Abstract
Co-delivery of anti-cancer drugs is promising to improve the efficacy of cancer treatment. This study was aiming to investigate the potential of concurrent delivery of resveratrol (RES) and docetaxel (DTX) via polymeric nanocarriers to treat breast cancer. To this end, methoxyl poly(ethylene glycol)-poly(d,l-lactide) copolymer (mPEG-PDLA) was prepared and characterized using FTIR and 1H NMR, and their molecular weights were determined by GPC. Isobologram analysis and combination index calculation were performed to find the optimal ratio between RES and DTX to against human breast adenocarcinoma cell line (MCF-7 cells). Subsequently, RES and DTX were loaded in the mPEG-PDLA micelles simultaneously, and the morphology, particle size distribution, in vitro release, pharmacokinetic profiles, as well as cytotoxicity to the MCF-7 cells were characterized. IC50 of RES and DTX in MCF-7 cells were determined to be 23.0 µg/ml and 10.4 µg/ml, respectively, while a lower IC50 of 4.8 µg/ml of the combination of RES and DTX was obtained. The combination of RES and DTX at a ratio of 1:1 (w/w) generated stronger synergistic effect than other ratios in the MCF-7 cells. RES and DTX loaded mPEG-PDLA micelles exhibited prolonged release profiles, and enhanced cytotoxicity in vitro against MCF-7 cells. The AUC(0→t) of DTX and RES in mPEG-PDLA micelles after i.v. administration to rats were 3.0-fold and 1.6-fold higher than that of i.v. injections of the individual drugs. These findings indicated that the co-delivery of RES and DTX using mPEG-PDLA micelles could have better treatment of tumors.
Keywords: Resveratrol; Docetaxel; Methoxyl poly(ethylene glycol)-poly(d,l-lactide) copolymer (mPEG-PDLA); Micelles; Drug resistance tumor
Graphical abstract
1. Introduction
The effect of chemo drug therapy for cancer is confined by the development of virulent multi drug resistant (MDR) phenotypes, which is a major impediment to cancer treatment. MDR is associated with acquired defense mechanisms, for instance blocked apoptosis and increased drug efflux [1]. Combination chemotherapy is one of the strategies used to overcome the cancer multidrug resistance, which could offer advantages such as targeting different signal pathways in cancer cells, and improving the therapeutic index either by increasing therapeutic efficacy or reducing toxicity [2], [3], [4], [5].
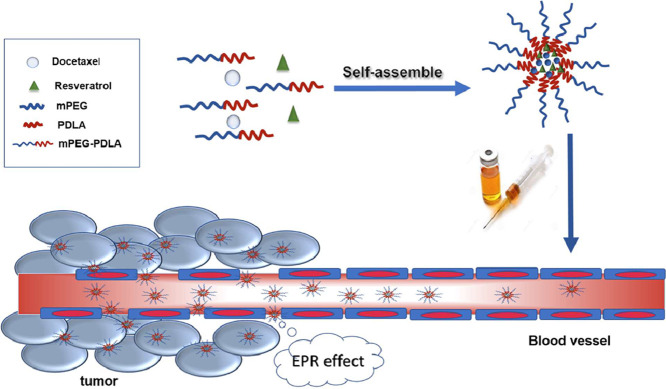
It has been reported that the co-delivery of combined anti-cancer drugs to tumor cells using nanocarriers is a hopeful strategy for treating MDR [6], [7]. Various nanocarriers have been investigated, including lipid nanoparticles [8], polymeric nanoparticles, polymeric micelles [9], [10], liposomes [11], and dendrimers [12], etc. Among them, self-assembly polymeric micelles are very attractive for co-delivery of multiple anticancer agents. This is because (i) the hydrophobic core could allow for loading hydrophobic drug compounds such as anticancer drugs; (ii) the outer hydrophilic shell could prolong circulation time; and (iii) they could provide sustained drug release profiles. In our previous work, resveratrol (RES) and docetaxel (DTX) have been loaded in mPEG-PDLA micelles separately [13]. The obtained mPEG-PDLA micelles exhibited prolonged release profiles for the two drug compounds. In this study, we intended to study the potential of co-delivery of RES and DTX using mPEG-PDLA micelles with respect to the treatment of breast cancer.
DTX is a widely used antineoplastic agent. However, it often suffers from drug resistance, resulting in a decrease in the anti-cancer effect. RES is a sort of phytochemical, which can be obtained from the roots of white hellebore and different food sources. It has been reported that RES can down regulate the expression of multidrug resistant genes encoded for P-gp, and inhibit the mammalian target of rapamycin via pyruvate kinase isoenzyme type M2, thereby preventing cancer cell metabolism [14]. It has been observed that RES reinforced cytotoxic properties of both anti-cancer drugs through increasing their intracellular level base on P-gp inhibition and downregulation of MDR1 [15]. We hypothesized that co-delivery of RES and DTX could provide synergistic anticancer effect and generate better treatment effect of cancer than the anti-tumor with each drug alone. In this study, the synergistic effects of co-administration of RES and DTX were first investigated. Subsequently, co-delivery of RES and DTX using mPEG-PDLA micelles was evaluated with respect to in vitro release, killing drug-resistance tumor cells, and pharmacokinetic profiles in rats.
2. Materials and methods
2.1. Materials
d,l-lactide was purchased from GLACO Ltd. (China). Polyethylene glycol (mPEG2000), stannous octoate (Sn(Oct)2) and 3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2-tetrazolium bromide (MTT) were purchased from Sigma (USA). Docetaxel was purchased from Shanghai Jinhe Bio-tech Co., Ltd. (China). Resveratrol was obtained from Guanyu Bio-tech Co., Ltd. (China). All other chemicals and solvents were of the highest reagent grade and used without further purification.
2.2. Preparation and characterization of mPEG-PDLA block copolymer
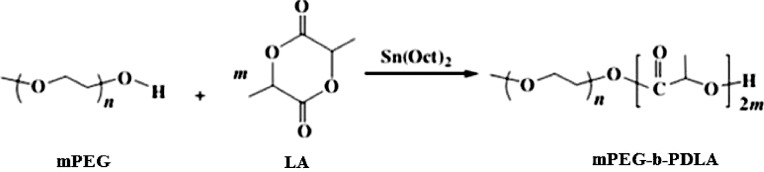
mPEG-PDLA block copolymer was prepared by ring-opening polymerization of appropriate amount of d,l-lactide monomer in the presence of polyethylene glycol (mPEG, Mn = 2000) using stannous octoate as the catalyst in a sealed tube (Fig. 1). The sealed tube was maintained under 130 °C for 12 h with continuous magnetic stirring. After that, the synthesized polymer was collected by adding some dichloromethane into the tube and then followed by precipitation in ice-cool ether for 48 h. The precipitate was filtered, dried at room temperature under vacuum [16].
Fig. 1.
Synthesis of mPEG-PDLA.
The molecular structure of the synthesized material was determined by using FTIR (Bruker, Switzerland), and proton nuclear magnetic resonance (1H NMR, Bruker ARX-300, Switzerland). FTIR spectrums were collected using a Bruker vector 22 analytical FTIR Spectrometer over the region of 400–4000 cm−1. The structure, average molecular weight and the mPEG content of the copolymer were determined by gel permeation chromatography (GPC, Waters, USA) and 1H NMR in CDCl3 at 300 Hz. The GPC measurements were conducted with a Waters 2414 GPC detector instrument equipped with Styragel® HR THF (7.8 mm× 300 mm) column. THF was used as eluent at a flow rate of 1.0 ml/min and column thermostat was set at 35 °C.
2.3. Screening the ratio of RES to DTX
The optimal ratio of RES to DTX with respect to antitumor effect was determined by incubating the two compounds with MCF-7 cells as reported previously [17]. Briefly, MCF-7 tumor cells were seeded in a 96-wells plate (6000 cells/well) 12 h prior to the experiment. Then, the cells were treated with vehicle Dulbecco's modified Eagle medium (DMEM) (control group), DTX (0–50.0 µg/ml, DTX group), RES (0–100.0 µg/ml, RES group), and combination of DTX and RES (combination group), respectively. For the combination group, 1.0 ml of DTX and RES with various concentrations were combined in the scheme of 2.5 + 2.5, 5 + 5, 10 + 10, 20 + 20, and 50 + 50 µg/ml and added into the wells. Two days after drug stimulation, the optical density (OD) of each well at 490 nm was measured using a full-wave length multi-function microplate reader (Thermo Scientific Corp., Waltham, USA). Each condition comprised six replicate wells with at least three independent duplicates.
2.4. Evaluation of synergistic effect of drug combinations using the CI method
The combination index (CI) was used to evaluate the synergistic, antagonistic or additive effects of drug combinations [18], which is calculated by using the following formula:
| CI = IC50ab1/IC50a + IC50ab2/IC50b |
where IC50ab1, IC50ab2 are the IC50 values when the drugs are administered in combination, while IC50a, IC50b are the IC50 values when the drugs are administered as single agents. CI > 1 indicates antagonism, CI < 1 indicates synergy and CI = 1 indicates an additive effect.
2.5. Preparation and characterization of drug-loaded mPEG-PDLA micelles
DTX and RES loaded mPEG-PDLA micelles were prepared by using the reported solvent casting method [19]. Briefly, DTX, RES and mPEG-PDLA were dissolved in acetonitrile and then added into a round-bottom flask. A thin and homogenous film containing the two drugs and polymer was formed after the solvent was removed with a rotary evaporator under vacuum in a 50 °C water bath. After the film was cooled down to room temperature, it was rehydrated in 2.0 ml of deionized water with gentle agitation to form the DTX/RES micelles spontaneously. The resultant micellar colloidal system was filtered through 0.22 µm filter.
The particle size and zeta potential of DTX/RES micelles were measured using Malvern Zetasizer Nano-ZS (Malvern Instruments Ltd., England). The morphology of DTX/RES micelles was observed by using a JEM-2100 transmission electron microscopy (TEM, JEOL Ltd., Tokyo, Japan). In sample preparation procedure, certain amount of DTX/RES micelles were attached to copper grids and negatively stained with 2% phosphotungstic acid for 15 s. After the removal of excessive liquid, the samples were dried in air and subjected to TEM examination.
2.6. In vitro release kinetics of DTX and RES from micelles
The release profile of DTX and RES from the mPEG-PDLA micelles was evaluated by using a previously reported method with slight modification [20]. In brief, 1.0 ml of DTX ethanol solution (1.0 mg/ml), RES ethanol solution (1.0 mg/ml) and DTX/RES mPEG-PDLA micelles were placed in dialysis bags with the cutting off MW of 3000 Da. These dialysis bags were incubated in 100 ml of pH 7.4 phosphate buffer containing 0.5% (w/w) Tween 80 to achieve the sink condition and were shaken in a horizontal shaking water bath (37 ± 0.5 °C) at 100 rpm for 72 h. At predetermined time intervals, 1.0 ml of sample was withdrawn and replaced with an equal volume of the fresh released medium. The collected samples were filtered through 0.22 µm filter and quantified by HPLC. In detail, the analysis was performed with Hitachi HPLC system (UV Detector L-2400, Pump L-2130, Hitachi, Tokyo, Japan) and a reversed-phase column (Diamonsil, C18, 4.6 mm× 250 mm, 5 µm). The mobile phase made up of acetonitrile and water (50:50; v/v) was injected at a flow rate of 1.0 ml/min, and RES and DTX was detected at a wavelength of 228 nm.
2.7. Pharmacokinetics studies
2.7.1. Animals
Sprague-Dawley rats (male, body weighing about 200 g) obtained from the Experimental Animal Center at Shenyang Pharmaceutical University (SPU) were employed in pharmacokinetic studies. All animal experiments were performed strictly in line with guidelines approved by the Life Science Research Center at SPU. All efforts were made to limit the number of animal used and to minimize animal suffering.
2.7.2. Experiment design
The rats were randomly divided into three groups (n = 4), i.e. DTX ethanol solution group, RES ethanol solution group and DTX/RES mPEG-PDLA micelles group. Each sample was intravenously administered at a dose of 10 mg/kg body weight, respectively. At given time points (0.083, 0.5, 1, 2, 4, 6, 8, 10, 12 h post injection), 0.5 ml of blood was drawn from the orbit venous plexus of the rats and placed in heparinized tubes. Plasma were immediately separated by centrifugation at 5000 rpm for 10 min and stored at −20 °C until further analysis.
2.7.3. Quantitative analysis of DTX and RES in plasma samples
For the first step of extraction procedure, 200 µl plasma was mixed with 20 µl of DTX (10.0 µg/ml) and carbamazepine (10.0 µg/ml) that was used as the internal standard. DTX and RES were extracted from plasma with 2.0 ml of methyl tert-butyl ether by vigorous mixing for 5 min followed by centrifugation at 12,000 rpm for 5 min to separate the organic phase. The organic phase was collected into a clean tube and evaporated to dryness under nitrogen gas flow at 35 °C. The residue was dissolved with 100 µl of acetonitrile by vortex-mixing for 5 min and centrifuged for 5 min at 12,000 rpm. The supernatant was analyzed using the HPLC analysis which was carried out with Hitachi HPLC system (UV Detector L-2400, Pump L-2130, Hitachi, Tokyo, Japan). A reversed-phase column (Diamonsil, C18, 4.6 mm× 250 mm, 5 µm) was used. The mobile phase for detecting DTX was made up of acetonitrile and water (50:50, v/v) eluted at a flow rate of 1.0 ml/min, and for RES methanol and water (50:50) was used at a flow rate of 1.0 ml/min. The DTX detection was performed at a wavelength of 228 nm while for RES a wavelength of 306 nm was used.
2.7.4. PK data analysis
The pharmacokinetic parameters including maximum concentration (Cmax), area under the drug concentration–time curve (AUC), and total clearance (CL was calculated by using DAS2.0 software (Mathematical Pharmacology Professional Committee of China, Shanghai, China).
2.8. Evaluation of the cytotoxicity of DTX/RES mPEG-PDLA micelles
The cytotoxicity of DTX/RES mPEG-PDLA micelles to MCF-7 cells was evaluated by MTT assay [21]. Free drugs (i.e. DTX and RES), DTX mPEG-PDLA micelles, and RES mPEG-PDLA micelles were used as control. The MCF-7 cells were seeded into sterile 96-wells plate (6000 cells/well) and cultured in a cell culture incubator at 37 °C under 5% CO2 for 16 h. Then the medium was replaced with 200 µl of fresh culture medium containing the same amount of free DTX, free RES, DTX mPEG-PDLA micelles, RES mPEG-PDLA micelles or DTX/RES mPEG-PDLA micelles. In addition, drug-free mPEG-PDLA micelles was also added as control. For the group of free DTX and RES, DMSO have to be used in order to dissolve the drugs, but the final DMSO concentration in culture medium was strictly controlled and limited to 0.02% to minimize its effects on cell viability. After further incubation for 48 h, the medium was removed and 100 µl of MTT solution (0.5 mg/ml) was added into each well. After 4 h, the medium was removed and 100 µl of DMSO was added to dissolve precipitated formazan crystals and the plate was shaken for 1 min. The viability of cells was measured with a microplate reader, and presented as a percentage of the viability ratio.
2.9. Statistical analysis
Data were generated in triplicates and expressed as mean ± S.D. Statistical analysis was performed using Student's t-test with the SPSS 17.0 software. Significance was determined by a P-value of 0.05 (demoted by *), P of 0.01 (denoted by **) and P of 0.001 (denoted by ***).
3. Results and discussion
The synthesis and characterization of the mPEG-PDLA can be found in the supplementary material.
In the following section the co-delivery of RES/DTX loaded mPEG-PDLA micelles was reported.
3.1. Evaluation of synergistic effect of DTX and RES
The potential synergistic effects of DTX and RES were investigated by measuring IC50 values of DTX solution, RES solution, and their combination at different ratio with MTT assay in MCF-7 cells. The IC50 of DTX, RES and their combination at different mass ratio listed in Table 1 suggested that co-administration of DTX and RES could enhance their cell proliferation inhibition efficiency. Furthermore, in order to find the optimal mass ratio between DTX and RES, CI values of DTX and RES at the ratio of 2:1, 1:1, 1:2 were calculated. The results showed that CI values varied with the weight ratio of DTX to RES and the lowest one was obtained at the ratio of 1:1. It indicated the combination at a mass ratio of 1:1 generated stronger synergistic effect than others. As the mass ratio increased from 1:1 to 1:2, the synergistic effect flipped and became an antagonism effect. Therefore, the mass ratio of 1:1 was chosen as the optimal ratio and was used in the subsequent preparation of drug-loaded mPEG-PDLA micelles.
Table 1.
CI values of different DTX and RES ratios. Data represent mean ± SD of three independent experiments from five samples for each group.
| DTX:RES (mass ratio) | IC50 (µg/ml) |
CI value | |
|---|---|---|---|
| DTX | RES | ||
| 2:1 | 7.82 ± 0.16 | 3.92 ± 0.12 | 0.92 |
| 1:1 | 4.80 ± 0.15 | 4.80 ± 0.17 | 0.64 |
| 1:2 | 6.57 ± 0.65 | 13.1 ± 0.24 | 1.20 |
| 1:0 | 10.37 ± 0.43 | – | – |
| 0:1 | – | 23.03 ± 0.54 | – |
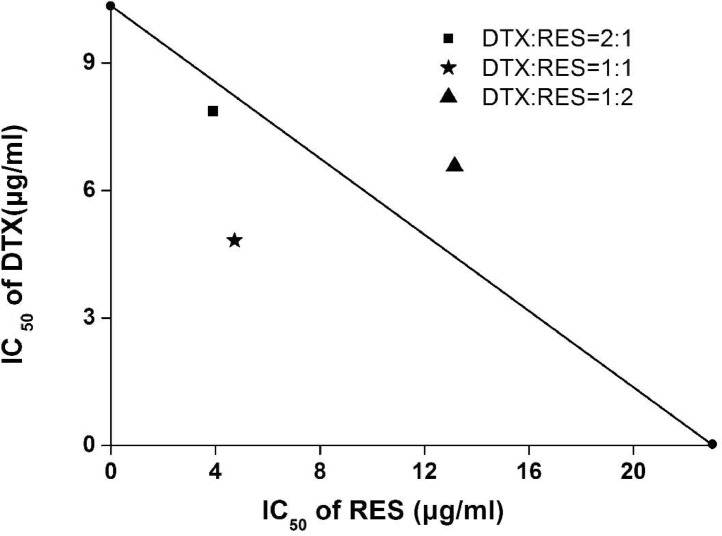
In addition, the isobologram analysis was used to evaluate the synergistic effect of the drug compounds (Fig. 2). As a mathematical approach, isobologram has been used to evaluate the drug interactive effects [22]. A particular effect level, such as 50% of the maximum was selected, and doses of drug A and B (each drug alone) that gave same effect were plotted. The straight line connecting A and B allows a comparison with the actual dose pair that produces this effect level experimentally. The best combinations can be distinguished. As shown in Fig. 2, the points below the straight line suggested that the combination of the two drugs (DTX:RES = 2:1 and 1:1) could generate a synergistic effect in MCF-7 cells. It was reported that RES could exhibit chemosensitization effect [23]. RES has been observed to be a chemosensitizer in docetaxel chemotherapy blocks upregulation and activation of human epidermal growth factor receptor-2 (HER-2) furthermore blocking downstream signaling pathways for instance Akt [24]. The possible explanation for the synergistic effects between DTX and RES could be that RES down regulated the expression of multidrug resistant gene encoding the membrane transporter P-gp [25] which inhibits the mammalian target of rapamycin through pyruvate kinase isoenzyme type M2, thus preventing cancer cell metabolism [26]. The reported treatment of combining RES upregulated the pro-apoptotic genes (BAX, BID, and BAK), cleaved PARP and down regulated the anti-apoptotic genes (MCL-1, BCL-2, BCL-XL) promoting apoptosis [27].
Fig. 2.
The isobologram of DTX and RES at different ratios. The straight line (additively line) connects the IC50 values of DTX and RES when the drugs were used alone, and the points plotted on the isobolograms were based on the result of Table 1.
3.2. Preparation and characterization of DTX/RES loaded mPEG-PDLA micelles
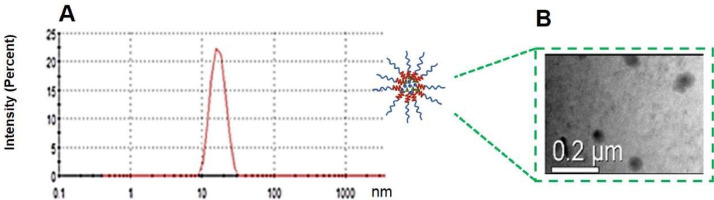
The effect of the process and formulation parameters including the film-forming temperature, the hydration temperature, and the drug to polymer ratio on the drug loading/entrapment efficiency, size, zeta potential of DTX/RES-loaded mPEG-PDLA micelles were investigated and reported in one of our previous article [28]. In the present study, DTX to RES was fixed at 1:1 and loaded into mPEG-PDLA micelles with the optimized process and formulation parameters obtained in the previous article, and the obtained micelles were characterized in terms of size, morphology, encapsulation efficiency and drug loading efficiency. As shown in Fig. 3A, DTX/RES-loaded mPEG-PDLA micelles had a mean particle size of 17.1 ± 3.2 nm, and PDI of 0.27 ± 0.01. The loadings of RES and DTX in the micelles are 16.89% and 16.87%, respectively. The TEM images depicted in Fig. 3B revealed that DTX/RES-loaded mPEG-PDLA micelles were spherical and uniform, and the particle size was approximately in the range of 20–50 nm, which are slightly larger than the value determined by Marlven Nano ZS Zetasizer Instrument.
Fig. 3.
Particle size distribution (A) and TEM image (B) of RES/DTX mPEG-PDLA micelles.
3.3. In vitro drug release of micelles
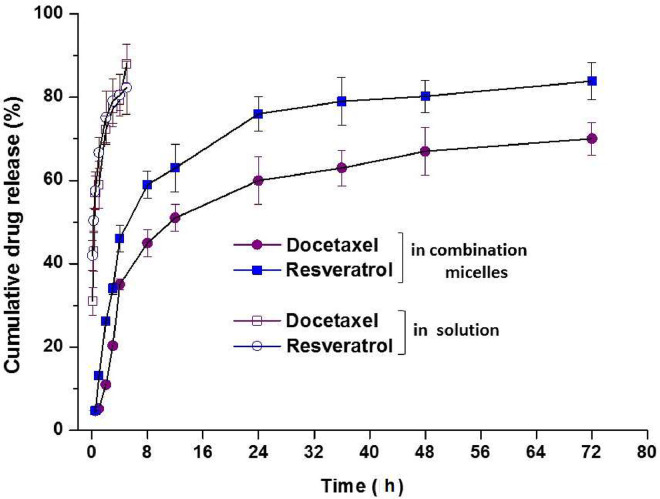
The in vitro release results are shown in Fig. 4. Both RES and DTX exhibited a continuous and fast release in the first 12 h. After that, the release rate was slowed down and finally the cumulative release of both DTX and RES reached nearly 80% at 72 h. Furthermore, it was noticed that although the absolute release percentage of RES was lower than that of DTX, the release rate of these two drugs was nicely kept to be synchronous, which was very important because it could ensure the ratio of DTX to RES (1:1) consistent in tissue to achieve the synergistic effect. In contrast to the sustained release profiles of DTX/RES loaded mPEG-PDLA, ca. 80% of DTX and RES dissolved within 5 h in the release medium. This extended release of RES and DTX from the micelles could be attributed to the hydrophobic interaction between the drugs and hydrophobic core. The release profiles were fitted with a few equations including Higuchi, Peppas and Sahlin model. It was found that the release of RES and DTX from the micelles was a combined effect of diffusion and disintegration. It might be explained to the diffusion of the drugs from the core and the disintegration of the outer polymeric shell [29].
Fig. 4.
Release profiles of DTX/RES mPEG-PDLA micelles (n = 4).
3.4. Cytotoxicity of DTX/RES-loaded mPEG-PDLA micelles on MCF-7 cells
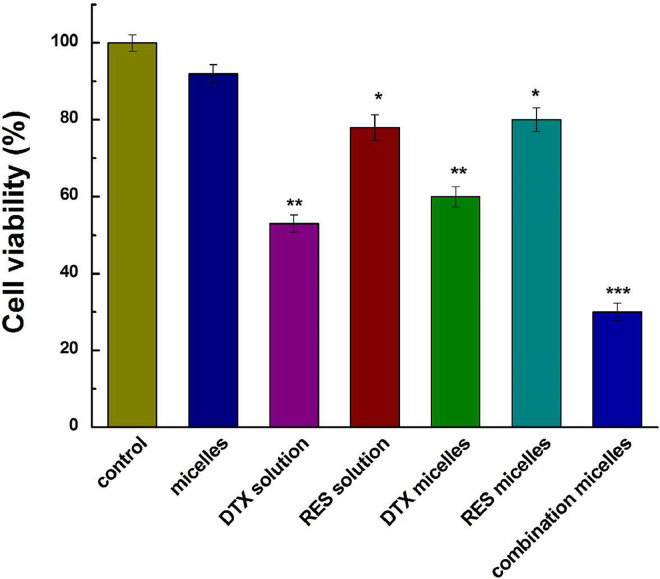
The cytotoxicity of DTX/RES loaded mPEG-PDLA against MCF-7 cells was assessed with MTT study. The results in Fig. 5 exhibited that the blank polymeric micelles did not have any cytotoxic effect on the MCF-7 cells, which suggested that the synthesized mPEG-PDLA polymer is safe and biocompatible with MCF-7 cell line. The DTX/RES loaded mPEG-PDLA showed the highest cytotoxic activity against MCF-7 cells among the all treatment groups and led to the death of 70% cells, which was higher than the effect of micelles containing either the same concentration of DTX or RES. The increased sensitivity of DTX/RES loaded mPEG-PDLA micelles in MCF-7 cells can be attributed to the presence of PEG shell on the surface which enhanced the uptake mediated through endocytosis. The copolymer micelles have been demonstrated to be potential nanocarriers for effective intracellular delivery drug to reverse tumor MDR [30], [31]. In addition, co-delivery of the two drugs acted on different pathways to evade cell resistance to DTX [32].
Fig. 5.
Cell viabilities of DTX/RES loaded mPEG-PDLA in MCF-7 cells. Significance was determined by P < 0.05 (demoted by *), P < 0.01 (denoted by **), P < 0.001 (denoted by ***) as compared to the control group.
3.5. In vivo pharmacokinetic study of DTX/RES-loaded mPEG-PDLA micelles
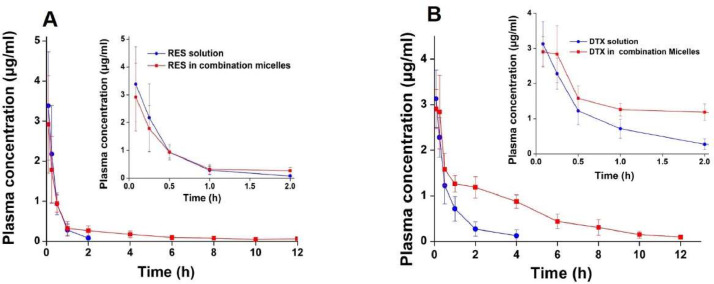
The PK parameters obtained from the in vivo study (Table 2) showed that mPEG-PDLA micelles largely extended the exposing time of RES and DTX in the blood circulation (Fig. 6). The half-lives of RES and DTX in mPEG-PDLA micelles were about 9 times and 4 times longer than the solutions. In addition, the AUC(0→t) value of RES and DTX when administered as mPEG-PDLA micelles was about 1.6 times and 3 times higher than the solutions. Hydro-PEG could prolong the half-life of drug in rats and enhance the targeting and residence time in tumor site [33]. The prolonged blood circulation of the mPEG-PDLA micelles can be attributed to its hydrophilic surface composed by PEG chain, which endowed the mPEG-PDLA micelles the ability of escaping from the reticuloendothelial system, exhibited a slower clearance. Since tumor tissue possess leaky blood vessels and poor lymphatic drainage, micelles may preferentially accumulate in the solid tumors due to the enhanced permeability and prolonged circulation time [34] and are expected to passive targeting to cancer cells to exert the anticancer effect.
Table 2.
Pharmacokinetic parameters of RES solution, DTX solution, and RSE/DTX mPEG-PDLA micelles after intravenous injection in rats at a dose of 10 mg/kg (n = 3).
| PK parameters | Formulations |
|||
|---|---|---|---|---|
| DTX solution | DTX in combination micelles | RES solution | RES in combination micelles | |
| t1/2 (h) | 0.66 ± 0.19 | 2.35 ± 0.44 | 0.34 ± 0.13 | 3.27 ± 0.35 |
| CL (l/h/kg) | 4.30 ± 2.11 | 1.37 ± 0.49 | 6.34 ± 3.54 | 4.009 ± 2.51 |
| V (l/kg) | 3.79 ± 0.83 | 4.286 ± 0.82 | 3.45 ± 2.93 | 18.29 ± 9.82 |
| AUC(0−t) (mg·h/l) | 2.59 ± 1.24 | 7.91 ± 2.78 | 1.84 ± 1.06 | 2.93 ± 1.79 |
| AUC(0−∞) (mg·h/l) | 2.64 ± 1.29 | 8.22 ± 3.05 | 1.87 ± 1.04 | 3.10 ± 1.94 |
Note: Data shown as mean ± standard deviation unless otherwise stated.
Fig. 6.
Plasma concentration–time curves of RES solution, DTX solution and RSE/DTX mPEG-PDLA micelles after i.v. administration to rats (n = 3). Plasma concentration–time curve of RES (A); Plasma concentration–time curves of DTX (B). The inserts are zoom-in of the plasma concentration at first 2 h.
4. Conclusions
This study demonstrated that the combination of resveratrol and docetaxel could generate synergistic effect against MCF-7 tumor cells. Co-delivery of resveratrol and docetaxel via methoxyl poly(ethylene glycol)-poly(d,l-lactide) micelles could not only extend the exposing time of the drug compounds in blood circulation in rats but also enhanced their AUC. In addition, the drug combination exhibited improved anticancer effect as compared to when each drug compound used alone in vitro. Our findings suggested that the co-delivery of resveratrol and docetaxel using methoxyl poly(ethylene glycol)-poly(d,l-lactide) micelles could be promising to treat drug-resistant tumors.
Conflicts of interest
The authors declare that there is no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
We are grateful to the Liaoning Province Pan Deng Xue Zhe Grant (M. Yang), Liaoning Provincial Education officer's Excellent Talents Supporting Plan (D. Cun) and National Natural Science Foundation of China (No. 81302720 and 81573380) for financial support.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2018.03.002.
Contributor Information
Mingxi Qiao, Email: qiaomingxi@163.com.
Mingshi Yang, Email: mingshi.yang@sund.ku.dk.
Appendix. Supplementary materials
References
- 1.Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53(4):615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez C.D., Clarke P.A., Al-Lazikani B., Workman P. Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance. Clin Pharmacol Ther. 2013;93(3):252–259. doi: 10.1038/clpt.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kummar S., Chen H.X., Wright J. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9(11):843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock J., Griffin J.P., Behrman R.E. Development of novel combination therapies. New Engl J Med. 2011;364(11):985–987. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 5.Wu L., Leng D., Cun D., Foged C., Yang M. Advances in combination therapy of lung cancer: rationales, delivery technologies and dosage regimens. J Control Release. 2017;260:78–91. doi: 10.1016/j.jconrel.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Lavan D.A., McGuire T., Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21(10):1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Gu F.X., Chan J.M., Wang A.Z., Langer R.S., Farokhzad O.C. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 8.Taratula O., Kuzmov A., Shah M., Garbuzenko O.B., Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349–357. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu C., Jung S., Luo S. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA–PCL–PDMAEMA triblock copolymers. Biomaterials. 2010;31(8):2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 10.Hasan W., Chu K., Gullapalli A. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2011;12(1):287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad M., Garbuzenko O.B., Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine. 2008;3(6):761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneshiro T.L., Lu Z.R. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials. 2009;30(29):5660–5666. doi: 10.1016/j.biomaterials.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Zeng J.F., Qiao M.X., Hong W. The preparation and in vitro evaluation of paclitaxel loaded polymeric micelles. J Shenyang Pharm Univ. 2015;1:002. [Google Scholar]
- 14.Iqbal M.A., Bamezai R.N. Resveratrol inhibits cancer cell metabolism by down regulating pyruvate kinase M2 via inhibition of mammalian target of rapamycin. PLoS ONE. 2012;7(5):e36764. doi: 10.1371/journal.pone.0036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alabd A.M., Mahmoud A.M., Elsherbiny G.A. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Proliferat. 2011;44(6):591–601. doi: 10.1111/j.1365-2184.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu C.F., Balakrishnan P., Cui F.D. The effects of mixed MPEG-PLA/pluronic copolymer micelles on the bioavailability and multidrug resistance of docetaxel. Biomaterials. 2010;31(8):2371–2379. doi: 10.1016/j.biomaterials.2009.11.102. [DOI] [PubMed] [Google Scholar]
- 17.Qiao H., Wang T.Y., Yan W. Synergistic suppression of human breast cancer cells by combination of plumbagin and zoledronic acid In vitro. Acta Pharmacol Sin. 2015;36(9):1085–1098. doi: 10.1038/aps.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou T.C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 19.Jie T., Zhang J., Yu H. A conformal hydrogel nanocomposite for local delivery of paclitaxe. J Biomater Sci Polym Ed. 2017;28(1):107–118. doi: 10.1080/09205063.2016.1250344. [DOI] [PubMed] [Google Scholar]
- 20.Phan Q.T., Le M.H., Le T.T., Tran T.H., Xuan P.N., Ha P.T. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano systems with various PLA:PEG ratios. Int J Pharm. 2016;507(1–2):32–40. doi: 10.1016/j.ijpharm.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Yin C., Tang G., Lin X., Wu Q. Glucose-functionalized multidrug-conjugating nanoparticles based on amphiphilic terpolymer with enhanced anti-tumorous cell cytotoxicity. Int J Pharm. 2013;441(1–2):291–298. doi: 10.1016/j.ijpharm.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Tallarida R.J. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298(3):865–872. [PubMed] [Google Scholar]
- 23.Piao L., Mukherjee S., Chang Q. TriCurin, a novel formulation of curcumin, epicatechin gallate, and resveratrol, inhibits the tumorigenicity of human papillomavirus-positive head and neck squamous cell carcinoma. Oncotarget. 2017;8(36):60025. doi: 10.18632/oncotarget.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinod B.S., Nair H.H., Vijayakurup V. Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis. Cell Death Discov. 2015;1:15061. doi: 10.1038/cddiscovery.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu F., Chou P.M., Zheng X., Mirkin B.L., Rebbaa A. Control of multidrug resistance genemdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res. 2005;65(22):10183–10187. doi: 10.1158/0008-5472.CAN-05-2002. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal M.A., Bamezai R.N. Resveratrol inhibits cancer cell metabolism by down regulating pyruvate kinase M2 via inhibition of mammalian target of rapamycin. PLoS ONE. 2012;7(5):e36764. doi: 10.1371/journal.pone.0036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S.K., Banerjee S., Acosta E.P., Lillard J.W., Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21waf1/cip1 and p27kip1 pathway. Oncotarget. 2017;8(10):17216–17228. doi: 10.18632/oncotarget.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X., Zhou L.H., Qiao M.X., Hai Y.H., Zhao X.L., Chen D.W. Application of Box–Behnken design response surface methodology in the formulation optimization of mPEG-PDLLA micelles loaded with docetaxel and resveratrol micelles. J Shenyang Pharm Univ. 2016;33(3):186–193. [Google Scholar]
- 29.Phan Q.T., Le M.H., Le T.T., Tran T.H., Xuan P.N., Ha P.T. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano systems with various PLA:PEG ratios. Int J Pharm. 2016;507(1–2):32–40. doi: 10.1016/j.ijpharm.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Jia N., Ye Y., Wang Q. Preparation and evaluation of poly(l-histidine) based pH-sensitive micelles for intracellular delivery of doxorubicin against mcf-7/adr cells. AJPS. 2017;12(5):433–441. doi: 10.1016/j.ajps.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J., Zhang H., Chen Z., Xu L., Zhang Z. A multi-functional nanoplatform for efficacy tumor theranostic applications. AJPS. 2016;12(3):235–249. doi: 10.1016/j.ajps.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill K.K., Kaddoumi A., Nazzal S. Mixed micelles of PEG2000-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosensitization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur J Pharm Sci. 2012;46(1-2):64–71. doi: 10.1016/j.ejps.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Li J., Li Z., Tang X., Zhang Z. Pharmacokinetics of a ternary conjugate based pH-responsive 10-hcpt prodrug nano-micelle delivery system. AJPS. 2017;12(6):542–549. doi: 10.1016/j.ajps.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alibolandi M., Abnous K., Hadizadeh F. Dextran-poly lactide-co-glycolide polymersomes decorated with folate-antennae for targeted delivery of docetaxel to breast adenocarcinoma in vitro and in vivo. J Control Release. 2016;241:45–56. doi: 10.1016/j.jconrel.2016.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.