Graphical Abstract
Keywords: Poorly water-soluble drug, Kaempferia parviflora, Solid dispersion, Solvent evaporation
Abstract
Kaempferia parviflora, a plant in the family Zingiberaceae, has been used in Thai traditional medicines for treating hypertension and promoting longevity with good health and well-being. However, its limited aqueous solubility and low dissolution restrict its bioavailability. The aim of the study was therefore to improve the dissolution rate of K. parviflora extracted with dichloromethane (KPD) by solid dispersions. Different water-soluble polymers were applied to improve dissolution of KPD. The solid dispersions in different ratios were prepared by solvent evaporation method. Only hydroxypropyl methylcellulose (HPMC) and polyvinyl alcohol-polyethylene glycol grafted copolymer (PVA-co-PEG) could be used to produce homogeneous, powdered solid dispersions. Physical characterization by scanning electron microscopy, hot stage microscopy, differential scanning calorimetry and powder X-ray diffractometry, in comparison with corresponding physical mixtures, showed the changes in solid state during the formation of solid dispersions. Dissolution of a selected marker, 5,7,4′-trimethoxyflavone (TMF), from KPD/HPMC and KPD/PVA-co-PEG solid dispersions was significantly improved, compared with pure KPD. The dissolution enhancement by solid dispersion was influenced by both type and content of polymers. The stability of KPD/HPMC and KPD/PVA-co-PEG solid dispersions was also good after 6-month storage in both long-term and accelerated conditions. These results identified that the KPD/HPMC and KPD/PVA-co-PEG solid dispersions were an effective new approach for pharmaceutical application of K. parviflora.
1. Introduction
Kaempferia parviflora Wall. Ex Baker is a member of the family Zingiberaceae and found in the northern part of Thailand. In Thai traditional medicine, rhizome of the plant has been used for treating hypertension, erectile dysfunction, inflammation and abdominal pain, as well as for the promotion of longevity with good health and well-being [1]. In Laos folk medicine, the rhizome has been used for treating high blood glucose level, increasing blood flow and improving vitality [2]. In Japan, K. parviflora extract is marketed as a nutrition supplement for the treatment of metabolic syndrome [3]. Several researchers have revealed the pharmacological activities of ethanol extract form K. parviflora such as aphrodisiac activity in male rats and prevention of myocardial ischemic-reperfusion injury in isolated rat heart [4]. Ethyl acetate extracted K. parviflora was investigated in an in vivo study, by Akase et al. [2] and Shimada et al [5]. The dichloromethane extracted K. parviflora (KPD) demonstrates the pharmacological activities such as decreasing vascular responsiveness to phenylephrine, decreasing visceral and subcutaneous fat and decreasing fasting serum glucose and triglyceride levels in middle-aged male rats [6].
The methoxyflavones, i.e., 5,7-dimethoxyflavone (DMF), 5,7,4′-trimethoxyflovone (TMF), and 3,5,7,3′,4-pentamethoxyflavone (PMF), were used as standard markers of K. parviflora. The major problem associated with methoxyflavones is inadequate solubility in biological fluids and their high lipophilicity with log P values ranging from 2.0 to 3.5 that finally limit their bioavailability and clinical utility after oral administration [7], [8]. Therefore, it is important to develop a suitable formulation to increase its solubility and bioavailability. A number of methods have been developed to improve the oral absorption of poorly water-soluble compounds such as milling [9], salt formation [10], micronization [11], solubilization using co-solvents, use of lipid-based formulations and solid dispersions [12], [13], etc. Solid dispersions are generally accepted as a method to enhance dissolution of poorly water-soluble drugs. The term solid dispersion refers to a group of solid substance consisting of at least two different components, generally a hydrophilic matrix and a hydrophobic drug. The drug can be homogeneously dispersed, in the form of amorphous particles or crystalline particles [14], [15]. Several methods have been used to produce the solid dispersions, i.e., melting, solvent evaporation and melting-solvent methods. Melting method is a process to prepare solid dispersions by mixing drug and carrier together, and heating them directly until they melted. The molten mixture is then solidified to get the solid mass. In solvent evaporation method, drug and carrier are dissolved in a common solvent and then the solvent is removed to form the solid mass. Nevertheless, the solvent evaporation method is a popular method because it uses low temperature to remove the solvent and requires simple equipment [16], [17], [18], [19]. Various carriers can be used to prepare solid dispersions [20], [21], [22], [23], i.e., polyethylene glycols, polyvinylpyrrolidone, polyols, polysaccharides, polyacids, etc. However, the study on development of herbal products using solid dispersion technique is still limited. To the best of our knowledge, solid dispersion containing K. parviflora extract is not available to date. Therefore, in this study, the solid dispersions of KPD were developed in order to improve the dissolution of KPD by solid dispersion using solvent evaporation method. The solid state characteristics and dissolution behavior of solid dispersions were investigated. The stability of KPD solid dispersions, after storage in long-term and accelerated conditions was also tested.
2. Materials and methods
2.1. Materials
KPD was extracted by maceration method as described previously [6]. Briefly, fresh rhizomes of K. parviflora were blended and extracted successively by macerating with 95% ethanol for 48 h, followed by extracting with dichloromethane. The dichloromethane-soluble part was filtered and then evaporated. The dried residue from the dichloromethane extract was further treated by vacuum condition in order to remove residual dichloromethane and then a yellowish gummy of KPD was obtained.
Polyvinyl alcohol polyethylene glycol graft copolymer (Kollicoat® IR, referred to as PVA-co-PEG) and polyethyleneglycol-polyvinyl acetate-polyvinylcaprolactame grafted copolymer (Soluplus®, referred to as PCL-PVAc-PEG) were a gift form BASF (Thai) Co., Ltd. (Bangkok, Thailand). Polyvinylpyrrolidone K30 (referred to as PVP), hydroxypropyl methylcellulose K4M (referred to as HPMC) and polyethylene glycol 4000 (referred to as PEG) were purchased form P.C. Drug Center (Bangkok, Thailand). Other chemicals were of pharmaceutical grade or analytical grade and used without further purification.
2.2. Preparation of solid dispersions
Solid dispersions of KPD and polymer were prepared by solvent evaporation method. The ratios of KPD to polymer are shown in Table 1. The KPD and polymer were separately dissolved in 5-ml dichloromethane. The clear solutions of KPD and polymer were mixed and the solvent was then evaporated in water bath at 50 °C for 24 h. The dried samples were kept in desiccator until further investigation.
Table 1.
Formulation of KPD solid dispersions.
| Ratio | KPD (mg) | Polymer (mg) |
|---|---|---|
| 1:0.5 | 90 | 45 |
| 1:1 | 90 | 90 |
| 1:2 | 90 | 180 |
| 1:4 | 90 | 360 |
2.3. Analysis of the content
The samples were dissolved in methanol and filtered with 0.45-µm nylon membrane filter. The filtered solution was analyzed by high performance liquid chromatography (HPLC) equipped with a photodiode array detector (model Agilent 1100 Series HPLC System, Agilent Technologies, USA) using Luna 5 µ C18 (5 µm, 4.6 nm × 25 cm, Phenomenex®, USA). The analysis asperformed at ambient temperature (25 °C). The analysis method from our previous report was applied [6]. The solvent system composed of solvent A (methanol) and solvent B (0.05% trifluoroacetic acid in water). A 40-min linear gradient from 10% A to 100% A was applied at flow rate of 1 ml/min, followed by a 10-min isocratic elution at 100% A, before returning to starting condition. The TMF was used as a marker in this study and detected by monitoring of UV absorbance at 254 nm; it was quantified by comparison of peak area to a standard curve.
2.4. Morphology examination of KPD and solid dispersions
The surface morphology of KPD and solid dispersions was investigated by a scanning electron microscope (SEM; model Maxim-2000, CamScan Analytical, England) with an accelerating voltage of 20 KeV. All samples were placed on the stub by double-sided adhesive carbon tape and coated in a vacuum with thin gold layer before investigation.
2.5. Hot stage microscopy
The properties of KPD and solid dispersions were investigated by hot stage microscopy. Approximately 10 mg of samples were placed on glass slides with cover glass, heated by a hot stage (model FP82HT, Mettler Toledo, Switzerland) at 1 °C/min and observed under an optical microscope (model CX41, Olympus, Japan). Changes in the morphology (melting) were noted as a function of temperature. The polarization properties of sample were observed during the experiment using a polarized filter (CX-AL, Olympus, Japan) to investigate the crystal properties.
2.6. Differential scanning calorimetry (DSC)
The thermal analysis of KPD, raw material, physical mixture of KPD and polymer, and solid dispersion was carried out with differential scanning calorimeter (model Sapphire, Perkin Elmer, USA). Samples (about 2.00 mg) were placed inside a standard crimped aluminum pan (Seiko Instruments, Horsham, PA, USA) and heated from 20 to 200 °C at a heating rate of 10 °C/min.
2.7. Powder X-ray diffractometry (PXRD)
PXRD patterns of KPD and solid dispersions were determined by powder X-ray diffractometer (model Miniflex II, Rigaku, Japan), at 15 mA, 30 KV and angle speed of 4°/min over the range of 2θ from 5 to 45°, using Cu Kα radiation wavelength of 1.5406 Å.
2.8. In vitro dissolution study
The dissolution study was performed by USP apparatus II (model PWS3C, Pharma Test, Germany) with 900 ml of simulated gastric fluid USP without pepsin (SGF, pH 1.2) as a dissolution medium that is intended to represent stomach acid secretion in fasted state. The paddle speed of 50 rpm was adjusted. The temperature was set at 37 ± 0.5 °C. The powders of KPD and solid dispersions (equivalent to 90 mg of TMF) were put into dissolution vessels. At predetermined time intervals (i.e., 5. 10, 15, 30, 60 and 120 min), 4 ml aliquots of medium were taken, filtered through 0.45-µm nylon membrane filter to eliminate the undissolved samples and analyzed for the TMF concentration by HPLC analysis as mentioned above. The equal volume (4 ml) of fresh medium was added to compensate for loss due to sampling. The experiments were performed in triplicate.
2.9. Stability of solid dispersion formulations
The selected solid dispersion formulations were stored for 6 months in two conditions, i.e., ambient condition (25 °C/68% RH) and accelerated condition (40 °C/75% RH), before the investigation of TMF content and dissolution. The analysis was performed in triplicate.
2.10. Statistical analysis
Analysis of variance (ANOVA) and Levene's test for homogeneity of variance were carried out using SPSS version 10.0 for Windows (SPSS Inc., USA). Post hoc testing (P < 0.05) of multiple comparisons was performed by either the Scheffé or Games–Howell test depending on whether Levene's test was insignificant or significant, respectively.
3. Results and discussion
3.1. Physical characterization of solid dispersions
Five different polymers, i.e., PEG, PVP, HPMC, PCL-PVAc-PEG and PVA-co-PEG, were examined in this study. The KPD solid dispersions were prepared by solvent method using dichloromethane as a solvent. Different ratios of KPD and various polymers were dispersed in dichloromethane. Solvent was evaporated for 24 h to remove dichloromethane. When using PEG, PVP and PCL-PVAc-PEG, the solid dispersions obtained were sticky and nonhomogeneous, for all ratios. The solid state of solid dispersions was obtained by using HPMC and PVA-co-PEG at KPD to polymer ratios of 1:0.5, 1:1, 1:2 and 1:4. From these results, the solid dispersions from HPMC and PVA-co-PEG were used for further investigation. The solubility of poorly-soluble water substance can be improved by decreasing inter-molecular binding.
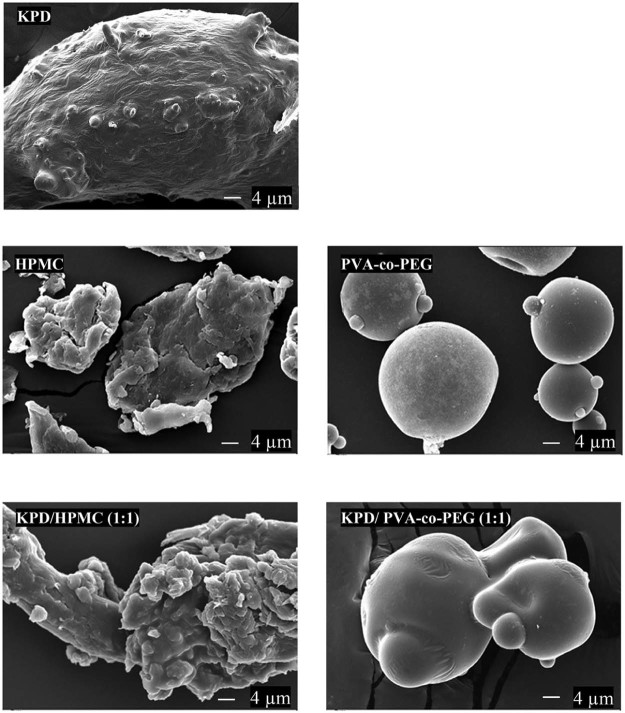
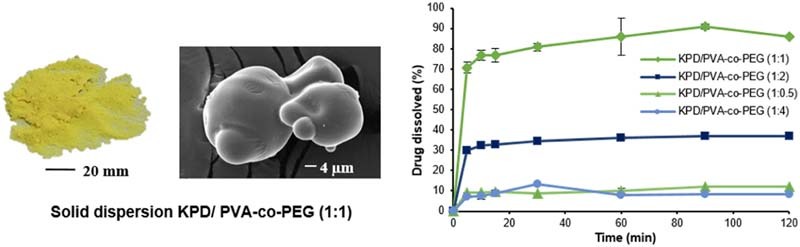
From the study, the solid state of solid dispersions was obtained while using the high Tm (Tm of PVA-co-PEG was more than 200 °C [24] and decomposition temperature of HPMC was 190–200 °C [25]). For PVP, PEG and PCL-PVAc-PEG, Tm was 90–140 °C [26], 53–59 °C [6] and 70 °C [27], respectively. This is probably due to the viscous condition after removing the solvent (Fig. 1). The sticky and tacky mass solid dispersions were obtained when using PVP, PEG and PCL-PVAc-PEG as a carrier. These results may be due to the intermolecular interaction between drug and polymer forming a eutectic system. These observations are consistent with other studies, which have shown that the drug can form eutectic system with PVP [28], [29], PEG [30] and PCL-PVAc-PEG [31]. The results also suggested thatthe Tm plays a significant role in the formation of a solid dispersion system [30], [32]. The SEM images of KPD, polymer and solid dispersions are shown in Fig. 2. SEM images for KPD, PVA-co-PEG and HPMC presented rough surface, rounded shape with smooth surface and irregular shape, respectively. The results also demonstrated that KPD in solid dispersions was homogeneously dispersed into polymeric carriers.
Fig. 1.
Photo images of KPD and solid dispersions of KPD.
Fig. 2.
SEM images of KPD and solid dispersions of KPD (magnification, 1000×).
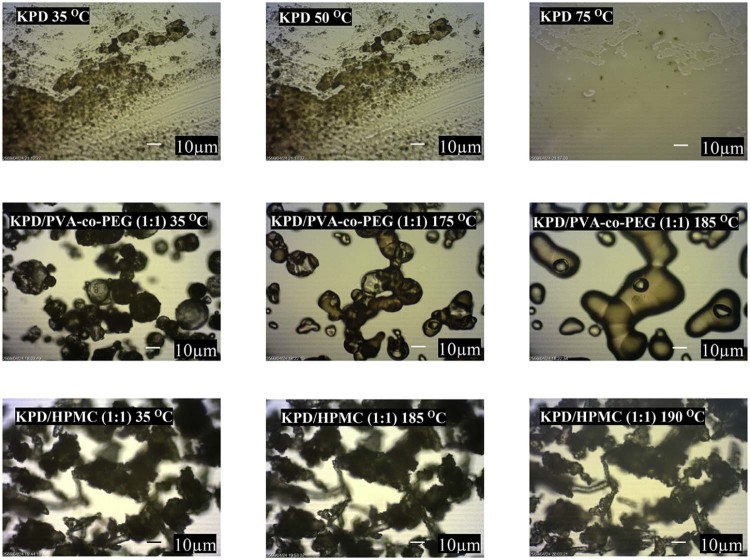
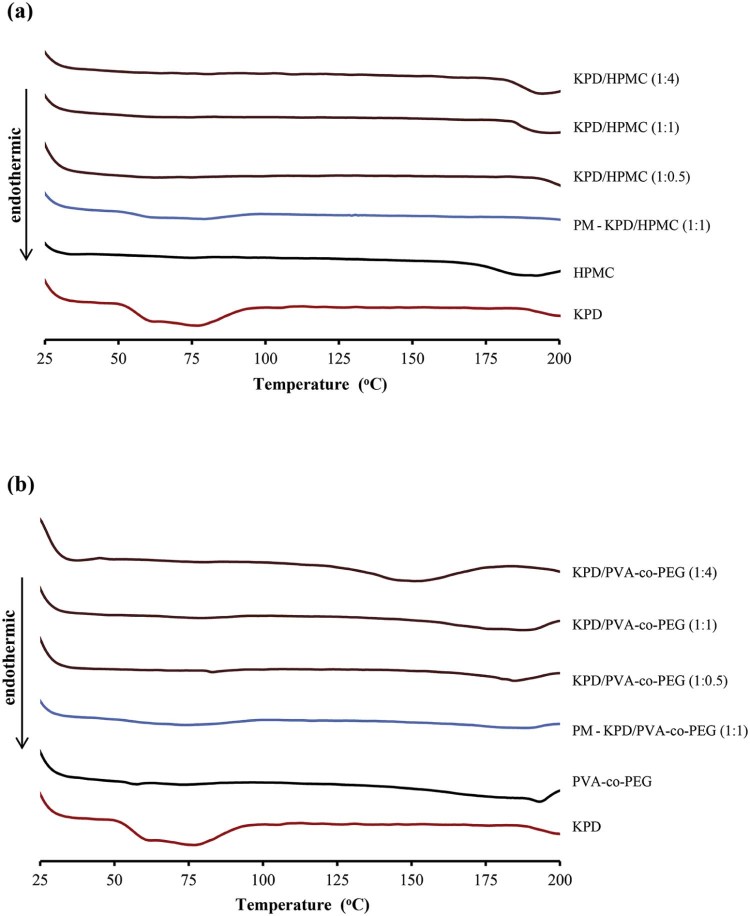
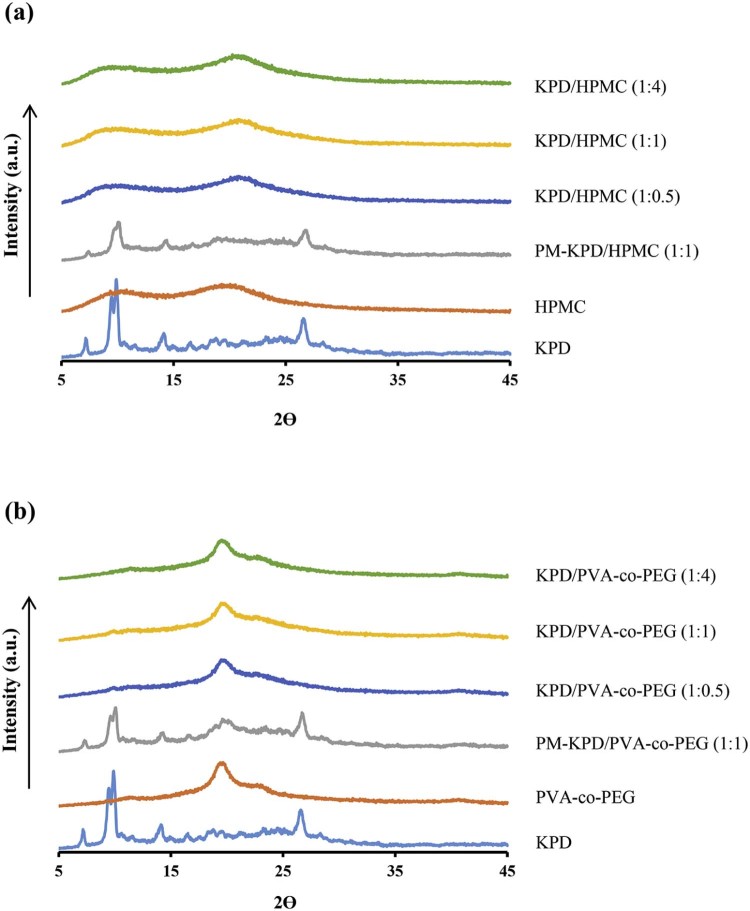
The hot stage microscopy was also used to observe the morphological change of KPD and solid dispersions under temperature changes (Fig. 3). The results from hot stage microscopy showed that KPD displayed melting at 50 °C. The morphological change of KPD/PVA-co-PEG (1:1) solid dispersions was observed at 175 °C. No thermal event was observed from KPD/HPMC (1:1) solid dispersions between 35 and 200 °C. The browning powder without changed structure of HPMC was observed at the decomposition temperature (190–200 °C), which is in agreement with the study by Enose et al. [25]. The DSC thermograms of KPD and solid dispersions are shown in Fig. 4. The thermogram of KPD showed broad endothermic peak at 45–100 °C, which correlates well with the melting observed by the hot stage microscopy (50 °C). No endothermic peak was presented in all ratios of KPD/HPMC solid dispersions andKPD/PVA-co-PEG solid dispersions, suggesting possible molecular dispersion of KPD in HPMC or PVA-co-PEG. Physical mixture of KPD and polymer showed a single broad peak with low intensity, similar to KPD alone. Fig. 5 shows PXRD patterns of KPD, polymer, physical mixture of KPD and polymer, and their solid dispersions. The PXRD pattern of KPD exhibited sharp and highly intense peaks at 2θ degree of 7.22, 9.46, 9.60, 14.90 and 26.56, indicating the crystalline nature of KPD. The physical mixture shows low intensity peaks of KPD along with peaks of HPMC (or PVA-co-PEG), suggesting no change in the crystallinity of KPD. The diffraction patterns of the solid dispersions showed no peak of KPD. The results indicated that the KPD in solid dispersions was molecularly dispersed in polymers (HPMC or PVA-co-PEG), which resulted in a higher KPD dissolution as compared to pure KPD (discussed later in 3.2). This confirmed the results from DSC study, which showed no endothermic peak of KPD in all solid dispersion formulations.
Fig. 3.
Hot stage microscopic images of KPD and solid dispersions of KPD, collected at the different temperatures (magnification, 400×).
Fig. 4.
Thermograms of KPD, polymer, physical mixture (PM) of KPD and polymer and solid dispersions.
Fig. 5.
Powder X-ray diffractograms of KPD, polymer and physical mixture (PM) of KPD and polymer and solid dispersions; using (a) HPMC and (b) PVA-co-PEG as a polymer.
3.2. Dissolution studies of TMF from KPD
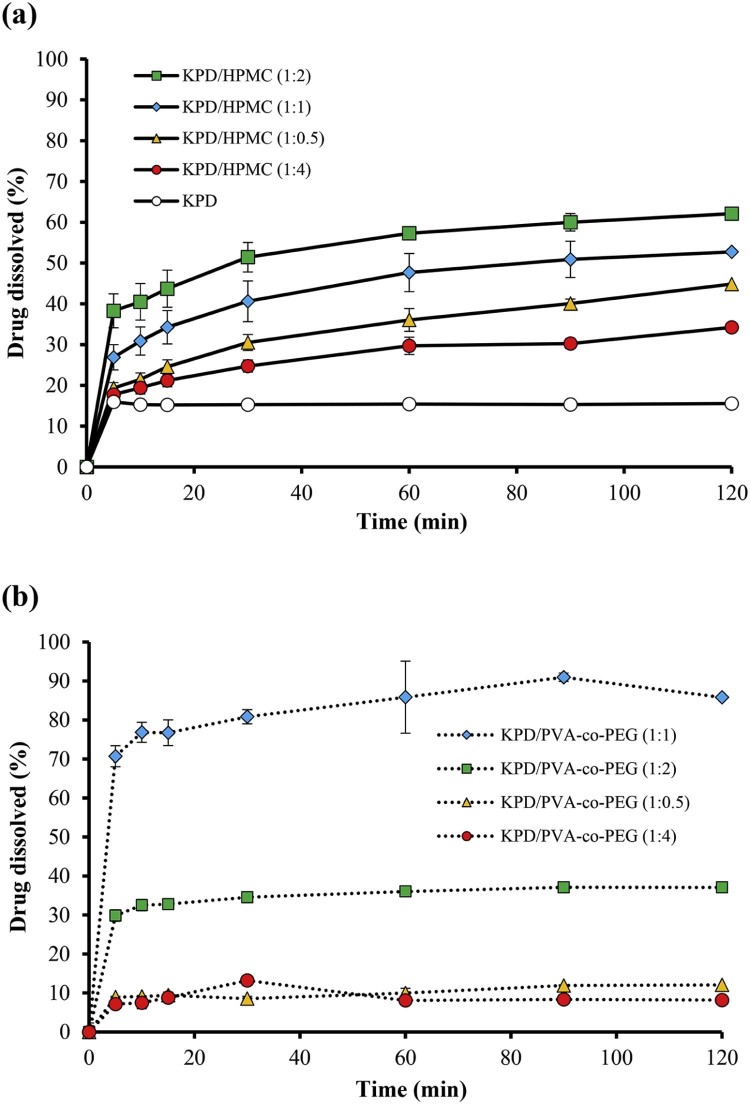
The compounds in KPD, including PMF, DMF and TMF [6], were analyzed by HPLC. In this study, the TMF was selected as a marker. The retention time of standard TMF and TMF in KPD was 27.07 and 27.05 min, respectively. The KPD/HPMC and KPD/PVA-co-PEG (1:2) solid dispersions showed the retention time of TMF at 26.97 and 27.17 min, respectively. Fig. 6 shows the dissolution profiles of KPD and solid dispersion formulations using HPMC and PVA-co-PEG. The TMF dissolved from KPD was about 15%. The dissolution at 15 min (Q15) of TMF from KPD/HPMC (at a ratio of 1:0.5, 1:1 and 1:2) and KPD/PVA-co-PEG (at a ratio of 1:1 and 1:2) was significantly higher than KPD (P < 0.05). At the end of dissolution test, the dissolution at 120 min (Q120) of TMF from KPD/HPMC (1:0.5, 1:1, 1:2 and 1:4) and KPD/PVA-co-PEG (1:1 and 1:2) was also significantly higher than KPD (P < 0.05). Q120 of TMF from KPD/HPMC solid dispersions at a ratio of 1:2 was 4-times higher than that of KPD while those at ratios of 1:0.5, 1:1 and 1:4 were 2.9, 3.3 and 2.2-times higher than that of KPD, respectively (Table 2). Q120 of TMF from KPD/PVA-co-PEG solid dispersions at a ratio of 1:1 was the highest, 5.4-times higher than KPD. Q120 of TMF from KPD/PVA-co-PEG solid dispersions at ratios of 1:0.5, 1:2 and 1:4 were 0.7, 2.4 and 0.5-times higher than that of KPD, respectively. The high dissolution of TMF from solid dispersion is mainly due to the crystallinity of KPD in solid dispersion [18]. Moreover, theincreased amount of polymer resulted in the increased dissolution of TMF. However, the excess amount of polymer (i.e., KPD/HPMC and KPD/PVA-co-PEG solid dispersions at a ratio of 1:4) decreased the dissolution of KPS, which may result from the increased viscosity.
Fig. 6.
Dissolution profiles in SGF of KPD and solid dispersion formulations using different polymers; (a) HPMC and (b) PVA-co-PEG.
Table 2.
Percentage TMF dissolved from KPD or solid dispersions at 15 min (Q15) and 120 min (Q120), n = 3.
| Formulation | Drug dissolved (%) ± S.D. | |
|---|---|---|
| Q15 | Q120 | |
| KPD | 15.21 ± 0.05 | 15.39 ± 0.14 |
| KPD/HPMC (1:0.5) | 24.58 ± 1.55 | 44.83 ± 1.09 |
| KPD/HPMC (1:1) | 34.27 ± 3.45 | 52.72 ± 4.45 |
| KPD/HPMC (1:2) | 43.71 ± 4.48 | 62.11 ± 2.17 |
| KPD/HPMC (1:4) | 21.20 ± 1.52 | 34.23 ± 1.23 |
| KPD/PVA-co-PEG (1:0.5) | 9.43 ± 0.10 | 12.07 ± 0.15 |
| KPD/PVA-co-PEG (1:1) | 75.74 ± 2.56 | 85.83 ± 1.10 |
| KPD/PVA-co-PEG (1:2) | 32.79 ± 1.46 | 37.08 ± 1.39 |
| KPD/PVA-co-PEG (1:4) | 8.83 ± 1.50 | 8.18 ± 0.08 |
In fact, the KPD:polymer ratio is an important practical consideration in solid dispersions. Typically, low KPD:polymer ratios led to enhanced drug dissolution but may result in large dosage units. In this study, at a ratio of 1:0.5, TMF dissolution was very low. This is probably due to the insufficient amount of PVA-co-PEG to form homogeneous solid dispersions. According to the properties of PVA-co-PEG, the dissolution of poorly water-soluble drugs could be improved when PVA-co-PEG content in solid dispersions was increased, for example, from 1:0.5 to 1:1, Q120 was increased about 7 times. However, solid dispersions of KPD/PVA-co-PEG at a ratio of 1:2 and 1:4 also showed low drug dissolution, resulting from the increased viscosity of dissolution surface on solid dispersions [24], [33], [34]. These results are in good agreement with other studies, which have shown that linearly increasing amount of PVA-co-PEG in solid dispersions is not directly improved drug dissolution [34], [35], [36]. To compare between the polymers used, Q120 of TMF from KPD/PVA-co-PEG 1:1 was higher than that from KPD/HPMC 1:2. Wlodarski et al. [19] reported that the solubility of tadalafil and its dissolution from solid dispersions using PVA-co-PEG are greater than HMPC.
3.3. Stability of solid dispersions
The stability of TMF in KPD and selected solid dispersion formulations was investigated under accelerated (40 °C/75% RH) and long-term storage conditions. After storage in both conditions for 6 months, the TMF content was analyzed (Table 3). TMF content was found to be the same; more than 99% of TMF was presented at the end of 6 months in both accelerated and long-term conditions. No significant difference in TMF loss was observed from the formulations tested. The formulations of KPD/HPMC and KPD/PVA-co-PEG solid dispersions did not show any physical change during the observation period. The physicochemical properties, i.e., DSC and PXRD, of selected solid dispersion formulations after storage under accelerated and long-term conditions for 6 months also demonstrated no significant change. No endothermic peak corresponding to the melting of crystalline KPD was observed, by DSC or PXRD, in both conditions (data not shown). The dissolution of solid dispersions after storage under accelerated and long-term conditions for 6 months was also tested. The dissolution profiles of KPD and solid dispersions kept in both storage conditions were nearly the same as the initial products. After storage under long-term stability condition, Q120 of KPD/HPMC (1:2) and KPD/PVA-co-PEG (1:1) solid dispersions was found to be 62.50% and 85.98%, respectively.
Table 3.
Stability test results of KPD and selected solid dispersion formulations, n = 3.
| TMF content (%) ± S.D. | ||
|---|---|---|
| KPD/HPMC (1:2) | KPD/PVA-co-PEG (1:1) | |
| Initial | 100.00 ± 0.25 | 100.00 ± 0.75 |
| Accelerated condition (40 °C/75%RH), 6 months | 99.84 ± 0.21 | 100.00 ± 0.25 |
| Long-term storage condition (25 °C), 6 months | 99.86 ± 0.50 | 99.96 ± 0.64 |
4. Conclusion
To increase dissolution of KPD, solid dispersions prepared by solvent evaporation were used. The solid dispersion formulations of KPD using different water-soluble polymers were carried out. The formulations with suitable polymers, e.g., HPMC and PVA-co-PEG, can improve the aqueous solubility of TMF for at least 5 times, compared to the KPD alone. The ratio of KPD to polymer of 1:2 and 1:1, for KPD/HPMC and KPD/PVA-co-PEG solid dispersions, respectively, was the most suitable ratio, providing the high TMF dissolution. From the results obtained, it could be concluded that solid dispersion technique can be applied for the production of herbal extracts.
Acknowledgments
The authors acknowledge Kanokwan Laohawong, Kanokkorn Liamdee and Pajaree Hongthipparat for laboratory assistance.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Sutthanut K., Sripanidkulchai B., Yenjai C. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatogr A. 2007;1143(1–2):227–233. doi: 10.1016/j.chroma.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Akase T., Shimada T., Terabayashi S. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J Nat Med. 2010;65(1):73–80. doi: 10.1007/s11418-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 3.Nakao K., Murata K., Deguchi T. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol Pharm Bull. 2011;34(7):1143–1146. doi: 10.1248/bpb.34.1143. [DOI] [PubMed] [Google Scholar]
- 4.Malakul W., Thirawarapan S., Ingkaninan K. Effects of Kaempferia parviflora Wall. Ex Baker on endothelial dysfunction in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2011;133(2):371–377. doi: 10.1016/j.jep.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T., Horikawa T., Ikeya Y. Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia. 2011;82(8):1272–1278. doi: 10.1016/j.fitote.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Yorsin S., Kanokwiroon K., Radenahmad N. Effects of Kaempferia parviflora rhizomes dichloromethane extract on vascular functions in middle-aged male rat. J Ethnopharmacol. 2014;156:162–174. doi: 10.1016/j.jep.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Kim M., Kim N., Han J. Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J Agric Food Chem. 2014;62(51):12377–12383. doi: 10.1021/jf504074n. [DOI] [PubMed] [Google Scholar]
- 8.Ta N., Walle T. Aromatase inhibition by bioavailable methylated flavones. J Steroid Biochem Mol Biol. 2007;107(1–2):127–129. doi: 10.1016/j.jsbmb.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh Z.H., Samanta A.K., Sia Heng P.W. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(4):255–274. [Google Scholar]
- 10.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junyaprasert V.B., Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(1):13–23. [Google Scholar]
- 12.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5(5):442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka M., Yano K., Hamatsu Y. Assessment of absorption potential of poorly water-soluble drugs by using the dissolution/permeation system. Eur J Pharm Biopharm. 2013;85:1317–1324. doi: 10.1016/j.ejpb.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Paudel A., Worku Z.A., Meeus J. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2013;453(1):253–284. doi: 10.1016/j.ijpharm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Dhirendra K., Lewis S., Udupa N. Solid dispersions: a review. Pakistan J Pharm Sci. 2009;22(2):234–246. [PubMed] [Google Scholar]
- 16.Mohammadi G., Hemati V., Nikbakht M.-R. In vitro and in vivo evaluation of clarithromycin–urea solid dispersions prepared by solvent evaporation, electrospraying and freeze drying methods. Powder Technol. 2014;257:168–174. [Google Scholar]
- 17.Brown C.K., Chokshi H.P., Nickerson B.Y. Acceptable analytical practices for dissolution testing of poorly soluble compounds. Dissolut Technol. 2005;12(4):6–12. [Google Scholar]
- 18.Loh G.O.K., Tan Y.T.F., Peh K.K. Hydrophilic polymer solubilization on norfloxacin solubility in preparation of solid dispersion. Powder Technol. 2014;256:462–469. [Google Scholar]
- 19.Wlodarski K., Sawicki W., Haber K. Physicochemical properties of tadalafil solid dispersions – impact of polymer on the apparent solubility and dissolution rate of tadalafil. Eur J Pharm Biopharm. 2015;94:106–115. doi: 10.1016/j.ejpb.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Sethia S., Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1–2):1–10. doi: 10.1016/j.ijpharm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Rashid R., Kim D.W., ud Din F. Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohyd Polym. 2015;130:26–31. doi: 10.1016/j.carbpol.2015.04.071. [DOI] [PubMed] [Google Scholar]
- 22.Mekjaruskul C., Jay M., Sripanidkulchai B. Modulatory effects of Kaempferia parviflora extract on mouse hepatic cytochrome P450 enzymes. J Ethnopharmacol. 2012;141(3):831–839. doi: 10.1016/j.jep.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Aller M., Guillarme D., Veuthey J.-L. Strategies for formulating and delivering poorly water-soluble drugs. J Drug Deliv Sci Technol. 2015;30:342–351. [Google Scholar]
- 24.BASF Technical information: Kollicoat® IR. 2013. https://industries.basf.com/en/documentDownload.8805700479445.Kollicoat%C2%AE%20IR%20-%20Technical%20Information.pdf [Accessed 5 June 2016]
- 25.Enose A.A., Dasan P.K., Sivaramakrishnan H. Formulation and characterization of solid dispersion prepared by hot melt mixing: a fast screening approach for polymer selection. J Pharm. 2014;2014:13. doi: 10.1155/2014/105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A., Jain C.P. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 27.BASF Technical information: Soluplus®. 2010. https://industries.basf.com/en/documentDownload.8805242743253.Soluplus%C2%AE%20-%20Technical%20Information.pdf [Accessed 5 June 2016]
- 28.Caron V., Hu Y., Tajber L. Amorphous solid dispersions of sulfonamide/Soluplus® and sulfonamide/PVP prepared by ball milling. AAPS PharmSciTech. 2013;14(1):464–474. doi: 10.1208/s12249-013-9931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorajana A., Rajendran A., Yew L.M. Preparation and characterization of cefuroxime axetil solid dispersions using hydrophilic carriers. Int J Pharm Investig. 2015;5(3):171–178. doi: 10.4103/2230-973X.160857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vippagunta S.R., Wang Z., Hornung S. Factors affecting the formation of eutectic solid dispersions and their dissolution behavior. J Pharm Sci. 2007;96(2):294–304. doi: 10.1002/jps.20754. [DOI] [PubMed] [Google Scholar]
- 31.Homayouni A., Sadeghi F., Nokhodchi A. Preparation and characterization of celecoxib dispersions in Soluplus®: comparison of spray drying and conventional methods. Iran J Pharm Res. 2015;14(1):35–50. [PMC free article] [PubMed] [Google Scholar]
- 32.Laitinen R., Priemel P.A., Surwase S. Theoretical considerations in developing amorphous solid dispersions. In: Shah N., Sandhu H., Choi D.S., editors. Amorphous solid dispersions: theory and practice. Springer; New York: 2014. pp. 35–90. [Google Scholar]
- 33.Huang Y., Dai W.-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4(1):18–25. doi: 10.1016/j.apsb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rane Y., Mashru R., Sankalia M. Effect of hydrophilic swellable polymers on dissolution enhancement of carbamazepine solid dispersions studied using response surface methodology. AAPS PharmSciTech. 2007;8(2):E1–E11. doi: 10.1208/pt0802027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssens S., de Armas H.N., Remon J.P. The use of a new hydrophilic polymer, Kollicoat® IR, in the formulation of solid dispersions of itraconazole. Eur J Pharm Sci. 2007;30(3–4):288–294. doi: 10.1016/j.ejps.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Xu L., Li S.M., Wang Y. Improvement of dissolution rate of ibuprofen by solid dispersion systems with Kollicoat® IR using a pulse combustion dryer system. J Drug Deliv Sci Technol. 2009;19(2):113–118. [Google Scholar]