Graphical Abstract
Keywords: Flavonoid, Activity, Structure, Pharmacokinetics, Bioavailability
Abstract
Flavonoids, a class of polyphenol secondary metabolites, are presented broadly in plants and diets. They are believed to have various bioactive effects including anti-viral, anti-inflammatory, cardioprotective, anti-diabetic, anti-cancer, anti-aging, etc. Their basic structures consist of C6—C3—C6 rings with different substitution patterns to produce a series of subclass compounds, and correlations between chemical structures and bioactivities have been studied before. Given their poor bioavailability, however, information about associations between structure and biological fate is limited and urgently needed. This review therefore attempts to bring some order into relationships between structure, activity as well as pharmacokinetics of bioactive flavonoids.
1. Introduction
Flavonoids are a class of compounds presented broadly in nature. Concerns about their extensive profitable bioactive benefits, including anti-viral/bacterial, anti-inflammatory, cardioprotective, anti-diabetic, anti-cancer, anti-aging, have long been received great attention and well supported by numerous studies [[1], [2], [3], [4]]. Till now, more than 9000 flavonoids have been reported [5], and their daily intake varies between 20 mg and 500 mg, mainly from dietary supplements including tea, red wine, apples, onions and tomatoes [[6], [7]]. Flavonoids are frequently found as glycosylated or esterified forms, consisting of C6—C3—C6 rings, namely rings A and B linked by three-carbon-ring C (Fig. 1) [8]. According to substitution pattern variations, flavonoids can thus be classified into different subclasses, providing an extremely diverse range of derivatives [8]. Although wide distribution and broad benefits, bioavailability of flavonoids is poor which may significantly influence the impact of nutritional effects, besides, information about pharmacokinetics in detail is limited. How to improve the issue is far from settled. This review attempts to bring some order into structure, activity as well as biological fate of flavonoids with particular emphasis on their relationships involved. Moreover, detailed information on structure-based drug design is crucial and required.
Fig. 1.
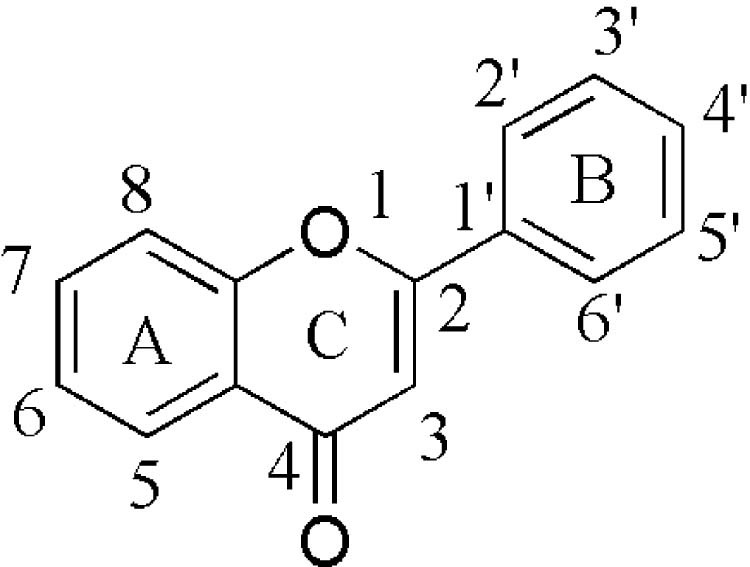
Basic skeleton or structure of flavonoids.
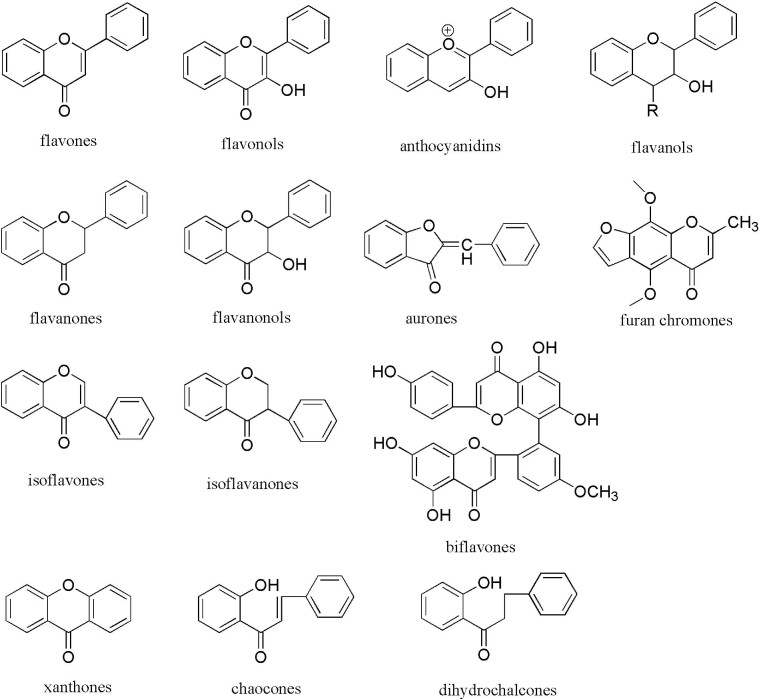
2. Chemical structure and classification of flavonoids
Flavonoids are a group of low molecular weight substances based on 2-phenyl-chromone nucleus (Fig. 2). They are biosynthesized from derivatives of acetic acids/phenylalanine by means of shikimic acid pathway. Traditionally, flavonoids are classified by oxidation degree, annularity of ring C, and connection position of ring B (Fig. 3). Flavones and flavonols contain the largest number of compounds, representing the narrow-sense flavonoids, namely 2-benzo-γ-pyrone category. Quercetin belongs to flavonol class, for example, has been studied most commonly. Flavanones and flavanonols possess saturated C2 C3 bonds, and often coexist with relevant flavones and flavonols in plants. Isoflavones, such as daidzein, are 3-phenyl-chromone substances. As key precursors of flavonoid biosynthesis, chalcones are ring C-opening isomers of dihydroflavones, responsible for color appearance of plants. Lacking typical structure of flavonoids, aurones are five-membered ring C benzofuran derivatives. Anthocyanidins are a group of important chromene pigments for characteristic color of plants, existing in the form of ions. Flavanols are reduction products of dihydroflavonols, especially with flavan-3-ols widely distribution in plant kingdom, also known as catechins. However, there are still other flavonoids without C6—C3—C6 skeleton, for instance, biflavones, furan chromones and xanthones. Glycosides, with different category, number and connecting pattern, are predominate existing forms of flavonoids. Preferred glycosylation sites are associated with the structure of aglycones.
Fig. 2.
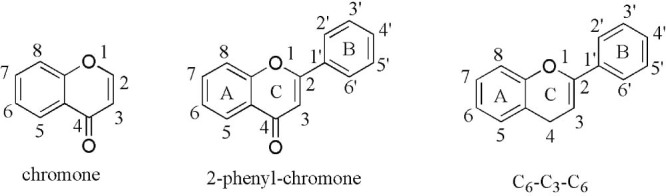
Chemical structures of the flavonoid classes.
Fig. 3.
Chemical structures and classification of flavonoids.
3. Structure activity relationship (SAR)
A myriad of epidemiological studies have suggested a negative correlation between medicinal flavonoids consumption and development of various diseases [[9], [10], [11]], thereinto, flavonoids with typical structures can interact with enzyme systems involved in crucial pathways, showing effective polypharmacological behaviors [[1], [6], [7]]. Thus, it is not surprising that the relationships between chemical structures and activities have been extensively studied.
3.1. SAR for anti-viral/bacterial activity
Nowadays, bioactive flavonoids have been investigated for potent anti-viral/bacterial activity. For instance, therapeutic activities against influenza virus [6], canine distemper virus [12], hepatitis C virus [9], and Escherichia coli [13], have been attributed, largely, to chemical structures in particular patterns of methoxylation, glycosylation and hydroxylation [[12], [14]]. Over years, related SAR researches have been characterized in diverse aspects. The C2 C3 double bond has been documented in most cases as a basic favorable element, which has been illustrated via the human fibroblast collagenase catalytic domain expression inhibitory activity loss of ampelopsin in comparison to quercetin [13].
In the case of hydroxylation, substitution style takes an important role. With regard to ring A hydroxylation, the positive role of 5-/7-hydroxyl derivatives has been suggested by six potential anti-H5N1 influenza A virus 5, 7-diOH flavonoid candidates [15], and less potent anti-human fibroblast collagenase catalytic domain (MMP1ca) effects of daidzein than quercetin [13]. Additionally, better MMP1ca inhibitory activity of 3′-OH ampelopsin/5′-OH gallocatechin gallate compared to daidzein/epicatechin gallate implys the contribution of hydroxylation in ring B [13]. Amongst others, a catechol group is the most common functional moiety. For example, better inhibitory activity of quercetin than morin in canine distemper virus inhibition [12], has provided a prominent therapeutic thought for novel drug synthesis. In the aspect of ring C, significant contribution of 3-OH has been observed (quercetin vs. luteolin) [16]. Apart from the site, the number of hydroxyl groups is another influencing factor. More hydroxyl groups results in lower hydrophobicity, which is obstructive for flavonoids to partition into biological membranes. Interestingly, sometimes certain hydroxyl group-rich-flavonoids do possess higher activity. The impact of hydrophobicity and electronic delocalization on the strength of hydroxylation assignment should be considered together, however. Additive hydroxyl groups might confer reduced hydrophobicity but higher C3 charges which is a direct indicator for pharmacological activity [16].
As for methoxylation, its influence on membrane fluidity increase is correlated a large extent to the pathopoiesia of some viruses/bacteria, decreasing activity is therefore obtained. On this occasion, two polymethoxy flavonoids (PMFs) have been observed to exhibit decreasing anti-E.coli activity compared with related aglycones [16]. The study of Amorpha fruticose L. flavanones corroborates the previous experiment that bacterial neuraminidase inhibition of compound 2 is 70-fold stronger than unmethylated compound 3 [14].
For flavonoid glycosides, greater anti-viral effects have been described and exemplified by puerarin and rutin/hesperidin with respect to daidzein and quercetin, which further provides favorable evidences for saccharides linkage with higher biological activity [13]. However, one deficiency about aforementioned structural influencing factors is the particular level of increase or reduction is not recorded in detail. The decipherment of SAR exerted by selected flavonoids in the context of anti-viral/bacterial effects may lead to screening of optimal compounds for dietetic therapy and/or medical treatment.
3.2. SAR for anti-cancer activity
By far, different mechanisms have highlighted the role of flavonoids in cancer-therapy, including induction of apoptosis [1], proteasome inhibition, nuclear factor signaling inhibition [17], differentiation induction [1], cell cycle arrest induction [9], receptor interaction [18], or interaction with carcinogenic associated enzymes [18]. Moreover, flavonoids could exhibit specific cytotoxicity on cancer cells, causing large attention focused on flavonoid-based cytostatics as anti-cancer agents [19].
The significant role of C2 C3 double bond contributes to molecular planarity and conjugation between rings C and A/B, which is essential for potent tumor inhibition (apigenin vs. naringenin) [[1], [18]]. In order to explore interactions between C2 C3 double bond and anti-cancer effects, tumor cell lines such as colon adenocarcinoma cells [20], and MDA-MB-231 breast cancer cells [17], have been utilized for in-depth analysis involving gene expression. Comparative enhancing tumor inhibitory effects about 2, 3-dihydrochrysoeriol and dihydroisorhamnetin with respect to unsaturated counterparts are further elucidated in detail by 65% and 82%, respectively [17]. Moreover, greater inhibition would occur with co-existence of C2 C3 unsaturation and two ring B hydroxyl groups [18].
Notably, many reports have provided evidences about influences of hydroxylation on tumor modulation. Specific hydroxylated flavonoids possess stronger inhibitory activity on cancer cells than permethoxylated counterparts. The contributive role of 6-OH and 5, 7-diOH has been disclosed [[20], [21]]. Ring B substitution such as a catechol moiety with vital influences has been proposed, and additional hydroxyl group substitution in ring B does not alter the activity [1]. In the case of ring C, 3-hydroxylation has been considered as a highly decisive moiety for improving biological effects (quercetin vs. kaempferol) [17]. Apigenin, for another example, lacking 3-OH possesses significantly lower antiproliferative activity than kaempferol [20]. Higher affinity between binding site and 3-OH has further been proposed [21].
Flavonoid derivatives of O-methylation are contributed to enhanced biological activity, which is frequently associated with ring A polymethoxylation. Out of several Ougan flavonoids tested in cell morphology change study, two A-ring PMFs, nobiletin and tangeretin, exhibit the highest proliferative inhibition, also suggesting the significance of C-8 position in anti-proliferative activity of flavonoids. Additionally, the anti-proliferative promoting effect of 3′-methoxy group has been disclosed from the higher inhibition of nobiletin than tangeretin [19]. In accordance with the previous study, glycosylation does not contribute to cell differentiation induction [1]. The antiproliferative weakening effects of flavonoid goycosides might be derived from steric blocking involved in cell entry and receptor binding [19]. Further explanation should be given, however.
3.3. SAR for anti-age-dependent-neuropathology activity
With respect to brain, flavonoid extracts or monomers have been effectively utilized, thus preventing neuro-degeneration [22]. For example, cholinesterase inhibition of fourteen Salvia species flavonoid extracts on Alzheimer's disease are explored [23]. Historically bioactivity of flavonoids against neurodegeneration is attributed to classical antioxidant effects, however, emerging evidences now have been attaching importance to interactions on acetylcholinesterace (AChE)/butyrylcholinesterase (BChE) [23], GABA-receptor [24], mitochondrial dysfunction [25], critical neuronal signaling pathways in controlling neuronal resistance to neurotoxical oxidants and inflammatory mediators [26], or through chelation of transition metal ions [22].
In order to delineate anti-age-dependent neuropathology more in-depth, structure-dependent stimulating manner of flavonoids has been investigated. As an example of this, beneficial role of ring B hydroxylation has been suggested at the side of galangin, kaempferol and myricetin, since only the latter two could significantly improve learning capability [27]. Besides, in light of differences in neuroprotective activity of 10 Rhus verniciflua Stokes flavonoids [28], the positive contribution of 5-dehydroxylation and 3′,4′-ortho-dihydroxylation in ring B have been proposed. As above-mentioned, it appears that hydroxylation takes an important role. Furthermore, a study of flavonoid-PI3-kinase interaction has further confirmed the pivotal role of ring B hydroxylation [29]. Pattern of hydroxylation in ring B and unsaturation degree of C2 C3 double bond account for a great deal. In light of this, it appears that interactions of flavonoids with some other receptors, downstream kinases or signaling pathways may be structure-dependent, since highly sensitive allosteric modulation has been proposed [24]. Meanwhile, a 12 times higher BChE binding ability of galangin than AChE, has supported the standpoint more concretely [30]. The researchers finally selected galangin as a promising potent therapeutic agent. In vivo, in a D-galactose-induced cognitive impairment model of mice, cognitive therapeutic mechanism of galangin has been demonstrated in the perspective of oxidative stress amelioration, Na+, K+-ATPase enhancement and regulation of ERK-CREB pathway expression [27]. Detailed SAR need further investigation, however.
3.4. SAR for cardioprotective activity
The pivotal role of flavonoids from apple peel [11], cranberry [31], onion [32], and herbs [3], in cardiovascular disease prevention, has been reported. In addition, evidences from studies in human, animal and cell model further suggest the contribution of flavonoid intake. In a recent cross-sectional study, higher dietary flavonoid intake is associated with improving lipid profile in a cohort of 1393 subjects in China [31].
In recent years, several SAR evidences such as specific alteration degree have been gathered from evaluation of flavonoid effects on eNOS transcription factor Krüpple like factor-2 expression [33]. In the light of the results, gene dependent effects have been provided that the presence of C2 C3 double bond results in a double efficient structure in terms of eNOS and ET-1 expression (apigenin vs. naringenin), substitution in position 3 including hydroxylation or glycosylation could decrease eNOS/ET-1 expression by 2 times or so (quercetin/rutin vs. luteolin), and a 4-carbonyl moiety leads to an approximately 1.35 fold gene expression (quercetin vs. epicatechin/catechin). Meanwhile, electronic distribution modification effects by those characteristic functional groups have been suggested in the case of SAR. More detailed, results from a SAR study of 12 flavonoids with paraoxonase1 (rePON1) has emphasized the ‘protein-binding’ mechanism which was due, moreover, at least partly, to different hydroxylation substitution, C2 C3 double bond and 4-carbonyl group in ring C [34]. Owing to C2 C3 double bond in ring C, PON1 interactions are higher for flavones and flavonols because of molecular planarity, which may contribute to electron delocalization between rings A and B, therefore 3-hydroxyl group and 4-carbonyl oxygen atom are coplanar. Accordingly, SAR is investigated into deeper levels. Apparently, to establish a putative relevance of flavonoids for cardiovascular protection, it is necessary to study different sub-class flavonoids with typical substitution on the top of pharmacological mechanism investigation.
3.5. SAR for anti-inflammatory activity
Generally, foremost role of flavonoids on inflammation involved diseases such as leukemia, sepsis, asthma, sclerosis, atherosclerosis, psoriasis, allergic rhinitis, ileitis/colitis, rheumatoid arthritis, etc. has been proposed [[8], [35], [36], [37], [38], [39], [40], [41]]. Thereinto, diverse inflammatory mediators have been studied including plasma proteases, prostaglandins, leukotrienes, interleukin, interleukins, nitric oxide, proinflammatory cytokines, chemokines [[42], [43], [44], [45], [46]], as well as relevance signaling pathways [47]. Taken together, anti-inflammation activity of flavonoids has been widely investigated, and specific mechanisms involved might not be united, which adds the urgency to explore SAR in-depth.
Generally, preferred structural aspects for anti-inflammatory effects of flavonoids are summarized as following: (a) The C2 C3 double bond might attribute to molecular planarity. Its absence results in a larger volume/surface ratio, since diosmetin shows stronger effects than hesperetin [48]. (b) Hydroxylation pattern, such as 3′-hydroxylation (since fisetin shows maximal effect), 5-hydroxylation in the case of isoflavones, especially ring B catechol moiety, provides effects for inducing cell differentiation (apigenin vs. chrysin) [48]. (c) Methoxylation greatly enhances anti-inflammatory property, probably through ionization of hydroxyl groups and more pronounced NF-kB signaling pathway inhibition (O-methylation of chrysin) [47]. (d) Glycosides with lower lipophilicity, showed lower anti-inflammatory property, which may be due to lower hydrophobicity as well as sterical hindrance, decreasing membrane permeability [8]. (e) Additionally, bulky substitution has been investigated. The presence of C7 C8 double bond, C-butyrolactone moiety and 5-acetic acid/lactone group have been recognized as possible taxonomic markers in an anti-inflammatory study of flavonoids from Cryptocarya chingii [49]. Of note, more compounds need evaluation in order to draw definitive conclusions about SAR in regard to flavonoids.
3.6. SAR for anti-diabetic activity
Diabetes mellitus (DM) is a multifactorial chronic hyperglycemic disease. Beneficial role of flavonoids in DM treatment is obvious on account of preeminent efficacy in terms of complications and decreased side reactions. Numerous cell, animal and epidemiological studies support the hypoglycemic activity of flavonoids [[4], [50]]. For bayberry flavonoids, anti-diabetic effects on glucose consumption have been well investigated in HepG2 cells [4]. In the 2007–2009 Korean National Health and Nutrition Examination Survey, 4186 Korean participants have been administrated with various subclass flavonoids, underlying relationships between DM risk factors and flavonoids intake [50].
As DM is concerned, anti-diabetic mechanism of flavonoids has been well known. Moreover, effects on various enzymes and molecular targets/signaling pathways, have been mentioned. It is therefore pertinent to point out a definite SAR to explain different actions of various flavonoids. Accordingly, it is of special interest that chalcones are found to be potential inhibitors of a-glucosidase, which is an effective target on glucose homeostasis. Phlorizin has been used as a classical SGLT-1 inhibitor clinically, which may link sugar to glucose site and bind the aglycone, thus affecting inhibitor binding [51]. Interestingly, hydroxylation and planarity in position 7 in several flavonoids provide capacity for PPAR activation [52]. The exploration of anti-diabetic ability of 44 flavonoids on adipogenesis of 3T3-L1 cells has shown positive contribution of methoxylation and inverse relationship between hydroxylation and anti-diabetic activity [53]. Unlike common SAR regarding hydroxylation and methoxylation, substitution of glycosylation especially glucosylation in position 3, has been demonstrated a lot. In a HepG2 cell model, significant hypoglycemic behavior of flavonoid-3-glucose has been observed instead of rhamnose [4]. Recently, effects of regulating blood glucose level and improving pancreatic β cell function of quercetin-3-glucoside have been observed in a diabetic KK-Ay mice model [54]. Detailed mechanism of C-3-Glu/Gly in regulating glucose consumption deserve further convincing support, however. Importantly, among synthesized novel anti-diabetic hybrids of 6-/7-OH flavones, compound 64 and 65 are evaluated as the most potent with bulky hydrophobic substitution in ring B as well as smaller functional groups like tertbutyl/isopropylamine at nitrogen atom. Collectively, relevant SAR studies of flavonoids remain elusive, requiring to precisely depict interactions of flavonoids with molecular targets.
3.7. SAR for anti-oxidant activity
Numerous studies have attributed abroad nutritional effects of flavonoids to anti-oxidant activity, and most anti-oxidant chemical assays are owing to free radical scavenging mechanisms [19]. However, those results are often ambiguous and incomparable based on different oxidant species or analytical methods applied. Generally, mechanisms underlying their antioxidant property are free radical scanvenging and transition metal ion chelating activity. Due to reducing activities of phenolic hydroxyl groups, flavonoids are able to donate hydrogen. Along with delocalization of phenoxy radical products, flavonoids can protect against various disease damage from ROS [55]. On the other hand, flavonoids can chelate transition metals which are able to promote hydroxyl radicals formation in reduced forms by virtue of Fenton reaction under abnormal conditions. Furthermore, considerable attention has been focused on SAR for antioxidant activity of flavonoids. In a zebrafish larvae organism, 15 commercially available flavonoids have been used to screen optimum radical oxygen scavenging compounds with lower toxicity as well as higher antioxidant activity [56]. In another example, with isoflavan showing the highest antioxidant capacity among the tested flavonoids, contribution of resorcinol moiety in ring A has been highlighted [34]. Those obtained results are clear evidences of basic structural elements for available anti-oxidant activity.
3.7.1. The C2 C3 double bond and 4-carbonyl group in ring C
A characteristic structural feature among flavonoids subclasses is the existence of a C2 C3 double bond in conjugation with a 4-carbonyl group in ring C, whose contribution to SAR has been investigated. However, several authors believe that there is no direct relationship between these moieties with anti-oxidant activity while other structural criteria are fulfilled. For example, although potent electron donating capacity has been obtained for certain selected flavonols, no significant deviation is observed between cellular ROS inhibition and characteristic structural moieties [55]. On the other hand, with other structural criteria fulfilled as the premise, the presence of a C2 C3 double bond in conjugation with a 4-carbonyl group plays an assisting role in anti-oxidant activity. For example, apigenin could bind rePON1 more effectively than naringenin with C2 C3 saturation, providing favorable evidence [34]. The presence of 4-carbonyl group is able to induce electron shifts via resonance effects, therefore influencing the dissociation constant of phenolic hydroxyl groups and phenoxy radical stability in ring B. Unsaturation of C2 C3 double bond provides planarity and electron coupling to the molecule so that conjugation between ring C and ring A/B could be obtained. Similarly, association with 5-OH often provides a hydrogen bond. Taken together, combination of 4-carbonyl group with C2 C3 double bond or other electron donating groups efficiently delocalizes ring B electron, thus significantly enhancing antioxidant activity.
3.7.2. Hydroxyl groups
Generally, position and number of hydroxylation correlate reasonably to anti-oxidation of flavonoids. Hydrogens and electrons are donated by ring B hydroxyl groups to hydroxyl, peroxyl, and peroxynitrite radicals, forming relatively stable flavonoid radicals. On the other side, flavonoids could scavenge the resulting radicals to neutralize the prior effect. The premise of at least two hydroxyl groups in ring B for anti-oxidant capacity is suggested on the basis of significantly improved anti-oxidant effects [48]. Amongst others, 3′, 4′-catechol group is recognized as the most significant responsible pharmacophore, producing extremely stable ortho–semiquinone radical via electron delocalization to confer high activity through intra-molecular hydrogen bonding between catechol hydroxyl groups. Apart from two hydroxyl groups in ring B, influence of only one substitution does make sense. In this perspective, apigenin with 4′-OH has been deduced to increase erythroid differentiation activity [48]. Besides, flavonoids with ortho-dihydroxyl group in ring B all possess stronger inhibitory effects than those with 4′-hydroxylation, which has been shown by mean Imax values as 36.2% and 22.5%, respectively. Ring A hydroxylation may contribute less to anti-oxidant activity than ring B, since ortho-dihydroxyl group in ring B is more easily oxidizable than ring A meta-dihydroxylation [57]. Anyway, 5, 7-diOH in ring A does interfere with anti-oxidant effects. Strong activities of luteolin, quercetin, kaempferol and apigenin emphasize the contribution of 5- and 7-OH combination as 2, 4-substituted resorcinol substructure [34]. Evidence has been proposed about ring C hydroxylation represented by 3-hydroxylation which is impaired by electron donating substitution in position 5 and position 7 in ring A. In the light of a comparison of antioxidant property of luteolin and quercetin, the presence of 3-OH clearly contributes to suppression of anti-oxidant activity [58]. Hypothesis of activity diminishment from 3-hydroxylation has been supplemented in the study of Haydar Çelik et al. [48]. Taken together, although individual influence of hydroxylation has been demonstrated, overall modulation on the molecule is more than just a collection. As for quercetin, 3-hydroxyl blocking in ring C and catechol moiety retaining in ring B do not promote antioxidant ability in brief [59]. Generally, electron transfer within the resonance system and total number of hydroxyl groups are usually considered while taking into account overall hydroxylation system. Since hydrophilicity is enhanced with hydroxyl number increasing, insertion of flavonoid nucleus with more hydroxyl groups is held up in the hydrophobic cavity which might constitute connection to the active site of relevance enzyme [58].
3.7.3. O-methylation
The influences of O-methylation include molecular hydrophobicity, electron donation and planarity. O-methyl substitution may cause steric hindrance, therefore decreasing anti-oxidant activity. Ring B is particularly sensitive to substitution position. Varying methylation on free hydroxyl groups in ring B alleviates anti-oxidant ability by altering coplanarity [60]. In the in vitro ferric reducing antioxidant power tests, inactivation of anti-oxidant property induced by ring B O-methylation has been proposed, however, increasing antioxidant property is obtained in methoxyl flavonoid derivates with flavonoid-flavonoid interaction under consideration [59]. It is rational to postulate that multiple methoxylation substitution in ring A would counterbalance the contribution of catechol moiety in ring B. Given the fact that radicals used may not always participate into hydrophobic membrane where polymethoxylated flavonoids accumulate, it is reasonable to suggest that the influence of O-methylation depends on many factors including substitution and lipophilicity of related substrates.
3.7.4. Glycosylation
Anti-oxidant ability of flavonoid glycosides in different forms such as O- or C-glycosides has been investigated. In the case of C-glycosides, whose antioxidant activities have been confirmed by chemical assays and elucidated with higher abilities in comparison to O-glycosides, moreover [2]. Almost total radical scavenging ability has been attributed to C-glycosyl flavonoids rather than O-glycosides in the study of Davide Barreca et al. [60]. Similar results have been obtained in another assay with C-glycosides responsible for nearly 50% antioxidant activity [60]. It is worth noting that aforementioned experiments about C-glycosides are carried out by virtue of chemical assays, in vivo data and in-depth interpretation are still required. Flavonoid glycosides occur in diet generally in ring A or C as O-glycosides [61], and corresponding substitution in ring A has a far greater impact on activity. Like methylation, coplanarity and electron delocalization are influenced by glycosylation, which confers decreasing activity. On the basis of anti-oxidant effects of fourteen structural different flavonoids, the significant role of 3-glycosylation has been pointed out in the case of quercetrin and rutin [48]. According to the author, those attenuating effects may be the result of enhancing polarity or increasing steric hindrance due to sugar moiety in position 3. Moreover, anti-oxidant property enhancing effect of 6-glucosylation and attenuating effect of 8-glucosylation in ring A, which are attributed to torsion angle and coplanarity broken, have been disclosed [57]. In spite of those results, influence of the number of glycosylation which is associated with lipophilicity has been considered. In addition, to the number of glycosylation, position and structure of saccharides are of great significance. Interestingly, different anti-oxidant inhibitory activities of eight Epimedium elatum flavonoids has been determined by 3- or 7- glycosylation with different number, position and structure, which may mainly stem from saccharide itself [61]. Although anti-oxidant activity of glycosides is weaker than corresponding aglycones, bioavailability is plausibly increased on account of cleavage of glycosidic bonds often occurred in vivo, thus raising anti-oxidant activity.
3.7.5. Summary of SAR for favonoid-induced anti-oxidant activity
Taken together, to flavonoids, the existence of a C2 C3 double bond in conjugation with a C4-carbonyl group, certain hydroxylation pattern especially a catechol moiety in ring B, methoxyl groups, and less saccharides connection confer higher anti-oxidant properties. Of which, the mechanism might involve planarity that is contributed to electron shifts across the molecule further influencing dissociation constants of phenolic hydroxyl groups, so that the whole molecule could bind to relevance molecular targets like enzymes in a more efficient pattern. Hydrophobicity is another consideration that relates to absorption across bio-membrane. As previously mentioned, however, there are discrepancies in SAR for flavonoid-induced anti-oxidant activity, which may probably stem from different mechanisms as well as various methods of detection/measurement of oxidative processes. Apart from those factors, influences among each functional moieties on final anti-oxidant property of the molecule cannot be ignored. In a Ceric Reducing/Antioxidant Capacity antioxidant test, the order of eight studied flavonoids has been established, and related anti-oxidant hierarchy of individual functional moiety is summarized owing to mutual correlations: 2′, 4′-diOH, 4′-OH ≈ 3′, 4′-diOH > 2, 3-double bond in conjugation with 4-carbonyl substitution, 3, 5-diOH in conjugation with 4-carbonyl substitution, 3-OH in conjugation with 4-carbonyl substitution, 5-OH in conjugation with 4-carbonyl substitution, and 3, 5-diOH [62]. It can be concluded, therefore, that contributions of each structural moiety are different, and their synergistic/antagonistic interactions might further influence interactions between flavonoids. Substantiating antioxidant activities of flavonoids with respect to structural characteristics is very challenging, nevertheless, which may definitely require further investigation and eventually lead to developing nutraceuticals for relieving oxidative stress in human.
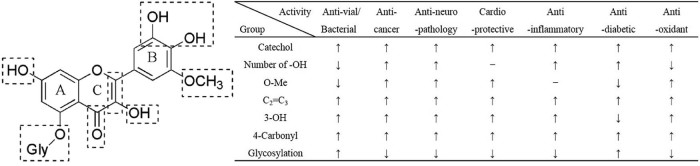
3.8. Summary of SAR
Cumulative findings concerning SAR derived from pharmacological studies have provided beneficial evidence of the role of various functional groups on nutritional utilities. Based on the foregoing, it's rational to conclude that a C2 C3 double bond, a 4-carbonyl group, and hydroxylation patterns especially 3-OH and a ring B catechol moiety, are major recognized beneficial determinants for various beneficial effects of flavonoids (Fig. 4). Apart from disagreements about SAR of certain biological activity, several inter-pharmacological crosslights may exist and originate in different mechanisms of action, diverse analytical methods and different subjective opinions. For example, O-methylation is beneficial to anti-viral/bacterial, anti-diabetic but adverse for anti-cancer and anti-inflammatory activities. Positive effects of hydroxylation have been delivered in the aspect of anti-viral/bacterial, anti-cancer, cardioprotective, ruling out anti-diabetic activity. Generally, glycosylation may decrease corresponding activity of anti-age-depentdent, but anti-viral/bacterial on the contrary.
Fig. 4.
Summary of SAR of flavonoids.
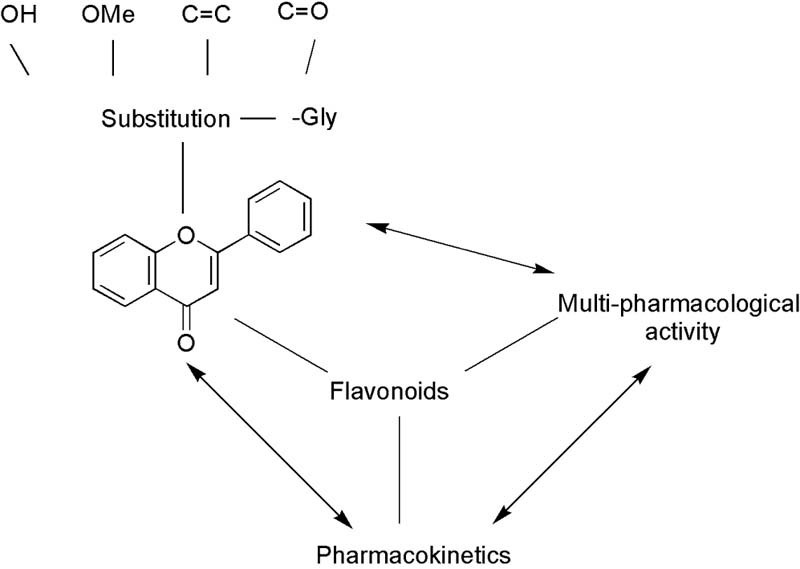
Centered on existing discussion about SAR of flavonoids, research status is as follows. First, the majority of studies have focused on characteristic functional groups that would alter related pharmacological activity, offering favorable reference for therapeutic substance screening. Nevertheless, specific influencing degree is rarely mentioned. Second, deeper investigation on interactions among various functional moieties is deficient. Last but not least, opinions about concrete mechanism of certain functional moiety, which undoubtedly are consequences of interactions of multiple factors, have been confined to surface phenomena. There are many changes resulted from the transformation of functional groups, such as the alteration of steric configuration, polarity of whole molecule, and physico-chemical property. In detail, the alteration of steric configuration induced by different functional groups, is a critical factor for evaluating suitability with target sites of action, thereinto, certain molecular size resulted from particular substitution is required for matching with the gap of target; the alteration of polarity of whole molecule is one of the decisive factors about electron distribution as well as interaction forces such as hydrogen bonding, which is the critical step for curative effects expression; physico-chemical property alteration could lead to variation of solubility and modification of in vivo absorption that active metabolites may be produced by given compounds with specific functional groups, which thus expands another research area for systematic elucidation of SAR of flavonoids thoroughly.
4. Absorption and metabolism
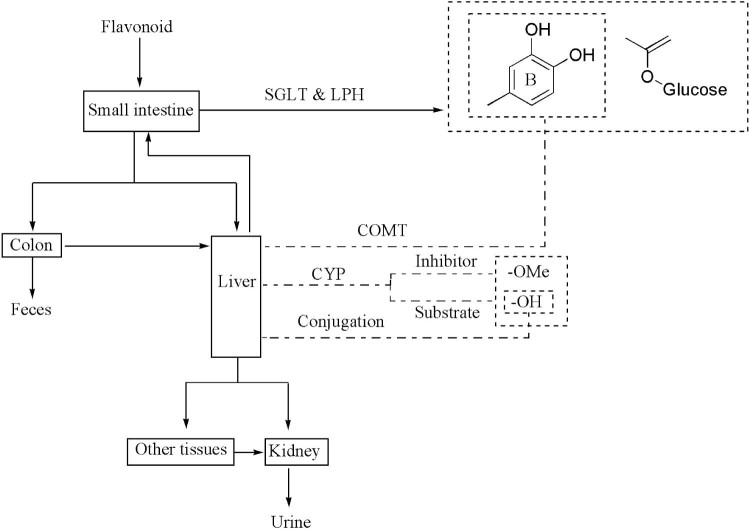
4.1. SAR for pharmacokinetics
Owing to broad-spectrum nutritional effects and wide-spread in diet, flavonoids have been administrated orally in the majority situation. As mentioned, their poor bioavailability has been the primary limitation for successful utilization. It is therefore necessary to study the pharmacokinetic profile and related influencing factors (Fig. 5). On this issue, structural influence is recognized as potential influencing factor. Intestinal mucosa and liver are major sites for biotransformation. Aglycones are absorbed into enterocytes in passive transport form. After oral administration, however, most flavonoids exist as glycosides. The first step to enter into systemic circulation may be deglycosylation through active uptake by sodium-dependent glucose transporter (SGLT1) with following deglycosylation by cytosolic β-glycosidase, or primarily undergo luminal hydrolysis by lactase phlorizin hydrolase (LPH) with subsequent passive absorption of released aglycones [63]. It is note-worthy and reasonable to point out that deglycosylation pattern may depend on the nature of aglycone and connected sugar. Absorption trend of flavonoid glycosides has been investigated and ascribed to absorption promotion effect of SGLT upon glucosides [64], however, SGLT may not the only explanation for better absorption, since glucosides are more hydrophilic, diffusion through unstirred water layer to LPH located brush border membrane is much easier. Furthermore, incorporation in polar-nonpolar interface of membrane, which is achieved by electrostatic and hydrophobic interactions with phospholipids, could be obtained by evaluating characteristic structures of flavonoids, so that bioavailability may be predicted. Aglycones are thus considered with higher bioavailability and earlier absorption than glycosides by virtue of better membrane interactions. In contrast to O-glycosides, C-glycosides are more resistant to hydrolysis. The pharmacokinetic profile of vicein-2(first and zero order absorption constant as 0.274/min and 16.3%/min respectively, and bioavailability as up to 40.2 ± 2.5%), which is a C-glycoside from Lychnophora ericoides leaf, has indicated relatively stable metabolic process [65]. In parallel, the findings of a comparative absorption study of apigenin and correlative glycosides, wherein apigenin 8-C-glucoside-2-O-xyloside is almost unchanged while major metabolites of apigenin/O-glycosides are related aglycone as well as glucuronides in portal blood, has further confirmed the metabolic stability of C-glycosides [66]. Without doubt, those studies have left room for detailed data about causal SAR for glycoside absorption.
Fig. 5.
Flavonoid biotransformation.
Followed by deglycosylation, phase 2 metabolism often occurs continuously in epithelial cells of small intestine. Then, further biliary excretion or enterohepatic cycling in colon occured. Corresponding aglycones are thus released and absorbed in large intestine, or undergo further degradation. As one of favorable phase 2 metabolic enzymes, catechol-O-methyltransferase catalyzes flavonoids with catechol moiety in ring B. Generally, methylated flavonoids exist predominantly in the form of 3′- rather than 4′- methyl metabolites. According to comparative pharmacokinetic behaviors of fisetin and three of its metabolites, geraldol as 3′-methylated product possess faster elimination process (t1/2 = 0.45 h) [67]. Flavonoids are able to act as CYP1 inhibitors of procarcinogens or substrates [[68], [69]]. Hydroxylation is attributed to inhibition, while methoxylation to metabolization [[69], [70]]. Therefore, it is reasonable to figure out bioavailability increasing effect of certain flavonoids as CYP substrates while co-administration with other flavonoids as CYP inhibitors, based on first-pass metabolism weakening effect. Flavonoids with hydroxyl groups are vulnerable to conjugation. In contrast, both absorption and excretion of O-methylated flavonoids are relatively slow thus facilitating better bioavailability, owing to delayed hepatic metabolism protective effects, increasing permeability across bio-membrane and more accumulation exerted by O-methylation [[70], [71]]. With comparison to hydroxyl flavonoids, 100-fold higher plasma concentration has been obtained on account of methoxylated ones [70]. In a comparative pharmacokinetic study of quercetin, kaempferol and isorhamnetin, the slowest process and the highest degree of absorption have been observed for isorhamnetin (tmax = 7.21 h, Cmax = 195.96 ng/ml), which is a methylated flavonoid [72]. It can be concluded that, due to extensive metabolism, no matter where flavonoids undergo absorption, intact flavonoids enter into systemic circulation rarely. In a pharmacokinetic/excreted model of rats, 0.81% and 0.05% of uncovered form of 5, 7, 3′, 4′-tetramethoxyflavone have been excreted in feces and urine, respectively, indicating metabolites are the main form of excretion [71]. Information from other experiments has further supported the thesis that nearly 74% luteolin formed glucuronide conjugates with hydroxyl groups substituted in 3′- (51%), 4′- (44%) and 7- (5%) position [73].
4.2. Pharmacological activities of aglycones and related metabolites
Not only biological fates but also nutritional effects of flavonoids may be influenced by metabolism, which may be related to structural moieties altering. Some authors believe that demethylation often confers more potent pharmacological activity. Identified as the major metabolite of nobiletin in mice urine, 3′, 4′-didemethyl-nobiletin, has been recognized with stronger anti-tumor property [70]. Similarly, O-methylation of 7-hydroxyflavone confers lower activity [74]. Moreover, the same research group has idtentified galangin with additional 3-hydroxylation as the most potent derivatives, suggesting the potential impact of hydroxylation on hydrogen bond and hydrophobic interaction. In addition, conjugated flavonoid metabolites could serve as storages of relevant aglycones, and the extent of which might be the result of therapeutic degree of active aglycones in certain tissues. Since hydroxyl groups are common reaction sites, several glucuronidated and sulfated conjugates of fisetin on 4-hydroxyl moiety have been detected at relative high levels in mice plasma, displaying anti-angiogenic effects in vivo [67]. Moreover, additional pharmacological properties may be provided by metabolites. In breast cancer cells, one CYP1 metabolite of nobiletin produces not only anti-invasive but also cytostatic effects [70]. In another model of human aortic endothelial cells, different anti-inflammatory and anti-oxidant effects of several major metabolites of quercetin and (-)-epigallocatechin-3-O-gallate in human versus their parent flavonoids have been obtained [75]. Above all, it is of interest, therefore, to attribute the nutritional behavior of flavonoids partly to bioactive metabolites in vivo, in the face of low bioavailability. On the other hand, relative low physiologic levels of certain flavonoids may also meet therapeutic requirements. For instance, a pharmacokinetic/pharmacodynamic study of Da-Cheng-Qi Decoction has suggested the direct acute pancreatitis therapeutic effects of prototypes of four major flavonoids detected in serum, in the light of the same time between maximal pharmacodynamic effects and maximum serum concentrations in rats [76].
Compared to various pharmacodynamic/pharmacokinetic studies, the reason for bioefficacy of flavonoids in vivo, although significant and discussed a lot, still remains detailed elucidation. Meanwhile, species differences about pharmacokinetics must be taken into account. Take quercetin, which is one of the most prevalent and documented flavonoids, as an example, its profile of absorption and metabolism varies significantly between different species. The bioavailability of quercetin in human is known as values ranging from 0.001 to 0.04% [77]. Bioavailability studies of quercetin aglycone and related glycosides in neonatal calves, cows, and rats, have provided illustrative information on discrepancy of bioavailability [[77], [78], [79]]. As a physiologically based kinetic model suggested, different metabolic systems (enzymatic metabolic rate and regioselectivity) may be responsible for observed inconsistent in vivo behaviors of various species [77]. Since major metabolites of quercetin in human are monoglucuronides (96%) and predominantly quercetin-3′-O-glucuronides, while di- and tri-glucuronic acid/sulfate/methyl conjugates in rats. A two time-higher rate of glucuronidation reported in rats (mainly at 4′-OH) rather than human (mainly at 3′-OH), has supported the point of view that animal models may not necessary be an adequate substitute to elucidate flavonoid behaviors in human, since significant species differences in pharmacokinetics of certain flavonoids do exist.
5. Conclusion
Concerning flavonoids, the significant role of pharmacokinetic behaviors in pharmacodynamics effects and utilization as nutritional supplements in the area of therapy, has been highlighted. However, pharmacokinetic profile of flavonoids with certain functional groups remains sysmetically elusive, which is important for screening out more common/easily synthetic flavonoids with better absorption and higher nutritional/therapeutic or less side effects. It is therefore important to elucidate biological fates and cellular metabolism of different subclass flavonoids, and investigate actions of mechanism at the molecular level as well as structure-activity-pharmacokinetics-relationships. Further investigation is required to elucidate biological fates and activities of flavonoids, to characterize their metabolites with special functional groups, thus meeting therapeutic and nutritional requirements.
Declaration of interest
The authors have declare that there is no conflicts of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NO. 81473324), National Key Scientific Project for China New Drug Discovery and Development during the Twelfth Five-Year Plan-preclinical study on suanzaoren granules (2014ZX09304306-007), National Key Scientific Project for China New Drug Discovery and Development during the Twelfth Five-Year Plan-Technology re-innovation of generic and superstar drugs (2014ZX09201-002), Key Technologies of Common Quality Evaluation of New Drugs (Grant No. 2015010201) and Liaoning Province Science and Technology Research Project (Grant No. 201610163L02).
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Krych J., Gebicka L. Catalase is inhibited by flavonoids. Int J Biol Macromol. 2013;58:148–153. doi: 10.1016/j.ijbiomac.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 2.Ragab F.A., Yahya T.A.A., El-Naa M.M., et al. Design, synthesis and structure–activity relationship of novel semi-synthetic flavonoids as antiproliferative agents. Eur J Med Chem. 2014;82(23):506–520. doi: 10.1016/j.ejmech.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Tian S.S., Jiang F.S., Zhang K., et al. Flavonoids from the leaves of Carya cathayensis Sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia. 2014;92:34–40. doi: 10.1016/j.fitote.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Huang H., Zhao X., et al. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J Funct Foods. 2015;14:144–153. [Google Scholar]
- 5.Wang Y., Chen S., Yu O. Metabolic engineering of flavonoids in plants and microorganisms. Appl Microbiol Biotechnol. 2011;91(4):949–956. doi: 10.1007/s00253-011-3449-2. [DOI] [PubMed] [Google Scholar]
- 6.Rakers C., Schwerdtfeger S.M., Mortier J., et al. Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg Med Chem Lett. 2014;24(17):4312–4317. doi: 10.1016/j.bmcl.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Giuliani C., Bucci I., Di Santo S., et al. The flavonoid quercetin inhibits thyroid-restricted genes expression and thyroid function. Food Chem Toxicol. 2014;66:23–29. doi: 10.1016/j.fct.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Isoda H., Motojima H., Onaga S., et al. Analysis of the erythroid differentiation effect of flavonoid apigenin on K562 human chronic leukemia cells. Chem Biol Interact. 2014;220:269–277. doi: 10.1016/j.cbi.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Shibata C., Ohno M., Otsuka M., et al. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology. 2014;462–463:42–48. doi: 10.1016/j.virol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Gao L., Li C., Yang R.-Y., et al. Ameliorative effects of baicalein in MPTP-induced mouse model of Parkinson's disease A microarray study. Pharmacol Biochem Behav. 2015;133:155–163. doi: 10.1016/j.pbb.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Balasuriya N., Rupasinghe H.P. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012;135(4):2320–2325. doi: 10.1016/j.foodchem.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho O.V., Botelho C.V., Ferreira C.G., et al. In vitro inhibition of canine distemper virus by flavonoids and phenolic acids: implications of structural differences for antiviral design. Res Vet Sci. 2013;95(2):717–724. doi: 10.1016/j.rvsc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T.T., Moon Y.H., Ryu Y.B., et al. The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. coli. Enzyme Microb Technol. 2013;52(1):26–31. doi: 10.1016/j.enzmictec.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.S., Ryu Y.B., Curtis-Long M.J., et al. Flavanones and rotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem Toxicol. 2011;49(8):1849–1856. doi: 10.1016/j.fct.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Sithisarn P., Michaelis M., Schubert-Zsilavecz M., et al. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013;97(1):41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Wu T., He M., Zang X., et al. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim Biophys Acta. 2013;1828(11):2751–2756. doi: 10.1016/j.bbamem.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Amrutha K., Nanjan P., Shaji S.K., et al. Discovery of lesser known flavones as inhibitors of NF-kappaB signaling in MDA-MB-231 breast cancer cells – a SAR study. Bioorg Med Chem Lett. 2014;24(19):4735–4742. doi: 10.1016/j.bmcl.2014.07.093. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z., Fang F., Wang J., et al. Structural activity relationship of flavonoids with estrogen-related receptor gamma. FEBS Lett. 2010;584(1):22–26. doi: 10.1016/j.febslet.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J., Wu Y., Zhao X., et al. Chemopreventive effect of flavonoids from Ougan (Citrus reticulata cv. Suavissima) fruit against cancer cell proliferation and migration. J Funct Foods. 2014;10:511–519. [Google Scholar]
- 20.Chidambara Murthy K.N., Kim J., Vikram A., et al. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. 2012;132(1):27–34. doi: 10.1016/j.foodchem.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Kothandan G., Gadhe C.G., Madhavan T., et al. Docking and 3D-QSAR (quantitative structure activity relationship) studies of flavones, the potent inhibitors of p-glycoprotein targeting the nucleotide binding domain. Eur J Med Chem. 2011;46(9):4078–4088. doi: 10.1016/j.ejmech.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Gopinath K., Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience. 2012;227:134–143. doi: 10.1016/j.neuroscience.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 23.Orhan I.E., Senol F.S., Ercetin T., et al. Assessment of anticholinesterase and antioxidant properties of selected sage (Salvia) species with their total phenol and flavonoid contents. Ind Crops Prod. 2013;41:21–30. [Google Scholar]
- 24.Eghorn L.F., Hoestgaard-Jensen K., Kongstad K.T., et al. Positive allosteric modulation of the GHB high-affinity binding site by the GABAA receptor modulator monastrol and the flavonoid catechin. Eur J Pharmacol. 2014;740(5):570–577. doi: 10.1016/j.ejphar.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Sandhir R., Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochim Biophys Acta. 2013;1832(3):421–430. doi: 10.1016/j.bbadis.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Lou H., Jing X., Wei X., et al. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology. 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Lei Y., Chen J., Zhang W., et al. In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 2012;135(4):2702–2707. doi: 10.1016/j.foodchem.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Cho N., Choi J.H., Yang H., et al. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem Toxicol. 2012;50(6):1940–1945. doi: 10.1016/j.fct.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 29.Spencer J.P., Vafeiadou K., Williams R.J., et al. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Katalinic M., Rusak G., Barovic J.D., et al. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem. 2010;45(1):186–192. doi: 10.1016/j.ejmech.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 31.Li G., Zhu Y., Zhang Y., et al. Estimated daily flavonoid and stilbene intake from fruits, vegetables, and nuts and associations with lipid profiles in Chinese adults. J Acad Nutr Diet. 2013;113(6):786–794. doi: 10.1016/j.jand.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Hamauzu Y., Nosaka T., Ito F., et al. Physicochemical characteristics of rapidly dried onion powder and its anti-atherogenic effect on rats fed high-fat diet. Food Chem. 2011;129(3):810–815. doi: 10.1016/j.foodchem.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Fernandez L., Pons Z., Margalef M., et al. Regulation of vascular endothelial genes by dietary flavonoids: structure-expression relationship studies and the role of the transcription factor KLF-2. J Nutr Biochem. 2015;26(3):277–284. doi: 10.1016/j.jnutbio.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Atrahimovich D., Vaya J., Khatib S. The effects and mechanism of flavonoid–rePON1 interactions. Structure–activity relationship study. Bioorg Med Chem. 2013;21(11):3348–3355. doi: 10.1016/j.bmc.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 35.Abdallah H.M., Almowallad F.M., Esmat A., et al. Anti-inflammatory activity of flavonoids from Chrozophora tinctoria. Phytochem Lett. 2015;13:74–80. [Google Scholar]
- 36.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen M., Wang T., Jiang Z.-Z., et al. Anti-inflammatory and hepatoprotective effects of total flavonoid C-glycosides from Abrus mollis extracts. Chin J Nat Med. 2014;12(8):590–598. doi: 10.1016/S1875-5364(14)60090-X. [DOI] [PubMed] [Google Scholar]
- 39.Shalini V., Bhaskar S., Kumar K.S., et al. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: possible role in the inflammatory signaling. Int Immunopharmacol. 2012;14(1):32–38. doi: 10.1016/j.intimp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Mascaraque C., Aranda C., Ocon B., et al. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol Res. 2014;90:48–57. doi: 10.1016/j.phrs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Mascaraque C., López-Posadas R., Monte M.J., et al. The small intestinal mucosa acts as a rutin reservoir to extend flavonoid anti-inflammatory activity in experimental ileitis and colitis. J Funct Foods. 2015;13:117–125. [Google Scholar]
- 42.Medda R., Lyros O., Schmidt J.L., et al. Anti inflammatory and anti angiogenic effect of black raspberry extract on human esophageal and intestinal microvascular endothelial cells. Microvasc Res. 2015;97:167–180. doi: 10.1016/j.mvr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung H.A., Jin S.E., Min B.S., et al. Anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem Toxicol. 2012;50(6):2171–2179. doi: 10.1016/j.fct.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Kang S.R., Park K.I., Park H.S., et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011;129(4):1721–1728. [Google Scholar]
- 45.Fu Y., Chen J., Li Y.-J., et al. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141(2):1063–1071. doi: 10.1016/j.foodchem.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 46.Das T., Mukherjee S., Chaudhuri K. Effect of quercetin on Vibrio cholerae induced nuclear factor-kappaB activation and interleukin-8 expression in intestinal epithelial cells. Microbes Infect. 2012;14(9):690–695. doi: 10.1016/j.micinf.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 47.During A., Larondelle Y. The O-methylation of chrysin markedly improves its intestinal anti-inflammatory properties: structure-activity relationships of flavones. Biochem Pharmacol. 2013;86(12):1739–1746. doi: 10.1016/j.bcp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Celik H., Kosar M. Inhibitory effects of dietary flavonoids on purified hepatic NADH-cytochrome b5 reductase: structure-activity relationships. Chem Biol Interact. 2012;197(2–3):103–109. doi: 10.1016/j.cbi.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Feng R., Guo Z.K., Yan C.M., et al. Anti-inflammatory flavonoids from Cryptocarya chingii. Phytochemistry. 2012;76:98–105. doi: 10.1016/j.phytochem.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Yeon J.Y., Bae Y.J., Kim E.Y., et al. Association between flavonoid intake and diabetes risk among the Koreans. Clin Chim Acta. 2015;439:225–230. doi: 10.1016/j.cca.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 51.Hummel C.S., Lu C., Liu J., et al. Structural selectivity of human SGLT inhibitors. Am J Physiol Cell Physiol. 2012;302:373–382. doi: 10.1152/ajpcell.00328.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matin A., Doddareddy M.R., Gavande N., et al. The discovery of novel isoflavone pan peroxisome proliferator-activated receptor agonists. Bioorg Med Chem. 2013;21(3):766–778. doi: 10.1016/j.bmc.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 53.Matsuda H., Kogami Y., Nakamura S., et al. Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorg Med Chem. 2011;19(9):2835–2841. doi: 10.1016/j.bmc.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 54.Zhang R., Yao Y., Wang Y., et al. Antidiabetic activity of isoquercetin in diabetic KK -Ay mice. Nutr Metab (Lond) 2011;8:85. doi: 10.1186/1743-7075-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma A.K., Singh H., Satyanarayana M., et al. Flavone-based novel antidiabetic and antidyslipidemic agents. J Med Chem. 2012;55(10):4551–4567. doi: 10.1021/jm201107g. [DOI] [PubMed] [Google Scholar]
- 56.Xie P.-J., Huang L.-X., Zhang C.-H., et al. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J Funct Foods. 2015;16:460–471. [Google Scholar]
- 57.Chen Y.H., Yang Z.S., Wen C.C., et al. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012;134(2):717–724. doi: 10.1016/j.foodchem.2012.02.166. [DOI] [PubMed] [Google Scholar]
- 58.Ribeiro D., Freitas M., Tome S.M., et al. Inhibition of LOX by flavonoids: a structure-activity relationship study. Eur J Med Chem. 2014;72:137–145. doi: 10.1016/j.ejmech.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 59.Zielińska D., Zieliński H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011;124(2):672–678. [Google Scholar]
- 60.Hidalgo M., Sánchez-Moreno C., de Pascual-Teresa S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010;121(3):691–696. [Google Scholar]
- 61.Androutsopoulos V.P., Tsatsakis A.M. Benzo[a]pyrene sensitizes MCF7 breast cancer cells to induction of G1 arrest by the natural flavonoid eupatorin-5-methyl ether, via activation of cell signaling proteins and CYP1-mediated metabolism. Toxicol Lett. 2014;230(2):304–313. doi: 10.1016/j.toxlet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Albishi T., John J.A., Al-Khalifa A.S., et al. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J Funct Foods. 2013;5(2):590–600. [Google Scholar]
- 63.Das S., Mitra I., Batuta S., et al. Design, synthesis and exploring the quantitative structure–activity relationship of some antioxidant flavonoid analogues. Bioorg Med Chem Lett. 2014;24(21):5050–5054. doi: 10.1016/j.bmcl.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 64.Guo Y., Bruno R.S. Endogenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem. 2015;26(3):201–210. doi: 10.1016/j.jnutbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Breiter T., Laue C., Kressel G., et al. Bioavailability and antioxidant potential of rooibos flavonoids in humans following the consumption of different rooibos formulations. Food Chem. 2011;128(2):338–347. doi: 10.1016/j.foodchem.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 66.Buqui G.A., Sy S.K.B., Merino-Sanjuán M., et al. Characterization of intestinal absorption of C-glycoside flavonoid vicenin-2 from Lychnophora ericoides leafs in rats by nonlinear mixed effects modeling. Rev Bras Farmacogn. 2015;25(3):212–218. [Google Scholar]
- 67.Touil Y.S., Auzeil N., Boulinguez F.O., et al. Fisetin disposition and metabolism in mice: identification of geraldol as an active metabolite. Biochem Pharmacol. 2011;82(11):1731–1739. doi: 10.1016/j.bcp.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 68.Kim H., Moon J.Y., Mosaddik A., et al. Induction of apoptosis in human cervical carcinoma HeLa cells by polymethoxylated flavone-rich Citrus grandis Osbeck (Dangyuja) leaf extract. Food Chem Toxicol. 2010;48(8–9):2435–2442. doi: 10.1016/j.fct.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 69.Androutsopoulos V.P., Papakyriakou A., Vourloumis D., et al. Dietary flavonoids in cancer therapy and prevention Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol Ther. 2010;126(1):9–20. doi: 10.1016/j.pharmthera.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Surichan S., Androutsopoulos V.P., Sifakis S., et al. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem Toxicol. 2012;50(9):3320–3328. doi: 10.1016/j.fct.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 71.Wei G.-J., Hwang L.S., Tsai C.-L. Absolute bioavailability, pharmacokinetics and excretion of 5,7,3′,4′-tetramethoxyflavone in rats. J Funct Foods. 2014;7:136–141. [Google Scholar]
- 72.Chen Z.P., Sun J., Chen H.X., et al. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia. 2010;81(8):1045–1052. doi: 10.1016/j.fitote.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 73.Wittemer S.M., Ploch M., Windeck T., et al. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine. 2005;12(1–2):28–38. doi: 10.1016/j.phymed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Lorendeau D., Dury L., Genoux-Bastide E., et al. Collateral sensitivity of resistant MRP1-overexpressing cells to flavonoids and derivatives through GSH efflux. Biochem Pharmacol. 2014;90(3):235–245. doi: 10.1016/j.bcp.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 75.Lotito S.B., Zhang W.-J., Yang C.S., et al. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51(2):454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J., Tang W., Wang J., et al. Pharmacokinetic and pharmacodynamic studies of four major phytochemical components of Da-Cheng-Qi decoction to treat acute pancreatitis. J Pharmacol Sci. 2013;122(2):118–127. doi: 10.1254/jphs.13037fp. [DOI] [PubMed] [Google Scholar]
- 77.Boonpawa R., Moradi N., Spenkelink A., et al. Use of physiologically based kinetic (PBK) modeling to study interindividual human variation and species differences in plasma concentrations of quercetin and its metabolites. Biochem Pharmacol. 2015;98(4):690–702. doi: 10.1016/j.bcp.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 78.Berger L.M., Wein S., Blank R., et al. Bioavailability of the flavonol quercetin in cows after intraruminal application of quercetin aglycone and rutin. J Dairy Sci. 2012;95(9):5047–5055. doi: 10.3168/jds.2012-5439. [DOI] [PubMed] [Google Scholar]
- 79.Maciej J., Schaff C.T., Kanitz E., et al. Bioavailability of the flavonol quercetin in neonatal calves after oral administration of quercetin aglycone or rutin. J Dairy Sci. 2015;98(6):3906–3917. doi: 10.3168/jds.2015-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]