Graphical Abstract
Keywords: Exosomes, RNA interfering, Small RNAs, Delivery, Neurological disorders, Tumors
Abstract
RNA interfering (RNAi), mediated by small interfering RNAs and microRNAs, is currently one of the most promising tools of gene therapy. Small RNAs are capable of inducing specific post-transcriptional gene silencing, providing a potentially effective platform for the treatment of a wide array of diseases. However, similar to other nucleic acid-based drugs, the major hurdle of RNAi therapy is lack of efficient and non-immunogenic delivery vehicles. Currently, viruses, synthetic polymers, and lipid-based carriers are among the most widely studied vehicles for small RNA delivery. However, many drawbacks are reported to be associated with these delivery vehicles. There is a pressing need to replace them with more efficient and better-tolerated approaches. Exosomes secreted from the endocytic compartment of live cells, are a subtype of endogenous extracellular vesicles that transfer genetic and biochemical information among different cells, thus playing an important role in cell-cell communication. Recently, accumulating attention has been focused on harnessing exosomes as nanaocarriers for small RNAs delivery. Due to their natural role in shuttling endogenous nucleic acid in our body, exosomes may exhibit higher delivery efficiency, lower immunogenicity, and better compatibility than existing foreign RNA carriers. Importantly, exosomes own intrinsic homing capacity that can guide small RNAs across natural membranous barriers. Moreover, such a capacity can be further improved by adding appropriate targeting moieties. In this manuscript, we briefly review the progress and challenges of RNAi therapy, and discuss the potential of exosomes' applications in small RNA delivery with focus on the most recent advances in exosome-based small RNA delivery for disease therapy.
1. Introduction
Gene therapy, including RNAi, has long been fascinating both scientific community and general public because of the potential for treating a diseases at its genetic roots [1]. RNAi mediated by small RNA molecules including small interfering RNA (siRNA) and micro RNA (miRNA), is a potent and specific post-transcriptional gene silencing tool by either catalytical degradation or translation arrest of targeted RNA, RNAi offers several advantages over conventional therapeutics based on small molecules or proteins. First, the mechanisms of gene silencing is well-known and consistent for each target [2]. Second, the binding of small RNAs with lead compound is relatively fast with high selectivity [3]. Third, RNAi therapeutics could target any genes, including those coding for undrugable protein products [[2], [3]]. In addition, RNAi therapeutics are more potent and specific than antisense oligonucleotides [[4], [5]]. Last but not the least, the production of small RNA molecules is relatively simple and efficient, as it does not involve with cellular expression systems, and complicated protein purification and refolding processes [[6], [7]]. Since the discovery of RNAi, small RNA-based therapeutics have brought gene therapy to the forefront as one of the most powerful tools for therapeutic applications in various refractory diseases, such as cancers and neurological disorders [8].
However, the major hurdle to move RNAi therapy from bench to bedside is the delivery issue. Naked small RNAs are hydrophilic and negative-charged, which prohibit them from crossing biological membranes. Apart from the biological barriers, small RNAs exhibit high sensitivity to RNases and may undergo rapid enzymatic degradation in vivo. Furthermore, the possibility of inducing immune responses and off-target gene silencing hinders the clinical application of RNA molecules. Over the past few decades, relentless search for efficient and safe delivery vectors has been done to improve the delivery efficiency of small RNAs. Virus vectors or synthetic polymeric nanoparticles and liposomes are among the most extensively explored vehicles. However, in spite of major advances in the delivery of RNAi, only a small number of clinical trials have been carried out. To date, there are still several disadvantages with these vehicles, such as issues related to delivery efficiency, immunogenicity, and toxicity. For example, poor safety profile, risk of insertional oncogenesis and immunogenicity, and high production cost limit the utilization of viral vectors for small RNA delivery. As for non-viral nanocarriers, low transfection efficiency, harmful immune responses, and poor biocompatibility have been reported [9]. As an example, when applying polyethylenimine (PEI), a promising polycationic polymer, for gene transfection, PEI with high molecular weight may achieve high transfection efficiency but also display high cytotoxicity. While PEI with low molecular weight shows lower cytotoxicity but also has limited transfection efficiency [10].
An emerging novel small RNA delivery vehicle is known as exosomes. There is asubtype of nanosized membrane vesicles secreted by almost all endogenous cells. Especially following the finding that exosomes are naturally-occurring RNA carriers that regulate the gene expression of recipient cells [11], the interest in exosomes for small RNA delivery has exploded. Exosomes compensate for the disadvantages of existing small RNA delivery systems and may represent a new avenue for future small RNA based therapeutics. These advantages include higher delivery efficiency, membrane permeation capacity, better biocompatibility, non-immunogenicity/toxicity, and better safety [[12], [13], [14], [15]]. Moreover, owing to the specific lipid and protein composition, exosomes have the inherent ability to fuse with cell membranes and directly deliver vesicular cargoes into the cytosol [13]. Therefore, this mode of internalization largely bypasses the endocytic pathway. Owing to these unique properties, exosomes offer a new avenue of opportunities for coping with the delivery dilemma of small RNAs.
In this review, we first give an overview of the progress and challenge of RNAi therapy. Then we discuss the potential role of exosomes in small RNA delivery with focus on the most recent advances and knowledge gaps in the area of exosome-based small RNA delivery for disease therapy.
2. Overview of RNAi therapy
RNAi is a widespread and natural process found in prokaryotic and eukaryotic organisms [16]. Initially, RNAs were identified to be intermediates carrying information between DNAs and proteins. About two decades ago, the functional repertoire of RNAs was expanded after the discovery that double-stranded RNAs (dsRNAs) could bind to complementary mRNAs and induce sequence-specific post-transcriptional gene silencing, and the term “RNAi” was first introduced [17]. Shortly thereafter, short dsRNAs or siRNAs could be synthesized artificially and their therapeutic potentials were soon realized and expanded. Since then, the knowledge that small RNAs play an important role in regulating gene expression has exerted a tremendous impact on the basic and applied research of RNAi therapeutics. The first clinical trial of RNAi therapeutics was conducted in 2004, where Bevasiranib, a vascular endothelial growth factor (VEGF) siRNA was tested for the repression of retinal neovascularization in patients with wet age-related macular degeneration (AMD) [18]. Moreover, with the development of RNAi technology, the first clinical trial of siRNA therapeutics via systematic administration for the treatment of tumor was carried out in 2010 [19]. Currently, Macugen, an anti-VEGF aptamer for the therapy of AMD and diabetic edema, is approved as the first therapeutic RNA aptamer [20]. These results suggested that much progress has been made in the realm of small RNA therapy over the last two decades, and RNAi therapy could be a powerful approach for disease therapy.
RNAi can be triggered by various source of RNA molecules, including siRNA, miRNA, long dsRNA, and short hairpin RNA (shRNA) [16]. Long dsRNA with a sequence length of 500 to 1000 is often utilized to study gene functions, and shRNAs is transcribed from a DNA vector in the nucleus of cell [3]. Long dsRNA and shRNA are not commonly used for therapeutic therapy, because dsRNA is prone to activate Dicer-related antiviral pathways and elicit antiviral immune response [16]. ShRNA is a DNA-based strategy depending on viral vectors which has a potential risk of genetic deregulation and insertional mutagenesis, thus leaving a safety concern to clinical application [[21], [22]]. Therefore, therapeutically investigated RNAi is mainly triggered by siRNA and miRNA, especially siRNA, as the most widely explored RNAi molecules for therapeutic use. In this review, small RNAs refer to siRNA and miRNA.
SiRNA is derived from exogenous dsRNA that enters cells via vectors, while miRNA is a naturally occurring non-coding RNA derived from primary miRNA (pre-miRNA) which is transcribed from endogenous miRNA gene in the nucleus [23]. After being processed into the cytoplasm of the cells, both dsRNA and pre-miRNA are processed by enzyme Dicer into mature siRNA (around 21 to 23 nucleotides in length) and miRNA (around 18 to 25 nucleotides in length), respectively. After siRNA or miRNA being incorporated into RNA-induced silencing complex (RISC), the sense strands (also called the passenger strand) undergo enzymatic degradation, whereas the antisense strand (also called the guide strand) could lead activated RISC to target mRNAs via complementary binding [[22], [23]]. There is an obvious difference in the binding characteristics between siRNA and miRNA. The antisense of siRNA could bind completely complementary to the target mRNA, leading to gene silencing via the cleavage of target mRNA. Unlike siRNA, miRNA binds partially complementary to the target mRNA and usually do not cleave the target mRNA, but instead suppresses mRNA translation by translational repression or mRNA deadenylation [[24], [25]].
Small RNA-based therapeutics have been regarded as the current “method of choice” and have several advantages over DNA based gene correction, since the latter may have a risk of inducing mutations [26]. Moreover, RNAi therapeutics are preferred from the delivery perspective, because it only requires the delivery of RNAi molecules into the cytoplasma for binding their intracellular target (RISC complex), which obviates the need for nuclear entry.
3. Challenges of RNAi therapy
Despite of many advances in RNAi therapy, only few clinical trials have been conducted. RNAi delivery issue remains the Achilles' heel for the clinical transformation of RNA therapeutics. For the treatment of different kinds of diseases, small RNAs need to go pass through both extracellular and intracellular membranous barriers to reach their target sites in the cytoplasm. First, small RNA molecules are susceptible to RNases and may undergo rapid enzymatic degradation in biological fluids. Indeed, unmodified small RNAs are reported to have an extremely short half-life of less than 15 minutes in the circulation system [27]. In addition, several physicochemical characteristics of naked RNA molecules, including large size, hydrophilicity, and negative charge make them essentially impermeable across plasma membranes.
Following reaching the interior of target cells, small RNA molecules need to overcome intracellular barriers. A broad range of endocytic mechanisms involve in the uptake of small RNA molecules, which directly influence their intracellular fate [[28], [29]]. Small RNA molecules are probably entrapped in the lysosomes as their final intracellular destinations, where progressively acidic degradation may occur [30]. Therefore, these RNA molecules should escape from endosomes to prevent being rapidly degraded in the acidic environment. In addition to these delivery issues, the presence of specific sequences in small RNA molecules may cause possible immune responses or off-target side effects [31]. These issues largely challenge the therapeutic application of small RNAs.
In order to overcome these challenges, various delivery strategies have been examined for boosting the cellular uptake of small RNA molecules. Currently, viral and non-viral delivery systems have been developed to improve the bioavailability of small RNAs. However, there are still safety concerns for viral vectors. While non-viral delivery vectors mainly include cationic polymers and liposomes which have been extensively reviewed [[32], [33], [34]]. Even though these delivery strategies have displayed some successes in gene silencing, as previously discussed, the promise of these systems are substantially nullified by issues such as immunogenicity and toxicity, low cellular uptake efficiency, and non-specificity [[35], [36]].
4. Exosomes offer a new avenue of opportunities for small RNA delivery
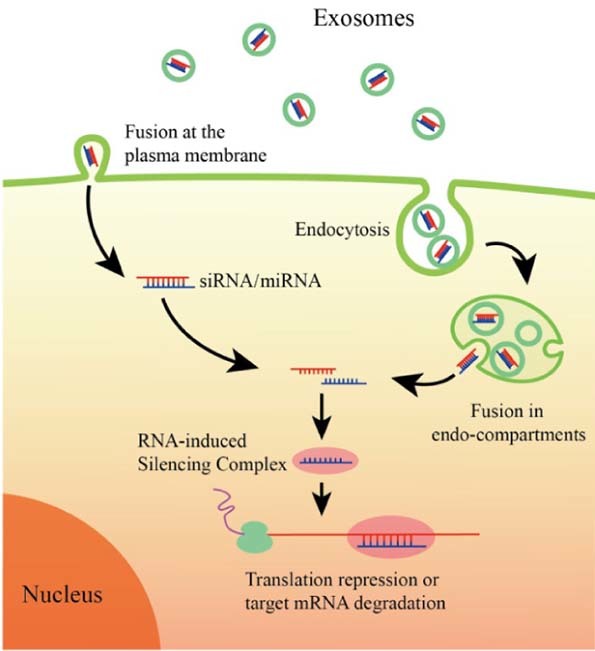
Almost all prokaryotic and eukaryotic cells secrete extracellular vesicles, a heterogeneous population of membrane derived vesicles that efficiently transfer their enclosed genetic and biochemical molecules from the cell of origin to the recipient cells for information exchange [37]. Exosomes are nanosized extracellular vesicles (40–100 nm) of endosomal origin. Specifically, exosomes are formed by inward budding of endosomal membrane, which generates multivesicular body (MVBs) containing multiple intraluminal vesicles (ILVs) [38]. Then, ILVs are released upon the fusion of MVBs with plasma membrane, and the released ILVs are called exosomes. The schematic representation of exosome biogenesis and secretion is shown in Fig. 1. Kowal et al. [39], provided a comprehensive review of current knowledge on the biogenesis and secretion of exosomes. Exosomes are present in almost all biological fluids (e.g., plasma, urine, saliva, cerebrospinal fluid) and conditioned cell cultured media [40]. Once targeting to recipient cells, exosomes uptake can be mediated by endocytosis and/or fusion with cell membrane for delivering their cargoes into the cytosol of target cells. The schematic picture of cellular uptake pathways of exosomes loaded with siRNA or miRNA is shown in Fig. 2 [41]. Accumulation studies indicated that exosomes play key roles in various physiological and pathological processes, including induction of stimulatory and suppressive immune responses, tumorigenesis, establishment of pre-metastatic tumor niche, virus spread, and neurodegenerative diseases progression [[42], [43], [44]]. Exosomes involve in these processes by ferrying different types of RNAs (mRNAs and noncoding regulatory RNAs), cytoplasmic proteins, membranous proteins, and lipids among different cell, tissues, and organs.
Fig. 1.
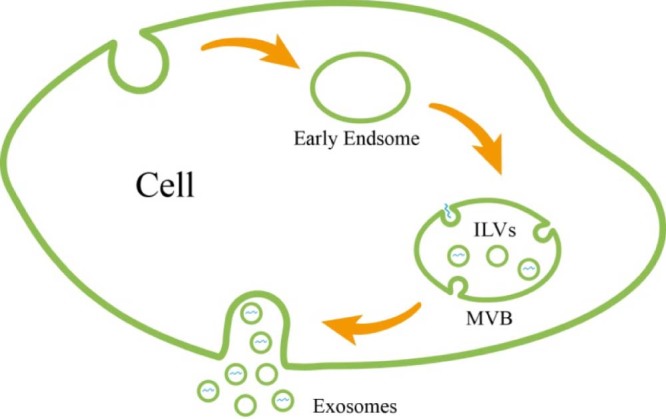
Schematic representation of exosome biogenesis and secretion. Exosomes are formed as ILVs by inward budding of endosomal membrane and released upon the fusion of MVBs with plasma membrane.
Fig. 2.
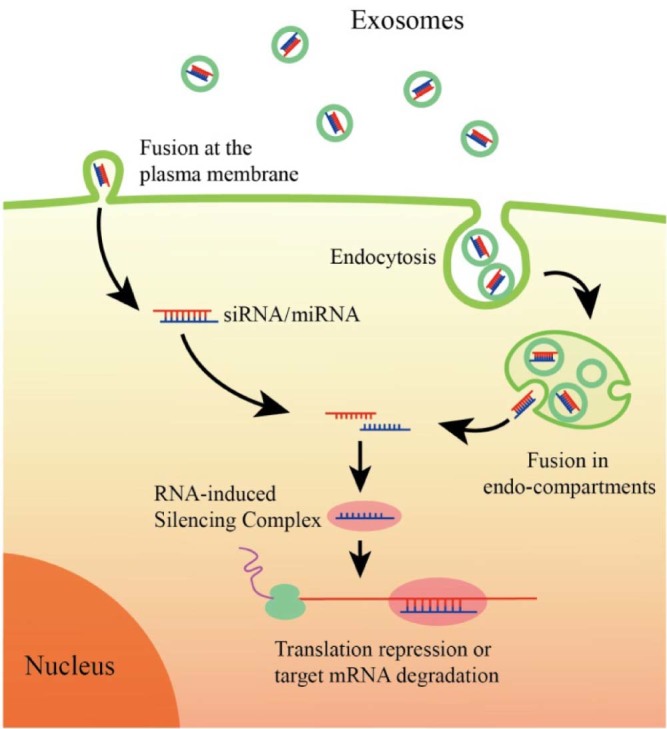
Schematic representation of uptake pathways of exosomes by recipient cells. Exosomes may either directly fuse with plasma membrane of recipient cells or be endocytosed by recipient cells followed by backfusion with the limiting membrane of endocyticcompartment. Both pathways lead to the release of small RNAs and mediate RNA interfering.
The great interest of harnessing exosomes for small RNA delivery derives from the intrinsic ability of exosomes to shuttle small RNA molecules among different cells. A number of advantageous features, including unique structure and physicochemical characteristics, high stability in circulation, intrinsic capacity to traverse biological barriers, potential targeting abilities, efficient cellular entry, lower immunogenicity and toxicity, enable exosomes to be a promising delivery system [[45], [46]]. Similar to liposomes, exosomes possess a lipid bilayer vesicular structure with an aqueous core and a lipophilic shell. The special structure enables exosomes to deliver both hydrophilic and hydrophobic payloads, which is quite desirable for the delivery of small RNA molecules. In addition, exosomes have an ideal particle size of 40 to 100 nm, making them possible to avoid rapid renal clearance and phagocytosis by mononuclear phagocyte system, and to enhance tumor accumulation via permeability and retention effect (EPR) [47]. It was demonstrated that charged nanoparticles, especially those positive-charged, are prone to be rapidly opsonized by reticuloendothelial systems. In contrast, neutral or slightly negative-charged nanoparticles are less possible to be cleared by reticuloendothelial systems [48]. Exosomes are shown to be slightly negative charged [[49], [50]], which offers explanations for their improved stability in blood circulation. Moreover, as natural biological delivery vehicles, exosomes are more compatible with the immune system and less likely to cause immune response and toxicity than artificially synthesized nanocarriers when used in vivo. Generally, delivery of pharmaceutical molecules into brain is challenging because of the selective nature of blood-brain barrier (BBB). Virtually, BBB prevents about 98% of all potent drugs from localizing in the brain [51]. Given that exosomes have the exceptional ability to pass across biological barriers, such as the BBB, the utilization of exosomes as nanocarriers of small RNAs may achieve a breakthrough for the treatment of neurological disorders. Additionally, exosomes possess intrinsic homing properties that can guide therapeutic cargoes to target tumors without eliciting immune responses and off-target adverse effects [[52], [53]]. Importantly, the highly expression of tetraspanin CD9 on the surface of exosomes derived from dendritic cells facilitates direct membrane fusion with target cells and cargo delivery into the cytosol, which largely bypasses the endocytic pathway and evades endosomal escape issues [13]. Finally, it worth mentioning that the safety of exosomes has been extensively tested and exosomes were shown to be relatively safe in numerous clinical trials [[54], [55]].
5. Exosomes for small RNA delivery – proof of concept
As described above, harnessing exosomes for the delivery of small RNA molecules is a promising strategy to cope with the obstacles of RNAi technology. To date, numerous studies have employed exosomes as carriers of small RNAs to treat various diseases, especially brain diseases and tumors. Due to the capacity to pass across the BBB, exosome are extensively tested as carriers of small RNAs for the treatment of neurological diseases. Similarly, exosomes have been widely tested to deliver small RNAs in cancer therapy due to their intrinsic homing selectivity. Furthermore, interest in treating other diseases, including ocular, liver, and kidney diseases, has exploded while incorporating multi-faceted unique properties of exosomes. In this section, we will provide an update and overview of new findings in exosome-based small RNA delivery for the treatment of these diseases. The current repertoire of diseases treated with exosome-based small RNA delivery systems is displayed in Fig. 3.
Fig. 3.
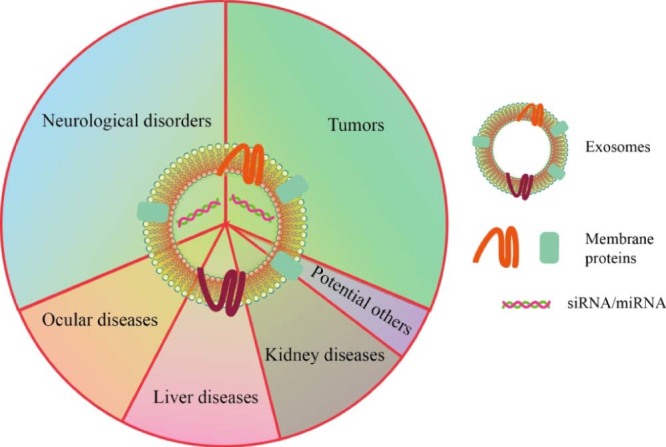
The current repertoire of diseases treated with exosome-based small RNA delivery systems.
5.1. Exosomes as carriers of small RNAs for the treatment of neurological disorders
The BBB is a tightly regulated biological barrier, composed of several cell types, including endothelial cells, basement membrane, pericytes, and astrocytes, that separate the blood from the brain parenchyma [56]. It is established that four fundamental properties of endothelalial cells, the central cells in the BBB, mainly contributes to BBB impermeability [[56], [57]]. First, brain endothelial cells tightly interconnect via tight junctions, forming an impermeable barrier against almost all substances, including small hydrophilic molecules and ions. Second, brain endothelial cells display low rate of transcytosis. Third, high levels of drug efflux proteins that prevent the entry of toxic substances are expressed in brain endothelial cells. Finally, pericytes provide structural support to vasculature by wrapping the entire surface of the BBB.
A growing pool of evidence suggests that many central nervous system diseases, such as Parkinson's disease, Alzheimer's disease, and Huntington's diseases, are caused by genetic abnormalities [58]. However, central nervous system-targeting delivery is notoriously difficult due to the impermeability of the BBB to most therapeutic agents. The inherent ability to cross the BBB makes exosomes an ideal delivery system for the targeting delivery of small RNAs into the brain. Given small particle size, exosomes pose little risk of embolism, enabling them advantageous for penetrating the BBB [59]. Yang et al. [60], recently demonstrated the capacity of exosomes to deliver paclitaxel and doxorubicin across the BBB in a zebra fish model. They reported that when administered free doxorubicin or paclitaxel, both drugs remained localized in the vasculature and did not penetrate the BBB, whereas exosomes significantly facilitated the two drugs across the BBB and inhibited tumor progression. In addition, several other studies have demonstrated the capacity of exosomes to traverse the BBB and deliver small RNAs into central nervous system for the treatment of neurological diseases [[61], [62], [63]]. In principle, two known mechanisms are proposed to be involved in exosomes entry into the brain [64]. First, exosome may enter brain endothelial cells via transcytosis. That is, after internalization into the MVB of recipient cells, exosomes could be released, and then enter the MVB of another receptor cells. As a consequence, exosomes successfully traverse the thick biobarriers via “jump” mode similar to some viruses. Alternatively, exosomes may be internalized by endothelial cells through intercellular junctions [64]. However, currently, there is no direct experimental proof to support these two modes of internalization.
Much progress has been made in utilizing exosomes to deliver small RNAs across the BBB during the past few years. An overview of current exosome-based small RNA delivery systems for the treatment of neurological diseases is shown in Table 1. Yang et al. [63], very recently performed a study employing brain endothelial exosomes to deliver siRNA across the BBB in zebra fish model of U87 glioblastoma. The in vitro and in vivo results showed that siRNA delivered by exosomes was efficiently transported across the BBB, significantly reduced VEGF gene level in brain neuronal glioblastoma-astrocytoma cells and inhibited the aggregation of xenograft brain tumors in zebrafish. These results indicated brain endothelial exosomes could deliver exogenous siRNA to the targeted site in the brain for the treatment of brain cancer. To improve the capacity of CNS-targeting, many studies engineered exosomes to express rabies virus glycoprotein peptide (RVG), a 29-mer peptide sequence that specifically binds to acetylcholine receptors expressed on neurological cells [69]. Generally, targeting moieties could either be expressed in the donor cells or directly fused onto exosome membrane surface. Alvarez-Erviti and coworkers [15], provided the first proof-of-concept of exploiting the RNA-transporting capacity of modified exosomes for the delivery of exogenous siRNA across the BBB. Given that siRNA lacks efficient carriers to traverse the BBB, the study set out to target small RNAs delivery into the brain via exosomes. Targeting of nerve cells was achieved by fusing exosomal protein lysosome-associated membrane glycoprotein 2b (Lamp2b) to brain-specific RVG peptide. Targeting exosomes were subsequently loaded with siRNA by electroporation. After intravenously injection, siRNA was specifically delivered to neurons, microglia, oligodendrocytes in the brain, and led to specific knockdown of target gene in these regions. Furthermore, the ability of targeting siRNA to the brain via RVG-exosomes was further tested by the delivery of BACE-1 siRNA (targeting the enzyme β-secretase, a therapeutic site in Alzheimer's disease). Strong RNAi responses were observed with up to 60% mRNA and protein knockdown of BACE1. Of note, RVG-exosomes were shown to almost completely bypass liver, displaying minor or no immunogenicity even after repeated administration. This is a critical feature for developing RNAi-based therapeutics. In a similar study by Liu et al. [65], neuron-specific RVG was also engineered onto exosome encapsulating siRNA via Lamp2b. The targeting exosomes were utilized to deliver opioid receptor mu (MOR) siRNA to the brain for the therapy of morphine addiction. After intravenously injection, MOR siRNA delivered by RVG-targeted exosomes strongly suppressed morphine relapse via down-regulation of MOR expression level in the brain. The results convincingly demonstrated that targeted RVG exosomes can efficiently and specifically deliver siRNA to the central nervous system for the therapy of drug relapse.
Table 1.
Exosome-based small RNA delivery for the treatment of neurological diseases.
| Authors | Exosome-based small RNA delivery systems | Neurological diseases | In vitro/in vivo | Therapeutic outcomes achieved | Ref. |
|---|---|---|---|---|---|
| Alvarez-Erviti (2011) | RVG-exosomes from mouse dendritic cells loaded with siRNA | Alzheimer's disease | In vivo | Strong knockdown of target mRNA and protein in Alzheimer's disease model | [15] |
| Liu (2015) | RVG-exosomes from HEK 293T cells loaded with MOR siRNA | Morphine relapse | In vivo | Specifically delivered MOR siRNA to the brain; Down-regulated MOR mRNA and protein level | [65] |
| Yang (2017) | RVG-exosomes from murine BM-MSCs loaded with miR-124 | Brain infarct | In vivo | MiR-124 was specifically ferried to the ischemic region; Protect against ischemic injury by robust cortical neurogenesis |
[66] |
| Lee (2017) | Exosomes from HEK 293 cells loaded with miR-124 | Huntington's disease | In vivo | Expression of target gene was reduced; However, no significant behavioral improvement was observed after exosomes miR-124 treatment |
[62] |
| Yang (2017) | Exosomes from brain endothelial bEND.3 cells loaded with VEGF siRNA | U-87 Glioblatoma | In vivo | Significant knockdown of VEGF gene in brain neuronal glioblastoma-astrocytoma cells; Effective inhibition of the aggregation of xenotransplanted cancer cells in zebrafish. |
[63] |
| Katakowski (2012) | Exosomes derived from miR-146-expressing MSCs | Glioma | In vivo | Significant reduction of glioma xenograft growth in rat model of glioma | [67] |
| Munoz (2013) | MSC derived exosomes loaded with anti-miR-9 | Glioblastoma multiforme | In vitro | Significantly reversed the chemoresistance of bioblastoma multiforme cells | [68] |
RVG: rabies virus glycoprotein peptide; MOR: opioid receptor mu; VEGF: vascular endothelial growth factor; MSC: marrow stromal cell; HEK: human embryonic kidney.
In addition to siRNA, exosomes can also be used for the brain delivery of miRNA. Recently, another research group evaluated the delivery capacity of exosomes to target exogenous miRNA to the brain for promoting neurogenesis after ischemia (i.e. brain infarction) [66]. Targeting exosomes, with RVG fused to Lamp2b, were then loaded with miR-124, which has a neuroprotective and neurorestorative potential against stroke. After systemically administered to mice, miR-124 was specifically ferried to the ischemic region, resulting in strong RNAi response and amelioration of brain injury caused by stroke [66]. MiR-146 has a potential of inhibiting the expression of EGFR and the malignant phenotype in glioma cell [67]. Katakowski et al. [67], found that exosomes released by miR-146-expressing marrow stromal cells (MSCs) significantly reduced glioma xenograft growth in a rat model of primary brain tumor. MiR-9 was observed to involve in the expression of p-glycoprotein, a drug efflux transporter [68]. Munoz et al. [68], found that MSCs derived exosomes could deliver anti-miR-9 to glioblastoma multiforme cells (GBM) and reversed the expression of p-glycoprotein, which improved the chemosensitivity of temozolomide against GBM. Given that Huntington's disease is a genetic neurodegenerative disease caused by abnormal gene regulation and altered miRNA expression, the delivery of being downregulated miRNAs might restore normal gene regulation. Recently, Lee et al. [62], harnessed exosomes as the delivery vehicles of miR-124, which was downregulated in Huntington's disease. MiR-124 incorporated with exosomes (Exo-124) were harvested from miR-124 overexpressing human embryonic kidney (HEK) 293 cells. After the injection into the striatum of transgenic Huntington's disease mice, Exo-124 could inhibit the expression of target gene, but could not produce significant behavioral improvement. Overall, the study provided a proof of concept for the therapeutic delivery of miR-124 in Huntington's disease model. However, it is recommended that the repression efficiency of target gene could be further compared with that from systemic administration.
The studies outlined above collectively revealed that exosome-based small RNA delivery systems have potentials to be a versatile strategy to treat neurological disease. In addition, the target efficiency of natural exosomes could be further improved by engineering exosomes to express specific surface ligands. The conjugation of exosomes to desirable surface moieties, such as RVG is an effective strategy to facilitate exosomes' penetration across the BBB. However, there exist several barriers that need to be overcome before clinical use. To date, a number of reports that exploit exosomes for the systemic delivery of therapeutic small RNAs to the brain still remain insufficient. Although the capacity of exosomes to traverse the BBB has been demonstrated, the exact mechanism of how they do it is not fully understood. Moreover, the CNS-targeting capacity of exosomes would be greatly enhanced by identifying more novel targeting moieties, other than RVG, specific for the brain. In this case, monoclonal antibodies against receptors or adhesion molecules that generally expressed on the BBB may be promising candidates. Overall, exosome-based small RNA delivery vehicles provide enormous potentials for the clinical application of RNAi in the treatment of brain disorders.
5.2. Exosomes as carriers of small RNAs for the treatment of tumors
Small RNAs are key regulators of gene expression in cancers [70]. Given that exosomes are able to transport nucleic acids to target cells, interest is fast growing in utilizing exosomes as vehicles to deliver therapeutic nucleic acids for the treatment of cancers. As a natural RNA carrier in our bodies, exosomes have high stability in the circulation and intrinsic homing ability, which is therapeutically advantageous compared to conventional anti-tumor delivery systems [71]. As a result, exosomes can help in the protection and specifically delivery of small RNAs to the targeted tumor cells when employed as therapeutic vehicles. In addition to the natural capacity to deliver RNAs, exosomes are non-viable, thus posing less risk of tumor formation [72]. Moreover, in contrast to viral vectors, exosomes delivery is notably safe without the risk of insertional oncogenesis [72]. Finally, the surface of exosomes may be modified to improve tumor-specific or even patient-specific capacity [73]. The current utilization of exosomes in RNAi for cancer therapy mainly focuses on testing their capacity to target small RNAs to the “disease sites”, alter gene expression in certain diseases, and enhance genetic therapy. A summary of research articles tailoring exosomes as delivery vehicles of small RNAs for treatment of tumors is provided in Table 2.
Table 2.
Exosome-based small RNA delivery for the treatment of tumors.
| Authors | Exosome-based small RNA delivery systems | Tumors | In vitro/in vivo | Therapeutic outcomes achieved | Ref. |
|---|---|---|---|---|---|
| Shtam (2013) | Exosomes from Hela and HT1080 human fibosarcoma cells loaded with siRNA | - | In vitro | Exosome-mediated siRNA delivery caused significant knockdown of target protein, and massive death of target cancer cells |
[14] |
| Banizs (2014) | Endothelial exosomes loaded with siRNA against luciferase | - | In vitro | Exosomes containing siRNA inhibited luciferase expression in target cells | [74] |
| Zhang (2014) | Exosomes from mouse fibroblast L929 cells loaded with TGF-β1 siRNA | Mouse sarcomas (S180) | In vivo | Strong suppression of TGF-β1 expression; Inhibition the growth and metastases of tumor cells in tumor bearing mice |
[75] |
| Ohno (2012) | Exosomes from HEK293 cells loaded with let-7a miRNA | Breast cancer | In vivo | Exosomes mediated specifically delivery of Let-7a miRNA to xenograft breast cancer tissue and strongly inhibited tumor growth | [76] |
| Fonsato (2012) | HLSCs derived exosomes loaded with miRNA | hepatocellular carcinoma | In vivo | Inhibit the growth and survival of hepatocellular carcinoma in mice | [77] |
TGF-β1: transforming growth factor β1; HLSCs: human adult liver stem cells.
Shtam et al. [14], for example, reported the use of exosomes to deliver siRNA against RAD51 (a prospective therapeutic target of cancer cells) to target cells. Successful siRNA delivery to recipient cells was observed by using confocal microscopy and flow cytometry. Moreover, the strong knockdown of pathologically relevant oncogenes, considerable reduction in RAD51 protein level, and massive death of cancer cells further demonstrated the efficient delivery of siRNA into target cells. Overall, the study provided in vivo evidence to use exosomes as carriers of small RNAs in cancer therapy. In another study, Banizs et al. [74], explored the capacity of endothelial exosomes to deliver exogenous siRNA to endothelial cells in vitro. After incubating luciferase-expression endothelial cells with siRNA containing exosomes, the expression of luciferase was suppressed by more than 40%, which was significantly lower than that from siRNA alone or control siRNA. Although exosomes from different cell lines can deliver exogenous siRNA to cancer cells in vitro, however, it remains largely unknown that whether exosomes are capable of systemically delivering siRNA to cancer cells and induce gene silencing effects. In a study by Zhang et al [75], exosomes were systematically employed to deliver transforming growth factor β1 (TGF-β1, a potential therapeutic target of cancers) siRNA for the suppression of tumor growth in mice. In this study, mouse fibroblast L929 cells were transfected with TGF-β1 siRNA via transfected agents. Exosomes containing TGF-β1 siRNA were then harvested from cell supernatant and tested for the inhibitory effect on the growth and metastasis of murine sarcomas cells in vitro and in vivo. The author found that exosomes containing TGF-β1 siRNA significantly reduced TGF-β1 level in the recipient tumor cells compared with free TGF-β1 siRNA and efficiently decreased the viability and migration of implanted mouse sarcomas 180 cells. In line with the in vivo results, the systemic anti-tumor activity was further confirmed by the effective inhibition of the growth and lung metastases of tumor cells in tumor bearing mice. Therefore, the study suggested that exosomes are effective carrier of therapeutic siRNA for the systemic suppression of tumor growth in mice, which pave the way for the clinical application of exosome-based RNAi therapeutics in controlling tumor cell growth and metastasis.
In addition to siRNA, exosomes can also be used as carriers of miRNA. A growing pool of studies suggest cancer progression is intimately related to the gain or loss-of functional mutations of miRNA [78]. For example, reduced expression level of let-7a has been observed in many cancer cells, such as lung, breast, colon, and ovary cells [78]. This, together with the natural role of shuttling miRNA, makes exosomes an attractive vehicle for miRNA in the miRNA replacement therapy of malignant neoplasms. Multiple research groups have gained successful results. For example, in a study performed by Ohno et al. [76], exosomes were used to deliver exogenous let-7a miRNA (a tumor inhibitor that suppresses the malignant growth of cancer cells) to epidermal growth factor receptor (EGFR) expressing breast cancer cells. In order to achieve targeting effect, EGFR-specific peptide (GE11) with the ability to bind specifically to EGFR, was incorporated onto the surfaces of exosomes. The in vitro assay results showed that, exosomes were taken up by various breast cancer cells, including HCC70, HCC1954, and MCF-7, via an EGFR-dependent mechanism. After intravenous injection into tumor-bearing RAG2-/- mice, let-7a miRNA delivered by exosome EGFR was observed to localize at xenograft breast cancer tissue and strongly inhibit tumor growth. These results indicated that exosomes targeted to EGFR-expressing cells may serve as a novel platform for miRNA replacement therapies in cancer treatment. All these studies collectively demonstrated the potential of exosomes to specifically deliver siRNA and exert gene silencing effects in cancerous tissues.
5.3. The potential of exosome-based small RNA delivery for the treatment of ocular diseases
Delivery of small RNA to the retina is a major challenge because it needs to circumvent physical and biochemical barriers to enter and diffuse throughout retinal tissues. In addition, it requires a delivery vehicle with better-compatibility and less immunogenicity in the eye. As exosomes have been shown to traverse biological barriers and display minor immunogenicity upon systemic administration, it is feasible to hypothesize that exosomes could efficiently and safely facilitate the permeation of small RNAs through ocular barriers, such as vitreous humour and inner limiting membrane. Adeno-associated viral vectors (AAVs) have been validated as powerful delivery vehicles for the treatment of retinal disorders [79]. However, intravitreal injection of AAV vectors often result in low delivery efficiency and high inflammatory risk from vectors or transgene antigens [79]. Very recently, Wassmer et al. [80], packaged AAV2 into exosomes and formed exosome-associated AAV2 vector (called vexosomes). The retinal tropism of vexosomes was evaluated by fundus image analysis and qRT-PCR. Vexosomes exhibited an increased level of retinal cell gene transduction compared with conventional AAV2, as vexosomes penetrated deeply in the retina and efficiently reached the inner nuclear and outer plexiform. Furthermore, these vexosomes were resistant to neutralizing antibodies due to the shielding of the AAV by exosomal membrane. In summary, the ability to improve delivery efficiency and reduce immunogenicity enables vexosomes to be an exciting and robust tool for retinal gene delivery and as such, could facilitate research on the treatment of ocular diseases.
5.4. Exosomes as potential carriers of small RNAs for the treatment of liver diseases
As important mediators that involve in liver physiology and pathophysiology, exosomes hold great potential as novel therapeutic modalities to treat liver diseases. It was reported that human adult liver stem cells (HLSC) derived exosomes loaded with a specific subset of mRNA could induce proliferation and apoptosis resistance of human and rat hepatocytes in vitro, and accelerate the hepatic regeneration in hepatectomized rats [81]. Another study by Fonsato et al. indicated the delivery of miRNA by HLSC derived exosomes could suppress the growth of hepatocellular carcinoma cells in vitro and induce the regression of ecptomic tumors in SCID mice after intratumor administration [77]. Pan et al. found that exosomes released from human hepatoma cells could shuttle siRNA against viral entry receptor CD8 between liver cells, and caused the suppression of CD81 expression in mouse hepatocytes without induction of obvious toxic responses [82]. These observations potentially extend the therapeutic realm of exosome-based small RNA delivery in the therapy of hepatocellular carcinoma, viral hepatitis, and other liver diseases.
5.5. Exosomes as potential carriers of small RNAs for the treatment of kidney diseases
Emerging evidences have showed that miRNA is responsible for post-transcriptional regulation of gene expression in the pathogenesis of chronic kidney diseases (CKD) [83]. In certain instances, up-regulation or down-regulation of miRNA has been identified in models of kidney fibrosis. MiRNA has a capacity to reduce fibrosis by modulating targets associated with collagen and extracellular matrix accumulation [83]. Therefore, exosome mediated delivery of miRNA may provide great promise for the treatment of kidney fibrosis. For example, let-7 was identified to be able to attenuate renal fibrosis by the repression of transforming growth factor beta (TGF-β) [84]. Moreover, according to Koh et al., MSC derived exosomes exhibited a high expression in let-7 family [85]. This allowed for the considerable potential of delivering let-7 miRNA by MSC-derived exosomes, where let-7 could be protected and delivered across the membrane of targeted cells and exert functional response. Recently, Wang et al. [86], reported that exosomes secreted from MSCs overexpressing let-7c miRNA could selectively transfer let-7c to damaged kidneys, up-regulate let-7c gene expression and reduce renal fibrosis. This finding could pave the way for therapeutic application of exosomes for small RNA delivery in treating kidney diseases.
6. Conclusion and perspectives
Over the last decades, RNAi technology has been rapidly developed. Parallelly, there has been an increasing interest in the therapeutic application of small RNAs for the treatment of a wide array of diseases. Although great outcomes have been accomplished, many obstacles still remain unsettled for small RNA delivery, such as fast clearance, low cellular uptake efficiency, and harmful immunogenicity, and toxicity. The concept of employing exosomes as carriers for small RNA delivery is fascinating due to multi-faceted nature of exosomes, including unique structure and physicochemical characteristics, increased stability in circulation, intrinsic traversing biological barriers ability, efficient cellular entry, inherent targeting ability, and low immunogenicity and toxicity. Currently, exosomes have been successfully tailored for small RNA delivery to treat various diseases, including neurological disorders, tumors, ocular, liver, and kidney diseases. However, to maximize the clinical potential of exosomes, further studies are pressingly needed to solve many contentious issues. Existing challenges for transforming exosome-based small RNA delivery from bench to bedside include choosing correct cell line, exploring isolation techniques with high efficiency and robust yield, scalable production, efficient loading methods without damaging exosome integrity, gaining an improved insight into the molecular mechanisms of such systems, and examining possible strategies to improve targeting ability. In addition, the potential application of exosome-based small RNA delivery to treat other diseases requires to be explored. Nevertheless, since the wisdom of nature inspired researchers to harness exosomes for nucleic acid delivery, the exciting research field is advancing rapidly. Given the enormous interest and continuous technological advancements, exosome-mediated delivery of small RNAs is firmly believed to reach the clinic in the future.
Declaration of interest
The authors declare that there is no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 81373335) and Liaoning Province Natural Science Foundation (Grant No. 20170541025).
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
Contributor Information
Dongkai Wang, Email: Wangdksy@126.com.
Xiaoyun Zhao, Email: 1014605559@qq.com.
References
- 1.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 2.Roberts T.C., Ezzat K., Andaloussi S.E. Synthetic siRNA delivery: progress and prospects. Methods Mol Biol. 2016;1364:291–310. doi: 10.1007/978-1-4939-3112-5_23. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y.S., Lam J.K.W., Leung S.W.S. Delivery of RNAi therapeutics to the airways from bench to bedside. Molecules. 2016;21:e1249. doi: 10.3390/molecules21091249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi X., Gatti P., Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov Today. 2017;5:823–833. doi: 10.1016/j.drudis.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand J.R., Pottier M., Vekris A. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296:1000–1004. doi: 10.1016/s0006-291x(02)02013-2. [DOI] [PubMed] [Google Scholar]
- 6.Xu C.F., Wang J. Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2015;10:1–12. [Google Scholar]
- 7.Birmingham A., Anderson E., Sullivan K. A protocol for designing siRNAs with high functionality and specificity. Nat Protoc. 2007;2:2068–2078. doi: 10.1038/nprot.2007.278. [DOI] [PubMed] [Google Scholar]
- 8.Castanotto D., Rossi J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng R., Yue Y., Jin F. Revisit the complexation of PEI and DNA – how to make low cytotoxic and highly efficient PEI gene transfection non-viral vectors with a controllable chain length and structure? J Control Release. 2009;140:40–46. doi: 10.1016/j.jconrel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Valadi H., Ekstrom K., Bossios A. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Haney M.J., Klyachko N.L., Zhao Y. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van den Boorn J.G., Schlee M., Coch C. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 14.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L., Seow Y., Yin H. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 16.Rana T.M. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 17.Fire A., Xu S., Montgomery M.K. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 18.OPKO Health I An open label study for the evaluation of tolerability of five dose levels of cand5 by single intravitreal injection in patients with wet age-related macular degeneration. 2004. https://clinicaltrials.gov/ct2/show/study/NCT00722384 Available from:
- 19.Davis M.E., Zuckerman J.E., Choi C.H.J. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morjaria R., Chong N.V. Pharmacokinetic evaluation of pegaptanib octasodium for the treatment of diabetic edema. Expert Opin Drug Metab Toxicol. 2014;10:1185–1192. doi: 10.1517/17425255.2014.922543. [DOI] [PubMed] [Google Scholar]
- 21.Karpala A.J., Doran T.J., Bean A.G.D. Immune responses to dsRNA: implications for gene silencing technologies. Immunol Cell Biol. 2005;83:211–216. doi: 10.1111/j.1440-1711.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- 22.Aagaard L., Rossi J.J. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam J.K.W., Chow M.Y.T., Zhang Y. SiRNA Versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T., Totoki Y., Toyoda A. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 25.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 26.Singh M.S., Peer D. SiRNA delivery: current trends and future perspectives. Ther Deliv. 2016;7:51–53. doi: 10.4155/tde.15.88. [DOI] [PubMed] [Google Scholar]
- 27.Haupenthal J., Baehr C., Kiermayer S. Inhibition of RNAse A family enzymes prevents degradation and loss of silencing activity of siRNAs in serum. Biochem Pharmacol. 2006;71:702–710. doi: 10.1016/j.bcp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Sahay G., Querbes W., Alabi C. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31 doi: 10.1038/nbt.2614. 653–U119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vercauteren D., Rejman J., Martens T.F. On the cellular processing of non-viral nanomedicines for nucleic acid delivery: mechanisms and methods. J Control Release. 2012;161:566–581. doi: 10.1016/j.jconrel.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Gilleron J., Querbes W., Zeigerer A. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31(7):638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 31.Naito Y., Ui-Tei K. Designing functional siRNA with reduced off-target effects. Methods Mol Biol. 2013;942:57–68. doi: 10.1007/978-1-62703-119-6_3. [DOI] [PubMed] [Google Scholar]
- 32.Jhaveri A.M., Torchilin V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead K.A., Dorkin J.R., Vegas A.J. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meade B.R., Gogoi K., Hamil A.S. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat Biotechnol. 2014;32:1256–1261. doi: 10.1038/nbt.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue H.Y., Liu S.M., Wong H.L. Nanotoxicity: a key obstacle to clinical translation of siRNA-based nanomedicine. Nanomedicine (Lond) 2014;9:295–312. doi: 10.2217/nnm.13.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y., Wang C.C., Choy K.W. Therapeutic potentials of gene silencing by RNA interference: principles, challenges, and new strategies. Gene. 2014;538:217–227. doi: 10.1016/j.gene.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 38.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 39.Kowal J., Tkach M., Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Kalani A., Kamat P.K., Chaturvedi P. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014;107:1–7. doi: 10.1016/j.lfs.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanez-Mo M., Siljander P.R.M., Andreu Z. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tkach M., Théry C. Communication by extracellular vesicles: where are we and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 44.Aryani A., Denecke B. Exosomes as a nanodelivery system: a key to the future of neuromedicine. Mol Neurobiol. 2016;53:818–834. doi: 10.1007/s12035-014-9054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andaloussi S.E., Lakhal S., Mager I. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Tan A., Rajadas J., Seifalian A.M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65:357–367. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Ren J., He W., Zheng L. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater Sci. 2016;4:910–921. doi: 10.1039/c5bm00583c. [DOI] [PubMed] [Google Scholar]
- 48.Lane L.A., Qian X.M., Smith A.M. Physical chemistry of nanomedicne: understanding the complex behaviros in vivo. Annu Rev Phys Chem. 2015;66:521–547. doi: 10.1146/annurev-physchem-040513-103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deregibus M.C., Figliolini F., D'Antico S. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38:1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saari H., Lázaroibáñez E., Viitala T. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Sekhar G.N., Watson C.P., Fidanboylu M. Delivery of antihuman African trypanosomiasis drugs across the blood-brain and blood-CSF barriers. Adv Pharmacol. 2014;71:245–275. doi: 10.1016/bs.apha.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Kooijmans S.A., Vader P., Dommelen S.M.V. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim M.S., Haney M.J., Zhao Y. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine (Lond) 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viaud S., Ploix S., Lapierre V. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-γ. J Immunother. 2011;34:65–75. doi: 10.1097/CJI.0b013e3181fe535b. [DOI] [PubMed] [Google Scholar]
- 55.Dai S., Wei D., Wu Z. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolburg H., Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 57.Chow B.W., Gu C.H. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu R.T., Liu J., Ji X.F. Synthetic nucleic acids delivered by exosomes: a potential therapeutic for generelated metabolic brain diseases. Metab Brain Dis. 2013;28:551–562. doi: 10.1007/s11011-013-9434-y. [DOI] [PubMed] [Google Scholar]
- 59.Katakowski M., Chopp M. Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol. 2016;36:343–352. doi: 10.1007/s10571-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang T.Z., Martin P., Fogarty B. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andaloussi S.E., Lee Y., Lakhal-Littleton S. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 62.Lee S.T., Im W., Ban J.J. Exosome-based delivery of miR-124 in a Huntington's disease model. J Mov Dis. 2017;10:45–52. doi: 10.14802/jmd.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang T.Z., Fogarty B., LaForge B. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19:475–486. doi: 10.1208/s12248-016-0015-y. [DOI] [PubMed] [Google Scholar]
- 64.Jarmalaviciute A., Pivoriunas A. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacol Res. 2016;113:816–822. doi: 10.1016/j.phrs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y., Li D., Liu Z. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J., Zhang X., Chen X. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katakowski M., Buller B., Zheng X.G. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munoz J.L., Bliss S.A., Greco S.J. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2(10):126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lentz T.L. Rabies virus binding to an acetylcholine receptor alpha-subunit peptide. J Mol Recognit. 1990;3:82–88. doi: 10.1002/jmr.300030205. [DOI] [PubMed] [Google Scholar]
- 70.Tomasetti M., Lee W., Santarelli L. Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med. 2017;49:285. doi: 10.1038/emm.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi Y., Nishikawa M., Shinotsuka H. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Chen T.S., Arslan F., Yin Y. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnsen K.B., Gudbergsson J.M., Skov M.N. A comprehensive overview of exosomes as drug delivery vehicles – endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Banizs A.B., Huang T., Dryden K. in vivo evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomed. 2014;9:4223–4230. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y., Li L., Yu J. Microvesicle-mediated delivery of transforming growth factor beta1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014;35:4390–4400. doi: 10.1016/j.biomaterials.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Ohno S., Takanashi M., Sudo K. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2012;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fonsato V., Collino F., Herrera M.B. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohno S., Kuroda M. Exosome-mediated targeted delivery of miRNAs. Methods Mol Biol. 2016;1448:261–270. doi: 10.1007/978-1-4939-3753-0_19. [DOI] [PubMed] [Google Scholar]
- 79.Carvalho L.S., Gyoergy B., Mu D. Retinal tropism of exosome-associated AAV vector via intravitreal delivery. Mol Ther. 2015;23:S61. [Google Scholar]
- 80.Wassmer S.J., Carvalho L.S., Gyorgy B. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci Rep. 2017;7:45329. doi: 10.1038/srep45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herrera M.B., Fonsato V., Gatti S. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan Q.W., Ramakrishnaiah V., Henry S. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61:1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 83.Yao K., Ricardo S.D. Mesenchymal stem cells as novel micro-ribonucleic acid delivery vehicles in kidney disease. Nephrology. 2016;21:363–371. doi: 10.1111/nep.12643. [DOI] [PubMed] [Google Scholar]
- 84.Wang B., Jha J.C., Hagiwara S. Transforming growth factor-beta1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int. 2014;85:352–361. doi: 10.1038/ki.2013.372. [DOI] [PubMed] [Google Scholar]
- 85.Koh W., Sheng C.T., Tan B. Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genomics. 2010;11:1–15. doi: 10.1186/1471-2164-11-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang B., Yao K. Huuskes BM. Mesenchymal stem cells deliver Exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24:1290–1301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]


