Graphical Abstract
Keywords: Porous silica nanoparticles, Thermoresponsive polymer, Drug delivery system, Ibuprofen, Drug loading, Drug release profile
Abstract
Hybrid drug delivery systems (DDS) have been prepared by grafting poly(NIPAM-co-MPS) chains on multimodal porous silica nanoparticles having an inner mesoporous structure and an outer thin layer of micropores. The hybrid thermoresponsive DDS were fully characterized and loaded with a model drug. The in vitro drug release tests are carried out at below and above the lower critical solution temperature (LCST) of the copolymer. The results have revealed that due to the presence of small diameter (~1.3 nm) micropores at the periphery of the particles, the collapsed globules of the thermoresponsive copolymer above its LCST hinders the complete release of the drug which resulted in a reverse thermoresponsive drug release profile by the hybrid DDS.
1. Introduction
Mesoporous silica nanoparticles (MSNs) have emerged as one of the most efficient drug delivery systems for the delivery of various drug molecules [1], [2], [3], [4]. Easy synthesis and surface modification methods, good stability and biocompatibility are the characteristic properties that make MSNs versatile material in drug delivery applications [5]. The transformation of bare MSNs into controlled thermosensitive drug delivery systems (DDS) is carried out by grafting of thermoresponsive polymers such as poly(N-isopropylacrylamide) or its various copolymers on the particle outer surface or/and on the inner pore walls [6], [7], [8]. Poly(N-isopropylacrylamide) (PNIPAM) is a watersoluble thermoresponsive polymer which shows a typical coil-to-globule transition at its lower critical solution temperature (LCST) which is around 32 °C [9], [10]. The LCST of the polymer can be tuned toward higher values by preparing various copolymers of PNIPAM [11]. Above LCST, the water soluble extended chains of the polymer collapse to form compact insoluble globules. As the volume occupied by the polymer in its hydrated coil form is much higher than the collapsed globules, this transition applies a pore opening–pore closing mechanism to the MSNs [6], [7], [8].
If the transition of the thermoresponsive polymer occurs inside the pores of the MSNs, at temperatures below the LCST the hydrated extended chains of the polymer block the pores, which prevent the release of the drug molecules loaded inside the MSNs. Instead upon heating above the LCST, the polymer chains collapse toward the pore walls which opens the pores and completely releases the drug. Thus a normal drug release profile of such thermosensitive DDS shows reduced or no release of the drug at temperatures below the LCST of the polymer and higher or complete release of the drug above the LCST of the polymer. However, the pore size can also play an important role in the pore opening/closing mechanism. This role of microporosity in thermoresponsive DDS needs to be investigated. Related to this in the present work we report about the influence of pore size in designing thermoresponsive DDS where the presence of micropores on the surface of MSNs can result in a reverse thermoresponsive drug release profile. This occurs due to the full or partial blockage of the micropores by the collapsed globules of the thermoresponsive copolymer. This work therefore points out an important consideration about the proper choice of MSNs and diameter of the pores present on them for their use for preparation of thermoresponsive DDS.
2. Materials and methods
2.1. Materials
Porous silica particles (particle size 200 nm, pore size 4 nm), N-isopropylacrylamide (NIPAM), azobisisobutyronitrile (AIBN), 3-(methacryloxypropyl) trimethoxysilane (MPS), ibuprofen (standard RS α-methyl-4-(isobutyl)phenylacetic acid, (±)-2-(4-isobutylphenyl) propanoic acid) were purchased from Sigma-Aldrich, Italy. All the solvents mentioned in the synthesis procedure were of high purity and used as received. Ethanol was kept on molecular sieves overnight before using for polymerization reaction.
2.2. Instruments and methods
High resolution transmission electron microscopy (HRTEM) images were obtained with a JEOL 2010 instrument (300 kV) equipped with a LaB6 filament. For specimen preparation powdery samples were supported onto holed carbon coated copper grids by dry deposition.
Fourier transform infrared spectroscopy (FTIR) spectra were collected with a Perkin Elmer FTIR Nexus instrument equipped with an attenuated total reflectance (ATR) devise (Thermo Nicolet Smart Endurance) and with a DTGS detector. The spectra were collected in the spectral range of 4000–670 cm−1 and with a resolution of 4 cm−1.
Gas-volumetric analysis, specific surface area (SSA), pore volume and size were measured by N2 adsorption–desorption isotherms at 77 K using an ASAP 2020 (Micromeritics) gas-volumetric analyzer. SSA was calculated using the Brunauer–Emmett–Teller (BET) method. Porosity distribution, allowing oneto estimate width and volume, was calculated with the non-local density functional theory (NLDFT), employing the N2-DTF model with slit pore geometry (regularization low). The samples were kept in an oven at 50 °C for 4 h and then outgassed at RT overnight before analysis.
Size exclusion chromatography (SEC) was performed with a Viscotek modular instrument equipped with a VE 1122 pump, a VE 7510 degasser, manual injection valve, VE 3580 refractive index detector, column oven and two PLgel 10 µm MIXED-B columns (Polymer Laboratories, UK). N,N-Dimethylformamide (DMF) (1.0 ml/min) was used as eluent. Due to the density of DMF, analyses were performed setting the column oven at 70 °C.
Thermogravimetric analyses (TGA) were carried out on a TA Q500 model from TA Instruments by heating samples contained in alumina pans at a rate of 10 °C/min from 25 to 800 °C in air. A differential scanning calorimeter (DSC Q200, TA Inc.) was used to collect DSC thermograms of the samples. Weighed quantity of samples was placed in sealed aluminum pans and DSC measurements were performed under nitrogen atmosphere with heating rate of 10 °C/min.
UV–visible analyses were performed using a Lambda 15 UV–Vis spectrophotometer (Perkin Elmer). For ibuprofen quantification a calibration curve (ε = 185.1 M−1, R2 = 0.99) was obtained by plotting absorbance vs. concentration of ibuprofen between 6.0 × 10−5 and 4.8 × 10−4 M.
2.3. Preparation of poly(NIPAM-co-MPS)-grafted MSNs
The copolymer-grafted mesoporous nanoparticles were obtained by carrying out the synthesis of the copolymer in ethanol in the presence of the particles. MSNs (280 mg) were dispersed in 10 ml of ethanol solution of NIPAM and AIBN by sonication at room temperature. The NIPAM/MSNs weight ratio was 1:4. After 30 min MPS was added into the suspension and dispersed evenly by sonication for another 30 min. The dispersion was then injected in a three neck round bottom flask equipped with condenser and kept under nitrogen atmosphere. The injected suspension was then heated at 70 °C under nitrogen for 16 h. The white residue of polymer grafted-particles obtained was transferred in a test tube and washed two times with 5 ml of deionized water and once with 5 ml of ethanol. Each time the liquid phase was separated by centrifugation. The final product obtained was dried under vacuum for several hours before characterization.
2.4. Drug loading and release tests
Ibuprofen was selected as model drug for loading and release tests. MSNs were outgassed at RT overnight to remove adsorbed water and then were dispersed under magnetic stirring in a hexane solution of ibuprofen (0.16 M) at 40 °C for 48 h. Then nanoparticles were separated by centrifugation, washed once with hexane and dried at RT. Ibuprofen loading was determined by TGA. The loading was calculated from TGA by subtracting from the total weight loss the contribution due to the copolymer, adsorbed water and dehydroxylation.
In vitro drug release tests were carried out in physiological saline solution (PSS). The release profiles in PSS were obtained by monitoring the absorbance peak of ibuprofen at 272 nm at different time intervals and at well below the LCST (4 °C) which is also the storage temperature of suspension of drugs and above the LCST (40 °C) of the copolymer.
3. Results and discussion
3.1. Synthesis and characterization of poly(NIPAM-co-MPS)-grafted MSNs
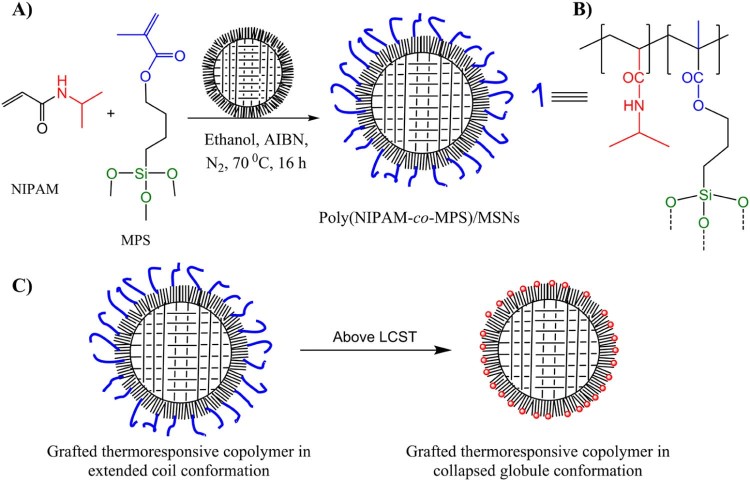
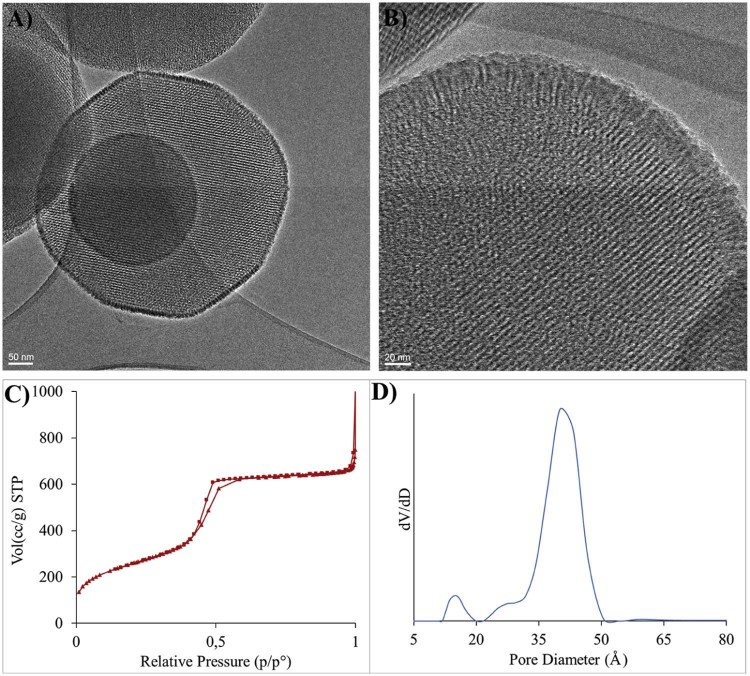
The scheme for the post-synthesis surface grafting of thermoresponsive random copolymer poly(NIPAM-co-MPS) on MSNs is shown in Fig. 1. The reactivity of surface silanol groups present on MSNs is exploited for anchoring of the copolymer [12], [13], [14]. The TEM images, N2 adsorption isotherms and pore size distribution of the MSNs used in this work are shown in Fig. 2. The average diameter of the particles is approximately 190–200 nm. The ordered porous structure is clearly visible, while the magnified TEM image of a single particle revealed that a border of microporous channels of approximately 15–25 nm in length is present at the periphery of the particles. This multimodal porous structure was further confirmed by porosimetry. Specific surface area of the particles calculated by BET method was 901 m2/g. The pore diameter distribution curve, calculated by NLDFT, clearly showed the presence of micropores (1.3 nm in diameter) and mesopores (4 nm in diameter) on the particles. During the copolymerization in the presence of MSNs diffusion of NIPAM and MPS monomers inside the pores may be limited due to the very small diameter of the porous channels at the periphery of the nanoparticles. This resulted in grafting of the copolymer mostly on the outer surface of the particles. The reaction conditions for the polymer grafting and properties of the copolymer are reported in Table 1. The copolymer grafting on MSNs was confirmed by ATR-FTIR spectroscopy. The results obtained are shown in Fig. 3 which shows ATR-FTIR spectra of bare and polymer-grafted nanoparticles. The characteristic peaks of the copolymer due to C—H (bend.) at 1370, CH3 (bend.) at 1391 cm−1, C—N (stretch.) at 1460, 1545 cm−1, amide C O (stretch.) at 1640 cm−1 and acryloxy C O (stretch.) from MPS at 1707 cm−1 were observed in the spectrum of poly(NIPAM-co-MPS)-grafted MSNs [6], [13]. While the spectra of ibuprofen-loaded hybrid MSNs showed the presence of characteristic peaks of ibuprofen. Especially the peak at 1719 cm−1 due to C O (stretch.) was clearly evident. This confirmed the loading of ibuprofen inside poly(NIPAM-co-MPS)-grafted MSNs.
Fig. 1.
(A) Grafting of the copolymer on MSNs, (B) structure of the copolymer and (C) coil-to-globule transition of the grafted copolymer on the surface of MSNs.
Fig. 2.
(A, B) TEM images of MSNs, (C) N2 adsorption desorption isotherms and (D) pore size distribution curve of the MSNs.
Table 1.
The synthesis conditions and properties of the copolymer.
| Polymer | NIPAM/MPS | NIPAM/AIBN | NIPAM/MSNs | Mn (Da) | Mw (Da) | PDI | LCST (°C) |
|---|---|---|---|---|---|---|---|
| Poly(NIPAM-co-MPS) | 20:1 | 30:1 | 1:4 | 8,500 | 19,000 | 2.2 | 36 |
Fig. 3.

ATR-FTIR spectra of (A) bare, (B) poly(NIPAM-co-MPS)-grafted MSNs, (C) ibuprofen loaded poly(NIPAM-co-MPS)-grafted MSNs and (D) ibuprofen reference spectrum.
3.2. Quantitative polymer grafting and drug loading
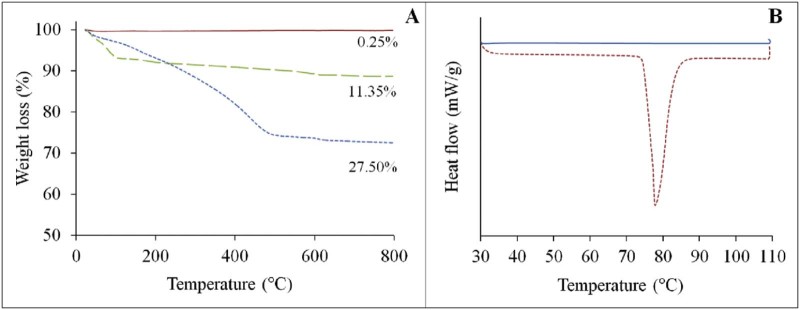
TGA curves of bare MSNs and poly(NIPAM-co-MPS)-grafted MSN samples are shown in Fig. 4A. Bare MSNs showed good thermal stability as upon programmed heating up to 800 °C only 0.25% of weight loss was observed for the particles. The weight loss for poly(NIPAM-co-MPS)-grated MSNs was 11.35% and for ibuprofen-loaded hybrid nanoparticles it was 27.5%. These data were used to calculate the amount of grafted copolymer and the drug loading. The calculated percentage of grafted copolymer was 11.1%, while the quantitative loading of ibuprofen obtained was 16.15%. The ibuprofen loaded inside the hybrid porous particles is in amorphous state as no signs of crystallization of the ibuprofen incorporated in hybrid porous silica were detected with DSC (Fig. 4B).
Fig. 4.
(A) TGA curves of bare MSNs (solid line), poly(NIPAM-co-MPS)-grafted MSNs (dashed line) and ibuprofen loaded poly(NIPAM-co-MPS)-grafted MSNs (dotted line). (B) DSC curves of ibuprofen loaded poly(NIPAM-co-MPS)-grafted MSNs (solid line) and ibuprofen (dotted line).
3.3. In vitro drug release tests
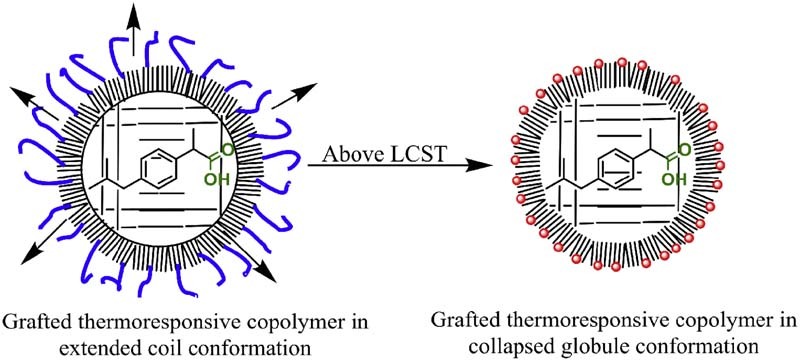
The drug release profiles at below and above the LCST of the thermoresponsive copolymer are shown in Fig. 5. An initial controlled release of ibuprofen at both temperatures by poly(NIPAM-co-MPS)-grafted MSNs was observed. The quantitative release at 40 °C is lower than at 4 °C. The reverse thermoresponsive drug release profile was observed due to partial blockage or plugging of the micropores at the periphery of the particles by the collapsed globules of the copolymer (refer to Fig. 1). Although a complete blockage of the pores was not observed, a significant difference in the total final quantitative release of ibuprofen was obtained. The more controlled and also less quantitative release above LCST of the copolymer clearly indicated the role of globules in hindering the release of drug. There are several studies which report the possible size, in the form of radius of gyration (Rg), of the PNIPAM globules formed above its LCST in water or water–solvent mixtures [15], [16], [17], [18]. A direct relation between the molecular weight of thermoresponsive polymers and size of globules formed is never reported. Also none of the studies speculate or directly report the approximate size or radius of gyration of the globules. However, some studies report a Rg of globules for free or end-grafted PNIPAM between 12 and 25 Å, which is very close to the diameter of the micropores present on MSNs used in this study [19], [20]. Hence the hypothesis of partial blocking of the micropores by the globules of the copolymer can be supported. The copolymer in its collapsed state on the micropores hinders the complete release of ibuprofen above its LCST. Instead the situation may be reversed in the case of MSNs with bigger pore diameters where a higher release of drug is observed above LCST of the polymer as the pore volume can be sufficiently higher to accommodate the globules [6], [7], [8]. The drug release profiles obtained thus point out that MSNs with very small pore diameters may not be a good choice for the preparation of thermoresponsive DDS. While on the other hand such reverse thermoresponsive delivery systems can be of great importance where a more controlled or less quantitative release of active molecules is desired at higher temperatures.
Fig. 5.
(A) Drug release profiles by poly(NIPAM-co-MPS)-grafted MSNs at 4 °C (solid line) and 40 °C (dotted line). (B) Magnification of the release profiles up to 7 h.
4. Conclusion
In this work thermoresponsive copolymer-grafted bimodal porous silica nanoparticles were synthesized and their in vitro drug release profiles were studied. It was observed that, due the presence of a microporous channel zone, the collapsed dehydrated state (globules) of the copolymer obstructs the release of ibuprofen molecules above the LCST of the copolymer. Hence reverse thermoresponsive drug release profiles were obtained. This highlights the importance of the pore diameters on MSNs for the preparation of thermoresponsive drug delivery systems. Instead such reverse thermoresponsive release behavior can be used in therapies where a sustained release of drug is desired at higher temperatures.
Acknowledgments
Dr. S. A. Jadhav thanks MIUR, Italy for the financial support. The authors thank Dr. Elena Ugazio for providing porous silica nanoparticles.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Argyo C., Weiss V., Brauchle C., et al. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater. 2014;26:435–451. [Google Scholar]
- 2.Hu L., Sun C., Song A., et al. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian J Pharm Sci. 2014;9:183–190. [Google Scholar]
- 3.Liu L., Li J., Zhao M., et al. Loading of tacrolimus containing lipid based drug delivery systems into mesoporous silica for extended release. Asian J Pharm Sci. 2016;11:751–759. [Google Scholar]
- 4.Jadhav S.A., Brunella V., Berlier G., et al. Effect of multimodal pore channels on cargo release from mesoporous silica nanoparticles. J Nanomater. 2016 1325174. [Google Scholar]
- 5.Jadhav S.A. Incredible pace of research on mesoporous silica nanoparticles. Inorg Chem Front. 2014;1:735–739. [Google Scholar]
- 6.Brunella V., Jadhav S.A., Miletto I., et al. Hybrid drug carriers with temperature-controlled on-off release: a simple and reliable synthesis of PNIPAM-functionalized mesoporous silica nanoparticles. React Funct Polym. 2016;98:31–37. [Google Scholar]
- 7.Ugazio E., Gastaldi L., Brunella V., et al. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int J Pharm. 2016;511:446–454. doi: 10.1016/j.ijpharm.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Jadhav S.A., Scalarone D., Brunella V., et al. Thermoresponsive copolymer-grafted SBA-15 porous silica particles for temperature-triggered topical delivery systems. Express Polym Lett. 2017;11:96–105. [Google Scholar]
- 9.Schild H.G. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci. 1992;17:163–249. [Google Scholar]
- 10.Taylor P., Heskins M., Guillet J.E. Chemistry solution properties of poly(N-isopropylacrylamide) J Macromol Sci A. 2006;8:37–41. [Google Scholar]
- 11.Jain K., Vedarajan R., Watanabe M., et al. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym Chem. 2015;6:6819–6825. [Google Scholar]
- 12.Jadhav S.A., Brunella V., Scalarone D. Polymerizable ligands as stabilizers for nanoparticles. Part Part Syst Charact. 2015;32:417–428. [Google Scholar]
- 13.Jadhav S.A., Miletto I., Brunella V., et al. Controlled post-synthesis grafting of thermoresponsive poly(N-isopropylacrylamide) on mesoporous silica nanoparticles. Polym Adv Technol. 2015;26:1070–1075. [Google Scholar]
- 14.Jadhav S.A., Brunella V., Miletto I., et al. Synthesis of poly(N-isopropylacrylamide) by distillation precipitation polymerization and quantitative grafting on mesoporous silica. J Appl Polym Sci. 2016;133:44181. [Google Scholar]
- 15.Wang X., Qiu X., Wu C. Comparison of the coil-to-globule and the globule-to-coil transitions of a single poly(N-isopropylacrylamide) homopolymer chain in water. Macromolecules. 1998;31:2972–2976. [Google Scholar]
- 16.Zeng F., Tong Z., Sato T. Molecular chain properties of poly(N-isopropyl acrylamide) Sci China B. 1999;42:290–297. [Google Scholar]
- 17.Rodríguez-Ropero F., Hajari T., Van Der Vegt N.F.A. Mechanism of polymer collapse in miscible good solvents. J Phys Chem B. 2015;119:15780–15788. doi: 10.1021/acs.jpcb.5b10684. [DOI] [PubMed] [Google Scholar]
- 18.Bischofberger I., Calzolari D.C.E., Trappe V. Co-nonsolvency of PNIPAM at the transition between solvation mechanisms. Soft Matter. 2014;10:8288–8295. doi: 10.1039/c4sm01345j. [DOI] [PubMed] [Google Scholar]
- 19.Alaghemandi M., Spohr E. A molecular dynamics study of poly(N-isopropylacrylamide) endgrafted on a model cylindrical pore surface. RSC Adv. 2013;3:3638–3647. [Google Scholar]
- 20.Deshmukh S.A., Li Z., Kamath G., et al. Atomistic insights into solvation dynamics and conformational transformation in thermo-sensitive and non-thermo-sensitive oligomers. Polymer (Guildf) 2013;54:210–222. [Google Scholar]