Abstract
Topical administration of phenylethyl resorcinol (PR) has attracted much attention as skin lightening agent with potent anti-tyrosinase activity. Two novel types of elastic carriers were developed to overcome the limitation of PR as topical delivery by increasing the solubility, stability and decreasing skin irritation compared to conventional liposomes. In addition, it also promotes skin penetration of PR to reach deep skin layer at the target site. The lead formulations were obtained from the invasomes containing 1% (w/v) d-limonene mixed with 10% (v/v) absolute ethanol as the skin enhancer, and transfersomes containing 15% (w/w) sodium deoxycholate (SDC) as edge activator. All formulations gave a vesicle size < 500 nm, polydispersity index (PDI) < 0.3, high zeta potential, entrapment efficiency > 50%, and good stability on storage at 30 °C at 75% RH for 4 months. Transfersomes have a lower degree of deformability (6.63%) than invasomes (25.26%). In contrast, the liposomes as rigid vesicles do not show a deformable property. This characteristic affects the skin permeation, and thus, transfersomes with high elastic property provided a significantly higher cumulative amount, steady state flux (Jss) and permeability coefficient (Kp) compared to other formulations. However, in vitro PR accumulation in full-thickness newborn pig skin demonstrated that the application of elastic carrier formulations gave significantly higher accumulation than liposomes, and gave anti-tyrosinase activity up to 80%. These results are straightforwardly related to the results of cellular level study. Transfersomes and invasomes showed higher tyrosinase inhibition activity and melanin content reduction when compared to liposomes in B16 melanoma cells. In addition, acute irritation test in rabbits confirmed that these formulations are safe for skin application. Therefore, elastic vesicle carriers have the efficiency to deliver PR into the deep skin in both quantity and effectiveness which are better than conventional liposomes and appropriate for a skin lightening product.
Keywords: Phenylethyl resorcinol, Transfersomes, Invasomes, Tyrosinase inhibition
Graphical abstract
1. Introduction
Melanin is a complex polymer derived from the amino acid tyrosine and produced by the melanogenesis pathway in melanocytes which is located in the basal layer of the epidermis. The light absorption of melanin in skin plays an important role for photo-protection from the ultraviolet (UV) radiation [1]. However, overproduction of melanin causes skin darkening and abnormal distribution of melanin in different and specific parts of the skin which in fact causes hyperpigmentation disorders including melasma, freckles, lentigines, etc. [2]. Tyrosinase inhibition is an effective strategy for treating hyperpigmentation because tyrosinase controls the rate-limiting steps of melanin synthesis.
Phenylethyl resorcinol (4-(1-phenylethyl) 1, 3-benzenediol, PR) is phenolic compound developed by Symrise (Holzminden, Germany) under trade name as SymWhite®377. PR, which is a new skin lightening agent, inhibits the tyrosinase activity by obstruction of the conversion of tyrosine to l-3,4-dihydroxyphenylalanine (L-DOPA) [3]. It reduced tyrosinase activity approximately 22 folds more effectively than kojic acid in vitro mushroom tyrosinase and in vitro epidermal model (MelanoDerma™), respectively [4]. It can increase the fairness of Asian human skin in vivo at a concentration of 0.5% [5]. In addition, PR has antioxidant property and improved UV protection activity when incorporated with inorganic titanium dioxide (TiO2) hybrid composites rather than bare TiO2 [6]. Recently, PR showed potential in antifungal activity. It was effective against the nine dermatomycoses: Microsporum gypseum, Microsporum canis, Trichophyton violaceum, Arthroderma cajetani, Trichophyton mentagrophytes, Epidermophyton floccosum, Nannizzia gypsea, Trichophyton rubrum and Trichophyton tonsurans which is highly active than antifungal agent fluconazole [7]. However, the poor aqueous solubility and low stability in light are the limitations of PR for the formulation as cosmetic and pharmaceutical dermal products. The color changes from white to pink tone when the formulation is exposed to light which may cause the application of PR to be ineffective whereas the poor aqueous solubility may reduce its absorption when used [8]. In addition, it has been reported that, a 1% PR in the formulation, causes skin irritation, and may lead to consumer rejection of the product [9].
Nanoencapsulation technique was used in liposomes [10], transfersomes [11], invasomes [12] and ethosomes [13] to increase the solubility and photo-stability, and decrease skin irritation of PR by protecting the encapsulated actives from external environment and from directly contact to the skin. Flexible or elastic liposomes are the type of phospholipid vesicles which were modified for improving skin drug delivery systems. Although it is similar to conventional liposomes in morphology, the permeability across skin is different. Transfersomes or ultra-flexible vesicles are the first generation of elastic liposomes. They were achieved by incorporating edge activators (EA) to the lipid bilayers. An edge activator is often a single-chain surfactant such as sodium cholate, sodium deoxycholate, dipotassium glycyrrhizinate, Spans and Tweens. A single-chain surfactant with a high radius of curvature and mobility, are able to destabilize the lipid bilayers of vesicles, and increase the elasticity and flexibility of the lipid bilayer which makes the vesicles have ultra-deformable property [14]. Several studies reported that transfersomes are efficient for transdermal and topical drug delivery [11]. Ethosomes are the fluid vesicles which contain ethanol at relatively high concentration (20%–45%) as skin enhancer. The elastic vesicle of ethosomes is an important feature related to skin permeability enhancement, which may be due to the synergistic mechanism between high concentration of ethanol, phospholipid vesicles, and lipid bilayers in stratum corneum. Therefore, they can penetrate into the skin and allow enhanced delivery of various drugs to deeper skin strata [15]. Invasomes are soft flexible liposome vesicles with very high membrane fluidity. They contain ethanol and terpenes which play the role of penetration enhancer. The mechanism of skin permeation of invasomes is similar to ethosomes [12].
Traditional or conventional liposome vesicles are of large size, more than 400 nm in diameter and have a rigid structure. Cholesterol is added in the formulation to stabilize the structure and generate more rigid liposomes. These are too large to fit within the intercellular lipid domains of the stratum corneum, and the liposomes are generally reported to accumulate in the stratum corneum, upper skin layers and in the appendages, with minimal penetration to deeper tissues or the systemic circulation [16]. Conventional liposomes remain near the skin surface, dehydrate and fuse with the skin lipids, whereas deformable transfersomes squeeze through stratum corneum lipid lamellar regions penetrating deeper by following the osmotic gradient to permeate to the deeper layers of the epidermis. Due to the flexibility conferred on the vesicles by the other vesicle compositions molecules such as ethanol and surfactant, elastic vesicle compositions have been investigated to develop systems that are capable of carrying drugs and macro- molecules to deeper tissues.
These vesicles have a unique skin permeation mechanism. Transfersomes can pass through the intact skin and can retain their shape, because of the presence of edge activators in the formulation, whereas the ethosomes have ethanol, and invasomes have combination of ethanol and terpenes in the formulation which play the role of penetration enhancer by disturbing the organization of the stratum corneum lipid bilayer. Therefore, the aim of this study was to develop and characterize phenylethyl resorcinol-loaded transfersome and invasome drug delivery systems for overcoming the limitation of PR as topical delivery, by increasing the solubility and light stability, and decreasing skin irritation. The stability, in vitro skin permeation, and skin irritation were assessed to evaluate the suitability of the formulations for skin lightening applications.
2. Materials and methods
2.1. Materials
PR was purchased from Starchem Enterprises Limited (Nanjing, China). l-α-phosphatidylcholine from soybean (SPC) and cholesterol (CHOL) were purchased from Sigma-Aldrich (Missouri, USA). Absolute ethanol was bought from RCI Labscan Limited (Bangkok, Thailand). Tween 20 and Tween 80 were purchased from P.C. Drug Center Co., Ltd. (Bangkok, Thailand). Span 20 and Span 80 was purchased from S. Tong Chemicals Co., Ltd. (Bangkok, Thailand). Kojic acid, tyrosinase enzyme and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (WGK, Germany). Citral, (R)-(+)-Limonene and (1R)-(−)-Fenchone were purchased from Sigma-Aldrich (WGK, Germany). Sodium deoxycholate were purchased from Homedia® (Mumbai, India). Milli-Q water was used throughout the experiment. All chemicals were used as received, without any modification.
2.2. Methods
2.2.1. Preparation of PR-loaded vesicle carrier
Preparation of PR-loaded transfersomes and invasomes was followed and modified from the method of Limsuwan et al. [13]. The PR in the formulations was fixed as 0.5% (w/v). The 0.5% (w/v) CHOL, 3% (w/v) SPC and water up to 100% (v/v) were the main composition of liposomes. The skin enhancers were varied in terms of concentrations and ratios as shown in the Table 1. The invasome formulations used fenchone, citral and d-limonene mixed with 10% (v/v) ethanol as skin enhancers. The transfersome formulations used Tween 80, Tween 20, Span 80, Span 20 and SDC as skin enhancers. All compositions were prepared by thin-film hydration method as follows: First, the oil phase which included SPC, CHOL, PR and skin enhancer were dissolved in absolute ethanol. The aqueous phase used was water for liposomes and transfersomes; a hydro-ethanolic solution consisting of water and 10% (v/v) absolute ethanol for invasomes. Second, the oil phase and aqueous phase were separately sonicated at 60 °C for 30 min until homogeneity. Then, the absolute ethanol was removed from oil phase via evaporation by a rotary evaporator (Model Eyela N-1000 series, Tokyo Rikakikai Co., Ltd., Japan) while the other components formed the thin lipid film. Afterward, the lipid film was hydrated with 10 ml of aqueous phase, followed by shaking for 5 min. Finally, the mixtures were sonicated at 60 °C for 30 min to obtain the complete formulations.
Table 1.
The composition of liposome, invasome and transfersome formulations.
| Composition |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Lipid phase (%w/v) |
Monoterpene (%, w/v) |
Surfactant (weight ratio) |
Aqueous phase (%, v/v) | |||||||
| SPC | CHOL | Fenchone | Citral | D-limonene | Tween 80 | Tween 20 | Span 80 | Span 20 | SDC | ||
| L | 3 | 0.5 | – | – | – | – | – | – | – | – | Water |
| IV-1 | 0.5 | – | – | – | – | – | – | – | 10% v/v Ethanol | ||
| IV-2 | – | 0.5 | – | – | – | – | – | – | |||
| IV-3 | – | – | 0.5 | – | – | – | – | – | |||
| IV-4 | 1 | – | – | – | – | – | – | – | |||
| IV-5 | 3 | 0.5 | – | 1 | – | – | – | – | – | – | |
| IV-6 | – | – | 1 | – | – | – | – | – | |||
| IV-7 | 1.5 | – | – | – | – | – | – | – | |||
| IV-8 | – | 1.5 | – | – | – | – | – | – | |||
| IV-9 | – | – | 1.5 | – | – | – | – | – | |||
| Tr-1 | – | – | – | 95/5 | – | – | – | – | Water | ||
| Tr-2 | – | – | – | – | 95/5 | – | – | – | |||
| Tr-3 | – | – | – | – | – | 95/5 | – | – | |||
| Tr-4 | – | – | – | – | – | – | 95/5 | – | |||
| Tr-5 | – | – | – | – | – | – | – | 95/5 | |||
| Tr-6 | – | – | – | 90/10 | – | – | – | – | |||
| Tr-7 | – | – | – | – | 90/10 | – | – | – | |||
| Tr-8 | 3 | 0.5 | – | – | – | – | – | 90/10 | – | – | |
| Tr-9 | – | – | – | – | – | – | 90/10 | – | |||
| Tr-10 | – | – | – | – | – | – | – | 90/10 | |||
| Tr-11 | – | – | – | 85/15 | – | – | – | – | |||
| Tr-12 | – | – | – | – | 85/15 | – | – | – | |||
| Tr-13 | – | – | – | – | – | 85/15 | – | – | |||
| Tr-14 | – | – | – | – | – | – | 85/15 | – | |||
| Tr-15 | – | – | – | – | – | – | – | 85/15 | |||
2.2.2. Characterization of the vesicle formulations containing PR
2.2.2.1. Evaluation of the physical appearances.
The color, phase separation, and precipitation of all formulations were visually observed. The formulations with good physical properties were selected to evaluate the other properties.
2.2.2.2. Determination of vesicle size, size distribution and zeta potential.
The vesicle size, size distribution and zeta potential of the formulations were evaluated at 25 °C using a Zeta Potential Analyzer (Model ZetaPALS, New York, USA) with a scattering angle (θ) of 90°. In all cases, 10 µl of samples were dispersed with 4 ml of Milli-Q water before measurement. All determinations were performed in triplicate and presented as mean ± standard deviation.
2.2.2.3. Chromatographic conditions for quantitative determination of PR.
Quantitative determination of PR in all studies was performed by the method described by Limsuwan et al. [17]. The instrument was coupled with the Agilent 1100 series photodiode-array detector (PDA) (Waldbronn, Germany), auto-samplers and Agilent Chemstation for LD 3D software. The reverse-phase chromatography was carried out with a BDS HYPERSIL C18 column (150 mm x 4.6 mm, 5 µm) at 25 °C. The mobile phase was a mixture of acetonitrile/methanol/milli-Q water (40:20:40, v/v/v) and maintained at 0.8 ml/min. The 20 µl sample solution was injected and the absorbance was detected at 254 nm.
2.2.2.4. Measurement of entrapment efficiency.
The ultracentrifugation technique was used to determine the percent entrapment efficiency (%EE) of PR in all formulations using Ultracentrifuge (Optima™L-100XP, Beckman, USA) equipped with SW60 Ti Rotor at 60 000 rpm at 4 °C for 1.5 h. The free PR was determined from supernatant solution after centrifugation and dilution with milli-Q water. The total PR amount in the formulation was measured after breaking the vesicles with methanol at ratio of 1:1 of sample and methanol. All samples were filtered through a 0.45 µm syringe filter membrane and PR content was analyzed using HPLC. All determinations were performed in triplicate and the %EE was calculated according to the Eq. (1):
| (1) |
where, % EE is the percent entrapment efficiency, T is the total amount of PR in the formulation and F is the free PR amount.
2.2.2.5. Measurement of elasticity and deformability.
The elasticity and deformability measurements were carried out by extrusion method which was modified from Singh et al. [18]. Amongst the transfersome and invasome vesicles extruded at 8.5 × 10–4 bar through the polycarbonate filter membrane (Sigma Chemicals, UK) having a pore diameter of 200 nm, the amount of vesicles which were extruded during 5 min was measured. The vesicle size before and after filtration was monitored by Zeta Potential Analyzer (Model ZetaPALS, New York, USA) at 25 °C with a scattering angle (θ) of 90°. Afterward, values of degree of deformability were calculated [19]. All determinations were performed in triplicate.
2.2.2.6. Visualization of structure and surface morphology.
The surface morphology of PR-invasome and -transfersome vesicles was observed by SEM (Quanta 400, FEI, Czech Republic) at an accelerating voltage of 20 kV. 100 µl of sample was diluted with 3 ml Milli-Q water, dropped on cover slip and dried. Then, the sample was stained with 2–3 drops of crystal violet solution and Gram's iodine solution. After that, the dried sample was gold coated in a sputter coater (Leica EMD005, Czech Republic) under an argon atmosphere (50 Pa) at 50 mA for 70 sec and observed at optimized magnification. The structure of PR loaded ethosome, invasome and transfersome vesicles were observed by TEM (TEM, JEM-2010, JEOL, Japan). The 10 µl of the formulation was diluted with 3 ml milli-Q water and dropped on the holey film grid. The dried coated grid was observed by TEM at optimized magnification with an acceleration voltage of 160 kV. In this study, the optimized composition was selected on the basis of a vesicle size less than 500 nm, narrow size distribution (pdI < 0.3), high absolute value of zeta potential and %EE > 50% [13], [20].
2.2.3. Evaluation of storage stability
The physicochemical stability of PR-loaded transfersomes and invasomes was evaluated after storing at 4 ± 1 °C, 30 ± 1 °C and 45 ± 1 °C under controlled humidity of 75% RH in a Constant Climate Chamber Model HPP260 (Memmert, Schwabach, Germany) for 0, 1, 2 and 4 months. The physicochemical stability was evaluated in term of the physical appearance, vesicle size, size distribution, zeta potential, total PR content and entrapment efficiency. Testing was carried out using triplicate samples at each storage condition.
2.2.4. In vitro skin permeation study
In vitro skin permeation and deposition of PR in the skin of transfersome and invasome systems were evaluated using modified Franz-diffusion cell (Model Hanson 57-6M, California, United States) with an effective diffusion area of 1.77 cm2. Newborn pig skin was used as the diffusion membrane because of its similarity to human skin in terms of general structure, thickness, hair follicle content and lipid component [20]. The methodology of this study was followed from the method of Limsuwan et al. [13], [20]. The skin diffusion membrane was placed between the donor and receptor compartments with the stratum corneum toward the donor compartment. The donor part was filled with 1 ml of the vesicle formulation whereas the receptor part was filled with 11 ml of a mixture of 0.44% w/v sodium chloride (NaCl) in phosphate buffer pH 7.4: propylene glycol (80:20, v/v) as receptor medium. In addition, water jacket of diffusion cell was maintained at 37 ± 1 °C to control the skin temperature to 32 ± 2 °C and a magnetic stirrer at 500 rpm was used throughout the experiment. At time intervals at 0.5, 1, 2, 4, 6, 8, 12 and 24 h, 1 ml of receiver medium was withdrawn and an equal volume of fresh medium at 37 ± 1 °C was replenished into the chamber to maintain sink conditions. The experiments were repeated five times, PR contents in receiver medium were analyzed in triplicate by HPLC and permeation data analysis was evaluated. The cumulative amount (Qt, µg/cm2) of PR that permeated through the pig skin per unit area of skin was calculated according to Eq. (2):
| (2) |
where, Qt is cumulative amount of PR permeated per unit area of skin (µg/cm2), Cn is concentration of PR determined at No.n sampling interval (µg/ml), Ci is concentration of PR determined at No.i sampling interval (µg/ml), V is volume of individual Franz diffusion cell (ml), S is volume of sampling aliquot and A is effective diffusion surface area, 1.77 cm2
The Qt amount was plotted as a function of time and the steady state flux (Jss) was calculated from the slope of the linear portion of the plot. The lag time (Tlag) of the PR to permeate through the pig skin before reaching the receptor fluid was calculated from the X-intercept of the plot. The value of permeation coefficient (Kp) for PR was calculated using the Eq. (3):
| (3) |
where C0 is the initial concentration of PR in the donor compartment.
2.2.5. Residual PR in the donor compartment
At the end of experiment, the excess formulation was removed from the membrane, cleaned with methanol (10 ml) and sonicated at room temperature (32 ± 2 °C) for 30 min. The clear supernatant that was obtained from centrifugation using a Hermle Z323K Centrifuge (Wehingen, Germany) at 6000 rpm, 4 °C for 30 min was lysed with methanol in the ratio of 1:1 (v/v) and analyzed using HPLC to determine the amount of residual PR in the donor compartment. All samples were analyzed in triplicate.
2.2.6. In vitro skin deposition study
The skin membrane after removal of residual PR in the donor compartment was cleaned, cut into small pieces and homogenized in methanol (5 ml) using homogenizer (Model PT 1200 E, Polytron, Switzerland) at room temperature (32 ± 2 °C) for 2 min. Then, the sample was sonicated at room temperature for 30 min and centrifuged at 6000 rpm at 4 °C for 30 min to separate the skin lipid. The clear supernatant was analyzed using HPLC to determine the amount of PR which had accumulated in the skin diffusion membrane. All samples were analyzed in triplicate.
2.2.7. Evaluation of anti-tyrosinase activity
The extracted PR from skin diffusion membrane after permeation testing as described above (2.2.6) was evaluated for tyrosinase activity using the dopachrome method. Mushroom tyrosinase was assayed with L-dopa as substrate and Kojic acid as a positive control. The reaction mixture contained 20 µl of test samples or positive controls in DMSO, 140 ??l of phosphate buffer (pH 6.8), 20 µl of mushroom tyrosinase (203.3 unit/ml) and 20 µl of 0.85 mM l-dopa. After the addition of L-dopa the reaction was measured for optical density with microplate reader at 492 nm wavelength, before incubation. Then, the reaction mixture was incubated at 25 °C for 20 min and the OD of dopachrome formation after incubation was measured. The % tyrosinase inhibition was calculated according to the Eq. (4):
| (4) |
where: A is the difference of OD before and after incubation at 492 nm (containing all reagents and enzyme except the test sample).
B is the difference of OD before and after incubation at 492 nm (containing all reagents except the test sample and enzyme).
C is the difference of OD before and after incubation at 492 nm (containing all reagents, test sample, and enzyme).
D is the difference of OD before and after incubation at 492 nm (containing all reagents and the test sample except enzyme).
2.2.8. Cell culture
B16 melanoma cell line was a kind gift from Assistant Prof. Dr. Sukanya Dej-adisai, Faculty of Pharmaceutical Sciences, Prince of Songkla University (Thailand). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) high glucose, supplemented with 10% fetal bovine serum (Gibco, USA), 2 mM glutamine, 1% penicillin/streptomycin and maintained at 37 °C in a humidified incubator with 10% CO2.
2.2.9. Quantification of cellular tyrosinase activity in B16 melanoma cells
Cellular tyrosinase activity was measured by adapting the assay established by Tomita et al. [21] with some modification. Briefly, cells (5 × 104 cells/well) were stimulated with α-MSH (100 nM) and then incubated in 24 well plates with different formulations containing 5 µM of PR for 72 h. After treatment, the cells were collected with trypsin-EDTA and centrifuged at 6000 rpm for 5 min to obtain cell pellets. The cell pellets were freeze-thaw lysed in 200 µl of 2% Triton X-100 in 0.1 M phosphate buffered saline (pH 7.2). After protein quantification by BCA method, 100 µl of cell lytic solution (100 µg/ml) were mixed with 100 µl of 0.2% l-Dopa in phosphate buffer solution (pH 7.2), and incubated for 1 h at 37 °C. The optical densities were measured as 475 nm using a microplate spectrophotometer. The tyrosinase activity of each formulation was presented as percentage against that of the control cells.
2.2.10. Determination of cellular melanin content in B16 melanoma cells
Cells (5 × 105 cells/dish) were stimulated with α-MSH (100 nM) and then incubated in 60 mm dishes with different formulations containing 5µM of PR for 72 h. After treatment, the cells were washed twice with ice-cold phosphate-buffered saline, and lysed in 200 µl of 1 N NaOH for 1 h at 95 °C and then vortexed to solubilize the melanin. Lytic solution (100 µl) was added in a 96 well plate. The total amount of melanin was measured at 405 nm wavelength. The melanin content was determined based on the absorbance divided by the protein concentration in the extract from each cell pellet. The melanin content of each formulation was then calculated and presented as percentage against that of the control cells.
2.2.11. Investigation of acute dermal irritation/corrosion
The effects of PR in transfersome and invasome formulations on skin irritation were evaluated using the Guidelines 404 of the OECD (Acute Dermal Irritation/Corrosion) by TISTR (Thailand Institute of Scientific and Technology Research).
Three healthy adult white albino rabbits, in range of 2–3 kg body weight were used in this study. One day before experimentation, the epidermal hair on dorsolumbar region was removed into a 10 × 10 cm2 area. The 2.5 × 2.5 cm2 of two hairless areas were selected for testing. 0.5 ml of the test sample and distilled water (negative control) were applied to each gauze patch. Then, the patch was applied on the selected skin sites and wrapped with adhesive tape to avoid movement. After 4 h, all patches were removed and the residual sample was removed gently with cotton wool. The skin irritation in terms of erythema and edema was subjectively examined at time 1, 24, 48, and 72 h, or, more than these time intervals, when the irritation still occurred, but not over 14 d of observation periods. The skin reactions were scored accounting for the numerical scoring system from 0 to 4, ranging from, none to severe signs, by two professional inspectors.
2.2.12. Statistical analysis
The results obtained in the study were presented as mean ± standard deviation (SD) or mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) followed by post hoc analysis was used to test the statistical significant of differences among groups. p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Characterization of vesicle carrier
3.1.1. Characterization of PR-liposomes
The main formulation was obtained from 0.5% (w/v) PR, 0.5% (w/v) CHOL, 3% (w/v) SPC and water up to 100% (v/v). In this study, the main composition was used to represent the liposome formulation which is the rigid vesicle, to compare with the developed vesicles. The physicochemical properties of developed vesicles, with other skin enhancers added in each formulation (Table 1) were evaluated. Fig. 1 and Table 2 show the physical appearance and the vesicle size, PDI, zeta potential and entrapment efficiency of all formulations. The liposome formulation (formulation code. L) showed a large vesicle size (641.52 ± 19.02 nm) with PDI (0.267 ± 0.22), absolute value of zeta potential (−27.32 ± 1.00 mV) and entrapment efficiency (53.55% ± 4.9%).
Fig. 1.
The physical appearance of liposome, invasome and transfersome formulations.
Table 2.
The physical appearance, particle size, size distribution, zeta potential and entrapment efficiency of liposomes, invasomes and transfersomes.
| Code | Physical property | Vesicle size (nm) | PDI | Zeta potential (mV) | Entrapment efficiency (%) |
|---|---|---|---|---|---|
| L | Yellow colloidal | 641.52 ± 19.02 | 0.267 ± 0.220 | −27.32 ± 1.00 | 53.55 ± 4.90 |
| IV-1 | Yellow cloudy | 403.40 ± 23.00 | 0.154 ± 0.038 | −53.58 ± 2.47 | 92.29 ± 0.02 |
| IV-2 | Yellow colloidal precipitate | ND | ND | ND | ND |
| IV-3 | Yellow cloudy | 560.50 ± 58.30 | 0.143 ± 0.040 | −55.66 ± 1.61 | 95.80 ± 0.02 |
| IV-4 | Yellow colloidal precipitate | ND | ND | ND | ND |
| IV-5 | Yellow colloidal precipitate | ND | ND | ND | ND |
| IV-6 | Yellow cloudy | 341.17 ± 54.11 | 0.188 ± 0.040 | −59.47 ± 2.66 | 91.32 ± 0.00 |
| IV-7 | Yellow colloidal precipitate | ND | ND | ND | ND |
| IV-8 | Yellow cloudy | 782.60 ± 75.60 | 0.117 ± 0.112 | −66.94 ± 1.68 | 100.05 ± 0.02 |
| IV-9 | Yellow cloudy | 819.30 ± 101.90 | 0.030 ± 0.025 | −65.82 ± 1.54 | 92.30 ± 0.04 |
| Tr-1 | Yellow turbid | 372.30 ± 8.00 | 0.225 ± 0.060 | −68.34 ± 2.28 | 55.70 ± 0.26 |
| Tr-2 | Yellow turbid | 368.30 ± 20.00 | 0.134 ± 0.045 | −68.55 ± 2.56 | 77.01 ± 0.04 |
| Tr-3 | Yellow colloidal precipitate | ND | ND | ND | ND |
| Tr-4 | Yellow turbid | 408.40 ± 25.90 | 0.259 ± 0.039 | −55.99 ± 2.89 | 57.57 ± 0.03 |
| Tr-5 | Yellow turbid | 392.10 ± 9.00 | 0.285 ± 0.020 | −56.09 ± 4.93 | 95.20 ± 0.00 |
| Tr-6 | Yellow turbid | 357.50 ± 9.10 | 0.100 ± 0.058 | −57.39 ± 3.42 | 53.33 ± 0.07 |
| Tr-7 | Yellow turbid | 320.10 ± 31.40 | 0.155 ± 0.044 | −63.25 ± 3.75 | 56.33 ± 0.21 |
| Tr-8 | Yellow colloidal precipitate | ND | ND | ND | ND |
| Tr-9 | Yellow turbid | 550.50 ± 39.10 | 0.106 ± 0.060 | −63.97 ± 1.47 | 38.72 ± 0.03 |
| Tr-10 | Yellow turbid | 395.20 ± 4.30 | 0.127 ± 0.041 | −42.04 ± 3.42 | 89.73 ± 0.03 |
| Tr-11 | Yellow turbid | 350.40 ± 4.60 | 0.093 ± 0.046 | −47.65 ± 2.29 | 75.74 ± 0.13 |
| Tr-12 | Yellow turbid | 387.50 ± 4.80 | 0.265 ± 0.027 | −66.43 ± 2.12 | 96.10 ± 0.12 |
| Tr-13 | Yellow colloidal precipitate | ND | ND | ND | ND |
| Tr-14 | Yellow turbid | 991.30 ± 330.60 | 0.179 ± 0.095 | −56.97 ± 2.79 | 35.76 ± 0.06 |
| Tr-15 | Yellow turbid | 353.70 ± 10.90 | 0.111 ± 0.038 | −41.86 ± 0.73 | 92.49 ± 0.01 |
ND means not determined
3.1.2. Characterization of PR-invasomes
In case of invasomes, they contain fenchone, citral and d-limonene which are monoterpenes mixed with 10% (v/v) absolute ethanol as the skin enhancer. At a high concentration of fenchone (1%–1.5% w/v) it gave the formulation with precipitate (IV-4 and IV-7), while the formulation using low concentration (0.5% w/v) gave a good physical appearance. On the other hand, citral gave an unstable formulation when low concentration was used (IV-2 and IV-5). The good physical property results were obtained from using d-limonene as skin enhancer, both high and low concentrations gave a yellow cloudy formulation with no precipitate of drug and other components.
Invasome size was distributed in the range of 208.10 ± 12.00 to 819.30 ± 101.90 nm. It was reported that the vesicle size increases with the increasing concentration of penetration enhancer [12]. The size of invasomes containing 0.5%, 1% and 1.5% (w/v) citral was 312.00 ± 10.30, 651.00 ± 161.80 and 782.60 ± 75.60 nm, respectively. The pdI of all invasomes was less than 0.3, indicating homogeneous population of the vesicles. The zeta potential of all invasome formulations showed high negative charge (−48.43 ± 4.64 to −71.36 ± 3.54) which represents the stability of the formulations (Table 2) due to low possibility of vesicle aggregation, and normally considered as a stable formulation [22].
The %EE of all invasomes was found in the range of 88.08% ± 0.02% to 98.23% ± 0.00%. It is related to log P value. The formulation containing monoterpene with the low log P gave more %EE than the monoterpene with the high log P. Table 2 showed that the formulation containing fenchone (log P = 2.13), citral (log P = 3.45) and d-limonene (log P = 4.58) gave a %EE value of 92.29% ± 0.02% (IV-1), 88.08% ± 0.02% (IV-4), and 84.34% ± 0.04% (IV-7), respectively. This result may be effect of the solubility of PR in the lipid bilayers. Since the log P of PR is 2.11, it may be soluble in the composition with nearly the same value. In this study, the optimized PR-invasome formulation was obtained from the formulation no. IV-6 with the 341.17 ± 54.11 nm vesicle size, 0.188 ± 0.040 PDI, −59.47 ± 2.66 mV zeta potential and 91.32% ± 0.00% entrapment efficiency.
3.1.3. Characterization of PR-transfersomes
Transfersome formulation had a yellowish turbid appearance with good physical property when prepared using Tween 20 and 80, Span 20 and 80, and sodium deoxycholate as edge activator, whereas the formulation containing Span 80 precipitated after preparation.
The size decreased together with the formulation containing surfactants with high HLB value. In this study it was found that the surfactant with high HLB (Tween 80, HLB = 15; SDC, HLB = 16; Tween 20, HLB = 16.7) gave smaller size than the surfactant with low HLB (Span 20, HLB = 8.6) as shown in Table 2. Transfersomes containing Tween 80, SDC and Tween 20 gave a mean vesicles size of 350.40 ± 4.60, 353.70 ± 10.90 and 387.50 ± 4.80 nm, respectively, while the vesicle size of 991.30 ± 330.60 nm was obtained from the formulation containing Span 20 when prepared by the same concentration. The surfactant with low HLB might be attributed to enhance surface free energy through increasing hydrophobicity, by interacting with lipid head groups of the membrane, which increase packing density of phospholipid layer leading to increase surface free energy that causes fusion between lipid bilayers to form a large vesicle [23]. However, Span 80 has low HLB with strongest hydrophobicity. That may cause the aggregation between vesicles, to form larger vesicle and tend to precipitate. In this study, the formulation containing Span 80 as edge activator becomes precipitated after preparation. The pdI, zeta potential and %EE of all transfersomes are as demonstrated in Table 2. The PDI of all formulations were in the range of 0.093 ± 0.046 to 0.285 ± 0.020 which were within 0.3 indicating that the population of the vesicles was homogeneous. The zeta potential of all transfersome formulations were high negative charge (−41.86 ± 0.73 to −68.55 ± 2.56). High negative zeta potential represents a good stability of the formulations.
The %EE of all transfersomes demonstrated in range of 35.76% ± 0.06% to 95.20% ± 0.00%. The highest %EE of transfersomes was observed in the formulation using SDC (95.20% ± 0.00%) when compared with other formulations. Since SDC is anionic surfactant, it may cause an increased electrostatic attraction with the positive charge of the drug, which causes an increased %EE. The optimized PR-transfersome formulation which matched with criteria set was the formulation no. Tr-15. It gave the small vesicle size (353.70 ± 10.90 nm) with low PDI (0.111 ± 0.038), high absolute value of zeta potential (−41.86 ± 0.73 mv) and high entrapment efficiency (92.49% ± 0.01%).
3.2. Elasticity and deformability of vesicle carrier
Table 3 shows the deformability results of all vesicle carriers before and after filtering through a 200 nm polycarbonate filter. The results demonstrated that invasomes (25.26%) have a higher degree of deformability than transfersomes (6.63%), while liposomes cannot pass 200 nm pore size. The results confirmed that all developed vesicles have elastic property, whereas, the liposome formulation is a rigid vesicle.
Table 3.
The deformability results of all formulations before and after filter through a 200 nm polycarbonate filter.
| Before filtration | After filtration | ||||
|---|---|---|---|---|---|
| Code | Vesicle size (nm) | PDI | Vesicle size (nm) | PDI | Deformability (%) |
| L | 600.23 ± 11.92 | 0.21 ± 0.07 | nil | nil | nil |
| IV-6 | 327.83 ± 25.19 | 0.24 ± 0.03 | 245.03 ± 2.44 | 0.24 ± 0.04 | 25.26 |
| Tr-15 | 398.37 ± 9.82 | 0.06 ± 0.08 | 371.97 ± 8.72 | 0.13 ± 0.09 | 6.63 |
In addition, the size of transfersome vesicles that was filtered through a polycarbonate filter is larger than the pore size of filter (200 nm). The sizes of transfersome vesicles before and after filter were 398.37 ± 9.82 nm and 371.97 ± 8.72 nm, respectively. The pdI of transfersomes in both before (0.06 ± 0.08) and after (0.13 ± 0.09) filter were less than 0.3, indicating homogeneous population of the vesicles. These results confirm that the transfersome vesicle has elastic and flexible properties because of retaining vesicle size and shape after filtration d = 6.63%. This characteristic of transfersomes is achieved by incorporating suitable edge activators to the lipid bilayers. In contrast, the invasomes which passed through 200 nm pore size show deformable property. The size of invasomes after filtration was smaller than the original vesicles size before filtration, but still larger than pore size of filter. The sizes of invasomes before and after filter were 327.83 ± 25.19 nm and 245.03 ± 2.44 nm, respectively d = 25.26%. Therefore, these results showed that invasomes only have deformability property. This property could be explained by effect of ethanol concentration, which reduced the interfacial tension of the vesicle membrane, leading to provide ability for small vesicles to extrude through the membrane [23].
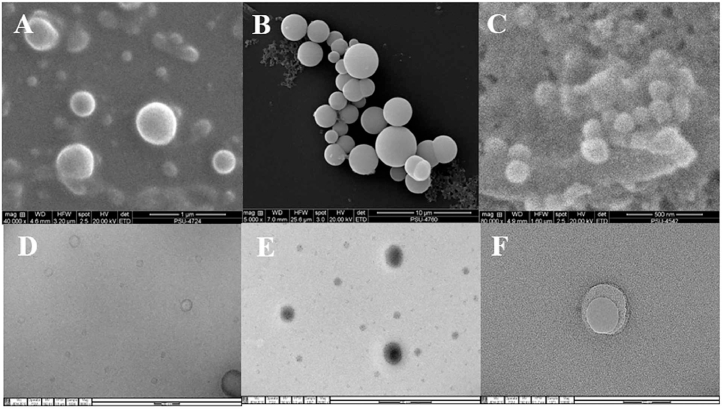
3.3. Visualization of structure and surface morphology of vesicle carrier
Fig. 2 shows the surface morphology of PR liposome, invasome and transfersome vesicles when observed by SEM and TEM. SEM photographs showed that all formulations revealed spherical structure with smooth surface (Fig. 2A–2C). TEM photographs indicated the surface morphology of liposomes and invasomes showed unilamellar vesicle structure (Fig. 1D and 1E), whereas, unilamellar to multilamellar was shown in case of transfersomes (Fig. 1F).
Fig. 2.
SEM photographs of liposome (A), invasome (B), and transfersome formulations (C), and TEM photographs of liposome (D), invasome (E) and transfersome formulations (F).
3.4. Storage stability of vesicle carrier
The storage stability of all vesicle carriers was evaluated in term of vesicle size, zeta potential, total active content and %EE after storage at 4 ± 1 °C, 30 ± 1 °C and 45 ± 1 °C under controlled humidity of 75% RH for 0, 1, 2 and 4 months as shown in Fig. 3.
Fig. 3.
The effect of storage condition on vesicle size (A and B), zeta potential (C and D), total active content (E and F), and entrapment efficiency (G–H) of the invasome and transfersome formulations.
Stability results indicated that the physical property of transfersome formulation unchanged over 4 months when stored at 4 ± 1 °C and 30 ± 1 °C. In contrast, invasome formulation shows only to be stable at 30 ± 1 °C. The zeta potential values of all systems were decreased when time increased (Fig. 3C to 3D). The zeta potential of invasomes and transfersomes decreased to −47.84 ± 13.20 and −40.76 ± 8.11 mV when stored at 30 ± 1 °C for 4 months, respectively. However, all zeta potential values were above 30 mV over 4 months which demonstrated the good stability of the formulation.
The vesicle size of all formulations showed that it increased with time in all conditions (Fig. 3A to 3B). The vesicle sizes of invasomes and transfersomes increased, by around 16.89% and, 8.87% after storage at 30 ± 1 °C over 4 months. It increased about 8.91% when the transfersome formulation was stored at 45 ± 1 °C for 3 months. In addition, it slightly increased approximately 6.28% when the transfersomes were kept under 4 ± 1 °C for 3 months. However, all formulations showed narrow size distribution (PDI < 0.3). The increase of size might be caused by the aggregation and fusion of vesicles itself.
However, the transfersome formulation showed higher percentage of total active content (Fig. 3F) and EE (Fig. 3H) at 4 ± 1 °C and 30 ± 1 °C, than at 45 ± 1 °C when tested after storing for 1, 2, and 4 months. It gave percentage of total active content more than 85% at 4 ± 1 °C and 30 ± 1 °C. This may be due to higher fluidity of lipid bilayers which leads to higher drug leakage when stored at higher temperature [24]. Therefore, the transfersome formulations in this study should be stored at 4 ± 1 °C and 30 ± 1 °C, whereas, the invasome formulation needs to be stored only at 30 ± 1 °C.
3.5. In vitro skin permeation and deposition study
Table 4 illustrates the percent recovery of PR in each compartment of the diffusion cell and skin permeation parameters after application of liposome, invasome and transfersome formulations. The amount of PR in the three compartments was analyzed following experimentation for 24 h and expressed as a percentage of the applied dose (0.5 mg/ml). The results showed that the liposome, invasome and transfersome formulations gave % recovery as 88.48 ± 4.29, 85.38 ± 3.86 and 92.47 ± 6.02% respectively, which were close to 90%–110%,which is the acceptable limit of OECD TG428 [25]. Thus, it was recognized that this method has performance and trustworthiness. In the percent recovery results, most of PR would not penetrate the skin. It accumulated at the donor compartment. The low penetration of PR may be because of the excellent barrier properties of the outermost skin layer named stratum corneum which had “the brick and mortar” structure [26].
Table 4.
The percent recovery of PR in each compartment of the diffusion cell and skin permeation parameters after application of liposome, invasome and transfersome formulations.
| Amount recovery (% of the applied dose) |
Skin permeation parameters |
||||||
|---|---|---|---|---|---|---|---|
| Formulation | Donor compartment | Newborn pig skin | Receptor compartment | Total | Jss (µg/cm2/h) | Kp (×10–3 cm/h) | Tlag (h) |
| L | 87.51 ± 4.41 | 0.56 ± 0.05b | 0.41 ± 0.17b | 88.48 ± 4.29 | 0.96 ± 0.42b | 0.19 ± 0.08b | 7 ± 1.50 |
| IV-6 | 83.33 ± 3.83 | 1.09 ± 0.09 b,c | 0.96 ± 0.07a | 85.38 ± 3.86 | 2.24 ± 0.20 a,b | 0.45 ± 0.04a,b | 5 ± 0.49 |
| Tr-15 | 89.59 ± 5.89 | 1.42 ± 0.17 c | 1.45 ± 0.21a | 92.47 ± 6.02 | 3.26 ± 0.45a | 0.65 ± 0.09a | 6 ± 0.00 |
Each data represents the mean ± SEM (n = 5). Jss, steady state flux; Kp, permeability coefficient; Tlag, lag time. a–c: means in the same row with different superscript letter differ significantly (P < 0.05).
Table 4, Figs. 4 and 5 show the skin accumulation and permeation results. Comparing with liposomes, all elastic carriers demonstrated that the application of them gave the PR accumulation, and permeation, higher than the application of liposomes. The skin permeation of PR after using liposomes was 20.65 ± 1.72 µg/cm2 while the elastic carriers showed the skin permeation of PR ranging from 47.99 ± 3.73 to 72.66 ± 4.66 µg/cm2. In addition, the skin accumulation in full-thickness newborn pig skin also showed that the elastic carriers gave higher PR accumulated than that of liposomes. The PR accumulations of liposome, invasome and transfersome formulations were 28.18 ± 2.59, 54.43 ± 4.62 and 71.21 ± 8.40 µg/cm2, respectively. The good results may be due to the special mechanism of permeation across skin of flexible or elastic carrier.
Fig. 4.
In vitro accumulation of PR in full-thickness newborn pig skin and receptor compartment after testing liposomes, invasomes and transfersomes for 24 h. Each bar represents the mean ± SEM (n = 5). *-***: means in the same color bar with different asterisk differ significantly (P < 0.05). Statistical significance was determined by one way ANOVA.
Fig. 5.
Cumulative permeation of PR across pig skin membranes following application of liposomes, invasomes and transfersomes. Each point represents the mean ± SEM (n = 5).
In case of ethosomes, they have deformability property which is an important feature related to enhancement of skin permeability. It penetrates skin using the synergistic mechanism between high concentration of ethanol, phospholipid vesicles and lipid bilayers in stratum corneum. Ethanol interacts with skin lipid molecules in the polar head group region, resulting in reducing the rigidity of the stratum corneum lipids, and increasing their fluidity, which may finally lead to an increase in skin permeability. In addition, the ethanol may provide the vesicles with soft and flexible characteristics which enable them to squeeze through the pores in stratum corneum which are much smaller than their diameter size [15], [27].
In case of invasomes, they are soft flexible carrier with very high membrane fluidity. The mechanism of skin permeation of invasomes is similar to ethosomes, by ethanol and terpenes disturbing the organization of the stratum corneum lipid bilayer. Ethanol interacts with polar head group region of the skin lipid molecules, terpenes break the interlamellar hydrogen bonding network of stratum corneum lipid bilayer which may finally lead to the openings creating new polar pathways. In addition, ethanol may provide the vesicles with soft and flexible characteristics which may finally lead to increase in skin permeability [12].
In case of transfersome carrier, it has ultra-elastic property [14] which allows it squeeze itself through the pores of the intact skin which is much smaller than its own diameter, into or through the deep skin layer. In addition, it penetrates across skin under the influence of a trans-epidermal water activity gradient in hydrophilic pathways of the skin. Moreover, it is able to retain its shape because of the presence of edge activators in the formulation [11].
Comparison within the flexible or elastic carriers, all the results showed that the highest amount of PR that permeate across pig skin membranes was obtained from the application of transfersome formulation (Figs. 4 and 5). In addition, the transfersomes with high flexible property provided a significantly higher cumulative amount, steady state flux ( Jss; 3.26 ± 0.45 µg/cm2/h) and permeability coefficient (Kp; 0.65 ± 0.09 × 10–3 cm/h) compared to other formulations. These results may be linked with the degree of deformability, since, invasomes (25.26%) have a higher degree of deformability than transfersomes (6.63%) (Table 3). In contrast, the liposomes as rigid vesicles did not show deformable and elastic property.
Moreover, TEM photographs indicated the surface morphology of transfersomes showed multilamellar structure (Fig. 2F), so they can entrap the more amount of PR for the topical delivery. Therefore, transfersomes with high elastic property [28] could penetrate across the skin more than liposomes and invasomes in both terms of the depth of skin layer, and amount of PR.
3.6. Anti-tyrosinase activity of vesicle carrier
Table 5 showed that all carriers exhibited tyrosinase inhibition activity in the range of 89.38% ± 0.42 to 95.54% ± 0.13% which is greater than kojic acid (87.35 ± 0.76), a potent tyrosinase inhibitor. Since skin accumulation study (Fig. 4) showed that the elastic vesicle formulations resulted in higher PR retention in the skin compared with liposomes, but tyrosinase inhibitory activity was similar. This result may be the effect of a shielding effect of vesicle systems. However, the tyrosinase inhibition activity of PR after application of liposomes, invasomes and transfersomes showed approximately 80%, which is estimated to result in highly efficient skin lightening activities.
Table 5.
Tyrosinase inhibitory activity of PR in liposomes, invasomes and transfersomes and pig skin following topical application in liposomes, invasomes and transfersomes.
| Formulations | Tyrosinase inhibition (%) |
|---|---|
| Liposomes | 95.54 ± 0.13 |
| Invasomes (IV-6) | 89.38 ± 0.42 |
| Transfersomes (Tr-15) | 91.09 ± 1.23 |
| PR from liposomes in pig skin | 79.53 ± 0.45 |
| PR from invasomes in pig skin | 79.82 ± 0.17 |
| PR from transfersomes in pig skin | 80.47 ± 0.22 |
| Kojic acida | 87.35 ± 0.76 |
PR concentration in test samples: 20 µg/ml. Each value represents the mean ± SD (n = 3).
positive control.
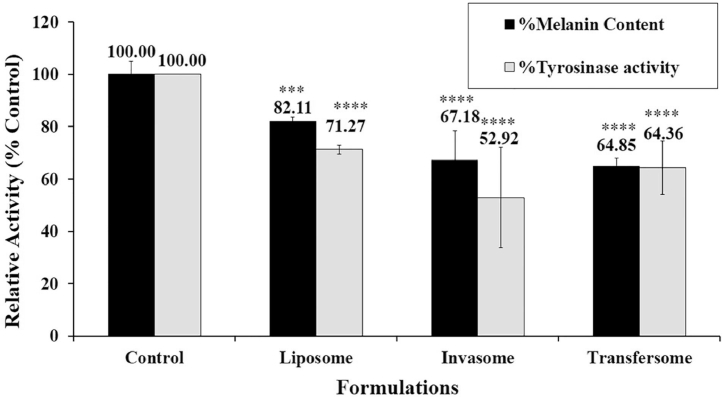
3.7. Effect of vesicle carrier on cellular melanin production and tyrosinase activity
Fig. 6 shows that the PR formulated in liposomes, invasomes, and transfersomes significantly inhibited the tyrosinase activity as well as melanin production in B16 melanoma cells compared with the control. Interestingly, both tyrosinase inhibition activity and melanin production of elastic vesicle carriers showed higher activity than liposomes. Consistent with skin accumulation study (Fig. 4.) shows that the elastic vesicle formulations exhibited high PR retention in the skin. The study in B16 melanoma cells also revealed that the elastic vesicle formulations displayed the higher tyrosinase inhibition activity and melanin content reduction when compared to liposomes. These findings confirmed that elastic vesicle carriers represent suitability for the PR delivery system, which proves it has highly effective skin lightening properties.
Fig. 6.
Effects of vesicle carriers containing PR on melanin production and tyrosinase activity in B16 melanoma cells. Cells were stimulated with 100 nM α-MSH and then treated with indicating formulations (each formulation containing 5 µM PR) for 72 h. Cellular melanin content and tyrosinase activity were measured and analyzed. Bars represent the means ± S.D. of three independent experiments (n = 3). **** P < 0.01, *** P < 0.05 compared with the control.
3.8. Acute dermal irritation testing of vesicle carrier
An acute irritation test in rabbits were carried out, based on erythema and edema formation systems after application PR in liposomes, invasomes and transfersomes in 72 h.
The slightly erythema formation was observed in case of the application of transfersomes at 1 h. The recovery of this skin reaction occurred within 24 h of the observation period. However, the skin area which was treated with the liposomes and invasomes gave no significant differences from the skin area which was treated with distilled water (controlled area). Therefore, the short-term exposure suggests that PR at 0.5% (w/v) was safe for using as topical products.
4. Conclusions
The main formulation was composed of 0.5% (w/v) CHOL, 3% (w/v) SPC and water up to 100% (v/v). The optimized vesicles formulations were obtained from adding skin enhancer into the main composition. The invasomes contained 1% (w/v) d-limonene mixed with 10% (v/v) absolute ethanol as the skin enhancer, and transfersomes contained 15% (w/w) sodium deoxycholate (SDC) as edge activator. All carriers gave a vesicle size < 500 nm, polydispersity index (PDI) < 0.3, high zeta potential, entrapment efficiency > 50%, and good stability on storage at 30 °C at 75% RH over 4 months. In addition, all elastic vesicles also showed a higher flexible property than liposomes. Moreover, transfersomes have a higher elasticity than invasomes and liposomes. It leads to provide a higher cumulative amount, steady state flux (Jss) and permeability coefficient (Kp), compared to other formulations. Moreover, the skin accumulation results exhibited that the elastic vesicle formulations which have the higher PR retention in the skin, showed the higher tyrosinase inhibition activity, and melanin content reduction when compared to conventional liposomes in B16 melanoma cells. Therefore, elastic vesicle carriers such as transfersomes and invasomes, presented suitability for the PR delivery system, which is highly effective for skin lightening products.
Acknowledgments
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgment
We are grateful to the Graduate School, Faculty of Pharmaceutical Sciences and Prince of Songkla University for providing financial support. Tunyaluk Limsuwan acknowledges the grant of the Prince of Songkla University Scholarship for her Ph.D. study.
References
- 1.D'Mello SAN, Finlay GJ, Baguley BC. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17(1144):1–18. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costin G-E, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21(4):976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 3.Linder J. Topical melasma treatments. J Pigm Disord. 2014;1(115):1–3. [Google Scholar]
- 4.Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10(6):2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vielhaber G, Schmaus G, Jacobs K. 4-(1-phenylethyl)1,3-benzenediol: a new, highly efficient lightening agent. Int J Cosmet Sci. 2007;29(1):65–66. [PubMed] [Google Scholar]
- 6.Leong HJ, Jang I, Hyun KS. Preparation of alpha-bisabolol and phenylethyl resorcinol/TiO2 hybrid composites for potential applications in cosmetics. Int J Cosmet Sci. 2016;38(5):524–534. doi: 10.1111/ics.12339. [DOI] [PubMed] [Google Scholar]
- 7.Romagnoli C, Baldisserotto A, Vicentini CB. Antidermatophytic action of resorcinol derivatives: ultrastructural evidence of the activity of phenylethyl resorcinol against Microsporum gypseum. Molecules. 2016;21:2–12. doi: 10.3390/molecules21101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan H, Liu G, Huang Y. Development of a nanostructured lipid carrier formulation for increasing photo-stability and water solubility of phenylethyl resorcinol. Appl Surf Sci. 2014;288:193–200. [Google Scholar]
- 9.Gohara M, Yagami A, Suzuki K. Allergic contact dermatitis caused by phenylethyl resorcinol [4-(1-phenylethyl)-1,3-benzenediol], a skin-lightening agent in cosmetics. Contact Dermatitis. 2013;69:311–322. doi: 10.1111/cod.12114. [DOI] [PubMed] [Google Scholar]
- 10.Fan H, Li Y, Huang Y. Preparation and evaluation of phenylethyl resorcinol liposome. Integr Ferroelectr. 2013;151:89–98. [Google Scholar]
- 11.Prisada R, Dinu-Parvuz C. Modalities to enhance the transepidermal penetration of therapeutics of topic usage. Pract Farm. 2012;1-2:17–22. [Google Scholar]
- 12.Lakshmi PK, Kalpana B, Prasanthi D. Invasomes-novel vesicular carriers for enhanced skin permeation. Sys Rev Pharm. 2013;4(1):26–30. [Google Scholar]
- 13.Limsuwan T, Boonme P, Khongkow P. Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. BioMed Research Int J. 2017;2017:1–12. doi: 10.1155/2017/8310979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YT, Xu YM, Zhang SJ. In vivo microdialysis for the evaluation of transfersomes as a novel transdermal delivery vehicle for cinnamic acid. Drug Dev Ind Pharm. 2014;40(3):301–307. doi: 10.3109/03639045.2012.756888. [DOI] [PubMed] [Google Scholar]
- 15.Touitou E, Dayan N, Bergelson L. Ethosomes: novel vesicular carriers for delivery. J Control Release. 2000;65:403–418. doi: 10.1016/s0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 16.Touitou E, Junginger HE, Weiner ND. Liposomes as carriers for topical and transdermal delivery. J Pharm Sci. 1994;83(9):1189–1203. doi: 10.1002/jps.2600830902. [DOI] [PubMed] [Google Scholar]
- 17.Limsuwan T, Boonme P, Amnuaikit T. The optimized HPLC method for quantitative analysis of phenylethyl resorcinol loaded in the novel vesicle carriers and permeated in in vitro skin permeation study. J Chromatogr Sci. 2017;55(10):992–999. doi: 10.1093/chromsci/bmx063. [DOI] [PubMed] [Google Scholar]
- 18.Singh HP, Utreja P, Tiwary AK. Elastic liposomal formulation for sustained delivery of colchicine: in vitro characterization and in vivo evaluation of anti-gout activity. AAPS J. 2009;11(1):54–64. doi: 10.1208/s12248-008-9078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goindi S, Kumar G, Kumar N. Development of novel elastic vesicle-based topical formulation of cetirizine hydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech. 2013;14(4):1284–1293. doi: 10.1208/s12249-013-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limsuwan T, Songkram C, Amnuaikit T. In vitro skin permeation study of ethosome containing mycophenolic acid. Isan J Pharm Sci. 2012;8(1):210–218. [Google Scholar]
- 21.Tomita Y, Maeda K, Tagami H. Melanocyte-stimulating properties of arachidonic acid metabolites: possible role in postinflammatory pigmentation. Pigm Cell Res. 1992;5(5):357–361. doi: 10.1111/j.1600-0749.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 22.Manwar JV, Patil SS, Jadhao RG. Diclofenac sodium loaded nanosized ethosomes: an investigation on Z-average, polydispersity and stability. J Pharma Res. 2017;1(3) [Google Scholar]
- 23.Lei W, Yu C, Lin H. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J Pharma Sci. 2013;8:336–345. [Google Scholar]
- 24.Girhepunje K, Pal R, Gevariya H. Thirumoorthy, “A novel vesicular carrier for enhanced dermal delivery of ciclopirox olamine. Der Pharm Lett. 2010;2(1):360–367. [Google Scholar]
- 25.OECD . Organisation for Economic Co-operation and Development; Paris: 2004. Test guideline 428: skin absorption: in vitro method. [Google Scholar]
- 26.High W, James QDR, Jacquelyn L. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 27.Touitou E, Alkabes M, Dayan N. Ethosomes: novel lipid vesicular system for enhanced delivery. Pharm Res. 1997;S14:305–306. [Google Scholar]
- 28.Duangit S, Opanasopit P, Rojanarata T. Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech. 2013;14(1):133–140. doi: 10.1208/s12249-012-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]