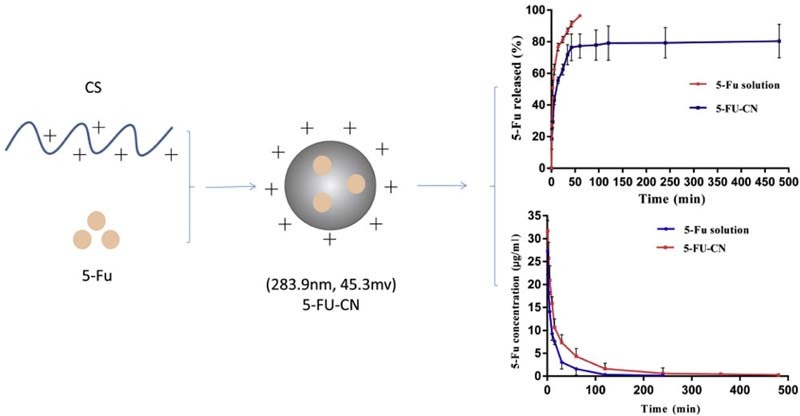
Graphical Abstract
The nanoparticles were prepared by chitosan as carrier and were characterized. In addition, in vitro release studies and in vivo pharmacokinetic studies of nanoparticles were performed.
Keywords: 5-Fluorouracil, Nanoparticles, Chitosan, Pharmacokinetics
Abstract
The sustained-release properties of the biodegradable nano-drug delivery systems were used to improve the residence time of the chemotherapeutic agent in the body. These drug delivery systems were widely used to deliver chemotherapeutic drugs. The 5-fluorouracil loaded chitosan nanoparticles prepared in this paper have the above advantage. Here, we found that when the mass ratio of 5-fluorouracil and chitosan was 1:1, the maximum drug loading of nanoparticles was 20.13 ± 0.007%, the encapsulation efficiency was 44.28 ± 1.69%, the particle size was 283.9 ± 5.25 nm and the zeta potential was 45.3 ± 3.23 mV. The prepared nanoparticles had both burst-release and sustained-release phases in vitro release studies. In addition, the inhibitory effect of the prepared nanoparticles on gastric cancer SGC-7901 cells was similar to that of 5-fluorouracil injection, and the blank vector had no obvious inhibitory effect on SGC-7901 cells. In the pharmacokinetic study of rats in vivo, we found that AUC (0−t), MRT (0−t) and t1/2z of nanoparticles were significantly increased in vivo compared with 5-fluorouracil solution, indicating that the prepared nanoparticles can play a role in sustained-release.
1. Introduction
Cancer has been a serious threat to human health and socio-economic development, and has become one of the world's major public health problems. Early invasion, early metastasis, immune escape and other biological behaviors are the difficulty of tumor therapy. When the tumor has been found in the advanced stage, the tumor has spread too extensively and transferred to a stage which is so difficult to remove clean by surgery and easy to relapse after surgery. The traditional treatment methods are surgery, radiation and chemical treatment [1]. Commonly used chemotherapy drugs are 5-fluorouracil (5-FU) [2], [3], gemcitabine [4], doxorubicin [5] and so on. 5-FU has been widely used in the treatment of cancer [2], but its half-life is only 10–15 min, and retention time is short in vivo [6]. The need for frequent administration limited its clinical application. Therefore, there is an urgent need to develop a new method for the treatment of cancer.
The development of nanotechnology has brought new opportunities for the treatment of cancer. The purpose of extending the release time of drugs in vivo can be achieved by loading the chemotherapeutic drug into the nano-carrier. Progress has been made in the treatment of cancer by incorporating chemotherapeutic agents into nanocarriers in recent years. Thioglycolic acid and glutathione-linked 5-FU gold nanoparticles have been used to study the treatment of rectal cancer, and have a sustained-release effect [7]. By loading the anti-chemotherapeutic drug 5-FU in the PLGA copolymer can delay drug release [8]. Studies have shown that 5-FU loaded glass nanoparticles can control drug release [9]. Thus, there is space for the development of other vectors used to encapsulate 5-FU to extend its release.
Chitosan (CS) is the basic polysaccharide obtained by deacetylation of chitin, which is the most abundant natural biopolyer on the earth except cellulose [10]. Due to its low toxicity, biodegradability and biocompatibility, chitosan has been widely used in the pharmaceutical industry [11]. In recent years, the use of chitosan as a carrier of sustained-release preparations to solve the problem of half-life of chemotherapy drugs has been reported. Chitosan-coated magnetic nanoparticles were used to prolong the release of 5-FU [10]. Chitosan and sodium alginate were used to prepare 5-FU-loaded nanoparticles by ion gel method and had a sustained release effect compared with 5-FU solution [12]. David et al. have reported chitosan nanoparticles loaded with quercetin and 5-FU had a sustained release effects in vitro release study [13]. Although a number of 5-FU-loaded chitosan nanoparticles have been developed, only a brief in vitro release experiment has been conducted to investigate its sustained-release effect, whereas little attention has been paid to chitosan nanoparticles in vivo. Therefore, the sustained release of chitosan nanoparticles as a chemotherapeutic drug carrier remains to be further studied.
The solid lipid nanoparticles loaded with gambogic acid were prepared in our laboratory and their sustained-release properties were evaluated in vitro and in vivo [14]. We also prepared biochanin A-loaded nanostructured lipid carriers and investigated their drug release behavior in rats after oral administration [15]. In this study, chitosan nanoparticles loaded with 5-FU (5-FU-CN) were prepared and their sustained-release behavior was investigated by in vitro release and in vivo initial pharmacokinetic studies.
2. Materials and methods
2.1. Materials
5-Fluorouracil powder was supplied by Aladdin Industrial Corporation (Shanghai, China), while chitosan (deacetylation degree 86%) and sodium tripolyphosphate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Methylthiazolyldiphenyl-tetrazolium bromide conversion (MTT) was purchased from Sigma (Sigma-Aldrich, Germany and USA). Dimethyl sulfoxide (DMSO) was obtained from Sigma (USA). Moreover, the human gastric cancer (SGC-7901) cell line was obtained from Cellbio (Shanghai, China). All other reagents used in the assay were of analytical grade, and the water used in the experiment was doubly distilled and deionized.
2.2. Preparation of 5-fluorouracil chitosan nanoparticles
The preparation method of the nanoparticles was ionic gelation. Briefly, a specific amount of CS (25 mg) was dissolved in 1% acetic acid solution and the pH was adjusted to 5.0. 5-FU powder which was accurately weighed was dissolved in water to prepare a concentration of 25 mg/ml solution. The prepared 5-FU solution was then slowly dropped into the CS solution using a micro-syringe. Finally, 2.5 ml of sodium tripolyphosphate solution having a concentration of 2.0 mg/ml was added to the above mixture under mechanical stirring (600 rpm). The reaction was kept for 1.5 h at room temperature.
Chitosan nanoparticles (CS-NPs) without 5-FU were prepared by the same method.
2.3. Encapsulation efficiency (EE) and loading capacity (LC)
The ultrafiltration centrifugation method was used to determine the encapsulation efficiency and loading capacity of 5-FU nanoparticles. The process was as follows: 1.0 ml of 5-FU-CN suspension was centrifuged (LC-4016, Anhui USTC Instruments Co., Ltd., China) at 3500 rpm for 10 min in an ultrafiltration centrifuge tube (100 kD, Amicon Ultra-4, Millipore, USA). Finally, the free drug which was removed from the bottom of the ultrafiltration centrifuge tube was measured by high performance liquid chromatography (HPLC, Shimadzu, Japan). The UV detection wavelength was 265 nm. WondaSil C18 column (4.6 mm × 250 mm, 5 µm, China) was used to analyze the samples. The column temperature was kept at 30 °C. The mobile phase was consisted of a mixture of methanol/0.1% phosphoric acid (5:95, v/v) and the flow rate was 0.8 ml/min. The EE and LC were calculated as follows:
2.4. Characterization of nanoparticles
Particle size, polydispersity index (PDI) and zeta potential of both CS-NPs and 5-FU-CN were determined by the Zetasizer (Nano-ZS90, Malvern Instruments, UK) at room temperature. Prior to measurement, each sample was diluted appropriately with double-distilled water.
Transmission electron microscopy (TEM, JEM-2100, Japan) was used to analyze the morphology of the 5-FU-CN. The formulation was diluted three-fold prior to assay. The sample for TEM analysis was loaded on copper grids, then evaporated in air at room temperature and 2.0% (w/v) phosphotungstic acid was added dropwise for observation.
2.5. In vitro drug release of nanoparticles
The in vitro release process of nanoparticles was as follows. 1.0 ml of the precision removal nanoparticle suspension was placed in a dialysis bag, the ends of the dialysis bag were tightened and then placed into a previously prepared PBS dialysis medium. The whole system was maintained at 37 °C and kept in a water bath shaker. During the release process, a dialysis medium of 1.0 ml was removed at a predetermined time point while adding the same volume of fresh medium. The released drug in each time point was determined by HPLC.
2.6. In vitro cytotoxicity study
MTT (methylthiazolyldiphenyl-tetrazolium bromide conversion) method was commonly used in cytotoxicity test. In this study, we selected gastric cancer SGC-7901 cells to study the inhibitory effect of 5-FU-CN. Cells were first seeded in 96-well plates (2 × 103 cells/well) and incubated for 24 h at 37 °C in 5% CO2 environment. Then the cells were treated with CS-NPs, 5-FU injection and 5-FU-CN, respectively. The untreated cells served as control, and the cells were cultured for 24 h, 48 h and 72 h. Then, 20 µl of MTT solution (5 mg/ml) was added to each well and the culture was continued for 4 h. The medium was then removed and 150 µl of DMSO was added per well and measured at 490 nm. The relative cell viability is calculated as follows:
2.7. Pharmacokinetic study
For all in vivo studies, Sprague-Dawley (SD) rats (200~250 g body weight, Laboratory Animal Center of Anhui Medical University, Hefei, Anhui, China) were carried out in accordance with the guidelines valuated and approved by the Institutional Animal Care and Use Committee of the Anhui Medical University Experimental Animal Center. All rats were given enough food and water and normal circadian circulation was ensured. Twelve SD rats (half male and female) were randomly divided into two groups for 5-FU solution and 5-FU-CN intravenous administration. Each formulation was administered at the 5-FU dose of 15 mg/kg. After administration, blood samples were gathered immediately by retroorbital venous plexus puncture. At defined time intervals (1, 3, 5, 10, 15, 30, 60, 120, 240, 360, 480, 600, 720 min), 0.5 ml of blood samples obtained from the angulus oculi of rats was collected and then centrifuged for 15 min at 3000 rpm. The plasma was stored frozen at −20 °C.
2.8. Statistical analysis
The figures involved in the paper were made by GraphPad Prism 6.02. The results were expressed as mean ± SD. The t-test was used for statistical analysis. P values less than 0.05 were considered to be a significant difference.
3. Results and discussion
3.1. Characterization of nanoparticles
The entrapment efficiency and drug loading were the two main indices for the evaluation of nanocarriers. Increasing the dosage was one of the main methods to improve the entrapment efficiency and drug loading. However, the effect was not very satisfactory. When the mass ratio of 5-FU and CS was 1:4 to 1:1, the encapsulation efficiency did not change significantly (P > 0.05), but the drug loading increased with the increase of 5-FU (Table 1). However, the formulation began to flocculate when the mass ratio was further increased. Therefore, in order to maximize the entrapment efficiency and drug loading, we selected 5-FU and CS as the 1:1 mass ratio for subsequent studies. In addition, although the encapsulation efficiency of 5-FU-CN was not high, the drug loading was higher than that of the previous reports [15], [16].
Table 1.
The encapsulation efficiency and loading capacity of 5-FU-CN.
| Mass ratio (5-FU:CS) | EE% | LC% |
|---|---|---|
| 1:4 | 42.15 ± 2.14 | 7.26 ± 0.003 |
| 1:2 | 43.34 ± 2.57 | 12.75 ± 0.007 |
| 1:1 | 44.28 ± 1.69 | 20.13 ± 0.007 |
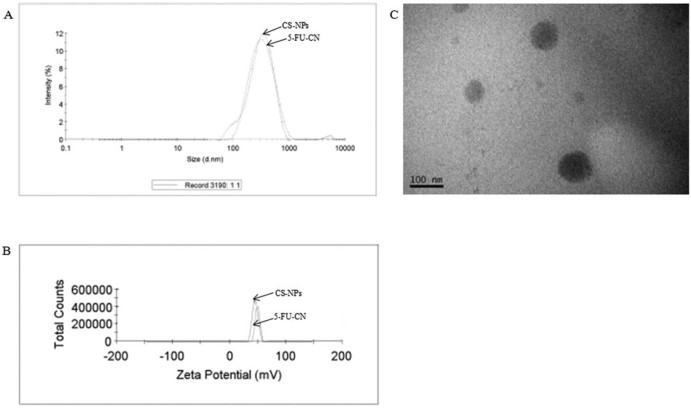
The particle size distributions of blank CS-NPs and 5-FU-CN were investigated as shown in Fig. 1. The average particle size of CS-NPs was 273.4 ± 7.24 nm, and its PDI was 0.252 ± 0.015, as for 5-FU-CN, its mean particle size was 283.9 ± 5.25 nm, and its PDI was 0.243 ± 0.012. The size of blank CS-NPs and the PDI did not have significant changes when combined with 5-FU. It was reported that particle size was associated with gastrointestinal uptake and clearance of the reticuloendothelial system [17], [18]. In addition, according to the enhanced permeability and retention effect, small particles tend to accumulate in the tumor site and large particles without this function [16]. But ultrafine particles were a risk with the induction of reactive oxygen species, oxidative stress inflammation and vasculature. So, it can be considered that the nanoparticles of this study can accumulate in the tumor sites and have no such harmful effects.
Fig. 1.
Size (A), Zeta potential (B) of blank CS-NPs and 5-FU-CN and the morphology (C) of 5-FU-CN.
In this work, the zeta potential of blank CS-NPs and 5-FU-CN were 49.9 ± 2.15 mV and 45.3 ± 3.23 mV, respectively. It was also known that the zeta potential of nanoparticles was higher than the value of 30 mV, implying the nanoparticles had good stability because the nature of the potential value is the electrostatic repulsion between the nanoparticles [19]. So these zeta potential values confirmed the good stability of both blank CS-NPs and 5-FU-CN, and the positive zeta potential indicated the positive surface charge in both of the systems.
The morphology of the nanoparticles was shown in Fig. 1C. The result of TEM showed that the morphology of 5-FU-CN was spherical and the surface was smooth.
3.2. Study on drug release in vitro
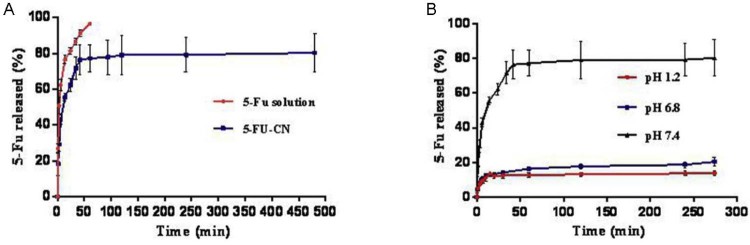
The drug release profile from the nanoparticles can occur in two parts: “sudden release” and “sustained release”. The initial burst phase is caused by drug adsorbed on the surface of the nanoparticles. While the sustained release of the drug is caused by the degradation of carrier matrix and the slow diffusion of drug from the polymer matrix [16]. Sudden release of drug in the body can quickly reach an effective therapeutic concentration, and sustained release can make the drug in the body to stay at the effective therapeutic concentration range. Fig. 2 shows in vitro the drug release pattern of 5-FU solution and 5-FU-CN in PBS 7.4. From the pattern (Fig. 2A), it was very clear that about 91% of the 5-FU was released at 0.7 h, whereas in the 5-FU-CN system, the release curve included sudden release phase and sustained release phase. As a result, 76% release occurred at the first 0.7 h, the duration of the sustained release was 0.7 to 8.0 h. From the above results, it was found that the nanoparticles had the effect of prolonging the drug release compared with the 5-FU solution. The drug release patterns of 5-FU from 5-FU solution and 5-FU-CN were discussed by zero-order kinetics, first-order kinetics and Higuchi equations. From Table 2, we could found that the 5-FU solution and 5-FU-CN were fitted into first-order kinetics model. The equations were Ln(1 − Q/100) = −2.5813t − 0.6425 (r = 0.9921) and Ln(1 − Q/80.4) = −0.6849t − 1.9168 (r = 0.9273), respectively. Among them, Q% and t respectively represent the cumulative release percentage of the drug and the time of drug release.
Fig. 2.
(A) In vitro release behaviors of 5-FU and 5-FU-CN in the PBS 7.4. (B) In vitro release behaviors of 5-FU-CN in different buffers.
Table 2.
The equation of 5-FU released from 5-FU solution and 5-FU-CN in vitro.
| Formulations | Model | Equation | Correlation coefficient (r) |
|---|---|---|---|
| 5-FU solution | Zero-order kinetics | Q = 37.42t + 62.68 | 0.9618 |
| First-order kinetics | Ln(1 − Q/100) = −2.5813t − 0.6425 | 0.9921 | |
| Higuchi | Q = 109.1 × t(1/2) + 1.56 | 0.9799 | |
| 5-FU-CN | Zero-order kinetics | Q = 2.39t + 67.05 | 0.4950 |
| First-order kinetics | Ln(1 − Q/80.4) = −0.6849t − 1.9168 | 0.9273 | |
| Higuchi | Q = 39.2s4 × t(1/2) + 11.22 | 0.8459 |
The release behaviors of 5-FU in PBS 6.8 (pH 6.8, imitated intestinal fluid environment) and PBS 1.2 (pH 1.2, simulation of gastric environment) were shown in Fig. 2B. From this figure we could find that the cumulative release percentage of the drug in PBS 6.8 and PBS 1.2 significantly decreased compared to PBS 7.4. The reason may be that 5-FU is an acidic drug; ionization under alkaline conditions increases its solubility [10]. In addition, it may also be due to the protonated amino groups of CS under acidic conditions which formed hydration layer on the surface of the nanoparticles, and the shielding effect of the hydration layer caused the drug to release less from the nanoparticles.
3.3. Cytotoxicity of nanoparticles
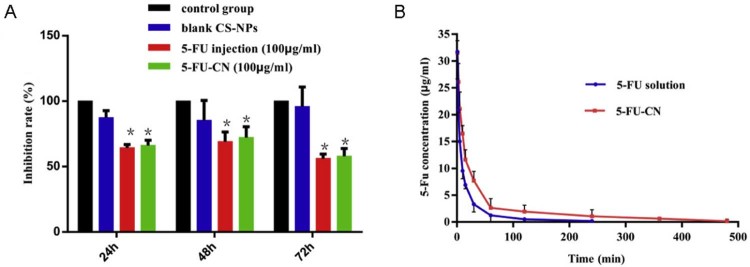
In this study, the potential cytotoxicity of 5-FU-CN on SGC-7901 cells was evaluated by MTT assay, the results were shown in Fig. 3A. In vitro MTT results showed that blank CS-NPs showed no cytotoxicity after 24 h, 48 h and 72 h of observation. In vitro MTT results showed that blank CS-NPs showed no significant cytotoxicity after 24 h, 48 h and 72 h, indicating chitosan as a carrier of cells without toxic side effects. It was consistent with the reports that chitosan is a natural carrier with low toxicity and biodegradability [20]. In addition, there was a significant difference between the 5-FU-CN group and the control group (P < 0.05), but no significant difference with the 5-FU injection group after 24 h, 48 h and 72 h (P > 0.05). The reason why there was no significant difference in cytotoxicity may be that the release of drug from nanoparticles was incomplete (eg, only about 80% of the drug was released from the nanoparticles in vitro release studies). These results indicate that the prepared 5-FU-CN has obvious inhibitory effect on SGC-7901 cells and has the same inhibitory effect as the 5-FU injection which has been used in clinic.
Fig. 3.
(A) Inhibition effect of 5-FU-CN (*P < 0.05). (B) Plasma concentration–time profiles of 5-FU solution and 5-FU-CN after the administration of intravenous in rats. The results were expressed as the mean ± SD of six rats.
3.4. Pharmacokinetic study
Pharmacokinetics of the drug in vivo were studied in rats in this work by intravenous administration at a dose of 15 mg/kg of 5-FU. And the pharmacokinetic parameters were calculated by establishing a mathematical model. Pharmacokinetic parameters can reflect the dynamic changes of drugs in the body of some of the parameters and also is the clinical formulation of one of the main bases for dosing. In 5-FU plasma drug concentration–time curve in vivo shown in Fig. 3B, the corresponding pharmacokinetic parameters were obtained by DAS2.0 software fitting, and the results are shown in Table 3.
Table 3.
The primary pharmacokinetic parameters of the 5-FU solution and 5-FU-CN after the administration of intravenous at a dose of 15 mg/kg (n = 6) (mean ± SD) (*P < 0.05, compared with 5-FU solution).
| Parameters | Unit | Formulations | |
|---|---|---|---|
| 5-FU solution | 5-FU-CN | ||
| AUC (0–t) | mg/l/min | 470.112 ± 23.306 | 1078.826 ± 307.302* |
| AUC (0–∞) | mg/l/min | 475.697 ± 17.925 | 1138.703 ± 307.836* |
| MRT (0–t) | min | 28.398 ± 10.49 | 81.226 ± 31.261* |
| t1/2z | min | 27.574 ± 10.411 | 84.636 ± 33.542* |
It was known from Fig. 3 that the plasma concentration of 5-FU-CN was significantly higher than that of 5-FU solution after intravenous administration. The size of the AUC value can reflect the size of the bioavailability. As shown in Table 3, we could find that the AUC(0−t) of 5-FU-CN was 1078.826 mg/l/min significantly increased compared with 470.112 mg/l/min of 5-FU solution (P < 0.05). This improvement may be due to the fact that nanoparticles were more likely to bind to plasma proteins in vivo although the release of the drug from nanoparticles was only 80% in vitro release studies. Similarly, MRT(0−t) for 5-FU-CN and 5-FU solutions were 81.228 min and 28.398 min, respectively. So the retention time of 5-FU-CN in vivo was significantly prolonged compared with 5-FU solution (P < 0.05). In addition, the t1/2z of 5-FU-CN was 84.636 min, which was about 3.06 times that of the 5-FU solution (27.574 min). The prepared 5-FU-loaded nanoparticles can delay the drug release in vivo, and the results were consistent with the release of 5-FU in vitro.
4. Conclusions
The 5-FU loaded nanoparticles were prepared by a simple and mild method of ionic gelation. When the mass ratio of 5-FU and CS was 1:1, the nanoparticles were prepared with the highest drug loading and the particle size was about 280 nm, the potential was 45.3 mV. The morphology of the prepared nanoparticles was observed by TEM, and the nanoparticles were spherical with a smooth surface. In vitro release studies found that nanoparticles can play a role in sustained-release. Moreover, the cytotoxicity of 5-FU-CN to SGC-7901 cells was similar to that of 5-FU injection. The results showed that 5-FU could not induce cytotoxicity after preparation of nanoparticles. The blank vector had no obvious inhibitory effect on gastric cancer SGC-7901 cells, which provided a basis for the clinical application of nanoparticles. In this work, we further studied the pharmacokinetics of 5-FU-CN in vivo. It was found that the half-life of 5-FU-CN was significantly increased after intravenous administration compared with 5-FU solution. The results further confirmed that the preparation of nanoparticles can extend the drug release time. The sustained release of 5-FU-CN can solve the short half-life problem of 5-FU in clinic and reduce the side effects caused by frequent administration. Therefore, 5-FU loaded nanoparticles have great potential for further clinical use.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Acknowledgments
This work has been supported by the Anhui Provincial Natural Science Foundation (grant number 1508085QH194).
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Sağir T., Huysal M., Durmus Z. Preparation and in vitro evaluation of 5-flourouracil loaded magnetite–zeolite nanocomposite (5-FU-MZNC) for cancer drug delivery applications. Biomed Pharmacother. 2016;77:182–190. doi: 10.1016/j.biopha.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Cheng M., He B., Wan T. 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047115. e47115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S., Kotla N.G., Tomar S. A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int J Nanomedicine. 2015;10:7175. doi: 10.2147/IJN.S89030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada K.I., Hirono S., Kawai M. Phase I study of nab-paclitaxel plus gemcitabine as neoadjuvant therapy for borderline resectable pancreatic cancer. Anticancer Res. 2017;37(2):853–858. doi: 10.21873/anticanres.11389. [DOI] [PubMed] [Google Scholar]
- 5.Han L., Tang C., Yin C. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and siRNA. Biomaterials. 2015;60:42–52. doi: 10.1016/j.biomaterials.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Xian X.S., Park H., Choi M.G. Cannabinoid receptor agonist as an alternative drug in 5-fluorouracil-resistant gastric cancer cells. Anticancer Res. 2013;33(6):2541–2547. [PubMed] [Google Scholar]
- 7.Safwat M.A., Soliman G.M., Sayed D. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int J Pharm. 2016;513(1):648–658. doi: 10.1016/j.ijpharm.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 8.Wamocha H.L., Misak H.E., Song Z. Cytotoxicity of release products from magnetic nanocomposites in targeted drug delivery. J Biomater Appl. 2013;27(6):661–667. doi: 10.1177/0885328211421989. [DOI] [PubMed] [Google Scholar]
- 9.El-Kady A.M., Farag M.M., El-Rashedi A.M.I. Bioactive glass nanoparticles designed for multiple deliveries of lithium ions and drugs: curative and restorative bone treatment. Eur J Pharm Sci. 2016;91:243–250. doi: 10.1016/j.ejps.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L., Ma J., Jia N. Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: preparation, characterization and cytotoxicity studies. Colloids Surf B Biointerfaces. 2009;68(1):1–6. doi: 10.1016/j.colsurfb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Arias J.L., López-Viota M., Gallardo V. Chitosan nanoparticles as a new delivery system for the chemotherapy agent tegafur. Drug Dev Ind Pharm. 2010;36(6):744–750. doi: 10.3109/03639040903517914. [DOI] [PubMed] [Google Scholar]
- 12.Nagarwal R.C., Kumar R., Pandit J.K. Chitosan coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: in vitro characterization and in vivo study in rabbit eye. Eur J Pharm Sci. 2012;47(4):678–685. doi: 10.1016/j.ejps.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 13.David K., I, Jaidev L.R., Sethuraman S. Dual drug loaded chitosan nanoparticles-sugar-coated arsenal against pancreatic cancer. Colloids Surf B Biointerfaces. 2015;135:689–698. doi: 10.1016/j.colsurfb.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Huang X., Chen Y.J., Peng D.Y. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids Surf B Biointerfaces. 2013;102:391–397. doi: 10.1016/j.colsurfb.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 15.Cheng M., Han J., Li Q. Synthesis of galactosylated chitosan/5-fluorouracil nanoparticles and its characteristics, in vitro and in vivo release studies. J Biomed Mater Res B Appl Biomater. 2012;100(8):2035–2043. doi: 10.1002/jbm.b.32767. [DOI] [PubMed] [Google Scholar]
- 16.Fan Y.L., Fan B.Y., Li Q. Preparation of 5-fluorouracil-loaded nanoparticles and study of interaction with gastric cancer cells. Asian Pac J Cancer Prev. 2013;15:7611–7615. doi: 10.7314/apjcp.2014.15.18.7611. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q., Cheng H., Zhou K. Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv. 2013;20:331–337. doi: 10.3109/10717544.2013.838716. [DOI] [PubMed] [Google Scholar]
- 18.Shahbazi B., Taghipour M., Rahmani H. Preparation and characterization of silk fibroin/oligochitosan nanoparticles for siRNA delivery. Colloids Surf B Biointerfaces. 2015;136:867–877. doi: 10.1016/j.colsurfb.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Da Silva S.B., Ferreira D., Pintado M. Chitosan-based nanoparticles for rosmarinic acid ocular delivery – in vitro tests. Int J Biol Macromol. 2016;84:112–120. doi: 10.1016/j.ijbiomac.2015.11.070. [DOI] [PubMed] [Google Scholar]
- 20.Kong F., Liu G., Sun B. Phosphorylatable short peptide conjugated low molecular weight chitosan for efficient siRNA delivery and target gene silencing. Int J Pharm. 2012;422(1):445–453. doi: 10.1016/j.ijpharm.2011.10.041. [DOI] [PubMed] [Google Scholar]