Abstract
Blindness and vision impairment are the most devastating global health problems resulting in a substantial economic and social burden. Delivery of drug to particular parts of the anterior or posterior segment has been a major challenge due to various protective barriers and elimination mechanisms associated with the unique anatomical and physiological nature of the ocular system. Drug administration to the eye by conventional delivery systems results in poor ocular bioavailability (<5%). The designing of a novel approach for a safe, simple, and effective ocular drug delivery is a major concern and requires innovative strategies to combat the problem. Over the past decades, several novel approaches involving different strategies have been developed to improve the ocular delivery system. Among these, the ophthalmic in-situ gel has attained a great attention over the past few years. This review discussed and summarized the recent and the promising research progress of in-situ gelling in ocular drug delivery system.
Keywords: In-situ gel, Ocular, Drug delivery, Bioavailability, Polymer, Corneal retention
Graphical abstract
1. Introduction
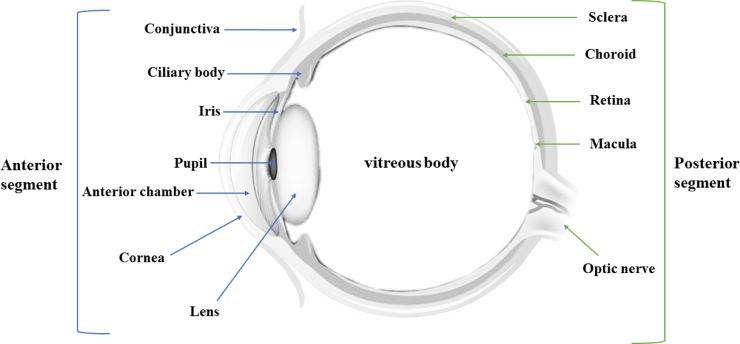
The eye is a complex and unique part of the human organs that has been considered as the window to the human soul. Broadly, the human eye is divided into two segments that are anterior and posterior segments (Fig. 1) [1]. The specific disease conditions of the eye are associated with each of these broad segments. For instance, conjunctivitis, glaucoma, blepharitis, and cataract are some of the diseases that affect the anterior segment of the eye, while diabetic retinopathy and age-related macular degeneration are known to affect the posterior segment [2].
Fig. 1.
The anatomy of ocular system: the anterior segment involves conjunctiva, ciliary body, iris, pupil, anterior chamber, cornea and lens; the posterior segment consists of sclera, choroid, retina, macula and optic nerve.
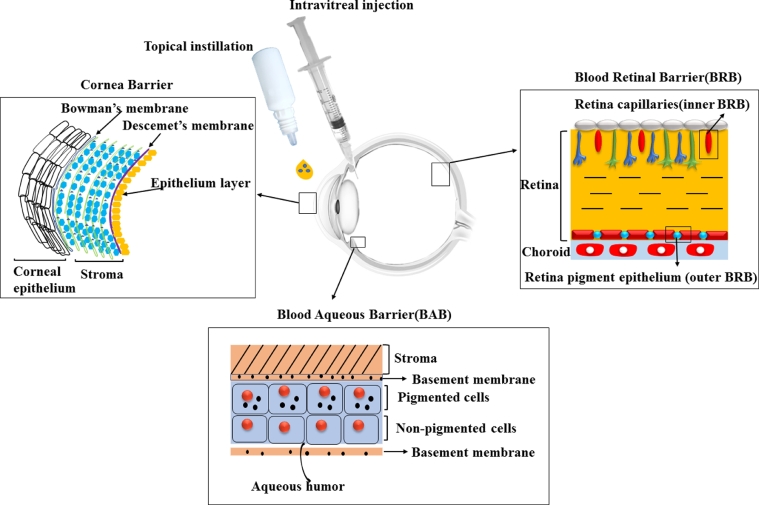
Due to the unique structure of the eye, which inhibits the entry of drug molecules into the desired site, the ophthalmic delivery of the drug has been one of the most challenging tasks for a pharmaceutical scientist. Eye drops accounts for more than 90% of ophthalmic preparations on the markets. However, they are washed away from the eye and results in low ocular bioavailability (<5%) after topical administration [3] by different elimination mechanisms. This elimination process includes tear turnover, nasolacrimal drainage, protein binding, systemic absorption, enzymatic degradation and complex penetration barriers (Corneal Barrier, Blood Aqueous Barrier (BAB), and Blood Retinal Barrier (BRB)) [4] (Fig. 2).
Fig. 2.
The critical barriers to ocular drug delivery systems: the Corneal Barrier: involves of epithelial layers attached together by tight junctions avoiding entry of drug particle followed by thick stroma and endothelial cells. The Blood Retinal Barrier (BRB): comprises of the inner BRB resulted from retinal capillaries. Blood Aqueous Barrier (BAB): made by the nonpigmented cells of the epithelium of the ciliary body, and the endothelium of the iris blood vessels.
One of the main drawbacks in ocular drug delivery is achieving and retaining of optimal concentration of drug at the desired site of action in the eye. Several ophthalmic dosage forms such as ointments, eye drop solutions, gels, and ocular inserts have been investigated in order to prolong the ocular residence time of drugs after the topical application to the eye. With these formulations, the corneal contact time has been increased to some extent. But, due to blurred vision and poor patient compliance resulted from ointments and inserts, respectively, they have not been fully accepted [5]. Furthermore, drugs that are administered systemically to exert their action in the ophthalmic system also have known to access poorly to the eye tissue [6]. Intravitreal and pertiocular routes are recommended in order to deliver drugs to the posterior part of the eye. However, there are disadvantages associated with these routes like the frequent intravitreal injections could be painful, thus affecting a patient compliance. The periocular route is easy for administration, but the static and dynamic barriers constitute a problem [7].
The low bioavailability of medications from the conventional delivery system is resulted from a great extent of precorneal drug loss by nasolachrymal drainage. The rapid clearance of the topically applied drug into the eye often results in a short duration of pharmacological activity and, therefore, the need for a frequent dosing regimen. Moreover, 50%−100% of an instilled dose could undergo systemic absorption through drainage via the nasolachrymal duct. This could lead to a possible increased risk of unwanted systemic toxic effects [8].
In last decades, various delivery systems such as using chemical permeability enhancers [6], prodrugs [9] stimuli-responsive in-situ gel [10], and drug delivery carriers such as liposomes [11] and nano- or microparticles [12], noisomes [13], dendrimers and microneedles [14] have been developed to increase ocular residence time, drug penetration across the ocular barriers and ophthalmic bioavailability. In-situ gelling system is one of the promising approaches to improve the retention time of drugs on the ocular surface. After instillation of the aqueous solution containing stimuli-responsive polymers such as pH-sensitive polymers, thermosensitive polymers, and ion-sensitive polymers, the viscous and mucoadhesive gels are formed on the eye surface [15], subsequently, ocular retention time and ocular bioavailability of the ophthalmic drugs are improved. Therefore, in this review, we summarized and discussed the most recent research innovations in stimuli-responsive in-situ gelling system for ocular drug delivery system.
2. Anatomy of the ocular system
Generally, the eye is divided into two important segments: (1) The anterior segment which involves the cornea, conjunctiva, iris, pupil, ciliary body, anterior chamber, aqueous humor, lens and trabecular meshwork. (2) The posterior segment includes vitreous humor, sclera, retina, choroid, macula and optic nerve (Fig. 1) [1].
The cornea is the transparent and clear avascular part of the ocular system that forms the anterior most coat of the eye. Anatomically, the cornea is consist of five major layers. Corneal epithelium is the first layer, which is the most exterior [16]. The other layers include Bowman's membrane, stroma, Descemet's membrane and the endothelium layer [1]. Corneal permeability is the most essential factor that determines drug concentration in aqueous humor. For most of hydrophilic drugs, the epithelium is a rate-limiting barrier of transcorneal diffusion of drugs [17]. The stroma is owing to the hydrophilic nature, it acts as a barrier for the diffusion of highly lipophilic drugs [17]. The corneal stroma is mainly consisting of charged and highly organized hydrophilic collagen that inhibit the diffusion of hydrophobic molecules [18].
Conjunctiva is a clear thin membrane that covers the sclera and lines the inner surface of the eyelid. It is consist of stratified epithelium (non-keratinized) and goblet cells. It provides protection to the eyes by secreting mucus that prevents entry of microorganisms and lubricating the eyes [1]. In humans, the conjunctiva occupies a 17-times larger surface area than the cornea. This allows for greater absorption of the drug to occur through the conjunctiva. Therefore, drugs are usually more permeable across the conjunctiva than the cornea. However, absorption of the drug via the conjunctiva is still not significant due to the existence of conjunctival blood capillaries and lymphatics, which leads to a considerable loss of drug into the systemic circulation, thereby reducing the overall ocular bioavailability [19].
Aqueous humor consists of clear liquid that fills both the posterior and anterior chambers of the eye. The aqueous humor is non-vascular structure that must be transparent to allow light transmission, which provides nutrition for the cornea [1]. It contains excessive concentration of ascorbate which is about 15-fold the concentration in the plasma, and has a pH of 7.2 [16]. Its main function is to provide nutrients, eliminate waste from non-vascular tissues and control the intraocular pressure that keeps the convex shape of the cornea [20].
The sclera is the whitish portion of the eye, opaque and elastic in nature, consisting of collagen fibers [1]. The solutes especially hydrophilic compounds are generally more permeable across the sclera than the cornea and the conjunctiva, because diffusion through sclera is mainly a matter of transport across an aqueous medium of proteoglycans or leaky spaces within the collagen network rather than diffusion across cellular membranes [19]. The sclera offers a protective outer layer, maintaining intraocular pressure and serving as the attachment portion for the extraocular muscles [17].
The retina is a multiple layers and complex structure that consists of vascular, glial and neural, cells and nerve fibers [16]. The retina is a major barrier to ocular delivery of drug with larger molecular weight [19].
3. In-situ gelling system
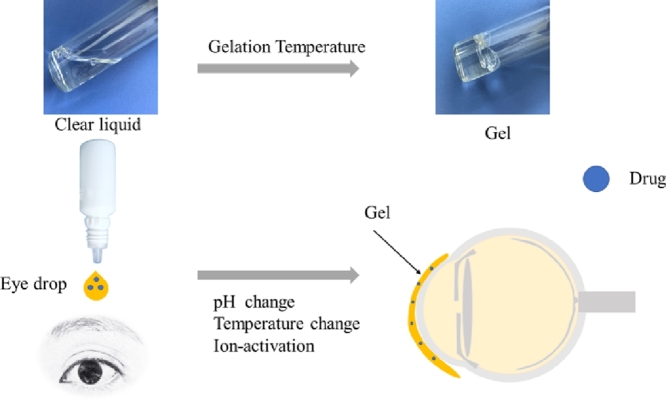
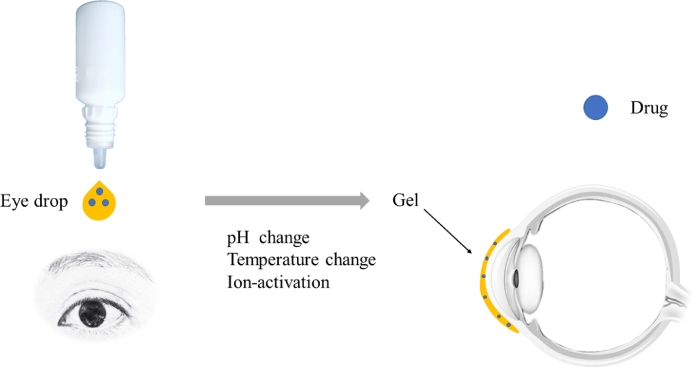
Ophthalmic in-situ gelling is comprising of environmentally sensitive polymers that will be altered structurally with the small changes in specific conditions like pH, temperature and ionic strength in the environment. In-situ forming gels are liquids during instillation into the eye and then undergoes rapid gelation in the cul-de-sac of the eye to form viscoelastic gels in response to environmental changes (Fig. 3); lastly release the drug slowly under physiological conditions [21]. Consequently, the residence time of the gel formed in-situ will be extended and the drug is released in a sustained manner which leads to enhanced bioavailability, minimized systemic absorption and reduced frequent dosing regimen resulting to improved patient compliance [22]. Furthermore, some other potential advantages such as simple manufacturing process, ease of administration, and deliverance of accurate dose have been exhibited by in-situ gelling systems [23].
Fig. 3.
In-situ forming gels process. The formulation is liquid when instilled into the eye which undergoes gel formation rapidly in the cul-de-sac of the eye in response to environmental changes such as pH, temperature and ion; finally release the drug slowly under physiological conditions.
3.1. Mechanisms of gelling system
In-situ gel formation may be achieved by a number of mechanisms including temperature- (Fig. 4), pH- and ion-activated systems. Temperature triggered in-situ gel system which utilizes the temperature sensitive polymers that exist as a liquid form below its low critical solution temperature (LCST) and undergoes gelation when the environmental temperature reaches or is above the LCST [24]. The pH induced in-situ gel contains polymers which possess acidic or alkaline functional groups within the chain molecule and undergoes a sol-gel phase transition on change from a low pH to high pH environment [23]. Ion-activated systems are also known as osmotically triggered in-situ gel systems wherein the polymer undergoes a sol-gel transition due to changes of ionic concentration, which is typically triggered by mono or divalent cations in tear fluid particularly Na+, Mg2+and Ca2+ [25]. In addition, sol-gel phase transition has known to be induced by enzymatic cross linking and photon polymerization [25], [26]. However, the pH, temperature, and ion-induced in-situ gel are the most extensively studied approaches of in-situ gel, and the concern of this review.
Fig. 4.
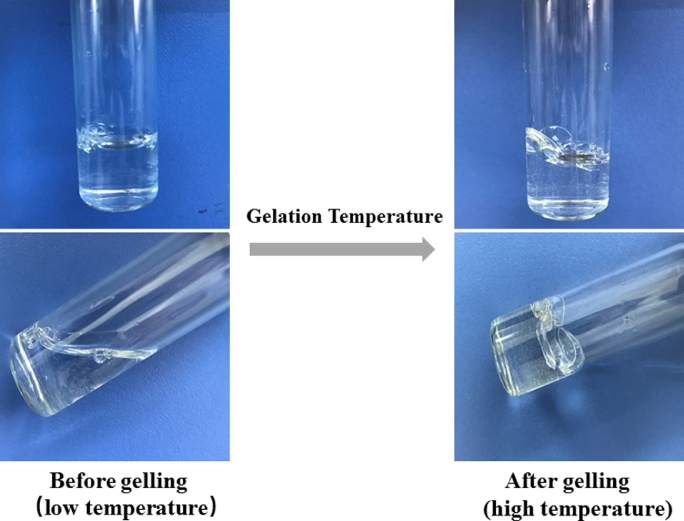
The gelation process of thermosensitive in-situ gelling. When the temperature is below the gelation temperature (GT), it is clear solution with low viscosity, upon heating it to GT, the solution is converted to the gel with high viscosity.
3.2. Stimuli-responsive in-situ gel system
3.2.1. Temperature-triggered in-situ gel systems
The temperature sensitive in-situ gel is the oldest, the most extensively studied and common type of stimuli-responsive gel. It can be easily and precisely introduced into the eye in liquid form without producing irritation or blurred vision. The gel is formed at the precorneal temperature (35 °C) to endure the lachrymal fluid dilution without rapid precorneal elimination of instilled drug after administration [27]. It has been recommended that a good thermo-responsive ocular in-situ gel should possess the gelation temperature above the room temperature and undergo gel-sol transition at a pre-corneal temperature in order to avoid storing in a refrigerator before instillation, which may sometimes result in eye irritation due to cold nature [28].
Polymers used in temperature triggered in-situ gel systems
Poloxamers (Pluronic)
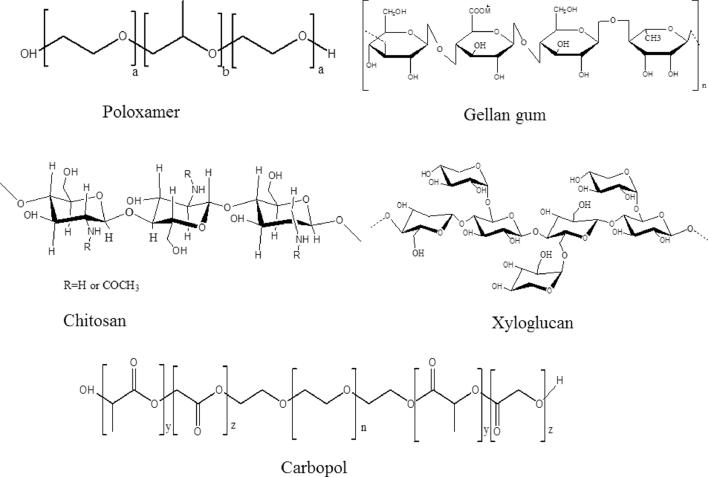
Poloxamers are a triblock copolymer poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) (PEO-PPO-PEO) exhibiting amphiphilic nature because of hydrophilic ethylene oxide domains and hydrophobic propylene oxide domains [29] (Fig. 5). The triple block of copolymers PEO-PPO-PEO (Pluronics or Poloxamers) undergo gelation at body temperature in concentrations above 15% (w/w) [30]. The principal possible mechanisms have been proposed to explain the sol-gel phase transition at an increased temperature are the gradual desolvation of the polymer, enhanced micellar aggregation, and the increased entanglement of the polymeric network [26]. The pluronic triblock copolymers are existing on the market in different grades with different physical forms and molecular weights. Depending upon the physical description for the grades are given as L for liquid, P for paste and F for flakes. The commonly used poloxamers are 188 (F-68), 237 (F-87), 338 (F-108) and 407 (F-127) [31]. Pluronic F-127 (F-127) or Poloxamer 407 (P407) (copolymer PEO106-PPO70-PEO106) consists of ethylene oxide (70%) which contributes to its hydrophilic property. F-127 is a copolymer with molecular weight of 12 000 Da, a PEO/PPO ratio of 2:1, nontoxic, with low viscosity below 4 °C and forming a semisolid gel at body temperature. Furthermore, F-127 has better solubility in cold water than in hot water because of the hydrogen linkages at low temperatures [31], [32].
Fig. 5.
The chemical structure of some in-situ gel polymers.
Xyloglucan
Xyloglucan is a polysaccharide obtained from tamarind seeds, therefore it is often named tamarind seed polysaccharide (TSP), which when partially degraded by β-galactosidase displays thermally reversible gel formation in diluted aqueous solution (Fig. 5). The sol-gel transition temperature is varying with the degree of galactose degradation [33]. TSP gels have been reported to have a potential for oral, ocular, intraperitonial and rectal drug delivery [23], [33]. TSP is highly water-soluble and gelation occurs when the galactose elimination exceeds 35% [34].
Cellulose derivatives
Cellulose is a polysaccharide containing a linear chain made up of several hundred to over ten thousand β (1→4) linked d-glucose units. The cellulose derivatives used in topical ophthalmic formulations are methyl cellulose, hydroxyethyl cellulose, hydroxypropyl methylcellulose (HPMC), sodium carboxymethyl cellulose (NaCMC) [34]. At low concentrations (1–10%), their aqueous solutions exist as a liquid but form gels upon heating. The high phase transition temperature exhibited by cellulose derivatives can be lowered by physical or chemical modification [35]. The transition temperature is between 40 and 50 °C for MC and between 75 and 90 °C for HPMC. Addition of sodium chloride is known to lowers the gelation temperature of MC to 32–34 °C, while the transition temperature of HPMC can be decreased to about 40 °C by lowering the hydroxypropyl molar substitution [36].
Chitosan
Chitosan is an aminopolysaccharide (Fig. 5) made from the partial deacetylation and depolymerization of chitin, which is found in the exoskeletons of arthropods, such as crustaceans [37]. Commercially, chitin is mainly derived from the shell wastes of shrimp, crab, krill, lobster, and squid [26]. Chitosan has been proven to possess many advantages in biomedical applications due to its biocompatibility, biodegradability, mucoadhesiveness with low immunogenicity and low cytotoxicity [38]. Recently, chitosan-based thermosensitive gels with different polyols such as ethylene glycol, glycerol, and sorbitol have attained much popularity [39].
The derivatization of primary amino groups of chitosan (CS) by thiol groups results in the formation of Thiolated Chitosan (TCS). TCS based drug delivery system is gaining attention because it exhibits high mucoadhesive strength and extended drug release properties. TCS shows in-situ gelling properties because of the formation of intra and intermolecular disulfide bonds as a result of oxidation of thiol groups at physiological pH-values [40].
Research progress in temperature triggered in-situ gel systems
Over the last decades, a large number of studies on temperature triggered in-situ forming system have been reported (Table 1). To mention some of them, Li et al. formulated Brinzolamide drug-resin in-situ thermosensitive gelling system, using Poloxamer F127 in combination with Carbopol 934P. The optimal formulation displayed a gel formation at 33.2 ± 1.1 °C and the diffusion-controlled release of the model drug over a period of 8 h. The in vivo study suggested that the in-situ gel demonstrated a better ability in retaining the drug than commercial formulations [41].
Table 1.
Some examples of thermo-sensitive in-situ gelling system.
| Model drugs | Polymers | Major finding | Ref. |
|---|---|---|---|
| Brinzolamide | Poloxamer F127 and carbopol 934P | A sol-gel at 33.2 ± 1.1 °C controlled release of drug over a period of 8 h. | [41] |
| Ofloxacin | Pluronic (PF-127 and PF-68) and sodium alginate | In vivo evalutaion in rabbits exhibited inproved retention performance of 20% (w/w) Pluronic F127 compared to Pluronic F68. | [42] |
| Ketorolac tromethamine | Pluronic F-127 HPMC K4M | Improved its ocular availability and prolonged its residence time. | [45] |
| Sparfloxacin | Pluronic (PF 127 and PF 68) | Showed promising antimicrobial activity in vitro and in vivo. | [51] |
| Fluconazole | Poloxamer/tween/carbopol | The in vivo ophthalmic absorption was superior to the conventional eye drop. | [52] |
| Lomefloxacin | Pluronic F127, Pluronic F68 and sodium alginate | Revealed a sustained release profile of 8 h. | [53] |
| Methazolamide | Poloxamer 407 and poloxamer P188 | Had a better ability to retain drug than the eyedrops. | [54] |
| Diclofenac sodium | Pluronic F127 | The bioavailability of diclofenac sodium in aqueous humor was significantly increased. | [55] |
| Dorzolamide hydrochloride | Poloxamer 407 and Poloxamer 188 | Better pharmacological effect, faster onset of action, and prolonged effect relative to either drug solution or the market product. | [56] |
Al-Khateb et al. also investigated the in-situ gelling system containing ofloxacin using a combination of Pluronic (PF-127 and PF-68) and sodium alginate. The incorporation of Pluronic F68 to Pluronic F127 solutions was found to rises the sol-gel temperature of binary formulation to above the physiological range of temperatures. The superior in vitro drug retention performance on glass surfaces and freshly excised bovine corn were exhibited by 20% (w/w) Pluronic F127 in comparison with other formulations. Additionally, in vivo evaluation in rabbits demonstrated that a retention performance of 20% (w/w) Pluronic F127 was higher than that of Pluronic F68. Furthermore, the slug mucosa irritation assay and bovine corneal erosion studies demonstrated no significant irritation was observed that resulted from these polymers and their combinations [42].
Osswald et al. prepared an injectable microsphere-hydrogel by loading the antivascular endothelial growth factor, anti-VEGF (ranibizumab or aflibercept) into poly (lactic-co-glycolic acid) microspheres that were then suspended within an injectable poly(N-isopropylacrylamide)-based thermo-responsive hydrogel DDS. The efficacy was evaluated in vivo in a laser-induced rat model of choroidal neovascularization (CNV). CNV lesion area was measured and quantified by fluorescein angiograms and a multi-Otsu thresholding technique, respectively. Intraocular pressure (IOP) and dark-adapted electroretinogram (ERG) were also measured pre- and post-treatment (1, 2, 4, 8, and 12 weeks). The result has shown that the anti-VEGF-loaded DDS group had exhibited significantly smaller CNV lesion areas than a non-treatment group of animals throughout the study, which suggests that the DDS offer a significant benefit in the management of posterior segment eye diseases [43].
The addition of cellulose derivates to Pluronic F12 hydrogels assist in increasing the bioavailability of the in-situ gel [44], Morsi et al., prepared Ketorolac tromethamine nanodispersions formulated into thermo-sensitive in-situ gel using Eudragit RL100. The study demonstrated that reducing the concentration of Pluronic F-127 was found to increase the gelation time and gelling temperature of the in-situ gels. The incorporation of HPMC to pluronic F12 hydrogels has significantly improved the mucoadhesive strength of the gel [45].
Addition of salts (NaCl and KCl) to in-situ gel system has known to decrease the gelation temperature. Bhowmik et al. examined the influence of different salts on the gelation properties, rheology and drug release of in-situ gel based on methylcellulose (MC). It was found that 5–7% (w/v) sodium chloride, 8–9% (w/v) potassium chloride, or 5% (w/v) sodium bicarbonate was capable of reducing the GT below physiological temperature, i.e. 37 °C. The duration of drug release increased from 1.5 h to 3–5 h from salt containing MC solutions depending on the concentration and the type of salt [46]. Similarly, Bhowmick et al. confirmed that the use of i-carrageenan with potassium chloride could effectively decrease the GT of the virgin MC solution from 60 °C to 33.5 °C which is below physiological temperature [47].
The mixture of poloxamer with a mucoadhesive agent (chitosan) is known to extend the retention time of drugs for the treatment of ophthalmic diseases. Gratieri et al. formulated in-situ forming gel consisting the combination of poloxamer and chitosan. The results demonstrated that the addition of chitosan could improve the mechanical strength as well as texture properties of poloxamer formulations and the in-situ gel increased a four-fold retention time in comparison with a conventional solution [48].
In addition to Poloxamer, Poly (N-isopropylacrylamide) (PN) has been widely used as thermo-responsive polymers. For instance, Hsiue et al. developed ophthalmic formulation using PN as the thermo-sensitive polymer. The clear solution of PN was known to form a gel upon the raising of temperature from the room temperature to about 32 °C. Epinephrine-loaded linear PN and crosslinked PN nanoparticles were developed and evaluated. The finding of the study showed that the pressure decreasing the activity of the formulation with linear PN and combination of linear PN and crosslinked nanoparticles lasted six-fold and eight-fold longer than that of the conventional eye drop, respectively [49].
Recently, the studies have shown that copolymerization of PN with hyaluronic acid (HA) has increased the LCST of PN from 32 °C to above body temperature, which is more appropriate for the ophthalmic application. With this aim, Zhu et al. developed thermo-sensitive in-situ forming gelling formulation of Ketoconazole (KCL) based on PN/HA. The in vitro gelation, drug release, and antifungal activity were evaluated for the developed formulations. The gelation temperature of the PN—HA thermo-gelling solution was found 33 °C. The moderate release of KCL from in-situ gels without burst effects was exhibited. No macroscopic signs of irritation, redness, or other toxic effects were observed. The in vivo antimicrobial study indicated that KCL PN—HA in-situ gels displayed an improved cure percent as compared with commercial eye drops [3].
Very recently, Iohara et al. developed a hydrophobically modified hydroxypropyl methylcellulose (HM-HPMC) gel formed thermo-responsive hydrogels by incorporation of small amount of α-Cyclodextrin (α-CD) into the solution. The formed HM-HPMC/α-CD gel exhibited a reversible sol-gel transition within the range of physiological temperature which was totally opposite to the temperature dependency has shown by the original HM-HPMC (without α-CD). The HM-HPMC/α-CD exhibited the rapid gelation on the ocular surface and a significantly improved ocular drug (diclofenac sodium) absorption [50].
3.2.2. pH triggered in-situ gelling systems
This in-situ gelling system consists of pH-sensitive polymers which are polyelectrolytes contain an acidic (carboxylic or sulfonic) or a basic group (ammonium salts) that either accept or release protons in response to alteration in pH in the surrounding environment. At lower pH (pH 4.4), the formulation exists as a regular solution, however, it undergoes gel formation at pH 7.4, that is the pH of tear fluid.
Polymers used in pH triggered in-situ gel systems
The most commonly used pH-responsive polymers in ophthalmic preparation are Polyacrylic acid (PAA, Carbopol 940), polycarbophil, and cellulose acetate phthalate (CAP) [17].
Carbopol (Polyacrylic acid)
Carbopol is a polyacrylic acid (PAA) polymer (Fig. 5), that displays a sol-gel phase transition in aqueous solution as a result of raising the pH above its pK of about 5.5 [57]. The carboxylic groups of PAA accept and release protons at low pH values and high pH values, respectively. Therefore, at high pH, the PAA swells due to the electrostatic repulsion of the negatively charged groups, releasing the drug molecules to the environment [17]. It is extensively exploited in ocular formulation with the aim of improving pre-corneal retention time of drugs. Carbopol provides the benefit of exhibiting superior mucoadhesive properties as compared to other polymers. Mucoadhesive properties of carbopol is attributed to the interaction of poly(acrylic acid) with mucin that occurs by four mechanisms viz. electrostatic interaction, hydrogen bonding, hydrophobic interaction and inter diffusion [35]. Despite carbopol displays excellent mucoadhesive properties, the acidic nature of the gel is a major drawback which leads to irritation and damage to the eye tissues. Therefore, combinations of carbopol with other polymers including chitosan and HPMC were subsequently developed to overwhelmed this problem [25].
Research progress in pH triggered in-situ gel systems
The in-situ pH-triggered gelling system has a great potential to keep drug product more stable and retain drug release (Table 2). With this aim, our research group (Wu et al.) designed and evaluated pH-triggered gel containing Baicalin for sustained ophthalmic drug delivery using Carbopol 974P as the gelling agent along with HPMC E4M (0.6%, w/v) that was a viscosity enhancing agent. The in vitro and in vivo evaluations were conducted using confocal scanning light microscopy, rheometry, Gamma scintigraphic technique and microdialysis method. The result of rheological study displayed that the gel strength was significantly enhanced under physiological condition. The gel could provide sustained release of the drug over an 8 h period. Furthermore, the AUC and Cmax values were found 6.1-times and 3.6-times higher than those of the control solution, respectively [58].
Table 2.
Some examples of pH-triggered in-situ gelling system.
| Model drugs | Polymers | Major finding | Ref. |
|---|---|---|---|
| Baicalin | Carbopol 974P with HPMC E4M | Better stability, ocular bioavailability and sustaining drug release compared to commercial baicalin eye drops. | [58] |
| Ciprofloxacin | Calcium alginate with HPMC K4M and E50LV | Added benefits of sustained drug release. | [5] |
| Norfloxacin | Carbopol 934P | Sufficiently mucoadhesive, antibacterial activity and free from ocular irritancy. | [60] |
| Timolol Maleate | Carbopol and chitosan | Showed a controlled type of release over 24 h periods. | [61] |
| Brimonidine | Carbopol 974 P and HPMC E4M | Increased efficacy and reduced systemic absorption of brimonidine tartrate. | [59] |
| Gatifloxacin | Carbopol 940 combined with HPMC and HPMC K15M | Provided sustained drug release over an 8-hour period. | [62] |
| Moxifloxacin | carbopol/HPMC | Showed increased in precorneal residence time, ocular bioavailability. | [63] |
In addition, our research group (Pang et al.) have confirmed that the ocular in-situ gel can reduce the systemic absorption of the drug and thus reduce the potential systemic toxicity. Brimonidine Tartrate in-situ gel was prepared using Carbopol 974 P and HPMC E4M, and its therapeutic efficacy and systemic absorption were compared with that of eye drop. The pharmacodynamics study on the eye of rabbit showed that the gel formulation could significantly decrease intraocular pressure (IOP) as compared to the eye drop. More importantly, the in vivo pharmacokinetic studies exhibited that the plasma AUC(0→∞) was found lower for the in-situ gel than the eye drop, which suggests the decreased systemic absorption [59].
3.2.3. Ion-activated in-situ gel system
Ion-activated in-situ gelling systems form a crosslink with cations exists in the tear fluid (Na+, Ca2+ and Mg2+), thus forming a gel on the ocular surface, which give rise to an extended corneal contact time (Table 3) [17], [64].
Table 3.
Some examples of ion-activated in-situ gelling system.
| Model drugs | Polymers | Major finding | Ref. |
|---|---|---|---|
| Gatifloxacin | Alginate with HPMC | A higher ocular bioavailability and extended residence time in aqueous humor than conventional ophthalmic solutions. | [75], [76] |
| Fluconazole | HPBCD complexed gellan gum and κ-carrageenan | Showed effective control of fluconazole release and good bioadhesive properties. | [66] |
| Acetazolamide | Gellan gum with xanthan gum, HPMC or carbopol. |
Enhanced therapeutic efficacy and more extended intraocular pressure lowering effect compared to that of marketed eye drops and oral tablet. | [77] |
| Terbinafine hydrochloride | Gellan gum | Significantly higher Cmax, delayed tmax, and prolonged mean residence time and increased bioavailability. | [74] |
| Antisense oligodeoxynucleotide | Gellan gum and carrageenan | The greatest reduction in wound size, the least stromal edema and hypercellularity | [78] |
Polymers used in Ion-activated in-situ gel system
The most commonly used ion-activated polymers in ocular formulations are gellan gum (Gelrite®), hyaluronic acid and sodium alginates [65].
Gellan gum
Gellan gum are polysaccharides that can be used to induce ion-sensitive hydrogels. It is a linear anionic heteropolysaccharide (Fig. 5) made up of a tetrasaccharide repeating unit of glucose, glucuronic acid and rhamnose in the ratio of 2:1:1 [66]. Gellan comprises hydroxyl and carboxylic functional groups, which may interact with other polymers via hydrogen bonding and/or electrostatic attractions [67]. A low-acetyl gellan gum is commonly available in the market as Gelrite®, which undergoes gelation in the presence of mono- or divalent cations. The electrolytes of the tear fluid especially Na+, Mg2+ and Ca2+ cations are particularly known to induce gel formation of the polymer upon instillation as a liquid solution into the cul-de-sac [68]. The incorporation of optimal quantities of calcium gluconate to gellan formulations lead to the formation of gellan calcium gluconate-simulated tear fluid (STF) gels with a significantly higher strength than when gellan alone was mixed with STF [67]. It undergoes gelation by both temperature sensitive or cations induced mechanism. The possible mechanism of gelation includes the formation of double helical junction zones followed by aggregation of the double helical segments to form a three-dimensional network by hydrogen bonding with water and complexation with cations [26].
Alginate/ Alginic acid
Alginate is a linear co-polysaccharide derived from brown seaweeds and some bacteria. Chemically it is a (1–4)-linked block copolymer of â-d-mannuronate (M) and its C-5 epimer R-l-guluronate (G), with residues arranged in homopolymeric sequences of both kinds and in region which approximate to the disaccharide repeating structure (MG) [69]. Sodium alginate undergoes gel formation as a result of calcium alginate formation by virtue of its interaction with a divalent cation (Ca2+) present in lachrymal fluid (pH 7.4) [5]. Various properties of the polymer such as mechanical strength, porosity, etc. are highly depend on the ratio of β-d-mannuronic acid and α-l-glucuronic acid. Alginate with a high guluronic acid content exhibit a better gelling properties and minimize the concentration of polymer required to form stiff gel [26].
Pectin
Pectins are a polysaccharides family, where the polymer backbone mostly consists of α-(1,4)-d-galacturonic acid residues. Low methoxy pectins which are with a degree of esterification <50% can form gel in aqueous solution in the existence of free calcium ions, that cross link the galacturonic acid chains. Its water solubility is one of the important advantages of pectin, therefore organic solvents can be avoided in the formulations [70]. The in-situ gelling of pectin induced by calcium ions exists in lacrimal fluid has been reported in a US patent. In addition, pectin based in-situ gel has been used to prolong drug release from the formulations such as theophylline, acetaminophen, and cimetidine [71].
Research progress in ion-activated in-situ gel systems
Various ion-activated in-situ gelling systems have previously been reported. Rupenthal et al. formulated ion-activated in-situ based on gellan gum, xanthan gum and carrageenan, and in vivo release, precorneal retention time and the ocular irritancy were characterized for the formulations. The results showed that the in-situ system was non-irritant with increased AUC and the miotic response of pilocarpine by 2.5-fold compared to an aqueous solution [64].
Zhu et al. also developed an ion-activated ketotifen ocular formulations using a natural polysaccharide which is deacetylase gellan gum. The study demonstrated that deacetylase gellan gum had a potential to prolong the residence time of the formulation. The in-situ gels exhibited a characteristic sustained and extended drug effects behavior compared with the conventional eye drops at the same dose [72].
Kesarla et al. formulated nanoparticles-loaded ophthalmic in-situ gel using the ion-sensitive polymer gellan gum used as a gelling agent which could form gel immediately and remained for the extended time of period. The developed formulation was found stable and displayed improved corneal contact time and minimizing the frequency of administration. The confocal microscopic study showed a clear cornea permeation of drug-loaded nanoparticles [73]. Tayel et al. developed a novel ion-sensitive in-situ ophthalmic nanoemulsion (NE) gels containing terbinafine hydrochloride. The optimized in-situ NE gel exhibited a significantly higher Cmax, delayed tmax, prolonged mean residence time and enhanced ocular bioavailability [74].
In the development of bioadhesive ion-sensitive hydrogels, the incorporation of the poorly water soluble drug is very challenging. Cyclodextrins (CDs) are beneficial pharmaceutical excipients that assist in the formulation of poorly aqueous soluble drugs. The inclusion of hydroxypropyl-β-cyclodextrin (HPBCD) in the in-situ formed gel has shown to allow a more effective control and a significant improvement in the fluconazole release [66].
3.2.4. Multi-stimuli responsive in-situ gel
One of the recent excellent strategies in ocular in-situ gelling system is the use of a combination of polymers with the different gelling mechanism, which have shown an improved therapeutic efficacy and better patient compliance [20]. Over last current years, a number of investigations that involved the combination of thermo-responsive polymers, pH-sensitive polymers or ion-activated polymers in the same ophthalmic formulation have been reported (Table 4).
Table 4.
Some examples of multi-stimuli responsive in-situ gelling system.
| Model drugs | Polymers | Stimuli | Major finding | Ref. |
|---|---|---|---|---|
| Sparfloxacin | Sodium alginate and methylcellulose | Ion and pH sensitive | Rapid gelation upon raising pH to 7.4, in vitro sustained drug release over period of 24 h, significantly enhanced corneal permeation. | [21] |
| Nepafenac | Carboxymethyl chitosan (CMC) and poloxamer | pH-induced and thermo-sensitive | The gelation temperature of 32–33 °C and retarding the drug diffusion rate was observed. | [79] |
| Timolol | Chitosan with gellan gum | pH-sensitive and ion-activated polymer | Enhanced transcorneal drug permeation and prolonged the retention at the corneal site. | [82] |
| Levofloxacin | Sodium alginate and chitosan | Ion and pH-triggered | Better retention time was observed. | [83] |
| Ciprofloxacin | Carbopol/HPMC and Poloxamer | pH-induced and thermo-sensitive | Emproved therapeutic efficacy and offers sustained release of the drug over an 8 h period. | [84] |
Khan et al. developed and evaluated sparfloxacin-loaded novel in-situ gelling system for sustained ophthalmic drug delivery using a combination of ion and pH activated gelling system, which were sodium alginate and methylcellulose, respectively. The formulation was in sol form at pH (4.7) and has undergone quick sol-gel transition upon raising pH to 7.4. The in-situ gel formulation demonstrated in vitro sustained release of sparfloxacin over a period of 24 h as compared to eye drop. The ex vivo corneal permeation study on goat eye revealed that a dramatically improved permeation as compared to eye drop [21].
In addition, Yu et al. reported nepafenac in-situ gel using carboxymethyl chitosan (CMC) and poloxamer composed of PEO-PPO-PEO block copolymer which was found to undergo a reversible sol-gel transition upon a change in a temperature and/or pH at a very low concentration. The result of a CCK-8 (Cell Counting Kit-8) study showed that the formulation was not toxic to human corneal epithelial cells at a low concentration. The formulation of poloxamer-CMC/NP showed a sustained release of nepafenac from the hydrogel. The release rate was found to be maximum at 35 °C and pH 7.4 [79].
Davaran et al. developed a dual thermo-/pH-responsive nanocarriers in-situ gel for ciprofloxacin. Ciprofloxacin released from the nanoparticles in-situ gelling system demonstrated an improved antimicrobial activity as determined by minimal inhibitory concentrations [80]. Gupta et al., also formulated in-situ gel using the combination of gellan gum (ion-sensitive) and chitosan (pH sensitive) so as to improve precorneal residence time of sparfloxacin. Accordingly, the developed sparfloxacin in-situ forming gel was found nonirritant and showed the prolonged retention at the corneal site with the prolonged drug release [81].
3.3. Nano-in-situ gelling systems
Nowadays nanotechnology is the most emerging concept in pharmaceutical sciences [85]. Several nano-technology based formulations have been developed to extend ocular residence time and to improve bioavailability of ophthalmic drugs. However, nanoparticles have not mucoadhesive property, so are cleared out of eyes rapidly [86]. The suspending of fabricated nanoparticles in an in-situ gelling vehicle which undergoes sol to gel phase transition upon exposure to physiological condition is known to solve this problem. The nanoparticulate in-situ gel was designed to explore the double benefit of nanoparticles and in-situ gelling system, for its ophthalmic delivery (Table 5) [60], [87]. This results in extending the pre-corneal residence time of the nanoparticles and improving ocular bioavailability [26].
Table 5.
Some examples of nanocarrier in-situ gelling system.
| Model drugs | Polymers | Type of stimuli-nanocarrier | Major finding | Ref. |
|---|---|---|---|---|
| Timolol | Gellan gum | Ion-triggered-liposome | Rapid reduction of intraocular pressure and significantly longer effective time. | [96] |
| Dorzolamide | Poloxamer 407 | Thermo-sensitive-nanoemulsion | Non-irritant and highly therapeutically efficient. | [56] |
| Loteprednol | Poloxamer 407 and 188 | Thermo-sensitive-nanoemulsion | Extended mean residence time and improved (2.54-times) bioavailability compared to marketed formulation. | [28] |
| Cyclosporine A | Deacylated gellan gum | Ion-triggered-microemulsion | Showed 3-fold greater bioavailability. | [97] |
| Acetazolamide | Carbopol 934 | pH-triggered-nanoparticles | Higher permeation, longer precorneal residence time and sustained release of the drug along with improved in vitro efficacy. | [98] |
| Acetazolamide | Gellan gum, xanthan gum, HPMC/carbopol | Ion-triggered-nanoemulsion | Showed higher therapeutic efficacy and more prolonged intraocular pressure lowering effect relative to that of commercial eye drops and oral tablet. | [77] |
| Ketorolac | Pluronic® F-127, HPMC K4M | Thermo-sensitive-nanodispersion | Sustained the release of drug, improved its ocular availability and prolonged its residence time without causing irritation to eye. | [45] |
| Curcumin | Poloxamer 188 and 407 | Thermo-sensitive-nanostructured lipid carriers | Significantly enhanced preocular retention and ocular permeation capacity. | [88] |
The formulations of in-situ gel in novel drug delivery system as colloidal carriers systems such as nanosuspensions, lipid-based nanocarriers, have proven to be the most effective strategy, causing an exponential increase in the bioavailability of the ophthalmic drugs. For instance, Liu et al. developed the curcumin (CUR)-loaded ocular nanogel by using cationic nanostructured lipid carriers (CNLC) and thermosensitive gelling agent. The in vitro release, corneal permeation, ocular irritation and preocular retention were evaluated. Also, the pharmacokinetic profile in the aqueous humor was evaluated by microdialysis technique. The AUC of the nanogel (CUR-CNLC-GEL) was found 9.24-times higher than those of curcumin solution (CUR-SOL), indicating a significantly improved bioavailability [88].
Pandurangan et al. formulated solid lipid nanoparticles (SLNs)-loaded in-situ gel with voriconazole which was found to be a promising vehicle for ocular delivery with good stability and excellent zone of inhibition in the microbial assay of voriconazole [89]. Paradkar et al. developed a niosomal in-situ gel using Poloxamer 407 and HPMC K4M. The prepared Natamycin niosomal in-situ gel formulation exhibited an increased corneal retention time due to bioadhesive property of gel and displayed extended drug release up to 24 h which as compared to marketed products. The formulation was also found to exhibit a better transcorneal permeation [90]. Shukr et al. also formulated voriconazole-loaded in-situ gelling noisome for ocular inserts using span 40 and span 60 with pluronic F127 and pluronic L64. The optimized in-situ gelling ocular insert showed a significantly higher Cmax, delayed tmax and increased bioavailability, and was found non-irritant [91].
Microsphere-loaded ion-activated in-situ gel of ofloxacin (OFX) was also formulated. In vivo results in rabbits exhibited that OFX-loaded microspheres in-situ gel could improve the relative bioavailability by 11.7-times relative to the commercial OFX eye drops. Furthermore, the extended duration of action of OFX-loaded microspheres in-situ gel preparation is thought to avoid frequent administration, which improves patient compliance [92].
Nofloxacin-loaded nanocarriers were designed utilizing chitosan as a matrix forming polymer, crosslinked by an anionic cross-linker sodium tripolyphosphate. The developed chitosan nanoparticulate in-situ gel exhibited superior performance over the marketed eye drops [60]. Levofloxacin nanoparticles laden in-situ gel was formulated and showed the improved ocular retention time. Gupta et al. designed nanoparticle laden in-situ gel encapsulated PLGA nanoparticle, containing levofloxacin, added into chitosan in-situ gel. Ocular retention was evaluated by gamma scintigraphy in rabbits. The developed nanoparticle laden in-situ gel formulation exhibited slow rate of clearance and retained at the corneal surface for more extended duration than commercially available formulation, in-situ gel and nanosuspension alone [93]. Furthermore, the same group of research confirmed for excellent sustained release of the nanoparticle in-situ gelling system containing sparfloxacin [94].
More recently, Ahmed et al. formulated ketoconazole poly(lactide-co-glycolide) nanoparticles with subsequent loading into in-situ forming gel for ophthalmic drug delivery system. The in vitro release of the drug from the formulations loaded with nanoparticles displayed a sustained and greater drug release compared to free drug formulations. In addition, the in-situ gelling with nanoparticles showed improved antifungal activity in comparison to pure drug formulations. Alginate-chitosan in-situ gel containing optimized ketoconazole nanoparticles displayed higher drug permeation via epithelial cell lines [95].
4. Clinical application of in-situ gelling
To date, some of in-situ gel formulations have been commercially available for ocular drug delivery (Table 6). For instance, Timoptic-XE®, containing timolol maleate (0.25% and 0.5%) in gellan gum has been available on market since 1994, which is applied topically on the eye to treat glaucoma. Furthermore, some of the patents on in-situ gel for ocular delivery system have been issued in the last decades, and are being summarized in Table 7.
Table 6.
List ocular in-situ gels approved for market.
| Name of the product | Polymer | The type of in-situ gelling system | Company | Ref. |
|---|---|---|---|---|
| Timoptic-XE® (Timolol maleate ophthalmic gel forming solution) | Gellan gum | Ion-induced | Merck Pharmaceuticals, USA | [99] |
| Pilopine HS® (pilocarpine hydrochloride ophthalmic gel) | Carbopol 940 | pH-triggered | Alcon laboratories, inc. USA | [26] |
| Akten® (Lidocaine hydrochloride) | HPMC | Temperature-triggered | Akorn Inc., Lake Forest, IL | [100] |
| AzaSite (azithromycin ophthalmic solution) | Poloxamer 407 | Temperature-triggered | InSite Vision | [101] |
| Timoptol-LA (Timolol maleate) | Gellan gum | Ion-activated | Laboratories Merck Sharp and Dohme | [25] |
| Virgan (Ganciclovir) | Carbopol® 974 | pH-triggered | Laboratoires THEA-France | [25] |
Table 7.
List of some patents of in-situ gelling system for ocular delivery.
| Patent Number | Title of the patent | Gelling agents | Year of publication | Ref. |
|---|---|---|---|---|
| US 2011/0 082 128 A1 | In-situ gel ophthalmic drug delivery system of estradiol or other estrogen for prevention of cataracts | Deacetylated gellan gum | 2011 | [102] |
| US 2002/0 114 778 A1 | Reversible gelling system for ocular drug delivery | A block copolymer of propylene oxide and ethylene oxide with HPMC | 2002 | [103] |
| US 8 343 471 B2 | Nanoparticulate in-situ gels of TPGS, gellan and PVA as vitreous humor substitutes | Gellan with PVA | 2013 | [104] |
| WO 2 011 018 800 A3 | In-situ gel forming solution for ocular drug delivery | A combination of natural polysaccharide, thermoreversible polymer | 2011 | [105] |
| US 6 703 039 B2 | Reversible gelling system for ocular drug delivery | A block copolymer of propylene oxide and ethylene oxide with HPMC | 2004 | [106] |
| US 6 511 660 B1 | Ophthalmic drug delivery formulations and method for preparing the same | Carbopol and Pluronic | 2003 | [107] |
5. Conclusions and future prospects
Despite the challenges in ocular drug delivery, over the past few years, many innovative approaches are being developed to overcome the problems associated with conventional of ophthalmic preparations. The in-situ gelling system is one the promising and extensively studied strategies that could prolong precorneal resident time and offer the sustained release drug delivery, thus improve ocular bioavailability and therapeutic efficacy and reduce systemic absorption and toxicity. Furthermore, due to its drug release sustaining ability and decrease the frequency of administration, in-situ gel could improve patient compliance. In in-situ gel formulation with different stimuli-responsive polymers that have high sensitivity to change in pH, temperature, and ion concentration are used. However, the combination of two or more stimuli-responsive polymers in the same formulation is known to exhibit greater compliance and improved therapeutic efficacy. Moreover, exploring the combination of different drug delivery approaches (i.e. nanoparticles loaded in-situ gelling) to develop in-situ gel has been the attractive strategies to improve ocular drug delivery system.
As the eye is the most essential and sensitive part of the body, the safety issues of ophthalmic formulations is critically important. The majorities of the cytotoxicity and irritability studies included in this review showed that no significant alterations or sign of toxicity due to the application of in-situ gel. However, further studies are required to evaluate the possible toxicity due to repeated and long term applications and materials for the preparation of nanoparticles in nano-gel systems. In addition, the increased viscosity of in-situ gel may cause some limitations like blurred vision and discomfort to patient resulting in a faster elimination due to reflex tears and blinks. Therefore, critical control of the viscosity should be taken into consideration during designing and optimization of in-situ gel formulation in order to reduce the limitations to the tolerable level.
Despite the promising potential of in-situ gel in ocular drug delivery, only a limited number of drugs in the form of in-situ gel are currently in clinical use. Consequently, further works should be done to explore this drug delivery system for the clinical application of other ophthalmic drugs.
At present, most of the ophthalmic in-situ gels were designed only for the formulations containing of single active ingredient. In the future, some more suitable strategies should be developed for the formula consisting of multiple ingredients such as Traditional Chinese Medicine in particular, which involves a multi-target approach to produce their action. Lastly, in the future, we expect the innovation of new and more reliable in-situ forming polymers which may be responsive to some biochemical markers associated with the disease conditions of the eye.
Conflict of interest
The authors affirm and confirm that there are no any conflict of interest issues with regard to the content of this article.
Contributor Information
Shouying Du, Email: dushouying@263.net.
Zhidong Liu, Email: lonerliuzd@163.com.
References
- 1.Addo E, Bamiro OA, Siwale R. Anatomy of the eye and common diseases affecting the eye. In: Addo RT, editor. Ocular drug delivery: Advances, challenges and applications. 2016. pp. 11–25. [Google Scholar]
- 2.Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer. Nanomedicine (Lond) 2017;12(6):683–702. doi: 10.2217/nnm-2016-0379. [DOI] [PubMed] [Google Scholar]
- 3.Zhu M, Wang J, Li N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/ hyaluronic acid of ketoconazole for ophthalmic delivery. Artif Cells Nanomed Biotechnol. 2017 doi: 10.1080/21691401.2017.1368024. [DOI] [PubMed] [Google Scholar]
- 4.Bisht R, Mandal A, Jaiswal JK, Rupenthal ID. Nanocarrier mediated retinal drug delivery: overcoming ocular barriers to treat posterior eye diseases. WIREs Nanomed Nanobiotechnol. 2018 doi: 10.1002/wnan.1473. [DOI] [PubMed] [Google Scholar]
- 5.Makwana SB, Patel VA, Parmar SJ. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016;6:1–6. doi: 10.1016/j.rinphs.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28(4):353–369. doi: 10.1081/ddc-120002997. [DOI] [PubMed] [Google Scholar]
- 7.Bamiro OA, Ubale RV, Addo RT. Background of Ocular Drug Delivery. In: Addo RT, editor. Ocular drug delivery: Advances, challenges and applications. Springer International Publishing; 2016. pp. 1–9. [Google Scholar]
- 8.Kotreka UK, Davis VL, Adeyeye MC. Development of topical ophthalmic in situ gel-forming estradiol delivery system intended for the prevention of age-related cataracts. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye T, Yuan K, Zhang W. Prodrugs incorporated into nanotechnology-based drug delivery systems for possible improvement in bioavailability of ocular drugs delivery. Asian J Pharmaceut Sci. 2013;8(4):207–217. [Google Scholar]
- 10.Liu Y, Liu J, Zhang X, Zhang R, Huang Y, Wu C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS PharmSciTech. 2010;11(2):610–620. doi: 10.1208/s12249-010-9413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan G, Yu S, Pan H. Bioadhesive chitosan-loaded liposomes: a more efficient and higher permeable ocular delivery platform for timolol maleate. Int J Biol Macromol. 2017;94(Pt A):355–363. doi: 10.1016/j.ijbiomac.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Addo RT, Yeboah KG, Siwale RC. Formulation and characterization of atropine sulfate in albumin-chitosan microparticles for in vivo ocular drug delivery. J Pharm Sci. 2015;104(5):1677–1690. doi: 10.1002/jps.24380. [DOI] [PubMed] [Google Scholar]
- 13.Biswas GR, Majee SB. Niosomes in ocular drug delivery. Eur J Pharmaceut Med Res. 2017;4(7):813–819. [Google Scholar]
- 14.Prausnitz MR, Jiang J, Pate SR. 2007. Ocular drug delivery using microneedles; p. 3191. ARVO Annual Meeting. [Google Scholar]
- 15.Duan Y, Cai X, Du H, Zhai G. Novel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcumin. Colloids Surf B Biointerfaces. 2015;128:322–330. doi: 10.1016/j.colsurfb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Malavade S. Overview of the ophthalmic system. In: Pathak Y, Sutariya V, Hirani AA, editors. Nano-Biomaterials for ophthalmic drug delivery. Springer International Publishing; 2016. pp. 9–35. [Google Scholar]
- 17.Almeida H, Amaral MH, Lobao P, Lobo JM. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today. 2014;19(4):400–412. doi: 10.1016/j.drudis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm Sin B. 2017;7(3):281–291. doi: 10.1016/j.apsb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D, Chen YS, Rupenthal ID. Overcoming ocular drug delivery barriers through the use of physical forces. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm. 2013;39(11):1599–1617. doi: 10.3109/03639045.2012.736515. [DOI] [PubMed] [Google Scholar]
- 21.Khan N, Aqil M, Imam SS, Ali A. Development and evaluation of a novel in situ gel of sparfloxacin for sustained ocular drug delivery: in vitro and ex vivo characterization. Pharm Dev Technol. 2015;20(6):662–669. doi: 10.3109/10837450.2014.910807. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Zhao H, Okeke CI. Comparison of systemic absorption between ofloxacin ophthalmic in situ gels and ofloxacin conventional ophthalmic solutions administration to rabbit eyes by HPLC-MS/MS. Int J Pharm. 2013;450(1-2):104–113. doi: 10.1016/j.ijpharm.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Devasani SR, Dev A, Rathod S, Deshmukh G. An overview of in situ gelling systems. Pharmaceut Biolog Evaluat. 2016;3(1):60–69. [Google Scholar]
- 24.Cao Y, Zhang C, Shen W, Cheng Z, Yu LL, Ping Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release. 2007;120(3):186–194. doi: 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Sheshala R, Kok YY, Ng JM, Thakur RR, Dua K. In situ gelling ophthalmic drug delivery system: an overview and its applications. Recent Pat Drug Deliv Formul. 2015;9(3):237–248. doi: 10.2174/1872211309666150724101227. [DOI] [PubMed] [Google Scholar]
- 26.Laddha UD, Mahajan HS. An insight to ocular in situ gelling systems. Int J Adv Pharmaceut. 2017;06(02):31–40. [Google Scholar]
- 27.Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83(1):65–74. doi: 10.1016/s0168-3659(02)00175-x. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Nakrani H, Raval M, Navin S. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016;23(9):3712–3723. doi: 10.1080/10717544.2016.1223225. [DOI] [PubMed] [Google Scholar]
- 29.Klouda L. Thermoresponsive hydrogels in biomedical applications: a seven-year update. Eur J Pharm Biopharm. 2015;97(Pt B):338–349. doi: 10.1016/j.ejpb.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Almeida H, Amaral MH, Lobao P. Temperature and pH stimuli-responsive polymers and their applications in controlled and selfregulated drug delivery. J App Pharm Sci. 2012;2(6):1–10. [Google Scholar]
- 31.Almeida H, Amaral MH, Lobao P, Sousa Lobo JM. Applications of poloxamers in ophthalmic pharmaceutical formulations: an overview. Expert Opin Drug Deliv. 2013;10(9):1223–1237. doi: 10.1517/17425247.2013.796360. [DOI] [PubMed] [Google Scholar]
- 32.Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9(3):339–358. [PubMed] [Google Scholar]
- 33.Miyazaki S, Suzuki S, Kawasaki N, Endo K, Takahashi A, Attwood D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int J Pharm. 2001;229(1):29–36. doi: 10.1016/s0378-5173(01)00825-0. [DOI] [PubMed] [Google Scholar]
- 34.Zambito Y, Colo GD. Polysaccharides as excipients for ocular topical formulations. InTech; 2011. Polysaccharides as excipients for ocular topical formulations; pp. 253–280. [Google Scholar]
- 35.Rajoria G, Gupta A. In-situ gelling system: a novel approach for ocular drug delivery. Am J PharmTech Res. 2012;2(4):24–53. [Google Scholar]
- 36.Pal K, Paulson AT, Rousseau D. Biopolymers in controlled-release delivery systems. In: Ebnesajjad S, editor. Handbook of biopolymers and biodegradable plastics properties, processing, and applications. Elsevier; 2013. pp. 347–348. [Google Scholar]
- 37.Mohammed S, Chouhan G, Anuforom O. Thermosensitive hydrogel as an in situ gelling antimicrobial ocular dressing. Mater Sci Eng C Mater Biol Appl. 2017;78:203–209. doi: 10.1016/j.msec.2017.04.065. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Gao Q, Lu X, Zhou H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J Pharmaceut Sci. 2016;11(6):673–683. [Google Scholar]
- 39.Chen X, Li X, Zhou Y. Chitosan-based thermosensitive hydrogel as a promising ocular drug delivery system: preparation, characterization, and in vivo evaluation. J Biomater Appl. 2012;27(4):391–402. doi: 10.1177/0885328211406563. [DOI] [PubMed] [Google Scholar]
- 40.Shastri DH. Thiolated chitosan: a boon to ocular delivery of therapeutics. MOJ Bioequiv Availab. 2017;3(2):1–5. [Google Scholar]
- 41.Li J, Liu H, Liu LL, Cai CN, Xin HX, Liu W. Design and evaluation of a Brinzolamide drug-resin in situ thermosensitive gelling system for sustained ophthalmic drug delivery. Chem Pharm Bull (Tokyo) 2014;62(10):1000–1008. doi: 10.1248/cpb.c14-00451. [DOI] [PubMed] [Google Scholar]
- 42.Al-Khateb K, Ozhmukhametova EK, Mussin MN. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int J Pharm. 2016;502(1-2):70–79. doi: 10.1016/j.ijpharm.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Osswald CR, Guthrie MJ, Avila A, Valio JJ, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res. 2017;42(9):1293–1301. doi: 10.1080/02713683.2017.1302590. [DOI] [PubMed] [Google Scholar]
- 44.Vodithala S, Khatry S, Shastri N, Sadanandam M. Development and evaluation of thermoreversible ocular gels of ketorolac tromethamine. Int J Biopharm. 2010;1(1):39–45. [Google Scholar]
- 45.Morsi N, Ghorab D, Refai H, Teba H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int J Pharm. 2016;506(1-2):57–67. doi: 10.1016/j.ijpharm.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Bhowmik M, Bain MK, Ghosh LK, Chattopadhyay D. Effect of salts on gelation and drug release profiles of methylcellulose-based ophthalmic thermo-reversible in situ gels. Pharm Dev Technol. 2011;16(4):385–391. doi: 10.3109/10837451003774369. [DOI] [PubMed] [Google Scholar]
- 47.Bhowmick B, Sarkar G, Roy I. Effect of carrageenan and potassium chloride on in-situ gelling ophthalmic drug delivery system based on methylcellulose. Rsc Adv. 2015;5(74):60386–60391. [Google Scholar]
- 48.Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de-Freitas O, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75(2):186–193. doi: 10.1016/j.ejpb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Hsiue GH, Hsu SH, Yang CC, Lee SH, Yang IK. Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials. 2002;23(2):457–462. doi: 10.1016/s0142-9612(01)00127-2. [DOI] [PubMed] [Google Scholar]
- 50.Iohara D, Okubo M, Anraku M. Hydrophobically modified polymer/α-cyclodextrin thermoresponsive hydrogels for use in ocular drug delivery. Mol Pharm. 2017;14(8):2740–2748. doi: 10.1021/acs.molpharmaceut.7b00291. [DOI] [PubMed] [Google Scholar]
- 51.Sawant D, Dandagi PM, Gadad AP. Formulation and evaluation of sparfloxacin emulsomes-loaded thermosensitive in situ gel for ophthalmic delivery. J Sol-Gel Sci Technol. 2016;77(3):654–665. [Google Scholar]
- 52.Wang L, Che X, Guo Y, Bian Y, Cheng G. Thermoresponsive ophthalmic poloxamer/tween/carbopol in situ gels of a poorly water-soluble drug fluconazole: preparation and in vitro-in vivo evaluation. Drug Dev Ind Pharm. 2014;40(10):1402–1410. doi: 10.3109/03639045.2013.828221. [DOI] [PubMed] [Google Scholar]
- 53.Gadad AP, Wadklar PD, Dandghi P, Patil A. Thermosensitive in situ gel for ocular delivery of lomefloxacin. Ind J Pharmaceut Educ Res. 2016;50(2):S96–105. [Google Scholar]
- 54.Qian Y, Wang F, Li R, Zhang Q, Xu Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev Ind Pharm. 2010;36(11):1340–1347. doi: 10.3109/03639041003801893. [DOI] [PubMed] [Google Scholar]
- 55.Asasutjarit R, Thanasanchokpibull S, Fuongfuchat A, Veeranondha S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int J Pharm. 2011;411(1-2):128–135. doi: 10.1016/j.ijpharm.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 56.Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Dev Ind Pharm. 2010;36(11):1330–1339. doi: 10.3109/03639041003801885. [DOI] [PubMed] [Google Scholar]
- 57.Lin HR, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release. 2000;69(3):379–388. doi: 10.1016/s0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 58.Wu H, Liu Z, Peng J, Li L, Li N, Li J. Design and evaluation of baicalin-containing in situ pH-triggered gelling system for sustained ophthalmic drug delivery. Int J Pharm. 2011;410(1-2):31–40. doi: 10.1016/j.ijpharm.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Pang X, Li J, Pi J. Increasing efficacy and reducing systemic absorption of brimonidine tartrate ophthalmic gels in rabbits. Pharm Dev Technol. 2018;23(3):231–239. doi: 10.1080/10837450.2017.1328693. [DOI] [PubMed] [Google Scholar]
- 60.Upadhayay P, Kumar M, Pathak K. Norfloxacin loaded pH triggered nanoparticulate in-situ gel for extraocular bacterial infections: optimization, ocular irritancy and corneal toxicity. Iran J Pharm Res. 2016;15(1):3–22. [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta S, Vyas SP. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci Pharm. 2010;78(4):959–976. doi: 10.3797/scipharm.1001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanoujia J, Sonker K, Pandey M, Kymonil KM, Saraf SA. Formulation and characterization of a novel pH-triggered in-situ gelling ocular system containing Gatifloxacin. Int Current Pharmaceut J. 2012;1(3):43–49. [Google Scholar]
- 63.Sheikh AA, Sheikh SR, Admane SS. Development and characterization of novel in situ gel of moxifloxacin hydrochloride. Asian J. Pharm. 2017;11(3):S616–S624. [Google Scholar]
- 64.Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. part 2: precorneal retention and in vivo pharmacodynamic study. Int J Pharm. 2011;411(1-2):78–85. doi: 10.1016/j.ijpharm.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 65.Agrawal AK, Das M, Jain S. In situ gel systems as 'smart'carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9(4):383–402. doi: 10.1517/17425247.2012.665367. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Ferreiro A, Fernandez Bargiela N, Varela MS. Cyclodextrin-polysaccharide-based, in situ-gelled system for ocular antifungal delivery. Beilstein J Org Chem. 2014;10:2903–2911. doi: 10.3762/bjoc.10.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reed K, Li A, Wilson B, Assamoi T. Enhancement of ocular in situ gelling properties of low acyl gellan gum by use of ion exchange. J Ocul Pharmacol Ther. 2016;32(9):574–582. doi: 10.1089/jop.2016.0084. [DOI] [PubMed] [Google Scholar]
- 68.Champalal KD, Sushilkumar SP. Current status of ophthalmic in-situ forming hydrogel. Int J Pharma Bio Sci. 2012;3(3):P372–P388. [Google Scholar]
- 69.Mishra DN, Gilhotra RM. Design and characterization of bioadhesive in-situ gelling ocular inserts of Gatifloxacin sesquihydrate. DARU: J Pharmaceut Sci. 2008;16(1):1–8. [Google Scholar]
- 70.Nirmal HB, Bakliwal SR, Pawar SP. In-situ gel: new trends in controlled and sustained drug delivery system. Int J PharmTech Res. 2010;2(2):1398–1408. [Google Scholar]
- 71.Vijaya C, Goud KS. Ion-activated in situ gelling ophthalmic delivery systems of Azithromycin. Indian J of Pharm Sci. 2011;73(6):615–620. doi: 10.4103/0250-474X.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu L, Ao J, Li P. A novel in situ gel base of deacetylase gellan gum for sustained ophthalmic drug delivery of ketotifen: in vitro and in vivo evaluation. Drug Des Devel Ther. 2015;9:3943–3949. doi: 10.2147/DDDT.S87368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesarla R, Tank T, Vora PA, Shah T, Parmar S, Omri A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016;23(7):2363–2370. doi: 10.3109/10717544.2014.987333. [DOI] [PubMed] [Google Scholar]
- 74.Tayel SA, El-Nabarawi MA, Tadros MI, Abd-Elsalam WH. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int J Pharm. 2013;443(1-2):293–305. doi: 10.1016/j.ijpharm.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315(1-2):12–17. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 76.Liu Z, Yang XG, Li X, Pan W, Li J. Study on the ocular pharmacokinetics of ion-activated in situ gelling ophthalmic delivery system for gatifloxacin by microdialysis. Drug Dev Ind Pharm. 2007;33(12):1327–1331. doi: 10.1080/03639040701397241. [DOI] [PubMed] [Google Scholar]
- 77.Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;104:302–314. doi: 10.1016/j.ejps.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Rupenthal ID, Alany RG, Green CR. Ion-activated in situ gelling systems for antisense oligodeoxynucleotide delivery to the ocular surface. Mol Pharm. 2011;8(6):2282–2290. doi: 10.1021/mp200140e. [DOI] [PubMed] [Google Scholar]
- 79.Yu S, Zhang X, Tan G. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr Polym. 2017;155:208–217. doi: 10.1016/j.carbpol.2016.08.073. [DOI] [PubMed] [Google Scholar]
- 80.Davaran S, Lotfipour F, Sedghipour N, Sedghipour MR, Alimohammadi S, Salehi R. Preparation and in vivo evaluation of in situ gel system as dual thermo-/pH-responsive nanocarriers for sustained ocular drug delivery. J Microencapsul. 2015;32(5):511–519. doi: 10.3109/02652048.2015.1065915. [DOI] [PubMed] [Google Scholar]
- 81.Gupta H, Malik A, Khar RK, Ali A, Bhatnagar A, Mittal G. Physiologically active hydrogel (in situ gel) of sparfloxacin and its evaluation for ocular retention using gamma scintigraphy. J Pharm Bioallied Sci. 2015;7(3):195–200. doi: 10.4103/0975-7406.160015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta H, Velpandian T, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target. 2010;18(7):499–505. doi: 10.3109/10611860903508788. [DOI] [PubMed] [Google Scholar]
- 83.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. An alternative in situ gel-formulation of levofloxacin eye drops for prolong ocular retention. J Pharm Bioallied Sci. 2015;7(1):9–14. doi: 10.4103/0975-7406.149810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Basaran B, Bozkir A. Thermosensitive and pH induced in situ ophthalmic gelling system for ciprofloxacin hydrochloride: hydroxypropyl-beta-cyclodextrin complex. Acta Pol Pharm. 2012;69(6):1137–1147. [PubMed] [Google Scholar]
- 85.Pardeshi C, Rajput P, Belgamwar V. Solid lipid based nanocarriers: an overview. Acta Pharm. 2012;62(4):433–472. doi: 10.2478/v10007-012-0040-z. [DOI] [PubMed] [Google Scholar]
- 86.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine. 2010;6(2):324–333. doi: 10.1016/j.nano.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Pitorre M, Gonde H, Haury C. Recent advances in nanocarrier-loaded gels: which drug delivery technologies against which diseases? J Control Release. 2017;266:140–155. doi: 10.1016/j.jconrel.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 88.Liu R, Sun L, Fang S. Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: development, characterization, in vitro permeation and in vivo pharmacokinetic studies. Pharm Dev Technol. 2016;21(5):576–582. doi: 10.3109/10837450.2015.1026607. [DOI] [PubMed] [Google Scholar]
- 89.Pandurangan DK, Bodagala P, Palanirajan VK, Govindaraj S. Formulation and evaluation of voriconazole ophthalmic solid lipid nanoparticles in situ gel. Int J Pharm Investig. 2016;6(1):56–62. doi: 10.4103/2230-973X.176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paradkar MU, Parmar M. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. Journal of Drug Delivery Science & Technology. 2017;39:113–122. [Google Scholar]
- 91.Shukr MH. Novel in situ gelling ocular inserts for voriconazole-loaded niosomes: design, in vitro characterisation and in vivo evaluation of the ocular irritation and drug pharmacokinetics. J Microencapsul. 2016;33(1):71–79. doi: 10.3109/02652048.2015.1128489. [DOI] [PubMed] [Google Scholar]
- 92.Sayed EG, Hussein AK, Khaled KA, Ahmed OA. Improved corneal bioavailability of ofloxacin: biodegradable microsphere-loaded ion-activated in situ gel delivery system. Drug Des Devel Ther. 2015;9:1427–1435. doi: 10.2147/DDDT.S80697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Nanoparticles laden in situ gel of levofloxacin for enhanced ocular retention. Drug Deliv. 2013;20(7):306–309. doi: 10.3109/10717544.2013.838712. [DOI] [PubMed] [Google Scholar]
- 94.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Nanoparticles laden in situ gel for sustained ocular drug delivery. J Pharm Bioallied Sci. 2013;5(2):162–165. doi: 10.4103/0975-7406.111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmed TA, Aljaeid BM. A potential in situ gel formulation loaded with novel fabricated poly(lactide-co-glycolide) nanoparticles for enhancing and sustaining the ophthalmic delivery of ketoconazole. Int J Nanomedicine. 2017;12:1863–1875. doi: 10.2147/IJN.S131850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu S, Wang Q, Wang X. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1-2):128–136. doi: 10.1016/j.ijpharm.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 97.Gan L, Gan Y, Zhu C, Zhang X, Zhu J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: In vitro and in vivo results. In J Pharm. 2009;365(1-2):143–149. doi: 10.1016/j.ijpharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Singh J, Chhabra G, Pathak K. Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm. 2014;40(9):1223–1232. doi: 10.3109/03639045.2013.814061. [DOI] [PubMed] [Google Scholar]
- 99.Ako-Adounvo AM, Nagarwal RC, Oliveira L. Recent patents on ophthalmic nanoformulations and therapeutic implications. Recent Pat Drug Deliv Formul. 2014;8(3):193–201. doi: 10.2174/1872211308666140926112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kant A, Reddy S, M.M S, J.S V, C N. In situ gelling system-An overview. Pharmacologyonline. 2011;2(1):28–44. [Google Scholar]
- 101.Jain D, Kumar V, Singh S, Mullertz A, Bar-Shalom D. Newer trends in in situ gelling systems for controlled ocular drug delivery. J Anal Pharm Res. 2016;2(3):00022. [Google Scholar]
- 102.Adeyeye MC, Davis VL, Kotreka UK. In-situ gel ophthalmic drug delivery system of estradiol or other estrogen for prevention of cataracts. US 2011/0082128 A1. 2011.
- 103.Xia E, Smerbeck RV. Reversible gelling system for ocular drug delivery. US 2002/0114778 A1. 2002.
- 104.Banerjee R, Carvalho E. Nanoparticulate in-situ gels of TPGS, gellan and PVA as vitreous humor substitutes. US 8,343,471 B2. 2013.
- 105.Chandavarkar NM, Jindal KC, Malayandi R. In-situ gel forming solution for ocular drug delivery. WO 2011018800 A3. 2011.
- 106.Xia E, Smerbeck RV. Reversible gelling system for ocular drug delivery. US 6,703,039 B2. 2004.
- 107.Lin HR, Sung KC. Ophthalmic drug delivery formulations and method for preparing the same. US 6,511,660 B1. 2003.