Abstract
Berberine chloride (BBR) is a pharmacokinetic profile of drug with poor bioavailability but good therapeutic efficacy, which is closely related to the discovery of BBR intestinal target. The major aim of this paper is to develop BBR intestinal retention type sustained-release pellets and evaluate their in vivo and in vitro behaviors base on the aspect of local action on intestinal tract. Here, wet milling technology is used to improve dissolution and dissolution rate of BBR by decreasing the particle size and increasing the wettability. The pellets are prepared by liquid layer deposition technology, and then the core pellets are coated with Eudragit® L30D-55 and Eudragit® NE30D aqueous dispersion. The prepared pellets show high drug loading capacity, and the drug loading up to 93%. Meanwhile, it possesses significant sustained drug release effect in purified water which is expected to improve the pharmacokinetic behavior of BBR. The pharmacokinetics results demonstrate that the half-life of BBR was increased significantly from 24 h to 36 h and the inter- and intra-subject variability are decreased compared to commercial BBR tablets. The retention test results indicate that the pellet size and Eudragit® NE30D plays an important role in retention time of the pellet, and it is found that the pellets with small particle size and high Eudragit® NE30D coating content can stay longer in the intestine than the pellets with large particle size. All in all, BBR intestinal retention type pellets are prepared successfully in this study, and the pellets show satisfactory in vivo and in vitro behaviors.
Keywords: BBR, Pellets, Sustained release, Intention, Pharmacokinetics
Graphical abstract
The major aim of this study is to prepare BBR intestinal retention type sustained-release pellets and evaluate their in vivo and in vitro behaviors. Preparation of BBR pellets by combined application of wet milling technology, liquid layer deposition technology and polymer coating technology. The pellets showed significant sustained release behavior and satisfactory gastrointestinal retention properties. Meanwhile, BBR pellets reduced individual differences significantly and showed more regular pharmacokinetic behavior compared with BBR tablets.
1. Introduction
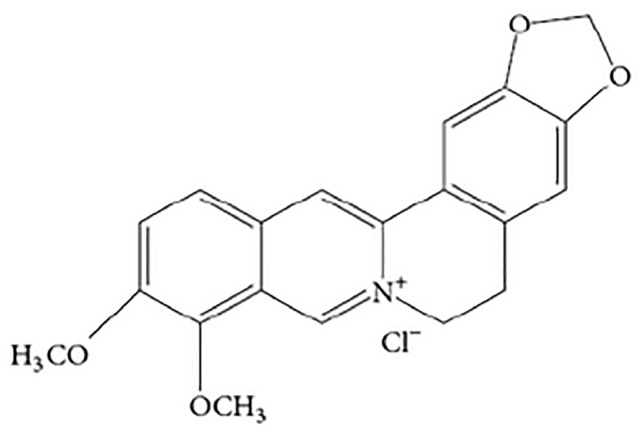
Berberine chloride (BBR, Fig. 1) is a well-known plant alkaloid isolated from medicinal herbs. It has been widely used to treat diarrhea, gastroenteritis and hepatic disorders, without apparent side-effects being reported from clinical use [1]. According to recent clinical application and experiment research reports, BBR has a significant effect of regulating lipid and glucose metabolism, suppressing tumor cell proliferation and inducing cell apoptosis, especially for glycolipid metabolism syndrome [2], [3], [4], [5]. Therefore, BBR has great development value and broad application prospects.
Fig. 1.
The chemical structure of Berberine (BBR).
Although BBR has recently caused widespread interest among researches due to good clinical efficacy feedback, pharmacokinetic studies have shown that BBR has a very low oral absorption and its application is greatly limited because of poor bioavailability [6]. Therefore, more and more studies have explored to improve the bioavailability of BBR by different absorption enhancement technologies such as the use of absorption enhancers, P glycoprotein inhibitors and various nanomedicine, but the extent of bioavailability enhancement is still limited [7], [8], [9]. Luckily, researchers found that one of the effect targets of BBR for antidiabetic and anti-hyperlipidemia effects is in the gut, which make BBR become a pharmacokinetic profile of drug with poor absorption but good therapeutic efficacy [10]. That is to say, plasma drug concentration is not the only criterion for evaluating the efficacy of BBR. Meanwhile, it is found that the efficacy of BBR is closely related to its residence time in the intestine and the mixing degree with the gut microbiota [11]. Accordingly, it seems more meaningful to develop intestinal retention preparation to improve the efficiency of BBR from the aspect of local effects on intestinal tract.
Pellets, one of multiple-unit drug delivery systems, have gained increasing attention as a platform to design intestinal retention dosages [12,13]. On the one hand, pellets possess smaller particle size and larger specific surface area than other commercially available preparations, which have a natural affinity for the intestinal tract. Generally speaking, Pellets with small particle size can stay in the gastrointestinal tract for long period of time through the interaction with goblet cells and gut microbiota. On the other hand, the intestinal retention capacity can be obtained by using adhesive materials, such as acrylic resin, chitosan, carbomer, cellulose, and so on [14,15]. The bioavailability and efficacy of the drugs would be improved because the residence time of the pellets at specific sites of the mucosa can be prolonged by utilizing the adhesion properties of these materials to the intestinal mucosal surface [16]. Additionally, pellets can reduce the variability in release profiles, providing less inter- and intra-subject variability in intestinal absorption compared to single unit dosage forms, such as coated tablets [17]. Meanwhile, pellets cause less mucosal irritation because individual sub-units are spread more broadly throughout the gastrointestinal tract. Thus, pellet is an ideal intestinal retention drug delivery system.
In order to ensure the sustainability of the therapeutic effect, it is necessary to introduce the sustained-release properties to intestinal retention pellets by adding one or more sustained release materials, or by coating the dry pellets with polymers. To date, the most common technique for controlling drug release is the application of polymer materials to coat the particles. We can choose different coating materials according to actual needs including control of the release rate, moisture proof, enteric or gastric material, taste masking material, dark [18,19]. In this paper, it was found that a satisfactory release curves cannot be obtained when Eudragit® NE30D and L30D-55 aqueous dispersion are applied alone. But it can acquire good results when Eudragit® L30D-55 and Eudragit® NE30D are used together [20], [21], [22]. Additionally, the flexibility of the film and intestinal retention time of pellets increased significantly with the introduction of Eudragit® NE30D. Meanwhile, triethyl citrate is used as a plasticizer to further increase film plasticity and stability and achieved good effects.
In this study, BBR intestinal retention type sustained-release pellets are prepared successfully by using wet grinding technology, liquid layer deposition technology and polymer coating. The preparation had several novelties and advantages: (1) a uniform BBR mixed suspension with high solubility and dissolution rate was prepared by using wet nanometer milling method to reduce the drug particle size and increase the wettability of the drug [23]. Combined the technology of fluidized bed spray liquid layer deposition, the BBR suspension is continuous sprayed on the surface of microcrystalline cellulose blank pellet core, and they could completely dissolute in 5 min. The drug release profile can be controlled by polymer coating, and the release type is close to Higuchi drug release model; (2) by reducing particle size and applying adhesive material simultaneously to prolong the intestinal retention time of the prepared pellets, and then make the drug release more regular; (3) with the residence time of the pellets prolonged, it can fully integrate with the intestinal target, improve the efficacy and reduce side effects. The powder properties of the pellets, concluding particle size, distribution, roundness and in vitro drug release are investigated. The pharmacokinetic profile of BBR sustained-release pellets is compared with that of commercial BBR tablets in beagle dogs. In addition, the retention test of the pellets in gastrointestinal tract is carried out in rabbits.
2. Materials and methods
2.1. Materials
BBR was purchased from Northeast Pharmaceutical Group Co., Ltd. (Shenyang, China). The internal standard, tetrahydropalmatine, was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Eudragit® NE30D and Eudragit® L30D-55 aqueous dispersions were obtained from Shanghai Chineway Pharmaceutical Accessories Technology Co., Ltd. Hydroxypropylmethylcellulose (HPMC), microcrystalline cellulose (MCC), and Triethyl citrate were purchased from Huzhou Zhanwang Pharmaceutical Co., Ltd. LC grade acetonitrile, methanol and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ). Purified water, obtained using a Milli-Q® ultrapure water system (USA).
2.2. Preparation of drug-loaded pellets
The uniform BBR mixed suspension (BBR and HPMC suspension) was prepared firstly by using wet nanometer milling method. an aqueous solution of HPMC was used as a grinding carrier at a concentration of 2.5% (w/v). Weigh 500 g of HPMC solution, 150 g of BBR into grinding cup and turn on the grinding machine. A suitable particle size BBR suspension can be obtained by grinding at 3600 rpm for 60 min. The mean meter was 0.905 µm and there was not any larger than 4 µm particle existing in the drug suspension. Combined the technology of fluidized bed spray liquid layer deposition, the BBR suspension was continuous sprayed on the surface of MCC blank pellet core of 80–100 mesh prepared by centrifugal granulation method, at last small particle size (40–60 mesh) pellets loaded drug were prepared. The drug-loaded pellets were dried in a 40 °C drying oven for 12 h and then the pellets were screened using 40–60 mesh sieves to obtain pellets of uniform particle size.
The acrylic resin Eudragit® NE30D and Eudragit® L30D-55 mixed at a certain proportion was used as the coating material. Triethyl citrate was chosen as a plasticizer to increase film plasticity and stability. BBR pellets of 400 g were coated in a fluidized bed with a bottom spray and the weight gain was 3.5%. The concrete parameters were as follows: inlet temperature was 25 °C, outlet temperature was 20 °C, product temperature was 20 ± 2 °C, spray rate was 2.0–2.5 ml/min, atomization pressure was 1.5 bar. And then, the pellets were further fluidized for 15 min. After the coating process, the pellets must be dried in the oven for at least 24 h to ensure the formation of an intact film.
2.3. Dissolution tests
In vitro drug release profile of BBR from the prepared pellets was evaluated according to the USPXXXIII apparatus 1. The pellets were placed in the 500 ml water and stirred at 50 rpm. The temperature of the water was kept at 37 ± 0.5 °C. At predetermined intervals aliquots (5 ml) were withdrawn and filtered using a 0.22 µm membrane syringe filter. An equal volume of purified water was added to the vessel. The samples were analyzed by HPLC. Chromatographic conditions: Proshell SB C18 column (4.6 µm, 150 mm × 4.6 mm); mobile phase (0.05 mol/l Potassium dihydrogen phosphate–0.05 mol/l sodium heptane sulfonate (1:1), adding 0.2% Triethylamine and applying phosphoric acid to adjust pH to 3.0)-acetonitrile (60:40); Flow rate and UV detector wavelength were 1 ml/min and 263 nm respectively. A good linear relationship of the peak areas of BBR vs the concentration of BBR can be obtained over the range 0.5–10.0 µg/ml.
2.4. Microscope and scanning electron microscope (SEM) studies
In this study, a scanning electron microscope (Motic DMBA450, Micro-Optic Industrial Group Co., Ltd) was used to record the structure of the prepared BBR sustained release pellet. It is well known that the preparation of the samples has a great influence on the final results, so the preparation method of the sample should be mastered. In general, the preparation process of the sample mainly includes the following steps: (1) It is extremely necessary to properly cut the sample to expose the section according to the needs of the experiment. (2) Fix the processed sample on the experiment board. (3) Spray metal on the surface of the sample to increase the conductivity of the sample. (4) Scan and get the picture.
2.5. Storage stability
The stability of BBR pellets was investigated by accelerated test (40 °C and 75% RH) and long-term stability test (25 °C and 60% RH). BBR released from the pellets was determined after 1, 2, 3 and 6 months.
2.6. Pharmacokinetic study
2.6.1. Animals
Six beagle dogs weighting 10–12 kg were acquired from the Laboratory Animal Center of Shengjing Hospital of China Medical University. All dogs were provided normal water and food throughout the in-life portion. Animals were housed in a room with standard feeding environment. All animals use procedures were in accordance with the regulation for animal experimentation issued by the State Committee of Science and Technology of the People's Republic of China.
2.6.2. Study design
A two-period, balanced, randomized cross-over pharmacokinetic study of the BBR pellets and the marketed tablets was performed in beagle dogs. The dogs were fasted overnight prior to drug dosing and were given in a single dose of 150, 300, 600 mg/dog respectively and multiple dose of 300 mg/kg with 50 ml water by oral administration. The multiple dose groups were administered continuously for 7 d. After a seven-day washout period the dogs were cross-overed.
2.6.3. Blood sampling
Blood samples were collected in heparinized tubes at appropriate intervals (single dose group: prior to the drug administration-zero time, and at 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0, 24.0, 36.0, 48.0, 60.0, 72.0, 96.0, 120 h; multiple dose group: −4d, −3d, −2d, −1d, the drug administration-zero time, and at 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0, 24.0, 36.0, 48.0, 60.0, 72.0, 96.0, 120 h). Blood samples (3.0 ml) were centrifuged (4 000 rpm/10 min) and the plasma collected was stored at −70 °C until determination.
2.6.4. UPLC-ESI-MS/MS method
Chromatography was performed on an Agilent 1290 UPLC system. The separation was carried out on an Agilent Proshell 120 SB C18 column (75.0 mm × 2.1 mm, 1.7 µm). The column temperature was 35 °C. The mobile phase consists of (A) acetonitrile and (B) water (containing 0.1% formic acid). The gradient conditions are shown in Table 1.
Table 1.
Gradient condition of HPLC.
| Time (min) | Flow rate (ml/min) | A (%)a | B (%)b |
|---|---|---|---|
| Initial | 0.60 | 70 | 30 |
| 0.8 | 0.60 | 70 | 30 |
| 1.0 | 0.60 | 20 | 80 |
| 2.5 | 0.60 | 20 | 80 |
| 2.6 | 0.60 | 70 | 30 |
| 3.0 | 0.60 | 70 | 30 |
0.1% formic acid water.
Methanol.
Mass spectrometer. The Agilent 1290 UPLC system was connected to AB Sciex Q-Trap-4500 instrument with an ESI source in positive ion mode. The MS detector was operated in MRM mode at unit mass resolution with a dwell time of 100.0 ms. The concrete mass spectrometric parameters are shown in Table 2.
Table 2.
Transition reactions of BBR and IS.
| Molecule | Transition | lonspray voltage (V) | Declustering potential (s) | Collision energy (eV) |
|---|---|---|---|---|
| BBR | 336.0→319.8 | 4500 | 65 | 40 |
| IS | 356.1→192.1 | 4500 | 85 | 37 |
2.6.5. Statistical analysis
Data was obtained and analyzed using Analyst version 1.6.2 software, from Applied Biosystems. Values were expressed as mean ± SD for all data. P < 0.05 was considered statistically significant.
2.7. Study on the retention of gastrointestinal tract in rabbits
2.7.1. Animals
Rabbits (weight: 4–5 kg) were supplied by the Laboratory Animal Center of Shengjing Hospital of China Medical University. Animals were housed in a room with standard feeding environment.
2.7.2. Study design
Eight rabbits were randomly divided into four groups each with 2 rabbits. The rabbits were fasted 48 h before administration, but free drinking water. Each group was given MCC blank pellets of different particle size (0.15–0.25 mm and 1.00–1.25 mm) and MCC pellets coated with Eudragit® NE30D or Eudragit® L30D-55. The rabbits were killed 8 h after administration, and collect contents in stomach and small intestine. Obtain the homemade pellets of different particle sizes stranded in the gastrointestinal tract (including the stomach and small intestine) in rabbits and count the number of the pellets in detail. Finally, we can evaluate the retention time in the gastrointestinal of different BBR pellets.
3. Results and discussion
3.1. Result of microscope and scanning electron microscope
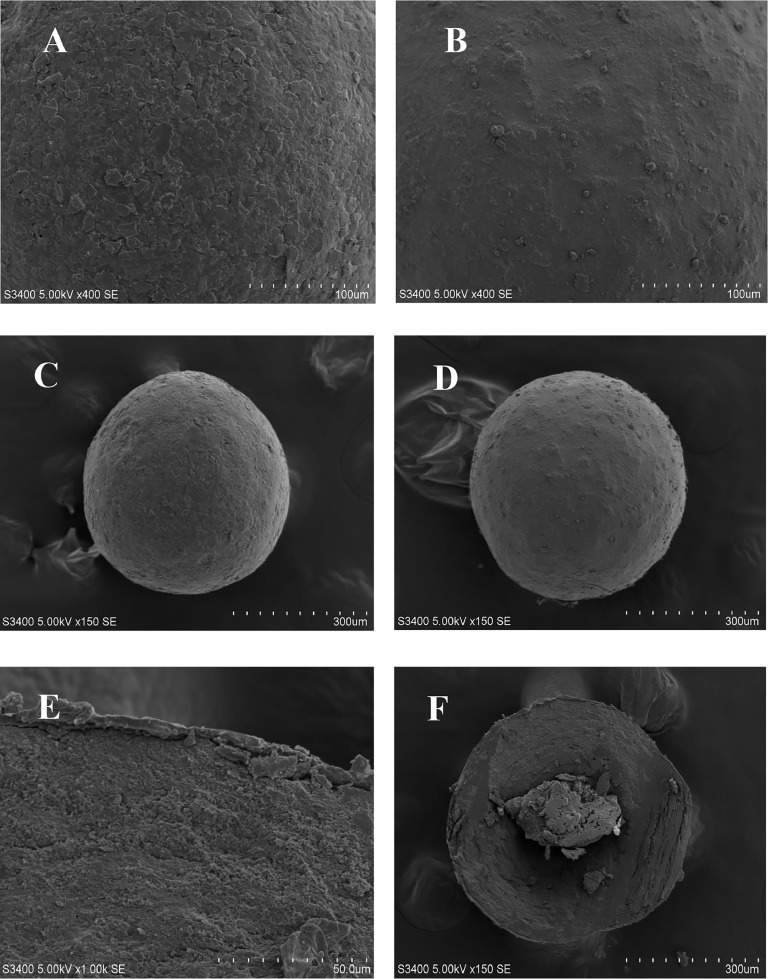
As a reservoir-type drug delivery system, BBR sustained release pellets consist of three parts, the inner layer is a blank MCC core, the middle is the drug loading layer, and the outer is the controlled release layer. The scanning electron microscope photographs of BBR pellets were shown in Fig. 2. It can be seen that the prepared pellets have a good roundness and a smooth surface. Additionally, the pellets showed a complete coating film, drug loading layer and blank core pellets.
Fig. 2.
Scanning electron microscope photographs of BBR sustained release coated pellets (A, C, E, and F) and pellets without coating (B, D).
3.2. Pretreatment of BBR on drug release
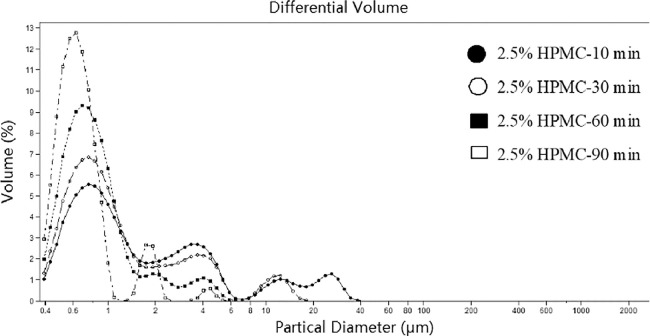
According to BCS, BBR is classified as class IV drug and has poorly aqueous solubility and permeability. It was proven by the experiment that BBR was sparingly soluble in water; thus it is necessary to improve the solubility and dissolution rate of BBR. While wet nanometer milling technology can improve solubility and dissolution rate of the poorly water-soluble drugs significantly. On the one hand, the particle size of BBR is reduced and its specific surface area is increased by wet milling, and then the solubility of BBR is significantly improved base on the principle of Noyes-Whitney equation. On the other hand, the introduction of a polymerduring the milling process not only reduced the particle size, but more importantly can maintain the high energy state by inhibiting the transformation of the drug crystal form. In this study, In the present study, an aqueous solution of HPMC was used as a grinding carrier at a concentration of 2.5% (w/v). The grinding time was determined by monitoring the particle size and particle size distribution of the BBR suspension, and the grinding time was 10 min, 30 min, 60 min, 90 min respectively. The results are shown in Fig. 3 and Table 3.
Fig. 3.
Particle size distribution of BBR suspensions with different wet milling time.
Table 3.
Parameters data of particle size distribution of BBR suspensions with different wet milling process time.
| Time (min) | Mean (μm) | SD (μm) | <1 µm (%) | <10 µm (%) | d 90 (µm) |
|---|---|---|---|---|---|
| 10 | 3.800 | 6.333 | 42.8 | 88.3 | 11.97 |
| 30 | 2.061 | 2.752 | 53.0 | 95.3 | 4.492 |
| 60 | 0.905 | 0.644 | 80.5 | 100 | 1.704 |
| 90 | 0.791 | 0.565 | 88.7 | 100 | 1.457 |
The results of the laser diffraction large particle mode showed that the particle size of the drug changed significantly after the suspension was milled for 60 min, and all the particles were reduced to less than 10 µm and the particle size distribution was uniform. As the milling time is extended, the particle size is gradually reduced, and the average particle diameter is about 1 µm, but the magnitude of the decrease is not obvious. Thus, the final grinding time was determined to be 60 min from the viewpoint of uniformity and time saving. The mean meter of BBR was 0.905 µm and there was not any larger than 4 µm particle existing in the BBR-HPMC suspension, and the drug could completely dissolute in 5 min. It can be concluded that pellets with BBR suspension have good solubility and dissolution rate.
3.3. Release of BBR from pellets coated with Eudragit ® L30D55/NE30D
Normal marketed BBR preparations are absorbed immediately after oral administration, and the plasma BBR concentration decreases rapidly, which significantly limit its potential therapeutic applications in clinic. In this study, Eudragit® L30D55 and Eudragit® NE30D were used to control the release of BBR from the pellets. Eudragit®L30D55 is an excellent enteric coating material. However, in our experiments, we found that Eudragit ®L30D55 alone could not effectively control the rapid release of BBR even if the coating weight is large because Eudragit® L30D55 is an aqueous dispersion and can dissolve quickly at pH above 5.5. While Eudragit® NE30D is an aqueous dispersion of a neutral copolymer and its permeability is independent of the pH, but the medium permeability of Eudragit® NE30D films would cause the drug release incompletely when using Eudragit® NE30D alone. Finally, an ideal release curve can be obtained when Eudragit® L30D55 and Eudragit® NE30D are used together, and the ratio of Eudragit® L30D55 and Eudragit® NE30D is 1:8. The coating formulations and the drug release curves of different coating weight gains are shown in Table 4 and Fig. 4.
Table 4.
Coating formulations of BBR pellets.
| Serial number | Coating formulations (w/w) | Weight gain (%) | Aging conditions | Existing problems |
|---|---|---|---|---|
| 1 | A: B = 30:1 | 6 | 40 °C, 24 h | Incomplete release drug |
| 2 | A: B = 10:1 | 2, 3, 4, 5 | 40 °C, 24 h | The release profile is acceptable when the weight gain is 4%. But the release is incomplete after 8 h |
| 3 | A: B = 8:1 | 2, 3, 4, 5 | 40 °C, 24 h | The release profile is acceptable when the weight gain is 4% |
| 4 | A: B = 8:1 | 4 | 40 °C, 48 h | The release rate is obvious slower as the aging time increases |
| 5 | A: B: C = 8:1:0.2 | 2, 3, 3.5, 4, 5 | 40 °C, 24 h | The release effect is ideal when the coating gain 3.5% |
| 6 | A: B: C = 8:1:0.2 | 3.5 | 40 °C, 48 h | The release rate did not change significantly compared with formulation 5 |
A represent Eudragit® NE30D, B represent Eudragit® L30D55, C represent triethyl citrate (TEC).
Fig. 4.
Effect of the coating level of Eudragit® L30D55 and Eudragit® NE30D on the drug release of pellets.
It can be seen from Fig. 4, BBR release rate gradually reduced as the weight gain of coating increased. Forming a complete film is a prerequisite for effective control of drug release. Therefore, it was not possible to control the prophase release of BBR from the pellets when the coating weight was 2% because the film was incomplete. When the weight gain was higher than 4%, BBR was not released completely from the pellets after 8 h. The reason may be due to the extension of the diffusion path, the increase of the diffusion channel and the repolymerization of the film leading to the lack of drug release powder. Additionally, the results showed that the pellets of coating formulation without plasticizer triethyl citrate (TEC) in accelerated stability test displayed slow drug release as the result of serious film continuing to aging and thin film becomes dense. Thus, triethyl citrate was added to the optimum coating formulation. Finally, the decided coating formulation was TEC:Eudragit® L30D55:Eudragit® NE30D = 0.2:1:8 (w/w/w) with 3.5% coating weight. The obtained goal BBR sustained-release pellets controlled by coating film presented good release results in the accelerated stability test and long-term storage conditions placed trial, and the release type was close to Higuchi drug release model in the drug release mechanism research.
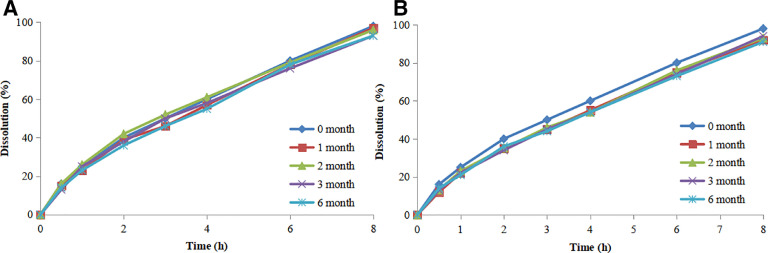
3.4. Stability studies
The stability of the prepared BBR has been investigated under stress and ambient conditions. The drug release results are shown in Fig. 5.
Fig. 5.
The drug release of BBR pellets stored at 25 °C/60%RH (A) and 40 °C/75%RH (B).
It can be seen in Fig. 5B, BBR release rate is gradually slowing during accelerated test, but the change still satisfies the requirements. This may be related to the film formation mechanism of Eudragit®L30D55 and Eudragit®NE30D aqueous dispersion. It is well known that temperature and humidity are important factors influence the stability of the film. In general, high temperature and humidity (40 °C/75%RH) can induce re-aggregation of the film. Thus, it is necessary to properly increase the curing time of the polymer coating films. In addition, plasticizer can lower the film formation temperature, increase the flexibility of the film, and further improve the stability of the pellets. Here, triethyl citrate was used as a plasticizer to increase film plasticity and stability. In contrast, the long-term stability of the pellets did not show significant changes owing to gentle conditions, the result was shown in Fig. 5A, and the release trend was similar to that of pellets stored at 40 °C/75%RH. Accordingly the long-term stability of the BBR pellets met the requirement specifications after 6 months’ storage.
3.5. UPLC-MS/MS method validation of BBR in dog blood
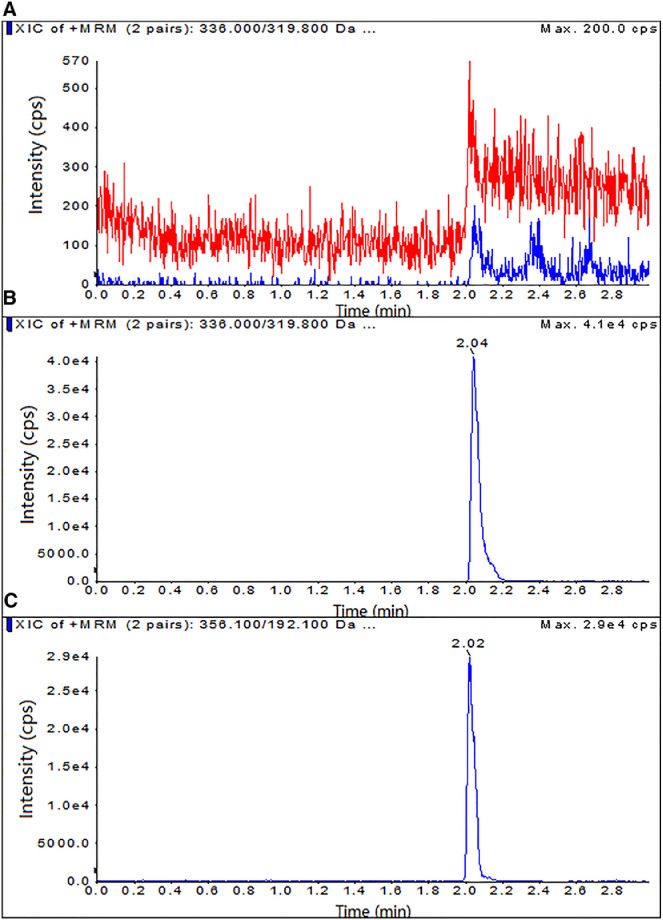
A novel UPLC-MS/MS method has been developed and validated to determine BBR concentrations in beagle dog blood. BBR and tetrahydropalmatine (internal standard), were extracted with diethyl ether-dichloromethane (2:3, v/v) and separated on an Agilent Proshell 120 SB C 18 column. The mobile phase consisted of acetonitrile and water. The representative chromatograms of blank blood sample, blank blood spiked with BBR or internal standard demonstrated the selectivity of the assay (Fig. 6). The calibration curves were obtained over the concentration ranges of 0.025–5.0 ng/ml. The intra- and inter-day precisions were < 11.5% and 11.9%, respectively. The accuracy was within 11.7% and 11.3%. The mean recoveries of BBR at three concentrations of 0.05, 0.5, 4.0 ng/ml were > 89.6%. Thus, the proposed method was applicable for in vivo study in Beagles.
Fig. 6.
Representative UPLC-MS/MS chromatograms of (A) blank plasma, (B) blank plasma spiked with BBR and (C) internal standard, respectively.
3.6. In vivo pharmacokinetic study in Beagles
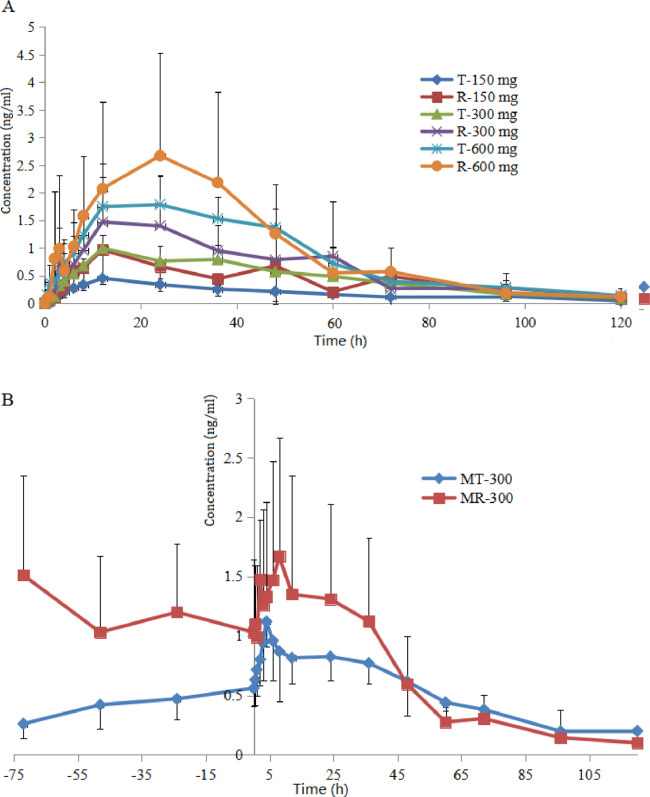
The mean plasma concentration-time curves of BBR after oral administration of the BBR pellets and commercial tablet to beagle dogs are depicted in Fig. 7. The corresponding pharmacokinetic parameters obtained are represented in Tables 5 and 6.
Fig. 7.
The drug concentration profiles in blood following oral administration of BBR pellets or commercial BBR tablets in beagle dogs under (A) single dose group or (B) multiple dose group. T represents BBR pellets, R represents BBR tablets. Data represent mean ± SD (n = 6).
Table 5.
Pharmacokinetic parameters of BBR preparations after single administration in dogs T represent BBR pellets, R represent BBR tablet.
| Single dose groups |
||||||
|---|---|---|---|---|---|---|
| Pharmacokinetic parameters | T-150 | R-150 | T-300 | R-300 | T-600 | R-600 |
| T1/2 (h) | 35.11 ± 6.32 | 26.97 ± 14.48 | 35.05 ± 10.28 | 20.02 ± 10.59 | 37.74 ± 12.33 | 24.52 ± 12.94 |
| Tmax (h) | 29.33 ± 9.35 | 15.33 ± 10.46 | 21.33 ± 10.01 | 13.83 ± 8.54 | 22.33 ± 13.54 | 18.33 ± 12.03 |
| Cmax (µg/l) | 0.45 ± 0.12 | 1.39 ± 0.54 | 1.04 ± 0.46 | 1.77 ± 0.91 | 1.97 ± 0.89 | 3.69 ± 1.14 |
| AUC0-t (µg/l·h) | 28.46 ± 8.34 | 40.30 ± 14.59 | 50.42 ± 20.61 | 72.93 ± 46.31 | 88.68 ± 27.77 | 118.74 ± 74.92 |
| AUC0-∞(µg/l·h) | 30.06 ± 9.31 | 41.29 ± 15.62 | 53.98 ± 21.65 | 77.68 ± 49.29 | 91.65 ± 30.23 | 126.43 ± 79.77 |
| MRT (h) | 39.23 ± 2.13 | 39.08 ± 11.20 | 36.68 ± 2.50 | 35.97 ± 3.69 | 36.28 ± 9.04 | 33.07 ± 4.65 |
| CL (L/h) | 10,415 ± 2123 | 3040 ± 850 | 7056 ± 3021 | 5977 ± 4657 | 6864 ± 2046 | 4251 ± 3970 |
Table 6.
Pharmacokinetic parameters of BBR pellet after multiple administration in dogs.
| Pharmacokinetic parameters | Multiple dose groups | |
|---|---|---|
| T1/2 (h) | 30.56 ± 11.82 | 20.57 ± 15.50 |
| Tmax (h) | 18.5 ± 10.45 | 4.75 ± 3.60 |
| Cmax | 1.89 ± 0.79 | 2.07 ± 0.83 |
| AUC0-t (µg/l·h) | 57.50 ± 23.45 | 72.22 ± 35.78 |
| AUC0-∞(µg/l·h) | 60.24 ± 24.58 | 75.39 ± 36.82 |
| MRT (0-t) (h) | 33.80 ± 3.16 | 31.18 ± 4.91 |
| CLz/F (l/h) | 4,541,933 ± 1,507,548 | 3,568,186 ± 1,368,923 |
| DF | 1.75 ± 0.80 | 0.91 ± 0.65 |
It can be seen that the blood concentration-time profiles of both BBR pellets and tablets were nonlinear pharmacokinetic characteristics, but the BBR pellets showed a higher linear correlation coefficient than BBR tablets. However, BBR pellets cannot improve the bioavailability significantly in contrast to BBR tablets. The reason may be as following: a high peak concentration can be obtained from BBR tablets because BBR were released and absorbed rapidly in beagle dogs. Meanwhile, BBR have obvious enterohepatic circulation effect and relatively long half-life, which leads to the reabsorption of BBR and increase of bioavailability. But the oral absorption of BBR tablet was irregular in beagle dogs with large intra- and inter-individual variations, especially in high dose group, which had affected its clinical use seriously.
In contrast to BBR tablets, BBR sustained release pellets showed a more stable plasma concentration, which avoided fluctuations in plasma concentration, and then reduce the incidence of side effects. The elimination T1/2 value of BBR pellets were 35.11, 35.05 and 37.74 h (single dose groups), respectively, which is longer than the corresponding BBR tablets groups (26.97, 20.02 and 24.52 h). This delayed absorption profile signifies that the delay of drug release from the dosage form predominately affected on absorption rate of BBR in gastrointestinal tract and blood concentration level. Meanwhile, the Tmax and CL of BBR pellet increased significantly due to the sustained release behavior. Thus, BBR pellets showed better in vivo pharmacokinetic behavior than the commercial BBR tablets. In addition, the effect targets for antidiabetic and anti-hyperlipidemia effects of BBR is in the gut, therefore, the pellets with sustained release effects might provide better therapeutic efficacy compared with other BBR preparations from the pharmacokinetic aspect. Compared with single dose groups, multi dose group can reduce the individual differences to some extent, and the drug accumulation of two BBR preparations was not obvious.
3.7. In vivo retention of gastrointestinal tract in rabbits
The absorption and therapeutic efficacy of BBR is closely related to intestinal retention time and gut microbiota mixed effect. While the residence time largely depends on the particle size and the properties of the materials used. In this study, pellets counting method was used to examine the pellets of different properties stranded in the gastrointestinal tract (including the stomach and small intestine) in rabbits, and the results are shown in Table 7.
Table 7.
The retention results of different pellets in stomach and small intestine of rabbits.
| No. | Number of pellets | Stomach | Small intestine | SUM | Percentage (%) |
|---|---|---|---|---|---|
| Pellet 1 | 300 | 144 | 130 | 274 | 91.3 |
| Pellet 2 | 100 | 45 | 0 | 45 | 45.0 |
| Pellet 3 | 200 | 109 | 40 | 149 | 74.5 |
| Pellet 4 | 200 | 100 | 10 | 110 | 55.0 |
Pellet 1 with particle size of 0.25–0.30 mm, without coating.
Pellet 2 with particle size of 1.00–1.25 mm, without coating.
Pellet 3 with particle size of 0.50–0.75 mm and Eudragit ® NE30D coating (3.5% weight gain); Pellet 4 with particle size of 0.50–0.75 mm and Eudragit ® L30D55 coating (3.5% weight gain).
The results show that 91.3% of pellets with small particle size (around 0.25–0.30 mm) was still in the gastrointestinal tract of rabbits after 8 h, while the pellets with big size (about 1.00–1.25 mm) only 45% and there was no pellets existed in small intestine tract. It can be concluded that the retention time of small size pellets in the gastrointestinal tract was longer than larger size pellets. This may be due to the pellets with small particle size can be trapped by the mucous layer of the small intestine mucosa, the annular gap and the cave formed by the submucosal gland [24,25]. While the above effects are not obvious on the pellets with large particle size because the large particles showed small specific surface area. In this paper, the particle size distribution of BBR pellets represented a narrow range of 250–450 µm with an average particle size of 325 µm. The experiment also found that Eudragit ® NE30D can prolong the residence time of the pellets. The pellets coated with Eudragit ® NE30D showed better residence effects than the pellets coated with Eudragit ® L30D55 (74.5% vs 55%). The reason may be due to Eudragit ® NE30D can form gel layer under the action of intestine fluid, which allows the pellets to adhere to the intestinal mucosa, while small particle size of the pellets further strengthens the retention capacity of Eudragit ® NE30D. At the same time, the side effects on the intestinal will be reduced because BBR pellets can release drugs evenly and slowly. Therefore, the BBR pellets have more clinical value than tablets.
4. Conclusions
A novel multiple-unit reservoir-type sustained release system of BBR base on the aspect of local action on intestinal tract was constructed successfully by combined application of wet milling technology, liquid layer deposition technology and polymer coating technology. The pellets showed good roundness, high drug loading, small particle size and uniform particle size distribution. Additionally, a significant sustained release behavior can be obtained from in vitro release tests compared with marketed BBR tablets. The accelerated stability and long-term stability of BBR pellets can meet the requirements of pharmacopoeia standards. A similar phenomenon was also observed in a pharmacokinetic study of beagle dogs, in which the mean T1/2 of the pellet formulation was 1.5-fold higher than that of corresponding BBR tablets. Meanwhile, BBR pellets reduced individual differences significantly and showed more regular pharmacokinetic behavior compared with BBR tablets. In addition, the prepared pellets can stay in the gastrointestinal tract longer than BBR tablets, which may reduce saccharides and fat absorption and ultimately achieve the efficient therapeutic action of saccharides and lipid metabolism sydrome.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
Dr. David B. Jack is gratefully acknowledged for correcting the manuscript.
References
- 1.Kumar A., Ekavali, Chopra K., Mukherjee M., Pottabathini R., Dhull D.K. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 2015;761:288–297. doi: 10.1016/j.ejphar.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz L.M., Lombardi P., Tillhon M., Scovassi A.I. Berberine, an epiphany against cancer. Molecules. 2014;19(8):12349–12367. doi: 10.3390/molecules190812349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N., Tan H.Y., Li L., Yuen M.F., Feng Y. Berberine and Coptidis Rhizoma as potential anticancer agents: recent updates and future perspectives. J Ethnopharmacol. 2015;1761:35–48. doi: 10.1016/j.jep.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Lan J., Zhao Y., Dong F. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Chen C., Yu Z., Li Y., Fichna J., Storr M. Effects of berberine in the gastrointestinal tract - a review of actions and therapeutic implications. Am J Chin Med. 2014;42(5):1053–1070. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 6.Mirhadi E., Rezaee M., Malaekeh-Nikouei B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed Pharmacother. 2018;104:465–473. doi: 10.1016/j.biopha.2018.05.067. [DOI] [PubMed] [Google Scholar]
- 7.Gupta L., Sharma A.K., Gothwal A. Dendrimer encapsulated and conjugated delivery of berberine: a novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int J Pharm. 2017;528(1–2):88–99. doi: 10.1016/j.ijpharm.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Cui Y.L., Gao L.N., Jiang H.L. Effects of β-cyclodextrin on the intestinal absorption of berberine hydrochloride, a P-glycoprotein substrate. Int J Biol Macromol. 2013;59:363–371. doi: 10.1016/j.ijbiomac.2013.04.074. [DOI] [PubMed] [Google Scholar]
- 9.Sahibzada M.U.K., Sadiq A., Faidah H.S. Berberine nanoparticles with enhanced in vitro bioavailability: characterization and antimicrobial activity. Drug Des Devel Ther. 2018;12:303–312. doi: 10.2147/DDDT.S156123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Feng R., Zhao Z.X., Ma S.R., Guo F., Wang Y., Jiang J.D. Gut microbiota-regulated pharmacokinetics of berberine and active metabolites in Beagle dogs after oral administration. Front Pharmacol. 2018;214:1–14. doi: 10.3389/fphar.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Zhao Y., Xu J. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M., Liew C.V., Heng P.W.S. Evaluation of the coat quality of sustained release pellets by individual pellet dissolution methodology. Int J Pharm. 2015;478(1):318–327. doi: 10.1016/j.ijpharm.2014.11.057. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W., Jia Z., Xiong P. Novel pH-responsive tobramycin-embedded micelles in nanostructured multilayer-coatings of chitosan/heparin with efficient and sustained antibacterial properties. Mater Sci Eng C Mater Biol Appl. 2018;90:693–705. doi: 10.1016/j.msec.2018.04.069. [DOI] [PubMed] [Google Scholar]
- 14.Bautzova T., Rabiskova M., Beduneau A., Pellequer Y., Lamprecht A. Bioadhesive pellets increase local 5-aminosalicylic acid concentration in experimental colitis. Eur J Pharm Biopharm. 2012;81(2):379–385. doi: 10.1016/j.ejpb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Kan S., Chen T., Liu J. Application of quality by design (QbD) to formulation and processing of naproxen pellets by extrusion-spheronization. Pharm Dev Technol. 2015;10(2):246–256. doi: 10.3109/10837450.2014.908300. [DOI] [PubMed] [Google Scholar]
- 16.Fabiano A., Piras A.M., Uccello-Barretta G. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur J Pharm Biopharm. 2018;130:281–289. doi: 10.1016/j.ejpb.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Miranda T., Montero I., Sepulveda F.J., Arranz J.I., Rojas C.V., Nogales S. A review of pellets from different sources. Materials. 2015;8(4):1413–1427. doi: 10.3390/ma8041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuereb M., Camilleri J., Attard N.J. Systematic review of current dental implant coating materials and novel coating techniques. Int J Prosthodont. 2015;28(1):51–59. doi: 10.11607/ijp.4124. [DOI] [PubMed] [Google Scholar]
- 19.Sharif Hossain A.B.M., Uddin M.M., Veetti V.N., Fawzi M. Nano-cellulose based nano-coating biomaterial dataset using corn leaf biomass: an innovative biodegradable plant biomaterial. Data Brief. 2018;17:162–168. doi: 10.1016/j.dib.2017.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todoran N., Ciurba A., Ai Hussein S., Lazar L., Ion V., Antonoaea P. Development of a modified-release pellets formulation–with metoprolol tartrat and kinetic aspects of in vitro release. Rev Med Chir Soc Med Nat Iasi. 2014;118(4):1143–1149. [PubMed] [Google Scholar]
- 21.Naiserova M., Kubova K., Vyslouzil J. Investigation of dissolution behavior HPMC/Eudragit®/Magnesium aluminometasilicate oral matrices based on NMR solid-state spectroscopy and dynamic characteristics of Gel layer. AAPS PharmSciTech. 2018;19(2):681–692. doi: 10.1208/s12249-017-0870-6. [DOI] [PubMed] [Google Scholar]
- 22.Rujivipat S., Bodmeier R. Moisture plasticization for enteric Eudragit ® L30D-55 coated pellets prior to compression into tablets. Eur J Pharm Biopharm. 2012;81(1):223–229. doi: 10.1016/j.ejpb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Li M., Alvarez P., Orbe P., Bilgili E. Multi-faceted characterization of wet-milled griseofulvin nanosuspensions for elucidation of aggregation state and stabilization mechanisms. AAPS PharmSciTech. 2018;19(4):1789–1801. doi: 10.1208/s12249-018-0993-4. [DOI] [PubMed] [Google Scholar]
- 24.Li Q., Huang W., Yang J. Gastric retention pellets of edaravone with enhanced oral bioavailability: absorption mechanism, development, and in vitro/in vivo evaluation. Eur J Pharm Sci. 2018;119:62–69. doi: 10.1016/j.ejps.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Severino P., Da Silva C.F., Dalla Costa T.C. In vivo absorption of didanosine formulated in pellets composed of chitosan microspheres. In Vivo. 2014;28(6):1045–1050. [PubMed] [Google Scholar]