Graphical Abstract
Keywords: Inflammation, Cyclooxygenase, NSAIDs, Serratiopeptidase, Steroids, Enzyme therapeutics
Abbreviations: COX, cyclooxygenase; t-PA, tissue plasminogen activator; NSAIDs, non-steroidal anti-inflammatory drugs; ALL, acute lymphoblastic leukemia; ADR, adverse drug reaction; EC, enzyme commission; IL, interleukins; PGs, prostaglandins; TXs, thromboxane; LOX, lipoxygenase; RA, rheumatoid arthritis; SPMs, specialized pro-resolvins mediators
Abstract
Inflammation remains a key event during most of the diseases and physiological imbalance. Acute inflammation is an essential physiological event by immune system for a protective measure to remove cause of inflammation and failure of resolution lead to chronic inflammation. Over a period of time, a number of drugs mostly chemical have been deployed to combat acute and chronic inflammation. Recently, enzyme based anti-inflammatory drugs became popular over conventional chemical based drugs. Serratiopeptidase, a proteolytic enzyme from trypsin family, possesses tremendous scope in combating inflammation. Serine protease possesses a higher affinity for cyclooxygenase (COX-I and COX-II), a key enzyme associated with production of different inflammatory mediators including interleukins (IL), prostaglandins (PGs) and thromboxane (TXs) etc. Currently, arthritis, sinusitis, bronchitis, fibrocystic breast disease, and carpal tunnel syndrome, etc. are the leading inflammatory disorders that affected the entire the globe. In order to conquer inflammation, both acute and chronic world, physician mostly relies on conventional drugs. The most common drugs to combat acute inflammation are Nonsteroidal anti-inflammatory drugs (NSAIDs) alone and or in combination with other drugs. However, during chronic inflammation, NSAIDs are often used with steroidal drugs such as autoimmune disorders. These drugs possess several limitations such as side effects, ADR, etc. In order to overcome these limitations and complications, enzyme based drugs (anti-inflammatory) emerged, and aim for a new high since the last decade. Serine protease, the largest proteolytic family has been reported for several therapeutic applications, including anti-inflammatory. Serratiopeptidase is a leading enzyme which has a very long history in medical as an effective anti-inflammatory drug. Current study emphasizes present scenario and future prospect of serratiopeptidase as an anti-inflammatory drug. The study also illustrates a comparative analysis of conventional drugs and enzyme based therapeutic to combat inflammation.
1. Overview
The enzyme-based therapeutics became an integral part of modern medicine mainly due to their selectivity and efficiency [1], [2]. In general, the enzymes are basically proteins that possess the tremendous catalytic capacity and offer robust implications in modern healthcare [3], [4]. Inflammation is a physiological response (immune response) against infection, injuries, autoimmune disorders and several diseases. In order to maintain physiological homeostasis, acute inflammation is essential and requires complete resolution [5]. The resolution of inflammation defines tissues homeostasis and balanced immune activity. However, failure of self-resolution of acute inflammation results in chronic inflammation and remains a major challenge [6]. Enzymes were first used as an anti-inflammatory in modern medicine in the 1950s when it was discovered in the United States that intravenous trypsin could relieve inflammation caused by rheumatoid arthritis, ulcerative colitis, and atypical viral pneumonia as well as post-surgical swelling and bruises caused by sports injuries [7], [8]. As per reports published, Japanese began using serratiopeptidase for inflammation in 1957. The increasing success of enzyme therapeutics leads to the application of empirically encapsulated enzyme, including trypsin, chymotrypsin, and bromelain via oral administration [6]. Over the 1980s and early 1990s, Japanese and European researchers compared several enzymes for potential anti-inflammatory activity, and their study indicated that serratiopeptidase was the most effective of all of them in reducing the inflammation response [9]. Currently, serratiopeptidase became widely used in Japan and Europe as the anti-inflammatory and pain treatment of choice [10].
2. Mechanism of inflammation
Inflammation is a protective measure which evolved in advance animal to combat primarily injury and infection [11]. The immune system rapidly responds to any foreign and unwanted change in the tissues, leading to recruitment of immune cells and several other inflammatory mediators. In another word, inflammation is a cleaning process of invading elements and noxious changes leading to maintenance of homeostasis [12]. The acute and chronic inflammation is categorized based on the intensity of trigger and a pathological condition of the tissues. The molecular biology of inflammation is quite complex and associated with enormous numbers of player, including infectious agents, proteins, short peptides, enzyme, and hormones, etc [13]. The external signal is the core element for causing both acute and chronic inflammation later. More important, internal triggers became more devastating in the modern period. Autoimmune disorders such as rheumatoid arthritis (RA) are much more difficult for treatment as our internal biomolecule start acting as a trigger for the immune system [14]. The inflammation lead to several life-threatening diseases and disorders became a major hallmark [15], [16]. However, the pathogenesis, i.e., both the cause and effect of inflammatory diseases, is difficult to pinpoint [17].
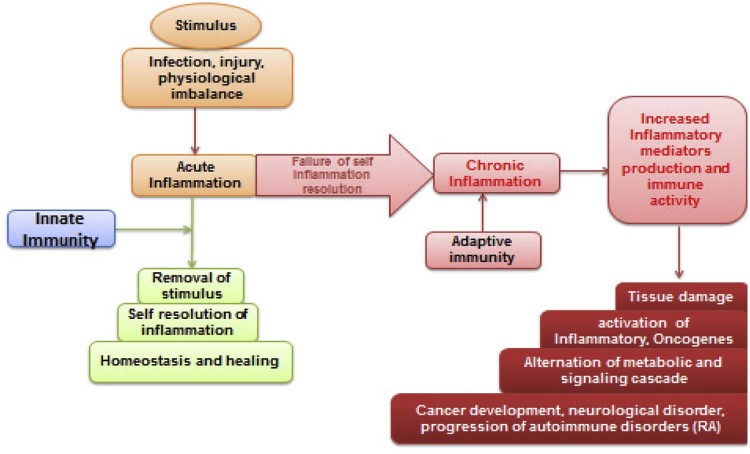
Modern research findings have revealed that inflammation plays a critical role in promoting cancer, in particular, the tumourigenesis, and a process of tumor formation [18], [19] (Fig. 1). In addition to cancer cells, various types of immune cells are commonly found in tumors. Inflammation, both acute and chronic, leads to the production of physiologically active bio-molecules like interleukins, cytokines and other short peptides like kallikreins associated with the fine tuning of the immune system [19], [20]. These pharmacologically active molecules further lead to the building of a microenvironment ideal of tumor growth. The most difficult scenario of inflammation and associated physiological changes has been reported in brain and another part of neuronal tissues [21]. An infection, injury, and entry of harmful molecules trigger the immune system rapidly. Meningitis is a serious disease in which there is inflammation of the meninges, caused by viral or bacterial infection, and marked by an intense headache and fever, sensitivity to light, and muscular rigidity [22]. The consequence is activation of several other metabolic pathways like IKK-β and JNK1-mediated chronic inflammation [23].
Fig. 1.
A detailed overview of various causes resulting in inflammation. Both environmental and endogenous factors are equally associated with the generation of inflammatory mediators and these mediators further affect tissues of normal homeostasis by affecting blood and lymph flow.
3. Therapeutics to combat inflammation
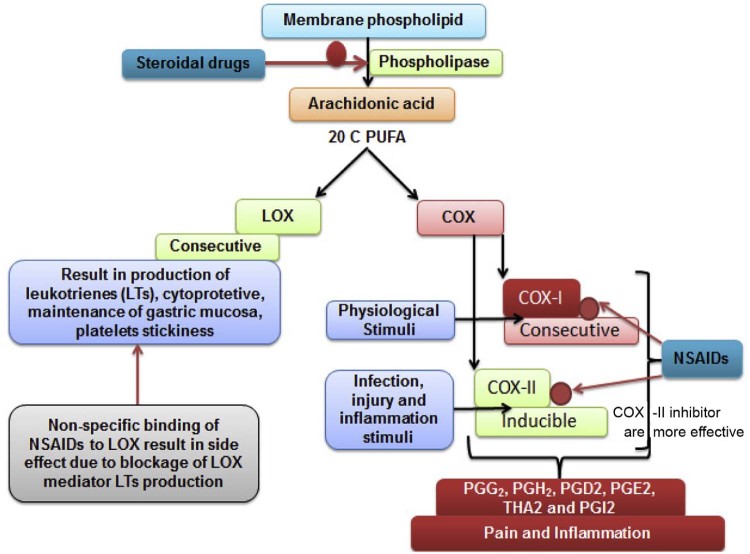
The NSAIDs are leading drugs prescribed to combat both acute and chronic inflammation in the modern times [24]. NSAIDs are chemically synthesized and designed to block the production of inflammatory mediators via competitive inhibition of COX-I [25]. COX-I is a key enzyme which catalyzes the breakdown of arachidonic acid, a 20 carbon fatty acid. The stimuli (external and endogenous) switch on the degradation of membrane phospholipid via activation of phospholipase [26]. Cyclooxygenase (COX-I) is primarily responsible for large scale production of cytokines and interleukins after mechanical injury and infection. The NSAIDs more likely provide a symptomatic relief rather than cure [27]. These medicines are also associated with several complications and limitations.
The long-term use of NSAIDs often leads to a negative impact on liver and renal system. These drugs are reported with several side effects and adverse drug reaction which can be mild to severe (Fig. 2). The most common side effect of long-term use of NSAIDs is gastric bleeding [28]. The etiology of infection is much more complex and at the molecular level, there are several players involved in different types of inflammation. For example, autoimmune disorders like rheumatoid arthritis further require an effective dose of steroids such as prednisolone in an advanced stage. The long-term use of steroid alone or in combination with NSAIDs affects immune system severely [29]. These existing complications of chemical based anti-inflammatory drugs led to the discovery of biological molecules, a special enzyme to replace conventional drugs [30].
Fig. 2.
A detailed mechanism of steroidal and NSAIDs used to combat inflammation. The inhibition of CoX I and lipoxygenase pathways is the primary cause of side effects by NSAIDs.
4. Enzymes as therapeutics
Enzymes play an integral role in a biological world by offering their potential as a biocatalyst [31]. With their broad substrate affinity and robust catalyst, activity enzyme has been used for many decades in different areas including industries, agriculture, research, and development [32], [33]. However, the therapeutic application of enzymes became vital in the current century as emerging novel diseases and failure of conventional drugs. Since the last few decades, different enzymes have fulfilled our therapeutic need in cancer treatment, curing blood vascular disorders, enzyme deficiency disorders and an alternate for anti-inflammatory drugs [34], [35]. It's quite interesting among various classes of enzyme one group called serine protease had shown tremendous scope in modern medicine. Serine proteases are widely present in the biological world, including animal, plant, and microbes. Serine proteases have a long history of therapeutic applications; tissue plasminogen activators either from animal or microbial sources are the most effective way to cure vascular disorders [36], [37]. Treatment of various kinds of tumor and cancers, including acute lymphoblastic leukemia (ALL), relies on enzyme like l-asparaginase and l-glutamine [38]. Caspase, a native proteolytic enzyme, had shown tremendous scope in the management of cancer and fighting against different classes of viruses [39], [40].
5. Serratiopeptidase as alternate anti-inflammatory drug
Serratiopeptidase (EC No 3.4.24.40) has a long history in medicine and is widely used to combat various kinds of inflammation and inflammatory disorders [41]. Serratiopeptidase or serrapeptase is a protein (proteolytic) enzyme isolated from the non-pathogenic enterobacteria Serratia E15 found in silkworms. Serratiopeptidase often prescribed in various specialties like surgery, orthopedics, otorhinolaryngology, gynecology and dentistry for its anti-inflammatory, anti-endemic and analgesic effects [42]. In the research in recent years, exploration illustrated enzyme also plays a vital role in the management of atherosclerosis as it does possess fibrinolytic and caseinolytic properties [43]. Like most enzymes, serratiopeptidase also possesses broad substrate affinity and has been to be reported therapeutically useful in the management of pain and inflammation. The concept of enzyme promiscuity [44] is not yet reported in serratiopeptidase but recent research finding suggests enzyme significantly binds with a different substrate.
5.1. Resolution of inflammation
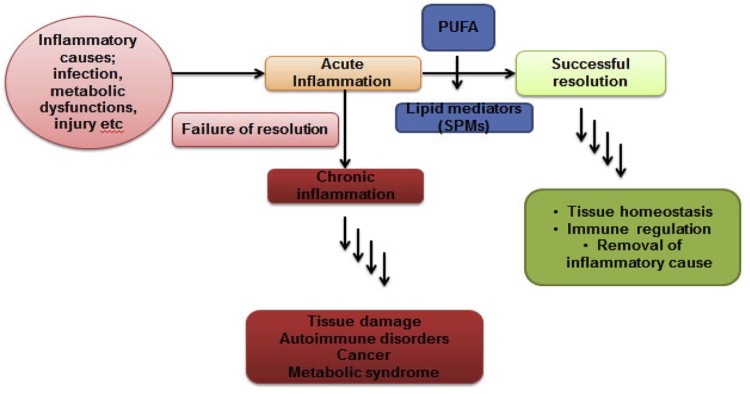
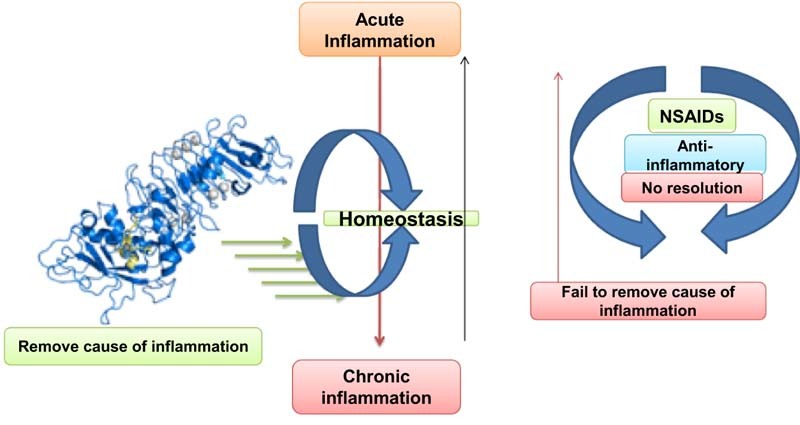
Every health system possesses both pro and anti-inflammatory molecules and balance between brings tissue homeostasis [45]. Lipid mediators primarily derived from polyunsaturated fatty acids (PUFAs) are a key player for resolution of inflammation (Fig. 3) [46]. These molecules known as specialized pro-resolvin mediators (SPMs) are lipoxin, resolvin, protectin and meresins [47]. The w-3 and w-6 PUFAs under LOX-mediated catalysis result in different kinds of lipid mediators. Among these, E (RvE) and D (RvD) series resolvin are leading pro-resolvin mediators to drive resolution of inflammation [48]. Eicosapentaenoic acid (EPA) derived resolvin E (RvE; RvE1, RvE2 and RvE3) and docosahexaenoic acid (DHA) derived resolvin D (RvD; EvD1-RvD5) are associated with a number of physiological events [49]. Now, LOXs (5, 12 and 15 LOX) are key enzymes which catalyze the biosynthesis of SPMs and non-specific inhibition of NSAIDs affects native inflammation resolution [10]. New generation NSAIDs are effective as COX-II specific but their clinical applications remain questionable. Hence, researchers find more concern on enzyme based agents and are seeking more specific drugs like anti-inflammatory agents. Here, serratiopeptidase and similar enzyme (enzyme) indirectly assist resolution of inflammation as they do not affect LOX-catalyzed SPMs production.
Fig. 3.
Scheme of resolution of inflammation: causes, acute and chronic inflammation.
5.2. Molecular mechanism
There is very little evidence about the molecular mechanism of serratiopeptidase as an anti-inflammatory agent. However, serratiopeptidase was reported to have a direct effect on the movement of immune cells. Enzyme regulates recruitment of PMMs and other lymphocytes at the site of inflammation [50]. A recent finding has suggested that serratiopeptidase reduces capillary permeability induced by histamine, bradykinin, and serotonin; breaks down abnormal exudates and proteins; facilitates the absorption of decomposed products through blood and lymphatics [48]. Further, enzyme promotes wound healing and repair and restores the skin temperature of the inflamed area, burn or trauma to normal. The activity of serratiopeptidase remains stable and offer more efficiency in combination with the addition of metal ions like zinc and manganese [51]. Serratiopeptidase has been shown to be absorbed from the digestive tract. Less is known about its absorption from intestine but clathrin mediated endocytosis could be involved in mechanism. On oral administration, it is unchanged when absorbed into the systemic circulation, from where it penetrates into all tissues. It reaches high concentrations in the inflamed tissues. It attains peak levels in one hour [52]. Unlike conventional anti-inflammatory drugs (NDAIDs), serratiopeptidase does not bind with LOX and block LOX-catalyzed SPMs biosynthesis [53]. More likely, serratiopeptidase was reported to have an effect in regulating immune cell migration from lymph node to inflamed and injured tissue [54]. Such unique mechanism and broad substrate affinity suggest a role of the enzyme in bringing tissue to normal condition i.e. maintaining homeostasis. Being a serine protease with immense proteolytic activity, the enzyme could participate in wound cleaning and healing [55].
A research finding in 2015 by Chappi et al. has concluded that methylprednisolone affords better pain relief while serratiopeptidase exerts better anti-inflammatory and anti-swelling effects in the postoperative period [50]. Further, synergistic combinations of methylprednisolone and serratiopeptidase proved to be more effective when extensive post-operative sequelae are expected. In the year 2015, serratiopeptidase loaded chitosan nanoparticles were analyzed for the effective anti-inflammatory drug in different inflammatory disease models [56]. The enzyme was also reported effective in the treatment of peri-implantitis in combination with several antibiotics. In the last few years, massive research findings have been made in finding novel drug delivery methods for serratiopeptidase [49]. The use of ciprofloxacin and serratiopeptidase periodontal solutions for extended drug delivery was studied in 2014. In a comparative study, the anti-inflammatory activity of serratiopeptidase with dexamethasone in the control of inflammation was demonstrated [57]. Serratiopeptidase along with broad spectrum antibiotics was employed in the treatment of osteoarticular infection. In a study, Okumura H et al. have illustrated proteolytic enzyme like serratiopeptidase is effective in the treatment of osteoarticular infection [58].
5.3. Therapeutic application of serratiopeptidase
The medical use of serratiopeptidase, primarily as an anti-inflammatory enzyme-based drug, has a very long history. Several findings have suggested enzyme alone or in combination with other drugs reported to have an effect on sinusitis and bronchitis, atherosclerosis, carpal tunnel syndrome, rheumatoid arthritis and other autoimmune diseases [59]. The exact molecular mechanism of serratiopeptidase is not known completely but research findings have demonstrated that enzyme possesses the unique ability to dissolve the dead and damaged tissue that is a by-product of the healing response without harming living tissues [60]. Serratiopeptidase also works by modifying cell-surface adhesion molecules. These cell surface adhesion molecules are directly and indirectly responsible for inflammation and bringing immune cells in damaged tissues [61]. A study by a team of Italian researchers suggests that proteolytic enzymes such as serratiopeptidase could significantly enhance the effectiveness of antibiotics against biofilm and can inhibit biofilm formation. Serratiopeptidase has been shown to enhance the activity of several antibiotics including ampicillin, ciclacillin, cephalexin, minocycline and cefotiam [62].
The clinical use of sserratiopeptidase during allergic conditions was studied and it actually reduces the thickness and viscosity of the mucus and improves the elimination of it through bronchopulmonary secretions [63]. The use sserratiopeptidase as an expectorant is rarely reported in any kind of literature but being a proteolytic enzyme it can replace the use of histamine and antihistamines in expectorant formulations. The enzyme serratiopeptidase was also used in the successful treatment of fibrocystic breast disease to help reduce swelling and pain with 25% of the patients receiving the enzyme reporting moderate to marked improvement with no adverse reactions [63]. Another promising area is the use of serratiopeptidase to break down atherosclerotic plaque. Khateeb et al. have demonstrated the role of serratiopeptidase in the management of ortho-dental inflammatory syndrome [64]. Because the enzyme digests non-living tissue and leaves live tissue alone; it may be effective in removing the deposits of fatty substances, cholesterol, cellular waste products, calcium and fibrin on the inside of the arteries. The fibrinolytic (clot removal) activity of serratiopeptidase may also be able to help with thickened blood, increased risk of stroke, and phlebitis/thrombophlebitis.
6. Conclusion
Inflammation and inflammatory disorders are the leading cause of death and physical deformities in the modern world. The most difficult challenge in the management of an inflammation is finding its exact cause at the molecular level and appropriate medication. As, immune system both cell mediate and humoral are centrally involve the inflammation hence management of inflammation remains complicated. In general, inflammation is defined as an excessive localized edema due to an enhanced vascular infiltration at the site of inflammation. Both acute and chronic inflammation are characterized by different sets of metabolic pathways triggered by different stimuli with external or endogenous or both. The autoimmune diseases and disorders are the most challenging in finding ideal therapeutics. Over many decades, symptomatic relief of inflammation was achieved by use of synthetic compounds like NDAIDs and steroids in chronic cases or both. As we stated above, the inflammation is a complex immune response based on various kinds of stimuli and may not be cured by common drugs. As a result, most of the anti-inflammatory drugs are associated with severe side effects and adverse drug reactions. These drugs are associated with symptomatic relief rather than cure.
In the last one decade, use of biological molecules like proteins (enzyme) as therapeutics emerged as one of major areas of modern medicine. Several enzymes are approved for clinical uses for many life-threatening diseases and disorders like t-PA and staphylokinase as clot buster and l asparaginase as an anticancer drug etc. Similarly, a serine protease from microbial sources has shown tremendous scope in the cure of various kinds of inflammation and inflammatory disorders. Serratiopeptidase is a serine protease with a molecular weight 60 kDa has been significantly reported for its potent anti-inflammatory activity. The clinical use of enzyme was reported for many diseases like arthritis, sinusitis, inflammatory bowel disease (IBD) and bronchitis etc. The current challenge toward developing serratiopeptidase into an effective broadspectrum anti-inflammatory drug is due to lack of precise molecular mechanism. This proteolytic enzyme was reported effective in many diseases precisely during surgical events for a long time, but there is a lack of research evidence and available literature. The current study again emphasizes the potential of the enzyme as broad spectrum anti-inflammatory drugs with minimal side effects and complications.
Acknowledgment
I would like to thank the Department of Biochemistry and Genetics, Barkatullah University, Bhopal, Madhya Pradesh, India for kind support providing resources and infrastructure for the study.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Kang T.S., Stevens R.C. Structural aspects of therapeutic enzymes to treat metabolic disorders. Hum Mutat. 2009;30(12):1591–1610. doi: 10.1002/humu.21111. [DOI] [PubMed] [Google Scholar]
- 2.Valayannopoulos V., Brassier A., Chabli A. Enzyme replacement therapy for lysosomal storage disorders. Arch Pediatr. 2011;18(10):1119–1123. doi: 10.1016/j.arcped.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Verma M.K., Pulicherla K.K. Enzyme promiscuity in Earthworm serine protease- Substrate versatility and therapeutic potential. Amino Acids. 2016;48(4):941–948. doi: 10.1007/s00726-015-2162-3. [DOI] [PubMed] [Google Scholar]
- 4.Li Y., Cirino P.C. Recent advances in engineering proteins for biocatalysis. Biotechnol Bioeng. 2014;111(7):1273–1287. doi: 10.1002/bit.25240. [DOI] [PubMed] [Google Scholar]
- 5.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiger S., Harper J.L. Mechanisms of spontaneous resolution of acute gouty inflammation. Curr Rheumatol Rep. 2014;16(1):392. doi: 10.1007/s11926-013-0392-5. [DOI] [PubMed] [Google Scholar]
- 7.Rainsford K.D. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 8.Bertolini A., Ottani A., Sandrini M. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr Med Chem. 2002;9(10):1033–1043. doi: 10.2174/0929867024606650. [DOI] [PubMed] [Google Scholar]
- 9.Joshi K.K., Nerurkar R.P. Anti-inflammatory effect of the serratiopeptidase–rationale or fashionable: a study in rat paw edema model induced by the carrageenan. Indian J Physiol Pharmacol. 2012;56(4):367–374. [PubMed] [Google Scholar]
- 10.Malshe P.C. Orally administered serratiopeptidase: can it work? J Assoc Physicians India. 1998;46(5):492. [PubMed] [Google Scholar]
- 11.Lomax A.R., Calder P.C. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des. 2009;15(13):1428–1518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Manwani B., Leng S.X. Frailty, inflammation, and immunity. Aging Dis. 2011;2(6):466–473. Epub 2011 Dec 2. [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence T., Gilroy D.W. Chronic inflammation: a failure of resolution? Int J Exp Pathol. 2007;88(2):85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidari B. Rheumatoid Arthritis: early diagnosis and treatment outcomes. Caspian J Intern Med. 2011;2(1):161–170. [PMC free article] [PubMed] [Google Scholar]
- 15.Gilroy D., De Maeyer R. Semin Immunol. 2015;27(3):161–168. doi: 10.1016/j.smim.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65(12 Pt 2):S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 17.Serhan C.N. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24(2):341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Bannenberg G.L. Resolvins: current understanding and future potential in the control of inflammation. Curr Opin Drug Discov Devel. 2009;12(5):644–658. [PubMed] [Google Scholar]
- 19.Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79(3–4):123–130. [PMC free article] [PubMed] [Google Scholar]
- 20.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrovolskaia M.A., Kozlov S.V. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. 2005;5(5):325–344. doi: 10.2174/1568009054629645. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman O., Weber R.J. Pathophysiology and treatment of bacterial meningitis. Ther Adv Neurol Disord. 2009;2(6):1–7. doi: 10.1177/1756285609337975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson A.R., Milner J.J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues E.B., Farah M.E., Bottos J.M. Nonsteroidal anti-inflammatory drugs in the treatment of retinal diseases. Dev Ophthalmol. 2016;55:212–220. doi: 10.1159/000431197. [DOI] [PubMed] [Google Scholar]
- 25.Dionne R.A., Berthold C.W. Therapeutic uses of non-steroidal anti-inflammatory drugs in dentistry. Crit Rev Oral Biol Med. 2001;12(4):315–330. doi: 10.1177/10454411010120040301. [DOI] [PubMed] [Google Scholar]
- 26.Lees P., Higgins A.J. Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs in the horse. Equine Vet J. 1985;17(2):83–96. doi: 10.1111/j.2042-3306.1985.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 27.Scott D.L., Berry H., Capell H. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford) 2000;39(10):1095–1101. doi: 10.1093/rheumatology/39.10.1095. [DOI] [PubMed] [Google Scholar]
- 28.Pasinetti G.M. From epidemiology to therapeutic trials with anti-inflammatory drugs in Alzheimer's disease: the role of NSAIDs and cyclooxygenase in beta-amyloidosis and clinical dementia. J Alzheimers Dis. 2002;4(5):435–445. doi: 10.3233/jad-2002-4510. [DOI] [PubMed] [Google Scholar]
- 29.Richy F., Bruyere O., Ethgen O. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63(7):759–766. doi: 10.1136/ard.2003.015925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imbimbo B.P. The potential role of non-steroidal anti-inflammatory drugs in treating Alzheimer's disease. Expert Opin Investig Drugs. 2004;13(11):1469–1481. doi: 10.1517/13543784.13.11.1469. [DOI] [PubMed] [Google Scholar]
- 31.Cooney D.A., Rosenbluth R.J. Enzymes as therapeutic agents. Adv Pharmacol Chemother. 1975;12:185–289. doi: 10.1016/s1054-3589(08)60222-7. [DOI] [PubMed] [Google Scholar]
- 32.Amadasi A., Bertoldi M., Contestabile R. Pyridoxal 5'-phosphate enzymes as targets for therapeutic agents. Curr Med Chem. 2007;14(12):1291–1324. doi: 10.2174/092986707780597899. [DOI] [PubMed] [Google Scholar]
- 33.Rossi J.J., Sarver N. RNA enzymes (ribozymes) as antiviral therapeutic agents. Trends Biotechnol. 1990;8(7):179–183. doi: 10.1016/0167-7799(90)90169-x. [DOI] [PubMed] [Google Scholar]
- 34.Tasaka K., Meshi T., Akagi M. Anti-inflammatory activity of a proteolytic enzyme, Prozime-10. Pharmacology. 1980;21(1):43–52. doi: 10.1159/000137414. [DOI] [PubMed] [Google Scholar]
- 35.Verma M.K., Sobha K. In-vitro evaluation of antioxidant and anti-inflammatory properties of autolysed extract of the Indian earthworm Pheretima Posthuma. Res J Pharm Biol Chem Sci. 2013;4(4):888–898. [Google Scholar]
- 36.Verma M.K., Pulicherla K.K. Targeting therapeutics across the blood brain barrier (BBB), prerequisite towards thrombolytic therapy for cerebrovascular disorders-an overview and advancements. AAPS PharmSciTech. 2015;16(2):223–233. doi: 10.1208/s12249-015-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma M.K., Sobha K. Understanding mechanism genetic risk factors in the beginning and progression of rheumatoid arthritis – current scenario and future prospect. Inflamm Res. 2015;64(9):647–659. doi: 10.1007/s00011-015-0843-8. [DOI] [PubMed] [Google Scholar]
- 38.Verma M.K., Verma Y.K. Conventional thrombolytic need to refine at molecular level for safe and efficient management of cerebrovascular disorders – an overview. Int J Pharm Sci. 2013;5(Suppl. 1):448–454. [Google Scholar]
- 39.Perchellet E.M., Wang Y., Weber R.L. Antitumor triptycene bisquinones induce a caspase-independent release of mitochondrial cytochrome c and a caspase-2-mediated activation of initiator caspase-8 and -9 in HL-60 cells by a mechanism which does not involve Fas signaling. Anticancer Drugs. 2004;15(10):929–946. doi: 10.1097/00001813-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Charalambous C., Pitta C.A., Constantinou A.I. Equol enhances tamoxifen's anti-tumor activity by induction of caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC Cancer. 2013;13:238. doi: 10.1186/1471-2407-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jadav S.P., Patel N.H., Shah T.G. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother. 2010;1(2):116–117. doi: 10.4103/0976-500X.72362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed A.U. An overview of inflammation: mechanism and consequences. Front Biol. 2011;6(4):274–281. [Google Scholar]
- 43.Bhagat S., Agarwal M., Roy V. Serratiopeptidase: a systematic review of the existing evidence. Int J Surg. 2013;11(2013):209–217. doi: 10.1016/j.ijsu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Panagariya A., Sharma A.K. A preliminary trial of serratiopeptidase in patients with carpal tunnel syndrome. J Assoc Physicians India. 1999;47:1170e2. [PubMed] [Google Scholar]
- 45.Klein G., Kullich W. Short-term treatment of painful osteoarthritis of the knee with oral enzymes. A randomized, double-blind study versus diclofenac. Clin Drug Invest. 2000;19:15e23. [Google Scholar]
- 46.Tsuyama N., Yoshio O., Makoto F. Clinical evaluation on anti-swelling drug-A- 4700 (Reparil tablet) in the orthopaedic field. A comprehensive double-blind controlled trial compared with serratiopeptidase and placebo in 20 orthopedic clinics. Rinsho Hyoka (Clin Eval) 1977;5:535e75. [Google Scholar]
- 47.Tachibana M., Mizukoshi O., Harada Y. A multi-centre, double-blind study of serrapeptase versus placebo in post-antrotomy buccal swelling. Pharmatherapeutica. 1984;3:526e30. [PubMed] [Google Scholar]
- 48.Nakamura S., Hashimoto Y., Mikami M. Effect of the proteolytic enzyme serrapeptase in patients with chronic airway disease. Respirology. 2003;8:316e20. doi: 10.1046/j.1440-1843.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 49.Sannino G., Gigola P., Puttini M. Combination therapy including serratiopeptidase improves outcomes of mechanical-antibiotic treatment of periimplantitis. Int J Immunopathol Pharmacol. 2013;26(3):825–831. doi: 10.1177/039463201302600332. [DOI] [PubMed] [Google Scholar]
- 50.Chappi D.M., Suresh K.V., Patil M.R. Comparison of clinical efficacy of methylprednisolone and serratiopeptidase for reduction of postoperative sequelae after lower third molar surgery. J Clin Exp Dent. 2015;7(2):e197–e202. doi: 10.4317/jced.51868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tachibana M., Mizukoshi O., Harada Y. A multi-centre, double-blind study of serrapeptase versus placebo in post-antrotomy buccal swelling. Pharmatherapeutica. 1984;3(8):526–530. [PubMed] [Google Scholar]
- 52.Jadav S.P., Patel N.H., Shah T.G. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother. 2010;1(2):116–117. doi: 10.4103/0976-500X.72362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mecikoglu M., Saygi B., Yildirim Y. The effect of proteolytic enzyme serratiopeptidase in the treatment of experimental implant-related infection. J Bone Joint Surg Am. 2006;88(6):1208–1214. doi: 10.2106/JBJS.E.00007. [DOI] [PubMed] [Google Scholar]
- 54.Flower R.J., Blackwell G.J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278(5703):456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- 55.Buckley C.D., Gilroy D.W., Serhan C.N. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 56.Flower R.J., Perretti M. Controlling inflammation: a fat chance? J Exp Med. 2005;201(5):671–674. doi: 10.1084/jem.20050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garg R., Aslam S., Garg A. A prospective comparative study of serratiopeptidase and aceclofenac in upper and lower limb soft tissue trauma cases. Int J Pharmacol Pharm Technol. 2012;1(2):11–16. [Google Scholar]
- 58.Okumura Y., Sato H., Seiki M. Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin- a possible cell surface activator. FEBS Lett. 1997;402:181–184. doi: 10.1016/s0014-5793(96)01523-2. [DOI] [PubMed] [Google Scholar]
- 59.Malshe P.C. Orally administered serratiopeptidase: can it work ? J Assoc Physicians India. 1998;46(5):492. [PubMed] [Google Scholar]
- 60.Ishihara Y., Kitamura S., Takaku F. Experimental studies on distribution of cefotiam, a new betalactam antibiotic, in the lung and trachea of rabbits. II. Combined effects with serratiopeptidase. Jpn J Antibiot. 1983;36(10):2665–2670. [PubMed] [Google Scholar]
- 61.Esch P.M., Gemgross H., Fabian A. Reduction of postoperative swelling. Objective measurement of swelling of the upper ankle joint in treatment with serrapeptase-a prospective study. Fortschr Med. 1989;107(4):67–68. 71-2. [PubMed] [Google Scholar]
- 62.Panagariya A., Sharma A.K. A preliminary trial of serratiopeptidase in patients with carpal tunnel syndrome. J Assoc Physicians India. 1999;47(12):1170–1172. [PubMed] [Google Scholar]
- 63.Klein G., Kullich W. Short-term treatment of painful osteoarthritis of the knee with oral enzymes. A randomized, double-blind study versus diclofenac. Chem Drug Invest. 2000;19(1):15–23. [Google Scholar]
- 64.Khateeb T.A., Nusair Y. Effect of the proteolytic enzyme serrapeptase on swelling, pain and trismus after surgical extraction of mandibular third molars. Int J Oral Maxillofac Surg. 2008;37:264e8. doi: 10.1016/j.ijom.2007.11.011. [DOI] [PubMed] [Google Scholar]