Graphical Abstract
Through formulation screening, the irregular-shaped azilsartan nanocrystals were prepared and optimized by bead milling method and solidified by freeze-drying. The best formulation which had the size of 300 nm was expressed in its state by the studies of morphology and crystal from. And azilsartan nanocrystals showed its improvement about in-vitro dissolution and in-vivo bioavailability with physical mixture as reference.
Keywords: Azilsartan, Nanocrystals, Sodium deoxycholate, Freeze drying, Dissolution, Oral bioavailability
Abstract
Azilsartan (AZL), a poorly soluble drug, was considered to be fit for nanocrystals to improve its solubility. Our study intended to prepare AZL nanocrystals by means of bead milling method. Eight stabilizers or their binary combination and the milling time were set to be variable factors to optimize AZL nanosuspension formulation, and six types of freeze-drying supports were investigated to reduce the aggregation of particles during the solidification. AZL nanocrystals with or without sodium deoxycholate (NaDC) as combined stabilizer with Poloxamer 188 (F68) were prepared owning mean particle sizes of about 300 nm and 460 nm. During the screening processes, the formulation containing NaDC showed a smaller particle size and better stability during lyophilization. The irregular shape and crystal form changing in AZL nanocrystals were discovered by various characterizations. And with physical mixture as reference, nanocrystals showed its improvement about in-vitro dissolution and in-vivo bioavailability. In conclusion, the nanocrystals of AZL could be prepared well in our study. Additionally, our results suggested that NaDC was an appreciated excipient on the nanocrystals platform, which can exhibit the abilities of size-reduction and stability-maintaining on freeze-drying.
1. Introduction
Nanocrystals, with surfactants or polymers as stabilizers [1], are becoming an effective strategy to improve the dissolution rate of poorly soluble drugs [2], [3]. By decreasing the particle size of drug to the scale of 1–1000 nm, this colloidal dispersion system would increase the drug surface area till a better dissolution. Top-down [4], [5] and bottom-up [6] were two methods used in preparing the nanocrystals. In our case, bead milling [7] technology was applied to nanosuspension preparation, which belongs to top-down. Subsequently, nanocrystal powders for characterization were obtained by freeze-drying process. Besides for improvement of dissolution, nanocrystals also could enhance the drug bioavailability of bioavailability classification system (BCS) II or IV. It may play its adhesion properties to surfaces in gastrointestinal tract (GIT), which leads to a prolonged residence and contact time in the GIT [8].
Azilsartan (AZL) is a potent and highly selective angiotensin II receptor blocker (ARB) being developed for hypertension treatment [9]. AZL was approved for marketing in Jan 2012 in Japan, and it was the active moiety of azilsartan medoxomil, which was a prodrug [10] that was explored systematically. AZL is a typical BCS II drug, which has low aqueous solubility and high permeability, and it is suitable to be a model drug for nanocrystals in our study.
The aim of this study is to prepare and characterize AZL nanocrystals for oral administration. We concentrated on controlling the particle size and polydispersity index (PDI) during the preparation of nanosuspension and lyophilization. And the emphasis of optimizing formulation was the combination of stabilizers, which helps in reducing particle size and assisting in keeping stability on freeze-drying. Morphology and crystal form of nanocrystals were evaluated and confirmed by transmission electron microscope (TEM), scanning electron microscope (SEM), X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC). Moreover, the in-vivo pharmacokinetics, relative bioavailability and in-vitro dissolution behaviors were investigated, compared with the physical mixture.
2. Materials and methods
2.1. Materials
AZL was purchased from Shandong Weigao Co., Ltd. (Weigao, China). Tween 80, sodium dodecyl sulfate (SDS), sucrose and glucose were purchased from Tianjin Bodi Chemical Holding Co., Ltd. (Tianjin, China). Sodium deoxycholate (NaDC) was purchased from Beijing Aoboxing Biotech Co., Ltd. (Beijing, China). PVP-K30, HPMC-E5 and HPMC-E50 were purchased from Anhui Sunhere Pharmaceutical Excipients Co., Ltd. (Huainan, China). Poloxamer 188 (F68) was gifted by BASF Co., Ltd. (Shanghai, China). TPGS was purchased from Wuhan GBD Pharm Chemical Co., Ltd. (Wuhan, China). Mannitol and lactose were purchased from Beijing Fengli Jingqiu Commerce and Trade Co., Ltd. (Beijing, China). Sorbitol was purchased from Sigma-Aldrich Co., Ltd. (Beijing, China). Maltose was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). Methanol and acetonitrile were purchased from Concord Technology Co., Ltd. (Tianjin, China). All other reagents used in this study were reagent grade.
2.2. Preparation of AZL nanosuspension
A planetary mill (Nanjing Shunchi Technology Development Co., Ltd., China) was used for preparing AZL nanosuspension. One kind of stabilizers or their combination was diluted with pure water totally. Then, AZL bulk drug was added into the aqueous solution of stabilizers with stirring (5%, w/v). Finally, the well mixed suspension was transferred into the mill and processed with 0.2 mm grinding beads.
In our study, we have investigated the eight kinds of stabilizers. Among them, SDS and NaDC were ionic surfactants, and the others were nonionic surfactants or polymers (PVP-K30, F68, Tween 80, TPGS, HPMC-E5 and HPMC-E50), which mainly play roles of electrostatic stabilization and steric stabilization respectively. Milling time was also studied as a technological factor.
2.3. Freeze-drying
Freeze-drying was applied to the solidification of nanosuspension using LAM-0.5F vacuum freeze dryer (Shinva Medical Devices Ltd., China). In our study, six kinds of sugars or polyols (glucose, maltose, sucrose, lactose, mannitol and sorbitol) as freeze-drying supports were added into the nanosuspension. And the freeze-drying process was as follows: first, the samples were further freeze-dried at −40 °C for 8 h. Then, under the vacuum, samples were lyophilized at a temperature of −20 °C for 6 h and −2 °C for 6 h, in sequence. Finally, the temperature returned to 25 °C for 6 h, then back to atmospheric pressure.
2.4. Characterization
2.4.1. Particle size and zeta potential
Particle size and zeta potential were determined using a Zetasizer Nano ZS90 instrument (Malvern Co., UK), which has a dynamic light scattering system. Before measurement, the nanosuspension was diluted in 500-fold, and similarly, about 30 mg freeze-drying nanocrystals was dissolved into 8 ml of deionized water and shaked well.
2.4.2. Microscopical observation
YS2-H microscope (Nikon Corporation, China) was used to observe the holistic morphology of the samples, and the magnification of 400-fold was chosen.
JEM-2100 transmission electron microscope (TEM) (Japan Electron Optics laboratory Co., Ltd., Japan) and SU8010N scanning electron microscope (SEM) (Hitachi High-Technologies Co., Japan) were respectively used to observe the partial individual morphology of the nanosuspension and freeze-drying of nanocrystal samples.
2.4.3. Differential scanning calorimeter (DSC) and X-ray powder diffraction (XRPD)
XRPD was measured for AZL bulk drug, freeze-drying nanocrystals, blank excipients and physical mixture of freeze-drying powders using a D\Max-2400 X-ray instrument (Rigaku Corporation, Japan), which was operated at a voltage of 40 kV. Samples were scanned from 3° to 50° (2θ) with a step size of 0.04°/min.
DSC experiments were performed using a DSC 1 (Mettler-Toledo International Inc., Switzerland). Aluminum oxide was used as a reference standard, and samples (about 3 mg) were accurately weighed and encapsulated in aluminum pans. Besides the same samples of XRPD measurement, the air-drying nanocrystals were also heated from 30 °C to 240 °C at a heating rate of 10 °C/min under nitrogen atmosphere.
2.4.4. Dissolution tests
Dissolution study was performed for the freeze-drying nanocrystals and the physical mixture of freeze-drying powders. According to the USP Apparatus II method using RC806D dissolution tester (Tianda Tianfa Technology Co., Ltd., China), the paddle rotation speed was set at 50 rpm. Samples (lyophilized powders or physical mixture powders), equivalent to 40 mg of AZL, were weighed accurately and filled into 2# gelatin capsules which were placed into sinkers. The dissolution tests were performed at 37 °C in 900 ml of phosphate buffers (pH 1.0, pH 4.5, pH 5.4, pH 6.0 and pH 6.8) and water. 10 ml of sample solutions was collected at 5, 10, 15, 20, 30, 45 and 60 min, and replaced with the same fresh medium. The collections of subsequent filtrate through 0.45 µm filter were measured at 249 nm using UV1102 ultraviolet spectrophotometer (Shanghai Techcomp Instrument Ltd., china). Each experiment was conducted in triplicate.
2.5. Pharmacokinetic study
Twelve SD rats (about 220 g) were supplied by the animal center of Shenyang Pharmaceutical University (Shenyang, China). All animal experiments were performed according to the guidelines for Animal Experimentation of Shenyang Pharmaceutical University. Before oral administration, rats were fasted for 12 h and provided water ad libitum. They were divided into two groups equally. One group received AZL nanosuspension and the other group received the physical mixture suspended in water of 0.5% sodium carboxymethylcellulose (CMC-Na). After administration, plasma samples were collected at different intervals (5 min, 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h and 72 h, n = 6). We got the supernatant plasma samples that were centrifuged at 13,000 rpm for 10 min. All the samples were stored at −20 °C until analysis.
Concentration of AZL in plasma was determined by HPLC. The analysis was carried out on a Waters HPLC 2695 system (Waters Technology Co., Ltd., China) with a Unitary-C18 column (250 mm × 4.6 mm, i.d. 5.0 µm). The mobile phase was consisted of water containing 0.1% glacial acetic acid and acetonitrile at a ratio of 50:50 (v/v). The injection volume was 20 µl and the column temperature was maintained at 35 °C. UV wavelength was 249 nm and rate was set to 1.0 ml/min. The internal standard (IS) solution was 1 µl/ml of diazepam. 100 µl treated plasma sample, 100 µl IS solution and 100 µl mobile phase were mixed. Then, the mixture was vortexed for 3 min, added with 400 µl methanol, vortexed for 3 min and centrifuged at 13,000 rpm for 10 min. The supernatant was removed. The extract was dried under nitrogen flow, redissolved in 100 µl mobile phase and finally injected for the analysis.
3. Results and discussion
3.1. Effects of stabilizers
3.1.1. Effects of single stabilizer
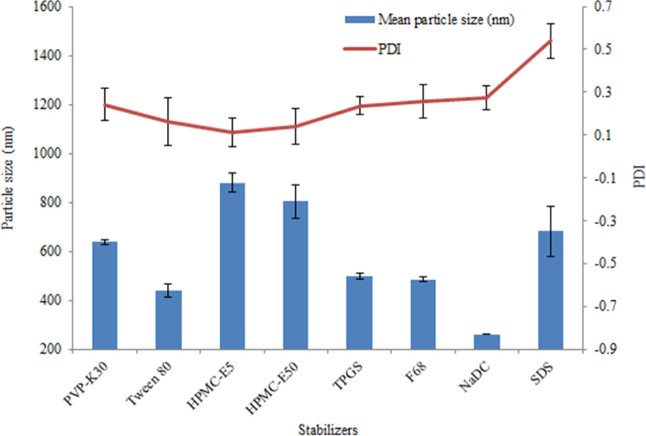
With bead milling method, eight kinds of stabilizers were investigated in our study. The mean particle size and PDI of nanosuspension were shown in Fig. 1. From the PDI values, SDS was removed because of being over 0.3, and from the size, HPMC-E5 and HPMC-E50 were also given up due to being over 700 nm. Through the microscope observation and the size distribution by intensity, we found that the nanosuspension stabilized by PVP-K30, Tween 80 or TPGS had big particles. Even the formulation of NaDC showed the small size of about 260 nm, but it had an unsatisfactory stability. All in all, F68 was considered as a suitable stabilizer with a mean particle size of 485.3 nm and PDI value of 0.258.
Fig. 1.
Mean particle size and PDI of AZL nanosuspension stabilized by single stabilizer.
3.1.2. Effects of combined stabilizers
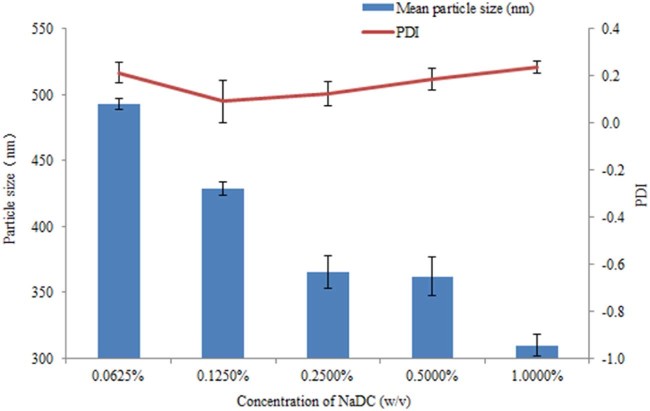
We have known that the combination of electrostatic stabilization and steric stabilization mechanisms can improve the stabilization of the nanosuspension [8]. Therefore we tried to unite the F68 and NaDC as combined stabilizers to prepare nanosuspension. We set the concentration of F68 as 1.250% (w/v). To avoid the Ostwald ripening, the quantity of NaDC cannot match F68 [11]. Concentrations of 1.0000%, 0.5000%, 0.2500%, 0.1250% and 0.0625% (w/v) NaDC were separately combined with F68 as stabilizers, and the results were shown in Fig. 2. We can see from the consequence that the particle size got lower with the increase of NaDC. The extreme quantities in our study of NaDC both cannot remain its stabilization, as the 1.0000% and 0.0625% (w/v) of NaDC had higher PDI values. From the size distribution by intensity, we found that the 0.2500% (w/v) of NaDC has big particles. The formulation of 0.50% (w/v) NaDC and 1.25% (w/v) F68 has a unimodal particle size distribution of about 360 nm, which was chosen to be a candidate of combined stabilizers.
Fig. 2.
Mean particle size and PDI of AZL nanosuspension stabilized by 1.250% F68 united with different concentration of NaDC.
3.2. Effects of milling time
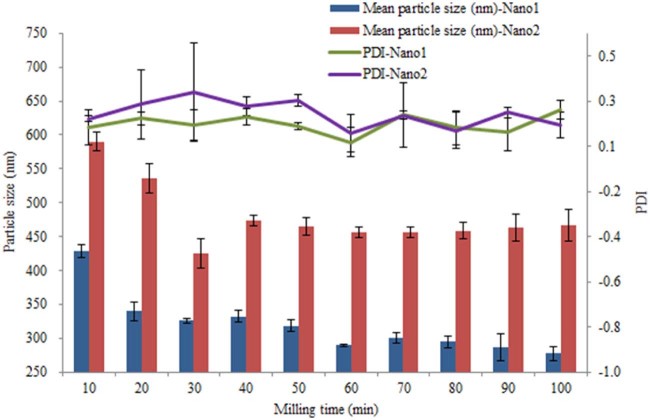
After screening the stabilizers, we got two formulations. The difference between the two was whether NaDC was added with F68 as stabilizers. The formulation of combined stabilizers was named Nano1, and the simple formulation was named Nano2. The bead milling time was another important factor to influence the quality of preparation. We collected samples while milling time was 10 min, 20 min, 30 min, 40 min, 50 min, 60 min, 70 min, 80 min, 90 min and 100 min. Then, we measured their mean particle size and PDI (Fig. 3). From the results, 60 min has been selected to be an appropriate milling time to get two formulations of Nano1 and Nano2.
Fig. 3.
Influence of the milling time on the mean particle size and PDI of Nano1 and Nano2.
3.3. Effects of freeze-drying
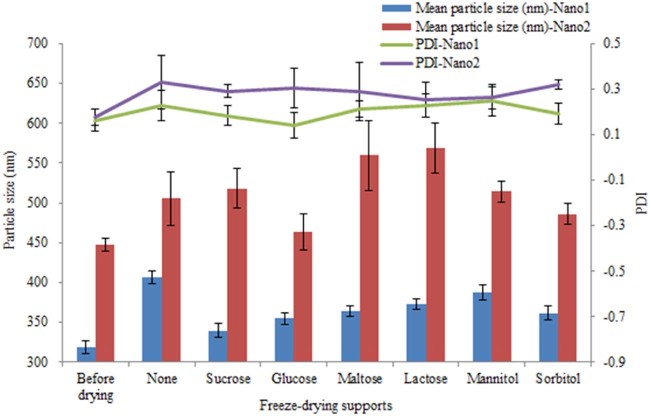
Six types of sugars and polyols were studied as freeze-drying supports (3%, w/v). The redispersed mean particle sizes and PDIs of nanocrystals before and after lyophilization were determined without or with different freeze-drying supports (Fig. 4). For particle size, it was obvious that the sizes of Nano1 with supports were less than without, which was not parallel with the consequences of Nano2. That means, for Nano1, all the supports mentioned helped to reduce the aggregation of particles during the solidification.
Fig. 4.
Influence of the different freeze-drying supporters on the mean particle size and PDI of Nano1 and Nano2.
We can also see that whether freeze-drying or not, and whether adding freeze-drying supports or not, the PDIs of Nano1 were lower than Nano2. And most of the PDIs of Nano2 were over 0.3, which means these particles of Nano2 hardly maintained their stabilization.
Apparently, sucrose was the most effective freeze-drying support from Fig. 4, which had a redispersed mean particle size of 339.6 nm and PDI of 0.183 of Nano1. Meanwhile, for Nano2, the above lyophilization process and supports cannot help to produce a fine freeze-drying powder of nanocrystals.
3.4. Roles of sodium deoxycholate (NaDC) in nanocrystals
Sodium deoxycholate (NaDC) was a kind of bile salt ionic surfactant [12], [13], which had the capacities of improving solubility [14], stability [15] and bioavailability [16] of drug, and keeping the stability of drug after reconstitution from a lyophilized dry powder [17]. In addition, as an adsorption promoter [18], NaDC could increase drug adsorption capacity of the membrane. In our case, firstly, it can help decrease the particle size of nanosuspension dramatically from Fig. 2. Then, during the lyophilization process, the formulation stabilized by F68 and NaDC (Nano1) displayed superior effects on maintaining an appropriate particle size and PDI. So the roles of sodium deoxycholate in AZL nanocrystals were demonstrated as a size-reducer and a stability-assistant on freeze-drying.
3.5. Particle size and zeta potential
According to the formulation screening processes previously studied, we choose the Nano1 to characterize the properties of nanocrystals. Before and after lyophilization, the mean particle sizes and zeta potentials were listed in Table 1. The freeze-drying nanocrystal powders obtained were loose, and it could be redispersed without remarkable distinguishability in size, PDI and zeta potential. And the values of zeta potential displayed that the formulation may have a pretty nice physical stabilization.
Table 1.
Comparison of mean particle size, PDI and zeta potential before and after lyophilization of Nano1 (mean ± SD, n = 3).
| Formulation | Before lyophilization | After lyophilization | ||||
|---|---|---|---|---|---|---|
| Mean particle size (nm) | PDI | Zeta potential (mV) | Mean particle size (nm) | PDI | Zeta potential (mV) | |
| Nano1 | 305.7 ± 9.47 | 0.126 ± 0.046 | −22.2 ± 0.66 | 314.7 ± 10.69 | 0.200 ± 0.026 | −23.1 ± 0.79 |
3.6. Morphology and crystal form of nanocrystals
The results of XRPD and DSC were shown in Fig. 5 and Fig. 6. In Fig. 5, we can see that AZL bulk drug plus blank excipients made physical mixture, which was different from the nanocrystals. Obviously, there were two sharp peaks at 38.70° and 47.13° in the nanocrystals pattern. And in Fig. 6, the two thermograms of nanocrystals were also totally dissimilar from the physical mixture and bulk drug. The comparison of two thermograms of nanocrystals (d and e) showed that the peak of 190 °C was the true peak of nanocrystals, while the freeze-drying nanocrystals' peak of 220 °C was the outcome of the support of sucrose.
Fig. 5.
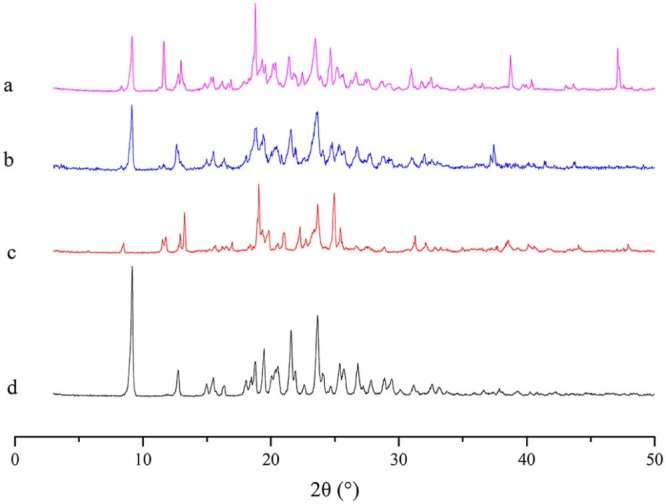
PXRD patterns for freeze-drying AZL nanocrystals (a), physical mixture of freeze-drying powder (b), blank excipients of freeze-drying powder (c) and AZL bulk drug (d).
Fig. 6.
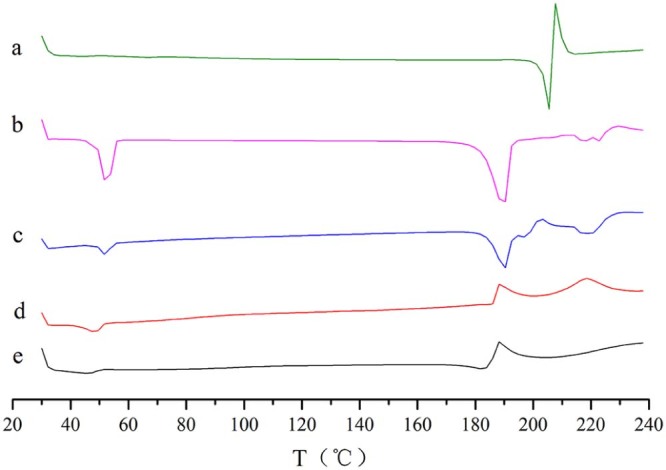
DSC thermograms of AZL bulk drug (a), blank excipients of freeze-drying powder (b), physical mixture of freeze-drying powder (c), freeze-drying AZL nanocrystals (d) and air-drying AZL nanocrystals (e).
XRPD and DSC are used to evaluate the crystal form. From Fig. 5 and Fig. 6, the freeze-drying nanocrystals cannot trace the features of bulk drug, maybe indicating that crystal form of AZL bulk drug was changing in the preparation of formulation. This change caused another crystal form instead of amorphous form, because it still had sharp peaks in XRPD pattern.
To further confirm the size of nanosuspension and freeze-drying nanocrystals, TEM and SEM were brought in. In addition, these two methods of observation also showed us the irregular shape of nanoparticles (Fig. 7 and Fig. 8). In micrographs, the nanoparticles were pretty uniform, and the size was parallel with the data using dynamic light scattering.
Fig. 7.
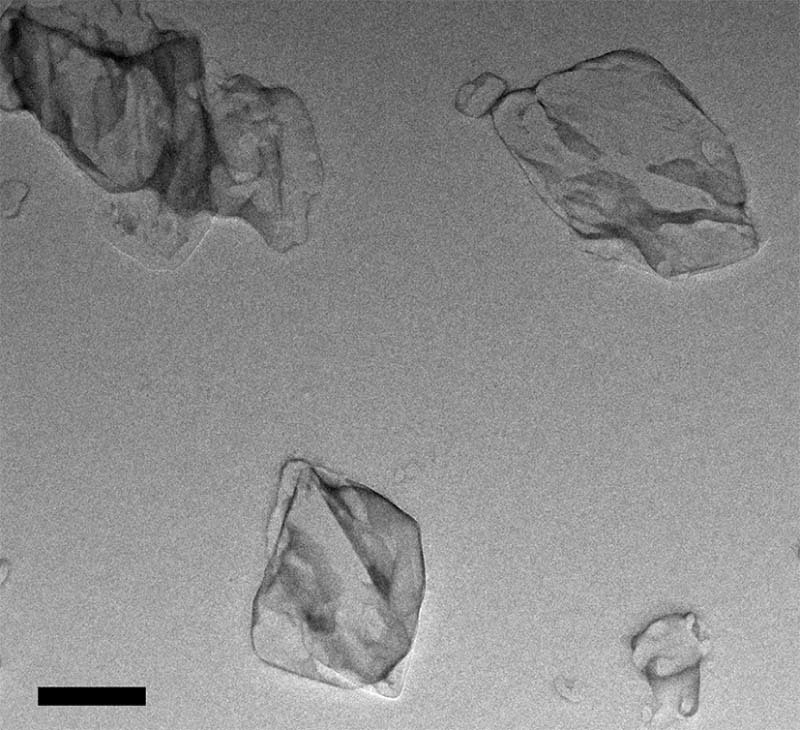
TEM image of AZL nanosuspension. The scale bar represents 200 nm.
Fig. 8.
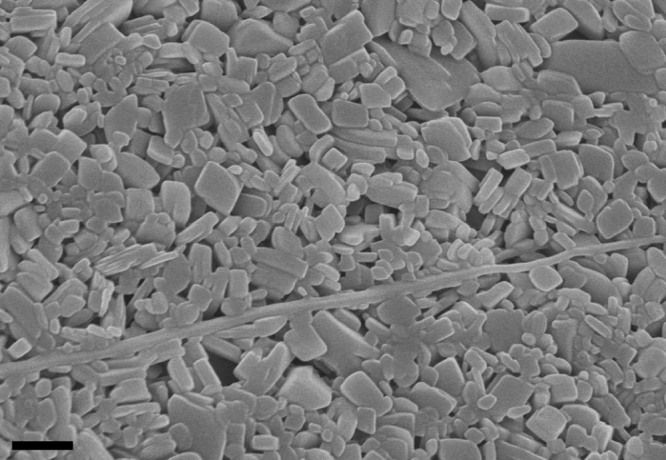
SEM image of AZL freeze-drying nanocrystals. The scale bar represents 500 nm.
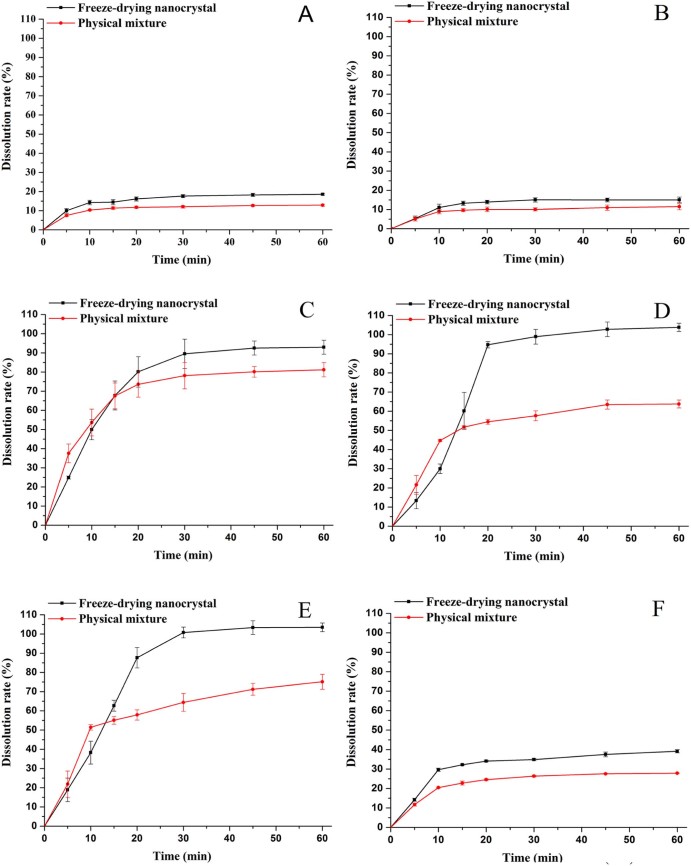
3.7. Dissolution study
On the basis of the Noyes–Whitney equation, reducing the size of drug could increase its surface area, which would improve the dissolution rate. As shown in Fig. 9, the freeze-drying nanocrystals (Nano1) exhibited better dissolution profiles than the physical mixture in every case, which proved that this technique of nanocrystals could enhance the in-vitro dissolution rate of AZL.
Fig. 9.
Dissolution behaviors of freeze-drying nanocrystals and physical mixture in different medium (a-pH 1.0, b-pH 4.5, c-pH 5.4, d-pH 6.0, e-pH 6.8, f-water) (mean ± SD, n = 3).
Due to the molecular structure of AZL, it belongs to acidic compound, whose dissolution displayed the property of pH-dependence. The dissolution rates of two samples in pH 1.0 and pH 4.5 were below 30%, far lower than that in pH 5.4, pH 6.0 and pH 6.8. The dissolution rates of Nano1 in pH 5.4, pH 6.0 and pH 6.8 were up to 100%, while the rates of the physical mixture were between 60% and 80%. Under these three conditions, the dissolution rates of the physical mixture in 5 min and 10 min were higher than the Nano1. But after 15 min, the differences of the dissolution profiles without or with nanocrystallization were widely notable. Maybe the bridges [19] of stabilizers and freeze-drying supports interconnected the nano-drugs, which need a short period to release the drug, compared with the direct release of physical mixture.
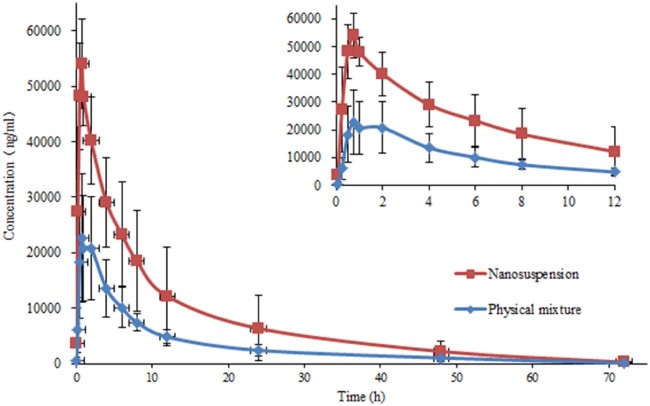
3.8. Pharmacokinetics
The pharmacokinetic performances were investigated for AZL nanosuspension (Nano1) and physical mixture in rats. The plasma concentration profiles of AZL versus time were plotted in Fig. 10 and the pharmacokinetic parameters were presented in Table 2. For nanosuspension, the Tmax (0.79 ± 0.19 h) was lower than physical mixture (1.17 ± 0.65 h), and its T1/2 (10.46 ± 2.51 h) was also lower than physical mixture (11.62 ± 1.43 h), which suggested that the AZL nanosuspension could slightly raise the rates of absorption and elimination.
Fig. 10.
Plasma concentration–time curves for the AZL nanosuspension (Nano1) and physical mixture after oral administration in rats (mean ± SD, n = 6).
Table 2.
Pharmacokinetic parameters following oral administration of AZL nanosuspension (Nano1) and physical mixture (mean ± SD, n = 6).
| Pharmacokinetic parameters | Physical mixture | Nanosuspension |
|---|---|---|
| Tmax (h) | 1.17 ± 0.65 | 0.79 ± 0.19 |
| Cmax (µg⋅ml−1) | 26.45 ± 8.01 | 56.50 ± 8.85 |
| T1/2 (h) | 11.62 ± 1.43 | 10.46 ± 2.51 |
| AUC0-72h (µg⋅h⋅ml−1) | 231.63 ± 72.50 | 545.49 ± 268.61 |
| AUC0-∞ (µg⋅h⋅ml−1) | 236.00 ± 71.19 | 555.46 ± 269.90 |
The relative bioavailability values (F) were calculated to be 235.50% for AZL nanosuspension (Nano1), in comparison with the physical mixture. Similarly, the Cmax and AUC0-∞ were 2.14-fold (P < 0.01) and 2.35-fold (P < 0.05) higher than physical mixture. These results proved that nanocrystallization of AZL helps this insoluble drug to strengthen the bioavailability by oral administration, which was in good agreement with the dissolution test.
4. Conclusion
In this work, the AZL nanocrystals and its lyophilization were prepared. Compared with the formulation of single stabilizer (Nano1), the formulation combined with NaDC (Nano2) showed smaller size during the bead milling and better stability on freeze-drying process. The nanocrystals state was characterized and confirmed by the studies of morphology and crystal form. And with the physical mixture as reference, the technology of nanocrystals exhibited its improvement of in-vitro dissolution and in-vivo bioavailability for poorly soluble drug.
Acknowledgements
This work was financially supported by Science research project of Department of Education Liaoning Province (No. L2013390).
References
- 1.Patravale V.B., Date A.A., Kulkarni R.M. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 2.Rabinow B.E. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 3.Fu Q., Guo M., He Z. Comparison of solid dispersion and nanosuspension for improvement of drug absorption. Asian J Pharm Sci. 2016;11:10–11. [Google Scholar]
- 4.Elsayed I., Abdelbary A.A., Elshafeey A.H. Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int J Nanomed. 2014;9:2943–2953. doi: 10.2147/IJN.S63395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Addio S.M., Prud'homme R.K. Controlling drug nanoparticle formation by rapid precipitation. Adv Drug Deliv Rev. 2011;63:417–426. doi: 10.1016/j.addr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Chan H.K., Kwok P.C. Production methods for nanodrug particles using the bottom-up approach. Adv Drug Deliv Rev. 2011;63:406–416. doi: 10.1016/j.addr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Inkyo M., Tahara T., Iwaki T. Experimental investigation of nanoparticle dispersion by beads milling with centrifugal bead separation. J Colloid Interf Sci. 2006;304:535–540. doi: 10.1016/j.jcis.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Müller R.H., Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237:151–161. doi: 10.1016/s0378-5173(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 9.Ojima M., Igata H., Tanaka M. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther. 2011;336:801–808. doi: 10.1124/jpet.110.176636. [DOI] [PubMed] [Google Scholar]
- 10.White W.B., Weber M.A., Sica D. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413–420. doi: 10.1161/HYPERTENSIONAHA.110.163402. [DOI] [PubMed] [Google Scholar]
- 11.Wu L., Zhang J., Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–469. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Hao F., He Y., Sun Y. Improvement of oral availability of ginseng fruit saponins by a proliposome delivery system containing sodium deoxycholate. Saudi J Biol Sci. 2016;23:S113–S125. doi: 10.1016/j.sjbs.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chingunpitak J., Puttipipatkhachorn S., Chavalitshewinkoon-Petmitr P. Formation, physical stability and in vitro antimalarial activity of dihydroartemisinin nanosuspensions obtained by co-grinding method. Drug Dev Ind Pharm. 2008;34:314–322. doi: 10.1080/03639040701662388. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi A.K., Mohapatra M., Mishra A.K. Fluorescence of N-acylated dansylamide with a long hydrophobic tail: sensitive response to premicellar aggregation of sodium deoxycholate. Phys Chem Chem Phys. 2015;17:29985–29994. doi: 10.1039/c5cp04263a. [DOI] [PubMed] [Google Scholar]
- 15.Salem H.F., Kharshoum R.M., Abdel Hakim L.F. Edge activators and a polycationic polymer enhance the formulation of porous voriconazole nanoagglomerate for the use as a dry powder inhaler. J Liposome Res. 2016;26:324–335. doi: 10.3109/08982104.2016.1140182. [DOI] [PubMed] [Google Scholar]
- 16.Froner E., D'Amato E., Adamo R. Deoxycholate as an efficient coating agent for hydrophilic silicon nanocrystals. J Colloid Interf Sci. 2011;358:86–92. doi: 10.1016/j.jcis.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Gangadhar K.N., Adhikari K., Srichana T. Synthesis and evaluation of sodium deoxycholate sulfate as a lipid drug carrier to enhance the solubility, stability and safety of an amphotericin B inhalation formulation. Int J Pharm. 2014;471:430–438. doi: 10.1016/j.ijpharm.2014.05.066. [DOI] [PubMed] [Google Scholar]
- 18.Ju J., He G., Duan Z. Improvement of bilirubin adsorption capacity of cellulose acetate/polyethyleneimine membrane using sodium deoxycholate. Biochem Eng J. 2013;79:144–152. [Google Scholar]
- 19.Kho K., Hadinoto K. Effects of excipient formulation on the morphology and aqueous re-dispersibility of dry-powder silica nano-aggregates. Colloids Surf A. 2010;359:71–81. [Google Scholar]