Abstract
Crude seed gum and their carboxymethyl derivatives from Tamarindus indica and Cassia fistula seeds were developed and characterized to apply as the pharmaceutical disintegrant in fast disintegrating Thai cordial tablet. The chemical structure of crude gum was chemically modified via carboxymethylation. Degree of substitution (DS) of carboxymethylated gums was determined. Carboxymethylated gums with minimum and maximum DS values were chosen for further application. IR absorption spectra of gum samples were observed to verify their chemical structure changes. In physical properties, the intrinsic viscosity and swelling property of all gum samples were evaluated. The results showed that carboxymethylated gums had higher intrinsic viscosity than those of crude gum. Moreover, they could swell and be soluble in cold water better than those of crude gums. In conclusion, the modified gums from both plants could provide higher hardness and be better used than that crude gums for fast disintegrating Thai cordial tablet. However, this is a preliminary assessment to expressing pharmaceutical application possibility of these gums as disintegrants, diluents and drug release controlling agents.
Keywords: Tamarindus indica, Cassia fistusla, Carboxymethylation, Thai cordial, Disintegrating agent, Fast disintegrating tablet
1. Introduction
Fast disintegrating tablets (FDTs) have received ever-increasing demand during the last decade and the field has become a rapidly growing area in the pharmaceutical industry. FDTs are those solid dosage forms disintegrate or dissolve instantaneously, releasing the drug in short period of time. Thus, important recipient for tablet formulation is disintegrating agent to release its medicinal substances on contact with moisture. Recently, natural disintegrating agent is alternative over synthetic substance because it is comparatively cheaper, easily available, non-irritating and nontoxic in nature. Therefore several natural gums have been extensively used in the field of novel drug delivery as disintegrating agent such as guar gum, tamarind gum, and karaya gum [1], [2], [3].
Seed gums are polysaccharides and hydrophilic biopolymer with various molecular weights and they offer viscose or gel solutions after dissolving or dispersing in water [4]. Different seed gums have been studied and applied for diverse purposes due to their nontoxicity. They have been widely used in the field of textile, paper, food, cosmetic and pharmaceutical industries as thickener, stabilizer, emulsifier, coating agent, gelling agent, binder, and drug release controller [3], [5], [6], [7], [8]. Seeds of Tamarindus indica and Cassia fistula belonging to Leguminous plant contain high amount of endosperm which is also source of gum. The structure of tamarind seed gum is based on a β 1,4-d-glucan backbone, substituted at position 6 of the glucopyranosyl units mainly by single α-d-xylopyranosyl residues [9]. While, the backbone of Cassia fistula gum is a linear chain of β 1,4-linked mannose residues to which galactose residues are 1,6-linked at mannose, forming short side-branches [10]. However, they often present low soluble in cold water, unpleasant odor and dull color. To improve and enhance the quality and acceptability of crude gum for a wider range of industries, the chemical structure of crude gum has been chemically modified.
Alternatively, carboxymethylation has been given interest as derivatization process that has been already successfully used for various polysaccharide gums [11], [12], [13], [14], [15]. The carboxymethylation of seed gum is the etherified gum. The hydroxyl groups of gum molecules are etherified by carboxymethyl groups. Gum is reacted with sodium monochloroacetic acid in presence of sodium hydroxide. In main reaction, sodium hydroxide first reacts with hydroxyl group of gum to give alkoxide groups (Eq. (1)). In the second step, glucose unit in gum molecules is etherified by carboxymethyl group (Eq. (2)). The side reaction takes place in both the liquid bulk and gum phase which results in the formation of sodium glycolate from monochloroacetic acid and sodium hydroxide (Eq. (3)).
| (1) |
| (2) |
| (3) |
In the present study, we aimed to observe the characterization of crude and modified gums from Tamarindus indica and Cassia fistula seeds as disintegrating agent for formulation of fast disintegrating Thai cordial tablet. Regarding Thai cordial, it is traditional Thai herbal medicine in original powder form. It has been widely used in disordered symptom groups concerning cardiovascular system in Thai society for long time. Nevertheless, application of Thai cordial is limited by its dosage form due to it is difficult to carry and measure for each dose. Hence, in order to develop Thai cordial in other form as fast disintegrating tablet would extend convenience and keep aromatic during dispersing in warm or cold water of the cordial. The seed gums were added as disintegrating agent for FDT by direct compression using super-disintegrants technique which is simple preparation process and can be used with heat and water unstable active ingredient.
2. Materials and methods
2.1. Plant materials
Crude gums were provided from Tamarindus indica and Cassia fistula seeds which were collected in Uthai thani and Chonburi provinces in Thailand, respectively. Thai cordial powder was kindly supported by Five-Pagoda (Thailand).
2.2. Modification
Carboxymethylated gums were prepared which adapted from the modification of Goyal and co-workers (2007) [12]. The used condition of reaction was at 70 °C for 1 h of reaction time. Ethanol was used as solvent media. Different mole ratios of sodium hydroxide (NaOH) to monochloroacetic acid (ClCH2COOH, MCA) as 1.00, 1.78 and 2.78 were used, while 0.56 mole of crude gum was fixed. The reaction product was precipitated and washed with ethanol to remove ionic salts. The carboxymethylated gum was removed by filtration and washed with 80% ethanol. The washed product was dried at 40 °C for 4 h. Then the degree of substitution (DS) of carboxymethylated gums was determined as followed by equations (4) and (5) [16].
| (4) |
| (5) |
where WA is the mass fraction of acetyl group, CNaOH and CHCl are the molar concentration of standard NaOH and HCl solutions, respectively, VNaOH and VHCl are the volume of standard NaOH and HCl solutions, respectively, m is the weight of sample taken, and 58 and 162 are the molar mass (g/mol) of acetyl group and anhydroglucose unit, respectively.
2.3. Chemical compositions
Moisture and ash contents are determined according to the American Society for Testing and Materials methods (ASTM-D2974-87) and AOAC Official Method 923.03, respectively. Protein content was obtained from the total nitrogen content (N x 5.7) by Kjeldahl method, as described in the AOAC Official Method of Analysis 981.10. Fat content was determined according to the AOAC Official Method of Analysis 923.06.
2.4. Fourier transform infrared (FTIR) spectroscopy
The crude and carboxymethylated gums were pulverized and blended with KBr then compressed. The measurements on prepared disks were carried out using a FT-IR spectrophotometer (Magna-IR system 750, Nicolet Biomedical Inc., USA).
2.5. Intrinsic viscosity
The intrinsic viscosity [η] of dilute solutions of gum samples was measured at 25 ± 0.1 °C with a Cannon-Fenske Routine Viscometer (9721-A53) (ASTM-D2515, ISO 3105, and Series 100). Solutions had relative viscosities from 1.2 to 2.0 to assure good accuracy and linearity of extrapolation to zero concentration. The intrinsic viscosity is conventionally obtained by double extrapolation to zero concentration of Huggins' (Eq. (6)) and Kraemer (Eq. (7)) equations, respectively.
| (6) |
| (7) |
where ηsp and ηrel are the (dimensionless) specific and relative viscosities, and are the Huggins's and Kraemer's coefficients, respectively, and C is the solution concentration.
2.6. Swelling and erosion behaviors
Swelling and erosion behaviors of tamarind gum tablet (without drug) were evaluated in order to reveal disintegrating mechanism of different seed gums in the fast disintegrating tablet. Measurements of swelling and erosion rates of the gum tablet were carried out, after immersion of tablet in the distilled water. The weighed tablets (W0) were placed in the closed glass containers at 37.0 ± 0.5 °C. After 5, 10, 20, 30, 40, 50 and 60 min, each container was removed from the incubator. The tablet with the mesh was withdrawn from the medium and blotted to remove excess water and then weighed (W1). The experiment was performed in triplicate for each time point and fresh samples were used for each individual time point. The percentage increase in weight due to absorbed liquid or water uptake was estimated at each time point from Eq. (8):
| (8) |
2.7. Preparation of fast disintegrating Thai cordial tablet by direct compression
Crude and carboxymethylated gums were mixed with Thai cordial powder at different formulations as shown in Table 1. Then, talcum (3%w) and magnesium stearate (1%w) as lubricants were blended by a cubic mixer for 15 min. The resulting blend was directly compressed into tablet at a fixed compression force using a hydraulic press (Specac Inc., USA) at fixed compression force of 2 tons for 60 s with 9.5 mm diameter flat-faced punch set for in vitro drug release test.
Table 1.
Different tablet formulations (F1–F4) of the fast disintegrating Thai cordial tablet.
| F1 (%) | F2 (%) | F3 (%) | F4 (%) | |
|---|---|---|---|---|
| Thai cordial | 91 | 86 | 81 | 76 |
| Gum* | 5 | 10 | 15 | 20 |
| Talcum | 3 | 3 | 3 | 3 |
| Magnesium stearate | 1 | 1 | 1 | 1 |
Note: *Both crude and carboxymethylated gums were observed.
2.8. Evaluation of the fast disintegrating Thai cordial tablet
The fracture strength of the tablet was monitored as the force required for breaking the tablet by radial compression. The tablet hardness, thickness and diameter were measured by using a tablet hardness tester (THB 325TD, Erweka, Germany).
The disintegration time was investigated using a disintegration tablet tester (ZT 220, Erweka, Germany). Purified water was used as the medium with temperature of 37 ± 2 °C. The dip speed was set at 30 dip/min. All measurements were performed in triplicate.
2.9. Statistical analysis
Analysis of variance (ANOVA) and Levine's test for homogeneity of variance were performed using SPSS version 10.0 for Windows (SPSS Inc., USA). Post hoc testing (P < 0.05) of the multiple comparisons was performed by either the Scheffé or Games–Howell test depending on whether Levine's test was insignificant or significant, respectively.
3. Results and discussion
3.1. Chemical compositions
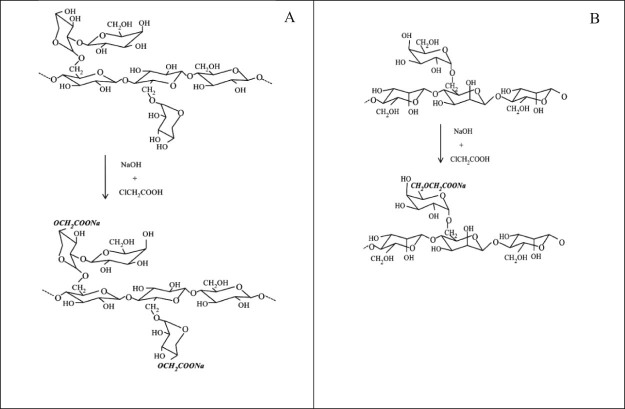
Carboxymethylation process was used to modify the chemical structure of crude gums from Tamarindus indica and Cassia fistula seeds. Due to investigation of the effect of NaOH adding on DS, carboxymethylation was carried out at different nNaOH/nMCA ratios (1, 1.78 and 2.78) and at fixed 0.56 mol. Two modified gums for each crude gum (Table 2) were then chosen from minimum and maximum values of degree of substitution (DS) which is defined as the amount of formed carboxymethyl groups (—CH2COO−) that substitutes per anhydroglucose unit and the monomer unit of polysaccharide. For our modified gum samples, DS value was obtained from 0.1711 to 0.1756 which was less than those of other researches [2], [12], [17]; however, this value strongly depends on the reaction conditions such as reagent condition, reaction medium and temperature [12], [15], [18], [19]. In theory, the maximum DS value in such molecules is thus three [20]. The general scheme as seen in Fig. 1 shows that the carboxymethyl group substitution onto side chain of polysaccharide.
Table 2.
DS value of carboxymethylated gums.
| Seed gum | Code | DS value |
|---|---|---|
| Tamarindus indica | ||
| Carboxymethylated gum a | Ta | 0.1711 |
| Carboxymethylated gum b | Tb | 0.1756 |
| Cassia fistula | ||
| Carboxymethylated gum a | Ca | 0.1717 |
| Carboxymethylated gum b | Cb | 0.1738 |
Fig. 1.
Depicts the substitution of carboxymethyl group onto side chain of polysaccharide as seed gums from Tamarindus indica (A) and Cassia fistula (B) via carboxymethylation.
The main chemical compositions of crude and carboxymethylate gum samples were analyzed. The impurities as protein and fat contents were eliminated by carboxymethylation process. Consequently, polysaccharide content of modified gums was enhanced as presented in Table 3.
Table 3.
Chemical compositions of crude and carboxymethylated gums.
| Seed gum | Code | Moisture | Ash | Protein | Fat | Polysaccharidea |
|---|---|---|---|---|---|---|
| Tamarindus indica | ||||||
| Crude | T | 3.61 | 0.12 | 3.58 | 15.25 | 81.05 |
| Carboxymethylated | Ta | 4.95 | 0.10 | 0 | 14.70 | 85.20 |
| Tb | 5.31 | 0.15 | 0 | 2.59 | 97.26 | |
| Cassia fistula | ||||||
| Crude | C | 4.29 | 0.09 | 1.03 | 10.04 | 88.84 |
| Carboxymethylated | Ca | 4.63 | 0.26 | 0 | 8.76 | 90.98 |
| Cb | 7.07 | 0.15 | 0 | 4.64 | 95.21 | |
Note: All values (%) on a dried weight basis are mean ± standard deviation of three determinations.
Polysaccharide values were calculated by difference.
3.2. Fourier transform infrared (FTIR) spectroscopy
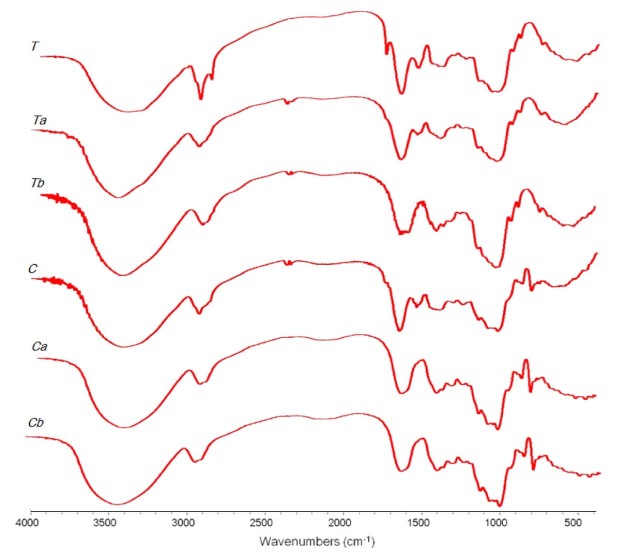
Infrared spectra of crude (T, C) and carboxymethylated (Ta, Tb, Ca, and Cb) gums from seeds of Tamarindus indica and Cassia fistula are shown in Fig. 2. All gums showed characteristic absorption bands associated with the stretching vibration of —OH in the 3500–3300 cm−1 region, —CH in the 3000–2800 cm−1 region and around 1400 cm−1 (bending vibration). The absorption bands appearing around 1020, 1070 and 1155 cm−1 were corresponding to the stretching vibration of C—OH from the mannose and glucan structures [13], [21]. The peak at 1748 cm−1 from crude gum of Tamarindus indica seed might be referred to C O stretching from impurity of protein. This peak disappeared after the chemical modification which is in agreement with the chemical compositions test result as presented in Table 3. The IR spectrum of carboxymethylated gums shows the higher wavelength shifting of the absorption band from the crude gums located at 3400 or 3370 cm−1 for Tamarindus indica and Cassia fistula seeds, respectively, due to OH stretching, indicating that some OH groups were carboxymethylated [22]. In addition, three new peaks due to carboxymethyl moiety emerged in the spectrum of the modified gums. A peak at 1650 cm−1 was due to asymmetric stretching vibration and peaks around 1398 and 1395 cm−1 for Ta and Tb and 1408 and 1400 cm−1 for Ca and Cb were concerned with the symmetrical stretching vibration of carboxylate ion in the modified gums [23].
Fig. 2.
IR spectra of crude (T, C) and carboxymethylated (Ta, Tb, Ca, and Cb) gums from seeds of Tamarindus indica (T, Ta, and Tb) and Cassia fistula (C, Ca, and Cb).
3.3. Intrinsic viscosity
The intrinsic viscosity of crude and modified gums was observed (Fig. 3). The parameters from intrinsic viscosity extrapolation are listed in Table 4. As expected, carboxymethylated gums exhibited high intrinsic viscosity when compared to the native gums. Due to carboxymethyl groups which hydrate well in aqueous solutions were obtained. In addition, the carboxymethylated gums with higher DS values as Tb and Cb resulted in higher intrinsic viscosity.
Fig. 3.
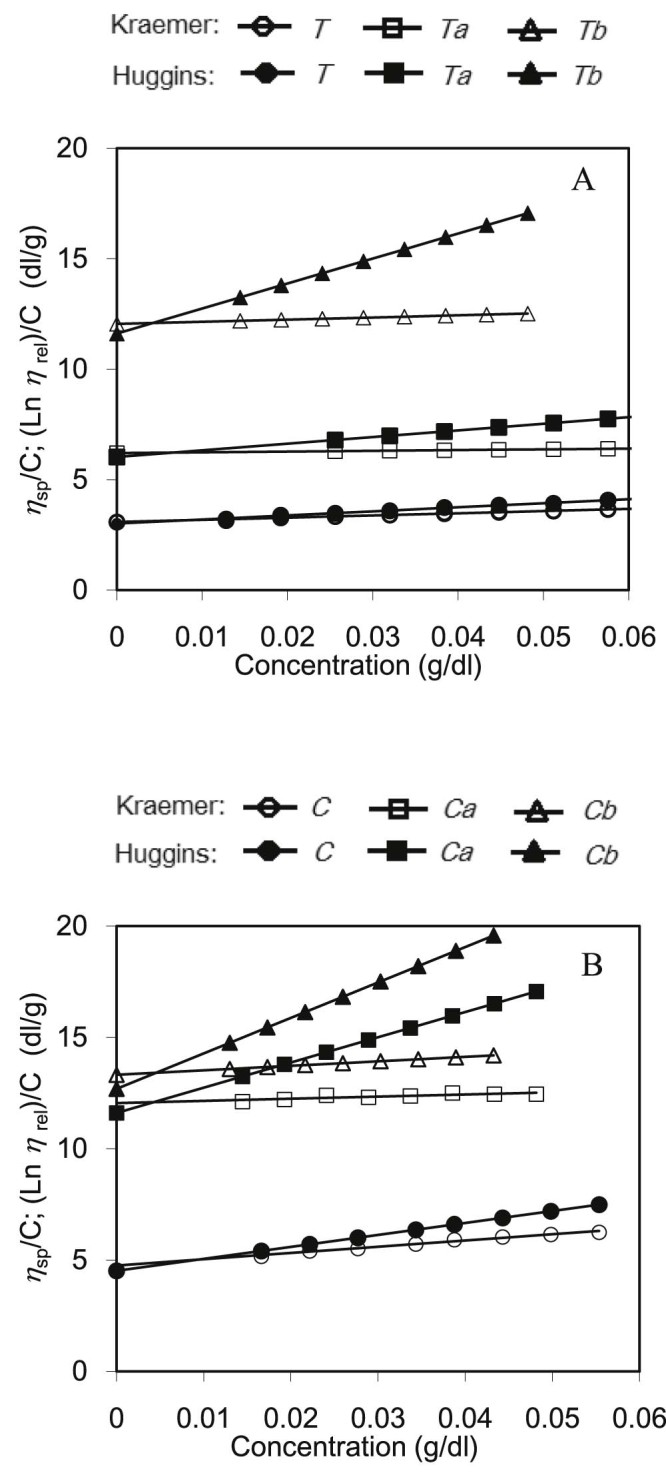
Extrapolation of intrinsic viscosity from Huggins' and Kraemer plots, ηsp/C and (lnηrel)/C against concentration for seed gums aqueous solutions at 25 °C. Tamarindus indica (A) and Cassia fistula (B).
Table 4.
Intrinsic viscosity of crude and carboxymethylated gums.
| Tamarindus indica | Cassia fistula | |||||
|---|---|---|---|---|---|---|
| T | Ta | Tb | C | Ca | Cb | |
| Intrinsic viscosity (dl/g), [η]H | 3.01 | 6.03 | 9.80 | 4.52 | 11.61 | 12.69 |
| Intrinsic viscosity (dl/g), [η]K | 3.08 | 6.02 | 10.51 | 4.76 | 12.05 | 13.32 |
| Huggins' coefficient, kH′ | 2.03 | 0.83 | 1.21 | 2.63 | 0.84 | 0.99 |
3.4. Swelling and erosion behavior
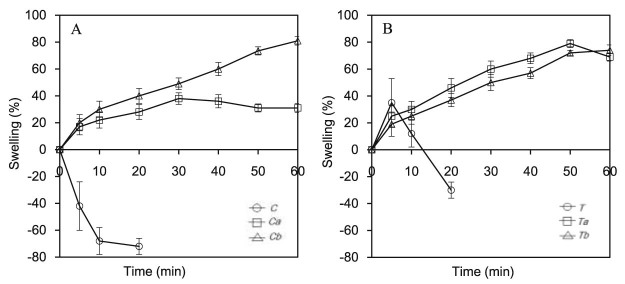
The swelling and erosion behaviors of the matrix tablet made of gum and its carboxymethylated forms, accessed by gravimetric technique, in purified water are illustrated in Fig. 4. The swelling profiles of those gum samples are also shown in Fig. 5. Crude did not swell but eroded instead, while carboxymethylated gums at both DS values showed high swelling profiles. Modified gum (Ta) gradually swelled until 30 min, and then the percentage of swelling did not increase and was stable around 31–38%. While the tablet of Tb swelled steadily and at 60 min the percentage of swelling was 81%. As shown in Fig. 5A and Fig. 5B, the swelling profiles of modified gums (Ca and Cb) were in the same direction with Ta and Tb. The crude of both types of modified gum absorbed water at first 5 min then completely disintegrated in 20 min. The swelling profiles of both Ca and Cb were not different statistically. The swelling percentages of both Ca and Cb increased constantly to about 72–79% at 50 min. The reason that carboxymethylated gums could swell better than those of crude gum might be due to the carboxymethylation process increased hydrophilic moiety in the polymer structure which enhanced polymer wetting and swelling [12], [13]. This reason also explains why Tb swelled greater than that of Ta. However, both modified gums were not significantly different. This might be because the DS level was not largely different [24].
Fig. 4.
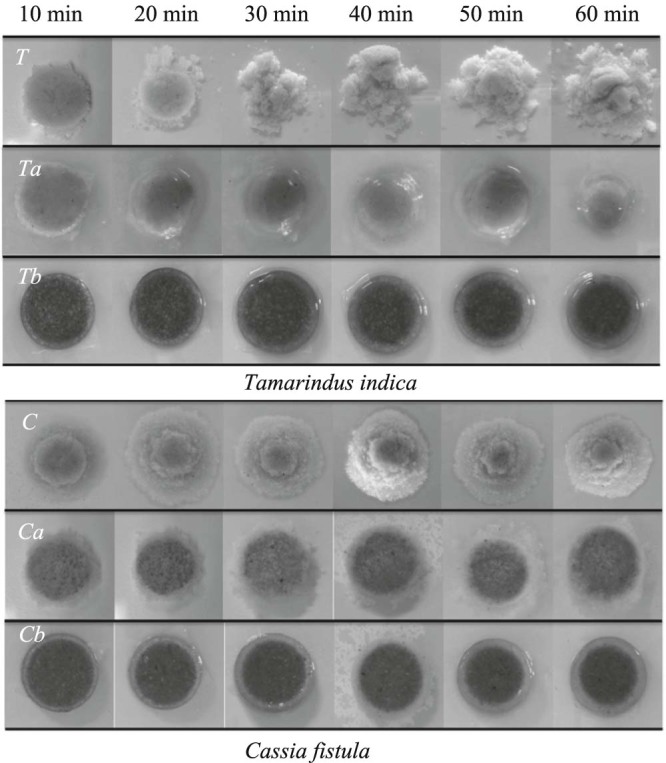
Swelling behaviors of crude (T and C) and carboxymethylated gums (Ta, Tb, Ca, and Cb).
Fig. 5.
Swelling profiles of crude and carboxymethylated gums from seeds of Tamarindus indica (A) and Cassia fistula (B).
3.5. Evaluation of the fast disintegrating Thai cordial tablet
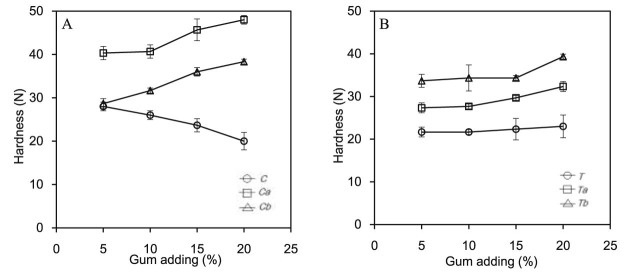
After physicochemical characterization of the crude and modified gums, both gums were formulated to the fast disintegrating Thai cordial tablet as indicated in Table 1. Thickness, diameter, hardness and disintegration time of tablet were evaluated to observe the effect of DS and amount of the gums. The average thickness and diameter of all prepared tablets were 4.71 ± 0.11 mm and 9.11 ± 0.02 mm, respectively. The hardness of the fast disintegrating Thai cordial tablet prepared by both crude gums was presented in Fig. 6A and 6B, respectively. For crude gum (T), the hardness slightly decreased from 28 to 20 N when the portion of the crude gum in the formation increased. On the other hand, the hardness of modified gums (Ta and Tb) extended in the same direction with gum amount in the formulation. This might be because the properties of gum powder were changed after chemical modification. Fat and protein contents from the crude gums were eliminated from the carboxymethylation process resulting in higher compactibility [25].
Fig. 6.
Hardness of the fast disintegrating Thai cordial tablet prepared by crude and carboxymethylated gums from seeds of Tamarindus indica (A) and Cassia fistula (B).
Regarding gum from Cassia fistula seed (Fig. 6B), the crude form offered the lowest hardness which was around 22–23 N. The hardness modified gums (Ca and Cb) was higher than that of C. Furthermore, increasing portion of the both modified gums in the formulation little accrued the tablet hardness. The reason is similar with those of gum from Tamarindus indica seed.
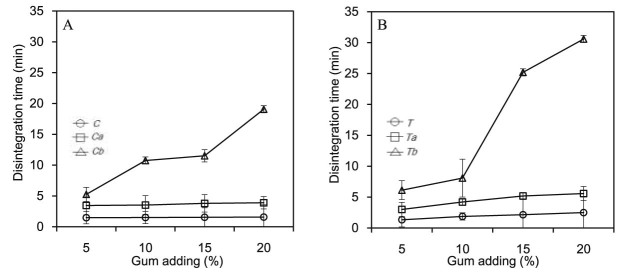
3.6. Disintegration time
Fig. 7A and 7B shows disintegration time of the fast disintegrating Thai cordial tablet prepared by both sources of gum at different amounts, respectively. The disintegration time result from the tablet fabricated by gums from Tamarindus indica was in the same direction with those of gums from Cassia fistula. The disintegration time increased when adding amount of gum with higher DS in the formulation. The disintegration times of crude gums were faster that the carboxymethylated gums due to low swelling and solubility properties of the crude gum as presented in previous study [26]. Based on the results, it can be concluded that adding crude gum can improve disintegration time of tablet by mechanism of particle repulsive forces as described by Caramella and co-workers [27]. However, adding the crude cannot improve tablet hardness due to the crude gum contains impurity such as fiber, fat and protein making surface properties of particle not suitable to compact together [28]. On the other hand, the modified gums could swell better than those of crude gum because the carboxymethylation process raised hydrophilic moiety in the polymer structure enhancing polymer wetting and swelling. This is in agreement with the result of tablet disintegration. Furthermore, disintegration of the tablet prepared from Tb and Cb trended to be longer than those of Ta and Ca. This might be due to the viscosities of Tb and Cb are higher than those of Ta and Ca resulting in retarding of water penetration in to the tablet. These can indicate that disintegrating mechanism of the carboxymethylated gums might be swelling and wicking. This finding is in the same direction with our previous study that the polymer in group of crosslinked cellulose expresses disintegration mechanism by swelling and wicking [29]. Moreover, the disintegration time depends on the swelling kinetics of polymer.
Fig. 7.
Disintegration time of the fast disintegrating Thai cordial tablet prepared by crude and carboxymethylated gums from seeds of Tamarindus indica (A) and Cassia fistula (B).
4. Conclusion
Crude and carboxymethylated gums from Tamarindus indica and Cassia fistula seeds were hence interesting to study as preliminary assessment to expressing pharmaceutical formulations. Carboxymethylation process could change the chemical structure and physical properties of crude gums. Swelling behaviors of both crude and carboxymethylated gums for both seed types were observed. The obtained results from swelling test showed that the carboxymethylated gums could be better used than that crude gums for further pharmaceutical application as disintegrant, diluent and drug release controlling agent.
Acknowledgments
Author (W. Sittikijyothin) thanks Higher Education Research Promotion-National Research Universities (HERP-NRU) for financial supporting under grant no. 2558A10862016. Chanisara Ngamsalak and Bantita Ponnikornkit are acknowledged for technical work.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Anand N., Singh L., Sharma V. Emergence of natural super-disintegrants in oro-dispersible tablets: an overview. Int Res J Pharm. 2013;4:33–37. [Google Scholar]
- 2.Singh V., Kumar P. Carboxymethyl tamarind gum–silica nanohybrids for effective immobilization of amylase. J Mol Catal B-Enzym. 2011;70:67–73. [Google Scholar]
- 3.Sumathi S., Ray A.R. Release behaviour of drugs from tamarind seed polysaccharide tablets. J Pharm Sci. 2002;5:12–18. [PubMed] [Google Scholar]
- 4.Sittikijyothin W., Torres D., Gonçalves M.P. Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohyd Polym. 2005;59:339–350. [Google Scholar]
- 5.Cerqueira M.A., Lima A.M., Teixeira J.A., et al. Suitability of novel galactomannans as edible coatings for tropical fruits. J Food Eng. 2009;94:372–378. [Google Scholar]
- 6.El-Siddig K. Internat. Centre for Underutilised Crops, University of Southampton; 2006. Tamarind: Tamarindus Indica L. [Google Scholar]
- 7.Mathur N.K. Taylor & Francis; 2011. Industrial galactomannan polysaccharides. [Google Scholar]
- 8.Osmałek T., Froelich A., Tasarek S. Application of gellan gum in pharmacy and medicine. Int J Pharm. 2014;466:328–340. doi: 10.1016/j.ijpharm.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Patel T.R., Morris G.A., Ebringerova A., et al. Global conformation analysis of irradiated xyloglucans. Carbohyd Polym. 2008;74:845–851. [Google Scholar]
- 10.Srivastava M., Kapoor V.P. Seed galactomannans: an overview. Chem Biodivers. 2005;2:295–317. doi: 10.1002/cbdv.200590013. [DOI] [PubMed] [Google Scholar]
- 11.Dodi G., Hritcu D., Popa M.I. Carboxymethylation of guar gum: synthesis and characterization. Cellulose Chem Technol. 2011;45:171–176. [Google Scholar]
- 12.Goyal P., Kumar V., Sharma P. Carboxymethylation of tamarind kernel powder. Carbohyd Polym. 2007;69:251–255. [Google Scholar]
- 13.Rajput G., Pandey I.P., Joshi G. Carboxymethylation of Cassia angustifolia seed gum: synthesis and rheological study. Carbohyd Polym. 2015;117:494–500. doi: 10.1016/j.carbpol.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 14.Sharma B.R., Kumar V., Soni P.L. Carbamoylethylation of Cassia tora gum. Carbohyd Polym. 2003;54:143–147. [Google Scholar]
- 15.Silva D.A., Paula R.C.M., Feitosa J.P.A., et al. Carboxymethylation of cashew tree exudate polysaccharide. Carbohyd Polym. 2004;58:163–171. [Google Scholar]
- 16.Gong H., Liu M., Chen J., et al. Synthesis and characterization of carboxymethyl guar gum and rheological properties of its solutions. Carbohyd Polym. 2012;88:1015–1022. [Google Scholar]
- 17.Pal S., Sen G., Mishra S., et al. Carboxymethyl tamarind: synthesis, characterization and its application as novel drug-delivery agent. J Appl Polym Sci. 2008;110:392–400. [Google Scholar]
- 18.Pushpamalar V., Langford S.J., Ahmad M., et al. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohyd Polym. 2006;64:312–318. [Google Scholar]
- 19.de Abreu F.R., Campana-Filho S.P. Characteristics and properties of carboxymethylchitosan. Carbohyd Polym. 2009;75:214–221. [Google Scholar]
- 20.Tijsen C.J., Kolk H.J., Stamhuis E.J., et al. An experimental study on the carboxymethylation of granular potato starch in non-aqueous media. Carbohyd Polym. 2001;45:219–226. [Google Scholar]
- 21.Banegas R.S., Zornio C.F., Borges A.M.G., et al. Preparation, characterization and properties of films obtained from cross-linked guar gum. Polímeros. 2013;23:182–188. [Google Scholar]
- 22.Yadav M., Srivastav A., Verma S.K., et al. Graft (partially carboxymethylated guar gum-g-poly vinyl sulfonic acid) copolymer: from synthesis to applications. Carbohyd Polym. 2013;97:597–603. doi: 10.1016/j.carbpol.2013.02.084. [DOI] [PubMed] [Google Scholar]
- 23.Singh R., Maity S., Sa B. Effect of ionic crosslink on the release of metronidazole from partially carboxymethylated guar gum tablet. Carbohyd Polym. 2014;106:414–421. doi: 10.1016/j.carbpol.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Huanbutta K., Sangnim T., Sittikijyothin W. Physicochemical characterization of gum from tamarind seed: potential for pharmaceutical application. Asian J Pharm Sci. 2016;11:176–177. [Google Scholar]
- 25.Youssef M.K., Wang Q., Cui S.W., et al. Purification and partial physicochemical characteristics of protein free fenugreek gums. Food Hydrocolloids. 2009;23:2049–2053. [Google Scholar]
- 26.Huanbutta K., Sangnim T., Sittikijyothin W. Development of tamarind seed gum as dry binder in formulation of diclofenac sodium tablets. Walailak J Sci Tech. 2016;13 (in press) [Google Scholar]
- 27.Caramella C., Colombo P., Conte U., et al. A physical analysis of the phenomenon of tablet disintegration. Int J Pharm. 1988;44:177–186. [Google Scholar]
- 28.Limnell T., Santos H.A., Makila E., et al. Drug delivery formulations of ordered and nonordered mesoporous silica: comparison of three drug loading methods. J Pharm Sci. 2011;100:3294–3306. doi: 10.1002/jps.22577. [DOI] [PubMed] [Google Scholar]
- 29.Huanbutta K., Sriamornsak P., Limmatvapirat S., et al. Swelling kinetics of spray-dried chitosan acetate assessed by magnetic resonance imaging and their relation to drug release kinetics of chitosan matrix tablets. Eur J Pharm Biopharm. 2011;77:320–326. doi: 10.1016/j.ejpb.2010.11.019. [DOI] [PubMed] [Google Scholar]