Graphical Abstract
Keywords: Food–drug interactions, Tomato juice, Grapefruit juice, Nifedipine, Midazolam, Pharmacokinetic interactions
Abbreviations: TJ, tomato juice; GFJ, grapefruit juice; NFP, nifedipine; MDZ, midazolam; AUC, area under the concentration–time curve; CYP, cytochrome P450; 9-oxo-ODA, 9-oxo-10,12-octadecadienoic acid; 13-oxo-ODA, 13-oxo-9,11-octadecadenoic acid
Abstract
We previously demonstrated that tomato juice (TJ) contains potent mechanism-based inhibitor(s) of CYP3A4. In this study, we investigated the effects of TJ and grapefruit juice (GFJ) on the pharmacokinetics of the CYP3A4-substrate drugs, nifedipine (NFP) and midazolam (MDZ), in male Wistar rats. Oral administration of GFJ 90 min before the intraduodenal administration of NFP or MDZ increased the area under the concentration–time curves (AUCs) of NFP and MDZ by 32.4% and 89.4%, respectively. TJ increased MDZ blood concentrations and AUC after intraduodenal MDZ administration; however, it had no effect on NFP. When MDZ and NFP were intravenously administered, GFJ significantly increased the AUC of MDZ, but only slightly increased that of NFP. In contrast, TJ only slightly increased the AUC of MDZ. These results suggest that, similar to GFJ, TJ influences the pharmacokinetics of CYP3A4-substrate drugs; however, it may be a drug-dependent partial effect.
1. Introduction
Many food and/or beverages have recently been found to influence drug metabolism and transport, sometimes resulting in clinically important drug interactions. These food–drug interactions are a critical aspect of pharmacotherapy, with potential impacts on the pharmacokinetics and pharmacodynamics of drugs. Pharmacokinetic interactions can involve enzymes and transporters that are involved in drug absorption, distribution, metabolism, and excretion. Pharmacodynamic interactions involve the pharmacological effects of a drug or the physiologic effect of a dietary constituent [1].
Foods and beverages that inhibit cytochrome P450 (CYP) drug metabolism enzymes can elevate the blood concentrations of co-administered drugs, resulting in food–drug interactions with potentially adverse effects [2], [3], [4]. In vitro screening assays that have evaluated various food and beverages, including beer, red wine, black and herbal teas, garlic, spices, mace, nutmeg, fruits, and fruit juices, have shown the ability of these to inhibit enzyme-mediated drug metabolism [5], [6], [7], [8], [9], [10]. In addition, several fruit juices have been reported to cause pharmacokinetic food–drug interactions in vivo, including grapefruit (GFJ) [11], [12], [13], [14], orange [12], star fruit [15], pomelo [16], cranberry [17], and pomegranate juice [18].
Recently, we reported that tomato juice (TJ) extract contains potent mechanism-based inhibitor(s) of CYP3A4 similar to GFJ [19]. However, whether TJ can alter the pharmacokinetic profile of CYP3A-substrate drugs remains unknown.
TJ is a very popular beverage; epidemiological studies have indicated that high consumption of tomato products is related to the reduced risk of prostate cancer [20], [21]. Lycopene, a major ingredient in tomato, shows promising anticancer effects, including antioxidant activity, inhibition of cell cycle progression, apoptosis induction, increased gap-junctional cell communication, inhibition of insulin-like growth factor I signal transduction, and inhibition of androgen activation and signaling [22], [23]. Furthermore, it has recently been reported that a conjugated linoleic acid (CLA) derivative, 9-oxo-10,12-octadecadienoic acid (9-oxo-ODA) [24], which is present in fresh tomato fruit, and 13-oxo-9,11-octadecadenoic acid (13-oxo-ODA) [25], which is an isomer of 9-oxo-ODA and present in only TJ but not in fresh tomato fruit, both serve as PPARa agonists. These reports suggest that 13-oxo-ODA is a more potent PPARa agonist than 9-oxo-ODA, and may improve obesity-induced dyslipidemia and hepatic steatosis.
To date, no studies have investigated whether TJ can cause adverse food–drug interactions in vivo. In this study, we evaluated the potential of TJ to influence the disposition of drugs that are metabolized by CYP3A4, such as nifedipine (NFP) and midazolam (MDZ).
2. Materials and methods
2.1. Materials
NFP was purchased from Sigma-Aldrich (MO, USA). MDZ was purchased from Wako Pure Chemical Ind., Ltd. (Osaka, Japan). Acetonitrile was purchased from Kanto Chemical (Tokyo, Japan) and was used for high performance liquid chromatography. All other reagents were of analytical grade.
2.2. Test samples
Additive-free TJ was purchased from Ito En Ltd. (Tokyo, Japan). Grapefruits (Citrus paradisi Macf.) were obtained from local commercial sources. Minced fresh grapefruit (without epicarp) was homogenized with an AM-8 homogenizer (Nihon Seiki Co., Ltd., Tokyo, Japan).
2.3. Animal experiments
Eight-week-old (180–200 g) male Wistar rats (Sankyo Labo Service Co., Tokyo, Japan) were given free access to water and a normal laboratory diet (MF, Oriental Yeast Co., Tokyo, Japan). All animals were acclimatized for 1 week and maintained in a room with controlled temperature (23 ± 3 °C), humidity (55 ± 10%), and a 12 h day/night cycle. All animal studies were performed in accordance with the “Standards Relating to the Care and Management of Experimental Animals” (Notice No. 6 of the Office of Prime Minister, dated March 27, 1980) and the guidelines of the Institutional Animal Care and Use Committee at the Josai University Life Science Center.
2.4. Pharmacokinetic studies
Rats were fasted overnight before experiments. For pharmacokinetic studies, rats were anesthetized with 20% urethane (1 g/kg body weight, intraperitoneally) 60 min after treatment with peroral administration (p.o.) of TJ, GFJ, or water. NFP (3 mg/kg/ml) or MDZ (20 mg/kg/ml) were then administered 30 min later via the duodenum or femoral vein. Blood samples (0.2 ml) were collected via the jugular vein at 2.5, 5, 10, 15, 30, 60, 90, 120, 180, and 240 min for intraduodenal administration (i.d.) or at 1, 5, 10, 30, 60, 90, 120, 180, and 240 min for intravenous administration (i.v.). Samples were immediately centrifuged at 15,000 rpm (4 °C) for 5 min, and plasma was separated. Plasma samples were stored at −40 °C until analysis.
2.5. HPLC analysis of NFP and MDZ
A 25-µl aliquot of each thawed plasma sample was transferred into a new tube with 50 µl acetonitrile containing internal standard (100 ng methylparaben for NFP, 100 ng diazepam for MDZ). After vigorous mixing, samples were centrifuged at 15,000 × g for 10 min at 4 °C. The supernatant (20 µl) was directly injected into the HPLC system, which consisted of a PU2089 pump, UV2075 UV absorbance detector, an AS2057 auto injector, and a ChromNAV system controller (JASCO). For NFP, the HPLC conditions included a Myghtysil RP-18GP column (5 µm, 4.6 mm × 250 mm; Kanto Chemical Co., Tokyo, Japan) connected to a precolumn (5 µm, 2 × 5 mm; Kanto Chemical Co, Tokyo, Japan), and eluted at a flow rate of 1.0 ml/min with a mobile phase of acetonitrile: 10 mM sodium phosphate [pH 6.1; 45:55 (v/v)]. Detection of NFP was performed by analyzing the UV absorbance at 236 nm. The retention time was 12.5 min for NFP and 5.4 min for methylparaben. For MDZ, the HPLC conditions included a Myghtysil RP-18GP column (5 µm, 4.6 mm × 150 mm; Kanto Chemical Co., Tokyo, Japan) connected to a precolumn (5 µm, 2 mm × 5 mm; Kanto Chemical Co, Tokyo, Japan), and eluted at a flow rate of 1.0 ml/min with a mobile phase of acetonitrile:10 mM sodium acetate [pH 4.7; 45:55 (v/v)]. Detection was performed by analyzing the UV absorbance at 220 nm. The retention time was 6.5 min for MDZ and 9.4 min for diazepam.
2.6. Data analysis
The blood concentration–time profile data (0–4 h) from each rat was analyzed by a model-independent method using the MULTI computer program [26]. We obtained following parameters: peak time (Tmax), maximum concentration (Cmax), elimination rate constant (kel), area under the curve (AUC), mean residence time from zero to infinity (MRT), elimination half-life (t1/2), total clearance (CLtot), and volume of distribution (Vd). The oral bioavailability (F) was calculated using AUCp.o./AUCi.v.. Statistical differences between the treatment and control groups were evaluated using Dunnett's test; a P value of <0.05 was considered significant.
3. Results and discussion
We have previously found that TJ extract contains potent mechanism-based inhibitor(s) of CYP3A4 similar to those of GFJ [19]. This study investigated whether TJ affects CYP3A4-substrate drug disposition in vivo. We employed two CYP3A4 substrate drugs NFP and MDZ to judge whether TJ influenced the pharmacokinetics of CYP3A4-substrate drugs compared with that of water, and the results were compared to those of GFJ.
3.1. Dosage of NFP, MDZ, and test samples
The doses of NFP [14], [27] and MDZ [28], [29] are similar to the doses used in previous studies on in vivo pharmacokinetics in rats. These doses can sufficiently detect plasma drugs by the HPLC method. In addition, we used 5 ml/kg dose of TJ, GFJ, and water in this study. This dose was appropriate as it corresponds to an intake of 300–400 ml for persons weighing 60–80 kg.
3.2. Effects of GFJ on intraduodenally administered NFP and MDZ pharmacokinetics
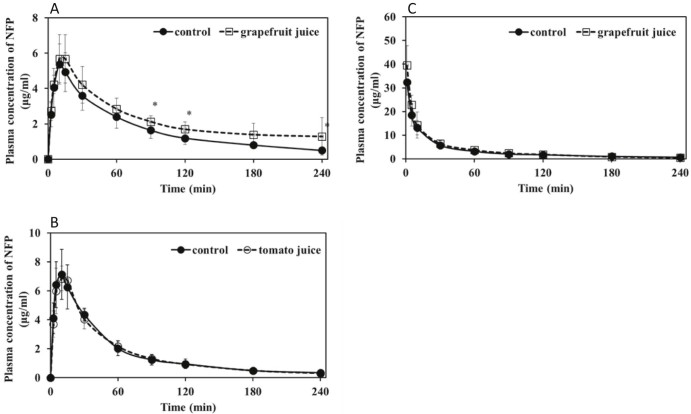
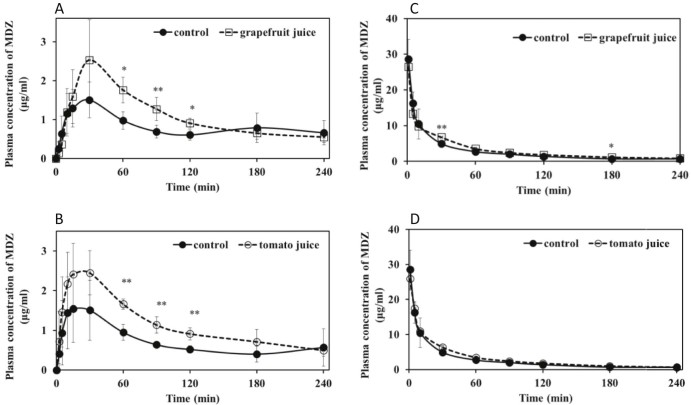
When GFJ (5 ml/kg) was orally administered 90 min before intraduodenal administration of NFP (3 mg/kg) or MDZ (20 mg/kg), the area under the concentration–time curve (AUC) of NFP (Fig. 1A and Table 1) and MDZ (Fig. 2A and Table 2) increased by 30.3% and 58.1%, respectively, compared with that of water. Consequently, GFJ significantly increased both the blood concentrations and AUC of intraduodenally administered NFP and MDZ. These findings were identical to those of previous reports [14], [30].
Fig. 1.
Plasma concentration–time profiles of rats treated with 3 mg/kg nifedipine 90 min after a single exposure to TJ, GFJ, or water (5 ml/kg, p.o.). A and B, intraduodenal administration; C, intravenous administration. Each point and bar represents the mean and SD of five or six rats. *P < 0.05 compared to control values.
Table 1.
Pharmacokinetic parameters of intraduodenally and intravenously administered NFP after pre-treatment GFJ or water.
| I.D. administration | I.V. administration | |||
|---|---|---|---|---|
| Control | GFJ | Control | GFJ | |
| Tmax (h) | 0.17 ± 0.02 | 0.20 ± 0.04 | - | - |
| Cmax (µg/ml) | 5.30 ± 1.14 | 5.92 ± 1.48 | - | - |
| Kel (h−1) | 0.50 ± 0.11 | 0.42 ± 0.20 | 0.57 ± 0.25 | 0.58 ± 0.07 |
| AUC (µg/ml ⋅ h) | 6.87 ± 1.61 | 8.95 ± 1.65a | 11.6 ± 0.76 | 13.7 ± 1.59 |
| MRT (h) | 1.27 ± 0.07 | 1.37 ± 0.36 | 0.91 ± 0.18 | 0.76 ± 0.22 |
| t1/2 (h) | 1.44 ± 0.31 | 1.95 ± 1.05 | 1.35 ± 0.58 | 1.21 ± 0.16 |
| CLtot (ml/h/kg) | 0.12 ± 0.02 | 0.09 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.02 |
| Vd (ml/kg) | 0.21 ± 0.02 | 0.21 ± 0.04 | 0.09 ± 0.20 | 0.08 ± 0.02 |
| F (%) | 59.2 | 65.3 | ― | ― |
Data represent means ± SD (n = 5–6).
P < 0.05 compared to control values.
Fig. 2.
Plasma concentration–time profiles of rats treated with 20 mg/kg midazolam 90 min after a single exposure to TJ, GFJ, or water (5 ml/kg, p.o.). A and B, intraduodenal administration; C and D, intravenous administration. Each point and bar represents the mean and SD of five or six rats. **P < 0.01, *P < 0.05 compared to control values.
Table 2.
Pharmacokinetic parameters of intraduodenally and intravenously administered MDZ after pre-treatment with GFJ or water.
| I.D. administration | I.V. administration | |||
|---|---|---|---|---|
| Control | GFJ | Control | GFJ | |
| Tmax (h) | 0.49 ± 0.21 | 0.50 ± 0.00 | - | - |
| Cmax (µg/ml) | 1.66 ± 0.64 | 2.53 ± 1.04 | - | - |
| Kel (h−1) | 0.44 ± 0.23 | 0.32 ± 0.14 | 0.53 ± 0.29 | 0.44 ± 0.07 |
| AUC (µg/ml⋅h) | 2.72 ± 0.79 | 4.30 ± 1.25a | 10.5 ± 2.80 | 13.5 ± 1.31a |
| MRT (h) | 1.34 ± 0.28 | 1.56 ± 0.08 | 0.85 ± 0.13 | 0.99 ± 0.09 |
| t1/2 (h) | 2.35 ± 1.41 | 2.13 ± 0.64 | 1.52 ± 0.74 | 1.61 ± 0.27 |
| CLtot (ml/h/kg) | 0.20 ± 0.08 | 0.15 ± 0.07 | 0.09 ± 0.02 | 0.07 ± 0.007a |
| Vd (ml/kg) | 0.61 ± 0.36 | 0.61 ± 0.31 | 0.13 ± 0.25 | 0.03 ± 0.009 |
| F (%) | 26.0 | 31.8 | ― | ― |
Data represent means ± SD (n = 5–6).
P < 0.05 compared to control values.
3.3. Effects of TJ on intraduodenally administered NFP and MDZ pharmacokinetics
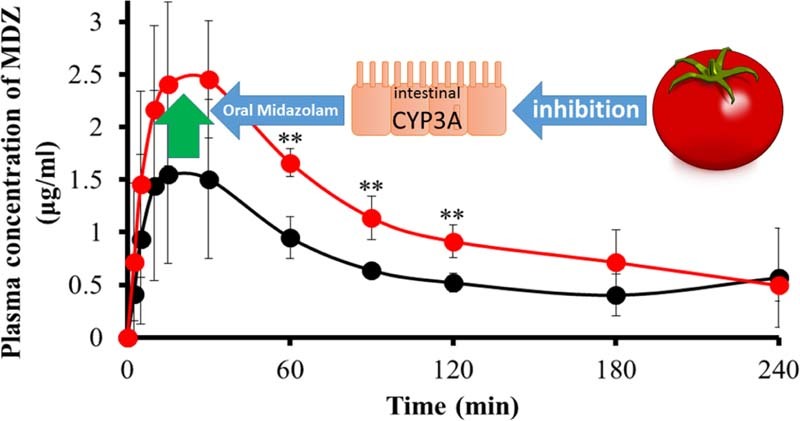
In contrast, TJ increased the MDZ blood concentrations and the AUC by 73.0% (Fig. 2B and Table 4) compared with water but did not influence NFP blood concentration and parameters (Fig. 1B and Table 3). These results demonstrate that TJ has the potential to influence the pharmacokinetics of some CYP3A4-substrate drugs in rats, similar to the findings for GFJ.
Table 4.
Pharmacokinetic parameters of intraduodenally and intravenously administered MDZ after pre-treatment with TJ or water.
| I.D. administration | I.V. administration | |||
|---|---|---|---|---|
| Control | TJ | Control | TJ | |
| Tmax (h) | 0.43 ± 0.13 | 0.40 ± 0.34 | - | - |
| Cmax (µg/ml) | 1.70 ± 0.67 | 2.76 ± 0.96 | - | - |
| Kel (h−1) | 0.46 ± 0.07 | 0.38 ± 0.34 | 0.53 ± 0.29 | 0.56 ± 0.11 |
| AUC (µg/ml⋅h) | 2.74 ± 1.05 | 4.74 ± 0.29a | 10.5 ± 2.80 | 12.8 ± 1.21 |
| MRT (h) | 1.44 ± 0.18 | 1.35 ± 0.42 | 0.85 ± 0.13 | 0.98 ± 0.04 |
| t1/2 (h) | 2.56 ± 0.34 | 1.86 ± 1.99 | 1.52 ± 0.74 | 1.29 ± 0.34 |
| CLtot (ml/h/kg) | 0.21 ± 0.04 | 0.17 ± 0.11 | 0.09 ± 0.02 | 0.07 ± 0.009 |
| Vd (ml/kg) | 0.68 ± 0.11 | 0.42 ± 0.49 | 0.13 ± 0.2 | 0.09 ± 0.004 |
| F (%) | 26.1 | 37.0 | ― | ― |
Data represent means ± SD (n = 5–6).
P < 0.01 compared to control values.
Table 3.
Pharmacokinetic parameters of intraduodenally administered NFP after pre-treatment with TJ or water.
| I.D. administration | ||
|---|---|---|
| Control | TJ | |
| Tmax (h) | 0.17 ± 0.04 | 0.17 ± 0.04 |
| Cmax (µg/ml) | 7.21 ± 1.65 | 6.97 ± 0.83 |
| Kel (h−1) | 0.62 ± 0.14 | 0.59 ± 0.07 |
| AUC (µg/ml⋅h) | 6.64 ± 1.68 | 6.72 ± 0.92 |
| MRT (h) | 0.95 ± 0.15 | 0.99 ± 0.07 |
| t1/2 (h) | 1.16 ± 0.23 | 1.18 ± 0.13 |
| CLtot (ml/h/kg) | 0.15 ± 0.04 | 0.14 ± 0.02 |
| Vd (ml/kg) | 0.17 ± 0.02 | 0.17 ± 0.02 |
Data represent means ± SD (n = 5–6).
However, TJ did not influence the pharmacokinetics of NFP and MDZ to the same extent as GFJ did. The reasons for this are not clear; however, differences in oral bioavailability (Table 1, Table 2, Table 4) of NFP (59.2%) and MDZ (26.0%–26.1%) may be a contributing factor. It has previously been reported that single-strength GFJ significantly increases the plasma concentrations of NFP in rats [14]. On the other hand, there is a report that while concentrated GFJ (2 × concentrations) significantly increases NFP bioavailability in rats, regular strength GFJ has no significant effect on NFP bioavailability [31]. Furthermore, it has been reported that pre-dosing with the GFJ constituent bergamottin increases the plasma level of diazepam, a substrate of CYP3A4 and CYP2C9 in male beagle dogs, but not in rats, indicating that the rat may not be an appropriate model [32]. In this study, these factors may have contributed to our findings that TJ did not influence NFP disposition. However, more research should be warranted.
The clinical significance of CYP3A4 inhibition by TJ is not well understood. In one study in which TJ was used as a ‘vehicle’, it was reported that TJ does not influence the pharmacokinetics of the proton pump inhibitor lansoprazole [33]. However, this is likely due to the fact that the study was not specifically designed to evaluate the inhibitory effect of TJ on CYP3A. In addition, lansoprazole is not an established CYP3A probe substrate, and the tomato juice product used was not characterized prior to use. The possibility of a food–drug interaction between tomato juice and appropriate CYP3A substrates needs further examination in clinical studies.
3.4. Effects of TJ and GFJ on intravenously administered NFP and MDZ pharmacokinetics
Another interesting result of our study was that when GFJ (5 ml/kg) was orally administered 90 min before the intravenous administration of MDZ (20 mg/kg), GFJ significantly increased the AUC of MDZ by 28.6% and that the systemic clearance (CLtot) of MDZ decreased by 25.0% compared with the oral administration of water (Fig. 2C and Table 2), but had no effect on NFP (Fig. 1C and Table 1). In comparison, TJ slightly increased the AUC (22.0%) of intravenously administered MDZ (Fig. 2D and Table 4); however, this effect was not significant. In the case of TJ, pharmacokinetic study of NFP by intravenous injection was not conducted because there was no difference in the elimination process in duodenal administration. However, these results demonstrate the possibility that GFJ and possibly TJ influenced not only the bioavailability but also the elimination of these drugs (i.e., GFJ and TJ inhibit not only intestinal but also hepatic drug metabolism). On the other hand, several studies have suggested that GFJ selectively inhibits intestinal CYP3A4, as opposed to hepatic CYP3A4 [34], [35], [36]. Thus, GFJ reportedly did not alter the pharmacokinetics of CYP3A4 substrates administered intravenously [30], [37], and little change in the elimination half-life was observed when the drugs were administered orally [37], [38]. On the other hand, it has been reported that the consumption of one glass of double-strength GFJ three times a day for 3 d significantly increases the AUC, Cmax, and t1/2 of MDZ and reduces the erythromycin breath test value [39]. These observations indicate that high doses of GFJ may inhibit both intestinal and hepatic CYP3A4 in vivo. In this study, appropriate experimental techniques and selection of MDZ as CYP3A substrate drug may have obtained high precision results. Further investigation is required to clarify whether GFJ and TJ influence the liver drug-metabolizing enzymes.
4. Conclusions
TJ increased MDZ blood concentrations and the AUC after intraduodenal MDZ administration similar to GFJ. Although it remains unclear why TJ did not influence NFP disposition in rats, it cannot be denied that TJ influences the pharmacokinetics of other CYP3A4 substrates in humans. In future studies, factors underlying the variability of TJ in influencing the pharmacokinetics of CYP3A4 substrates should be investigated, particularly for humans in order to perform inter-species comparisons.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Boullata J.I. An introduction to drug-nutrient interactions. In: Boullata J.I., Armenti V.T., editors. Handbook of drug-nutrient interactions. Humana Press; New York: 2010. pp. 3–26. [Google Scholar]
- 2.Spence J.D. Drug interactions with grapefruit: whose responsibility is it to warn the public? Clin Pharmacol Ther. 1997;61:395–400. doi: 10.1016/S0009-9236(97)90189-2. [DOI] [PubMed] [Google Scholar]
- 3.Dresser G.K., Bailey D.G., Carruthers S.G. Grapefruit juice–felodipine interaction in the elderly. Clin Pharmacol Ther. 2000;68:28–34. doi: 10.1067/mcp.2000.107524. [DOI] [PubMed] [Google Scholar]
- 4.Dreier J.P., Endres M. Statin-associated rhabdomyolysis triggered by grapefruit consumption. Neurology. 2004;62:670. doi: 10.1212/wnl.62.4.670. [DOI] [PubMed] [Google Scholar]
- 5.Foster B.C., Vandenhoek S., Hana J. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine. 2003;10:334–342. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 6.Sand P.G., Dreiseitel A., Stang M. Cytochrome P450 2C19 inhibitory activity of common berry constituents. Phytother Res. 2010;24:304–307. doi: 10.1002/ptr.2910. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y., Ito H., Hatano T. Effects of mace and nutmeg on human cytochrome P450 3A4 and 2C9 activity. Biol Pharm Bull. 2010;33:1977–1982. doi: 10.1248/bpb.33.1977. [DOI] [PubMed] [Google Scholar]
- 8.Fujita T., Kawase A., Niwa T. Comparative evaluation of 12 immature citrus fruit extracts for the inhibition of cytochrome P450 isoform activities. Biol Pharm Bull. 2008;31:925–930. doi: 10.1248/bpb.31.925. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald L., Foster B.C., Akhtar H. Food and therapeutic product interactions – a therapeutic perspective. J Pharm Pharm Sci. 2009;12:367–377. doi: 10.18433/j30p4c. [DOI] [PubMed] [Google Scholar]
- 10.Hidaka M., Fujita K., Ogikubo T. Potent inhibition by star fruit of human cytochrome P450 3A (CYP3A) activity. Drug Metab Dispos. 2004;32:581–583. doi: 10.1124/dmd.32.6.581. [DOI] [PubMed] [Google Scholar]
- 11.Bailey D.G., Malcolm J., Arnold O. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998;46:101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Marco M.P., Edwards D.J., Wainer I.W. The effect of grapefruit juice and seville orange juice on the pharmacokinetics of dextromethorphan: the role of gut CYP3A and P-glycoprotein. Life Sci. 2002;71:1149–1160. doi: 10.1016/s0024-3205(02)01799-x. [DOI] [PubMed] [Google Scholar]
- 13.Bailey D.G., Dresser G.K. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4:281–297. doi: 10.2165/00129784-200404050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mohri K., Uesawa Y. Effects of furanocoumarin derivatives in grapefruit juice on nifedipine pharmacokinetics in rats. Pharm Res. 2001;18:177–182. doi: 10.1023/a:1011028401189. [DOI] [PubMed] [Google Scholar]
- 15.Hidaka M., Okumura M., Ogikubo T. Transient inhibition of cyp3a in rats by star fruit juice. Drug Metab Dispos. 2006;34:343–345. doi: 10.1124/dmd.105.006486. [DOI] [PubMed] [Google Scholar]
- 16.Grenier J., Fradette C., Morelli G. Pomelo juice, but not cranberry juice, affects the pharmacokinetics of cyclosporine in humans. Clin Pharmacol Ther. 2006;79:255–262. doi: 10.1016/j.clpt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Uesawa Y., Mohri K. Effects of cranberry juice on nifedipine pharmacokinetics in rats. J Pharm Pharmacol. 2006;58:1067–1072. doi: 10.1211/jpp.58.8.0007. [DOI] [PubMed] [Google Scholar]
- 18.Voruganti S., Yamsani S.K., Ravula S.K. Effect of pomegranate juice on intestinal transport and pharmacokinetics of nitrendipine in rats. Phytother Res. 2012;26:1240–1245. doi: 10.1002/ptr.3704. [DOI] [PubMed] [Google Scholar]
- 19.Sunaga K., Ohkawa K., Nakamura K. Mechanism-based inhibition of recombinant human cytochrome P450 3A4 by tomato juice extract. Biol Pharm Bull. 2012;35:329–334. doi: 10.1248/bpb.35.329. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E., Rimm E.B., Liu Y. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 21.Etminan M., Takkouche B., Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–345. [PubMed] [Google Scholar]
- 22.Sharoni Y., Danilenko M., Dubi N. Carotenoids and transcription. Arch Biochem Biophys. 2004;430:89–96. doi: 10.1016/j.abb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Wertz K., Siler U., Goralczyk R. Lycopene: modes of action to promote prostate health. Arch Biochem Biophys. 2004;430:127–134. doi: 10.1016/j.abb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.I., Hirai S., Takahashi H. 9-oxo-10(E),12(E)-Octadecadienoic acid derived from tomato is a potent PPAR alpha agonist to decrease triglyceride accumulation in mouse primary hepatocytes. Mol Nutr Food Res. 2011;55:585–593. doi: 10.1002/mnfr.201000264. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.I., Hirai S., Goto T. Potent PPARalpha activator derived from tomato juice, 13-oxo-9,11-octadecadienoic acid, decreases plasma and hepatic triglyceride in obese diabetic mice. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0031317. e31317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaoka K., Nakagawa T.A. nonlinear least squares program based on differential equations, MULTI (RUNGE), for microcomputers. J Pharmacobiodyn. 1983;6:595–606. doi: 10.1248/bpb1978.6.595. [DOI] [PubMed] [Google Scholar]
- 27.Mohri K., Uesawa Y., Sagawa K. Effects of long-term grapefruit juice ingestion on nifedipine pharmacokinetics: induction of rat hepatic P-450 by grapefruit juice. Drug Metab Dispos. 2000;28:482–486. [PubMed] [Google Scholar]
- 28.Zhang W., Tan T.M., Lim L.Y. Impact of curcumin-induced changes in P-glycoprotein and CYP3A expression on the pharmacokinetics of peroral celiprolol and midazolam in rats. Drug Metab Dispos. 2007;35:110–115. doi: 10.1124/dmd.106.011072. [DOI] [PubMed] [Google Scholar]
- 29.Li W.L., Xin H.W., Su M.W. Inhibitory effects of continuous ingestion of Schisandrin A on CYP3A in the rat. Basic Clin Pharmacol Toxicol. 2012;110:187–192. doi: 10.1111/j.1742-7843.2011.00787.x. [DOI] [PubMed] [Google Scholar]
- 30.Kupferschmidt H.H., Ha H.R., Ziegler W.H. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58:20–28. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 31.Grundy J.S., Eliot L.A., Kulmatycki K.M. Grapefruit juice and orange juice effects on the bioavailability of nifedipine in the rat. Biopharm Drug Dispos. 1998;19:175–183. doi: 10.1002/(sici)1099-081x(199804)19:3<175::aid-bdd85>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Sahi J., Reyner E.L., Bauman J.N. The effect of bergamottin on diazepam plasma levels and P450 enzymes in beagle dogs. Drug Metab Dispos. 2002;30:135–140. doi: 10.1124/dmd.30.2.135. [DOI] [PubMed] [Google Scholar]
- 33.Chun A.H., Erdman K., Chiu Y.L. Bioavailability of lansoprazole granules administered in juice or soft food compared with the intact capsule formulation. Clin Ther. 2002;24:1322–1331. doi: 10.1016/s0149-2918(02)80036-4. [DOI] [PubMed] [Google Scholar]
- 34.Lown K.S., Bailey D.G., Fontana R.J. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C.Y., Benet L.Z., Hebert M.F. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: studies with cyclosporine. Clin Pharmacol Ther. 1995;58:492–497. doi: 10.1016/0009-9236(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 36.Ameer B., Weintraub R.A. Drug interactions with grapefruit juice. Clin Pharmacokinet. 1997;33:103–121. doi: 10.2165/00003088-199733020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Ducharme M.P., Warbasse L.H., Edwards D.J. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther. 1995;57:485–491. doi: 10.1016/0009-9236(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 38.Bailey D.G., Arnold J.M., Bend J.R. Grapefruit juice-felodipine interaction: reproducibility and characterization with the extended release drug formulation. Br J Clin Pharmacol. 1995;40:135–140. [PMC free article] [PubMed] [Google Scholar]
- 39.Veronese M.L., Gillen L.P., Burke J.P. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol. 2003;43:831–839. doi: 10.1177/0091270003256059. [DOI] [PubMed] [Google Scholar]