous methanol). A solution of l-ascorbic acid (100 µl), at concentrations between 10 and 50 µg/ml in methanol was used as a positive control. The radical scavenging activity of the extract solution was expressed as the SC50 value. All experiments were determined in triplicate.
2.8.2. FRAP assay
The FRAP assay was conducted using the same procedure described in a previous report with some modifications [12]. This assay measures the antioxidant potencies in samples through the reduction of the iron ions from Fe3+ to Fe2+. After contact with an antioxidant, the light blue reagent containing Fe3+-TPTZ changes to a dark blue solution owing to the formation of Fe2+-TPTZ. The FRAP reagent was prepared by mixing 25 ml of 0.3 mol/l acetate buffer (pH 3.6), 2.5 ml of 0.1 mol/l 2,4,6-Triphridyl-s-triazine (TPTZ) in 0.4 mol/l hydrochloric acid, and 2.5 ml of 0.2 mol/l ferric chloride hexahydrate in distilled water. This reagent had to be prepared freshly and then warmed to 37 °C prior to use. The FRAP reagent (100 µl) was mixed with 10 mg/ml aqueous solution of a sample extract (100 µl). After incubation at 37 °C for 30 min, the increase in absorbance was recorded at 593 nm against the reagent blank, using a microplate reader (Fusion universal microplate analyzer, Model A153601, Packard BioScience Company, USA). Ascorbic acid was used as a reference standard to obtain the standard curve, and the results were expressed as milligrams of ascorbic acid equivalent per gram of dried extract (mg AAE/g extract). All assays were performed in triplicate.
2.9. Cytotoxicity test
The cytotoxicity test was performed according to the original assay with some modifications [13]. A keratinocyte cell line (HaCaT) was kindly provided by Professor Dr. Tamaki Okabayashi from MOCID, FTM, Mahidol University, and RIMD, Osaka University. HaCaT cells were cultured in a medium containing DMEM, 0.25 µg/ml amphotericin B, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% FBS at 37 °C in a 5% CO2 incubator (Heraeus, Germany) until reaching 80%–90% confluence. The cells were subsequently seeded on 96-well plates at 2.5 × 104 cells/well and incubated under the same condition for 24 h. After removing the medium, the sample solutions (200 µl) with varying concentrations (50–3200 µg/ml) were added to each well and then the plates were kept under the same condition for the same period. The viability of the cells was determined using an MTT colorimetric assay. After treatment, the solutions were removed and then 100 µl of 0.5 mg/ml MTT in PBS was added to each well. The plates were kept under the same condition for 4 h. The MTT was then replaced by 100 µl of DMSO in each well to dissolve formazan crystals. The absorbance of the reduced MTT in each well was measured at 550 nm using a microplate reader (Fusion universal microplate analyzer, Model A153601, Packard BioScience Company, USA). The percentages of cell viability were expressed as comparisons with the untreated control cultures. The experiment was carried out in three independent wells.
2.10. Cytoprotective effect
t-BHP, an organic lipid hydroperoxide analogue, was used as a pro-oxidant in this cell-based assay. After routine sub-culture, HaCaT cells were grown into 96-well plates and pretreated with 200 µl of sample solutions (50–3200 µg/ml) at 37 °C, 5% CO2 for 24 h, followed by treatment with 200 µl of 2 mmol/l t-BHP for 2 h. Then cell viability was measured using an MTT assay, as mentioned above. The test was performed in triplicate.
2.11. Measurement of reactive oxygen species (ROS) production
The generation of ROS by HaCaT cells was determined using a peroxide/redox-sensitive fluorescent dye (DCF-DA) as previously described [14]. The capacity of the cells to generate ROS was measured by 2′,7′-dichlorofluorescein (DCF) fluorescence. Following exposure to t-BHP for 2 h, the cells were stained with 100 µl of 20 µmol/l DCF-DA in Hank's balanced salt solution for 30 min in the dark. The cells were then rinsed two times with PBS, and their fluorescence intensity was recorded at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a microplate reader (Fusion universal microplate analyzer, Model A153601, Packard BioScience Company, USA). The experiment was performed in triplicate.
2.12. Heavy metal determination
The concentrations of toxic heavy metals including Cd, As, Pb, and Hg in the sample extract were analyzed by ICP-MS using microwave digestion as in our previous report [15]. One gram of the extract was digested in triplicate with 7 ml of 65% (v/v) nitric acid solution using a microwave digester Model ETHOS ONE (Milestone Corporation, Sorisole, Italy), then further diluted to 25 ml with ultrapure water for heavy metal determination using an ICP-MS spectrometer, Model 7500ce (Agilent Technologies, Santa Clara, USA). The experiment was done in triplicate. The calibration curves were prepared at five different concentrations of an ICP multi-element standard solution XIII in 5% v/v nitric acid solution.
2.13. Formulation of hydroalcoholic gels containing the coconut kernel extract
At first, base gel formulations (without coconut kernel extract) were prepared by using 3 types of polymers in different concentrations as gelling agents including Carbopol Ultrez 21 (0.5%, 0.75%, 1%, and 1.25%, w/w), Carbopol 940 (0.5%, 0.75%, 1%, and 1.25%, w/w), and Poloxamer 407 (15%, 18%, 20%, and 22%, w/w). The gels were then comparatively evaluated for various parameters including homogeneity, pH, spreadability, consistency coefficient (K), and flow behavior index (n), to select the best formulation for further study.
With regard to the results from above parameters, the most suitable gel base formulation (1% w/w Carbopol Ultrez 21) was selected for incorporation of the coconut kernel extract (3% w/w of TC06 extract). The details of gel preparation containing TC06 extract were described as follow. A 1 g of Carbopol Ultrez 21 (gelling agent) was dispersed in 50 ml of distilled water containing 4 g of glycerin (emollient) with vigorous stirring and kept overnight at 4 °C to attain complete hydration. Subsequently, 3 g of TC06 extract, 10 ml of 95% (v/v) ethanol, 1.5 g of triethanolamine (to control the pH), and paraben preservatives were added to the gel base. In most cosmetics, parabens are used at very low concentrations between 0.01% and 0.3%. Under FDA regulation, methylparaben and propylparaben are generally recognized as safe (GRAS) when used as chemical preservatives in finished cosmetic products, with a use limit of 0.19% for each [16]. For this study, the final concentrations of methyl paraben and propyl paraben used in gels containing coconut kernel extract were of 0.10% and 0.02% (w/w), respectively. The final weight of the gel product was adjusted to 100 g with distilled water, and further submitted for evaluation of the antioxidant activities and stability according to the method described in 2.8 and 2.14, respectively. An ascorbic acid gel was also prepared using the same procedures, but 1 g of ascorbic acid was added instead of 3 g of the coconut extract and used for comparison.
2.14. Stability test
Stability studies were carried out according to the previous methods [17] with a few modifications. Hot and cold temperature cycling was used for stability determination of the gel containing 3% (w/w) of TC06 extract and the gel containing 1% (w/w) of ascorbic acid. Each gel was kept alternately at 4 ± 1 °C (24 h) and 45 ± 1 °C (24 h) with 75% ± 2% RH (relative humidity) for 6 cycles in airtight glass containers. Furthermore, each gel was divided into three samples, and those samples were each kept under different storage conditions, being 4 ± 1 °C, 25 ± 1 °C, and 45 ± 1 °C with 75% ± 2% RH, and observed for the same period as that of the temperature cycling test. Before and after the stability test, the physical properties including color and homogeneity, viscosity, and pH values of the sample gels were determined using visual observation, a Kinexus rheometer (Malvern, UK), and a pH meter (First Clean Corporation, USA), respectively. Antioxidant activities were also performed for those samples using the DPPH method, as mentioned above.
2.15. Microbial limit test
Methods used for the total aerobic microbial count (TAMC) and the total yeasts and molds count (TYMC) of the formulated gel were modified from the United States Pharmacopeia (USP) 38 [18]. For the TAMC, the samples were inoculated on plates of soybean–casein digest agar using a spread plate method and then the plates were incubated at 35 ± 1 °C for 24 h. In the case of the TYMC, the samples were inoculated on plates of sabouraud dextrose agar using a spread plate method and the plates were subsequently incubated at 25 ± 1 °C for 3 d After the incubation period, the plates were examined for microbial growth by comparing it with the negative control. Each experiment was performed in triplicate.
2.16. Statistical analysis
All determinations were repeated three times and then the mean values and standard deviations were reported. One-way analysis of variance (ANOVA) in SPSS 16.0 was used for data analysis. Differences between any two means would be accomplished using a Duncan's multiple range test (DMRT). The significance level was set at P-value of less than 0.05.
3. Results and discussion
3.1. Extract yields and phytochemical constituents
The percentage yields of coconut kernel extracts from various extraction conditions are shown in Table 2. The highest extraction yield (TC09) was obtained by soaking dried kernels in hot water for 30 min. Extracts prepared in ethanol or aqueous ethanol such as TC01, TC02, TC04, TC05, TC07, and TC08 existed in the form of viscous liquids at room temperature while those in distilled water such as TC03, TC06, and TC09 existed in the form of semisolids as shown in Fig. 1. The oily portions in ethanolic extracts were prone to become rancid as they contained free fatty acids. These results demonstrated that the yield and physical appearance might partially be affected by the polarity of the solvents.
Table 2.
Extract yields, total phenolic contents, and antioxidant activities of coconut kernel extracts.
| Code of extracts | Extract yields (%) | Total phenolic contents (mg GAE/g extract) | DPPH SC50 (mg/ml) | FRAP reducing power values (mg AAE/g extract) |
|---|---|---|---|---|
| TC01 | 1.88 | 3.39 ± 0.34b | 2.63 ± 0.20bcd | 4.06 ± 0.21e |
| TC02 | 1.32 | 2.55 ± 0.19a | 3.06 ± 0.39bcd | 2.24 ± 0.37bcd |
| TC03 | 3.63 | 4.73 ± 0.27d | 3.42 ± 0.42cd | 1.55 ± 0.99bc |
| TC04 | 1.11 | 4.37 ± 0.32cd | 2.56 ± 0.43bc | 2.28 ± 0.33cd |
| TC05 | 1.59 | 3.39 ± 0.32b | 2.62 ± 0.48bcd | 1.47 ± 0.42b |
| TC06 | 1.66 | 6.98 ± 0.30e | 2.38 ± 0.14b | 4.12 ± 0.16e |
| TC07 | 1.70 | 3.56 ± 0.50b | 3.25 ± 0.19bcd | 2.13 ± 0.09bcd |
| TC08 | 2.15 | 2.55 ± 0.19a | 3.32 ± 0.66cd | 2.47 ± 0.39d |
| TC09 | 3.88 | 4.11 ± 0.14c | 3.47 ± 0.56d | 0.63 ± 0.10a |
| l-ascorbic acid | n/a | n/a | 0.02 ± 0.00a | n/a |
* SC50: Concentration that produces 50% scavenging of the DPPH; Reducing property: milligram of ascorbic acid equivalent per gram of an extract (mg AAE/g extract); n/a: not applicable.
Each value is represented as mean ± SD (n = 3).
a–eValues in the same column followed by different letters are significantly different (P < 0.05).
Fig. 1.
Physical appearances of coconut kernel extracts (TC01–TC09).
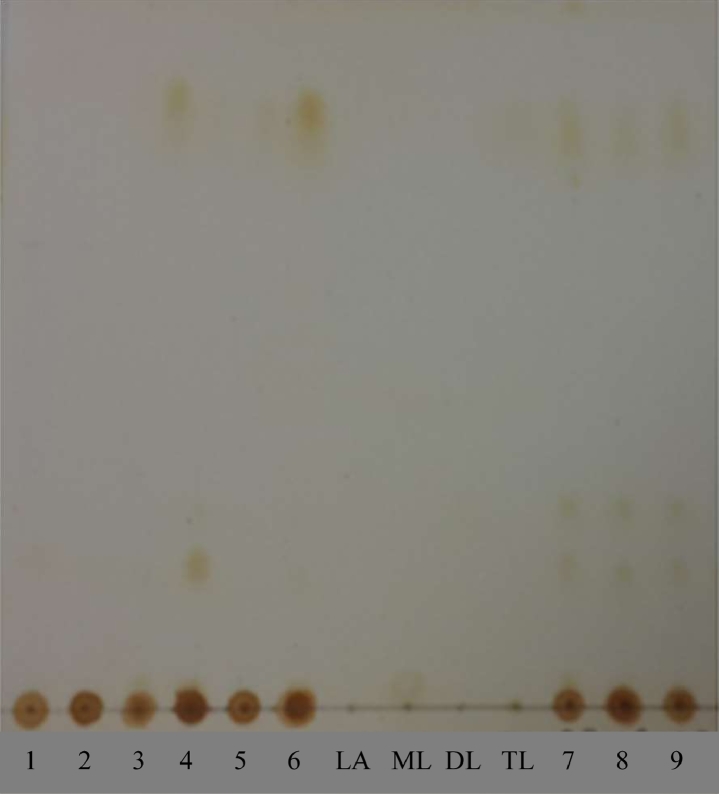
The results of phytochemical screening based on colorimetric reactions and spraying TLC plates with p-anisaldehyde-sulfuric acid reagent exhibited that all crude extracts (TC01–TC09) contained terpenoids, flavonoids, and carbohydrates. The main components of all extracts were phenolic compounds. After exposure to iodine vapor and p-anisaldehyde-sulfuric acid reagent, the TLC patterns of TC03–TC06 extracts carried out with silica gel 60 F254 TLC plates revealed the presence of trilaurin (Fig. 2) at an Rf value of 0.82 in the mobile phase system containing hexane: dichloromethane: ethyl acetate (8: 1: 1, v/v/v).
Fig. 2.
TLC patterns of TC01–TC09 extracts (1–9) and reference standards (LA, lauric acid; ML, monolaurin; DL, dilaurin; TL, trilaurin) developed in a mobile phase containing hexane: dichloromethane: ethyl acetate (8:1:1, v/v/v) and exposed to iodine vapor.
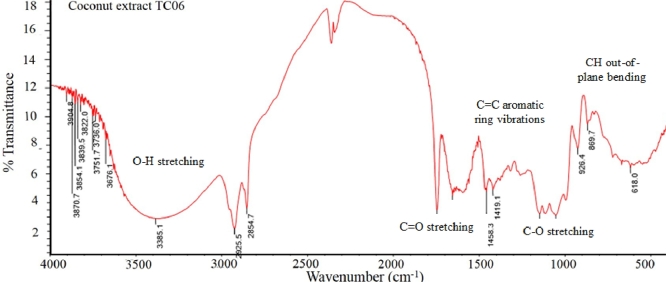
In accordance with a previous work [19], the FTIR spectrum of TC06 extract (Fig. 3) presented band characteristics of phenolics. The broad band at 3385 cm−1 with high intensity was assigned to O—H stretching. Besides, peaks at 1458 and 1419 cm−1 related to the C C aromatic ring vibrations were also seen, as well as the C—O stretching bands at 1147 and 1054 cm−1. Finally, the aromatic CH out-of-plane bending vibrations were observed at 926, 869, and 618 cm−1. It was however found that signals around 1746 and 1654 cm−1 were identified as belonging to the C O stretching absorptions of ketone, carboxylic, ester or amide groups and those around 2925 and 2854 cm−1 were defined as N—H stretching of amine, amide groups or symmetric and asymmetric C—H stretching.
Fig. 3.
FTIR spectrum of TC06 extract.
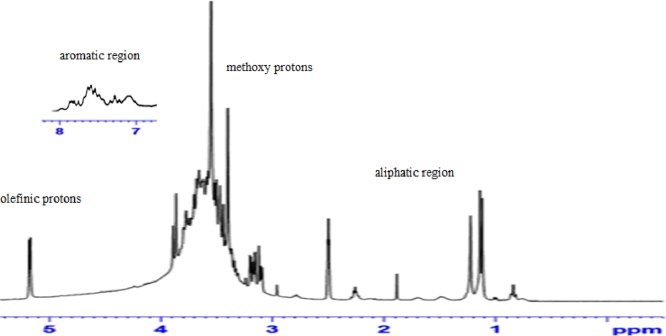
The 1H NMR spectrum of TC06 extract (Fig. 4) exhibited an aromatic region from δ 7.1 to 7.8 ppm of phenolic compounds. In the aliphatic region from δ 1.1 to 3.9 ppm, most signals of polyphenolic compounds are concealed under the signals of fatty acids. The signals at δ 3.40 and δ 3.55 were attributed to the methoxy protons. Furthermore, the signal at δ 5.2 ppm appears to be a doublet corresponding to the olefinic protons of unsaturated fatty acids.
Fig. 4.
1H NMR spectrum of TC06 extract.
3.2. Total phenolic contents and antioxidant activities
The results of the total phenolic contents using the Folin–Ciocalteu assay and antioxidant activities using DPPH and FRAP assays of all extracts (TC01–TC09) are given in Table 2. The total phenolic contents ranged from 2.55 to 6.98 mg GAE/g extract, the DPPH radical scavenging activities (SC50) ranged between 2.38 and 3.47 mg/ml, and the FRAP reducing power values ranged from 0.63 to 4.12 mg AAE/g extract. The TC 06 extract presented the highest total phenolic content (6.98 ± 0.30 mg GAE/g extract) and the strongest antioxidant properties as indicated by the lowest value of DPPH SC50 (2.38 ± 0.14 mg/ml) and the highest value of FRAP reducing power (4.12 ± 0.16 mg AAE/g extract). It could be concluded from these results that ethanolic and aqueous extracts of coconut kernels were composed of polyphenol antioxidants, especially the TC06 extract which had a significant amount (P < 0.05) of phenolic content and also exhibited antioxidant activities that are crucial for pharmaceutical uses. Therefore, the TC06 extract was chosen for further studies.
3.3. Cell cytotoxicity, cytoprotective effect, and inhibition of ROS production
As TC06 extract exhibited the highest radical scavenging activity, we next tested the cellular viability and the ability of this extract to scavenge t-BHP-induced cellular ROS. An MTT proliferation assay was used to determine the effect of the TC06 extract on the growth of the HaCaT cell lines. The TC06 extract had no significant cytotoxic effect on HaCaT cells even after 24 h at concentrations up to 3200 µg/ml (Table 3). The result exhibited that the TC06 extract at 3200 µg/ml had no effect on HaCaT cell viability. Therefore, subsequent experiments were performed at concentrations that were not cytotoxic to the cells.
Table 3.
Effects of TC06 extract on cell cytotoxicity, cytoprotection, and ROS production of HaCaT cells.
| Concentrations of TC06 extract (µg/ml) | Cell viability on HaCaT cells (% of control) |
ROS production (% of control) | |
|---|---|---|---|
| Cell cytotoxicity pretreated with TC06 extract | Cytoprotective effect exposed to t-BHP | ||
| Control | 100.00 ± 0.00b | 100.00 ± 0.00e | 100.00 ± 0.00a |
| Treatment with t-BHP | n/a | 48.02 ± 6.01ab | 172.15 ± 29.67b |
| 50 | 91.12 ± 2.35ab | 45.15 ± 5.74a | 173.27 ± 27.34b |
| 100 | 92.37 ± 6.43ab | 53.69 ± 9.40abc | 168.73 ± 29.92b |
| 200 | 91.56 ± 2.74ab | 56.09 ± 6.97bcd | 164.08 ± 29.97b |
| 400 | 92.38 ± 6.37ab | 58.52 ± 5.40cd | 157.99 ± 28.44b |
| 800 | 94.43 ± 7.86ab | 64.25 ± 3.65d | 147.56 ± 26.46ab |
| 1600 | 90.22 ± 4.87a | 65.93 ± 4.06d | 136.74 ± 24.71ab |
| 3200 | 91.77 ± 2.11ab | 65.63 ± 1.99d | 134.67 ± 23.64ab |
*n/a: not applicable.
Each value is represented as mean ± SD (n = 3).
a-eValues in the same column followed by different letters are significantly different (P < 0.05).
A pro-oxidant t-BHP was used as a compound for evaluation of mechanisms of oxidative stress-induced cell damages. As shown in Table 3, 2 h after HaCaT cells had been exposed to t-BHP, a significant decrease in cell viability was seen from the 400 µg/ml concentration compared to the untreated control group (P < 0.05). The cytotoxicity of t-BHP (2 mmol/l) was also significantly ameliorated by treatment with the TC06 extract at the concentration higher than 400 µg/ml. These results indicated that the TC06 extract had some potential to protect HaCaT cells from t-BHP induced oxidative damage although the dose response relationship was not clearly observed.
Treatment of HaCaT cells with t-BHP increased ROS levels, as evaluated using the cell-permeable, non-fluorescent probe DCFDA which is de-esterified intracellularly and changes into a highly fluorescent DCF. There was no statistically significant decrease in intracellular ROS formation as shown in Table 3, therefore pretreatment with the TC06 extract did not affect the t-BHP-induced ROS.
Because no particular assay is recommended to be the most applicable to each experimental situation as described in the experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance [20], we decided to run some assays to prove the changes of oxidative stress. However, well-designed experiments using the suitable concentration of the TC06 extract can prove hypotheses. Therefore, our experiment should be designed carefully. Finally, an experimental plan should also include assays to confirm the efficiency and safety of the extract in animal and human tissues. Nevertheless, the TC06 extract was selected for the preparation of gels due to its nontoxicity, water solubility, and chemical constituents as mentioned in the previous paragraphs.
3.4. Heavy metal determination
Heavy metals including Cd, As, Pb, and Hg are affiliated with possible toxicity in herbal extracts [15]. The concentrations of heavy metals in the digested solution of the TC06 extract were therefore analyzed in triplicate using an ICP-MS spectrometer. The concentrations of Cd (0.051 ± 0.002 mg/kg), As (1.016 ± 0.082 mg/kg), Pb ( < 4.45 mg/kg), and Hg (0.029 ± 0.004 mg/kg) in the TC06 extract were within permissible limits (Cd 0.3, As 4, Pb 10, and Hg 0.5 mg/kg) according to Thai Herbal Pharmacopoeia [21] and the prevention of food adulteration act [22]. The results showed that the amounts of heavy metals in the TC06 extract were found to be safe and acceptable.
3.5. Evaluation and stability of hydroalcoholic gels containing the coconut kernel extract
The TC06 aqueous extract showed the highest total phenolic content and the strongest antioxidant activity among all other extracts. Furthermore, it was nontoxic for HaCaT cells. Therefore, we decided to formulate gels containing the TC06 extract. Studies of the gel base formulation showed that 20% (w/w) Poloxamer 407 and 1% (w/w) Carbopol Ultrez 21 were the suitable gelling agents because the gel bases showed good physical appearance (homogeneous, clear, and transparent) and non-greasy characteristic. However, after adding 1%–3% (w/w) TC06 extract, the 20% (w/w) Poloxamer 407 could not form gels while those containing 1% (w/w) Carbopol Ultrez 21 demonstrated acceptable gel characteristic although the reduced viscosity was still observed. After adding the TC06 extract, it was found that the consistency coefficients of the 1% (w/w) Carbopol Ultrez 21 gel bases loaded with 1%, 2%, and 3% (w/w) TC06 extract were significantly (P < 0.05) decreased to 88.45 ± 3.17, 59.71 ± 5.30, and 49.95 ± 4.90, respectively and therefore need further development based on achieving the highest concentration of TC06 extract in the stable gel base.
The next study was to investigate the influence of ethanol on the rheological properties of formulated gels containing 3% w/w TC06 extract and 1% (w/w) Carbopol Ultrez 21. We examined the effect of ethanol content on the consistency and flow behavior of systems containing 0, 5, 10, 20, and 30 ml of 95% v/v ethanol in 100 g formulated gel. All hydroalcoholic gels showed qualitatively similar flow behavior indices (between 0.22 ± 0.01 and 0.28 ± 0.06) and characteristics of pseudoplastic flow. The consistency coefficients of the hydroalcoholic gels containing 0, 5, 20, and 30 ml of 95% v/v ethanol in 100 g formulated gel were relatively low (34.50 ± 3.64, 56.24 ± 4.76, 52.31 ± 3.25, and 40.22 ± 3.22, respectively) while that comprising 10 ml of 95% v/v ethanol in 100 g formulated gel (9.5% v/w ethanol) exhibited the highest consistency coefficient (83.06 ± 1.61). The result suggested the influence of percent ethanol on the viscosity of formulated gels and the most suitable concentration of ethanol was 9.5% (v/w) to attain the maximum viscosity for gel containing 3% (w/w) TC06 extract.
In this study, triethanolamine, a strong tertiary amine base, was used as a neutralizing agent in the 3% (w/w) TC06 extract hydroalcoholic gel. Carboxylic acid groups of Carbopol Ultrez polymers are neutralized by triethanolamine resulting viscous gels [23]. Additionally, methyl paraben and propyl paraben were used as preservatives in this hydroalcoholic gel. The hydroalcoholic gel composed of 3% (w/w) TC06 extract, 1% (w/w) Carbopol Ultrez 21, 9.5% (v/w) ethanol, 1.5% (w/w) triethanolamine, 0.10% (w/w) methyl paraben, and 0.02% (w/w) propyl paraben demonstrated good physical appearance (Fig. 5) and gave a comfortable smooth non-sticky feeling and a physical cooling effect. The spreadability and homogeneity of the gel preparation were applicable as revealed by a sufficient consistency coefficient (83.06 ± 1.61).
Fig. 5.
Physical appearances of formulated gels (A) containing 3% w/w TC06 extract and gels (B) containing 1% (w/w) ascorbic acid at different storage conditions.
The prepared formulation was subjected to a stability test under 4 conditions including 4 °C (24 h)/45 °C (24 h) with 75% RH for 6 cycles, and at three different temperatures being 4 °C, 25 °C, and 45 °C at the same RH and period of time. Gel batches containing 3% (w/w) TC06 extract did not exhibit any marked changes in odor or color throughout the storage period at any storage conditions apart from at 45 °C (Fig. 5). However, the control formulation containing 1% (w/w) ascorbic acid showed a yellow brown discoloration over the entire storage period (Fig. 3). During the storage period in all conditions, the formulated gel exhibited only minor changes in its consistency coefficients (74.58–83.06), flow behavior indices (0.20–0.25), and pH values (5.49–5.77), whereas the control formulation containing 1% (w/w) ascorbic acid showed a marked decrease in its consistency coefficients (from 21.52 to 1.36) and flow behavior indices (from 0.31 to 0.25) but less so for the pH values (from 5.76 to 5.49). The results showed that the formulated gel was more stable than the control gel in all storage conditions throughout the period of study.
After the analysis period of all storage conditions, the antioxidant activities of the TC06 extract in formulated gels showed no significant changes in SC50 values (2.63–2.96 mg/ml), whereas those activities of the ascorbic acid in gel preparations exhibited significant increases in SC50 values ranging from 12.13 ± 0.81 to 513.20 ± 9.75 mg/ml as shown in Table 4. In the case of the microbial test for the formulated gel containing 3% w/w TC 06 extract in all storage conditions, the results conformed to the pharmacopoeia requirements (not more than 100 CFU/g for bacteria and fungi) [18].
Table 4.
DPPH antioxidant activities of the formulated gel containing 3% w/w TC06 extract and gel containing 1% w/w ascorbic acid after stability test.
| Stability conditions | DPPH SC50 (mg/ml) |
|
|---|---|---|
| Formulated gel containing 3% (w/w) of TC06 extract | Control gel containing 1% (w/w) of ascorbic acid | |
| Freshly prepared | 2.63 ± 0.20a | 12.13 ± 0.81a |
| Six cycles of temperature cycling test | 2.94 ± 0.28a | 145.96 ± 0.12d |
| At 25 °C with 75% RH | 2.91 ± 0.25a | 223.42 ± 0.72c |
| At 4 °C with 75% RH | 2.65 ± 0.26a | 52.46 ± 0.06b |
| At 45 °C with 75% RH | 2.96 ± 0.47a | 513.20 ± 0.09e |
*SC50: Concentration that produces 50% scavenging of the DPPH.
Each value is represented as mean ± SD (n = 3).
a-eValues in the same column followed by different letters are significantly different (P < 0.05).
4. Conclusions
Coconut kernels obtained from tall palms were extracted under various conditions. The extracts were determined in terms of phytochemical compounds, total phenolic contents, antioxidant activities, cell cytotoxicities, cytoprotective effects, ROS-inhibitory activities, and heavy metal contents. The study found that the TC06 aqueous extract was composed of terpenoids, flavonoids, and carbohydrates. Among all other extracts, the TC06 extract showed the strongest antioxidant activity based on the DPPH and FRAP assays and the highest total phenolic content. It was not cytotoxic to HaCaT cells. Moreover, the concentrations of heavy metals including Cd, As, Pb, and Hg in the TC06 extract were below acceptable limits. The water compatible TC06 extract was therefore formulated as a hydroalcoholic gel using Carbopol Ultrez 21. Finally, a stability study was carried out under 4 conditions including 4 °C (24 h)/45 °C (24 h) with 75% RH- for 6 cycles and at three different temperatures, being 4 °C, 25 °C, and 45 °C at the same RH and period of time. The formulated gel containing 3% (w/w) TC06 extract and the control gel containing 1% (w/w) ascorbic acid were evaluated for appearance, pH values, consistency coefficients, flow behavior indices, and antioxidant activities at the end of the storage period. The results revealed that the formulated gel was stable at 4 °C and 25 °C with 75% RH throughout the period of study. By means of a microbial test, the TAMC and TYMC of the formulated gel were in compliance with pharmacopoeia requirements. The hydroalcoholic gel composed of TC06 extract, Carbopol Ultrez 21, ethanol, and triethanolamine appeared to be a suitable formulation owing to physicochemical characteristics, stability, and safety. However, some potential antioxidants especially from natural sources should be added into the formulated gel containing TC06 extract to potentiate its biological efficiency.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgments
This research was supported by the Faculty of Pharmacy, Silpakorn University, Thailand; Silpakorn University Research and Development Institute and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (HERP grant number 109201). We thank Professor Dr. Tamaki Okabayashi from MOCID, FTM, Mahidol University, and RIMD, Osaka University for providing keratinocytes cell lines (HaCaT cells). The authors would like to thank Mr. Henry Erskine-Hill for proofreading and editing the manuscript.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
Contributor Information
Wantanwa Krongrawa, Email: song-pt@hotmail.com.
Sontaya Limmatvapirat, Email: limmatvapirat_s@su.ac.th.
Nushjira Pongnimitprasert, Email: pongnimit_n@su.ac.th.
Paranee Meetam, Email: meetam_p@su.ac.th.
Chutima Limmatvapirat, Email: limmatvapirat_c@su.ac.th.
References
- 1.Odenigbo UM, Otisi CAO. Fatty acids and phytochemical contents of different coconut seed flesh in Nigeria. Int PPB. 2011;3:176–182. [Google Scholar]
- 2.Yalegama LL, Nedra Karunaratne D, Sivakanesan R, Jayasekara C. Chemical and functional properties of fibre concentrates obtained from by-products of coconut kernel. Food Chem. 2013;141:124–130. doi: 10.1016/j.foodchem.2013.02.118. [DOI] [PubMed] [Google Scholar]
- 3.Salil G, Nevin KG, Rajamohan T. Arginine rich coconut kernel protein modulates diabetes in alloxan treated rats. Chem Biol Interact. 2011;189:107–111. doi: 10.1016/j.cbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Zakaria ZA, Reezal I, Mat Jais AM, et al. The anti-inflammatory, anti-pyretic and wound healing activities of Cocos nucifera (MATAG Types) fresh juice and kernel extract in experiment animals. J Pharmacol Toxicol. 2006;1:516–526. [Google Scholar]
- 5.DebMandal M, Mandal S. Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med. 2011;4:241–247. doi: 10.1016/S1995-7645(11)60078-3. [DOI] [PubMed] [Google Scholar]
- 6.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Haneefa KPM, Hanan KS, Saraswathi R, Mohanta GP, Nayar C. Formulation and evaluation of herbal gel of Pothos scandens Linn. Asian Pac J Trop Med. 2010;3:988–992. [Google Scholar]
- 8.Chomchalow N. Coconut varieties in Thailand. Au J Tech. 1999;3:19–30. [Google Scholar]
- 9.Ahuja J, Suresh J, Deep A, Ravi MP. Phytochemical screening of aerial parts of Artemisia parviflora Roxb.: a medicinal plant. Der Pharm Lett. 2011;3:116–124. [Google Scholar]
- 10.Harborne JB, Mabry TJ. Chapman & Hall; London; New York: 1982. The flavonoids advances in research. [Google Scholar]
- 11.Pengon S, Ponphaiboon J, Chaidedgumjorn A, Limmatvapirat C, Sriamornsak P, Limmatvapirat S. Comparison of solvent miscibility of coconut oil and its modified forms. Adv Mater Res. 2015;1060:151–154. [Google Scholar]
- 12.Manivannan A, Bhardwaj R, Padmanabhan S, Suneja P, Hebbar KB, Kanade SR. Biochemical and nutritional characterization of coconut (Cocos nucifera L.) haustorium. Food Chem. 2018;238:153–159. doi: 10.1016/j.foodchem.2016.10.127. [DOI] [PubMed] [Google Scholar]
- 13.Yang JH, Hwang YH, Gu MJ, Cho WK, Ma JY. Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine. 2015;22:1262–1268. doi: 10.1016/j.phymed.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Lundvig DM, Pennings SW, Brouwer KM, et al. Cytoprotective responses in HaCaT keratinocytes exposed to high doses of curcumin. Exp Cell Res. 2015;336:298–307. doi: 10.1016/j.yexcr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Limmatvapirat C, Limmatvapirat S, Charoenteeraboon J, et al. Comparison of eleven heavy metals in Moringa oleifera lam. products. Indian J Pharm Sci. 2015;77:485–490. doi: 10.4103/0250-474x.164782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Final report of the cosmetic ingredient review expert panel, amended safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben, June13, 2006. [DOI] [PubMed]
- 17.Maske PP, Lokapure SG, Nimbalkar D, Malavi S, D'souza JI. In vitro determination of Sun protection factor and chemical stability of Rosa kordesii extract gel. J Pharm Res. 2013;7(6):520–524. [Google Scholar]
- 18.Siddiqui N, Rauf A, Latif A, Mahmood Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth) J Taibah Univ Med Sci. 2017;12:360–363. doi: 10.1016/j.jtumed.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christophoridou S, Dais P. Detection and quantification of phenolic compounds in olive oil by high resolution 1H nuclear magnetic resonance spectroscopy. Anal Chim Acta. 2009;633:283–292. doi: 10.1016/j.aca.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Powers SK, Smuder AJ, Kavazis AN, Hudson MB. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int J Sport Nutr Exerc Metab. 2010;20(1):2–14. doi: 10.1123/ijsnem.20.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department of Medical Sciences, Ministry of Public Health . II. Prachachon Co., Ltd.; Thailand: 2000. (Thai herbal pharmacopoeia). [Google Scholar]
- 22.Ministry of Public Health . Thailand; 1986. The regulation of the prevention of food adulteration act. [Google Scholar]
- 23.The Lubrizol Corporation Formulating semisolid products. Pharm Bull. 2011;21:1–7. [Google Scholar]