Abstract
Aim:
To identify differential patterns of brain activation between adolescents with bipolar disorder and adolescents with attention-deficit hyperactivity disorder (ADHD) to better understand the neurophysiology of both disorders. We hypothesized that subjects with ADHD would show altered activation in brain regions involved in executive and sustained attention. In contrast, we hypothesized that bipolar subjects would show altered brain activation in regions responsible for emotionally homeostasis, including the striatum and amygdala.
Methods:
Functional magnetic resonance imaging was performed during a continuous performance task with a response inhibition component in 11 adolescents with bipolar disorder during a manic episode, 10 adolescents with ADHD, and 13 healthy adolescents.
Results:
There were no differences in behavioural performance among the three groups. Compared with bipolar subjects, subjects with ADHD showed increased activation in the superior temporal lobe during successful response inhibition. Although bipolar subjects did not show activation differences in the striatum or amygdala compared with ADHD subjects, increased left parahippocampal activation in the bipolar group was associated with increased manic symptoms.
Conclusions:
The patterns of brain activation observed in the current study support divergent patterns of neurophysiological dysfunction in individuals with bipolar disorder as compared with those with ADHD. Therefore, the impulsive behaviour seen in both disorders may be the consequence of dysfunction in different brain regions, and further research may help identify neurobiological markers that are specific to each condition.
Keywords: attention, attention-deficit hyperactivity disorder, bipolar disorder, fMRI, neurophysiology
INTRODUCTION
Despite the high co-occurrence of adolescent bipolar and attention-deficit hyperactivity disorders (ADHD), the relationships between these conditions remain unclear.1 Adolescents with bipolar disorder are often initially misdiagnosed with ADHD and treated with medications that may exacerbate their mood symptoms.2 Impulsivity and inattention are two underlying features associated with bipolar disorder and ADHD.3–6 Mayberg and colleagues suggest that in the presence of a mood disorder, attentional disturbances may represent a marker of dysfunction in brain regions that are primarily involved in mood regulation.7 In contrast, in primary disorders of attention such as ADHD, neuronal dysfunction may primarily involve brain regions associated with attention.8 Given the reciprocal connections between emotional and cognitive brain systems, impaired cognition could then lead to dysregulation of mood. Comparing brain networks associated with inattention and impulse control in adolescents with bipolar disorder versus adolescents with ADHD may help to identify neurobiological markers that are specific to each condition.
Prior research has shown that bipolar patients in a manic episode and patients with ADHD have significant difficulty with inhibitory control.9–11 The majority of functional magnetic resonance imaging (fMRI) studies of ADHD and several studies of bipolar disorder have used response inhibition tasks to examine brain regions involved in attention and impulsivity.8,12–15 However, a significant limitation of these studies is that none of them directly compared subjects with ADHD with those with bipolar disorder; rather, they compared each group to healthy subjects. In a more direct comparison of bipolar and ADHD patients, Adler et al.16 examined neurophysiological differences between these groups by comparing bipolar adolescents with and without co-morbid ADHD during a manic episode. The authors were interested in brain activation differences in the two groups during sustained attention and therefore examined subjects with fMRI during a continuous performance task (CPT). Bipolar subjects with co-morbid ADHD showed greater brain activation in posterior brain regions, including the temporal cortex during the task.16 Strakowski et al.17 then added a stop signal component to this CPT task to measure response inhibition. This modified CPT task allows for the measure of response inhibition within a background of sustained attention and was proposed as a more challenging and ecologically valid measure of response inhibition.
To test the hypothesis that different brain regions contribute to the deficits in response inhibition seen in ADHD and bipolar disorder, we studied adolescent subjects with each disorder along with healthy subjects while subjects performed the modified CPT task during fMRI. Based on prior neuroimaging work in ADHD,15,18 we hypothesized that compared with bipolar and healthy subjects, ADHD subjects would show altered activation in the anterior cingulate and dorsal prefrontal regions involved in executive attention. Based on the findings by Adler et al.16, we also hypothesized that subjects with ADHD would show increased activation in the temporal cortex compared with bipolar subjects. In bipolar patients, because attentional deficits may represent problems with emotional regions of the brain, we hypothesized that compared with ADHD and healthy subjects, bipolar patients would exhibit altered activation in regions responsible for emotional homeostasis.19 Specifically, we predicted bipolar subjects would show increased activation in the amygdala and striatum. Prior studies in adolescents with bipolar disorder have consistently found altered activation in these two regions.20–24
METHODS
Subjects
Adolescents with bipolar disorder (n = 14) and adolescents with ADHD (n = 14) were recruited from the Cincinnati Children’s Hospital Medical Centre. Subjects with bipolar disorder were included if they met DSM-IV criteria for bipolar I disorder, current episode manic or mixed, did not have a history of co-occurring ADHD and had a Young Mania Rating Scale (YMRS)25 score ≥ 20. Subjects with ADHD were recruited if they met DSM-IV criteria for ADHD and had no history of a mood or psychotic disorder themselves or in any first-degree relative. Overall, there was very little co-morbidity in either patient group. In the bipolar group, four subjects had co-morbid diagnoses: one subject with alcohol dependence, one subject with simple phobia, one subject with conduct disorder and one subject with generalized anxiety disorder. In the ADHD group, only four patients had co-morbid diagnoses: two subjects with transient tic disorder and two subjects with oppositional defiant disorder.
Demographically matched healthy adolescents (n = 14) were also recruited. Healthy subjects had no history of Axis I psychiatric disorders themselves or in any first-degree relative. All subjects were between the ages of 11 and 18 years, were physically and neurologically healthy, and if female, had a negative urine pregnancy test. Other exclusion criteria were any lifetime substance dependence or illicit substance use within the last 3 months, medical or neurological illnesses that might influence brain structure or function, contraindications to receiving an MRI, and diagnosis of mental retar-dation or a documented IQ below 70 using the Wechsler Abbreviated Scale of Intelligence.
Bipolar, ADHD and healthy subjects were not taking medications at the time of the fMRI scan. Eight of the ADHD subjects were taking stimulants, which were held the morning of the scan. All bipolar subjects had been off atypical anti-psychotics for at least 72 h and had undetectable levels of mood stabilizers. All medications were self-discontinued, and no medications were stopped for the study. Healthy subjects were not taking medications. Adolescents provided written assent, and their legal guardians provided written informed consent for study participation after study procedures were fully explained. The study was approved by the Investigational Review Boards of the University of Cincinnati and the Cincinnati Children’s Hospital Medical Centre and conformed to the provisions of the Declaration of Helsinki. Diagnoses were made using the Washington University at St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia26 by trained child and adolescent psychiatrists with established diagnostic reliability (kappa = 0.94).2 Depressive and manic symptoms were evaluated using the Children’s Depression Rating Scale-Revised Version (CDRS) and YMRS, respectively.25,27 The interrater reliability for total scores on the CDRS and YMRS was measured with the interclass correlation coefficient (ICC) and was judged to be good (ICC > 0.80).28
CPT-X task
During the MRI scan, all subjects performed a CPT-X task. This task builds on a CPT task that is purposefully undemanding and incorporates a stop signal component (Strakowski go-no-go 2008 and Birkett et al.). Sustained attention is engaged by having subjects press on the target, a blue X presented in the centre of the visual field. Blue Xs were randomly interspersed with other blue letters that were not targets. The response inhibition component was added by including a ‘stop’ signal, a red X that replaced the blue X after variable time intervals (0, 50, 100 or 150 ms). A measure of inhibitory control is then provided by the percentage of correct stops.
All stimuli were presented for 450 ms, with an inter-trial interval of 50 ms. Responses were counted as part of the trial if they were recorded within 800 ms of the stimulus onset. Subjects were instructed to press a button as quickly and accurately as possible every time a blue X occurred, but to resist responding if the colour of the target changed to red. A total of 1919 trials in a 16-min fMRI scanning run were presented, including 197 ‘go’ trials (blue X), 64 ‘stop’ trials (red X) and 1658 non-targets. The task was written and displayed using PsyScope software29 (http://psy.ck.sissa.it/). Responses were recorded using a MRI-compatible button box. One subject with ADHD could not be used in the analysis because behavioural data were not recorded in the scanner due to a hardware malfunction. Two subjects with bipolar disorder were not further analysed because they did not correctly respond to greater than 10% of the blue X trials.
Imaging
All subjects were scanned at the University of Cincinnati College of Medicine’s Centre for Imaging Research using a 4.0 Tesla Varian Unity INOVA Whole Body MRI/MRS system (Varian Inc., Palo Alto, CA, USA). Following a three-plane gradient echo scan for alignment and brain localization, a shim procedure was performed to generate a homogeneous magnetic field. To provide anatomical localization for activation maps, a high-resolution, T1-weighted, 3-D brain scan was obtained using a modified driven equilibrium Fourier transform (MDEFT) sequence (TMD = 1.1 s, time to repetition (TR) = 13 ms, echo time (TE) = 6 ms, field of view (FOV) = 25.6 × 19.2 × 19.2 cm, matrix 256 × 192 × 96 pixels, flip angle = 20 degrees). A mid-sagittal localizer scan was obtained to place 30 contiguous 5-mm axial slices extending from the inferior cerebellum to encompass the entire brain. Subjects then completed an fMRI session in which scans were acquired while performing the response inhibition task using a T2*-weighted gradient-echo echoplanar imaging (EPI) pulse sequence (TR/TE = 2000/30 ms, FOV = 25.6 × 25.6 cm, matrix 64 × 64 pixels, slice-thickness=5 mm, flip angle = 75 degrees).
fMRI analysis
The fMRI data were analysed using AFNI (Analysis of Functional NeuroImages; http://afni.nimh.nih.gov/afni).30 Following acquisition, the MRI images were reconstructed using in-house software developed in IDL (interactive data language), which converts raw scanner data into AFNI format. In AFNI, MDEFT (structural) and EPI (functional) images were co-registered using scanner coordinates. Average motion was calculated for each subject, and all subjects had less than 4 mm of uncorrected movement across the run before motion correction. There were no differences in average uncorrected motion among the three groups (F = 1.175, P = 0.32). Functional images were corrected for motion using a six-parameter rigid body transformation.31 In addition, each volume was inspected for signal artefact using a semi-automated algorithm in AFNI to identify questionable TRs. These volumes were removed from further analysis if visual inspection indicated uncorrectable head movement, that is within-TR head motion, or greater than 30% of voxels were greater than 2 standard deviations from the mean signal intensity. On average, we removed 10 volumes (4%) from each person, and two ADHD and one healthy subject were excluded from further analysis because greater than 40% of the TRs were removed.
Using tools in AFNI, anatomical and functional maps were transformed into stereotaxic Talairach space using the ICBM452 template and spatially blurred to twice the voxel dimensions. Binary masking was applied to each image to remove pixels outside the brain. Individual activation maps were then created for each subject using a deconvolution algorithm that compares the actual hemodynamic response to a canonical hemodynamic response function (a gamma function), creating voxel-wise t-maps. Event-related hemodynamic response functions were calculated for correct delayed STOPs (red X) and correct non-responses to distracter stimuli (non-X). General linear tests were then performed on each subject to determine contrasts of correct delayed red X’s versus correct non-responses to non-X’s. Individual activation maps were then averaged across subjects, and the final regression analysis was performed to contrast the three groups with 11 bipolar, 10 ADHD and 13 healthy subjects. Two bipolar and one ADHD subject were lost because of poor (less than 15% correct on Blue-X trials) or missing behavioural data, and one bipolar, two ADHD and one healthy subject were excluded because of within-TR head motion. Group activation maps were corrected to P ≤ 0.05 using a threshold probability of P ≤ 0.05 and a voxel-level correction of 137 contiguous voxels, as determined by Monte Carlo simulations within AFNI.32,33
RESULTS
There were no statistically significant group differences in age, sex, race or IQ among the three groups (see Table 1). There were statistically significant group differences in YMRS and CDRS scores, essentially by definition (see Table 1). Post-hoc tests (Tukey’s honestly significant differences) were performed and indicated that bipolar subjects had significantly higher YMRS scores than ADHD (P ≤ 0.001) and healthy subjects (P ≤ 0.001), and ADHD subjects had significantly higher YMRS scores than healthy subjects (P ≤ 0.002). Bipolar subjects had significantly higher CDRS scores than ADHD (P ≤ 0.001) and healthy (P ≤ 0.001) subjects, but there were no statistically significant difference in CDRS scores between ADHD and healthy subjects (P = 0.347).
TABLE 1.
Demographic measures in patients with BPD, ADHD and healthy comparison subjects
| BPD (N = 11) | ADHD (N = 10) | Healthy subjects (N = 13) | |
|---|---|---|---|
| Gender, N (%) female | 7 (64) | 3 (30) | 6 (46) |
| Ethnicity, N (%), white | 9 (82) | 9 (90) | 11 (85) |
| Mean | SD | Mean | SD | Mean | SD | F | P | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 14.2 | 1.5 | 14.0 | 2.0 | 14.5 | 1.9 | 0.27 | 0.77 |
| Education (years) | 8.1 | 104 | 7.8 | 2.0 | 8.5 | 2.0 | 0.47 | 0.63 |
| IQ (WASI)† | 105 | 8.2 | 104 | 15.2 | 112 | 14.0 | 1.29 | 0.29 |
| YMRS‡ | 26 | 6.3 | 8.6 | 7.5 | 0.2 | 0.6 | 67 | 0.00 |
| CDRS§ | 43 | 12.2 | 22.2 | 3.5 | 18 | 2.3 | 41 | 0.00 |
Three of the bipolar subjects were lost to follow up and did not receive IQ measures.
Post hoc Tukey’s HSD tests showed significant differences between bipolar and ADHD subjects (P ≤ 0.001), between bipolar and healthy subjects (P ≤ 0.001), and between ADHD and healthy subjects (P ≤ 0.002).
Post hoc Tukey’s HSD tests showed significant differences between bipolar and ADHD subjects (P ≤ 0.001), and between bipolar and healthy subjects (P ≤ 0.001).
ADHD, attention-deficit hyperactivity disorder; BPD, bipolar disorder; CDRS, Children’s Depression Rating Scale-Revised Version; HSD, honestly significant differences; SD, standard deviation; WAIS, Wechsler Abbreviated Scale of Intelligence; YMRS, Young Mania Rating Scale.
Regarding behavioural performance, there were no statistically significant differences in reaction time or percent accuracy of correct blue-X trials or delayed red-X trials among the three groups (see Table 2). In addition to total performance, percent accuracy scores were also calculated for each half of the scan to determine if performance varied during the 16 minutes task. Repeated measures ANOVAs were performed, and there was a main effect of trial time for blue-X (F = 37, P ≤ 0.001) and red-X (F = 15, P ≤ 0.001) trials. All subjects had significantly lesser accuracy on blue-X trials and significantly greater accuracy on delayed red-X trials during the final 8 minutes. There were no between group differences over time. Finally, to further assess performance, discriminability and bias were calculated. There were no significant differences in either measure (see Table 2).
TABLE 2.
Behavioural performance measures in patients with BPD, ADHD and healthy comparison subjects
| Behavioural measure | BPD (N = 11) | ADHD (N = 10) | Healthy subjects (N =13) | F | P | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Total accuracy blue X (%) | 42 | 19 | 45 | 14 | 50 | 16 | 0.76 | 0.48 |
| Total accuracy delayed red X (%) | 83 | 13 | 79 | 8 | 84 | 10 | 0.74 | 0.49 |
| 1st 8 minutes accuracy blue X (%)† | 47 | 18 | 52 | 11 | 53 | 19 | - | - |
| 1st 8 minutes accuracy delayed red X (%) | 78 | 14 | 71 | 13 | 78 | 16 | - | - |
| 2nd 8 minutes accuracy blue X (%)‡ | 36 | 22 | 38 | 17 | 47 | 20 | - | - |
| 2nd 8 minutes accuracy delayed red X (%) | 87 | 13 | 86 | 8 | 90 | 8 | - | - |
| RT correct blue X (ms) | 581 | 34 | 587 | 30 | 579 | 19 | 1.24 | 0.302 |
| RT incorrect delayed red X ms | 551 | 23 | 562 | 26 | 548 | 17 | 1.25 | 0.298 |
| Discriminability red X | 0.72 | 0.10 | 0.71 | 0.09 | 0.78 | 0.06 | 2.52 | 0.097 |
| Bias red X | −0.14 | 0.13 | −0.17 | 0.08 | −0.10 | 0.14 | 1.01 | 0.377 |
| Discriminability non-X | 0.84 | 0.05 | 0.84 | 0.04 | 0.86 | 0.04 | 1.01 | 0.375 |
| Bias non-X | 0.12 | 0.03 | 0.12 | 0.05 | 0.14 | 0.03 | 1.20 | 0.316 |
Repeated measures ANOVA revealed a significant main effect of trial time for red X (F = 15, P ≤ 0.001) trials. There were no between group differences across trial time (group × time F = 0.9, P = 0.42).
Repeated measures ANOVA revealed a significant main effect of trial time for blue X (F = 37, P ≤ 0.001) trials. There were no between group differences across trial time (group × time F = 1.0, P = 0.39).
ADHD, attention-deficit hyperactivity disorder; BPD, bipolar disorder; SD, standard deviation.
Significant differences in brain activation occurred during successful response inhibition as measured by the contrast of correct red X (STOP) trials versus correct non-responses to distracter stimuli (non-X) (see Table 3 and Fig. 1). Compared with bipolar subjects, ADHD subjects showed increased activation in the left superior temporal gyrus, right posterior cingulate gyrus, right lingual gyrus, right cuneus, left middle temporal gyrus, right cerebellar tonsil and left insula. See Table 3 for regions that were significant between each patient group and the healthy comparison subjects.
TABLE 3.
Regions of significant brain activation in each of the three group comparisons
| Region of interest | Activation | Laterality | Brodmann area(s) | Centre of mass talairach coordinates (X, Y, Z) | Volume (3-mm3 voxels) | ||
|---|---|---|---|---|---|---|---|
| Bipolar versus healthy | |||||||
| Cerebellar vermis | Decreased | Bilateral | −2.6 | −33 | −31 | 505 | |
| Parahippocampal gyrus, hippocampus, amygdala | Decreased | Left | −37 | −9 | −18 | 429 | |
| Postcentral gyrus | Decreased | Left | −42 | −9 | 48 | 426 | |
| Precentral gyrus | Decreased | Right | 58 | −7 | 32 | 310 | |
| Middle and superior temporal gyrus | Increased | Right | 22 | 52 | −50 | 15 | 254 |
| Fusiform gyrus, inferior temporal gyrus | Decreased | Right | 20 | 44 | 7 | −26 | 167 |
| ADHD versus healthy | |||||||
| Anterior cingulate | Decreased | Left | 24 | −3 | 30 | 2 | 256 |
| Superior temporal gyrus | Increased | Left | 42, 22 | −64 | −17 | 12 | 174 |
| Insula | Increased | Right | 30 | −11 | 20 | 169 | |
| ADHD versus bipolar | |||||||
| Superior temporal gyrus, insula | Increased | Left | 22 | −53 | −6 | 11 | 630 |
| Cuneus, posterior cingulate | Increased | Right | 31, 30, 18 | 21 | −70 | 11 | 250 |
| Middle occipital gyrus, lingual gyrus, cuneus | Increased | Left | 30 | −38 | −64 | 10 | 245 |
All regions were significantly at P ≤ 0.05 and with a cluster-level correction of 137 contiguous voxels.
ADHD, attention-deficit hyperactivity disorder
FIGURE 1.
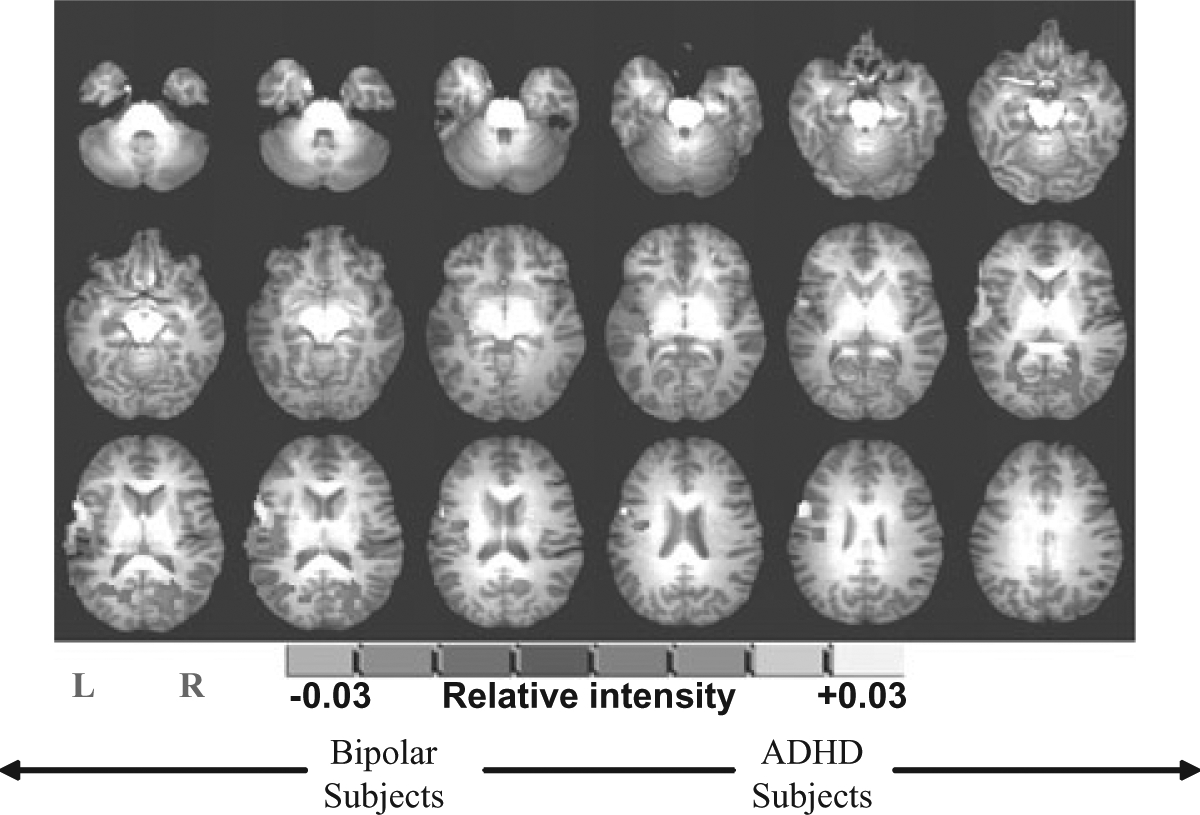
Functional activation map showing relative brain activation during response inhibition between patients with attention-deficit hyperactivity disorder (ADHD) and bipolar disorder (all regions were significant at P ≤ 0.05). Orange areas represent significantly greater brain activation in ADHD subjects.
Although gender distribution was not significantly different among the groups, gender was further controlled for by inclusion as a covariate in the group analysis. None of the activation differences in Table 2 overlapped with brain regions associated with gender. Additional analyses were also performed in the bipolar subjects to determine if any of the brain regions activated during successful inhibition in bipolar subjects were correlated with mood state. YMRS and CDRS scores were used as covariates in a regression analysis performed in AFNI. Increased YMRS scores were associated with increased activation in the left parahippocampal gyrus, the right superior temporal gyrus (BA 22) and the right cerebellar tonsil. Increased CDRS scores were associated with decreased activation in the bilateral thalamus, right superior temporal lobe (BA 22) and right posterior cingulate gyrus.
DISCUSSION
To our knowledge, this is the first fMRI study to directly compare adolescents with ADHD with those with bipolar disorder. Adolescents with ADHD and bipolar disorder show unique alterations in brain activation during successful response inhibition despite a lack of differences in behavioural performance on this task. Consistent with our initial hypothesis, subjects with ADHD exhibited increased activation in the superior temporal lobe as compared with subjects with bipolar disorder and healthy subjects, and decreased anterior cingulate activation compared with healthy subjects. Contrary to our hypothesis, there were no differences in striatum or amygdala between the patient groups.
There was a sustained attention decrement over time as expected for CPT performance.34,35 There was also a high rate of successful ‘stops’, suggesting that each group inhibited motor responses after the stop signal as appropriate. All three groups showed relatively poor response rates to targets (blue-Xs), suggesting that they adopted a conservative strategy of withholding responses to achieve a high level of stop-signal performance. Group similarities on the signal detection measure of discriminability and bias further confirm that subjects in all three groups were using a similar conservative strategy, that is, delaying their responses to avoid incorrect response on the delayed red-X trials. Another possibility is that the subjects may have inconsistently attended to the task, leading to a low rate of successful target presses and a correspondingly high rate of apparent successful inhibition to the delayed red-X trails. In addition, there may have been some difficulty in clearly distinguishing the letters and colours inside the scanner. However, the fact that response times were longer than the presentation interval supports the first possibility. In addition, our previous research using a CPT task showed that bipolar and healthy subjects were able to consistently attend to the task.34,35 To further distinguish between these possibilities, behavioural performances were compared between the first and second half of the task. All three groups had significantly lower accuracy on blue-X trials (i.e. a sustained attention deficit) and significantly greater accuracy on delayed red-X trials the final 8 min. This suggests a practice effect, with subjects using a more conservative strategy as the task progressed and provides evidence that subjects were paying attention throughout the entire task.
The fMRI findings in subjects with ADHD were consistent with previous studies.
Altered activation in the anterior cingulate cortex (ACC) is a common finding in ADHD. The majority of prior studies (9 out of 13) showed decreased ACC activation compared with controls, consistent with the present findings.15 Increased brain activation in the superior temporal lobe (BA 39), a region important for the alerting and orienting components of attention,36,37 was also found in ADHD subjects. Altered activation in the superior temporal lobe is a common finding in ADHD, although most often, this region shows decreased activation compared with healthy subjects.15 However, these studies used a variety of tasks. When prior studies using response inhibition tasks are examined, ADHD subjects consistently show increased activation in the superior temporal lobe compared with healthy subjects.8,13,14 Subjects with bipolar disorder also showed increased activation in the superior temporal lobe (BA 39) compared with healthy subjects, but in the opposite hemisphere. Given the equal performance in all groups, increased temporal activation may have been needed in both patient groups to compensate for deficits in sustained attention during the CPT portion of the task. It is also possible that increased temporal activation leads to attentional problems. These possibilities could not be differentiated in the current study.
Although bipolar subjects did not show activation differences in the striatum or amygdala compared with ADHD subjects, increased left parahippocampal activation in the bipolar group was associated with increased YMRS scores. The amygdala/hippocampal complex has been hypothesized to regulate emotional homeostasis.19,38 Activation within the amygdala may increase with worsening mood symptoms and contribute to cognitive symptoms via reciprocal connections to the prefrontal regions. Further studies with larger number of subjects are needed to determine whether amygdala differences exist between the two patient groups.
Several limitations of this study should be considered when interpreting the results. First, the study is limited by the small sample and may not have had sufficient power to detect differences that might be present. Further studies with larger sample sizes are therefore needed. Second, it may be useful to use a more challenging cognitive task to be able to differentiate the patient groups from healthy subjects, although these creates a further confound of performance differences. Third, in the current study, all bipolar patients were manic so that the findings in subjects with bipolar disorder may reflect mood state effects related to mania rather than bipolar disorder per se. Further studies of subjects with bipolar disorder during depression and euthymia are needed to determine if these brain activation patterns are simply effects of mania. Another potential limitation was the poor performance on the task. This lowered the power of the study, and if this poor performance was related to lack of attention on the task, it may have also biased the neuroimaging results. Finally, while none of the subjects with ADHD were on medications at the time of the scan, eight of the subjects were taking stimulants daily. Although these medications were held the morning of the scan, 24 h off stimulants may not have been long enough to remove the acute effects of these medications on brain activation. Nevertheless, the current study is unique in comparing adolescent subjects with ADHD and bipolar disorder off medication and scanning both groups during a cognitive task. The results indicate there are unique alterations in brain activation patterns in the two patient groups. Therefore, the impulsive behaviour seen in both disorders may be the consequence of dysfunction in different brain regions.
ACKNOWLEDGEMENTS
Supported in part by NARSAD Young Investigator Award (DelBello) and MH63373.
Declaration of conflicts of interest:
Michael A. Cerullo, Martine Lamy, and David E. Fleck have no conflicts to disclose.
James C. Eliassen consulted on an fMRI task design and data analysis for Brown University from 2003-2008.
For Conflicts of interest for Stephen M. Strakowski, Caleb M. Adler and Melissa P. DelBello, refer to the following:
Calendar year 2008 to date
Stephen M. Strakowski, MD
Recent Research Support (grants to the UC Academic Health Center): Eli Lilly, Janssen, Pfizer, Forrest, AstraZeneca, Bristol-Myers Squibb, Martek Biosciences, Nutrition 21, Repligen, Johnson and Johnson, Shire, Somerset, NIDA, NIAAA, NARSAD, Thrasher Foundation.
Consultant: Pfizer (paid through Kendle).
Speaker’s bureaus: France Foundation (CME company).
The CME companies get support from different pharmaceutical companies that can and does change. I am aware of AstraZeneca support to the France Foundation.
Calendar year 2007
Stephen M. Strakowski, MD
Recent Research Support (grants to the UC Academic Health Center): Eli Lilly, Janssen, Pfizer, Forrest, AstraZeneca, Bristol-Myers Squibb, Martek Biosciences, Nutrition 21, Repligen, Shire, NIDA, NIAAA, NARSAD, Thrasher Foundation.
Consultant: Pfizer, Lilly, Tikvah.
Speaker’s bureaus: France foundation (CME company); DiMedix (CME company).
The CME companies get support from different pharmaceutical companies that can and does change. I am aware of AstraZeneca support to the France Foundation and Bristol-Myers Squibb support to DiMedix. I also gave grand rounds/CME talks at Dalhousie University, University of Michigan and the American Psychiatric Association annual meeting, in which I do not know the source of funding that they used.
Calendar year 2006
Stephen M. Strakowski, MD
Recent Research Support (grants to the UC Academic Health Center): Eli Lilly, Janssen, Pfizer, Forrest, AstraZeneca, Bristol-Myers Squibb, Martek Biosciences, Nutrition 21, Repligen.
Consultant: Kendle (for work chairing DSMB’s at Pfizer); AstraZeneca; Pfizer; Solvay.
Speaker’s bureaus: Peerview; Pharmanet; PIM; France Foundation; ConsumerMed; DLN.
The CME companies get support from different pharmaceutical companies that can and does change. I am aware of AstraZeneca support to the France Foundation and Bristol-Myers Squibb support to DiMedix. I am uncertain of the support to Peerview, Pharmanet, ConsumerMed and DLN. I also gave grand rounds/CME talks at MGH in Boston, Vanderbilt, Ohio State University, and LIJ on Long Island, in which I do not know the source of funding that they used.
Caleb M. Adler, MD
Research Support
Abbott Laboratories
AstraZeneca
Eli Lilly
Johnson and Johnson
Shire
Janssen
Pfizer
Bristol Myers Squibb
Repligen
Martek
Somerset
Lecture Bureau
AstraZeneca
Consulting
AstraZeneca
Although research support is difficult to quantify, I have received ~$50 000 from AstraZeneca over the last 12 months for consulting and honoraria.
Melissa DelBello, MD
Research Support
AstraZeneca
Eli Lilly
Johnson and Johnson
Shire
Janssen
Pfizer
Bristol Myers Squibb
Repligen
Martek
Somerset
NIDA
NIMH
NIAAA
NARSAD
Thrasher Foundation
Lecture Bureau
Bristol-Myers Squibb, AstraZeneca, France Foundation
Consulting/Advisory Board/Honoraria
AstraZeneca, GlaxoSmithKline, Eli Lilly, France Foundation, Kappa Clinical, NIDA, Pfizer, medical communications media/
Footnotes
The work was carried out at the University of Cincinnati Department of Psychiatry, 231 Albert Sabin Way (ML0559), Cincinnati, OH 45267-0559.
REFERENCES
- 1.Singh M, DelBello M, Kowatch R, Strakowski S. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord 2006; 8: 710–20. [DOI] [PubMed] [Google Scholar]
- 2.DelBello M, Soutullo C, Hendricks W, Niemeier R, McElroy S, Strakowski S. Prior Stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001; 3 (2): 53–7. [DOI] [PubMed] [Google Scholar]
- 3.Swann A, Pazzaglia P, Nicholls A, Dougherty D, Moeller F. Impulsivity and phase of illness in bipolar disorder. J Affect Disord 2003; 73: 105–11. [DOI] [PubMed] [Google Scholar]
- 4.Christodoulou T, Lewis M, Ploubidis G, Frangou S. The relationship of impulsivity to response inhibition and decision-making in remitted patients with bipolar disorder. Eur Psychiatry 2006; 21: 270–3. [DOI] [PubMed] [Google Scholar]
- 5.Swanson J Role of Executive Function in ADHD. J Clin Psychiatry 2003; 64 (Suppl. 14): 35–9. [PubMed] [Google Scholar]
- 6.Furman L What is attention-deficit hyperactivity disorder (ADHD)? J Child Neurol 2005; 20: 994–1002. [DOI] [PubMed] [Google Scholar]
- 7.Mayberg H, Liotti M, Brannan S et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 165: 675–82. [DOI] [PubMed] [Google Scholar]
- 8.Durston S, Tottenham N, Thomas K et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 2003; 53: 871–8. [DOI] [PubMed] [Google Scholar]
- 9.Bearden C, Hoffmann M, Cannon T. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord 2001; 3: 106–50. [DOI] [PubMed] [Google Scholar]
- 10.Rubia K, Oosterlaan J, Sergeant J, Brandeis D, Van Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behav Brain Res 1998; 94: 25–32. [DOI] [PubMed] [Google Scholar]
- 11.Barkley R Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121: 65–94. [DOI] [PubMed] [Google Scholar]
- 12.Leibenluft E, Rich B, Vinton D et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry 2007; 164 (52): 52–60. [DOI] [PubMed] [Google Scholar]
- 13.Tamm L, Memon V, Ringel J, Reiss A. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2004; 43: 1430–40. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya C, Bunge S, Dudukovic N, Zalecki C, Elliot G, Gabriele J. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry 2005; 162 (48): 1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paloyelis Y, Mehta M, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother 2007; 7: 1337–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler C, DelBello M, Mills N, Schmithorst V, Holland S, Strakowski S. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord 2006; 7: 577–88. [DOI] [PubMed] [Google Scholar]
- 17.Strakowski S, Adler C, Cerullo M et al. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Intervent Psychiatry 2008; 2: 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger A, Posner M. Pathologies of brain attentional networks. Neurosci Biobehav Rev 2000; 24 (1): 3–5. [DOI] [PubMed] [Google Scholar]
- 19.Strakowski S, DelBello M, Adler C. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry 2005; 10: 105–16. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg H, Martin A, Kaufman J et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 2003; 160: 1345–7. [DOI] [PubMed] [Google Scholar]
- 21.Chang K, Adleman N, Dienes K, Simeonova D, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Arch Gen Psychiatry 2004; 61: 781–92. [DOI] [PubMed] [Google Scholar]
- 22.Pavuluri M, O’Connor M, Harral E, Sweeney J. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 2007; 62: 158–67. [DOI] [PubMed] [Google Scholar]
- 23.Dickstein D, Rich B, Roberson-Nay R et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord 2007; 9: 679–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rich B, Vinton D, Roberson-Nay R et al. Limbic Hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA 2006; 103: 8900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–35. [DOI] [PubMed] [Google Scholar]
- 26.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord 1998; 51: 93–100. [DOI] [PubMed] [Google Scholar]
- 27.Poznanski E, Cook S, Carrol B. A depression rating scale for children. Pediatrics 1979; 64: 442–50. [PubMed] [Google Scholar]
- 28.Strakowski S, Williams J, Sax K, Fleck D, DelBello M, Bourne M. Is impaired outcome following a first manic episode due to mood-incongruent psychosis? J Affect Disord 2000; 61: 87–94. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput 1993; 25: 257–71. [Google Scholar]
- 30.Cox R AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 2007; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- 31.Cox R, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic resonance in medicine. 1999; 42: 1014–18. [DOI] [PubMed] [Google Scholar]
- 32.Xiong J, Gao J, Lancaster J, Fox P. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp 1995; 3: 287–301. [Google Scholar]
- 33.Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33: 636–47. [DOI] [PubMed] [Google Scholar]
- 34.Fleck D, Shear P, Strakowski S. Processing efficiency and sustained attention in bipolar disorder. JINS 2005; 11: 49–57. [DOI] [PubMed] [Google Scholar]
- 35.Fleck D, Sax K, Strakowski S. Reaction time measures of sustained attention differentiate bipolar disorder from schizophrenia. Schizophr Res 2001; 52: 251–9. [DOI] [PubMed] [Google Scholar]
- 36.Posner M, Sheese B, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Netw 2006; 19: 1422–9. [DOI] [PubMed] [Google Scholar]
- 37.Fan J, McCandliss B, Fossella J, Flombaum J, Posner M. The activation of attentional networks. NeuroImage 2005; 26: 471–9. [DOI] [PubMed] [Google Scholar]
- 38.Adolphs R Neural systems for recognizing emotion. Curr Opin Neurobiol 2002; 12: 169–77. [DOI] [PubMed] [Google Scholar]


