Abstract
Until now, there are no publications about the preformulation studies on (S)-zaltoprofen ((S)-ZPF). Hence, we first investigated the solubility of (S)-ZPF, screened solubilizers and performed the pharmacokinetic study of (S)-ZPF in the presence of the solubilizers. The measurement of the solubility of (S)-ZPF in 26 different solvents was carried out, including d-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS), 2-hydroxypropyl-β-cyclodextrin (HPCD), and mixtures of individual solvent. The plasma concentration of (S)-ZPF and the amount of (S)-ZPF retained in stomach were determined after oral (35.0 mg/kg) and intravenous (5.0 mg/kg) administration. The solubility of (S)-ZPF showed an increase of 484-fold in TPGS compared to its aqueous solubility. There was a significant increase of AUC0-24 h for pure (S)-ZPF in the TPGS group (813.59 ± 64.17 µg⋅h/ml) in comparison with AUC0-24 h in the HPCD group (595.57 ± 71.76 µg⋅h/ml) and water group (465.57 ± 90.89 µg⋅h/ml). In addition, the Tmax of (S)-ZPF in the TPGS group was 2 h, much faster than that in the HPCD or water groups (5.50 or 5.67 h, respectively). This suggested that TPGS played a significant role in the increase of solubility and bioavailability of (S)-ZPF.
Keywords: (S)-zaltoprofen, Solubility, Bioavailability, D-alpha tocopheryl polyethylene glycol 1000 succinate, 2-hydroxypropyl-β-cyclodextrin
Graphical abstract
1. Introduction
Recently, stereoselectivity for the development of chiral drugs has been focused because it might have a significantly different pharmacological effect [1], toxicological [2] based on pharmacodynamic and pharmacokinetic behaviour [3], [4]. Consequently, the pure enantiomer might be preferred for safety and efficacy. For example, the (S) configuration such as (S)-ketoprofen [5] or (S)-ibuprofen [6], [7] exclusively expresses the ability to the prostaglandin-inhibitory activities. Recently, dexibuprofen ((S)-isomer of ibuprofen) was developed for arthritis, inflammation, pain, and fever [8].
There were publications that (S)-zaltoprofen ((S)-ZPF) displays greater anti-inflammatory and analgesic activities than racemic (RS)-ZPF in rodents and has substantially higher bioavailability than (R)-ZPF [9], [10]. Interestingly, they insisted that absorption of (R)-ZPF is higher than that of (S)-ZPF in a racemic mixture. Subsequently, it resulted in slightly higher initial plasma concentration of (R)-ZPF than that of (S)-ZPF. However, higher plasma concentration of (S)-ZPF at 4 h after oral administration indicated that metabolism of (R)-ZPF was faster than that of (S)-ZPF from (RS)-ZPF. In addition, the pharmacokinetics profiles of (R)-ZPF and (S)-ZPF were considerably different in experimental rats by stereoselective metabolism of the enantiomers [9]. Also, there was a report that only (S)-ZPF might exhibit anti-inflammatory activity in racemic (RS)-ZPF [10].
However, no studies have examined the solubility, partition coefficient, assay validation, and pharmacokinetic parameters of (S)-ZPF in experimental animals so far. Hence, we focused on these investigations as the preformulation work of (S)-ZPF in this study.
2. Materials and methods
2.1. Materials
(S)-ZPF, (R)-ZPF and (RS)-ZPF were synthesized from the laboratory of Prof. S.H. Jung (Chungnam National University, Daejeon, Korea). d-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS) and 2-hydroxypropyl-β-cyclodextrin (HPCD) were purchased from Sigma-Aldrich (Steinheim, Switzerland). Poloxamer 188 and poloxamer 407 were purchased from BASF SE (Ludwigshafen, Germany). Cremophor EL, polyethylene glycol (PEG) 400, PEG 600, Tween 80, and propylene glycol (PG) were purchased from Samchun Chemical Co., Ltd. (Pyungtaek, Korea). HPLC-grade acetonitrile, methanol, n-hexane, and 2-propanol were from JT Baker (Phillipsburg, NJ, USA). Distilled water (DW) was used in this study. All solvents were analytical grade and used without further purification.
2.2. Measurement solubility and partition coefficient
Excess (S)-ZPF was added to 15-ml centrifuge tubes containing 1 ml of phosphate buffers with different pH values or solvents at concentration of 10% (w/v) including water; nonionic surfactants: poloxamer 188, poloxamer 407, TPGS, Tween 80 and cremophor EL; some solubilizers: PEG 400, PEG 600 and HPCD; double mixtures containing TPGS and other individual solubilizers; and two triple mixtures of TPGS, PEG 400, and PG. The samples, then, were kept on an end-to-end labquake rotator (Barnstead Thermolyne, Sparks, NV, USA) at rotation level 8 continuously for 72 h at ambient temperature. The samples after rotating were filtered with 0.45-µm membrane filters (Whatman, Dismic-25, Japan), and the solubility of (S)-ZPF was identified using reversed-phase HPLC. All solubility determinations were performed in triplicate. The octanol/water partition coefficient (log P) of (S)-ZPF was also determined by the shake-flask method [11]. One mg of (S)-ZPF was dissolved in the same volume of n-octanol and water in a 15-ml centrifuge tube covered with Parafilm to prevent solvent loss. The flasks were then shaken horizontally at ambient temperature on an end-to-end labquake rotator (Barnstead Thermolyne, Sparks, NV) at rotation level 8 continuously for 72 h and then allowed to settle for 30 min. Subsequently, the mixtures were then centrifuged at 9425 × g for 10 min. Each phase was analyzed by reversed-phase HPLC. Each determination was performed three times, and the mean values were used to calculate the log P values.
2.3. Surface tension measurement
A Sigma 703D force tensiometer (Biolin Scientific, Stockholm, Sweden) was used to measure the surface tension of TPGS solution, and the double mixtures, which was TPGS/Tween 80 mixture, TPGS/Cremophor EL mixture, TPGS/poloxamer 188 mixture, TPGS/HPCD mixture, and TPGS/PEG 600 mixture. The surface tension of 30 ml of each solution was measured at 25 ± 1 °C with a ring (width = 19.6 mm, thickness = 0.1 mm).
2.4. HPLC analysis
2.4.1. Reversed-phase HPLC
HPLC analysis was carried out on an Agilent HP 1100 HPLC. Elution was performed using an isocratic mobile phase of acetonitrile and water (55:45, v/v) at a flow rate of 1.2 ml/min. The column temperature was 25 °C, and the injection volume was 20 µl. A reversed-phase C18 column (4.6 mm × 150 mm, 5 µm, Bischoff Chromatography, Leonberg, Germany) was used, and the chromatograms were monitored by an ultraviolet detector set at 240 nm.
2.4.2. Chiral HPLC
Chiral HPLC analysis was carried out on an Agilent HP 1100 HPLC using a Chiralcel OJ-H column (4.6 mm × 150 mm, 5 µm, Daicel Corp., Tokyo, Japan). The mobile phase, hexane-isopropanol-triflouroacetic acid (90:10:0.1, v/v/v), was filtered (0.45 µm), degassed, and delivered at a flow rate of 0.8 ml/min. The column temperature was maintained at 25 °C, and the injection volume was 20 µl. (RS)-ZPF, (R)-ZPF, and (S)-ZPF were detected at 240 nm.
2.5. Calibration curves
Stock solutions of (S)-ZPF enantiomer and racemic ketoprofen (internal standard, IS) were prepared in methanol at 1000 µg/ml. Standard solutions (0.4, 4.0, 20.0, 40.0, 200.0, and 300.0 µg/ml) of (S)-ZPF were also prepared by diluting the stock solution. Working IS solutions were prepared at 200 µg/ml by diluting the stock solution in methanol. Then, 25 µl of each standard solution was transferred to a glass tube containing 50 µl blank rat plasma, and then 25 µl IS solution was added to the glass tube. The resulting plasma containing 0.1, 1.0, 5.0, 10.0, 50.0, or 75.0 µg/ml of (S)-ZPF was processed as described below (2.6. Sample preparation). Quality control (QC) solutions were prepared similarly. For the (R)- and (S)-ZPF enantiomers from (RS)-ZPF, calibration curves were constructed as for pure (S)-ZPF but without using IS.
2.6. Sample preparation
For pure (S)-ZPF, 100 µl of blank rat plasma, (S)-ZPF and IS mentioned above was placed into a glass tube with 10 µl of 10% phosphoric acid and then vortexed for 1 min. The extraction solvent was then added into the glass tube in the order as follows: 0.5 ml of acetonitrile and then 3 ml of dichloromethane. The process of liquid–liquid extraction (LLE) was continually performed with extraction by vortexing samples for 15 min. After that, the tubes were centrifuged at 2874 × g for 10 min, and the organic phase was carefully removed to another set of clean glass tubes and dried under a gentle nitrogen stream at 40 °C. Residues in these tubes were dissolved in 200 µl of a mixture of acetonitrile and water (55:45, v/v), centrifuged at 9425 × g, and then 20 µl of the upper layer was used for HPLC analysis. The other QC solutions were also prepared in the same method.
For (R)-ZPF and (S)-ZPF from (RS)-ZPF, method for preparation of calibration samples was similar to the method for pure (S)-ZPF above but without IS. Final residues in glass tubes were dissolved in 200 µl of methanol, and then 20 µl of the upper layer war measured using a chiral HPLC column.
2.7. Validation of the HPLC method
2.7.1. Extraction recovery
Two sets of standards containing blank plasma, (S)-ZPF and IS at five different concentrations (0.1, 1.0, 5.0, 10.0 and 50.0 µg/ml of (S)-ZPF) were prepared. At 0.1 µg/ml of (S)-ZPF, one set was standard solution processed via sample preparation method above. The other set as a control, blank plasma experienced through the LLE procedure was added (S)-ZPF and IS and again measured by reversed-phase HPLC. Extraction recoveries of (S)-ZPF followed the LLE procedure were calculated as the ratio of the peak area ratio of (S)-ZPF to IS from the spiked plasma samples to those of the controls. The extraction recoveries for other concentrations, 1.0, 5.0, 10.0 and 50.0 µg/ml of (S)-ZPF were calculated in the same manner.
2.7.2. Accuracy and precision
The within-run accuracy and precision of the method were assessed by analyzing three replicates containing (S)-ZPF at five different QC levels, including 0.1, 1.0, 5.0, 10.0 and 50.0 µg/ml on a single day. The between-run accuracy and precision were determined by analyzing three replicates of each of the QCs of (S)-ZPF described above for three consecutive days. Accuracy was calculated as the percent of (S)-ZPF found in the intra-day and inter-day samples to that of the nominal concentration. Precision was expressed as the coefficient of variation (CV, %), and calculated as the ratio of the standard deviation to the measured mean drug concentration. The within-run and between-run accuracy and precision value should not exceed 15% for the QC samples, except for the lower limit of quantification (LLOQ) which should not exceed 20%. The sensitivity of detection of this method was estimated as the LLOQ, which is the concentration of drug corresponding to a peak area five times higher than the baseline noise.
2.7.3. Stability test
The stability of (S)-ZPF in rat plasma was studied using the QC samples at three levels: 1.0, 10.0 and 50.0 µg/ml. We prepared the QC samples in sufficient volumes to divide into multiple aliquots to be investigated in triplicate for each condition. The results obtained by reversed HPLC column were then compared with those of freshly prepared QC samples described above. Stability studies were performed under the following conditions: (1) three freeze/thaw cycles on 3 consecutive days; and (2) exposure to 4 °C (refrigerator) for 24 h. In all tested conditions, the transformation of (S)-ZPF into (R)-ZPF was also evaluated using the chiral HPLC column.
2.8. Pharmacokinetics studies
All animal studies were carried out according to the “Guiding Principles in the Use of Animals in Toxicology” adopted by the Society of Toxicology (USA), and the experimental protocols were approved by the Animal Care Committee of Chungnam National University. Twelve male Sprague–Dawley (SD) rats were purchased from the Samtako (Chungbuk, Korea). Three rats were housed per cage in laminar flow, and the cages were maintained at 22 ± 2 °C and 50%–60% relative humidity. The rats were kept in these conditions for at least 1 week and were fasted for at least 24 h before performing the experiment. For oral administration, the suspension of (S)-ZPF in DW, 2% HPCD, or 2% TPGS used was prepared. The suspension was then vortexed and placed in an ultrasonic bath for 30 min and 14.0 mg of (S)-ZPF was administrated to each rat. To prepare the solution for IV administration, 16 mg of (S)-ZPF was completely dissolved in 0.4 ml of ethanol, and the solution was 10-fold diluted with 10% (w/v) cremophor EL solution before filtration through a 0.2-µm filter. The amount of (S)-ZPF for oral and IV administration were 35.0 mg/kg and 5.0 mg/kg, respectively. Blood samples (0.8 mL) were collected from the orbital vein before drug administration (0 h) and at 0.5, 1, 2, 4, 8, 12, and 24 h following oral administration of (S)-ZPF and at 5, 15, 30, 45, 1, 2, 4, 8, 12, and 24 h after IV administration. Within 30 min following blood withdrawal, the samples were centrifuged at 21 206 × g, 4 °C for 15 min. The plasma was collected, labeled and stored at − 80 °C until HPLC analysis.
2.9. (S)-ZPF retained in the stomach
Rats were sacrificed, and the stomachs were excised immediately after completed pharmacokinetics studies. Stomachs were divided into small pieces with scissors and placed in glass bottles with 10 mL of methanol. The continuous stirring was carried out to extract (S)-ZPF from stomach tissues for 24 h. And then, the glass bottles were placed in an ultrasonic bath for 1 h. The extraction solution was centrifuged at 9425 × g for 10 min and then purified through 0.45 µm filters. The final solution was analyzed by HPLC.
2.10. Pharmacokinetic analysis
Non-compartmental pharmacokinetic analysis was performed using the WinNonlin Professional 2.1 software (Pharsight, Mountain View, CA, USA). Pharmacokinetic parameters were calculated from the observed data, including the area under the curve (AUC), the maximum plasma concentration (Cmax), the time to reach the maximum plasma concentration (Tmax), the half-life (T1/2), volume of distribution (Vd), clearance (CL), absolute bioavailability (AB), and relative bioavailability (RB) for oral route administration.
2.11. Statistical analysis
Student's t-test was used to compare two different groups of samples. A P-value < 0.05 was considered significant.
3. Results and discussion
3.1. Solubility of (S)-ZPF in various solvents
(S)-ZPF was practically insoluble in DW with water solubility of 13.79 ± 0.78 µg/ml. The log P of (S)-ZPF was 2.68, indicating its strong hydrophobicity. Therefore, we measured the solubility of (S)-ZPF in various nonionic surfactants including poloxamer, TPGS, Tween 80 and cremophor EL as well as some solubilizers such as PEG 400, PEG 600 and HPCD. Also, the solubility of (S)-ZPF was examined in double mixtures of nonionic surfactants containing 5%, 6% and 7% (w/v) TPGS. As well as, the solubility of (S)-ZPF was observed in triple mixtures of nonionic surfactants containing 1.43% and 2.61% (w/v) TPGS [12]. In PEG 400 or PEG 600, the solubility of (S)-ZPF was 17.59 ± 0.94 µg/ml or 30.79 ± 0.97 µg/ml, respectively. The solubility of (S)-ZPF in poloxamer 407 solution was 2.44-fold higher than that of (S)-ZPF in poloxamer 188 solution, which was 274.67 ± 12.45 µg/ml. The solubility of (S)-ZPF in cremophor EL was lower than that of (S)-ZPF in Tween 80 but about 3.2-fold higher than that of (S)-ZPF in HPCD. Interestingly, TPGS exhibited the highest solubility of (S)-ZPF at 6675.82 ± 141.22 µg/ml, that is 484-fold greater than aqueous solubility of (S)-ZPF.
The solubility of (S)-ZPF in TPGS/Tween 80 mixture was rather decreased compared to the solubility of (S)-ZPF in single TPGS and comparable the solubility of (S)-ZPF in single Tween 80. The similar results were obtained in TPGS/cremophor EL mixture.
However, the solubility of (S)-ZPF in TPGS/HPCD mixture was increased compared to that of (S)-ZPF in single HPCD. Also, the solubility of (S)-ZPF in TPGS/HPCD mixture was rather decreased compared to the solubility of (S)-ZPF in single TPGS. This phenomenon was similar in TPGS/poloxamer 188 mixture as well as TPGS/PEG 600 mixture (Fig. 1).
Fig. 1.
Solubility of (S)-ZPF according to the different solvents.
The surface tension was examined to figure out the solubilization mechanism. Surface tension was reported to affect the solubility of drugs due to the interfacial tension between the aqueous solution and hydrophobic solute [13], [14], [15]. In aqueous solutions with low surface tension, solutions contact with hydrophobic compounds tends to be easier than that in solutions with higher surface tension. Thus, aqueous solutions with reduced surface tension probably enhance drug solubility compared to those with higher surface tension. This argument was consistent with data on the solubility of (S)-ZPF when comparing between surfactants and HPCD, or PEG600 (Fig. 2). This knowledge can also be applied to interpret solubility outcomes in cases of the mixtures of TPGS/HPCD, and TPGS/PEG 600 (Fig. 2).
Fig. 2.
Surface tension of double mixtures against content of TPGS in these mixtures.
Although the surface tension of poloxamer 188 was lower than the surface tension of TPGS solution (Fig. 2), the solubility of (S)-ZPF in TPGS was significantly higher than that in poloxamer solutions. This was likely resulted from the significant differences in hydrophilic–lipophilic balance (HLB) values of poloxamers and TPGS. The HLB values of poloxamer 188 and poloxamer 407 were significantly higher than the figure for TPGS (29, 22, and 13.2, respectively) [16], [17], [18]. A surfactant with an HLB value over 20 is hydrophilic/lipophobic. Therefore, poloxamers have difficulties in dissolving hydrophobic molecules like (S)-ZPF (log P = 2.68), whereas TPGS can uptake (S)-ZPF into its micelles easier. This assumption might also explain the differences in the solubility of (S)-ZPF in the double mixtures of TPGS and poloxamer 188.
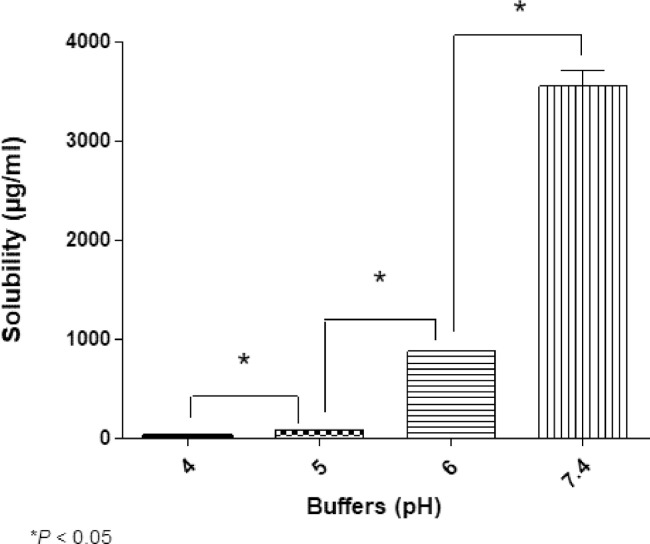
Cremophor EL and Tween 80 have insignificant differences in HLB values compared with TPGS (13.9, 15, and 13.2, respectively [19], [20]), and the surface tension of cremophor EL and Tween 80 even is lower than the figure for TPGS (Fig. 2). However, TPGS outstripped these nonionic surfactants in the solubility of (S)-ZPF. Because (S)-ZPF is weakly acidic (pKa of 4.21) [21], we considered pH as a factor to elucidate significant differences in the solubility of (S)-ZPF between TPGS, cremophor EL and Tween 80. Fig. 3 shows that the solvent pH varied from 4.06 ± 0.03 in PEG 400 solution to 7.05 ± 0.04 in poloxamer 188 solution and Fig. 4 illustrates the solubility of (S)-ZPF according to buffers whose pH range from pH 4.0 to 7.4. Generally, the solubility of (S)-ZPF strongly increased over buffer pH from 4.0 to 7.4. This result was consistent with the results of Li and Zhao on the solubilization of flurbiprofen (pKa of 4.17) [22]. The pH values of cremophor EL or Tween 80 were markedly lower than that of TPGS solution. These significant falls in pH values, as well, possibly could be employed to explain for the significant gap between the solubility of (S)-ZPF in TPGS solution and that in the double mixtures containing TPGS/Tween 80, TPGS/cremophor EL. A similar correlation between pH and the solubility of (S)-ZPF in PEG 400 was observed. Decreases in pH value might also explain the gap between the solubility of (S)-ZPF in the double mixtures containing TPGS/Tween 80, TPGS/cremophor EL.
Fig. 3.
pH values of investigated solvents.
Fig. 4.
Solubility of (S)-ZPF according to buffers whose pH range from pH 4.0–7.4.
Moreover, the differences in solubilization principles of surfactants (by micelles), HPCD (by central cavities), and PEGs (disrupting waters self-association, reducing waters ability to squeeze out nonpolar, hydrophobic compounds) maybecontributed to the differences in the solubility of (S)-ZPF.
From all data above, TPGS indicated as the best solubilizer in 26 investigated solvents due to possessing a set of critical properties which facilitate the solubility of (S)-ZPF (self-formed micelles, low surface tension, HLB value of 13.2, and pH of 6.47 ± 0.08).
For triple mixtures of TPGS/PEG 400/PG, the mixture containing more TPGS had greater capacity to dissolve (S)-ZPF. This finding, continually, revealed the important role of TPGS in enhancing (S)-ZPF solubility.
3.2. Linearity and sensitivity
We measured the peak area ratios of pure (S)-ZPF to the IS and used the ratio as a surrogate for quantitation. A calibration curve was prepared for (S)-ZPF over a wide range of concentrations from 0.10 to 75.00 µg/ml, which covered the enantiomer levels expected following oral and IV administration of a single dose of 35.0 mg/kg and 5.0 mg/kg of (S)-ZPF to rats, respectively. The limit of detection was 0.06 µg/ml at a signal to-noise ratio of 3:1. The LLOQ for (S)-ZPF in plasma was 0.1 µg/ml (Table 1). Using reversed-phase HPLC, IS and (S)-ZPF were detected separately at 2.95 and 4.16 min, respectively (Fig. 5).
Table 1.
Calibration curves, linear range, limit of detection (LOD), and lower limit of quantification (LLOQ) for pure (S)-ZPF, (R)-ZPF and (S)-ZPF from (RS)-ZPF (n = 3).
| Parameters | pure (S)-ZPF | (R)-ZPF from (RS)-ZPF | (S)-ZPF from (RS)-ZPF |
|---|---|---|---|
| Slope | 0.0209 ± 0.0030 | 88.3937 ± 0.9394 | 91.2043 ± 1.2548 |
| Intercept | 0.0028 ± 0.0007 | −50.8197 ± 22.9106 | −60.5433 ± 22.8994 |
| Correlation coefficient | 0.9998 | 0.9996 | 0.9997 |
| LOD (µg/ml) | 0.06 | 0.3 | 0.3 |
| LLOQ (µg/ml) | 0.1 | 0.5 | 0.5 |
| Linear range (µg/ml) | 0.1–75 | 0.5–37.5 | 0.5–37.5 |
Fig. 5.
The reversed HPLC chromatograms of (A) rat blank plasma, (B) blank plasma spiked with ketoprofen (IS), and (C) blank plasma spiked with (S)-ZPF and IS.
For (R)-ZPF and (S)-ZPF in (RS)-ZPF, chiral HPLC results exhibited linearity in the calibration range of 0.5–37.5 µg/ml with R² = 0.9996 and 0.9997 for (R)-ZPF and (S)-ZPF, respectively. The LLOQ was 0.5 µg/ml for both (R)-ZPF and (S)-ZPF.
The validated methods were successfully applied to the quantification and determination of pure (S)-ZPF as well as (R)-ZPF and (S)-ZPF in the racemic mixture in a wide range of concentrations in the pharmacokinetic study.
3.3. Extraction recovery
After investigating different extraction solvents, the mixture of acetonitrile and dichloromethane at a ratio 1:6 (v/v) exhibited the best extraction recovery. The extraction method was followed as reported previously with some modification [23]. Acetonitrile was used first as the protein precipitation reagent, and then dichloromethane was added to efficiently extract (S)-ZPF from plasma. Thus, the LLE procedure eliminated the formation of an irregular protein emulsion, which has been the major obstacle for use of dichloromethane alone as the extract solvent. Because only a limited amount of acetonitrile was used for the extraction, the extracts were still clean. Furthermore, the addition of acetonitrile did not substantially increase the evaporation time. The efficiency of the solvent for the extraction of the (S)-ZPF enantiomer ranged from 85.24% ± 4.87% to 91.83% ± 0.50% across the concentrations examined (Table 2). The results revealed that the recovery (%) of the extraction method was high and reproducible.
Table 2.
Recovery (%) of (S)-ZPF (n = 3).
| Conc. (µg/ml) | Recovery (%) |
|---|---|
| 0.1 | 85.24 ± 4.87 |
| 1 | 88.32 ± 2.65 |
| 5 | 90.65 ± 2.24 |
| 10 | 90.95 ± 1.15 |
| 50 | 91.83 ± 0.50 |
3.4. Accuracy and precision
The intra-day and inter-day accuracy and precision were estimated from standard curves prepared from three concentrations of standard solutions of (S)-ZPF. These results revealed that the method was quite precise. The intra- and inter-day accuracies were from 92.65% at 5.00 µg/ml intra-day to 118.70% at 0.1 µg/ml inter-day. The intra- and inter-day precisions were in the range of 0.82%–5.64% (Table 3).
Table 3.
Intra-day and inter-day precision and accuracy of (S)-ZPF (n = 3).
| Spiked (µg/ml) | Intra-day |
Inter-day |
||||
|---|---|---|---|---|---|---|
| Found (µg/ml) | Accuracy (%) | Precision (%) | Found (µg/ml) | Accuracy (%) | Precision (%) | |
| 0.1 | 0.12 | 117.28 | 4.14 | 0.12 | 118.70 | 5.64 |
| 1 | 1.11 | 110.81 | 2.66 | 1.10 | 110.87 | 2.69 |
| 5 | 4.63 | 92.65 | 1.86 | 4.62 | 92.77 | 2.16 |
| 10 | 9.76 | 97.57 | 1.11 | 9.50 | 95.12 | 1.18 |
| 50 | 48.19 | 96.38 | 0.82 | 47.59 | 95.20 | 1.15 |
3.5. Stability of (S)-ZPF
3.5.1. Freeze–thaw stability
Table 4 shows the stability of the QC samples following three freeze-thaw cycles. There were insignificant increases in the concentration of (S)-ZPF after one or three freeze-thaw cycles for 1.0 µg/ml (S)-ZPF. For 10.0 µg/ml (S)-ZPF, the concentrations in fresh samples were measured at 9.49 ± 0.14 µg/ml and 10.88 ± 0.14 µg/ml after the first freeze-thaw cycle and 10.91 ± 0.17 µg/ml after the third cycle. Similarly, for 50.0 µg/ml (S)-ZPF, the concentration was a little higher across the order of freeze-thaw cycle. In general, (S)-ZPF was stable in the frozen plasma at − 80 °C during at least three freeze-thaw cycles.
Table 4.
Stability of (S)-ZPF enantiomer in rat plasma (n = 3).
| Spiked (µg/ml) | Freshly (µg/ml) | Once (µg/ml) | Three times (µg/ml) | 4 °C/24 h (µg/ml) | Transformation |
|---|---|---|---|---|---|
| 1 | 1.07 ± 0.04 | 1.10 ± 0.06 | 1.12 ± 0.08 | 1.19 ± 0.05 | None |
| 10 | 9.49 ± 0.14 | 10.88 ± 0.14 | 10.91 ± 0.17 | 11.28 ± 0.11 | None |
| 50 | 48.60 ± 0.21 | 56.45 ± 0.66 | 56.70 ± 0.7 | 56.75 ± 0.41 | None |
Each value represents the means ± SD of three determinations.
Freshly: initial concentration of (S)-ZPF.
Once: concentration of (S)-ZPF after one time of freeze-thaw.
Three times: concentration of (S)-ZPF after three time of freeze-thaw
4 °C/24h: concentration of (S)-ZPF after storing at 4 °C for 24 h.
Interconversion: interconversion from (S)-ZPF to (R)-ZPF.
3.5.2. Stability in refrigerator
There was no degradation of (S)-ZPF for QC samples stored at 4 °C for 24 h and no transformation of (S)-ZPF into (R)-ZPF (Table 4). Using chiral HPLC, (R)-ZPF and (S)-ZPF were detected separately at 10.5 and 11.4 min, respectively. The HPLC chromatogram of (R)-ZPF and (S)-ZPF using the chiral column is shown in Fig. 6. The stability data demonstrated that (S)-ZPF was stable and did not interconvert either after three freeze-thaw cycles or after storage at 4 °C for 24 h.
Fig. 6.
The chiral HPLC chromatograms of (A) rat blank plasma, (B) blank plasma spiked with (RS)-ZPF, (C) blank plasma spiked with (R)-ZPF, (D) rat blank plasma spiked with (S)-ZPF, and (E) plasma sample spiked with (S)-ZPF under a storage condition of three cycles of freeze-thaw.
3.6. Pharmacokinetic study of (S)-ZPF
After single oral administration of 35 mg/kg (S)-ZPF or single IV administration of 5 mg/kg (S)-ZPF to male SD rats, the plasma concentrations of (S)-ZPF were determined by the validated method. For a racemic drug or stereo chemically pure enantiomer, this indicates knowledge of the in vivo behavior of the stereoisomers. Therefore, we first determined whether there was any existence of the metabolic chiral inversion of (S)-ZPF to (R)-ZPF after oral and IV administration of pure (S)-ZPF in all plasma samples. The chiral HPLC chromatogram revealed no interconversion of (S)-ZPF to (R)-ZPF in experimental rats. This finding was consistent with the report from Chu and co-workers [9]. Similarly, scientific evidence of (S)-enantiomer inversion to the (R)-enantiomer among 2-APAs is rare [24].
The plasma concentration of pure (S)-ZPF was in the linear range of the validated analytical method for the entire test period. The pharmacokinetic parameters were calculated using a non-compartmental analysis. To investigate the correlation of solubilization of (S)-ZPF and its oral bioavailability, three groups of rats were orally administered different (S)-ZPF suspensions according to the different solubility of (S)-ZPF in TPGS, HPCD, and DW at the same dosing regimens (35.0 mg/kg).
We prepared suspensions at 2% TPGS or HPCD [25] using sonication to increase dissolution of (S)-ZPF. The final concentrations of dissolved (S)-ZPF in DW, 2% (w/v) TPGS, and 2% (w/v) HPCD were 23.70 ± 3.91, 3140.86 ± 94.94, and 680.57 ± 27.58 µg/ml, respectively (Fig. 7).
Fig. 7.
Dissolved concentration of (S)-ZPF in water, 2% HPCD, and 2% TPGS suspension. Total amount of (S)-ZPF was administrated to each rat: 14.0 mg.
Fig. 8 shows the plasma (S)-ZPF concentration over time. The AUC0-24 h for (S)-ZPF after IV administration was 149.96 ± 8.23 µg·h/ml. For oral administration, AUC0–24 h of DW group was the lowest at 465.57 ± 90.89 µg·h/ml, whereas that of TPGS group was the highest at 813.59 ± 64.17 µg·h/ml and that of HPCD group was 595.57 ± 71.76 µg·h/ml. Table 5 shows similar trends in the Cmax and T1/2 value with the highest values in TPGS group (55.40 ± 7.51 µg/ml and 8.37 ± 0.92 h, respectively), followed by HPCD group (41.87 ± 4.11 µg/ml and 6.32 ± 0.65 h, respectively) and DW group (35.78 ± 4.81 µg/ml and 4.89 ± 0.53 h, respectively). The longer time for (S)-ZPF circulated in rat blood in TPGS group compared to HPCD group and Water group was probably explained by the enhancement in the clearance of (S)-ZPF over formulations (0.015 ± 0.001 l/h in TPGS, 0.021 ± 0.002 l/h in HPCD, and 0.029 ± 0.005 l/h in water). The relative bioavailability of (S)-ZPF from TPGS group and HPCD group were approximately 1.75- and 1.23-fold higher than that from DW group. There was a strong correlation coefficient between the dissolved amount of (S)-ZPF in the suspension and the oral bioavailability of (S)-ZPF (AUC0-24 h) (R2 = 0.97, y = 0.1097x + 488.16). A previous report indicated that the bioavailability of paclitaxel prepared in three different groups, including paclitaxel alone, paclitaxel co-administered with verapamil, and paclitaxel with TPGS, also showed the highest increase in the bioavailability of paclitaxel when administered with TPGS as the solubilizing agent [26]. In addition to working as a solubilizer, the considerable rise in AUC, the shortening of Tmax, and the prolongation of the half-life in oral bioavailability of (S)-ZPF with TPGS in comparison with HPCD and water might be due to p-gp inhibition and permeation enhancement [27], [28].
Fig. 8.
Plasma concentration versus time following iv dosing at 5.0 mg/kg and oral dosing of the three groups to rats at 35.0 mg/kg.
Table 5.
Pharmacokinetic parameters of (S)-ZPF (n = 3).
| TPGS group | HPCD group | Water group | IV | ||
|---|---|---|---|---|---|
| AUC0-24 h(µg⋅h/ml) | 813.59 ± 64.17*a | 595.57 ± 71.76 | 465.57 ± 90.89 | 149.95 ± 8.23 | |
| Cmax (µg/ml) | 55.40 ± 7.51*a | 41.87 ± 3.1 | 35.78 ± 4.8 | – | |
| Tmax (h) | 2.00 ± 1.73 | 5.5 ± 4.33 | 5.67 ± 4.04 | – | |
| T1/2 (h) | 8.37 ± 0.92*a | 6.32 ± 0.65* | 4.88 ± 0.53 | 14.91 ± 5.52 | |
| Vd (l) | 0.18 ± 0.03 | 0.20 ± 0.04 | 0.21 ± 0.06 | 0.20 ± 0.04 | |
| CL (l/h) | 0.015 ± 0.001*a | 0.021 ± 0.002 | 0.029 ± 0.005 | 0.010 ± 0.002 | |
| AB (%) | 77.51 | 56.74 | 44.35 | 100 | |
| RB (%) | 174.75 | 127.92 | 100 | ||
Each value represents the means ± SD of three determinations.
*P < 0.05 compared to water group; aP < 0.05 compared to HPCD group.
AB: absolute bioavailability to IV group.
RB: relative bioavailability to water group.
Oral dose = 35.0 mg/kg.
IV dose = 5.0 mg/kg.
Interestingly, the pure (S)-ZPF suspension in TPGS group in this study exhibited a considerably faster Tmax (2.00 ± 1.73 h) than those of (R)-ZPF (4.89 ± 1.03 h) and (S)-ZPF (6.06 ± 0.74 h) enantiomers from commercial (RS)-ZPF (CJ Pharm. Co., Ltd., Seoul, Korea) in the study of Chu and colleagues [9]. Additionally, also in comparison with the PK parameters from Chu group, the half-life of pure (S)-ZPF in TPGS (8.37 ± 0.92 h) was significantly longer than those of (R)-ZPF and (S)-ZPF in the (RS)-ZPF (3.39 ± 0.71 h and 4.20 ± 0.52 h, respectively) [9]. These indicated that not only the therapeutic concentration of (S)-ZPF in the TPGS suspension of the pure (S)-ZPF enantiomer was probably reached earlier than the figure for the racemic mixture but also this concentration will be remained longer than that of (RS)-ZPF in rat plasma. Therefore, the dosing interval could be reduced when replacing the (RS)-ZPF with pure (S)-ZPF prepared with TPGS for oral administration.
The plasma profiles of (S)-ZPF showed biphasic elimination after oral administration, consistent with the plasma concentration decreases of some other NSAIDs such as ibuprofen, meloxicam, and etoricoxib [29], [30], [31].
3.7. (S)-ZPF retained in the stomach
Fig. 9 provides the amount of (S)-ZPF remained in the stomachs of SD rats after the last time point of the pharmacokinetic study. Water group provided the highest percentage of (S)-ZPF recovered from the stomach tissue at 1.38% ± 0.16%, while the value was lowest at 0.28% ± 0.01% in TPGS group. The increase in the amount of (S)-ZPF in stomach tissues across TPGS group, HPCD group, and Water group was in line with the growth in the volume of distribution (Vd) of (S)-ZPF over formulations (Table 5).
Fig. 9.
Correlation between AUC0–24 h and amount of (S)-ZPF retained in the rat stomach against different groups after a 24 h-period pharmacokinetic study.
The greater plasma level of (S)-ZPF was, the lower (S)-ZPF level in the stomach was examined (Fig. 9). The amount of (S)-ZPF in the stomach of DW group was about five-fold higher than that in TPGS group. Thus, TPGS facilitated the absorption of (S)-ZPF into plasma. On the other hand, among the various risk factors identified for NSAID-related GI effects, high dosage was a key factor [32], [33]. Thus, TPGS could play an important role in decreasing the side effects of (S)-ZPF on the GI tract because less (S)-ZPF was retained in the stomach.
4. Conclusion
TPGS showed the highest capability to completely dissolve (S)-ZPF among the 26 solvents studied, and the oral bioavailability of (S)-ZPF was highest in the TPGS group. There was no chiral inversion of (S)-ZPF to (R)-ZPF after oral or IV administration of pure (S)-ZPF in experimental rats. In addition, the results of this study revealed the strong correlation between solubilization and oral bioavailability of (S)-ZPF. Therefore, TPGS will be a potential surfactant for preparing formulations containing (S)-ZPF in the future.
Conflicts of interest
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by the Basic Science Research Program (2016R1A2B4011294) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2018.10.002.
Appendix. Supplementary materials
References
- 1.Islam M.R., Mahdi J.G., Bowen I.D. Pharmacological importance of stereochemical resolution of enantiomeric drugs. Drug Saf. 1997;17(3):149–165. doi: 10.2165/00002018-199717030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Jozwiak K., Lough W.J., Wainer I.W. 3rd ed. CRC Press; Boca Raton: 2012. Drug stereochemistry: Analytical methods and pharmacology. [Google Scholar]
- 3.Drayer D.E. Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview. Clin Pharmacol Ther. 1986;40(2):125–133. doi: 10.1038/clpt.1986.150. [DOI] [PubMed] [Google Scholar]
- 4.Midha K.K., McKay G., Rawson M.J., Hubbard J.W. The impact of stereoisomerism in bioequivalence studies. J Pharm Sci. 1998;87(7):797–802. doi: 10.1021/js9703683. [DOI] [PubMed] [Google Scholar]
- 5.Reddy I.K., Mehvar R. 1st ed. CRC Press; Boca Raton: 2004. Chirality in drug design and development. [Google Scholar]
- 6.Yoon J.S., Jeong D.C., Oh J.W. The effects and safety of dexibuprofen compared with ibuprofen in febrile children caused by upper respiratory tract infection. Br J Clin Pharmacol. 2008;66(6):854–860. doi: 10.1111/j.1365-2125.2008.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landoni M.F., Soraci A. Pharmacology of chiral compounds: 2-Arylpropionic acid derivatives. Curr Drug Metab. 2001;2(1):37–51. doi: 10.2174/1389200013338810. [DOI] [PubMed] [Google Scholar]
- 8.Kaehler S.T., Phleps W., Hesse E. Dexibuprofen: Pharmacology, therapeutic uses and safety. Inflammopharmacology. 2003;11(4):371–383. doi: 10.1163/156856003322699555. [DOI] [PubMed] [Google Scholar]
- 9.Chu V.M., Kim K.T., Kim S.H. Chiral pharmacokinetics of zaltoprofen in rats by HPLC with solid-phase extraction. J Pharm Biomed Anal. 2012;70:567–573. doi: 10.1016/j.jpba.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Cho I.J., Lee C.W., Lee M.Y. Differential anti-inflammatory and analgesic effects by enantiomers of zaltoprofen in rodents. Int Immunopharmacol. 2013;16(4):457–460. doi: 10.1016/j.intimp.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 11.de Mello H., Echevarria A., Bernardino A.M., Canto-Cavalheiro M., Leon L.L. Antileishmanial pyrazolopyridine derivatives: Synthesis and structure-activity relationship analysis. J Med Chem. 2004;47(22):5427–5432. doi: 10.1021/jm0401006. [DOI] [PubMed] [Google Scholar]
- 12.Strickley R.G. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 13.Pham C.V., Cho C.W. Application of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) in transdermal and topical drug delivery systems (TDDS) J Pharm Investig. 2017;47(2):111–121. [Google Scholar]
- 14.Nayak A.K., Panigrahi P.P. Solubility enhancement of etoricoxib by cosolvency approach. ISRN Phys Chem. 2012;820653:1–5. [Google Scholar]
- 15.Son G.H., Lee B.J., Cho C.W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J Pharm Investig. 2017;47(4):287–296. [Google Scholar]
- 16.Wu S.H., Hopkins W.K. Characteristics of d-a-tocopheryl PEG1000 succinate for applications as an absorption enhancer in drug delivery systems. Pham Tech. 1999;23(10):52–68. [Google Scholar]
- 17.Rowe C.R., Sheskey J.P., Quinn E.M. 6th ed. Pharmaceutical Press; London: 2009. Handbook of pharmaceutical excipients. [Google Scholar]
- 18.Schmidts T., Dobler D., Nissing C., Runkel F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J Colloid Interface Sci. 2009;338(1):184–192. doi: 10.1016/j.jcis.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Irini M., Panagiotis B., Andrea S., Ioannis N. The influence of surfactant HLB and oil/surfactant ratio on the formation and properties of self-emulsifying pellets and microemulsion reconstitution. AAPS PharmSciTech. 2012;13(4):1319–1330. doi: 10.1208/s12249-012-9855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarvand R., Moghadam S.H., Sheikhi A., Atyabi F. Effect of surfactant HLB and different formulation variables on the properties of poly-D,L-lactide microspheres of naltrexone prepared by double emulsion technique. J Microencapsul. 2005;22(2):139–151. doi: 10.1080/02652040400026392. [DOI] [PubMed] [Google Scholar]
- 21.Cui H., Quan P., Zhao H. Mechanism of ion-pair strategy in modulating skin permeability of zaltoprofen: Insight from molecular-level resolution based on molecular modeling and confocal laser scanning microscopy. J Pharm Sci. 2015;104(10):3395–3403. doi: 10.1002/jps.24543. [DOI] [PubMed] [Google Scholar]
- 22.Li P., Zhao L. Solubilization of flurbiprofen in pH-surfactant solutions. J Pharm Sci. 2003;92(5):951–956. doi: 10.1002/jps.10360. [DOI] [PubMed] [Google Scholar]
- 23.Xue Y.J., Pursley J., Arnold M.E. A simple 96-well liquid-liquid extraction with a mixture of acetonitrile and methyl t-butyl ether for the determination of a drug in human plasma by high-performance liquid chromatography with tandem mass spectrometry. J Pharm Biomed Anal. 2004;34(2):369–378. doi: 10.1016/S0731-7085(03)00520-X. [DOI] [PubMed] [Google Scholar]
- 24.Fournel S., Caldwell J. The metabolic chiral inversion of 2-phenylpropionic acid in rat, mouse and rabbit. Biochem Pharmacol. 1986;35(23):4153–4159. doi: 10.1016/0006-2952(86)90689-1. [DOI] [PubMed] [Google Scholar]
- 25.Maga A.J., Tu T.A. 1st ed. CRC Press; Boca Raton: 1994. Food additive toxicology. [Google Scholar]
- 26.Varma M.V., Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005;25(4–5):445–453. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Bogman K., Erne-Brand F., Alsenz J., Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J Pharm Sci. 2003;92(6):1250–1261. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- 28.Cornaire G., Woodley J., Hermann P., Cloarec A., Arellano C., Houin G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharm. 2004;278(1):119–131. doi: 10.1016/j.ijpharm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal N.G., Porras A.G., Matthews C.Z. Single- and multiple-dose pharmacokinetics of etoricoxib, a selective inhibitor of cyclooxygenase-2, in man. J Clin Pharmacol. 2003;43(3):268–276. doi: 10.1177/0091270003251122. [DOI] [PubMed] [Google Scholar]
- 30.Busch U., Schmid J., Heinzel G. Pharmacokinetics of meloxicam in animals and the relevance to humans. Drug Metab Dispos. 1998;26(6):576–584. [PubMed] [Google Scholar]
- 31.Cole E.T., Scott R.A., Cade D., Connor A.L., Wilding I.R. In vitro and in vivo pharmacoscintigraphic evaluation of ibuprofen hypromellose and gelatin capsules. Pharm Res. 2004;21(5):793–798. doi: 10.1023/b:pham.0000026430.73789.e6. [DOI] [PubMed] [Google Scholar]
- 32.Schlansky B., Hwang H.J. Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy. J Gastoenterol. 2009;44:44–52. doi: 10.1007/s00535-008-2275-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Cun D., Kong X., Fang L. Design and evaluation of a novel transdermal patch containing diclofenac and teriflunomide for rheumatoid arthritis therapy. Asian J Pharm Sci. 2014;9(5):251–259. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.