Graphical Abstract
Keywords: Metformin hydrochloride, Hydroxypropylcellulose, High drug loaded tablet, Melt granulation
Abstract
The present work explores the application of melt granulation technology to develop a high drug loaded sustained release matrix tablet of Metformin HCl using hydroxypropylcellulose (HPC) as a hydrophilic binder and stearic acid as an extrusion aid for producing cohesive granules. This novel approach allowed the use of a minimum number of excipients to reduce the tablet size, and to enhance compressibility of the drug. This also offered a cost effective method owing to the elimination of a ‘drying step’ prevalent in wet granulation method. Moreover, this research also focuses on resolving the processability issues associated with the use of HPC Nisso-H at high drug loading. The thermal lubricants were screened for this purpose and evaluated for their impact on extrudability, granule and tablet characteristics. Stearic acid was selected as the thermal lubricant, which not only contributed to the inhibition of burst release, but also improved the flow property of the granules.
The developed matrix tablet (75% drug loading) resulted in 670 mg of weight for 500 mg dose strength and showed sustained drug release over 10 h. When compared, with conventional granulation techniques, it was observed that, under identical compression force, the tablet prepared by MG exhibited superior compactibility along with tablet hardness and optimal drug release profile. FTIR suggested nonexistence of chemical interaction between the drug and the other excipients while XRD and DSC analysis revealed the crystalline state of the drug. Furthermore, the results obtained from Raman spectroscopy proved the uniform distribution of the Metformin HCl and polymer in the final dosage form. This technology leads to the manufacture of sustained release matrix formulation with reduced tablet size of a high dose, highly water soluble drug otherwise difficult to process using standard batch-granulation.
1. Introduction
Conventionally, to achieve the sustained release profile of a highly water soluble drug, it is necessary to use a large amount of release-controlling polymers which in turn increases the tablet weight [1], [2], [3], [4], [5]. Conversely, high-dose drug products require minimal use of excipients to keep the tablet size small enough to allow ease of swallowing. Metformin HCl is a highly water soluble drug with high dose and also has significant processing challenges due to its poor compressibility and moisture sensitivity. In addition, Metformin HCl is commonly used in combination with other hypoglycemic agents to develop a fixed dose combination which makes the tablet size even larger and might worsen its compactibility. Employing wet granulation (WG) to overcome these challenges may result in formation of solid bridges in Metformin HCl particles, poor powder flow as well as sticking problems during tableting due to consequent moisture migration or desorption during the granulation process [6], [7].
MG can be explored to overcome these formulation challenges of Metformin HCl. It is advantageous over traditional granulation techniques as it is a solvent free green technology with a closed process unit and easy to scale up. In addition, it produces granules that contain fewer fines, reduces the number of processing steps and requirements of inventory. Moreover, in MG extensive solid bridges are formed between the drug and excipient particles resulting in a smaller pore radius and a more tortuous network, thereby facilitating densification of the molten mass to a higher extent and a less porous matrix, thus capable of reducing burst release in the early hours [8], [9]. On this basis, MG technology can be used to formulate sustained release matrix with high drug loading of water soluble drugs.
Literature data have reported several approaches to formulate sustained release Metformin HCl. However, as per reports to date, no literature data are available on the application of continuous granulation process using hot melt extrusion to develop sustained release formulation of Metformin HCl with minimal use of excipients.
In this study, hydroxypropylcellulose (HPC) Nisso-H polymer was used as meltable binder owing to its thermoplastic nature, low softening point and high swelling capacity [10], [11], [12], [13]. However, these properties vary from manufacturer to manufacturer. Although reports citing the use of HPC Klucel are available in literature, the current research work further deals with the challenges of extruding HPC Nisso-H. It was observed that extrusion of HPC Nisso-H by itself is very challenging and the challenge becomes even more difficult as the objective was to extrude the blend with a high ratio of crystalline drug. Furthermore, the research work deals with further development of the extruded material into a sustained release tablet dosage form.
Based on these premises, the present work aimed to explore feasibility of MG technology to improve physiochemical properties of Metformin HCl such as poor flow and compressibility and to formulate sustained release matrices with maximum drug loading. For this purpose, the potential of HPC Nisso-H was explored as a binder for sustained release formulation. Moreover, the present work also comprises estimation of robust manufacturing process for high-dose Metformin HCl formulation by comparison of matrices prepared by MG and other conventional granulation methods.
2. Materials and methods
2.1. Materials
Metformin HCl was purchased from Sohan Healthcare Pvt. Ltd. (Pune, India). Hydroxypropylcellulose (HPC) Nisso H was provided by Nippon Soda Co. Ltd. (Tokyo, Japan) as a free sample. Propylene glycol, Polyethylene glycol 400 (PEG 400), Polyethylene glycol 1500 (PEG 1500), Polyethylene glycol 4000 (PEG 4000), Triethylcitrate (TEC), Stearic acid and Glyceryl monostearate (GMS) were purchased from S.D. Fine Chemicals. All other excipients used were of analytical grade.
2.2. Preparation of matrix tablets
The drug and the thermoplastic binders were first delumped by passing through #60 and mixed for 10 min to form a uniform powder blend. The powder blends were processed using three different methods like direct granulation (DG), wet granulation (WG) and melt granulation (MG). The screened granules obtained for various tablet formulations were compressed using 9 × 17 mm (drug loading: 50%) and 8 × 16 mm for higher drug loading on a rotary tablet press (Rimek Minipress I, Ahmedabad, India).
2.2.1. Influence of thermal aid
To improve the processability of melt extrusion various thermal aids were evaluated. Table 1 enlists the different thermal aids screened and the concentration optimization.
Table 1.
Screening and optimization of concentration of thermal aid.
| Ingredients | Formulation code | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
| Metformin HCl | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Hydroxypropylcellulose HPC Nisso-H |
40 | 40 | 40 | 40 | 40 | 40 | 40 | 45 | 43 | 38 | 35 |
| Propylene glycol | 10 | – | – | – | – | – | – | – | – | – | – |
| PEG 400 | – | 10 | – | – | – | – | – | – | – | – | – |
| PEG 1500 | – | – | 10 | – | – | – | – | – | – | – | – |
| PEG 4000 | – | – | – | 10 | – | – | – | – | – | – | – |
| GMS | – | – | – | – | 10 | – | – | – | – | – | – |
| TEC | – | – | – | – | – | 10 | – | – | – | – | – |
| Stearic acid | – | – | – | – | – | – | 10 | 5 | 7 | 12 | 15 |
All quantities are in percentage (w/w).
2.2.2. Influence of drug loading
Tablet formulation comprising different drug loadings (50–85%) was prepared using MG method. All the ingredients were mixed as per the specified ratio (Table 2). The MG process was performed using a laboratory scale, single screw hot melt extruder (Lab Model, S.B. Panchal & Co.) equipped with a 100 mm long and 20 mm diameter screw using the following parameters: processing temperature: 110 ± 2 °C (much below the melting temperature of drug), screw speed: 50 rpm. The obtained product was then cooled to ambient temperature and subjected to milling and sieving through a screen of #40. The powdered extrude was then subjected to tablet compression.
Table 2.
Composition of blend for SR matrix tablet prepared by various methods and its process parameters.
| Ingredients | Drug loading | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 a | F2a | F3a | F4a | F5a | F6a | F7a | F8a | F9b | F10c | |
| Metformin HCl | 50 | 55 | 60 | 65 | 70 | 75 | 80 | 85 | 75 | 75 |
| HPC Nisso-H | 40 | 35 | 30 | 25 | 20 | 15 | 10 | 5 | 15 | 15 |
| Stearic acid | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Purified water | – | – | – | – | – | – | – | – | – | q.s.d |
| Extrusion with die | + | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Extrusion without die | NA | + | + | + | + | + | + | + | NA | NA |
All quantities are in percentage (w/w).
Melt granulation with HPC Nisso-H.
Dry granulation with HPC Nisso-H.
Wet granulation with HPC Nisso-H.
Water used for granulation and removed by drying.
2.2.3. Effect of method of preparation
The tablet containing 75% loading of Metformin HCl was prepared by different techniques like MG (F6), DG (F9) and WG (F10) (Table 2). The MG process was carried out as mentioned above. In DG, all ingredients were accurately weighed and passed through a #60 sieve and mixed in geometric proportion for 10 min to form a uniform blend. For WG, water (8% w/w) was sprinkled to form a wet mass which was passed through #20 to obtain granules, which were then subjected to drying at 40 °C until the desired loss on drying (LOD) level (less than 2%) was obtained. The blends prepared by different methods were compressed to obtain tablets.
2.3. Micromeritics of the formulation blend
The products obtained from all the batches were evaluated for their physicochemical properties. The angle of repose (α) was determined by the static funnel method and calculated using the formula
| (1) |
Bulk density (BD) was measured by filling a preweighed 100 ml glass cylinder to its capacity with the granules and then weighing it. The tap density (TD) was calculated by dividing the fill weight of the powder by its new reduced volume after tapping it 100 times on a tap density apparatus. The compressibility index (CI) and Hausner's ratio (HR) were measured by USP method I using a graduated cylinder and were calculated using the following formulas:
| (2) |
| (3) |
2.4. Evaluation of tablets
2.4.1. Physical properties of tablets
The compressed tablets were evaluated for hardness, friability, weight variation, thickness and content uniformity using USP methods. Hardness (n = 10) was measured using a Dr. Schleuniger 6D hardness tester (Schleuniger Pharmatron, New Hampshire, USA) and friability (n = 10) was tested using a Roche friabilator (F. Hoffmann-La Roche Ltd, Basel, Switzerland). The dimensions of the tablets were determined using a Vernier caliper (Mitutoyo Corp, Kawasaki, Japan). Tablet weight variation was evaluated by weighing individual tablets (n = 20) and the variability (% RSD) in tablet weights was reported as percent of average tablet weight. Individual tablets (n = 10) sampled from each batch were individually assayed by a UV spectrophotometer to determine the content uniformity.
2.4.2. Evaluation of in-vitro drug release
Dissolution testing was performed in accordance with the USP 31 monograph. 1000 ml of pH 6.8 phosphate buffer was used as a dissolution medium at 37 °C by using a USP dissolution test apparatus II (Electrolab, TDT-08L, India) at a paddle speed of 50 rpm with sinkers. Sink condition was maintained throughout the study. An aliquot of 10 ml was withdrawn at 1, 3, 5, 7 and 10 h intervals and replaced with the same amount of fresh media. All the samples were filtered through a 0.45 µm filter paper, appropriately diluted and analyzed for drug release spectrophotometrically (UV1650PC, Schimadzu Corporation, USA) at λ max of 233 nm. The USP limits for a sustained release profile of Metformin HCl are depicted in Table 3.
Table 3.
The USP limits for a sustained release profile of Metformin HCl.
| Time (h) | USP limit |
|---|---|
| 1 | 20%–40% |
| 3 | 45%–65% |
| 10 | NLT 85% |
2.4.3. Kinetic analysis and mechanism of drug release
In order to elucidate the drug release mechanism from the matrices of a solid dispersion tablet, release data of selected formulations were examined according to different mathematical equations such as Zero order (M = kt) equation, First-order equation, (lnM = kt), Higuchi model (M = k√t) and Korsemeyer–Peppas equation (Power law), (M = ktn). M is the amount of drug (%) released after time t. k is the release rate constant and n is the diffusional exponent. A value of n = 0.45 indicates case I (Fickian) diffusion, 0.45 < n < 0.89 anomalous (non Fickian) diffusion, n = 0.89 for the case II transport and n > 0.89 for the supercase II transport. The best fit was decided on the basis of regression values [14].
In order to characterize the drug release process further time point approach was used. With this approach, the mean dissolution time (MDT) was determined as it provides a more accurate view of the drug release behavior. MDT is determined as the sum of the individual periods of time during which a specific fraction of the total drug dose is released, calculated using following equation [15]:
| (4) |
where Mt is the fraction of dose released in time ti, ti = (ti + ti−1)/2, and M∞ corresponds to the loading dose.
In addition, to compare differences in the drug release rate and extent among the prepared formulas, dissolution efficiency (%DE) was calculated using the following equation. %DE is defined as the area under the dissolution curve up to the time “t,” expressed as percentage of the area of a rectangle described by 100% dissolution in the same time [16], [17].
| (5) |
where Y is the percent drug release as the function of time, t; T is the total time of drug release and Y100 is 100% drug release.
2.4.4. Fit factors
Fit factors are composed of difference (f1) and similarity components (f2) which compare and establish similarities and differences between two dissolution curves obtained from a set of experimental data [15], [16]. For this study, f1 and f2 were calculated by comparing in vitro drug release profiles obtained under similar conditions of the formulations (test) with the release profile of marketed SR tablet as reference (Gluformin XR®, Abbott Healthcare Pvt Ltd, India). f1 measures the percent error between the two curves over all time points and was calculated by using Eq. (6).
| (6) |
where Ri and Ti are the percent dissolved of the reference and test products at each time point i respectively.
ƒ2 was used to compare the dissolution behavior of reproducibility batches and compared with that of marketed and innovator's products. f2 is a logarithmic transformation of the sum of squared error of differences between the test Ti and the reference products Ri over all time points. It was calculated by using Eq. (7).
| (7) |
As per the recommendations made by the FDA Guidance for Industry, an f2 value between 50 and 100 indicates similarity between two dissolution profiles [15], [18] and f1 values up to 15 ensure sameness or equivalence of the test (post change) and reference (pre-change) products.
2.4.5. Stability studies
The optimized formulations were stored in high density polyethylene (HDPE) bottles and subjected to stability studies as per International Conference on Harmonization (ICH) guidelines for a period of six months at 25 °C/RH 60%, 30 °C/RH 65% and 40 °C/RH 75%, and were monitored for changes in physical attributes, drug content and in vitro release.
2.5. Characterization
2.5.1. Fourier-transform infrared (FTIR) analysis
FTIR studies were performed using a Perkin Elmer FTIR spectrophotometer (Spectrum RX1, USA) to investigate the interaction of Metformin HCl with the polymer or plasticizer. The samples were pelletized with KBr and the pellet was analyzed at a scanning range of 450–4000 cm−1. Change in characteristic peaks of the respective samples was recorded.
2.5.2. Differential scanning calorimetry (DSC) analysis
DSC analysis was carried out using Perkin Elmer, Pyris 6 DSC (Perkin Elmer life & Analytical Sciences Inc., USA) system in the temperature range 30–250 °C at a heating rate of 10 °C/min in a dynamic nitrogen atmosphere (18 ml/min). The samples were ground into fine powders with a pestle and mortar. Approximately 5 mg of sample was accurately weighed and crimped in an aluminum lid, and an empty sealed aluminum pan was used as the reference.
2.5.3. X-ray diffraction analysis
X-ray diffraction (XRD) analysis was performed for raw materials, physical mixture and powdered extrudate using Bruker AXS D8 Advance powder diffractometer (Germany) with Cu–Kα radiation source (λ = 1.5406 A°) at 25 °C operated at 40 kV and 40 mA, using a 0.02° step size and 2θ/min scan speed.
2.5.4. Scanning electron microscopy
The morphological characteristics of the matrix tablet prepared by melt granulation, dry and wet granulations were studied by scanning electron microscopy (Model JSM-6380LA, JEOL, Japan). The samples were mounted on an aluminum stage using adhesive carbon tape and were coated with a platinum plasma beam using JFC-1600 auto fine coater to make a layer of 2 nm thickness above the sample. Scanning electron microphotographs were obtained by applying electron current under a voltage difference of 20 kV.
2.5.5. Raman spectral analysis
The Raman spectra of the individual components of the tablet formulation (Metformin HCl, HPC Nisso-H and stearic acid) along with that of the optimized formulation were recorded with a LabRamHR800 (Horiba Jovan Yvon). The spectrometer was equipped with a 1800 groove/mm holographic grating, a holographic notch filter and a Peltier-cooled charge-coupled device (CCD, Andor Technology PLC), which was employed as a detector. The laser excitation was focused using a microscope objective of 50× (Olympus Corporation). An Ar:Ne laser was employed during the experiments with a wavelength of 633 nm. Raman spectroscopic imaging or mapping was used to evaluate the distribution of Metformin HCl in the matrix tablet prepared by melt granulation.
3. Results and discussion
3.1. Preparation of matrix tablets
3.1.1. Influence of thermal aid
Sustained release matrix formulation of a high dose drug poses a major challenge of maintaining the polymer matrix integrity since the release rate is dependent on it. The processing temperature should be above the Tg or melting temperature of the polymeric carrier and typically required to soften the polymer by lowering its melt viscosity which allows adequate flow through the extruder. However, it was evident from preliminary experiments that the HPC Nisso-H was difficult to extrude and required thermal aid to ease the granulation process. So, the initial challenge was to plasticize HPC Nisso-H to overcome processing of the powder blend with high ratio of the drug. In order to extrude HPC Nisso-H, various thermal aids were studied at a concentration of 10% w/w.
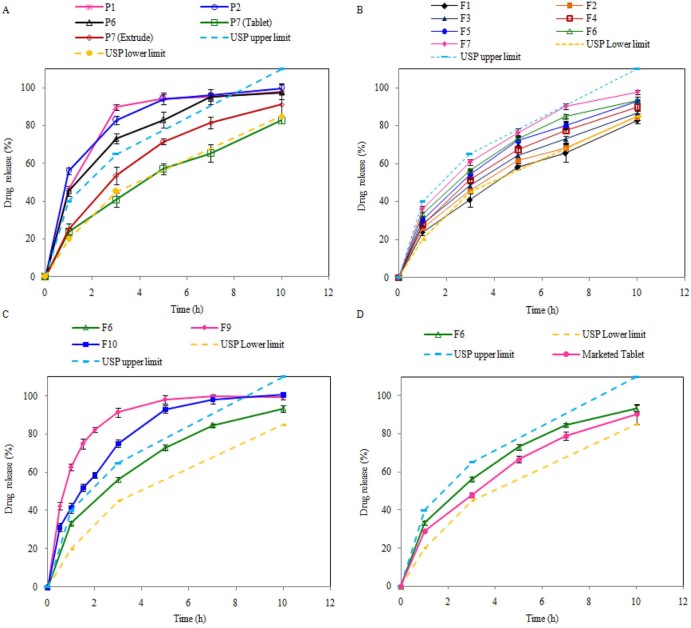
Parameters for screening the thermal aid for melt granulation include ease of granulation, powder and tablet properties and dissolution profile. Though thermal aid like Propylene Glycol, PEG 400 and TEC showed an effective plasticizing effect in the extrusion process, the pulverized powder of the extrudates showed poor compressibility with sticking problem due to hygroscopicity. Moreover, these thermal aids were unable to retard drug release sufficient to ensure a sustained release profile of Metformin HCl within USP limits, due to its hydrophilic nature (Fig. 1A). In case of PEG 1500 and PEG 4000, the high molecular weight of these plasticizers may interfere in the melting of physical mixture, and hence did not act as good plasticizers in the presence of Metformin HCl. In contrast, stearic acid as a solid thermal aid was easy to process since it blended easily with the polymer and the drug, and unlike liquid thermal aids, it did not make a tacky blend with HPC Nisso-H, which is difficult to feed in the extruder uniformly. Further, extrusion of the blend with 10% stearic acid was carried out without producing any torque on machine with processing temperature of 110 °C ± 2 °C. In addition, the extrudates obtained were easy to pulverize and the powder of the extrudates was not hygroscopic at all and gave excellent flow.
Fig. 1.
Effect on dissolution profile of Metformin HCl: (A) due to different thermal aids, (B) due to different drug loadings, (C) due to different methods of granulation and (D) comparison of optimized formulation with marketed tablet.
In order to evaluate the optimum concentration of stearic acid as thermal aid for the extrusion process, trial batches were prepared with different concentrations. At 15% concentration of stearic acid, the material was discharged as soft rubbery extrudate resulting from the lowering of the viscosity of the mixture at the increased fatty acid level. These extrudates remained soft even after cooling at ambient temperature and were not suitable for milling. At the levels of 12%, 10% and 7% of stearic acid, extrudates were found to be suitable for extrusion without creating any excess friction and forming soft rubbery extrusions. The powder blend extruded smoothly, and the obtained extrudates were sufficiently brittle for milling. However, it was observed that the increase in drug loading was not supported by 7% of the stearic acid and higher amounts of it were required. Both 10% and 12% concentrations of stearic acid underwent extrusion at 110 ± 2 °C, however considering the objective of high drug loading, 10% stearic acid concentration was fixed for further study.
3.1.2. Influence of drug loading
MG was performed by varying drug loading (50–85%) to investigate the feasibility of loading higher concentrations of the drug. The formulation containing 85% drug load resulted in excessive friction between the screw and wall of the barrel, and the powder blend was not discharged. The extrudability of the material is generally characterized by extrusion torque during the process, the material output rate and the physical appearance of the extrudes. Initial processability trials in the absences of a plasticizer with 50% drug loading resulted in charring of HPC Nisso-H in physical mixture due to the long residence time and exposure to heat. This trial indicated that melt granulation with HPC Nisso-H leads to poor extrudability and hence mandates the use of a plasticizer for ease of extrusion purpose. The flow properties of the optimized formulation are presented in Table 4. The angle of repose of the melt granulated formulation (F6) showed excellent flowability (25 < angle of repose < 30). Further, Carr's index and Hausner's ratio of melt granulated formulation (F6) are between 10.0 and 18.0% and less than 1.25 respectively, confirming the good flow of granules. It indicates that the addition of stearic acid not only served as a thermal lubricant, but also improved the flow property of the granules, thereby avoiding capping and sticking problems. This obviated the need for further lubrication of granules with magnesium stearate or talc and helped to reduce tablet weight further.
Table 4.
Micromeritics of the pre-compression blend prepared by different granulation processes with HPC Nisso-H.
| Properties | Metformin HCl | F6 | F9 | F10 |
|---|---|---|---|---|
| Angle of repose (o) | Very very poor | 23.91 ± 0.61 | 38.89 ± 0.42 | 32.65 ± 0.58 |
| Bulk density (g/ml) | 0.4545 ± 0.082 | 0.4376 ± 0.012 | 0.4219 ± 0.029 | 0.4444 ± 0.020 |
| Tapped density (g/ml) | 0.6667 ± 0.031 | 0.5217 ± 0.017 | 0.5732 ± 0.021 | 0.5882 ± 0.038 |
| Carr's index | 31.82 ± 9.15 | 16.11 ± 0.434 | 26.45 ± 2.366 | 24.38 ± 1.488 |
| Hausner ratio | 1.49 ± 0.204 | 1.19 ± 0.006 | 1.36 ± 0.044 | 1.32 ± 0.026 |
The effect of drug loading on tablet properties is depicted in Table 5. The data demonstrate that tablets of all batches with successive increase in drug loading had friability and weight variation within acceptable limits and a tablet hardness range of 14–17 kN. Overall, tablets with reduced weight and dimensions and with improved hardness and friability have been achievable by performing melt granulation with a single screw extruder at an extruder speed of 50 rpm, and using a combination of HPC Nisso-H with 10% stearic acid at the temperature (110 ± 2 °C). Further, all the formulations processed by melt granulation showed drug content of >96%. This is indicative of a robust formulation and process. The dissolution profile of Metformin HCl from matrices containing varying drug loadings is shown in Fig. 1B. As seen in Fig. 1B, the drug release profiles were greatly influenced by the amount of drug loading. By simply combining a water-insoluble retardant like stearic acid and a water-soluble polymer, HPC Nisso-H, the extent of release retardation was modified. Interestingly, as seen in Fig. 1B, the dissolution profile at different levels of drug loading showed that loading up to a level of 80% was feasible and gave release profiles within USP limits. However, at the level of 80%, the drug release profile was found to be on the higher range of the USP limit. At a 75% w/w drug loading it can be reasonablyexpected that numerous and large channels will rapidly develop as the matrix tablet hydrates. If there is a sufficient polymer within the matrix to maintain structural integrity and prevent tablet disintegration, then rapid drug dissolution and diffusion of the Metformin HCl can be overcome. It may, however, be possible that when used in combination, HPC Nisso-H might have swollen within the porous shell of stearic acid, thereby creating a more effective barrier for drug release. In addition, it has been also reported that melt extrusion resulted in a lower free-volume, a small pore radius, and a more tortuous pore network of the extrudates, thus reducing the drug release rates [8], [19]. On the basis of these data, 75% drug loading with 10% stearic acid and 15% HPC Nisso-H as a binder was chosen to minimize the overall tablet size while still achieving acceptably robust tableting properties.
Table 5.
Properties of tablets produced by the melt granulation process with various drug loadings.
| Formulation code | Tablet properties | |||||||
|---|---|---|---|---|---|---|---|---|
| Weight (mg)a | Dimensions (L × B × T) (mm)b | Hardness (kN)b | Friability (%)b | Assay (%)b | Moisture contentb (%) | |||
| L | B | T | ||||||
| F1 | 1015.8 ± 2.65 | 17.71 ± 0.01 | 9.51 ± 0.02 | 6.62 ± 0.01 | 16.53 ± 0.26 | 0.32 ± 0.31 | 97.22 ± 1.89 | 0.572 ± 0.06 |
| F2 | 920.34 ± 2.46 | 16.84 ± 0.03 | 8.94 ± 0.02 | 7.23 ± 0.02 | 14.97 ± 0.58 | 0.38 ± 0.26 | 99.63 ± 0.61 | 0.688 ± 0.01 |
| F3 | 844.17 ± 3.39 | 16.88 ± 0.01 | 9.07 ± 0.03 | 6.92 ± 0.02 | 15.08 ± 0.65 | 0.27 ± 0.21 | 98.93 ± 0.47 | 0.739 ± 0.02 |
| F4 | 779.77 ± 3.47 | 16.90 ± 0.03 | 8.97 ± 0.02 | 5.99 ± 0.04 | 15.54 ± 0.36 | 0.39 ± 0.36 | 96.32 ± 1.12 | 0.489 ± 0.03 |
| F5 | 732.06 ± 5.63 | 16.89 ± 0.03 | 8.96 ± 0.03 | 5.46 ± 0.02 | 16.42 ± 0.40 | 0.28 ± 0.18 | 97.38 ± 0.71 | 0.871 ± 0.07 |
| F6 | 669.93 ± 2.48 | 16.91 ± 0.02 | 8.95 ± 0.05 | 5.20 ± 0.02 | 15.51 ± 0.39 | 0.23 ± 0.29 | 99.09 ± 1.07 | 0.792 ± 0.03 |
| F7 | 635.15 ± 3.27 | 16.90 ± 0.02 | 8.99 ± 0.01 | 4.84 ± 0.04 | 14.48 ± 0.97 | 0.28 ± 0.21 | 98.93 ± 1.33 | 0.972 ± 0.05 |
| F9 | 680.65 ± 7.79 | 16.96 ± 0.01 | 8.97 ± 0.01 | 5.24 ± 0.01 | 7.46 ± 0.28 | 1.85 ± 1.31 | 99.17 ± 1.58 | 1.894 ± 0.13 |
| F10 | 679.41 ± 7.20 | 16.96 ± 0.01 | 9.02 ± 0.02 | 5.19 ± 0.02 | 12.95 ± 0.34 | 0.81 ± 1.14 | 97.23 ± 1.36 | 2.172 ± 0.31 |
| Marketed tablet | 753.80 ± 5.21 | 16.09 ± 0.03 | 8.10 ± 0.04 | 6.45 ± 0.02 | 14.02 ± 0.16 | 0.17 ± 0.11 | 97.31 ± 1.19 | – |
± Standard deviation, n = 20.
± Standard deviation, n = 10.
3.1.3. Effect of method of preparation
At higher drug loading (75%), the method of granulation has an effect on granule flowability and compactability, which further affects the die filling and tablet mechanical characteristics. Hence, the micromeritic properties of granules were evaluated and presented in Table 4. The flow of granules prepared by DG (F9) and WG (F10) was poor even in the presence of stearic acid, and hence additional lubrication was employed by the addition of magnesium stearate and talc. On this basis, it can be concluded that MG (F6) showed improved flow properties and compressibility in comparison with DG (F9) and WG (F10). The dissolution profiles of tablets prepared by wet, dry and melt granulations using Nisso HPC-H can be seen in Fig. 1C. Tablet prepared by WG (F10) and DG (F9) techniques showed 42.19% and 63.12% drug release within one hour respectively. At the end of 5 hours more than 90% of the drug was released from these tablets due to poor matrix integrity and high porosity. It was observed that the conventional wet granulation method was not a robust one at such a high drug loading of Metformin HCl. Granules prepared by wet massing consisted of intact drug particles held together in a sponge-like matrix of the binder of HPC Nisso-H and stearic acid. However, drug loading to a level of 75% resulted in tablets with greater porosity and failed to form a smooth, even surface. This theory confirmed the latter by scanning electron microscopy analysis. Similar observations can be made with tablets prepared by DG technique. Properties of the tablet produced by MG, WG and DG processes are mentioned in Table 5. It indicates that tablets prepared by the MG process are relatively hard with less moisture content. It is clear that MG is superior to WG and DG as it allowed for substantially better compaction profile, friability and dissolution profile. MG was clearly superior to WG and DG. Fig. 1D shows the comparative dissolution profile of optimized (F6) and marketed formulations. The optimized formulation provided the release profile within USP limit and was found to be comparable with that of marketed formulation.
3.2. Kinetic analysis and mechanism of drug release
To interpret the mechanism of drug release from these formulations, the data were subjected to different mathematical models, such as zero-order, First-order, and Higuchi's and Korsmeyer's equations. In Table 6, the kinetic parameters for Metformin HCl release from the matrix tablets are presented. It revealed that in vitro release profiles of drugs from the formulations with successive loading (F1–F7) follow Higuchi's release model as the plots showed high linearity (Rh2: 0.9895–0.9981). Drug release from a matrix tablet containing hydrophilic polymers generally follows diffusion. Diffusion is related to transport of the drug from the dosage matrix into the in vitro dissolution medium depending on the concentration. As the concentration gradient varies, the drug is released, and the distance for diffusion increases. This may possibly explain drug diffusion at a comparatively slower rate as the distance for diffusion increases, which is referred to as square root kinetics or Higuchi's kinetics. To confirm the diffusion mechanism, the data were fitted into Korsmeyer's equation to calculate np values [20]. In our experiments, the formulations (F1–F7) showed np values ranging from 0.553 to 0.431, indicating that the diffusion is the dominant mechanism of drug release from these formulations. Further, np values were decreasing for the successive increase in drug loading, which indicates a direct relationship of np values with the concentration of HPC Nisso-H. For matrix tablets, an np value of near 0.5 indicates diffusion control, and an np value of near 1.0 indicates erosion or relaxation control. Intermediate values suggest that diffusion and erosion contribute to the overall release mechanism [21].
Table 6.
Data from regression fitting between dissolution profiles (obtained from different matrix tablets prepared by different methods and several kinetic models).
| Formulation code | Parameters of model dependent factors | ||||
|---|---|---|---|---|---|
| Zero | First | Higuchi | Korsmeyer–Peppas | ||
| R02 | R12 | Rh2 | Rp2 | np | |
| F1 | 0.8664 | 0.9805 | 0.9953 | 0.9981 | 0.553 |
| F2 | 0.8221 | 0.9799 | 0.9974 | 0.9975 | 0.509 |
| F3 | 0.8025 | 0.9823 | 0.9981 | 0.9982 | 0.492 |
| F4 | 0.7740 | 0.9855 | 0.9968 | 0.9979 | 0.470 |
| F5 | 0.7665 | 0.9893 | 0.9953 | 0.9968 | 0.466 |
| F6 | 0.7337 | 0.989 | 0.992 | 0.9963 | 0.442 |
| F7 | 0.7173 | 0.9873 | 0.9895 | 0.9958 | 0.431 |
| F9 | 0.3825 | 0.9962 | 0.6327 | 0.9756 | 0.376 |
| F10 | 0.3009 | 0.9866 | 0.9326 | 0.9756 | 0.376 |
The np values for F1–F4 are ranged 0.509–0.466, indicating a non-Fickian/anomalous diffusion (0.45 < np < 0.89), and tablets of F5 (np = 0.466), F6 (np = 0.442) and F7 (np = 0.431) follow a Fickian diffusion during the entire period of drug release (10 h). The principle of diffusion is based on Fick's laws, which illustrate the macroscopic transport of molecules by a concentration gradient. Drug release from the matrix tablet generally follows diffusion for water-soluble drugs and erosion or relaxation for water insoluble drugs.
The dissolution model independent factors (MDT, h, and %DE) were calculated by analyzing in vitro dissolution data of all batches and considering the release profile of marketed tablets as reference (Table 7). Dissolution efficiency (%DE) and mean dissolution time (MDT, h) were used as the criteria for comparing the effect of different granulation methods on the drug release profile. %DE values for tablets prepared by MG were remarkably less as compared to tablets prepared by other granulation methods. The %DE and MDT (h) values for F6 (%DE = 66.364, MDT = 2.953 h) formulations were comparable with the reference tablet (%DE = 60.584, MDT = 3.305 h). Hence, by comparing the %DE and MDT of the different formulations, it was clear that drug release was effectively controlled by melt granulation process.
Table 7.
Analysis of in vitro dissolution data (model independent factors).
| Parameter | F6 | F9 | F10 | Reference |
|---|---|---|---|---|
| MDT (h) | 2.953 | 1.135 | 2.025 | 3.305 |
| %DE | 66.364 | 88.910 | 80.238 | 60.584 |
| f1 | 9.369 | 45.183 | 30.764 | – |
| f2 | 61.986 | 27.658 | 36.449 | – |
3.3. Fit factors
The results of similarity (f2) and difference (f1) factors (Table 7) revealed that the drug release profile of tablets prepared by melt granulation method F6 (f1 = 9.369 and f2 = 61.986) was found to be best fitted to the drug release profile of the reference tablet (marketed tablet) compared to tablets prepared by WG and DG.
3.4. Stability study
The optimized formulation was found to be stable after six months as per ICH guidelines with no significant changes in dissolution behavior (Fig. 2), drug content (Table 8) and physical stability.
Fig. 2.
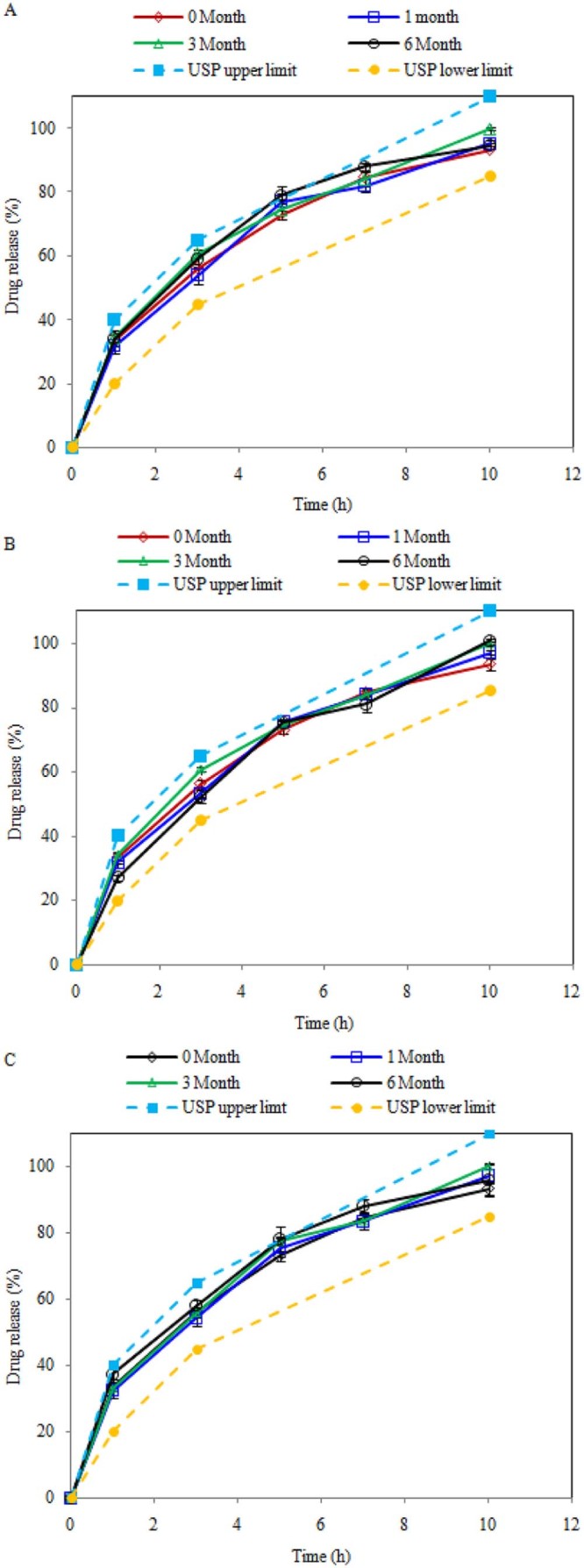
Dissolution profile of Metformin HCl at initial and after 1, 3, 6 months: (A) at 25 °C ± 2 °C/RH 60 ± 5%, (B) at 30 °C ± 2 °C/RH 65 ± 5% and (C) at 40 °C ± 2 °C/RH 75 ± 5%.
Table 8.
Effect of storage on tablet prepared by melt granulation with HPC Nisso-H.
| Parameter | 25 °C/RH 60% | 30 °C/RH 65% | 40 °C/RH 75% |
|---|---|---|---|
| Drug content (%) | 98.23 ± 1.95 | 99.40 + 0.80 | 97.73 ± 1.14 |
| Moisture (%) | 0.99 ± 0.21 | 0.91 ± 0.09 | 1.28 ± 0.18 |
| Hardness(kN) | 14.63 ± 0.61 | 14.93 ± 0.64 | 15.1 ± 0.7 |
| Friability (%) | 0.23 | 0.33 | 0.21 |
3.5. Characterization
3.5.1. Fourier-transform infrared analysis (FTIR)
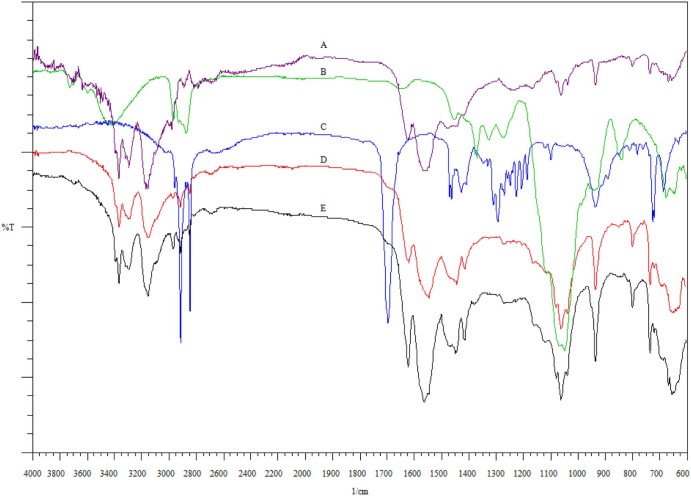
Fig. 3A–C shows the FTIR spectra of pure Metformin HCl, HPC Nisso-H and stearic acid respectively. Metformin HCl (Fig. 3A), being a biguanide, has strong absorption bands at 1634, 1573, and 1562 cm−1 due to the presence of C—N stretching vibrations. It has been reported that C—N stretching of aliphatic diamine is generally weak and occurs in the region of 1220–020 cm−1. Hence, weak intensity bands were found at 1062 and 1242 cm−1. N—H stretching of C—N—H groups occurs in the region of 3400–3100 cm−1. So medium intensity peaks appeared in the region of 3369, 3292, 3169, and 3153 cm−1 respectively because of N—H asymmetric and symmetric stretching vibrations. Fig. 3D and E shows the infrared spectrum of physical mixture and optimized formulation (F6) respectively. Fig. 3E reveals all the characteristic peaks of Metformin HCl, which clearly shows the nonexistence of any chemical interaction between the drug and the other excipients even at elevated operating temperatures during the melt granulation process.
Fig. 3.
FTIR spectrum of (A) Metformin HCl, (B) HPC Nisso-H, (C) stearic acid, (D) physical mixture and (E) melt granulated extrudes.
3.5.2. Differential scanning calorimetry (DSC)
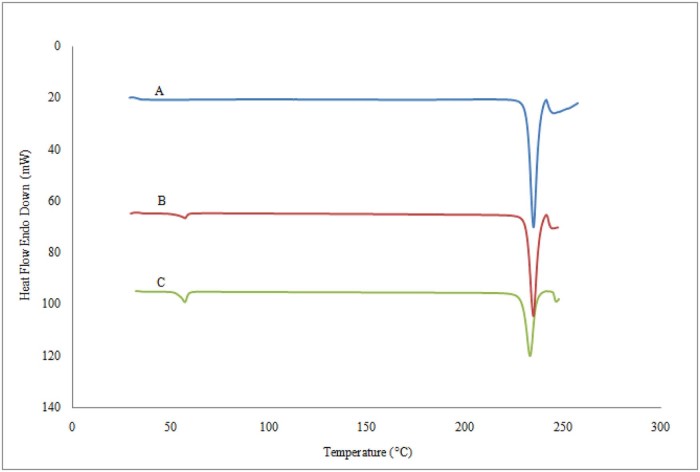
DSC analyses were performed in order to evaluate possible solid-state interactions between the components and, consequently, to assess the actual drug–excipient compatibility in all the examined formulations. As shown in Fig. 4A, the melting point depression of pure Metformin HCl was 234.54 °C. The onset and end of the endothermic peak were observed at 231.28 °C and 237.65 °C respectively. The DSC plot of physical mixture of Metformin HCl, stearic acid and HPC Nisso-H (Fig. 4B) showed two distinct endothermic peaks of Metformin HCl at 233.12 °C and stearic acid at 53.38 °C respectively. The DSC plot of melt granulated extrudes (F6) (Fig. 4C) did not exhibit any significant difference near the melting endotherm of the individual component. The endothermic peak of Metformin HCl (233.73 °C) and stearic acid (56.93 °C) did not show any significant change in melting point, however the peak height of Metformin HCl was observed to reduce which likely reflects the slight melting point depression. This reveals that there was a partial conversion of the crystalline drug into an amorphous form; in other words crystallinity of the drug reduced, but still the presence of the crystalline drug after MG resulted in a two-phase solid dispersion in which Metformin HCl was present in both amorphous and crystalline forms, but no evidence of any other physicochemical interaction was observed. However, there is no conversion of crystalline drug substance to amorphous form [22], [23].
Fig. 4.
Differential scanning calorimetry thermograms of (A) pure Metformin HCl, (B) physical mixtures and (C) melt granulated powdered granules.
3.5.3. Powder X-ray diffraction studies (PXRD)
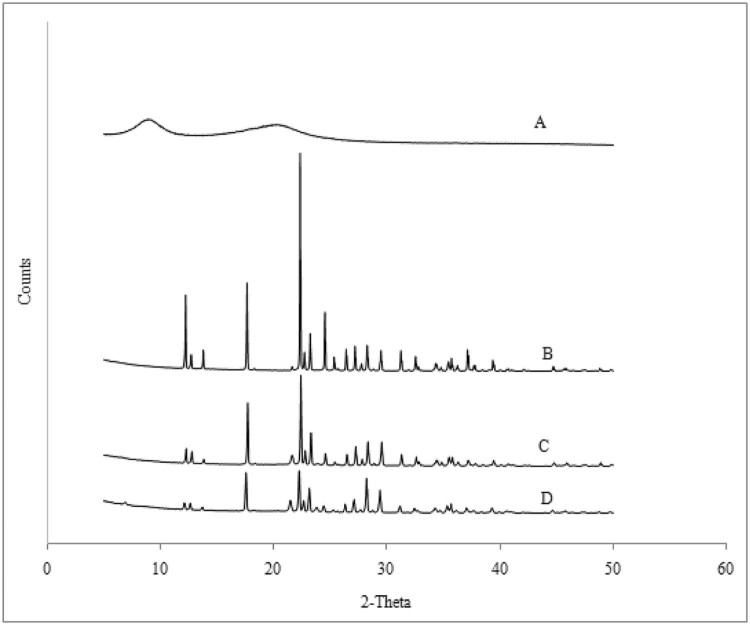
The X-ray diffraction patterns of pure Metformin HCl, HPC Nisso-H, physical mixture and powdered extrudes are shown in Fig. 5. The XRD of plain HPC Nisso-H showed (Fig. 5A) an amorphous state as indicated by the presence of a background bump in the powder X-ray diffraction (PXRD). Background bump is often indicative of the non-crystalline composition of a material, whereas peak position describes the phases present in the sample. Pure Metformin HCl (Fig. 5B) showed peaks at 2θ values of 6.94, 5.01, 3.96, 3.82, and 3.50, signifying the presence of a highly crystalline nature, which is in good agreement with literature values. The crystallinity of Metformin HCl was found to progressively decrease from pure Metformin HCl, physical mixture (Fig. 5C) to melt granulated extrudates (Fig. 5D) indicated by a low peak intensity. This reveals that the melt granulation process decreased the crystallinity of Metformin HCl in the powdered extrudates as an effect of high shear mixing of melted physical mixture with amorphous HPC Nisso-H, resulting in improved compressibility even at such a high drug loading.
Fig. 5.
X-ray diffraction profiles of (A) HPC Nisso-H, (B) Metformin HCl, (C) physical mixture and (D) melt granulated extrudes.
3.5.4. Scanning electron microscopy
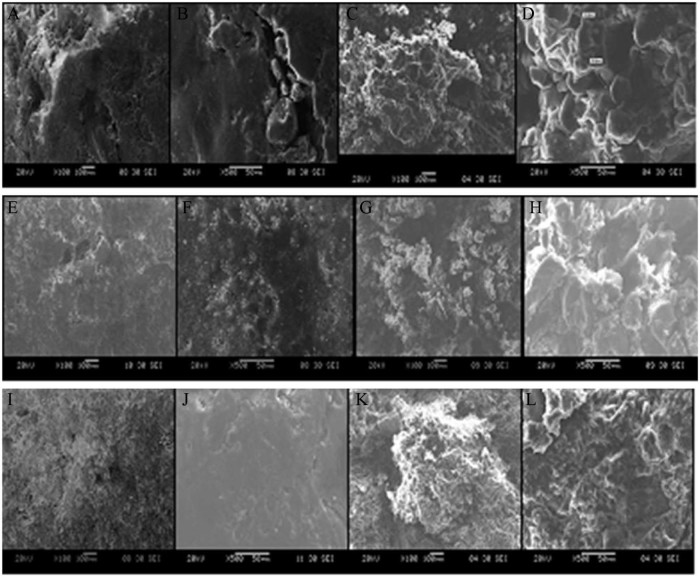
The present study of scanning electron microscopy images revealed microstructures of the surfaces and interiors of a sustained release tablet of Metformin HCl prepared by three different granulation techniques. It was observed that tablets prepared by DG and WG processes failed to produce a smooth surface area at such high drug loading (75%). This can be elucidated from the uneven surface and cracks present on the surfaces of the tablet (Fig. 6A, B, E, F). Also, a cross sectional view of these tablets showed a sufficiently high porous network with large void spaces (Fig. 6C, D, G, H). In contrast, tablets prepared by MG (Fig. 6I, J, K, L) with a single screw extruder possess a densified material with improved compressibility of the drug, which resulted in a tablet with a smooth surface area and less void spaces. On the basis of these observations, it can be concluded that at high drug loading due to the compressibility issue of Metformin HCl, tablets prepared with DG or WG were unable to maintain their integrity for a long period of time and failed to retard the drug profile.
Fig. 6.
Dry granulation surface area: (A) at 100×, (B) at 500×, and cross sectional area: (C) at 100×, (D) at 500×. Wet granulation surface area: (E) at 100×, (F) at 500×, and cross sectional area: (G) at 100×, (H) at 500×. Melt granulation surface area: (I) at 100×, (J) at 500×, and cross sectional area: (K) at 100×, (L) at 500×.
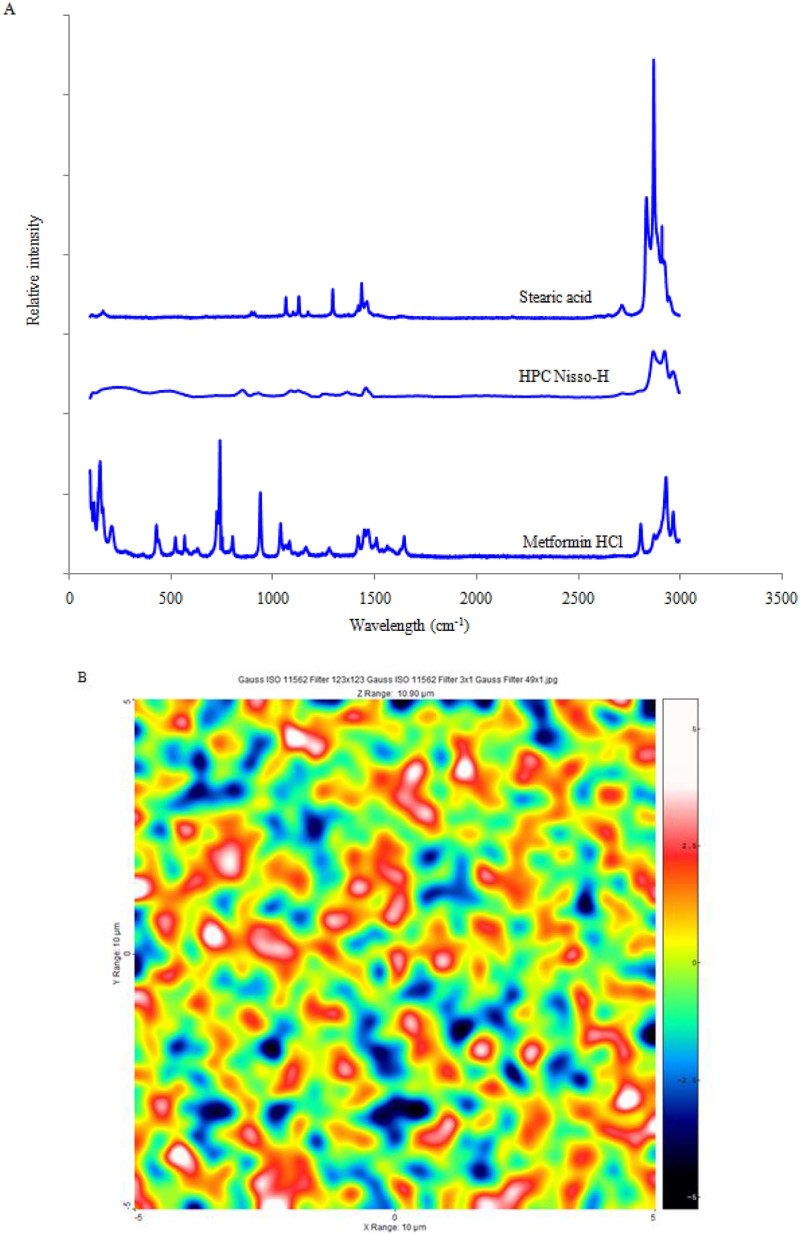
3.5.5. Raman spectral analysis
In the present study, Raman spectroscopy was applied to evaluate the drug distribution in the matrix tablet. Fig. 7A illustrates the Raman spectra of individual components of optimized formulation. The spectrum of Metformin HCL displayed intense bands at 425 cm−1, 739 cm−1and 938 cm−1, whereas stearic acid and HPC Nisso-H had a different spectral profile from that of Metformin HCl. These peaks of Metformin HCl were monitored to map the distribution of the drug in the matrix because there were no spectral interferences from the other excipients in this region. Fig. 7B displays their concentration distribution, wherein the more reddish areas correspond to higher concentration and the bluish color signifies lower concentration. Interestingly, the concentration map of the melt granulation processed tablet showed better uniformity and distribution of the tablet's components, suggesting very high mixing efficiency at such a high drug loading. In addition, Raman spectroscopy confirmed the existence of crystalline Metformin HCl in the matrix, and such an intimate distribution of the drug and excipients appears to be responsible for better compactibility and sustained release profile of Metformin HCl.
Fig. 7.
(A) Raman spectra of pure reference materials: Metformin HCl, HPC Nisso-H, stearic acid and (B) distribution mapping of the melt granulation tablet of optimized formulation.
4. Conclusion
The present study showed that reduction in tablet weight can be achieved by higher drug loading (75%) to prepare Metformin HCl 670 mg sustained release tablets. Such a formulation will lead to improved patience compliance due to ease of swallowing. Combination of cellulose based hydrophilic binder with hydrophobic excipient can be successfully employed to formulate a sustained release matrix tablet of Metformin HCl using MG technology. Addition of stearic acid lubricates the extrusion screw, thereby decreasing the pressure on the machine. Additionally, intense mixing of stearic acid with HPC Nisso-H and the drug resulted in effective lubrication of the blend thereby ruling out the need for other excipients to lubricate the granules or enhance compactibility of the drug substance. Moreover, the MG process decreases the number of the processing steps as granules obtained after melt granulation were subjected to only milling before compression. This can substantially reduce the manufacturing cost of tablets due to the use of lesser excipients and unit operations. In addition, the superiority of MG technology with enhanced sustained effect was also apparent from its comparison with the conventional granulation methods with equivalent composition. In light of the above discussion, MG technology holds significant potential as a continuous granulation process to develop a sustained release system for highly water soluble drugs with minimal use of excipients.
Acknowledgement
The authors acknowledge the financial support received from University Grants Commission (UGC), for the support and encouragement in carrying out this work.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Furlanetto S., Cirri M., Maestrelli F. Study of formulation variables influencing the drug release rate from matrix tablets by experimental design. Eur J Pharm Biopharm. 2006;62:77–84. doi: 10.1016/j.ejpb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y.B., Tsai Y.H., Yang W.C. Once daily propranolol extended-release tablet dosage form: formulation design and in vitro/in vivo investigation. Eur J Pharm Biopharm. 2004;58:607–614. doi: 10.1016/j.ejpb.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y.B., Tsai Y.H., Lee S.H. Optimization of pH dependent release of nicardipine hydrochloride extended release matrix tablets using response surface methodology. Int J Pharm. 2005;289:87–95. doi: 10.1016/j.ijpharm.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Siepe S., Lueckel B., Kramer A. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int J Pharm. 2006;316:14–20. doi: 10.1016/j.ijpharm.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Sung K.C., Nixon P.R., Skoug J.W. Effect of formulation variables on drug and polymer release from HPMC-based matrix tablets. Int J Pharm. 1996;142:53–60. [Google Scholar]
- 6.Bika D., Tardos G.I., Panmai S. Strength and morphology of solid bridges in dry granules of pharmaceutical powders. Adv Powder Technol. 2005;150:104–116. [Google Scholar]
- 7.Billings S.W., Bronlund J.E., Paterson A.H.J. Effects of capillary condensation on the caking of bulk sucrose. J Food Process Eng. 2006;77:887–895. [Google Scholar]
- 8.Crowley M., Schroeder B., Fredersdorf A. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004;269:509–522. doi: 10.1016/j.ijpharm.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Dalziel G., Nauka E., Zhang F. Assessment of granulation technologies for an API with poor physical properties. Drug Dev Ind Pharm. 2012:1–11. doi: 10.3109/03639045.2012.687744. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Lorenzo C., Go'mez-Amoza J.L., Martı'nez-Pacheco R. The stability of theophylline tablets with a hydroxypropylcellulose matrix. Drug Dev Ind Pharm. 2000;26:13–20. doi: 10.1081/ddc-100100322. [DOI] [PubMed] [Google Scholar]
- 11.Repka M.A., Gerding T.G., Repka S.L. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev Ind Pharm. 1999;25:625–633. doi: 10.1081/ddc-100102218. [DOI] [PubMed] [Google Scholar]
- 12.Repka M.A., McGinity J.W. Influence of chlorpheniramine maleate on topical hydroxypropylcellulose films produced by hot-melt extrusion. Pharm Dev Technol. 2001;6:297–304. doi: 10.1081/pdt-100002610. [DOI] [PubMed] [Google Scholar]
- 13.Quinten T., Gonnissen Y., Adriaens E. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur J Pharm Sci. 2009;37:207–216. doi: 10.1016/j.ejps.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Siepmann J., Peppas N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48:139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 15.Adeleke O.A., Choonara Y.E., Kumar P. Evaluation of the impacts of formulation variables and excipients on the drug release dynamics of a polyamide 6,10-based monolithic matrix using mathematical tools. AAPS PharmSciTech. 2013;14:1349–1359. doi: 10.1208/s12249-013-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson N.H., Bauer M., Boussac N. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J Pharm Biomed Anal. 1998;17:811–822. doi: 10.1016/s0731-7085(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 17.Khan K.A. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 18.Shah V.P., Tsong Y., Sathe P. In vitro dissolution profile comparison statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15:889–896. doi: 10.1023/a:1011976615750. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y.E., Tchao R., Schwartz J.B. Effect of processing methods and heat treatment on the formation of wax matrix tablets for sustained drug release. Pharm Dev Technol. 2001;6:131–144. doi: 10.1081/pdt-100000736. [DOI] [PubMed] [Google Scholar]
- 20.Korsmeyer R.W., Gurny R., Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. [Google Scholar]
- 21.Peppas N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–111. [PubMed] [Google Scholar]
- 22.Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm. 2002;54:107–117. doi: 10.1016/s0939-6411(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 23.Crowley M.M., Zhang F., Repka M.A. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;339:909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]