Abstract
Introduction:
The optimal radiation dose in locally advanced non-small cell lung cancer (NSCLC) is not known for patients who receive sequential chemoradiation or definitive radiation only. Our objective was to determine whether there is a benefit to radiation dose escalation for these patients.
Methods:
Patients included in our retrospective analysis received radiation treatment for NSCLC between 2004 and 2013, did not have surgery, and received a dose ≥50.0 Grays (Gy). Patients who received concurrent chemoradiation were excluded from the analysis, leaving 336 patients included. The primary outcomes were overall survival, local failure, and distant failure.
Results:
On multivariate analysis, after adjusting for age, Karnofsky performance status, gross tumor volume, and treatment modality, patients treated with radiation dose >66 Gy had significantly improved overall survival compared to those treated with <60 Gy (HR 0.58, 95%CI 0.39 to 0.87, p=0.008). After adjusting for smoking history and radiologic size of the tumor, patients treated with radiation dose >66 Gy had a significantly decreased risk of local failure compared to those treated with <60 Gy (HR 0.59, 95%CI 0.38 to 0.91, p=0.02). Radiation dose was not an independent prognostic factor of distant failure on multivariate analysis.
Conclusions:
When controlling for tumor volume and/or dimensions and other independent prognostic factors, patients with locally advanced NSCLC who are not candidates for concurrent chemoradiation benefit from a radiation dose >66 Gy versus <60 Gy to improve overall survival and reduce local failure. Increasing radiation dose does not appear to affect distant failure.
Keywords: Inoperable tumor, dose escalation, sequential chemoradiation, definitive radiation, RTOG 0617
MicroAbstract:
The optimal radiation dose for patients with inoperable, locally advanced non-small cell lung cancer who are ineligible for concurrent chemoradiation remains unclear. In this retrospective, multivariate analysis of 336 patients treated with sequential chemoradiation or definitive radiation only, radiation dose >66 Grays (Gy) was superior to radiation dose <60 Gy for the endpoints of overall survival and local failure.
Introduction
Lung cancer continues to be the number one cause of cancer death in the United States.1 For patients with inoperable locally advanced non-small cell lung cancer (NSCLC), radiotherapy (RT) is the definitive treatment modality, given in conjunction with chemotherapy in eligible patients. Multiple studies have endeavored to determine the optimal radiation dose in patients with unresectable NSCLC. A phase I dose escalation study at our institution suggested that dose could be safely escalated up to 84 Gy.2 Another study found that patients who received an increased dose had improved overall survival, with patients receiving up to 102 Gy.3 A phase I-II dose escalation trial sponsored by the Radiation Therapy Oncology Group (RTOG) escalated doses to 83.8 Gy and 77.4 Gy for patients with a V20 less than 25% and between 25% and 36%, respectively.4 These studies showed that radiation dose could be safely escalated beyond 60 Gy and suggested that patients receiving higher doses experienced greater tumor control and overall survival. However, these early studies included patients with and without concurrent chemotherapy.
Adding chemotherapy to radiation in the treatment of locally advanced NSCLC was shown to result in improved survival compared to RT only.5,6 Subsequently, concurrent chemoradiation (CRT) was established as the standard of care for inoperable stage III NSCLC. In an RTOG randomized controlled trial, patients with locally advanced inoperable NSCLC were randomized to receive either sequential CRT or concurrent CRT. Patients who received concurrent CRT experienced significantly improved overall survival compared to patients who received sequential CRT.7 Auperin and colleagues conducted a meta-analysis of six randomized trials that compared concurrent and sequential CRT, finding a significant benefit in locoregional control and overall survival associated with concurrent CRT.8
In a recent phase III randomized trial (RTOG 0617), patients with unresectable stage III NSCLC were randomized to receive either 60 Gy or 74 Gy, delivered with concurrent paclitaxel and carboplatin chemotherapy.9 Somewhat surprisingly, this study found higher overall survival in the lower dose (60 Gy) arm compared to the 74 Gy arm--a median of 28.7 months versus 20.3 months, respectively. These findings suggest that increased radiation dose beyond 60 Gy may not be associated with an added benefit in patients receiving concurrent CRT.
Although concurrent CRT is the standard of care for eligible patients, in practice many patients are ineligible for concurrent CRT. The recent RTOG study enrolled patients at a median age of 64 years,9 but the median age of diagnosis for NSCLC is 7010; this makes evident a common clinical challenge of treating elderly patients with locally advanced NSCLC. Elderly patients may be poor candidates for concurrent CRT due to medical comorbidities, e.g., limited kidney function, peripheral neuropathy, cardiac impairment, and poor performance status. For these patients, concurrent CRT could potentially cause unacceptable toxicity. Patients who receive non-concurrent CRT generally experience lower toxicities compared to patients who receive concurrent CRT, making the former a potentially safer option for elderly patients and patients with poor performance status.7,8 In a study conducted on all patients in the Netherlands with a diagnosis of stage III lung cancer between 2002 and 2005, 59% of patients were deemed ineligible for concurrent CRT.11 A group of Canadian investigators conducted a retrospective review of patients treated at their institution, which showed that only 55% of patients receiving curative therapy for stage III NSCLC were receiving concurrent CRT.12 It remains unclear whether radiation doses >60 Gy would benefit the large subset of patients with locally advanced NSCLC who are ineligible for concurrent CRT.
We performed a retrospective analysis of patients treated at our center for locally advanced NSCLC who were not candidates for concurrent CRT. We hypothesized that these patients might experience improved tumor control and overall survival from increased radiation dose.
Materials and Methods
Study Design
We obtained a waiver from the Institutional Review Board of Memorial Sloan Kettering Cancer Center prior to beginning this retrospective analysis of existing patient data. Patient and treatment characteristics included demographics; Karnofsky performance status (KPS); tumor histology and staging; and treatments received. Patients were treated for inoperable locally advanced NSCLC between 2004 and 2013 and had stage II or III disease at the time of diagnosis based on the 7th edition TNM guidelines from the American Joint Committee on Cancer. All patients received definitive RT to a dose ≥50.0 Gy and did not undergo surgery. Patients who received concurrent CRT were excluded. A total of 336 patients were analyzed who received sequential CRT or definitive RT only.
All patients underwent simulation using CT imaging for treatment planning at our institution. Patients were immobilized supine with their arms raised above their head. Simulation CT images were acquired at 3mm slice thickness. Patients simulated since 2008 were imaged using a 4D-CT scan to assess tumor motion during the respiratory cycle. The gross tumor volume (GTV) was contoured based on a free-breathing CT scan. A PET scan was also used to determine GTV for 325 (97%) of the 336 patients. An internal target volume (ITV) margin was added based on the observed respiratory motion. The ITV was typically expanded by 7 to 10 mm. We added a further 5 mm expansion to account for uncertainty in the day-to-day setup of the patient, resulting in the final planning target volume (PTV). Treatment was typically delivered using 3D conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) with four to seven coplanar 6 MV beams. After treatment completion, patients were followed with chest CT scans every 3–4 months for two years; then every 6 months for one year; then annually in subsequent years.
We queried the radiation treatment plans for the patients in our analysis to determine the gross tumor volume (GTV) of both the primary tumor and lymphadenopathy used in radiation planning. In our study, GTV is the combined value of the primary tumor and primary nodal disease. Data on GTV were available for 308 of 336 patients. Radiographic tumor size was the size of the primary tumor in the diagnostic CT scan.
Statistical Methods
Primary endpoints were overall survival (OS), local failure (LF)--defined as local nodal or tumor failure within the primary treatment field, and distant failure (DF)--defined as distant metastasis. OS was analyzed using the Cox proportional hazards regression model. One hundred fifty patients were alive at the time of last follow-up and thus were censored from the OS analysis. LF and DF were analyzed using a Fine and Gray competing risk regression model and treating death without event as a competing risk. For these two endpoints, patients were censored who were alive at last follow up and had not experienced LF (111 patients) or DF (102 patients). Radiation prescription dose was classified as <60.0 Gy, 60.0 to 66.0 Gy, or >66.0 Gy. We set p<0.05 as the threshold for statistical significance.
For each endpoint (OS, LF, and DF), any factor with p ≤0.1 on univariate analysis was considered as a candidate for a stepwise multivariate regression. For those variables included in the stepwise multivariate analysis final models, we reported estimated 3-year Kaplan-Meier OS rates and 3-year cumulative incidence of LF and DF using Gray’s method. Data on GTV and radiographic tumor size were log-transformed for analysis to reduce skewness. Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc. Cary, NC), and R 3.1.2 package “cmprsk.”
Results
Patient Characteristics
Patient characteristics are shown in Table 1. Three hundred thirty six patients were included in the analysis. Of these, 235 (70%) received sequential CRT and 101 (30%) received definitive RT only. Median OS was 20.2 months (range 3.0 to 118.8 months). The median age was 78 years for RT only patients and 71 years for sequential CRT patients (Table 1). Median KPS at diagnosis was 80 in both groups of patients, but KPS was <70 in 47 RT only patients (47%) compared to 59 sequential CRT patients (25%). All patients had biopsy-proven NSCLC: adenocarcinoma was the most common histology, followed by squamous cell and other/not otherwise specified. Most patients with stage II disease received RT only (Table 1). Of the 308 patients for whom GTV data were available, the median combined GTV of primary tumor and lymphadenopathy was 91.9 cc in RT only patients (range 1.9 to 630.4 cc) and 67.4 cc in sequential CRT patients (range 2.5 to 762.2 cc).
Table 1.
Patient Characteristics.
| Characteristic | Definitive RT only (N=101) | Sequential CRT (N=235) |
|---|---|---|
| Gender, No. (%) | ||
| Male | 49 (49) | 129 (55) |
| Female | 52 (51) | 106 (45) |
| Age, median (range), y | 78 (51–93) | 71 (32–91) |
| Patients aged >70, No. (%) | 80 (79) | 119 (51) |
| KPS, median (range) | 80 (40–90) | 80 (40–100) |
| No. patients with KPS <80 | 47 (47) | 59 (25) |
| Smoking history, No. (%) | ||
| Former | 71 (70) | 149 (63) |
| Current | 24 (24) | 70 (30) |
| Never | 6 (6) | 16 (7) |
| Stage, No. (%) | ||
| IIA | 20 (20) | 7 (3) |
| IIB | 14 (14) | 8 (3) |
| IIIA | 46 (46) | 135 (57) |
| IIIB | 21 (21) | 85 (36) |
| Primary tumor radiographic size, median (range), cm | 3.9 (0.6–12.2) | 4.1 (0.9–12.4) |
| GTV, median (range), cc | 91.9 (1.9–630.4) | 67.4 (2.5–762.2) |
| Histology, No. (%) | ||
| Squamous cell | 47 (47) | 77 (33) |
| Adenocarcinoma | 42 (42) | 123 (52) |
| Other/NOS | 12 (12) | 35 (15) |
| Radiation treatment, No. (%) | ||
| Definitive RT only | 101 (100) | - |
| Sequential CRT | - | 235 (100) |
| Chemotherapy regimen in sequential CRT patients, No. (%) | ||
| Platinum doublet | - | 220 (94) |
| Platinum-gemcitabine | - | 42 (19) |
| Platinum-pemetrexed | - | 60 (27) |
| Carboplatin-paclitaxel | - | 49 (22) |
| Other platinum doublet | - | 69 (31) |
| Other chemotherapy | - | 14 (6) |
| Radiation technique, No. (%) | ||
| 3D-CRT | 30 (30) | 47 (20) |
| IMRT | 71 (70) | 188 (80) |
| Radiation dose, median (range), Gy | 66.0 (50.0–80.0) | 64.8 (50.0–80.0) |
| Radiation dose No. (%), Gy | ||
| <60.0 | 13 (13) | 42 (18) |
| 60.0 to 66.0 | 40 (40) | 122 (52) |
| >66.0 | 48 (48) | 71 (30) |
| Overall survival, median (range), mo | 20.2 (3.0–118.8) | |
| Follow-up time for survivors, median (range), mo | 38.6 (3.4–118.8) | |
KPS: Karnofsky performance status, GTV: primary and nodal gross tumor volume, NOS: not otherwise specified, RT: radiotherapy, CRT: chemoradiation, 3D-CRT: three-dimensional conformal radiation therapy, IMRT: intensity-modulated radiation therapy, Gy: Gray
Compared to RT only patients, a lower proportion of sequential CRT patients received a radiation dose >66.0 Gy (Table 1). The overall median radiation dose was 64.8 Gy (RT only: 66.0 Gy, CRT: 64.8 Gy).
Outcomes
A table of univariate analysis results is available in the Appendix (Appendix A, Table A1)
Overall Survival
On univariate analysis, improved OS was observed with radiation dose >66.0 Gy compared to dose <60.0 Gy (HR 0.67, 95%CI 0.48 to 0.93). No significant difference was observed between the reference <60.0 Gy and 60.0 to 66.0 Gy (p=0.055). Other factors associated with improved OS were sequential CRT compared to RT only (HR 0.54, 95%CI 0.42–0.70) and adenocarcinoma compared to squamous cell carcinoma (HR 0.73, 95%CI 0.57 to 0.95). Poorer OS was associated with: larger GTV (HR per log unit increment 1.45, 95%CI 1.28 to 1.66), 3D-CRT rather than IMRT (HR 1.47, 95%CI 1.12 to 1.92), older age (HR per year increment 1.02, 95%CI 1.01 to 1.03), and stages T3 (HR 1.55, 95%CI 1.10 to 2.19) and T4 (HR 1.76, 95%CI 1.20 to 2.59) compared to T1.
On multivariate analysis for OS, after adjusting for other independent prognostic factors age, KPS, treatment modality, and combined GTV, we found a significant benefit for doses >66.0 Gy compared to doses <60.0 Gy (HR 0.58, 95%CI 0.39 to 0.87, p=0.008) (Table 2). There was no significant difference in OS for doses of 60.0 to 66.0 Gy compared to the reference <60.0 Gy on multivariate analysis (p=0.12).
Table 2.
Stepwise multivariate analysis final model for the endpoints of overall survival, local failure, and distant failure.
| Overall Survival | Local Failure | Distant Failure | ||||
|---|---|---|---|---|---|---|
| Factor | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age at diagnosis | 1.01 (1.001–1.03) | 0.03 | − | − | − | − |
| KPS | ||||||
| <80 | 1 [Reference] | − | − | − | − | |
| ≥80 | 0.73 (0.56–0.97) | 0.03 | − | − | − | − |
| History of smoking | ||||||
| Former | − | − | 1 [Reference] | − | − | |
| Current | − | − | 0.97 (0.68–1.38) | 0.85 | − | − |
| Never | − | − | 1.69 (1.04–2.75) | 0.04 | − | − |
| T stage | ||||||
| T1 | 1 [Reference] | − | − | − | ||
| T2 | 1.08 (0.75–1.57) | 0.67 | − | − | − | |
| T3 | 1.35 (0.91–2.00) | 0.14 | − | − | − | |
| T4 | 1.69 (1.09–2.61) | 0.02 | − | − | − | |
| Tx | 0.83 (0.39–1.78) | 0.64 | − | − | − | |
| Histology | ||||||
| Squamous cell | − | − | − | − | 1 [Reference] | |
| Adenocarconima | − | − | − | − | 1.79 (1.26–2.54) | 0.001 |
| Other/NOS | − | − | − | − | 1.52 (0.88–2.62) | 0.13 |
| Histologic grade | ||||||
| Moderate | − | − | − | − | 1 [Reference] | |
| Poor | − | − | − | − | 0.71 (0.45–1.13) | 0.15 |
| Unknown | − | − | − | − | 0.62 (0.39–0.97) | 0.04 |
| Radiation dose, Gy | ||||||
| <60.0 | 1 [Reference] | 1 [Reference] | − | − | ||
| 60.0 to 66.0 | 0.76 (0.53–1.08) | 0.12 | 0.71 (0.48–1.07) | 0.10 | − | − |
| >66.0 | 0.58 (0.39–0.87) | 0.008 | 0.59 (0.38–0.91) | 0.02 | − | − |
| GTV | 1.34 (1.17–1.55)* | <0.001* | − | − | 1.24 (1.07–1.44)* | 0.004* |
| Primary tumor radiographic size | − | − | 1.75 (1.28–2.39)* | <0.001* | − | − |
| Treatment modality | ||||||
| Definitive RT only | 1 [Reference] | − | − | − | − | |
| Sequential CRT | 0.56 (0.41–0.75) | <0.001 | − | − | − | − |
KPS: Karnofsky performance status, NOS: not otherwise specified, Gy: Gray, GTV: primary and nodal gross tumor volume, RT: radiotherapy, CRT: chemoradiation.
based on log transformed data.
Local Failure
On univariate analysis, improved LF was observed with radiation dose >66.0 Gy compared to dose <60.0 Gy (HR 0.57, 95%CI 0.36 to 0.88). Worse LF was associated with larger tumor size on CT imaging (HR per log unit increment 1.73, 95%CI 1.28 to 2.35, p=0.004), and never smoker status compared to former smoker status (HR 1.88, 95%CI 1.19 to 2.98). Although we detected a significant difference in local failure based on radiographic tumor size, we did not observe a significant difference in LF based on GTV (HR per log unit increment 1.08, 95%CI 0.93 to 1.25, p=0.30). The Pearson correlation coefficient for GTV and radiographic tumor size was 0.52 (p<0.001).
On multivariate analysis for LF, after controlling for other independent prognostic factors smoking history and radiologic size of the tumor, we found a significant benefit for RT doses >66.0 Gy compared to RT doses <60.0 Gy (HR 0.59, 95%CI 0.38 to 0.91, p=0.02) (Table 2). There was no significant benefit detected for doses of 60.0 to 66.0 Gy compared to doses <60.0 Gy (p=0.10) (Table 2).
Distant Failure
On univariate analysis, improved DF was observed with radiation dose >66.0 Gy compared to dose <60.0 Gy (HR 0.62, 95%CI 0.41 to 0.95). Worse DF was associated with increased GTV (HR per log unit increment 1.22, 95%CI 1.06 to 1.41), adenocarcinoma compared to squamous cell carcinoma (HR 1.68, 95%CI 1.21 to 2.33), and N3 disease compated to N0 disease (HR 1.99, 95%CI 1.06 to 3.71).
On multivariate analysis, no effect of radiation dose on DF was observed after controlling for the independent prognostic factors of histology, histologic grade, and combined GTV (Table 2).
Discussion
In this retrospective analysis of patients who were not candidates for concurrent CRT, we found that radiation dose >66 Gy is superior to dose <60 Gy for the endpoints of OS and LF after controlling for other independent prognostic factors. A number of earlier studies had suggested that dose escalation could be beneficial.
Prior Studies
We had previously investigated the impact of radiation dose escalation from 70.2 to 90.0 Gy in a phase I trial of patients with inoperable NSCLC.2 Unacceptable toxicities occurred at doses of 90.0 Gy, but patients treated with 84.0 Gy experienced improved OS and acceptable toxicities. Patients with stage III NSCLC who received >80.0 Gy experienced improved overall survival compared with patients who received 51.0 to 80.0 Gy. In a related retrospective analysis of NSCLC patients who received a dose ≥80 Gy, patients with stage III NSCLC had OS rates of 59% and 31% at 2 and 5 years, respectively.13 Similar to our present analysis, patients in both of these analyses received either sequential CRT or RT only.
In a phase I-II dose escalation study known as RTOG 9311, 177 patients were assigned to radiation doses ranging from 70.9 Gy to 90.3 Gy within an isotoxic dose escalation scheme.4 Similar to our present study, patients received sequential chemo-RT or RT only. Patients with a V20 less than 25% were escalated to 83.8 Gy with minimal toxicities. In patients with a V20 between 25% and 36%, dose was escalated safely to 77.4 Gy.4
In a phase I-II dose-escalation study of 106 patients with stage I to III NSCLC treated with either sequential CRT or RT only, increased radiation dose was associated with improved survival.3 With a median OS of 19 months, the study reports a multivariate HR for death of 0.97 per Gy. Patients who received 92.0 to 103.0 Gy appeared to have superior OS compared to those who received 74.0 to 84.0 Gy. However, patients were not randomized to radiation prescription dose, but were assigned based on an isotoxic dose escalation scheme which included the estimated risk of radiation pneumonitis and the volume of normal lung available (Veff). In this scheme, the patients with smaller tumors received higher doses of radiation, because larger volumes of normal lungs were more easily spared. The additional survival advantage to high doses observed could be due to the isotoxic assignment of patients, resulting in smaller tumors being treated to higher radiation doses.
RTOG 0617 and Concurrent vs. Non-Concurrent CRT
These single institution and early phase cooperative group studies led to the design of RTOG 0617, which compared 74 Gy versus 60 Gy in the setting of concurrent chemoradiation.9 The protocol was designed in light of evidence that patients who receive concurrent CRT experience improved overall survival versus sequential CRT.7,8,14 Interestingly, RTOG 0617 found worse OS and increased toxicities in the higher dose (74.0 Gy) grouping compared to 60.0 Gy, calling into question the benefit of dose escalation beyond 60 Gy when giving concurrent chemotherapy.9 Much speculation has been entertained regarding the reasons behind this observation.15
In contrast to the early dose escalation studies and RTOG 0617, we present data on a more homogenous subset of patients who were not candidates for concurrent CRT. It is unlikely that the optimal RT dose in patients who are too frail for concurrent CRT will be determined in a prospective randomized trial. We observed benefit to dose escalation beyond 66.0 Gy for the clinical endpoints of OS and LF. Our data suggest that the optimal radiation dose for these patients may be greater than 66 Gy. This could represent a mild escalation over the 60 Gy tested in RTOG 0617. However, our analysis does not clearly indicate whether doses >66 Gy are superior to doses between 60 and 66 Gy.
A recent meta-analysis of 21 randomized trials included 15 studies of definitive RT alone, 4 trials with concurrent CRT, and 2 trials with sequential CRT.16 The analysis showed a benefit for dose escalation in the setting of definitive RT only, consistently favoring higher doses. This was true even when all dose groupings in a trial were relatively high, for example, 76 Gy versus 70 Gy.16 Based on the two trials of sequential CRT in the meta-analysis (a total of 179 patients), no significant benefit to dose escalation was detected in the setting of sequential CRT.16 Even after adjusting for treatment modality (sequential CRT versus RT only), we observed a benefit to escalation >66 Gy versus <60 Gy for the endpoints of OS and LF.
GTV and Radiation Dose
One of the strengths of our study is that we investigated dose escalation in the context of tumor volume. Our analysis showed that increasing GTV conferred a significant risk in OS (HR per log unit increment 1.43, 95% CI 1.25 to 1.64) and DF (HR per log unit increment 1.24, 95% CI 1.07 to 1.44). Greater tumor volume is a negative prognostic factor. On multivariate analysis of a cohort of 191 patients who received concurrent CRT for stage III NSCLC, higher GTV conferred a disadvantage in OS, LF, and progression-free survival.17 A secondary analysis of RTOG 0235 analyzed tumor volumes acquired using PET imaging, finding that pretreatment metabolic tumor volume was a negative factor for OS.18 Tumor volume was also a negative factor for LF. Despite the known prognostic value of tumor volumes, few dose escalation studies have taken GTV into account. In a secondary analysis of RTOG 0911, 161 patients were separated into groups of GTV >45 cm3 and ≤45 cm3.19 In the multivariate analysis for both OS and progression-free survival, when controlling for GTV, radiation dose did not have a significant effect on either endpoint.19 This remained true when different GTV cutoff values were used to separate patients.
On our multivariate analysis for LF, radiation dose escalation was beneficial even when controlling for tumor radiologic size. GTV was not an independent prognostic factor in the multivariate analysis for LF. However, we observed a moderate correlation between the tumor radiologic size and the GTV (Pearson correlation coefficient =0.52, p<0.001). In our analysis, GTV included primary tumor and nodal disease whereas radiologic tumor size was the size of the primary lesion on CT imaging. This difference could partially explain the discrepancy. A recent analysis was conducted of locoregional failures in patients who received concurrent CRT for locally-advanced NSCLC.20 The univariate analysis showed that the primary tumor was more likely to be a source of LF compared to lymph nodes that were in the radiation field.20 When controlling for combined tumor volume, primary tumor failure was not more likely than local nodal failure. The primary tumor generally has a higher density of clonogenic tumor cells, so it is possible that primary tumor size, as opposed to primary tumor plus lymphadenopathy, could be a better predictor of local failure. For distant failure, histology, histologic grade, and GTV were highly significant, suggesting that DF is more dependent on tumor biology and characteristics, not radiation dose.
Limitations
There are some limitations that should be considered when interpreting our data. Although our analysis included multivariate analyses, the retrospective nature of our study raises the potential for selection bias. Although all patients were treated without concurrent chemotherapy, there remains heterogeneity in our dataset due to the fact that 30% of patients received RT only. In addition, GTV data were not available for the complete dataset, but only for 308 patients.
Conclusion
When controlling for tumor volume and/or dimensions and other independent prognostic factors, patients with locally advanced NSCLC who are not candidates for concurrent chemoradiation benefit from a radiation dose >66 Gy versus <60 Gy to improve overall survival and reduce local failure. Increasing radiation dose does not appear to affect distant failure.
Figure 1.
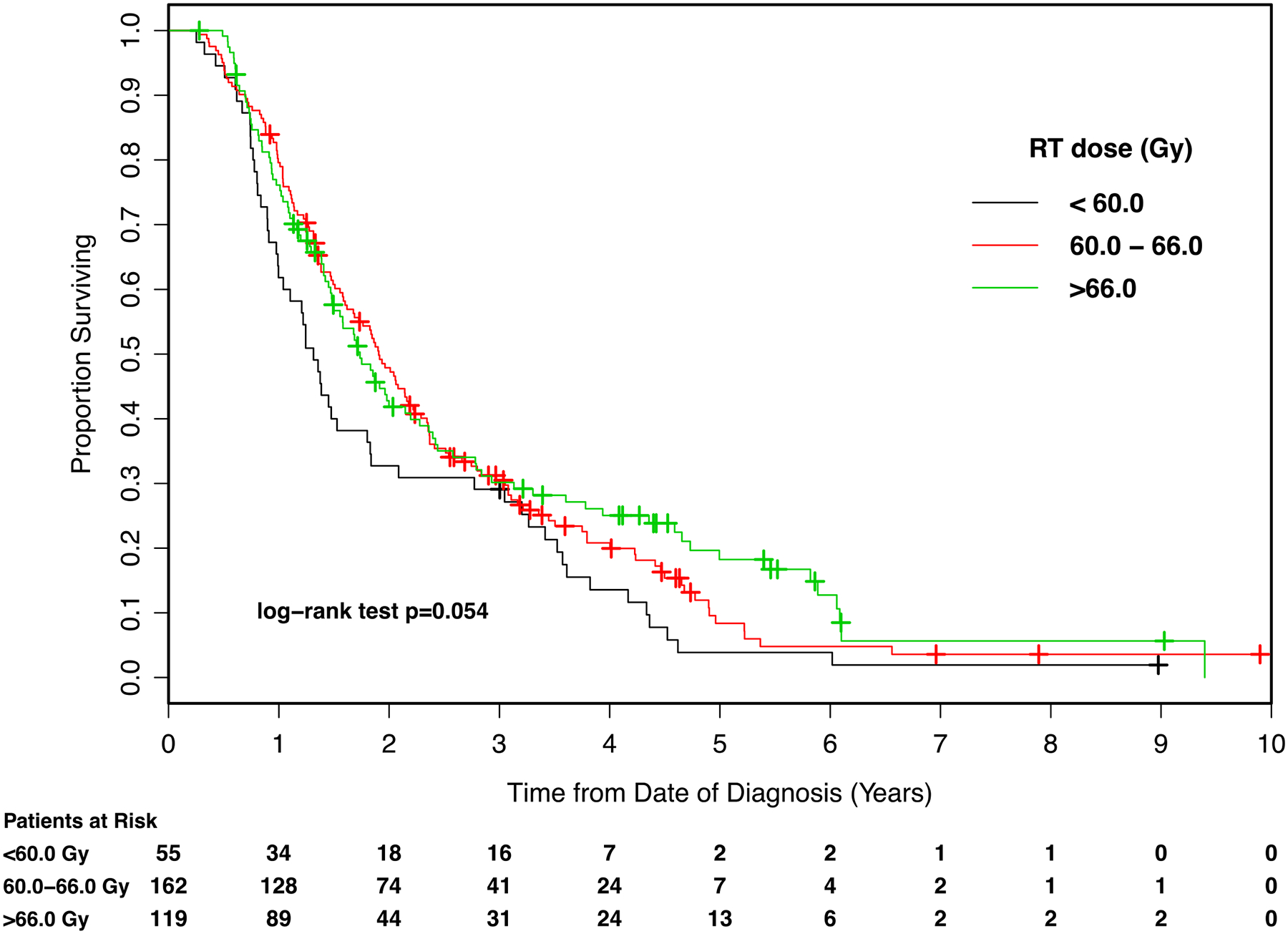
Kaplan-Meier survival curves for patients enrolled in the analysis, stratified by radiotherapy (RT) dose. Gy: Gray.
Figure 2.
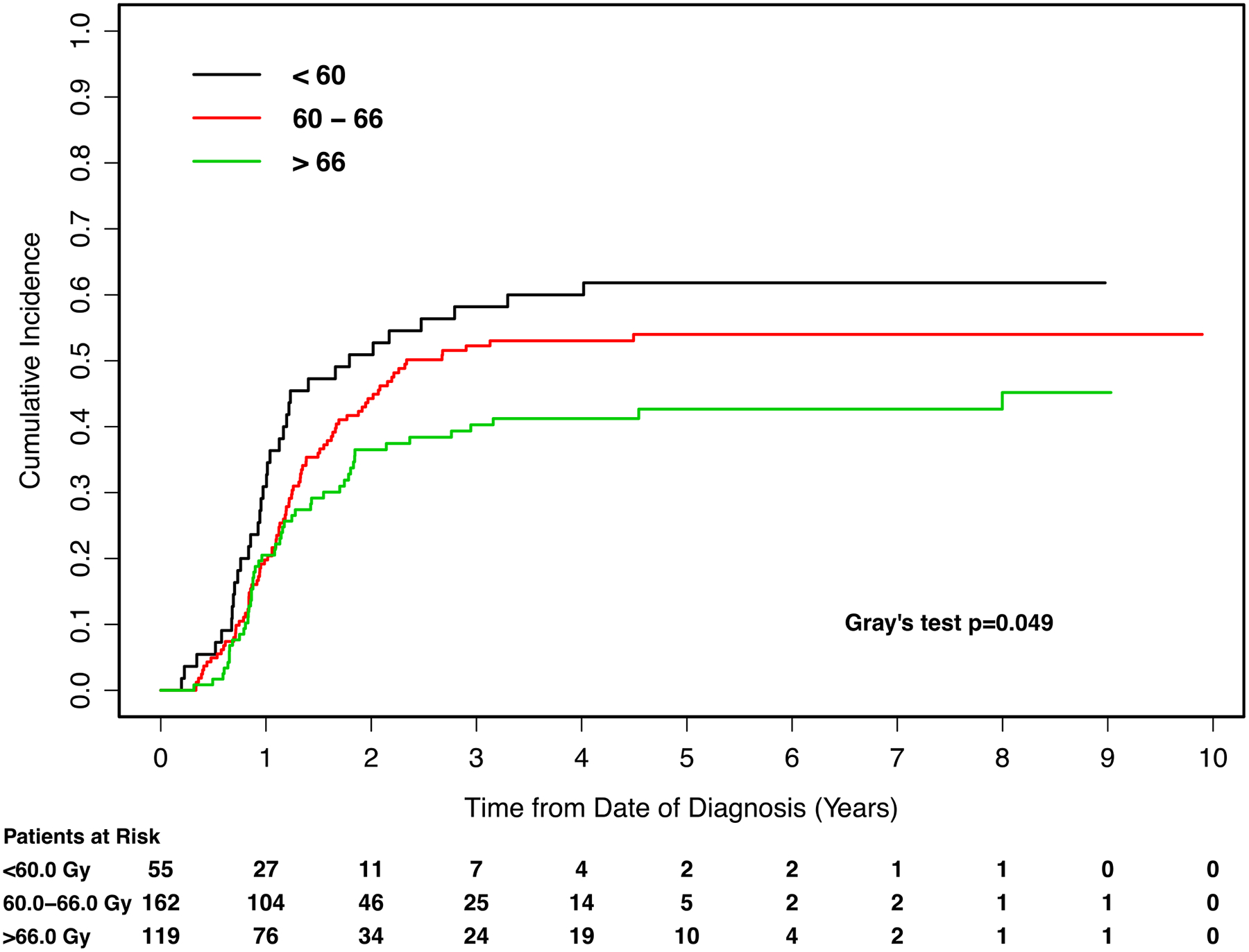
Cumulative incidence curves for the endpoint of local failure, with patients stratified by radiotherapy (RT) dose. Gy: Gray.
Figure 3.
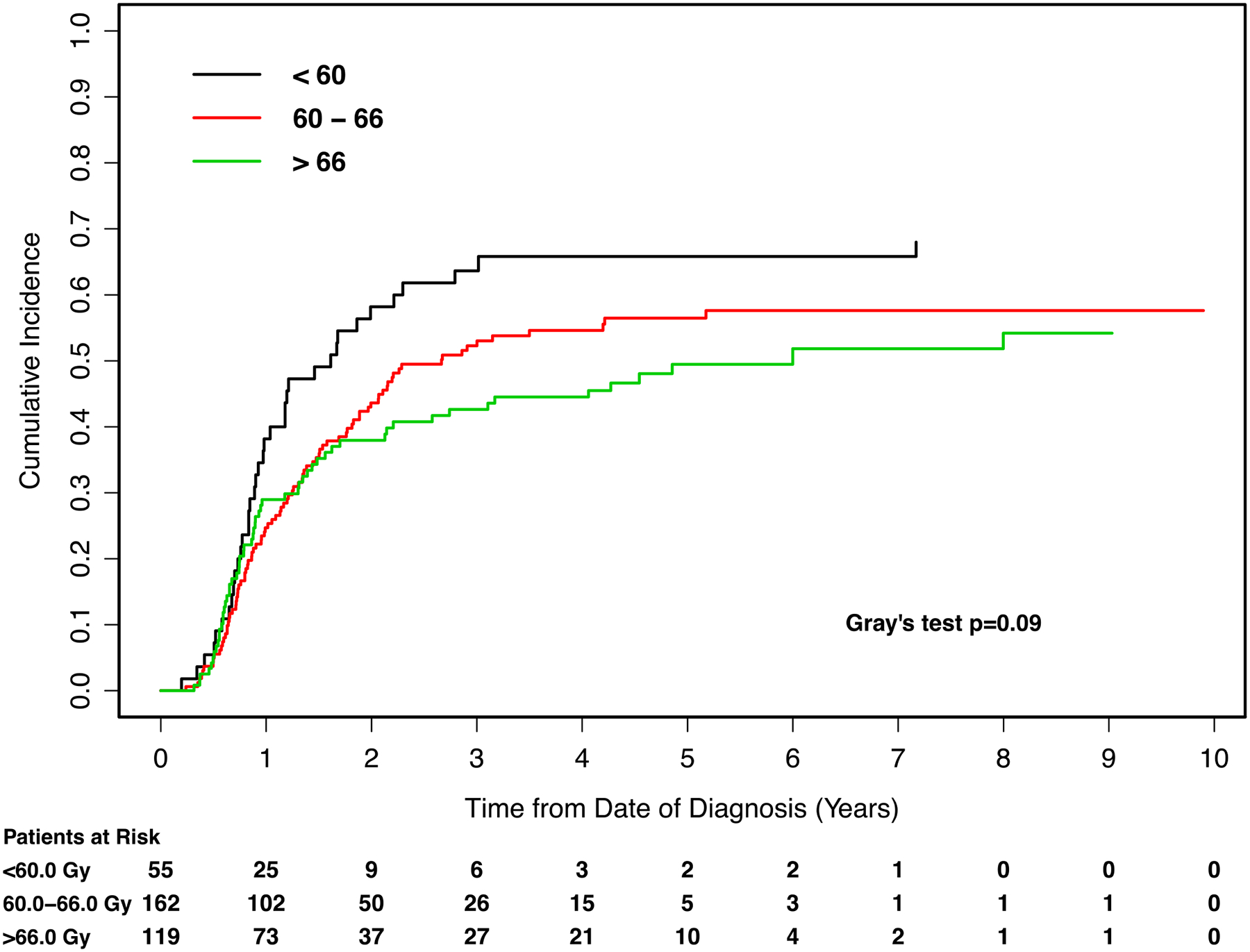
Cumulative incidence curves for the endpoint of distant failure, with patients stratified by radiotherapy (RT) dose. Gy: Gray.
Table 3.
Estimated three-year overall survival rate, and three-year cumulative incidence of local failure, and distant failure according to variables included in the multivariate analysis final models.
| Overall Survival | Local Failure | Distant Failure | |
|---|---|---|---|
| Factor | Three-year rate (95% CI) | Three-year cumulative incidence (95% CI) | Three-year cumulative incidence (95% CI) |
| Age at diagnosis | NA1 | − | − |
| KPS | |||
| <80 | 14.5% (8.3–22.3%) | − | − |
| ≥80 | 37.3% (30.9–43.7%) | − | − |
| History of smoking | |||
| Former | − | 46.6% (39.9–53.3%) | − |
| Current | − | 50.6% (40.3–60.9%) | − |
| Never | − | 69.5% (47.6–91.4%) | − |
| T stage | |||
| T1 | 43.9% (32.2–55.0%) | − | − |
| T2 | 29.6% (21.3–38.3%) | − | − |
| T3 | 22.5% (13.7–32.6%) | − | − |
| T4 | 17.9% (8.9–29.4%) | − | − |
| Tx | 54.5% (22.9–78.0%) | − | − |
| Histology | |||
| Squamous cell | − | − | 37.7% (28.9–46.5%) |
| Adenocarconima | − | − | 60.3% (52.7–67.9%)) |
| Other/NOS | − | − | 51.1% (36.4–65.7%) |
| Histologic grade | |||
| Moderate | − | − | 61.5% (45.3–77.6%) |
| Poor | − | − | 52.5% (44.0–61.0%) |
| Unknown | − | − | 46.3% (38.2–54.3%) |
| Radiation dose, Gy | |||
| <60.0 | 29.1% (17.8–41.3%) | 58.2% (44.8–71.6%) | − |
| 60.0 to 66.0 | 30.5% (23.4–37.9%) | 52.3% (44.4–60.1%) | − |
| >66.0 | 30.2 (21.8–38.9%) | 40.3% (31.2–49.4%)) | − |
| GTV | NA1 | − | NA1 |
| Primary tumor radiographic size | − | NA1 | − |
| Treatment modality | |||
| Definitive RT only | 14.2% (7.9–22.1%) | − | − |
| Sequential CRT | 36.9%(30.6–43.2%) | − | − |
NA: not available, KPS: Karnofsky performance status, NOS: not otherwise specified, Gy: Gray, GTV: primary and nodal gross tumor volume, RT: radiotherapy, CRT: chemoradiation.
Based on log transformed data.
Estimate is not available for a continuous variable.
Clinical Practice Points.
Conflicting data have been published regarding the optimal radiation dose in the treatment of inoperable, locally advanced non-small cell lung cancer (NSCLC). Early studies established that a dose as high as 83.8 Grays (Gy) could be safely administered and suggested that dose escalation was beneficial. However, the randomized controlled trial RTOG 0617 reported worse overall survival in a high-dose cohort receiving 74 Gy compared to a low-dose cohort receiving 60 Gy. Importantly, the randomized controlled trial included only patients receiving concurrent chemoradiation, a treatment which many patients are ineligible for due to comorbidities. We report the results of a large retrospective analysis of 336 patients with locally advanced, inoperable NSCLC who received sequential chemoradiation or definitive radiation only. On multivariate analysis, a dose >66 Gy rather than dose <60 Gy was associated with improved overall survival (HR 0.58, 95%CI 0.39 to 0.87, p=0.008). A dose >66 Gy rather than a dose <60 Gy was also associated with a decreased risk of local failure (HR 0.59, 95%CI 0.38 to 0.91, p=0.02). Therefore, we recommend that patients with locally advanced NSCLC who are ineligible for concurrent chemoradiation receive a dose of at least 66 Gy for optimal overall survival and local control.
Acknowledgements:
This work was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant No. P30 CA008748.
Non-standard abbreviations:
- RT
radiotherapy
- RTOG
Radiation Therapy Oncology Group
- CRT
chemoradiation
- GTV
gross tumor volume
- ITV
initial target volume
- PTV
planning target volume
- 3D-CRT
three-dimensional conformal radiation therapy
- IMRT
intensity-modulated radiation therapy
- OS
overall survival
- LF
local failure
- DF
distant failure
Appendix A
Table A1.
Univariate analysis results.
| Overall Survival | Local Failure | Distant Failure | ||||
|---|---|---|---|---|---|---|
| Factor | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age at diagnosis | 1.02 (1.01–1.03) | 0.005 | − | − | − | − |
| KPS | ||||||
| <80 | 1 [Reference] | − | − | − | − | |
| ≥80 | 0.58 (0.45–0.75) | <0.001 | − | − | − | − |
| History of smoking | ||||||
| Former | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Current | 1.04 (0.81–1.35) | 0.75 | 1.04 (0.7– 1.46) | 0.83 | 1.14 (0.83–1.57) | 0.43 |
| Never | 0.85 (0.52–1.41) | 0.53 | 1.88 (1.19–2.98) | 0.007 | 1.63 (0.98–2.70) | 0.06 |
| T stage | ||||||
| T1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| T2 | 1.34 (0.97–1.83) | 0.08 | 1.58 (1.04–2.41) | 0.03 | 1.19 (0.82–1.73) | 0.37 |
| T3 | 1.55 (1.10–2.19) | 0.01 | 1.87 (1.21–2.88) | 0.005 | 1.23 (0.82–1.86) | 0.32 |
| T4 | 1.76 (1.20–2.59) | 0.004 | 1.30 (0.78–2.19) | 0.32 | 1.02 (0.63–1.66) | 0.94 |
| Tx | 0.69 (0.35–1.40) | 0.30 | 0.61 (0.19–1.93) | 0.40 | 0.38 (0.13–1.11) | 0.08 |
| N stage | ||||||
| N0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| N1 | 0.79 (0.48–1.30) | 0.35 | 0.76 (0.42–1.38) | 0.36 | 1.61 (0.81–3.19) | 0.17 |
| N2 | 0.89 (0.59–1.34) | 0.58 | 0.75 (0.46–1.22) | 0.25 | 1.48 (0.80–2.74) | 0.21 |
| N3 | 0.66 (0.43–1.03) | 0.07 | 0.96 (0.58–1.59) | 0.88 | 1.99 (1.06–3.71) | 0.03 |
| Stage | ||||||
| IIA | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| IIB | 1.39 (0.75–2.58) | 0.30 | 2.68 (1.25–5.74) | 0.01 | 0.83 (0.36–1.90) | 0.65 |
| IIIA | 0.96 (0.61–1.50) | 0.86 | 1.10 (0.58–2.08) | 0.76 | 0.84 (0.49–1.44) | 0.53 |
| IIIB | 0.90 (0.56–1.43) | 0.65 | 1.51 (0.80–2.87) | 0.21 | 1.25 (0.73–2.15) | 0.42 |
| Histology | ||||||
| Squamous cell | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Adenocarconima | 0.73 (0.57–0.95) | 0.02 | 0.89 (0.64–1.24) | 0.50 | 1.68 (1.21–2.33) | 0.002 |
| Other/NOS | 0.91 (0.64–1.31) | 0.63 | 1.12 (0.70–1.79) | 0.65 | 1.36 (0.82–2.24) | 0.23 |
| Histologic grade | ||||||
| Moderate | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Poor | 1.27 (0.86–1.87) | 0.22 | 1.03 (0.63–1.68) | 0.91 | 0.73 (0.48–1.11) | 0.14 |
| Unknown | 1.08 (0.74–1.59) | 0.68 | 0.98 (0.61–1.58) | 0.93 | 0.61 (0.40–0.91) | 0.02 |
| Primary site | ||||||
| RML/RLL/LLL | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| RUL/LUL | 1.08 (0.84–1.39) | 0.54 | 1.01 (0.73–1.38) | 0.96 | 1.20 (0.88–1.62) | 0.25 |
| Radiation dose, Gy | ||||||
| <60.0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| 60.0 to 66.0 | 0.73 (0.53–1.01) | 0.055 | 0.76 (0.51–1.13) | 0.17 | 0.71 (0.49–1.04) | 0.08 |
| >66.0 | 0.67 (0.48–0.93) | 0.02 | 0.57 (0.36–0.88) | 0.01 | 0.62 (0.41–0.95) | 0.03 |
| RT technique | ||||||
| IMRT | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| 3D-CRT | 1.47 (1.12–1.92) | 0.005 | 0.91 (0.63–1.31) | 0.61 | 0.67 (0.46–0.97) | 0.03 |
| GTV | 1.45 (1.28–1.66)* | <0.001* | 1.08 (0.93–1.25)* | 0.30* | 1.22 (1.06–1.41)* | 0.006* |
| Primary tumor radiographic size | 0.93 (0.76–1.13)* | 0.46* | 1.73 (1.28–2.35)* | 0.004* | 1.31 (0.99–1.74)* | 0.06* |
| Treatment modality | ||||||
| Definitive RT only | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Sequential CRT | 0.54 (0.42–0.70) | <0.0001 | 1.20 (0.84–1.71) | 0.32 | 1.57 (1.09–2.25) | 0.02 |
KPS: Karnofsky performance status, GTV: primary and nodal gross tumor volume, NOS: not otherwise specified, RT: radiotherapy, CRT: chemoradiation, 3D-CRT: three-dimensional conformal radiation therapy, IMRT: intensity-modulated radiation therapy, Gy: Gray
based on log transformed data.
Footnotes
Conflicts of Interest: authors have completed the ICJME Conflict of Interest Form. Dr. Sonnick has nothing to disclose. Dr. Oro has nothing to disclose. Ms. Yan has nothing to disclose. Mr. Desai has nothing to disclose. Dr. Wu reports grants from CivaTech Oncology, outside the submitted work. Ms. Shi has nothing to disclose. Dr. Zhang has nothing to disclose. Dr. Gelblum has nothing to disclose. Dr. Paik has nothing to disclose. Dr. Yorke has nothing to disclose. Dr. Rosenzweig has nothing to disclose. Dr. Chaft has nothing to disclose. Dr. Rimner reports grants from Varian Medical Systems, grants from Boehringer Ingelheim, personal fees from Bristol Myers-Squibb, personal fees from General Electric, outside the submitted work.
References:
- 1.United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2016. http://www.cdc.gov/uscs.
- 2.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103(10):2118–2127. [DOI] [PubMed] [Google Scholar]
- 3.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–333. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61(2):318–328. [DOI] [PubMed] [Google Scholar]
- 5.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323(14):940–945. [DOI] [PubMed] [Google Scholar]
- 6.Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88–08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst. 1995;87(3):198–205. [DOI] [PubMed] [Google Scholar]
- 7.Curran WJ Jr., Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. [DOI] [PubMed] [Google Scholar]
- 9.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco R, Maestu I, de la Torre MG, Cassinello A, Nunez I. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26(3):451–463. [DOI] [PubMed] [Google Scholar]
- 11.De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20(1):98–102. [DOI] [PubMed] [Google Scholar]
- 12.Al-Shamsi HO, Al Farsi A, Ellis PM. Stage III non-small-cell lung cancer: Establishing a benchmark for the proportion of patients suitable for radical treatment. Clin Lung Cancer. 2014;15(4):274–280. [DOI] [PubMed] [Google Scholar]
- 13.Sura S, Yorke E, Jackson A, Rosenzweig KE. High-dose radiotherapy for the treatment of inoperable non-small cell lung cancer. Cancer J. 2007;13(4):238–242. [DOI] [PubMed] [Google Scholar]
- 14.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–2699. [DOI] [PubMed] [Google Scholar]
- 15.Hong JC, Salama JK. Dose escalation for unresectable locally advanced non-small cell lung cancer: end of the line? Transl Lung Cancer Res. 2016;5(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramroth J, Cutter DJ, Darby SC, et al. Dose and Fractionation in Radiation Therapy of Curative Intent for Non-Small Cell Lung Cancer: Meta-Analysis of Randomized Trials. Int J Radiat Oncol Biol Phys. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo TR, Moon SH, Lim YJ, et al. The effect of tumor volume and its change on survival in stage III non-small cell lung cancer treated with definitive concurrent chemoradiotherapy. Radiat Oncol. 2014;9:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohri N, Duan F, Machtay M, et al. Pretreatment FDG-PET metrics in stage III non-small cell lung cancer: ACRIN 6668/RTOG 0235. J Natl Cancer Inst. 2015;107(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner-Wasik M, Swann RS, Bradley J, et al. Increasing tumor volume is predictive of poor overall and progression-free survival: secondary analysis of the Radiation Therapy Oncology Group 93–11 phase I-II radiation dose-escalation study in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70(2):385–390. [DOI] [PubMed] [Google Scholar]
- 20.van Diessen JN, Chen C, van den Heuvel MM, Belderbos JS, Sonke JJ. Differential analysis of local and regional failure in locally advanced non-small cell lung cancer patients treated with concurrent chemoradiotherapy. Radiother Oncol. 2016;118(3):447–452. [DOI] [PubMed] [Google Scholar]


