Graphical Abstract
Keywords: PLGA, Nanoparticles, Topical instillation, Retina, Surface modification
Abstract
We investigated the delivery of drugs to the posterior segment of the eye by non-invasive topical instillation using submicron-sized poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles (NPs). Surface-modified PLGA NPs were developed to improve the drug delivery efficiency to the retina and were administered as topical eye drops to mice. Chitosan (CS) and glycol chitosan (GCS), which are mucoadhesive polymers, and polysorbate 80 (P80) were used as surface modifiers, and have been reported to increase the association of NPs with cells. Coumarin-6 was used as a model drug and fluorescent marker, and after ocular administration of PLGA NP eye drops, the fluorescence intensity of coumarin-6 was observed in the retina. The fluorescence image analysis indicated that there are several possible routes to the retina and fates of PLGA NPs in ocular tissue, and that these pathways involved the corneal, non-corneal, or uveal routes. Delivery to the mouse retina segments after topical administration was increased by surface modification with CS, GCS, or P80. Surface-modified PLGA NPs are a promising method for retinal drug delivery via topical instillation.
1. Introduction
Diseases of the posterior segment of the eye related to the retina, including age-related macular degeneration (AMD), diabetic macular edema (DME), and retinal vein occlusion (RVO), require chronic therapy and sometimes result in blindness. These diseases are treated clinically with anti-vascular endothelial growth factor drugs, such as ranibizumab and aflibercept, that prevent eye neovascularization and are usually administered via intravitreal injection to the eye [1]. Although intravitreal injections deliver therapeutic amounts of drugs, this administration method carries risks, like pain, bleeding, cataracts, and infection. Therefore, non-invasive alternative methods for self-medication, particularly topical instillation, should be developed to avoid the risk of intravitreal injections. However, achieving sufficient drug delivery efficiency to the posterior segment of the eye after topical eye drop instillation is challenging because of biological barriers such as the tear film and anterior epithelium layers.
Nanosized liposomes are a promising drug carrier for drug delivery to the posterior segment of the eye following topical instillation [2], [3]. The intraocular behaviors of the drug and the carrier are strongly affected by the physicochemical properties of liposomes, such as particle size, lipid composition, and surface modification by functional materials [4], [5].
Another type of colloidal drug delivery system is biodegradable polymeric nanoparticles (NPs), such as poly(d,l-lactide-co-glycolide) (PLGA), which is approved for clinical use by the U.S. Food and Drug Administration. We have prepared PLGA NPs by emulsion solvent diffusion (ESD) and have demonstrated that they are suitable as drug carriers [6]. Surface modification of the polymer NPs by functional materials improves the interaction of NPs with cells [6]. Furthermore, a PLGA-based formulation for treating the posterior segment of the eye is commercially available. Ozurdex (Allergan Inc., Irvine, CA), an intravitreal implant for the sustained release of the steroid dexamethasone, has been developed to maintain therapeutic drug concentrations in the eye over the long-term to treat DME, RVO, and uveitis [7]. Therefore, PLGA-based formulations have commercial and practical advantages for ocular drug delivery over other drug carriers. However, there are few studies focusing on the treatment of the posterior segment of the eye including the retina with PLGA NPs. In the present study, we investigated drug delivery by PLGA NPs to the retina via topical instillation and proposed a mechanism for the retinal drug delivery of PLGA NPs based on in vivo experiments. Subsequently, we examined the improvement of delivery efficiency achieved by the surface modification of PLGA NPs. Based on our previous work, chitosan (CS) and glycol chitosan (GCS), which are biodegradable mucoadhesive polymers, and polysorbate 80 (P80), which is nonionic surfactant, were used as surface modifiers to improve interactions with ocular tissue [6].
2. Materials and methods
2.1. Preparation of surface-modified PLGA NPs
Information about the materials used in this study is given in the Supplementary materials. PLGA NPs were loaded with coumarin-6 (C6) as the fluorescent label and prepared by ESD [6], [8]. Briefly, C6 (1 mg) and PLGA (100 mg) were dissolved in acetone/ethanol (2/1, 3 ml). The solution was poured into a polyvinyl alcohol (PVA; 25 ml) solution (2% w/v, in distilled water) and stirred at room temperature at 400 rpm with a propeller agitator with three blades. The dispersion was centrifuged (43,400 × g for 10 min), the sediment was resuspended in distilled water, and the process was repeated. The dispersion was freeze-dried for 24 h. Surface-modified PLGA NPs were prepared using CS (0.05% w/v, in 100 mM acetate buffer, pH 4.4)-PVA (1% w/v), GCS (0.25% w/v, in acetate buffer)-PVA (1% w/v), or P80 (0.5% w/v, in distilled water)-PVA (1% w/v) as an antisolvent. The particle size and zeta potential were determined with Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK).
2.2. In vivo evaluation of drug delivery efficiency to mouse retina by image analysis
We used four-week-old male albino ddY mice (Japan SLC, Hamamatsu, Japan). All experiments were approved and monitored by the Institutional Animal Care and Use Committee of Gifu Pharmaceutical University. The delivery efficiency to the retina following topical instillation of the PLGA NPs suspension (3 µl, 0.05 g/l C6 in phosphate-buffered saline; PBS) was determined by fluorescence image analysis of slices of frozen eyeball. The fluorescence intensity of C6 in the specific region of the inner plexiform layer (IPL) close to the optic disc was measured by image analysis software (NIH Image, ImageJ) [2].
3. Results and discussion
3.1. Characterization of surface-modified PLGA NPs
We expected that similar to nanoliposomes, PLGA NPs would be suitable for retinal drug delivery following topical instillation [2], and that the surface modification using functional materials would improve the interaction with eye tissue and the delivery efficiency. Table 1 shows the physicochemical properties of the surface-modified C6-labeled PLGA NPs. Fig. 1 shows the particle size distributions of the different surface-modified PLGA NPs measured by dynamic light scattering. The polymers CS, GCS, and P80 were used as surface modifiers. CS is generally water soluble under acidic conditions, whereas GCS, which is CS conjugated with ethylene glycol, is water soluble over the whole pH range and retains its positive charge at physiological pH. P80 was used as a surface modifier to increase the cell association of NPs, because P80-modified NPs have a higher uptake into cells compared with unmodified NPs [6].
Table 1.
Characterization of surface-modified PLGA NPs.
| Surface modifier | Suspension after preparation | Resuspension in PBS of freeze-dried powder | Zeta potential (mV) | ||
|---|---|---|---|---|---|
| Particle size (nm) | Polydispersity index | Particle size (nm) | Polydispersity index | ||
| Unmodified | 224.5 | 0.068 | 219.2 | 0.161 | −41.3 ± 9.5 |
| P80 | 240.7 | 0.129 | 227.3 | 0.111 | −44.6 ± 7.0 |
| CS | 332.7 | 0.339 | 330.0 | 0.314 | −9.34 ± 6.5 |
| GCS | 518.6 | 0.296 | 588.7 | 0.483 | 39.9 ± 4.2 |
Fig. 1.
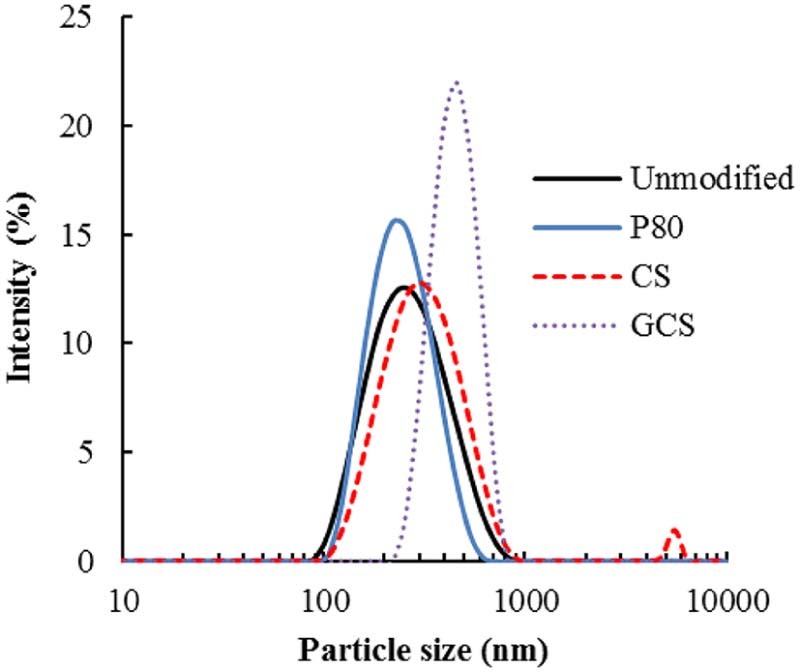
Particle size distributions of surface-modified PLGA NP suspension redispersed in PBS after freeze-drying.
The zeta potential of unmodified-PLGA NPs was −40 mV because of the carboxyl group of the PLGA polymer and the particle size was 200 nm. Freeze-dried NPs were readily dispersed in PBS by manual shaking, and the particle diameter was similar to that before drying. The range of particle sizes was 250 to 500 nm depending on the surface modifier. The particle sizes of CS- or GCS-PLGA NPs were larger because of the layers of CS or GCS molecules adsorbed on the PLGA NP surfaces [6]. Cationic polymer modified-PLGA NPs had increased zeta potentials because the amino groups were protonated. A small peak in the micrometer range was observed in the particle size distribution of CS- or GCS-PLGA NPs, owing to bridging between the loosely polymers on the surface (Fig. 1). The physicochemical properties of unmodified PLGA NPs and P80-modified PLGA NPs were similar; however, we have previously confirmed the surface modification of NPs with P80 by quantifying the amount of P80 with cobalt thiocyanate reagent [6].
3.2. Topical instillation of unmodified-PLGA NPs for drug delivery to the retina
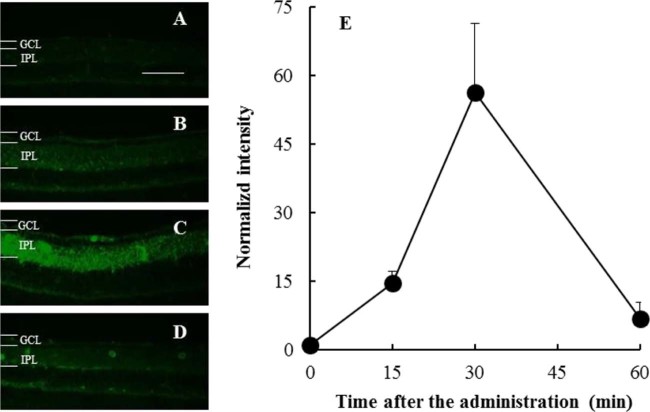
C6 was used as a fluorescence marker for tracking PLGA NPs by fluorescence microscopy. C6 release would not be detected in ocular tissue during an experiment because in an in vitro test, the leakage of C6 from NPs in PBS was not observed [6]. In the time-course experiments, microscopic images of the retina from the eye slices taken 0 (untreated), 15, 30, and 60 min after the topical instillation of unmodified PLGA NPs was administered are shown in Fig. 2A–D, respectively. The time-course of the normalized intensity calculated using the gray values of C6 converted from the IPL region in the retina image is shown in Fig. 2E. Up to 30 min, the normalized intensity of the marker C6 in the IPL of the retina increased, and then it decreased to the baseline level. The disappearance of fluorescence at 60 min suggested that the NPs may have been cleared from the retina, which means that NPs diffused to the periocular (scleral and choroidal) circulatory systems [2].
Fig. 2.
C6 fluorescence intensity time course in the IPL after administration of the unmodified PLGA NP suspension. Representative fluorescence microscopy images of the retina (A) 0 min (untreated), (B) 15 min, (C) 30 min, and (D) 60 min after administration. Scale bar: 50 µm. (E) Changes in the median fluorescence intensity in the IPL. Data are shown as mean ± SEM (n = 5–6).
3.3. Effect of surface modifiers of PLGA NPs on the drug delivery efficiency to the retina
We confirmed that surface-modified PLGA NPs did not induce cytotoxicity at the concentrations used in the in vitro study [6]. Surface modification improved the delivery efficiency of NPs although there was no statistically significant difference (P-value was over 0.05) between the formulations (Fig. 3). The optimization of surface-modified PLGA NPs, such as the concentration of surface modifiers, should be investigated before they are used clinically.
Fig. 3.
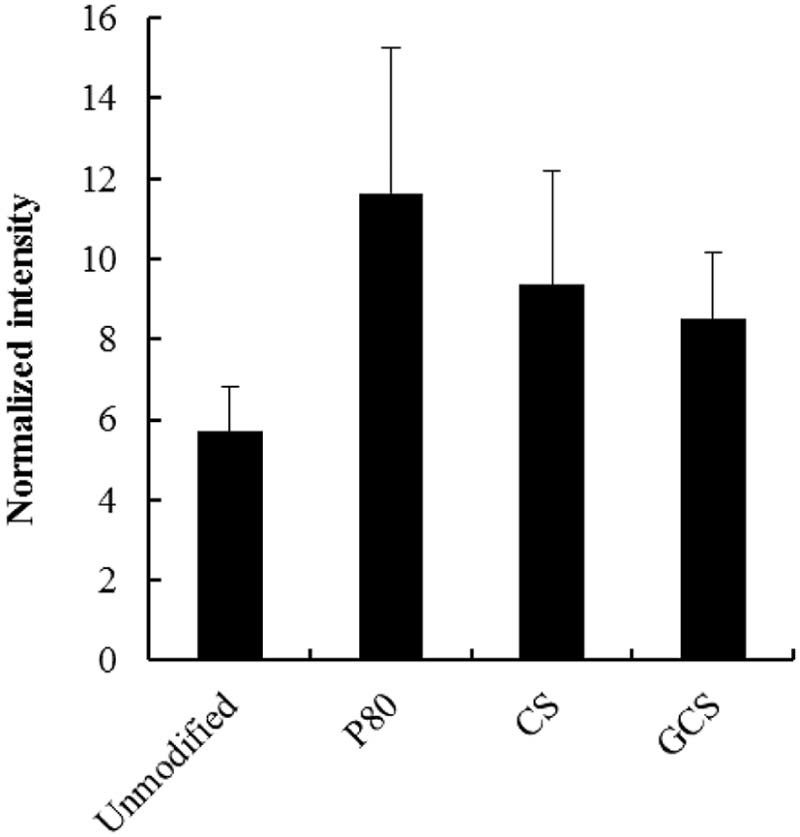
Effect of surface modification of PLGA NPs on the drug delivery efficiency to the retina at 30 min after topical instillation of a surface-modified PLGA NP suspension. Data are shown as mean ± SEM (n = 8).
The residual CS or GCS amino acid groups on the PLGA NPs may interact electrostatically with eye mucus. PLGA NPs may interact with the mucus layer on the surface of the eye because of the mucoadhesive properties of CS or GCS on the NP surface, resulting in long-term NP retention in the ocular tissues. GCS-modified PLGA NPs showed the highest positive zeta potential, suggesting that they interact electrostatically with cells. However, the delivery efficiency of GCS-NPs and CS-NPs to the retina was lower than that for the P80-PLGA NPs. In the in vitro cellular uptake study using conjunctival cells, P80-PLGA NPs showed stronger fluorescence in the cytoplasm than unmodified NPs (Supplementary Fig. S1). One possible mechanism is that cell membrane fluidity may be increased by the P80 on the surfaces of PLGA NPs, triggering endocytosis [6]. Generally, association with cell membranes was increased by the positive zeta potential. However, the resulting increase in the size of PLGA NPs after CS or GCS modification could outweigh the advantages of increased cell association of positively charged NP surfaces to improve delivery efficiency to the back of the eye. The balance between smaller particle size and cell association properties involved in the surface characteristics of PLGA NPs, such as mucoadhesive properties and electrostatic interactions with cells, is important in determining the ocular delivery efficiency to the retina following topical instillation [4].
3.4. Delivery route of PLGA NPs to mouse retina following topical instillation
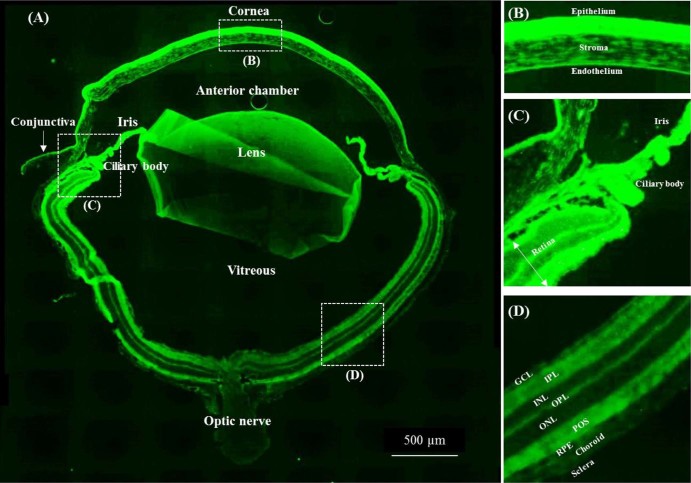
The entire sliced mouse eye was observed 30 min after instillation to examine the fate of PLGA NPs in the retina after the NPs were administered to the ocular surface (Fig. 4A). Auto-fluorescence was observed around the photoreceptor outer segments as shown in the image of the eyeball treated with PBS as a control under the specific microscopic condition (Supplementary Fig. S2). Fluorescence intensity from C6 was observed throughout the eyeball (Fig. 4A), in the anterior segment, including the cornea (Fig. 4B) and conjunctiva, lens, iris/ciliary body (Fig. 4C), and retina (Fig. 4D). The overall fluorescence image of the eyeball after topical administration of C6 labeled PLGA NPs was similar to that reported for liposomal administration [2]. Fluorescence mediated by NPs was visible in the anterior segment, the cornea surface, and in the iris/ciliary body and retina.
Fig. 4.
Representative fluorescence image of the entire eyeball (A) 30 min after topical instillation of unmodified PLGA NPs. The figures on the right show magnifications of the (B) cornea, (C) iris/ciliary body, and (D) posterior segment. Abbreviations in the images: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; POS, photoreceptor outer segments; RPE, retinal pigment epithelium.
Several papers have suggested plausible drug delivery routes to the posterior segment following topical instillation [1], [9], [10]: (1) trans-corneal route: cornea → anterior chamber → vitreous → posterior retina; (2) periocular (trans-sclera) route: conjunctiva → periocular Tenon tissue → posterior sclera → posterior choroid → retina; and (3) uveal route: cornea → anterior chamber → anterior choroid → posterior choroid → retina. The average particle sizes of PLGA NPs were over 200 nm, which suggests they may not pass through the sclera because the scleral pore is hydrophilic and the diameter varies between 20 and 80 nm [11]. However, the smaller size fraction in PLGA NP suspension may only pass through the sclera following the association with the conjunctiva, and then reach the ciliary body/posterior retina. Based on the entire eye image, particularly the corneal images after the administration of PLGA NPs (Fig. 4B), corneal transport may also occur because C6 fluorescence was visible even in the corneal stroma, endothelium, and lens. In another possible mechanism, intense C6 fluorescence was observed around the tissue region containing the iris root, trabecular meshwork, and pars plana, suggesting the uveal route or that the NPs were delivered to the iris/ciliary body (Fig. 4C) via the interaction with the conjunctiva.
Lajunen et al. reported that conjunctival capillaries are fenestrated and that there are few barriers preventing the transfer of macromolecules or NPs from the epithelium to the blood vessels [3]. Below the retinal pigment epithelium, the capillaries are densely fenestrated with pores with diameters of 75–85 nm, which may limit the size of the particles that can pass through. In this study, the small-sized fraction of NPs in the suspension of PLGA NPs might pass through these pores. Previously, we suggested that the main liposome delivery route for topical instillation to the posterior segment of the eye could be a non-corneal pathway [2]. A plausible route for PLGA NPs to the retina following eye drop administration is also a non-corneal pathway, related to the conjunctiva. Therefore, the interaction of the particles with the conjunctiva in the anterior segment could be an important factor affecting the drug delivery efficiency to the retina.
In conclusion, in relation to the fate of NPs after administration, none of the possible routes can be ruled out based on the fluorescence images of the eyes to determine the specific pathway to the retina. A homogeneous NP suspension with a smaller average particle size of less than 100 nm should be administered to the eye in future studies to elucidate the delivery route to the posterior segment and improve the NP delivery efficiency to the retina. Additional studies quantifying drugs in the ocular tissue or performing high resolution imaging of eyeball, such as autoradiographs, are also required to determine the NP delivery route to the posterior segment following topical instillation [10].
4. Conclusions
We have demonstrated using fluorescence image analysis that PLGA NPs could be used as drug carriers to the posterior segment following topical administration. Surface modification of the NPs with mucoadhesive polymers or P80 increased the interaction with cells, and improved the delivery efficiency of NPs to the retina. In future work, we will optimize the particle design for targeting the retina and for the in vivo pharmacological effect by using surface-modified PLGA NPs loaded with a model active pharmaceutical agent to demonstrate their clinical utility.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP16K18948.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
Supplementary data to this article can be found online at doi:10.1016/j.ajps.2017.03.002.
Appendix. Supplementary material
The following is the supplementary data to this article:
Supplementary materials and methods, and Figs. S1 and S2.
References
- 1.Kaur I.P., Kakkar S. Nanotherapy for posterior eye diseases. J Control Release. 2014;193:100–112. doi: 10.1016/j.jconrel.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Hironaka K., Inokuchi Y., Tozuka Y. Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J Control Release. 2009;136:247–253. doi: 10.1016/j.jconrel.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Lajunen T., Hisazumi K., Kanazawa T. Topical drug delivery to retinal pigment epithelium with microfluidizer produced small liposomes. Eur J Pharm Sci. 2014;62:23–32. doi: 10.1016/j.ejps.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Inokuchi Y., Hironaka K., Fujisawa T. Physicochemical properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eyedrop administration. Invest Ophthalmol Vis Sci. 2010;51:3162–3170. doi: 10.1167/iovs.09-4697. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki H., Karasawa K., Hironaka K. Retinal drug delivery using eyedrop preparations of poly-l-lysine-modified liposomes. Eur J Pharm Biopharm. 2013;83:364–369. doi: 10.1016/j.ejpb.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Tahara K., Yamamoto H., Kawashima Y. Cellular uptake mechanisms and intracellular distributions of polysorbate 80-modified poly (d,l-lactide-co-glycolide) nanospheres for gene delivery. Eur J Pharm Biopharm. 2010;75:218–224. doi: 10.1016/j.ejpb.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Lee S.S., Hughes P., Ross A.D. Advances in biodegradable ocular drug delivery systems. In: Kompella U.B., Edelhauser H.F., editors. Drug product development for the back of the eye. Springer US; Boston (MA): 2011. pp. 185–230. [Google Scholar]
- 8.Tahara K., Yamamoto H., Hirashima N. Chitosan-modified poly(d,l-lactide-co-glycolide) nanospheres for improving siRNA delivery and gene-silencing effects. Eur J Pharm Biopharm. 2010;74:421–426. doi: 10.1016/j.ejpb.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes P.M., Olejnik O., Chang-Lin J.E. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Mizuno K., Koide T., Shimada S. Route of penetration of topically instilled nipradilol into the ipsilateral posterior retina. Invest Ophthalmol Vis Sci. 2009;50:2839–2847. doi: 10.1167/iovs.08-2922. [DOI] [PubMed] [Google Scholar]
- 11.Cholkar K., Gunda S., Earla R. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech. 2015;16:610–622. doi: 10.1208/s12249-014-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods, and Figs. S1 and S2.