Graphical Abstract
For improving the utilization of NK, a complex of NK-TUDCA to reduce the toxicity of intravenous NK injection, was designed by using NK as the cationic moiety and TUDCA as the anionic moiety. The NK-TUDCA reduced toxicity by the delayed release of NK from the complex and still possessed the inherent powerful efficiency of NK.
Keywords: Nattokinase, Tauroursodeoxycholate, Complex, Toxicity test, In vitro thrombolytic test
Abstract
Nattokinase (NK), which has been identified as a potent fibrinolytic protease, has remarkable potential in treatment of thrombolysis, and even has the ability to ameliorate chronic vein thrombosis. To reduce the hemorrhagic risk from an intravenous injection of NK, nattokinase-tauroursodeoxycholate (NK-TUDCA) complex was prepared at different pH values and with different ratios of NK and TUDCA. When assessing survival time, survival state, tail injury, and the body weight of mice, it was found that the NK-TUDCA complex (NK: 10 kIU/ml; TUDCA: 10 mg/ml; pH 5.0) had a lower toxicity when administered at an NK dosage of 130 kIU/kg in the acute toxicity test and 13 kIU/kg in the repeated low-dose challenge. From the results of the in vitro thrombolytic test and characterization of NK-TUDCA, we speculated that the delayed release of NK-TUDCA might be the main cause of toxicity reduction by the complex. This study described the preparation of an NK complex with low toxicity following intravenous administration, which could be utilized for further clinical study of NK.
1. Introduction
In recent years, cardiovascular diseases (CVDs) have been a major cause of death worldwide. Vein thromboses are one kind of CVDs, which inevitably lead to a high death rate owing to their origin from the disorders of the heart and blood vessels. For example, deep vein thromboses and pulmonary embolisms can be dislodged and move to the heart and lungs, which present a danger to health. It has been reported that thrombotic embolisms are responsible for the death of more than 26 million people worldwide annually [1]. In addition, the death rate may rise in future because of the increasingly unhealthy eating and living habits. Therefore, the discovery of effective treatments for thrombotic diseases is important to decrease the morbidity and mortality associated with thrombosis.
Currently, various agents have been used in the clinical treatment of thrombosis, including urokinase, streptokinase, tissue plasminogen activator (t-PA) [2], anisoylated plasminogen streptokinase activator complex (APSAC) [3], and single-chain urokinase-type plasminogen activator (SCUPA) [4]. However, all of these agents function by converting plasminogen to plasmin [3]; hence, they may be ineffective for the therapy of thrombosis that has developed beyond a certain extent, such as chronic vein thrombosis, which is associated with a lower plasminogen level than that reported for other common thromboses. Moreover, these thrombolytic agents exhibit many limitations in clinical applications, including a low specificity to fibrin [5], [6], severe toxic effects such as hemorrhage [4], [7], [8], and a short half-life in the body, which influences the fibrinolytic efficiency of the drug [9], [10], [11]. Additionally, these drugs are generally expensive because of the scarcity of the source [12], [13].
Nattokinase (NK, 27728 Da [14]), produced by Bacillus subtilis natto, has been identified as a polypeptidase with strong thrombolytic efficiency. It comprises 275 amino acid residues [15], which contain eight lysine residues and no disulfide bonds in the structure [16]. The thrombolytic mechanisms of NK have been studied clearly and the following steps were reported: (1) direct degradation of the cross-linked fibrin of blood clots [10], [17]; (2) conversion of pro-urokinase to urokinase [18]; (3) activation of vascular endothelial cells to produce t-PA [19], [20]; (4) degradation of plasminogen activator inhibitor-1 (PAI-1) and the consequent reduction in the hydrolysis of t-PA [21], [22]. Based on these mechanisms, it is clear that NK can not only activate the fibrinolytic system, but also act directly on cross-linked fibrin, which confers NK with remarkable thrombolytic efficiency and an excellent ability to consistently enhance fibrinolytic activity. It is important to note that owing to this property, NK may be effective in the treatment of certain types of thrombosis, such as chronic vein thrombosis, which do not respond to other thrombolytic agents. Moreover, other properties of NK are also advantageous for its potential use as a highly effective thrombolytic agent. First, it was reported that NK showed no genotoxicity in vitro or adverse effects in male and female SD rats at an oral dosage of 2000 mg/kg [23]; therefore, NK is a relatively safe proteinase with a strong foundation to support its clinical use in the future. Second, NK possessed properties that confer sustained efficiency in the body, including the resistance to trypsin hydrolysis, stabilization in the gastrointestinal tract [24], and a long half-life [25]. Third, owing to the possibility of a production method involving bacterial fermentation, NK is suitable for industrial production; moreover, the production process has a short production cycle, high yield, and low cost [13]. Therefore, it is clear that NK is an ideal novel biochemical agent for the prevention and treatment of thrombosis.
However, at present, the commercially available formulations of NK are predominantly oral preparations, which have many disadvantages. It has been reported that the gastrointestinal uptake of intact nattokinase is limited because of its large molecular mass and low absolute oral bioavailability (less than 1%) [23]; additionally, an increase in the serum activity of the intestinal isoform of alkaline phosphatase (ALP) was observed in rats that were fed NK. This might be attributable to the effect secondary to the test substance-related hepatobiliary effects, such as cholestasis or biliary hyperplasia [23]. Furthermore, oral administration is unsuitable in patients who are in a critical condition, coma, or have symptoms such as vomiting. To improve the utilization of NK, an injectable formulation is necessary. Additionally, although NK is relatively safe, there is a risk of hemorrhage following administration via intravenous injection, owing to the stronger thrombolytic activity of NK than that of other fibrinolytic agents. In summary, the development of an injectable formulation of NK with a relative high thrombolytic efficiency, but low toxicity, is required.
NK is as an alkaline protease with multiple positively charged amino acid residues on its surface [16] and an isoelectric point (pI) of 8.6 ± 0.3 [26], [27]. We aimed to exploit these characteristics, by the preparation of NK in a complex with an anionic substance, to obtain a formulation with lower toxicity and a higher thrombolytic efficiency.
Bile salts exist mostly in the form of glyco- or tauro-conjugates in vivo [28]. As the main components of bile, they perform important roles in the digestive system, have multiple functions in the emulsification and solubilization of lipids that promote the digestion and absorption of fat, affect phospholipids and proteins of cell membranes, and disrupt cellular homeostasis to resist aggression from pathogens and commensals [29]. Among bile salts, the tauro-conjugates, which predominantly comprise sodium tauroursodeoxycholate (TUDCA), sodium taurocholate (TCA), sodium taurochenodeoxycholate (TCDCA) and sodium taurodeoxycholate (TDCA) [30], contain many sulfonate groups [30] and are regarded as promising candidates for complex formation with NK. Of these, TUDCA shows higher safety and better effect. Narain et al. reported that TUDCA had a lower hemolytic risk than TCA, TCDCA, and TDCA did [31]. From a pharmacological perspective, TUDCA serves as the main conjugate of ursodeoxycholic acid (UDCA), has beneficial effects against cholestasis and liver dysfunction [32], [33], and has demonstrated potent clinical efficacy as a choleretic (marketed as Taurolite® in Italy) to treat patients with biliary atresia [32], [34]. Furthermore, TUDCA demonstrated improved efficacy compared with UDCA in the treatment of cholestasis and primary biliary cirrhosis [34], [35], [36].
As the structure of NK possesses an abundance of positive charges, we attempted to prepare a complex of NK with reduced toxicity, in which the NK was the cationic moiety of the complex when the pH value of the solution was below its pI value (8.6 ± 0.3) [26], [27]. TUDCA was selected as the anionic moiety for conjugation with NK because it has negative charges at pH values above its pKa (<2) [37]. In particular, the addition of TUDCA might contribute to a solution of the problem of cholestasis or biliary cirrhosis that occurred after oral NK administration [23], although it was not clear if this problem was caused by NK, or the oral administration of NK, or both factors. Therefore, it was feasible to form a novel complex of NK in which the toxicity of NK was reduced by the addition of TUDCA in order to achieve better treatment of thrombosis. Acute toxicity analysis and repeated low-dose challenge were performed to evaluate the toxicity of NK-TUDCA; an in vitro thrombolytic test was used to verify the thrombolytic efficiency and uncover the mechanism responsible for toxicity reduction.
2. Materials and methods
2.1. Materials
NK (17.9 kIU/ml) was provided by Sungen Biotech Co., Ltd. (Guangdong, China). TUDCA was purchased from Aike Reagent Co., Ltd. (Sichuan, China). The 5% glucose injection (5% Glu) was from Cisen Pharmaceutical Co., Ltd. (Shandong, China) and the 50% glucose injection (50% Glu) was from Tianjin Pharmaceutical Group Xinzheng Co., Ltd. (Tianjin, China). Trisodium phosphate (Na3PO4 ⋅ 12H2O) was provided by Sinopharm Chemical Reagent Co., Ltd. (Beijing, China), and sodium dihydrogen phosphate (NaH2PO4 ⋅ 2H2O) was purchased from Xilong Chemical Co., Ltd. (Guangdong, China).
2.2. Animals
Male Kunming mice (weight: 18–22 g) and male New Zealand white rabbits (weight: 2.0–2.5 kg) were purchased from the Experimental Animal Center of Shenyang Pharmaceutical University (Liaoning, China). All mice and rabbits were given free access to food and water and all animal experiments were in compliance with the guidelines for the Care and Use of Laboratory Animals at Shenyang Pharmaceutical University.
2.3. Preparation of solutions
2.3.1. Preparation of NK and TUDCA solutions
NK solution (2.8 ml; 17.9 kIU/ml) and TUDCA (500.00 mg) were added to volumetric flasks which were with filled with an appropriate volume of sterile water in advance. The pH of the solutions was adjusted by the addition of NaH2PO4 solution (833 mM) or Na3PO4 solution (158 mM). These solutions were diluted with 0.5 ml 50% Glu and sterile water to a total final volume of 5 ml and mixed for 2 min. The solutions were filtered through a 0.22 µm membrane filter to obtain solutions of NK (10 kIU/ml) or TUDCA (100 mg/ml) with different pH values (5.0, 6.0, 7.0, and 8.0). In addition, solutions of NK (500 IU/ml, pH 5.0) and TUDCA (5.00 mg/ml, pH 5.0) were obtained by further dilution. Moreover, a TUDCA solution (100 mg/ml) or NK solution (10 kIU/ml) without pH adjustment was prepared by the same method but without the addition of the compounds for pH adjustment.
2.3.2. Preparation of NK-TUDCA solution
TUDCA (17.90 mg) was added into NK solution (1 ml; 17.9 kIU/ml) and mixed for 2 min. The solutions were adjusted to different pH values (5.0, 6.0, 7.0, and 8.0) by the addition of NaH2PO4 solution (833 mM) or Na3PO4 solution (158 mM). The solutions with different pH values were diluted with 180 µl 50% Glu and sterile water to a total final volume of 1.79 ml and mixed for a further 2 min. The solutions of NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml) at different pH values (5.0, 6.0, 7.0, and 8.0) were obtained after filtration through a 0.22-µm membrane filter.
The NK-TUDCA solutions (NK: 10 kIU/ml, TUDCA: 1, 5, 15 mg/ml; pH 5.0) were obtained using the method described and by adjusting the added amount of TUDCA to 1.79, 8.95, and 26.85 mg. Additionally, these solutions were subjected to a 20-fold dilution to obtain NK-TUDCA solutions (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0).
2.3.3. Preparation of mixed solution of NK and TUDCA
First, the NK solution (NK: 1 kIU/ml, pH 5.0) and the TUDCA solutions (0.10, 0.50, 1.00, 1.50 mg/ml, pH 5.0) were prepared by the dilution of NK solution (NK: 10 kIU/ml, pH 5.0) and TUDCA solution (100 mg/ml, pH 5.0) with 5% Glu, respectively. Then, an equal volume of NK solution (NK: 1 kIU/ml, pH 5.0) and TUDCA (0.10, 0.50, 1.00, 1.50 mg/ml, pH 5.0) were mixed together for 2 min. Finally, the mixtures were filtered through a 0.22-µm membrane filter to obtain the mixed solutions of NK and TUDCA (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.5, 0.75 mg/ml; pH 5.0).
2.4. Acute toxicity of NK and TUDCA
2.4.1. NK toxicity with different concentrations
Male Kunming mice were randomly divided into five groups of three animals each and injected with NK solution (100 mg/ml) via the tail veins at dosages of 150, 130, 100, 80, 650, 40 kIU/kg. During the experimental period, the survival time, survival status, and tail injury of surviving mice at 48 h after injection were recorded to assess the toxicity of NK solution.
2.4.2. TUDCA toxicity with different concentrations
Male Kunming mice were randomly divided into six groups of three animals each and injected with TUDCA solution (100 mg/ml) via the tail veins at dosages of 1300, 1040, 910, 780, 650, 260 mg/kg. The toxicity of TUDCA was recorded similarly as described above, to assess the toxicity of the TUDCA solution.
2.5. Acute toxicity of NK-TUDCA
2.5.1. NK-TUDCA toxicity at different pH values
Male Kunming mice were randomly divided into eight groups of three animals each and injected with NK solutions (10 kIU/ml, pH 5.0, 6.0, 7.0, 8.0) and NK-TUDCA solutions (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0, 6.0, 7.0, 8.0) via the tail veins at a dosage of 130 kIU/kg NK. The toxicity of NK-TUDCA solution at different pH values was recorded as described in section 2.4.
2.5.2. NK-TUDCA toxicity with different concentrations of TUDCA
Male Kunming mice were randomly divided into five groups of three animals each and injected with NK solution (10 kIU/ml, pH 5.0) and NK-TUDCA solutions (NK: 10 kIU/ml, TUDCA: 1, 5, 10, and 15 mg/ml; pH 5.0) via the tail vein at an NK dosage of 130 kIU/kg. The toxicity of NK-TUDCA solutions at different concentrations of TUDCA was recorded as described in section 2.4.
2.6. Repeated-dosage toxicity of NK-TUDCA
Male Kunming mice were divided into three groups of six animals each and injected with the solutions of NK (13 kIU/kg, pH 5.0) and NK-TUDCA (NK: 13 kIU/kg, TUDCA: 10 mg/ml; pH 5.0) diluted in 5% Glu. The mice received daily injections for 7 d of the different preparations via tail veins. The survival rate, body weight, and food uptake were monitored during the experimental period. The average daily food uptake was calculated by the following equation: .
2.7. In vitro thrombolytic activity of NK-TUDCA
First, the blood clot was prepared. Briefly, blood was collected from the heart of New Zealand white rabbits into a medical sampling tube with pre-added bacteriostatic agent and stored at 37 °C for 4 d under an atmosphere of nitrogen to enable the successful formation of a blood clot. The blood clot was then cut into small patches of approximately 0.2 g, cleaned using 5% Glu, and accurately weighted after removal of the liquid on the surface of the blood clot by filter paper. The small blood clots were transferred into 1 ml of the following solutions, all prepared in 5% Glu: TUDCA solution (5.00 mg/ml, pH 5.0), NK solution (500 IU/ml, pH 5.0), NK-TUDCA solutions (NK: 10 kIU/ml, TUDCA: 1, 5, 10, 15 mg/ml; pH 5.0), NK-TUDCA solutions (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0), the mixture of NK and TUDCA solutions (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0), and then incubated for 0.5, 2, 4, 6, 8, 10, 18, and 24 h at 37 °C. After incubation, the blood clots were removed and accurately weighed after the liquid on the surface of the blood clots was removed by filter paper. The relative dissolution percentage was calculated using the following equation: where ms is the weight of blood clot before dissolution and mn is the weight of blood clot at different time points.
2.8. Characterize of NK-TUDCA
The zeta potential of NK solution (10 kIU/ml; pH 5.0), TUDCA solution (10 mg/ml; pH 5.0), and NK-TUDCA solution (NK: 10 kIU/ml, TUDCA: 10 mg/ml, pH 5.0) was estimated by using a dynamic laser light scattering instrument (Nicomp™ 380 Submicron Particle Sizer; Particle Sizing Systems, Port Richey, FL, USA). The particle sizes of these solutions were measured by using a dynamic laser light scattering instrument (Nicomp™ 380 Submicron Particle Sizer; Particle Sizing Systems, Port Richey, FL, USA) at a wavelength of 632.8 nm. The morphology of NK-TUDCA was observed by transmission electron microscopy (TEM, JM-1200EX, JEOL Ltd., Japan) as follows. NK-TUDCA was dropped on a formvar-coated copper grid (300-mesh, hexagonal fields) and stained with phosphotungstic acid solution (2%, w/v). The sample was allowed to air-dry overnight at room temperature before measurement.
3. Results and discussions
3.1. Toxicity of NK and TUDCA
3.1.1. Toxicity of NK
The toxicity of NK was assessed through the administration of different dosages of NK solution to mice. The results of survival time, survival state, and tail injury of surviving mice at 48 h after injection are presented in Table 1.
Table 1.
The toxicity of NK after a single intravenous injection to mice (n = 3).
| Injection dosage (kIU/kg) | Death rate (%) | Survival time (min) | State |
|---|---|---|---|
| 150 | 100 | 0.25, 0.33, 0.42 | ddd |
| 130 | 100 | 1, 3, 2 | ddd |
| 100 | 100 | 150, 258, 204 | ccc |
| 80 | 0 | a, a, a | bbb |
| 40 | 0 | a, a, a | bbb |
The mouse was not dead at 48 h after injection.
The mouse exhibited shortness of breath, movement reduction, and drowsiness.
The mouse exhibited shortness of breath, movement reduction, drowsiness, and tetany.
The mouse exhibited shortness of breath, tetany, bleeding from the mouth and nose, and death.
As shown in Table 1, the survival time of the mice was extended by a decrease in the dosage of injected NK solution. The mice immediately died after injections of NK solution at 150 and 130 kIU/kg. While the mice were still alive at about 240 min at 100 kIU/kg, they exhibited symptoms before death, such as shortness of breath, movement reduction, drowsiness and tetany. When the injection dosage was decreased to 80 kIU/kg, all mice survived.
3.1.2. Toxicity of TUDCA
The toxicity of TUDCA was assessed through the administration of different dosages of TUDCA solution to mice. The results of survival time, survival state, and tail injury of surviving mice at 48 h after injection are presented in Table 2 and Fig. 1.
Table 2.
The toxicity of TUDCA after a single intravenous injection to mice (n = 3).
| Injection dosage (mg/kg) | Death rate (%) | Survival time (s) | State |
|---|---|---|---|
| 1300 | 100 | 6, 7, 7 | ddd |
| 1040 | 100 | 20, 18, 23 | ddd |
| 910 | 0 | a, a, a | ccc |
| 780 | 0 | a, a, a | bbc |
| 650 | 0 | a, a, a | bbb |
| 260 | 0 | a, a, a | bbb |
The mouse was not dead at 48 h after injection.
The mouse exhibited shortness of breath, movement reduction, and drowsiness.
The mouse exhibited shortness of breath, movement reduction, drowsiness, and tetany.
The mouse exhibited symptoms of tetany and death.
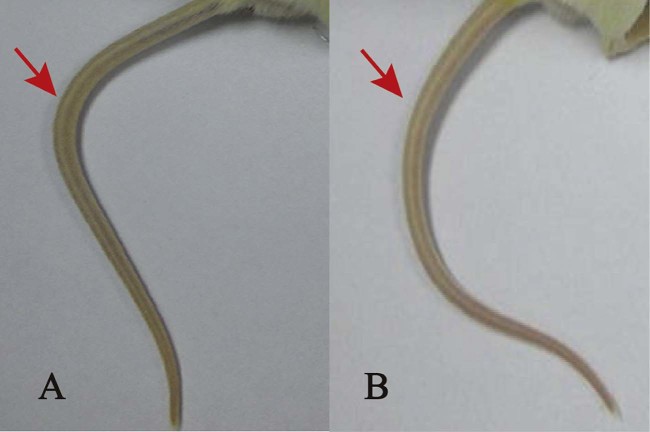
Fig. 1.
Tail injuries of mice administered 5% Glu (A) and TUDCA solution at dosages of 780 mg/kg (B), 650 mg/kg (C), and 260 mg/kg (D).
As shown in Table 2, the survival time of the mice was extended by a decrease in the dosage of injected TUDCA solution. The mice immediately died after injection of TUDCA solution at 1300 and 1040 mg/kg. When the injection dosage was decreased to 910 mg/kg, all mice survived, but presented some symptoms indicative of death, such as shortness of breath, movement reduction, drowsiness and tetany, and gradually recovered to normal after 30 min. At an injection dosage of 780 mg/kg, all mice survived, but one exhibited symptoms similar to that observed at the dosage of 910 mg/kg and the remaining mice presented mild symptoms, such as shortness of breath, movement reduction, and drowsiness, and recovered quickly. Notably, the mice administered with 650 and 260 mg/kg of TUDCA solutions only exhibited the mild symptoms observed in the injection dosage of 780 mg/kg.
The extent of tail injury was also correlated with the administered dosage of TUDCA solution. Fig. 1 shows that the tails were inflamed after 48 h for dosages up to 780 mg/kg, but this was not observed when the dosages of TUDCA solution were 650 and 260 mg/kg.
3.2. Acute toxicity of NK-TUDCA
3.2.1. NK-TUDCA toxicity at different pH values
The influence of pH on the toxicity of NK-TUDCA was evaluated. The results of survival time, survival status, and tail injury of the surviving mice at 48 h after injection are shown in Table 3 and Fig. 2.
Table 3.
The toxicity of NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml) with different pH values after a single intravenous injection of 130 kIU/kg NK (n = 3).
| Preparation | Death rate (%) | Survival time (min) | State |
|---|---|---|---|
| NK (pH 5.0) | 100 | 1, 2, 3 | ddd |
| NK (pH 6.0) | 100 | 2, 2, 1 | ddd |
| NK (pH 7.0) | 100 | 1, 2, 1 | ddd |
| NK (pH 8.0) | 100 | 1, 1, 1 | ddd |
| NK-TUDCA (pH 5.0) | 0 | a, a, a | bbb |
| NK-TUDCA (pH 6.0) | 100 | 7, 5, 3 | ccc |
| NK-TUDCA (pH 7.0) | 100 | 3, 3, 2 | ddd |
| NK-TUDCA (pH 8.0) | 100 | 1, 1, 2 | ddd |
The mouse was not dead at 48 h after injection.
The mouse exhibited shortness of breath, movement reduction, and drowsiness.
The mouse exhibited shortness of breath, movement reduction, drowsiness, and tetany.
The mouse exhibited shortness of breath, tetany, bleeding from the mouth and nose, and death.
Fig. 2.
Tail injuries of mice administered 5% Glu (A) and NK-TUDCA solution (10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) (B).
As shown in Table 3, pH greatly influenced the toxicity of NK-TUDCA. For the group administered NK-TUDCA solution at pH of 5.0, no mice died within the 48 h observation period and only mild symptoms appeared after injection, such as shortness of breath, movement reduction, and drowsiness, and the recovery period was short. In addition, Fig. 2 shows that the tails of the mice from this group also showed no injury during 48 h after administration. However, in other groups, all mice died within 7 min after administration; all mice treated with the NK solutions and NK-TUDCA solutions at pH 7.0 and pH 8.0 even bled from the mouth and nose before death. These results showed that the toxicity of NK-TUDCA was lowest at pH 5.0. Thereby, the pH of the NK-TUDCA was fixed at 5.0 in the subsequent experiments in our study.
Combining these results, we can analyze from the aspect in the formation of NK-TUDCA. Owing to the formation of NK-TUDCA through the electrostatic interaction between NK and TUDCA, the stoichiometric composition of NK and TUDCA will be greatly influenced by the pH of the preparative conditions. NK is stable in the pH range 5.0–9.0 [38], [39]; thus, the pH range of the conditions in which the complex was prepared was set between 5.0 and 8.0 to ensure NK was in a cationic state. Furthermore, NK was more positively charged with a decrease in the pH value of preparative conditions. TUDCA is a bile salt that contains a sulfonate group with a low pKa (<2) [37]; hence, the charge was negative and hardly affected in the pH range 5.0–8.0. Consequently, there was a stronger binding force between NK and TUDCA when the pH of the preparation conditions was at 5.0; a tightly-bound complex (NK-TUDCA) by electrostatic interaction was obtained successfully.
3.2.2. NK-TUDCA toxicity at different concentrations of TUDCA
The influence of the concentration of TUDCA on the toxicity of NK-TUDCA was evaluated. The results of survival time, survival status, and tail injury of the surviving mice at 48 h after injection are shown in Table 4 and Fig. 3.
Table 4.
The toxicity of NK-TUDCA (NK: 10 kIU/ml; pH 5.0) with different concentrations of TUDCA after a single intravenous injection of 130 kIU/kg NK (n = 3).
| Preparation (pH 5.0) | Injection dosage of TUDCA (mg/kg) | Death rate (%) | Survival time (min) | State |
|---|---|---|---|---|
| NK | 0 | 100 | 1, 1, 1 | ccc |
| NK-TUDCA (TUDCA: 1 mg/ml) | 13 | 100 | 2, 2, 4 | ccc |
| NK-TUDCA (TUDCA: 5 mg/ml) | 65 | 0 | a, a, a | bbb |
| NK-TUDCA (TUDCA: 10 mg/ml) | 130 | 0 | a, a, a | bbb |
| NK-TUDCA (TUDCA: 15 mg/ml) | 195 | 0 | a, a, a | bbb |
The mouse was not dead within 48 h after injection.
The mouse exhibited shortness of breath, sports reduction, and drowsiness.
The mouse exhibited symptoms of shortness of breath, tetany, bleeding from mouth and nose, and death.
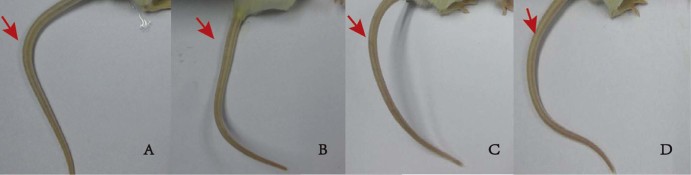
Fig. 3.
The tail injuries of mice administered 5% Glu (A) and NK-TUDCA solution (NK: 10 kIU/ml; pH 5.0) with TUDCA concentrations of 5 mg/ml (B), 10 mg/ml (C), and 15 mg/ml (D).
As shown in Table 4, the concentration of TUDCA directly influenced the toxicity of NK-TUDCA. When the concentration of TUDCA in the NK-TUDCA solution (NK: 10 kIU/ml; pH 5.0) was 1 mg/ml, the mice died within 1 min of administration, and exhibited severe symptoms such as tetany, and bleeding from the mouth and nose. Fig. 3 shows that all mice survived without inflammation or necrosis in their tails when the concentration of TUDCA was increased to 5, 10, or 15 mg/ml. To ensure the reduced toxicity was caused by the addition of TUDCA, the concentration of TUDCA in the NK-TUDCA solution (NK: 10 kIU/ml; pH 5.0) was fixed at 10 mg/ml in the following experiments for our further study.
Considering the different effects of reducing toxicity, we speculate that the micellar structure of TUDCA may mainly contribute to this phenomenon. TUDCA, a surfactant, will spontaneously form micelles when the concentration was above its critical micelle concentration (CMC). The results of the toxicity test of NK-TUDCA with different concentrations of TUDCA showed that the toxicity was significantly reduced when the concentration of TUDCA was above the CMC (<5 mg/ml) [40], but was unchanged at a TUDCA concentration of 1 mg/ml. Therefore, we speculated that the toxicity reduction may be related to the packaging effect of TUDCA micelles on the surface of NK.
3.3. Repeated-dosage toxicity of NK-TUDCA
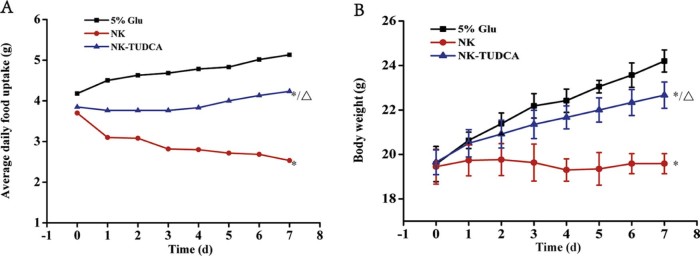
The parameters of body weight and food uptake were used as indicators of the toxicity of NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) following repeated injection of an NK dosage of 13 kIU/kg. The results are shown in Fig. 4A and B.
Fig. 4.
The average daily food uptake (A) and body weight (B) of the mice after repeated injection of NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) at a dosage of 13 kIU/kg NK. (n = 6). * P < 0.001 compared with 5%Glu, △P < 0.001 compared with NK.
Fig. 4A shows that the average daily food uptake from the 5% Glu group was above 4.5 g throughout the entire experiment period and slightly increased with time. For the mice in the NK-TUDCA group, the food uptake remained at approximately 4.0 g and was significantly different from that reported for 5% Glu (P < 0.001), but also showed a slight increase over time. However, the mice in the NK group had a lower food uptake (2.81 g) and showed a continuous decrease, which was significantly different from the results obtained for the 5% Glu (P < 0.001) and NK-TUDCA (P < 0.001) groups.
The trends in body weight were similar to those of food uptake. As shown in Fig. 4B, throughout the entire experimental period, the body weights of the mice in the groups of 5% Glu and NK-TUDCA were increased steadily (up to 24.2 and 22.7 g, respectively, on day 7), and the growth rates of 5% Glu and NK-TUDCA were significantly different (P < 0.001). For the mice in the NK group, the body weight was almost unchanged (19.6 g) throughout the entire administration period and was significantly different to the 5% Glu (P < 0.001) and NK-TUDCA (P < 0.001) groups.
3.4. The in vitro thrombolytic activity of NK-TUDCA
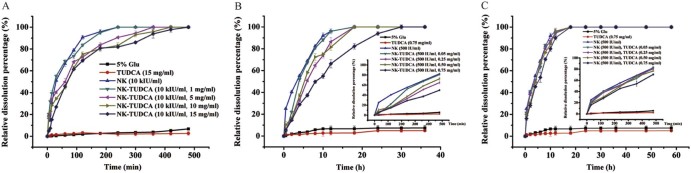
Fig. 5 shows that the relative dissolution percentage of the NK-TUDCA solutions was calculated at different times to evaluate the influence from TUDCA on the fibrinolytic efficiency of NK.
Fig. 5.
The efficiency of blood clot dissolution of NK-TUDCA solutions (NK: 10 kIU/ml, TUDCA:1, 5, 10, 15 mg/ml; pH 5.0) (A), NK-TUDCA solutions (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0) (B) and the mixed solutions of NK and TUDCA (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0) (C).
Fig. 5A shows that all positive control groups clearly displayed thrombolytic activity. The blood clots dissolved completely because of the efficiency of NK and the dissolution time was 240 min. The data from the NK-TUDCA solutions (NK: 10 kIU/ml, TUDCA: 1, 5, 10, 15 mg/ml; pH 5.0) clearly showed that the addition of TUDCA affected the thrombolytic activity of NK. As the concentration of TUDCA increased in the NK-TUDCA solutions (1, 5, 10, 15 mg/ml), the dissolution rate slowed, and the time required to dissolve the entire clot increased to 240, 360, 420, and 480 min, respectively.
For further study of the delayed release effect of NK-TUDCA in dilute conditions, a comparison of the thrombolytic efficiency of the 20-fold diluted complex of NK-TUDCA and mixture of NK and TUDCA with the same concentration (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0) was conducted. It was important to note that all concentrations of TUDCA were below the CMC [40]. Fig. 5B shows that the NK-TUDCA solutions (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0) demonstrated delayed release with an increase in the concentration of TUDCA. Except for NK-TUDCA with 0.75 mg/ml TUDCA, all blood clots were dissolved within 18 h and the dissolution rate was slower in the higher concentrations of TUDCA. The dissolution percentage of NK-TUDCA (NK: 500 IU/ml, TUDCA: 0.75 mg/ml; pH 5.0) reached 100% when the dissolution time was extended to 30 h. In contrast, Fig. 5C shows that the physical mixture of NK and TUDCA (NK: 500 IU/ml, TUDCA: 0.05, 0.25, 0.50, 0.75 mg/ml; pH 5.0) showed only a slightly delayed release effect on the clot dissolution behavior; the dissolution process was similar to that for NK solution (NK: 500 IU/ml; pH 5.0).
Overall, the study of the in vitro thrombolytic activity of NK-TUDCA illustrated that there was a delayed release effect during the period of thrombolysis when the concentration of TUDCA was increased, but the effect was not apparent when the concentration of TUDCA was lower than the CMC. Furthermore, this delayed release of NK-TUDCA still occurred even after a 20-fold dilution, which resulted in a concentration of TUDCA below the CMC. We accounted for this phenomenon by considering that the disruption of TUDCA micelles was a time-consuming process as this delayed release effect did not appear in the physical mixture with the same concentration as NK-TUDCA after a 20-fold dilution. These results may demonstrate that the delayed release effect arises from the formation of a protective layer of TUDCA micelles on the surface of NK.
3.5. Characterize of NK-TUDCA
3.5.1. Determination of zeta potential
The zeta potential of the solutions of NK (10 kIU/ml), TUDCA (10 mg/ml), and NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) were estimated and the results are shown in Table 5. The mean zeta potentials of the solutions of NK, TUDCA, and NK-TUDCA were 2.11 ± 0.30 mV, −6.45 ± 0.74 mV, and -0.94 ± 0.88 mV, respectively. It was found that the positive charge of NK could be covered by the conjugation with the negative charged TUDCA micelles, and the absolute value of TUDCA solutions were also decreased by the electrostatic binding of NK.
Table 5.
The zeta potentials of NK (10 kIU/ml; pH 5.0), TUDCA (TUDCA: 10 mg/ml; pH 5.0) and NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0).
| Sample | ζ-potential (mV) |
|---|---|
| NK | 2.11 ± 0.30 |
| TUDCA | −6.45 ± 0.74 |
| NK-TUDCA | −0.94 ± 0.88 |
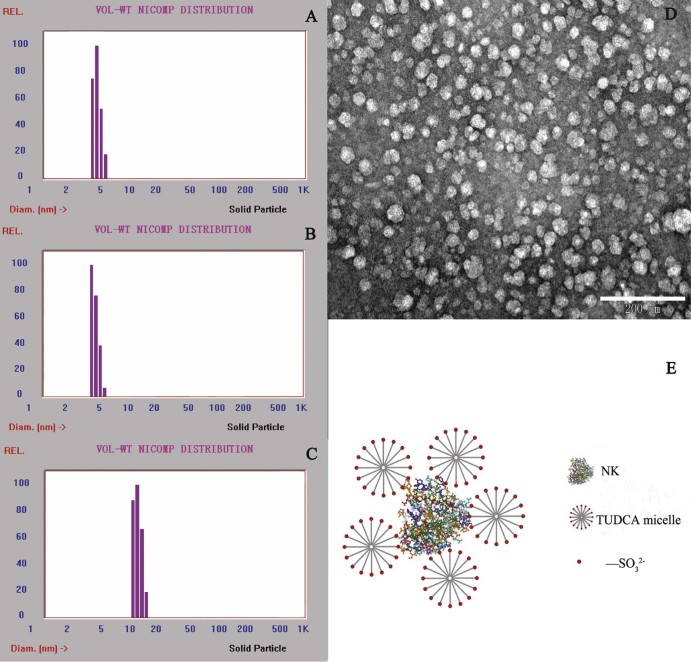
3.5.2. Determination of particle size and morphology
The particle sizes of the solutions of NK (10 kIU/ml), TUDCA (10 mg/ml), and NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) were measured and the results are shown in Fig. 6A, B and C. The mean sizes of particles in the solutions of NK, TUDCA, and NK-TUDCA were 4.1 ± 0.4 nm, 3.4 ± 0.6 nm, and 11.8 ± 0.7 nm, respectively. The particle size in NK-TUDCA was significantly increased compared with NK or TUDCA (P < 0.001), which demonstrated the successful formation of the NK-TUDCA complex. The diameter of NK-TUDCA was equal to the sum of the diameter of one NK particle and two TUDCA particles. The morphology of NK-TUDCA by TEM presented in Fig. 6D showed that NK-TUDCA existed as homogeneous spheres with a mean diameter of about 20 nm, which matched the results of the determination of particle size to some extent. When the hypothesis of reducing the toxicity explained above was combined with the results of particle size determination and morphology, the structure of NK-TUDCA can be clearly elucidated as the schematic depiction exhibited in Fig. 6E.
Fig. 6.
The Nicomp diagram of the particle size distribution of NK (10 kIU/ml; pH 5.0) (A), TUDCA (TUDCA: 10 mg/ml; pH 5.0) (B), NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) (C), the morphology of NK-TUDCA (NK: 10 kIU/ml, TUDCA: 10 mg/ml; pH 5.0) by TEM (scale bar, 200 nm) (D), and the schematic depiction of NK-TUDCA (E).
From the above, the toxicity reduction can be summarized by the following process steps. Initially, the positive charge of NK was increased by lower pH conditions, which allowed the strong interaction with the TUDCA micelles. Secondly, the micelles possessed a better packaging effect compared with single TUDCA and served as a protective layer for NK to cover the enzyme activity center. Consequently, NK-TUDCA reduced the risk of hemorrhage following NK administration by causing the slow release of NK and preventing the release of a high concentration of free NK in plasma. During this process, the release of NK became increasingly delayed by the tighter packing effect of an increased amount of TUDCA micelles. The thrombolytic efficiency of NK in NK-TUDCA was activated to a reduced extent, but not weakened, and still possessed the inherent powerful efficiency of NK.
4. Conclusions
In this study, the complex of NK-TUDCA was designed to reduce the toxicity of intravenous NK injection, which would allow a better utilization of NK that shows tremendous potential in thrombolysis. Both the acute toxicity test and repeated-dosage toxicity test showed that NK-TUDCA (NK: 10 kIU/ml; TUDCA: 10 mg/ml; pH 5.0) was less toxic than the NK solution. The thrombolytic test in vitro and characteristics of NK-TUDCA of NK-TUDCA showed that the reduced toxicity of NK-TUDCA might be caused by the delayed release of NK from the complex, which resulted in the reduction of free NK in plasma. Therefore, the toxicity evaluation of NK-TUDCA complex demonstrated the advantage of NK-TUDCA in lowering toxicity compared with NK, and this advantage will enable NK-TUDCA to make the treatment of thrombosis better.
Conflicts of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
We are grateful to Sungen Biotech Co., Ltd. (Shantou, China) for providing the material and technical guidance.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Guan C.L., Zhang A.D., Guo J. Application of antiplatelet drugs in cardiovascular and cerebrovascular diseases. Chin J Integr Med. 2016;14(3):197–200. [Google Scholar]
- 2.Chandrasekaran S.D., Vaithilingam M., Shanker R. Exploring the in vitro thrombolytic activity of nattokinase from a new strain Pseudomonas aeruginosa CMSS. Jundishapur J Microbiol. 2015;8(10):e23567. doi: 10.5812/jjm.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali M.R., Salim H.M., Islam M.A. Aspect of thrombolytic therapy: a review. Sci World J. 2014;2014:586510. doi: 10.1155/2014/586510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding B.S., Hong N., Murciano J.C. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111(4):1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugimoto S., Fujii T., Morimiya T. The fibrinolytic activity of a novel protease derived from a tempeh producing fungus, Fusarium sp. BLB. Biosci Biotechnol Biochem. 2007;71(9):2184–2189. doi: 10.1271/bbb.70153. [DOI] [PubMed] [Google Scholar]
- 6.Chiou J.F., Woon M.D., Cheng S.N. Staphylokinase-annexin XI chimera exhibited efficient in vitro thrombolytic activities. Biosci Biotechnol Biochem. 2007;71(5):1122–1129. doi: 10.1271/bbb.60279. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Palasubramaniam J., Gkanatsas Y. Towards effective and safe thrombolysis and thromboprophylaxis: preclinical testing of a novel antibody-targeted recombinant plasminogen activator directed against activated platelets. Circ Res. 2014;114(7):1083–1093. doi: 10.1161/CIRCRESAHA.114.302514. [DOI] [PubMed] [Google Scholar]
- 8.Yue R., Li D., Yu J. Atrial fibrillation is associated with poor outcomes in thrombolyzed patients with acute ischemic stroke: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95(10):e3054. doi: 10.1097/MD.0000000000003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moukhametova L.I., Aisina R.B., Lomakina G.Y. Properties of the urokinase-type plasminogen activator modified with phenylglyoxal. Russ J Bioorganic Chem. 2002;28(28):278–283. [PubMed] [Google Scholar]
- 10.Mahajan P.M., Gokhale S.V., Lele S.S. Production of nattokinase using Bacillus natto NRRL 3666: media optimization, scale up, and kinetic modeling. Food Sci Biotechnol. 2010;19(6):1593–1603. [Google Scholar]
- 11.Alkawi A., Kirmani J.F., Janjua N. Advances in thrombolytics and mechanical devices for treatment of acute ischemic stroke. Neurol Res. 2005;27(Suppl. 1):42–49. doi: 10.1179/016164105X25306. [DOI] [PubMed] [Google Scholar]
- 12.Kim W., Choi K., Kim Y. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl Environ Microbiol. 1996;62(7):2482–2488. doi: 10.1128/aem.62.7.2482-2488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Y., Yang X., Zhang Y. Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol. 2005;69(2):126–132. doi: 10.1007/s00253-005-0159-7. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M., Nomura K., Hong K. Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun. 1993;197(3):1340–1347. doi: 10.1006/bbrc.1993.2624. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M., Hong K., Ito Y. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull. 1995;18(10):1387–1391. doi: 10.1248/bpb.18.1387. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Z.L., Zuo Z.Y., Liu Z.G. Construction of a 3D model of nattokinase, a novel fibrinolytic enzyme from Bacillus natto: a novel nucleophilic catalytic mechanism for nattokinase. J Mol Graph Model. 2005;23(4):373–380. doi: 10.1016/j.jmgm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Omura K., Hitosugi M., Zhu X. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J Pharmacol Sci. 2005;99(3):247–251. doi: 10.1254/jphs.fp0050408. [DOI] [PubMed] [Google Scholar]
- 18.Sumi H., Yanagisawa Y., Yatagai C. Natto Bacillus as an oral fibrinolytic agent: nattokinase activity and the ingestion effect of Bacillus subtilis natto. Food Sci Technol Res. 2004;10(1):17–20. [Google Scholar]
- 19.Peng Y., Huang Q., Zhang R.H. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Comp Biochem Physiol B Biochem Mol Biol. 2003;134(1):45–52. doi: 10.1016/s1096-4959(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 20.Agrebi R., Haddar A., Hmidet N. BSF1 fibrinolytic enzyme from a marine bacterium Bacillus subtilis A26: purification, biochemical and molecular characterization. Process Biochem. 2009;44(11):1252–1259. [Google Scholar]
- 21.Choi K.S., Fitzpatrick S.L., Filipenko N.R. Regulation of plasmin-dependent fibrin clot lysis by annexin II heterotetramer. J Biol Chem. 2001;276(27):25212–25221. doi: 10.1074/jbc.M101426200. [DOI] [PubMed] [Google Scholar]
- 22.Urano T., Ihara H., Umemura K. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001;276(27):24690–24696. doi: 10.1074/jbc.M101751200. [DOI] [PubMed] [Google Scholar]
- 23.Lampe B.J., English J.C. Toxicological assessment of nattokinase derived from Bacillus subtilis var. natto. Food Chem Toxicol. 2015;88:87–99. doi: 10.1016/j.fct.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Sumi H., Hamada H., Tsushima H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Jiang S., Wang X. Preparation and evaluation of nattokinase-loaded self-double-emulsifying drug delivery system. Asian J Pharm Sci. 2015;10(5):386–395. [Google Scholar]
- 26.Liu J.G., Xing J.M., Shen R. Reverse micelles extraction of nattokinase from fermentation broth. Biochem Eng J. 2004;21(3):273–278. [Google Scholar]
- 27.Chen J., Wei Q., Jiang F. 2008. Bacillus Subtilis Strain and its Use in Preparing Pharmaceuticals for Treating Thrombosis. EP. US20080193973. [Google Scholar]
- 28.Matsuoka K., Maeda M., Moroi Y. Micelle formation of sodium glyco- and taurocholates and sodium glyco- and taurodeoxycholates and solubilization of cholesterol into their micelles. Colloids Surf B Biointerfaces. 2003;32(2):87–95. [Google Scholar]
- 29.Begley M., Gahan C.G.M., Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29(4):625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Funasaki N., Nomura M., Seiji Ishikawa A. Hydrophobic self-association of sodium taurochenodeoxycholate and tauroursodeoxycholate. J Phys Chem B. 2000;104(32):7745–7751. [Google Scholar]
- 31.Narain P.K., Demaria E.J., Heuman D.M. Lecithin protects against plasma membrane disruption by bile salts. J Surg Res. 1998;78(2):131–136. doi: 10.1006/jsre.1998.5364. [DOI] [PubMed] [Google Scholar]
- 32.Ishizaki K., Kinbara S., Miyazawa N. Effect of sodium tauroursodeoxycholate (UR-906) on liver dysfunction in bile duct-ligated rats. Eur J Pharmacol. 1997;333(2–3):207–213. doi: 10.1016/s0014-2999(97)01143-6. [DOI] [PubMed] [Google Scholar]
- 33.Ishizaki K., Kinbara S., Hirabayashi N. Effect of sodium tauroursodeoxycholate on phalloidin-induced cholestasis in rats. Eur J Pharmacol. 2001;421(1):55–60. doi: 10.1016/s0014-2999(01)00996-7. [DOI] [PubMed] [Google Scholar]
- 34.Boatright J.H., Nickerson J.M., Moring A.G. Bile acids in treatment of ocular disease. J Ocul Biol Dis Infor. 2009;2(3):149–159. doi: 10.1007/s12177-009-9030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crosignani A., Battezzati P.M., Setchell K.D.R. Tauroursodeoxycholic acid for treatment of primary biliary cirrhosis: a dose-response study. Dig Dis Sci. 1996;41(4):809–815. doi: 10.1007/BF02213140. [DOI] [PubMed] [Google Scholar]
- 36.Gallo V., De Micheli A., Chiandussi L. Tauro-ursodeoxycholic acid vs. ursodeoxycholic acid in the dissolution of biliary calculi. Results of a single blind study. Clin Ter. 1993;143(5):421–428. [PubMed] [Google Scholar]
- 37.Black R.B., Naylor F., Stenhouse N.S. Gastric mucosal damage by taurine and glycine conjugates of chenodeoxycholic acid[J] Am J Dig Dis. 1977;22(12):1106–1108. doi: 10.1007/BF01072866. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen T.T., Quyen T.D., Le H.T. Cloning and enhancing production of a detergent- and organic-solvent-resistant nattokinase from Bacillus subtilis VTCC-DVN-12-01 by using an eight-protease-gene-deficient Bacillus subtilis WB800. Microb Cell Fact. 2013;12(1):232–241. doi: 10.1186/1475-2859-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin H.T.V., Wu G.J., Hsieh M.C. Purification and characterization of Nattokinase from cultural filtrate of red alga porphyra dentata fermented by Bacillus subtilis N1. J Marine Sci Technol. 2015;23(2):240–248. [Google Scholar]
- 40.Matsuoka K., Suzuki M., Honda C. Micellization of conjugated chenodeoxy- and ursodeoxycholates and solubilization of cholesterol into their micelles: comparison with other four conjugated bile salts species. Chem Phys Lipids. 2006;139(1):1–10. doi: 10.1016/j.chemphyslip.2005.08.006. [DOI] [PubMed] [Google Scholar]