Abstract
Rapid prototyping based on in silico design and 3D printing enables fast customization of complex geometries to multiple needs. This study utilizes, additive manufacturing for rapid prototyping of elements for continuously operating mixing geometries including interfaces with process analytical technology (PAT) tools, to show that 3D printing can be used for prototyping of both parts of production line and PAT interfacing solution. An additional setup was designed for measuring the dynamic calibration samples for a semi-quantitative near infrared (NIR) spectroscopic model. The powder was filled in a small calibration chamber and in-line NIR spectra of calibration samples were collected from moving material while mimicking the powder flow dynamics in a typical continuous mixer. This dynamic powder mixing system was compared with a static powder calibration model where the NIR probe was placed at different positions on a static sample. Principal component analysis (PCA) revealed that the 3D printed device with dynamic measurement of the NIR spectra had more potential for quantitative analysis. With the prototype continuous mixer, two differently placed process interfaces for NIR spectroscopic monitoring of the powder mixing were evaluated. With this approach, the importance of positioning the process analytical tools to assess the blend uniformity could be demonstrated. It was also observed that with the longer mixing geometry, a better mixing result was achieved due to a larger hold up volume and increased residence time.
Keywords: 3D printing, Continuous mixing, Near-infrared spectroscopy, Additive manufacturing
Graphical abstract

1. Introduction
The interest for continuous manufacturing of solid dosage forms is growing within pharmaceutical sciences. However, the transition from traditional batch to batch manufacturing to continuous manufacturing is challenging, although continuous manufacturing has well documented advantages [1], [2], [3], [4]. The regulatory burden related to implementation of these principles is making many pharmaceutical companies hesitant to change towards continuous manufacturing [1].
Engineering of the mechanical parts of continuous manufacturing lines for solid dosage forms have been investigated with a focus on the powder blending process, the granulation or drying process and the tableting or capsule filling part [4], [5], [6], [7], [8], [9], [10]. It is crucial to identify critical engineering parameters to effectively implement continuous manufacturing and potentially, experimentally investigate different engineering solutions. Continuous mixing has been intensively studied, taking into consideration environmental factors, like controlling humidity and temperature, the effect of material properties and the influence of the setup (feeder, mixing chamber and screw) on the mixing process [7], [11]. Modifying the existing engineering solution of mechanical parts is challenging and often limited to the already existing options available from the manufacturer of the specific production line. Rapid prototyping provides a fast approach for investigating different engineering solutions at smaller scale and implementation of manufacturing innovation based on computational approaches.
Previous work has been focusing on assessing how different process parameters and powder properties influence the mixing result. Mean residence time and residence time distribution (RTD) are the key engineering terms used, to characterize the continuous mixing processes. [12]. Gao et al. found that the RTD is more sensitive to changes in blade speed than to the powder feed rate into the system or the blade configuration. They further identified that a low blade speed and a lower flow rate lead to a higher mean residence time and a wider RTD, while with increasing feed rate the RTD becomes more narrow [5]. A narrow RTD and a long mean residence time increase the chance of achieving a consistent homogeneous powder mixture [7]. Different types of formulations have different challenges during the mixing process. High dose formulations have typically been shown to be mixed well at high mixer speeds [13], whereas, low dose formulations are a challenge in pharmaceutical development and production as the concentration can be too low to be detected by in-line process analytical tools. However, it was shown that during continuous mixing the paddle orientation and the mixer speed did not have an influence on the critical quality attributes of the tablets with a low content [14]. Besides the design of the mixer, the material properties largely dominate the mixing performance. Especially to break clumps or agglomerates of cohesive powder, a certain minimum of shear rate is required [7]. During the continuous mixing process, real-time monitoring of the mixing is important to ensure a homogeneous blend and, if necessary, adjustment of the process parameters to ensure the quality of the blend. Real-time in-line monitoring with spectroscopic methods such as near-infrared (NIR) spectroscopy [2], [15] and Raman spectroscopy have been proven to show good results [16]. NIR spectroscopy has a wide range of applications during solid dosage form manufacturing. It can be used indirectly to monitor continuous powder flow in the feeders, by analyzing the density of the powder [17]. Granulation and drying processes can be monitored using NIR spectroscopy [18], [19], [20]. The powder mixture can be monitored right before tableting by placing a NIR probe before the tablet punches [21] and the content uniformity of tablets can be determined using NIR spectroscopy [22], [23], [24]. Previous work has shown that the process measurements with an in-line NIR spectrometer during continuous mixing results in comparable results with measurements after the mixing process [25]. It is, however, crucial that the NIR probe is properly positioned in the system, and it might even be needed to identify and evaluate multiple positions in the mixing chamber [26], [27], [28], [29]. Developing a quantitative model for the concentration of a given analyte is an important part of a functional continuous production line. Alam et al. found that a NIR calibration based on only the raw material in the formulation gave a similar prediction performance, compared to a full factorial calibration set with eleven different samples [30]. Also moving the powder blend manually in a vessel before taking each spectrum resulted in a good calibration method [31]. Evaluation of new ideas for mechanical parts (screw design) or a modification of a continuous mixing setup can be challenging and expensive using existing solutions.
In silico design and modelling of pharmaceutical processes are gaining increasing interest [32]. The behavior of powder and granular material can be modelled during different unit operations and the expected outcome can be predicted [33], [34], [35]. Another approach is the in silico design of process geometries to optimize the design and to modify it to its own needs and by modeling, identify the optimal geometry. 3D printing provides a platform for rapid prototyping of the designed geometries and testing these in a small scale [36]. Additionally, interfaces for process analytical tools and the behavior of material of interest at the process measurement interface can be optimized without destroying an existing equipment. Furthermore, these different in silico designs can be directly printed and new prototype ideas tested with minimal cost [32].
In this study, computer aided design (CAD) and 3D printing were first used to design a novel calibration setup for NIR spectroscopy. This new calibration setup enables dynamic mixing of the powder sample during the measurement of the calibration samples with a final goal to reduce the spectral variation between the calibration samples. Additionally, a prototype production geometry for a continuous mixing line was designed and 3D printed with two interfaces for an NIR probe to monitor the powder blending process in this continuous mixer. This approach enables an efficient optimization of production geometries including dimensions of equipment and further, location of process analytical interfaces.
2. Material and methods
2.1. Material
FlowLac 100 SD (spray dried lactose monohydrate, lactose monohydrate, Meggle Pharm, Wasserburg, Germany) and Pearlitol 100 SD (spray dried mannitol, mannitol, Barentz ApS, Breda, The Netherlands) were used as model excipients in this study. Polylactic acid (PLA, Innofil 3D, Emmen, Netherlands) and polyvinyl alcohol (PVA, Ultimaker, Geldermalsen, Netherlands) were used to 3D print the prototype elements of continuous production lines.
2.2. Methods
2.2.1. Sample preparation
All used powder samples were sieved (500 µm) before they were used in an experimental setup. For the NIR calibration samples, lactose monohydrate and mannitol were weighed in different weight ratios: 0/100, 20/80, 40/60, 60/40, 80/20 and 100/0, respectively, with a total mass of 100 g. The mixtures were blended in a Turbula mixer (Type T2F, System Schatz, Willy A. Bachhofen AG, Maschinenfabrik, Switzerland) for 2 min at 32 rounds per minute (rpm).
2.2.2. Powder characterization
The powder flowability and the wall friction were analyzed using a ring shear tester (RST-Xs., Dr. Dietmar Schulze, Wolfenbüttel, Germany). A shear cell type XS-Mr. was used for the flowability measurements and a shear cell type XS-WM for the wall friction measurements (both: Dr. Dietmar Schulze, Wolfenbüttel, Germany). The powder flowability was analyzed using a preshear of 1 kPa with normal stresses set to 200, 500 and 800 Pa. The flow function coefficient (ffc) and the bulk density were analyzed with this method. The wall friction was analyzed against a steel wall plate and a 3D printed wall plate, printed from the same material as the later used setups. The applied normal stresses ranked from 1400 to 200 Pa with a 200 Pa distance. The orientation of the print layers of the wall friction plate is not parallel to the shear movement as the wall plate was printed using horizontal nozzle moves as opposed to circular nozzle moves.
The flow rate was further analyzed using a FlowPro (SAY-group, Finland). Approximately 5 mL of sample was filled in the sample holder and tapped against the top of the instrument with a frequency of 1 s−1. The mass of a sample released through a 3 mm hole on a balance was recorded.
The particle size distribution was analyzed using a laser diffraction particle size analyzer (MasterSizer 2000, Malvern Instruments, Worcestershire, UK) equipped with a dry powder feeder (Scirocco 2000, Malvern Instruments, Worcestershire, UK). An air pressure of 3 bar was used to disperse the powder samples. Scanning electron microscopy (SEM, TM3030 Tabletop Microscope, HITACHI, Tokyo, Japan) gave an indication about the particle shape. The samples were prepared on carbon sticky stubs and coated with gold (Cressington sputter coater 108, Cressington Scientific Instruments, Watford, UK). The picture was taken with a voltage of 15 kV and 300× magnification.
The dynamic vapor sorption of the excipients was analyzed using a VTI-SA+ (TA-instruments, Worcestershire, UK). The sample was dried at 60 °C for a maximum time of 180 min or until weight equilibrium was established (< 0.001 wt% in 5 min). The relative humidity was increased from 0 to 95% and decreased to 0% in 10% steps (5% step from 90%–95% relative humidity). Equilibrium time for each step was set to 150 min or until weight-equilibrium was reached (< 0.001 wt% in 5 min). Data points were recorded every 2 min or at a weight change of 0.010 wt%.
2.2.3. 3D printing
The structures were designed by computer aided design (CAD) using the online platform tinkercad.com (Autodesk, CA, US) and were exported as stl. files. A MakerBot Replicator 2 (MakerBot, New York, NY, US) was used to print structures from PLA, with a layer height of 200 µm. An Ultimaker 3 extended (Ultimaker, Geldermalsen, Netherlands) was used to print structures with PLA and PVA as support structure, with a layer height of 150 µm. No post processing was performed after printing. Before printing, the files were converted using Cura 2.4.0 (Ultimaker, Geldermase, Netherlands) to create a readable file for the printer.
2.2.4. Near infrared spectroscopy
For spectroscopic analysis a NIR spectrometer (NIR-256L-2.2T2) equipped with a reflectance probe (7-400-SMA, fiber diameter 400 µm) and the light source LS-E-NIR (all Control Developments Inc., IN, US) was used. A spectroscopic range of 1100–2200 nm was applied to the sample. The integration time per spectrum was set to 0.01 s and an average of 32 measurements was used to record one spectrum. The spectra were recorded using Spec 32 (version 1.5.4.2, Control Development Inc, IN, US). After each measurement cycle, it was visually verified that powder residues were not blocking the probe.
2.2.5. Calibration setup
Calibration samples for the NIR spectroscopic evaluation were prepared according to Section 2.2.1 and emptied on a flat surface. Spectra were measured recorded at nine different positions in the powder sample, by placing the NIR-probe in a 90° angle in the powder. This method is referred to as the static method.
Using the 3D printed calibration setup (Fig. 1), the pre-mixed samples were filled into the sample chamber. The rotation of the screw was controlled with a power supply (303DD, ISO-TECH, Ahaus, Germany) connected to a motor 919D series (MFA/Como Drills, Kent, UK), set to 10 V, corresponding to 17 rpm. Process monitoring was performed using NIR spectroscopy and nine spectra were measured.
Fig. 1.
Calibration setup left: CAD of the calibration setup, right: 3D printed calibration setup, consisting of chamber, lid, screw and backstopper.
2.2.6. Powder mixing
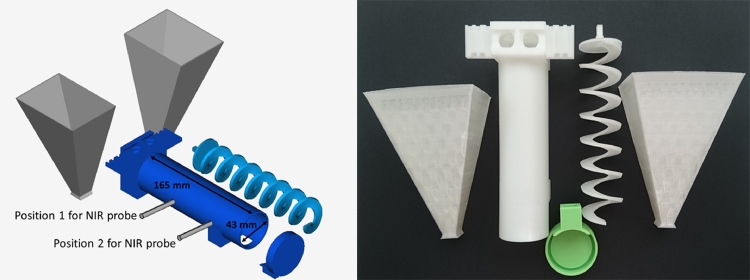
A prototype continuous mixing setup was designed using CAD and 3D printed (Fig. 2). In the dynamic mixing experiments, this setup was used to mix lactose monohydrate and mannitol. Two identical hoppers were attached to the mixing chamber and filled with powder. The rotation was controlled with a power supply (303DD, ISO-TECH, Ahaus, Germany) connected to a motor 919D series (MFA/Como Drills, Kent, UK), set to 15 V, corresponding to 25 rpm. The same weight of lactose monohydrate and mannitol (100 g each) was filled into funnels, which could be attached to the mixing chamber. The integrity of the mixing screw was evaluated visually after every experiment, to detect possible deformations or defects in the screw. Applying an axial load of 1 kg to the screw, resulted in a deformation of approx. 1%.
Fig. 2.
left: CAD of the continuous single-screw mixing prototype chamber and screw, right: 3D printed single-screw mixing prototype setup for continuous mixing.
2.2.7. Data processing
The obtained spectral data were transformed using MATLAB R2014a (Mathworks, Natick, MA, US) and multivariate data analysis was performed using SIMCA (Umetrics, Umeå, Sweden). The spectra were pre-treated by calculating the first derivate, performing standard normal variate (SNV) normalization and mean centering. A spectral range of 1440–2200 nm was chosen. Principal component analysis (PCA) was performed to capture the difference between the used calibration samples and to evaluate the recording during the mixing experiments. Global models taking all calibration spectra and the measured spectra of the sample were always created including the calibration spectra. Graphs were plotted using Origin Pro 9.1 (Origin Lab Cooperation, MA, US).
3. Results and discussion
3.1. Powder characteristics
To understand the powder behavior during processing it is necessary to know a range of properties at the particulate level and on the bulk level of the powder. Different properties of the two model powders (lactose monohydrate and mannitol) were assessed. The size and the shape of the particles influence the bulk powder behavior. When comparing mannitol with lactose monohydrate, it can be observed that mannitol had a larger particle size (d(50), Table 1). The SEM images (data not shown) display that both lactose monohydrate and mannitol had spherical particle shapes. The dynamic vapor sorption profiles showed that both materials adsorb less than 0.2% water below a relative humidity (RH) of 70%. The deliquesce points of lactose monohydrate and mannitol are at 95% RH and 96% RH, respectively [37]. It can be expected that below the deliquescence point the physicochemical properties of these materials are not dramatically affected by changes in the relative humidity.
Table 1.
Summary of powder characteristics for lactose monohydrate and mannitol, all results are indicated as mean ± standard deviation (n = 3/n = 5 for flow rate).
| Bulk density (kg/m³) | Flow function coefficient – ffc (a.u.) | d(50) (µm) | Flow rate (mg/s) | |
|---|---|---|---|---|
| Lactose monohydrate | 640 ± 0 | 9.4 ± 0.3 | 45 ± 0 | 301 ± 18 |
| Mannitol | 470 ± 0 | 12.7 ± 0.3 | 60 ± 1 | 324 ± 13 |
The flowability measurements (ffc and flow rate) indicate that mannitol had better flowability than lactose monohydrate (flow rate: P < 0.05, α = 0,05) (Table 1). The flowability of the model powders was essential in this study, because in the later mixing experiments (Section 3.3) no active powder feeding was used. Powder flow in the dynamic experimental setup occurred by gravitational force from two comparable hoppers with the same design and a same hopper opening. Due to the higher flow rate of mannitol (Table 1), a higher concentration of mannitol can be expected in the dynamic blending experiments. The friction between the powder and the surrounding wall material influences to a high degree the powder behavior within the geometry [38]. In this study, the setups were 3D printed using polymeric materials and not manufactured, as common industrial practice, from stainless steel. Therefore, it was important to understand the behavior of the model powders against the printing polymer, PLA. A wall plate fitting for the wall friction cell of the used ring shear tester was designed and 3D printed.
The wall friction experiments indicated that there was less friction between lactose monohydrate and both wall materials (PLA and stainless steel) than between mannitol and the respective wall material. With increasing normal stress during the wall friction measurements, the difference in wall friction between the stainless steel wall plate and the 3D printed plate increases. At low normal stresses (200 Pa), there is only a small difference between the two different wall materials (Fig. 3), indicating that at low stresses, as can be present in small scale setups [39], the choice of wall material has no practical influence on the powder behavior. In this study no post treatment was performed to smoothen the surface of the printed wall plate. By performing post treatment of the printed wall plate and printed geometries using solvent vapor based approaches or mechanical approaches, a smoother surface can be obtained and thereby the wall friction can be decreased [40].
Fig. 3.
Wall friction of lactose monohydrate and mannitol against stainless steel and 3D printed wall plates printed with PLA, mean ± standard deviation (n = 3).
A similar bulk behavior of both materials is expected in later experiments. Due to the lower particle–particle interactions (a higher ffc value), mannitol might express an advanced flow over lactose monohydrate. However, due to the similar physicochemical and bulk properties a good mixability of lactose monohydrate and mannitol can be expected [7], [41]
3.2. Calibration setups
The spectral range of 1440–2200 nm showed most variation between lactose monohydrate and mannitol and, therefore, was identified as the most useful area for multivariate modeling (Fig. 4). In this range, lactose monohydrate has the relatively sharp absorption maxima related to water (1900–1950 nm).
Fig. 4.
SNV normalized and mean centered NIR spectra of the raw materials, lactose monohydrate and mannitol at the spectral range 1440–2200 nm.
The calibration setup was 3D printed using 3D printers based on the fused deposition modelling method. The resolution that can be reached with those printers is moderate. However, as the same printers, with the same settings were used throughout the study, no differences in print quality can be expected between the geometries.
Calibration samples for NIR spectroscopic analysis were measured with the two different experimental setups. The main difference between the two methods to measure the calibration spectra is that in the static method, the NIR probe is moved in a powder sample and when using the calibration setup, the NIR probe is on a fixed position, while the powder sample is being moved. Both datasets were preprocessed by calculating the first derivate, subsequent SNV normalization and mean centering of the data. This combination of pre-treatment has been shown to be suitable for NIR spectra analysis [2]. A PCA plot was then calculated and plotted, using the first two principal components, to compare the performance of the two methods to record the calibration samples (Fig. 5). PCA scores for NIR spectra have been shown to be a good way to compare the performance of different recorded calibration sample sets [42].
Fig. 5.
A: principal component analysis (PCA) of the calibration blends left: NIR spectra of the premixed powders measured at different positions of the blend right: NIR spectra of the moving calibration powder blends, measured in the 3D printed calibration device; B: loadings of the principal components of the corresponding PCA models.
As for both the used methods, the first two principal components explain over 99% of the variation observed in the spectra, further components were not taken into consideration. The loadings for both methods to record the spectra of the calibration samples are comparable and are related to the same variation in the samples (Fig. 5B). The largest variation in the loading can be seen between 1900 and 2000 nm, which corresponds to a combination band of water, related to the water in lactose monohydrate. The scores plot of the PCA indicate that the data points of the spectra recorded using the 3D printed device are closer to each other in the PC1–PC2 space, compared to the spectra measured on a static powder bed (Fig. 5A). This indicates that dynamic movement of the powder while measuring the calibration samples can result in a better calibration model for NIR spectroscopy, which can result in a lower prediction errors for quantitative analysis, e.g., while monitoring powder mixing using for example PLS regression of the data. Movement of powder while recording the samples was shown to improve the calibration of a NIR method before [31]. While Berntsson et al. moved their powder by shaking the vessel manually; the approach presented in this study automates the process and thereby makes the recording of spectra of calibration samples independent from the operator. Furthermore, the 3D printed device is mimicking the mixing chamber in which the powder blend is monitored later, giving the same conditions for recording the calibration spectra and the spectra while mixing the powder.
3.3. Continuous powder mixing in the single-screw mixing prototype geometry
A continuously operating mixing line has several specific requirements that limit the design and setup of the line: powder is constantly fed into the blender and the blend is constantly released from the process and directly transferred into the next unit operation. Most commonly a loss in weight feeder is used to control the feed of powder into the mixing chamber [12]. In this study, a gravitational feeder is used instead. Since it is expected that no interruption will occur during the production, in-line process monitoring and control of the powder blending process are crucial. Near IR (NIR) spectroscopy is a well-established method and broadly applied in different applications, as it is a non-destructive method and can measure through a sight glass without disturbing the material flow [15]. In this study, a 3D printed single-screw prototype of a mixing line was used with two interfaces for a NIR probe (Fig. 2, Position 1 and Position 2). The powder feed into the system was not weight controlled, but based on gravitational flow from two similar hoppers with a similar hopper opening. Due to the similar bulk properties of lactose monohydrate and mannitol, a similar behavior during the process and a good mixability was expected. During the experiments, it was observed that mannitol had better flow properties in this geometry into the mixing chamber compared to lactose monohydrate, which can be explained by the better flowability of mannitol (see Section 3.1).
The NIR probe was positioned in two different positions in the mixing setup to monitor the powder blend and to explore the role of process interfacing of an in-line PAT tool. In position 1 the observed NIR spectra were mainly related to mannitol and only low concentrations of lactose monohydrate can be observed in the blend (Fig. 6A, left). The mannitol was fed into the mixing setup on the same side, the NIR probe was positioned at, which further explains the poor quality of mixing at this point and indicates that this position should not be used for process monitoring. After switching the lactose and the mannitol feed to opposite hoppers, a high concentration of lactose could be observed (data not shown). This further indicates the wrong location of the NIR probe and confirms that the two powders were not yet mixed well at Position 1. At position 2, the lactose monohydrate content in the blend was up to 40% (w/w). It can be concluded from these observations, that the positioning of the NIR probe is crucial for monitoring of continuous processes, which is comparable with other studies, where a NIR probe was placed at different positions in the vessel during blending [26], [27], [28], [29].
Fig. 6.
Principal component analysis (PCA) of the NIR data recorded during continuous mixing in the continuous single-screw mixing prototype geometry; Ieft: position 1 for the NIR probe and right: position 2 for the NIR probe. Red symbols referring to dynamic mixing samples and other samples referring to the calibration samples.
During this study, the feed of raw material was not controlled and thereby the blend ratio was at a same volumetric ratio. By choosing raw materials with similar bulk properties fast mixing was expected. The observed higher amount of mannitol in the blend was expected, due to the superior flowability of mannitol (Table 1). Overall, the use of 3D printed prototypes is a promising approach for rapid prototyping of continuously operating production geometries. The design can be changed and adapted to specific needs fast at a very low cost. Multiple interfaces for process analytical technology can be implemented in the process, thereby helping to improve the design without destroying expensive existing equipment.
4. Conclusion
It was demonstrated that computer aided design (CAD) and 3D printing is a valuable starting point for designing and prototyping continuously operating production geometries. These 3D printed geometries enable fast testing of different continuous powder mixing setups. By designing a process interface with a possibility for measuring, e.g., the NIR spectra in a dynamic mode gives an opportunity to mimic different process dynamics and directly measure the effect on the process performance. The powder movement and dynamic blending during the measurement of calibration samples can also be modified in custom-made calibration geometries, which improves the calibration models. Furthermore, it was shown that a prototype of a continuously operating single-screw mixing line can be used to follow the mixing process in this 3D printed setup. Hence, 3D printing provides a possibility for rapid prototyping of different process interface and production equipment geometries guiding the design of new setups and process innovation.
Acknowledgments
Conflicts of interest
The authors declare that there is no conflict of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This study was funded by Innovation Fund Denmark; Project: High Quality Dry Products with Superior Functionality and Stability – Q-Dry; File No: 5150-00024B
References
- 1.Plumb K. Continuous processing in the pharmaceutical industry. Chem Eng Res Des [Internet] 2005;83:730–738. [Google Scholar]
- 2.Vanarase AU, Alcalà M, Jerez Rozo JI, Muzzio FJ, Romañach RJ. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem Eng Sci. 2010;65:5728–5733. [Google Scholar]
- 3.Rantanen J, Khinast J. The future of pharmaceutical manufacturing sciences. J Pharm Sci. 2015;104:3612–3638. doi: 10.1002/jps.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khinast J, Bresciani M. Continuous manufacturing: definitions and engineering principles. In: Kleinebudde P, Khinast JG, Rantanen J, editors. Continuous manufacturing pharmaceutical. 1st ed. John Wiley & Sons; 2017. pp. 1–31. [Google Scholar]
- 5.Gao Y, Vanarase A, Muzzio F, Ierapetritou M. Characterizing continuous powder mixing using residence time distribution. Chem Eng Sci. 2011;66:417–425. [Google Scholar]
- 6.Thompson MR, Sun J. Wet granulation in a twin-screw extruder: implications of screw design. J Pharm Sci. 2009;99:2090–2103. doi: 10.1002/jps.21973. [DOI] [PubMed] [Google Scholar]
- 7.Vanarase AU, Muzzio FJ. Effect of operating conditions and design parameters in a continuous powder mixer. Powder Technol. 2011;208:26–36. [Google Scholar]
- 8.Osorio JG, Muzzio FJ. Effects of processing parameters and blade patterns on continuous pharmaceutical powder mixing. Chem Eng Process Process Intensif. 2016;109:59–67. [Google Scholar]
- 9.Mendez R, Muzzio F, Velazquez C. Study of the effects of feed frames on powder blend properties during the filling of tablet press dies. Powder Technol. 2010;200:105–116. [Google Scholar]
- 10.Vanhoorne V, Vanbillemont B, Vercruysse J. Development of a controlled release formulation by continuous twin screw granulation: influence of process and formulation parameters. Int J Pharm. 2016;505:61–68. doi: 10.1016/j.ijpharm.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Ierapetritou M, Sebastian Escotet-Espinoza M, Singh R. Process simulation and control for continuous pharmaceutical manufacturing of solid drug products. In: Kleinebudde P, Khinast JG, Rantanen J, editors. Continuous manufacturing pharmaceutical. 1st ed. John Wiley & Sons; 2017. pp. 33–105. [Google Scholar]
- 12.Pernenkil L, Cooney CL. A review on the continuous blending of powders. Chem Eng Sci. 2006;61:720–742. [Google Scholar]
- 13.Ervasti T, Simonaho S-PP, Ketolainen J. Continuous manufacturing of extended release tablets via powder mixing and direct compression. Int J Pharm. 2015;495:290–301. doi: 10.1016/j.ijpharm.2015.08.077. [DOI] [PubMed] [Google Scholar]
- 14.Roth WJ, Almaya A, Kramer TT, Hofer JD. A demonstration of mixing robustness in a direct compression continuous manufacturing process. J Pharm Sci. 2017;106:1339–1346. doi: 10.1016/j.xphs.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Martinez L, Peinado A, Liesum L, Betz G. Use of near-infrared spectroscopy to quantify drug content on a continuous blending process: Influence of mass flow and rotation speed variations. Eur J Pharm Biopharm. 2013;84:606–615. doi: 10.1016/j.ejpb.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 16.De Beer T, Burggraeve A, Fonteyne M, Saerens L, Remon JP, Vervaet C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int J Pharm. 2011;417:32–47. doi: 10.1016/j.ijpharm.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Román-Ospino AD, Singh R, Ierapetritou M. Near infrared spectroscopic calibration models for real time monitoring of powder density. Int J Pharm. 2016;512:61–74. doi: 10.1016/j.ijpharm.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Rantanen J, Jørgensen A, Räsänen E. Process analysis of fluidized bed granulation. AAPS PharmSciTech. 2001;2:13–20. doi: 10.1007/BF02830562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rantanen J, Yliruusi J. Determination of particle size in a fluidized bed granulator with a near infrared set-up. Pharm Pharmacol Commun. 1998;4:73–75. [Google Scholar]
- 20.Rantanen J, Räsänen E, Tenhunen J, Känsäkoski M, Mannermaa JP, Yliruusi J. In-line moisture measurement during granulation with a four-wavelength near infrared sensor: an evaluation of particle size and binder effects. Eur J Pharm Biopharm. 2000;50:271–276. doi: 10.1016/s0939-6411(00)00096-5. [DOI] [PubMed] [Google Scholar]
- 21.Wahl PR, Fruhmann G, Sacher S, Straka G, Sowinski S, Khinast JG. PAT for tableting: inline monitoring of API and excipients via NIR spectroscopy. Eur J Pharm Biopharm. 2014;87:271–278. doi: 10.1016/j.ejpb.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Järvinen K, Hoehe W, Järvinen M, Poutiainen S, Juuti M, Borchert S. In-line monitoring of the drug content of powder mixtures and tablets by near-infrared spectroscopy during the continuous direct compression tableting process. Eur J Pharm Sci. 2013;48:680–688. doi: 10.1016/j.ejps.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 23.Moes JJ, Ruijken MM, Gout E, Frijlink HW, Ugwoke MI. Application of process analytical technology in tablet process development using NIR spectroscopy: blend uniformity, content uniformity and coating thickness measurements. Int J Pharm. 2008;357:108–118. doi: 10.1016/j.ijpharm.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Bagnol L, Berman M, Chiarella RA, Gerber M. Applications of NIR in early stage formulation development. Part II. Content uniformity evaluation of low dose tablets by principal component analysis. Int J Pharm. 2009;380:49–54. doi: 10.1016/j.ijpharm.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Vanarase AU, Järvinen M, Paaso J, Muzzio FJ. Development of a methodology to estimate error in the on-line measurements of blend uniformity in a continuous powder mixing process. Powder Technol. 2013;241:263–271. [Google Scholar]
- 26.Scheibelhofer O, Balak N, Wahl PR, Koller DM, Glasser BJ, Khinast JG. Monitoring blending of pharmaceutical powders with multipoint NIR spectroscopy. AAPS PharmSciTech. 2013;14:234–244. doi: 10.1208/s12249-012-9910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Hagrasy AS, Delgado-Lopez M, Drennen JK. A process analytical technology approach to near-infrared process control of pharmaceutical powder blending: part II: qualitative near infrared models for prediction of blend homogeneity. J Pharm Sci. 2006;95:407–421. doi: 10.1002/jps.20466. [DOI] [PubMed] [Google Scholar]
- 28.Mendez ASL, de Carli G, Garcia CV. Evaluation of powder mixing operation during batch production: application to operational qualification procedure in the pharmaceutical industry. Powder Technol. 2010;198:310–313. [Google Scholar]
- 29.Shi Z, Cogdill RP, Short SM, Anderson CA. Process characterization of powder blending by near-infrared spectroscopy: blend end-points and beyond. J Pharm Biomed Anal. 2008;47:738–745. doi: 10.1016/j.jpba.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Alam MA, Shi Z, Drennen JK, Anderson CA. In-line monitoring and optimization of powder flow in a simulated continuous process using transmission near infrared spectroscopy. Int J Pharm. 2017;526:199–208. doi: 10.1016/j.ijpharm.2017.04.054. [DOI] [PubMed] [Google Scholar]
- 31.Berntsson O, Danielsson LG, Lagerholm B, Folestad S. Quantitative in-line monitoring of powder blending by near infrared reflection spectroscopy. Powder Technol. 2002;123:185–193. [Google Scholar]
- 32.Boetker J, Raijada D, Aho J. In silico product design of pharmaceuticals. Asian J Pharm Sci. 2016;11:492–499. [Google Scholar]
- 33.Cleary PW, Sawley ML. DEM modelling of industrial granular flows: 3D case studies and the effect of particle shape on hopper discharge. Appl Math Model. 2002;26:89–111. [Google Scholar]
- 34.González-Montellano C, Ramírez Á, Gallego E, Ayuga F. Validation and experimental calibration of 3D discrete element models for the simulation of the discharge flow in silos. Chem Eng Sci. 2011;66:5116–5126. [Google Scholar]
- 35.Balevičius R, Kačianauskas R, Mróz Z, Sielamowicz I. Analysis and DEM simulation of granular material flow patterns in hopper models of different shapes. Adv Powder Technol. 2011;22:226–235. [Google Scholar]
- 36.Hirschberg C, Boetker JP, Rantanen J, Pein-Hackelbusch M. Using 3D printing for rapid prototyping of characterization tools for investigating powder blend behavior. AAPS PharmSciTech. 2018;19(2):941–950. doi: 10.1208/s12249-017-0904-0. [DOI] [PubMed] [Google Scholar]
- 37.Mauer LJ, Taylor LS. Deliquescence of pharmaceutical systems. Pharm Dev Technol. 2010;15:582–594. doi: 10.3109/10837450903397594. [DOI] [PubMed] [Google Scholar]
- 38.Søgaard SV, Olesen NE, Hirschberg C. An experimental evaluation of powder flow predictions in small-scale process equipment based on Jenike's hopper design methodology. Powder Technol. 2017;321:523–532. [Google Scholar]
- 39.Søgaard SV, Pedersen T, Allesø M, Garnaes J, Rantanen J. Evaluation of ring shear testing as a characterization method for powder flow in small-scale powder processing equipment. Int J Pharm. 2014;475:315–323. doi: 10.1016/j.ijpharm.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 40.Kuo CC, Mao RC. Development of a precision surface polishing system for parts fabricated by fused deposition modeling. Mater Manuf Process. 2016;31:1113–1118. [Google Scholar]
- 41.Vanarase AU, Osorio JG, Muzzio FJ. Effects of powder flow properties and shear environment on the performance of continuous mixing of pharmaceutical powders. Powder Technol. 2013;246:63–72. [Google Scholar]
- 42.Mantanus J, Ziémons E, Rozet E. Building the quality into pellet manufacturing environment - Feasibility study and validation of an in- line quantitative near infrared (NIR) method. Talanta. 2010;83:305–311. doi: 10.1016/j.talanta.2010.09.009. [DOI] [PubMed] [Google Scholar]