Abstract
Papillary craniopharyngiomas (PCPs) are characterized by the presence of BRAF V600E mutations, which are emerging as a useful guide for diagnosis and treatment decision making. The ongoing multicenter phase 2 Alliance A071601 trial is evaluating the efficacy of BRAF and mitogen-activated protein kinase kinase (MEK) inhibitors for patients with PCPs. With continued successful responses, it is proposed that BRAF (and MEK) inhibitors be evaluated for the neoadjuvant treatment of patients with PCPs.
Keywords: craniopharyngioma, papillary, BRAF, surgery, clinical trial
Craniopharyngiomas are primary brain tumors of the sellar region that are presumed to arise from embryologic remnants of Rathke’s pouch.1,2 Although craniopharyngiomas have benign histology and 10-year survival is over 90%, quality of life is frequently impaired by involvement of neurohormonal structures, including the optic nerves, the pituitary gland and the hypothalamus.3, 4 Complete surgical resection is attempted when possible, but partial resection is favored to preserve function when the tumor involves the hypothalamus. Radiation therapy is commonly used to manage residual tumor and recurrence.5 Currently, US Food and Drug Administration-approved systemic treatments are not available for patients in whom craniopharyngiomas recur after surgery and radiation.
Craniopharyngiomas are divided in 2 main subtypes – adamantinomatous craniopharyngiomas and papillary craniopharyngiomas (PCPs) – that exhibit different clinical and molecular features (Table 1).6, 7
Table 1:
Comparisons of Different Clinical and Molecular Features of Adamantinomatous and Papillary Craniopharyngiomas
| Adamantinomatous Craniopharyngiomas | Papillary Craniopharyngiomas | |
|---|---|---|
| Age with highest prevalence | Bimodal: incidence peaks (5–15 and 40–60 y) | Primarily adults (40–55 y) |
| Location | Supra- and/or infradiaphragmatic | Mainly supradiaphragmatic |
| Calcifications | Frequently present | Rare |
| Tumor signaling pathway | Wnt pathway | MAPK pathway |
| Frequent genomic alterations | CTNNB1 mutations | BRAFV600E mutations |
Abbreviations: CTNNB1, catenin β1; MAPK, mitogen-activated protein kinase.
A hallmark of PCPs is their supradiaphragmatic location. Surgical resection is challenging because of the proximity to critical structures and is typically performed via a traditional transcranial approach or an endoscopic endonasal approach.8 The extent of resection varies with the tumor size and location. Despite the benign nature of these tumors, recurrences are common and necessitate further surgery or radiation. Consequently, morbidity due to neurological and endocrinological complications is high, with potentially devastating effects, including visual loss and hypothalamic dysfunction.9, 10
BRAF Inhibitors in PCPs
Five years ago, we reported that nearly all PCPs have BRAF V600E mutations (BRAFV600E), which constitutively activate the mitogen-activated protein kinase signaling pathway.7 BRAFV600E mutations are recurrent aberrations in neoplasms such as melanoma that can demonstrate remarkable sensitivity to BRAF inhibitors.11 Similarly, our group and others have published case reports of dramatic responses in patients with BRAFV600E-mutant PCP treated with BRAF and/or mitogen-activated protein kinase kinase (MEK) inhibitors.
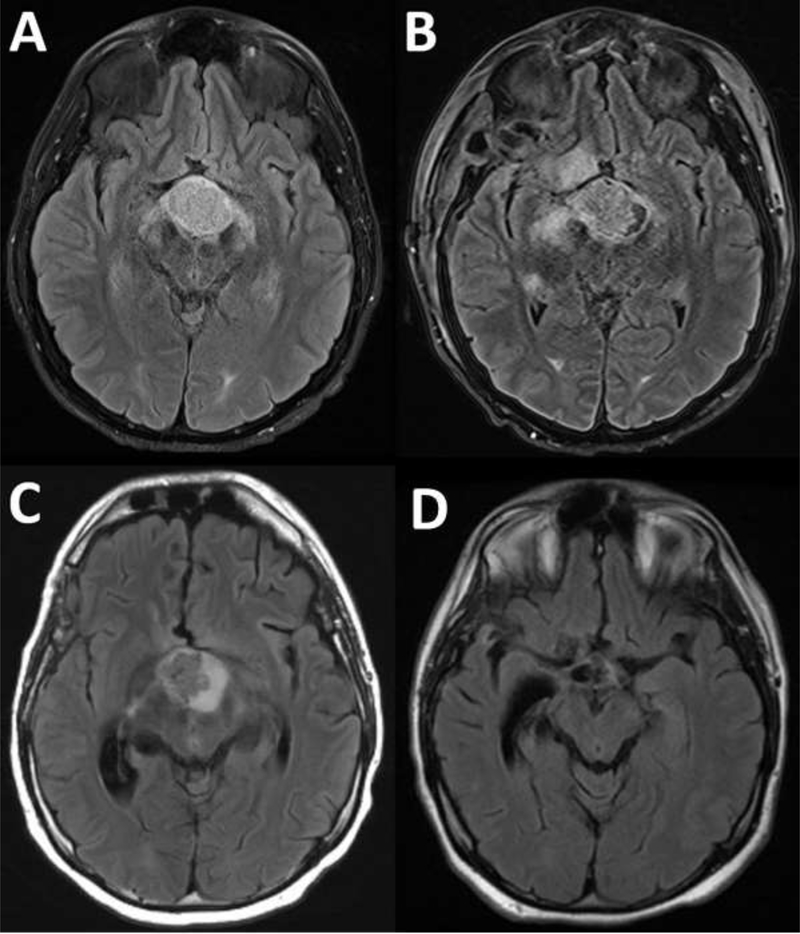
In this article, authors from all 5 previously published reports share their collective experience, provide updated follow-up on their patients, and thus generate an overview of all currently available information on targeted therapy in patients with BRAFV600E-mutant PCP (Table 2).12–16 We have also included information on an additional patient with PCP recently treated at Massachusetts General Hospital (patient PCP6). He was 21 years old when he presented with several months of intermittent headaches and progressive fatigue. He also reported difficulty in concentrating, weight gain, and nausea. An ophthalmologic evaluation identified visual field deficits, and brain magnetic resonance imaging (MRI) revealed an enhancing suprasellar mass. He underwent biopsy of the lesion, with pathology demonstrating BRAFV600E-mutant PCP. The surgery was complicated by infarcts involving the right anterior choroidal artery territory as well as panhypopituitarism. One month after the operation, imaging revealed continued tumor growth. He was admitted to our institution and started on dabrafenib and trametinib. Serial MRI scans over the course of 6 months demonstrated a significant reduction in the tumor size with corresponding improvements in his mental status and headaches (Fig. 1). This is the first example of successful neoadjuvant treatment in a patient with BRAFV600E-mutant PCP. At the time of treatment, the tumors in patients PCP1 to PCP5 had recurred after they had undergone surgical resection(s) and/or radiation therapy, whereas treatment in PCP6 was performed after biopsy alone and without any other therapy; this highlights the potential of neoadjuvant treatment. Four patients (PCP1, PCP3, PCP4, and PCP6) received both BRAF and MEK inhibitors, whereas 2 patients (PCP2 and PCP5) received BRAF inhibitor monotherapy (vemurafenib or dabrafenib). Despite different treatment strategies, in all cases, complete or nearly complete tumor regression was achieved, mostly within just a few months after the start of treatment. Both solid and cystic components were responsive. The treatment was well tolerated in most cases, but therapy was temporarily held for 1 patient who developed a cerebrospinal fluid (CSF) leak (PCP2) and for another patient who developed pyrexia (PCP3).
Table 2:
An overview of currently available and updated information on targeted therapy in patients with BRAFV600E mutant, papillary craniopharyngioma.
| Case no. | Age, y/ sex | Histology and Molecular Status | Tumor Size Before Treatment, cm | Initial Treatment | Agents | Regimen | Response | Reference |
|---|---|---|---|---|---|---|---|---|
| PCP1 | 39/male | rPCP, BRAFV600E mutant | 4.4 × 2.7 × 4.2 | Multiple surgeries | Dabrafenib and Trametinib | 150 mg and 2 mg bid | PR (85% decrease) after 1 mo | 12 |
| PCP2 | 57/ female | rPCP, BRAFV600E mutant | 2 × 3 × 2 | Multiple surgeries | Vemurafenib | 960 mg bid | Near CR after 3 mo | 13 |
| PCP3 | 65/male | rPCP, BRAFV600E mutant | 2.15 × 2.64 × 2.2 | Multiple surgeries | Dabrafenib and Trametinib | 150 mg bid and 2 mg qd | PR (91% decrease) after 4 mo | 14 |
| PCP4 | 47/ female | rPCP, BRAFV600E mutant | 2.6 × 2.3 × 3.2 | Surgery and RTx | Dabrafenib and Trametinib | 150 mg bid and 2 mg qd | CR after 7 mo | 15 |
| PCP5 | 52/male | rPCP, BRAFV600E mutant | 1.9 × 1.8 × 1 | Surgery and RTx | Dabrafenib | 150–225 mg qd | CR after 9 mo | 16 |
| PCP6 | 21/male | Residual PCP, BRAFV600E mutant | 3.1 × 2.6 × 2.3 | Surgery (biopsy) | Dabrafenib and Trametinib | 150 mg bid and 2 mg qd | PR (> 80% decrease) after 6 mo | Recent case |
Abbreviations: bid, twice daily; CR, complete response; PCP, papillary craniopharyngioma; PR, partial response; qd, every day; rPCP, recurrent papillary craniopharyngioma; RTx, radiation therapy.
Figure 1.
T2/fluid-attenuated inversion recovery-weighted magnetic resonance imaging sequences demonstrating cystic and solid suprasellar mass: (A) on initial presentation, (B) after subtotal resection, (C) after 2 weeks of dabrafenib and trametinib, and (D) after 6 months of therapy.
In the patient who developed a CSF leak and meningitis (PCP2) while on vemurafenib monotherapy, the tumor had responded to therapy with a nearly complete response and regrew 3 months after treatment was discontinued because of the complications. Tumor control was again achieved with vemurafenib monotherapy but lasted only 7 months, after which the tumor progressed. A significant reduction in performance status occurred after the CSF leak, so further active treatment was considered inappropriate, and she died 6 months after treatment was discontinued. In 1 patient (PCP1), a dramatic response was achieved after only one month of BRAF/MEK inhibitor dual therapy, after which the remaining tumor was resected, and the area was treated with radiation. The patient was well 12 months later and was subsequently lost to follow-up. Similarly, a substantial treatment response was achieved in another patient (PCP4) using BRAF/MEK inhibitor dual therapy, and the residual tumor was treated with radiation. This patient was tumor-free and remained on dual therapy with dabrafenib and trametinib at the time of this writing. In 1 case (PCP5), minimal tumor remained after 9 months of targeted monotherapy with dabrafenib, at which point treatment was discontinued. With the patient off therapy, the tumor had not recurred 18 months later. The dramatic responses achieved in these cases highlight the transformative potential of BRAF-targeted therapy for patients with PCP. The most recently treated patient (PCP6) had a rapid response in the form of an approximately 80% to 90% reduction of the solid and cystic portions within 6 months.
Although these 6 cases are highly informative for guiding patient treatment, uncertainty remains concerning the optimal timing, the particular agents (single-agent or dual therapy) to be used, and the duration of treatment. A combination of dabrafenib and trametinib has been used most frequently (4 of 6 patients); however, more data are needed to decide on the optimal drug combination. The ongoing multicenter phase 2 Alliance A071601 trial () of vemurafenib and cobimetinib for patients with biopsy-proven residual or recurrent PCP should provide additional information to help to inform patient management.
In addition, the remarkable responses in patients PCP1 to PCP5 with recurrent disease and in PCP6 with neoadjuvant treatment after biopsy signal the potential for using targeted therapy before any surgery. Algorithms for discriminating PCPs from adamantinomatous craniopharyngiomas have been proposed on the basis of retrospective analyses of MRI data.17–19 Such approaches will require further refinement and testing in prospective studies. Further diagnostic approaches, including the detection of BRAF mutations in serum, may contribute to preoperative diagnostic evaluations. Circulating BRAF in the blood of a patient with a PCP (PCP2) was previously reported.12 This liquid biopsy approach would allow not only the noninvasive detection of BRAF mutations but also monitoring of treatment responses. However, until such noninvasive techniques can be demonstrated to be reliable, the diagnosis of a papillary subtype of craniopharyngioma will be made through biopsy, histology review, and molecular assessment. A feasible strategy may be conservative biopsy of a craniopharyngioma that has radiological features suggestive of the papillary subtype with the potential for targeted therapy. In the future, if achievable with high sensitivity and specificity, the noninvasive or minimally invasive diagnosis of PCP would lead to neoadjuvant targeted therapeutic approaches that could further reduce the significant lifelong morbidity associated with these challenging neoplasms beyond what has already been achieved for patients with recurrent disease.
Summary
PCPs are characterized by the presence of BRAFV600E mutations, which are emerging as a useful guide for diagnosis and treatment decision making. The ongoing multicenter phase 2 Alliance A071601 trial is evaluating the efficacy of BRAF and MEK inhibitors for patients with PCP. With continued successful responses, we propose that BRAF (and MEK) inhibitors be evaluated for the neoadjuvant treatment of patients with PCP.
Acknowledgments
Funding Support
Sandro Santagata is supported by the National Institutes of Health (U54CA225088) and the Ludwig Center at Harvard. Priscilla K. Brastianos receives salary support from the National Institutes of Health (1R01CA227156-01), the Breast Cancer Research Foundation, Susan G. Komen, and Damon Runyon Cancer Research.
This study was conducted according to the guidelines of the Declaration of Helsinki and has been approved by the local ethics committee of our faculty.
We thank Dr. Joon H. Uhm (Mayo Clinic, Rochester, Minnesota) for contributing to the clinical care of the patients.
Conflict of Interest Disclosures
Yazmin Odia reports grants from Bristol-Myers Squibb and grants and other from Novocure outside the submitted work. Daniel P. Cahill reports receiving honoraria from Merck and Lilly outside the submitted work. Eva Galanis reports grants and personal fees from MedImmune and Genentech; grants from Bristol-Myers Squibb, Tracon, and Denovo Biopharma; and personal fees from Vyriad, Celgene, KIYATEC, Tactical Therapeutics, and Oncorus outside the submitted work. Sandro Santagata is a consultant for RareCyte. Priscilla K. Brastianos reports honoraria for consulting from Tesaro, Lilly, Angiochem, and Genentech-Roche; speaker’s honoraria from Genentech-Roche and Merck; and research funding (to Massachusetts General Hospital) from Merck and Pfizer. The other authors made no disclosures.
References
- 1.Schlaffer SM, Buchfelder M, Stoehr R, Buslei R, Holsken A. Rathke’s Cleft Cyst as Origin of a Pediatric Papillary Craniopharyngioma. Front Genet. 2018;9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89: 547–551. [DOI] [PubMed] [Google Scholar]
- 3.Yamini B, Narayanan M. Craniopharyngiomas: an update. Expert Rev Anticancer Ther. 2006;6 Suppl 9: S85–92. [DOI] [PubMed] [Google Scholar]
- 4.Lustig RH, Post SR, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88: 611–616. [DOI] [PubMed] [Google Scholar]
- 5.Smee RI, Williams JR, Kwok B, Teo C, Stening W. Modern radiotherapy approaches in the management of craniopharyngiomas. J Clin Neurosci. 2011;18: 613–617. [DOI] [PubMed] [Google Scholar]
- 6.Holsken A, Sill M, Merkle J, et al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun. 2016;4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brastianos PK, Taylor-Weiner A, Manley PE, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46: 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg. 1999;90: 237–250. [DOI] [PubMed] [Google Scholar]
- 9.Muller HL. Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Metab. 2011;96: 1981–1991. [DOI] [PubMed] [Google Scholar]
- 10.Mortini P, Losa M, Pozzobon G, et al. Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg. 2011;114: 1350–1359. [DOI] [PubMed] [Google Scholar]
- 11.Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brastianos PK, Shankar GM, Gill CM, et al. Dramatic Response of BRAF V600E Mutant Papillary Craniopharyngioma to Targeted Therapy. J Natl Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aylwin SJ, Bodi I, Beaney R. Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary. 2016;19: 544–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostami E, Witt Nystrom P, Libard S, Wikstrom J, Casar-Borota O, Gudjonsson O. Recurrent papillary craniopharyngioma with BRAFV600E mutation treated with neoadjuvant-targeted therapy. Acta Neurochir (Wien). 2017;159: 2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roque A, Odia Y. BRAF-V600E mutant papillary craniopharyngioma dramatically responds to combination BRAF and MEK inhibitors. CNS Oncol. 2017;6: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himes BT, Ruff MW, Van Gompel JJ, et al. Recurrent papillary craniopharyngioma with BRAF V600E mutation treated with dabrafenib: case report. J Neurosurg. 2018: 1–5. [DOI] [PubMed] [Google Scholar]
- 17.Fujio S, Juratli TA, Arita K, et al. A Clinical Rule for Preoperative Prediction of BRAF Mutation Status in Craniopharyngiomas. Neurosurgery. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Omay SB, Chen YN, Almeida JP, et al. Do craniopharyngioma molecular signatures correlate with clinical characteristics? J Neurosurg. 2018;128: 1473–1478. [DOI] [PubMed] [Google Scholar]
- 19.Yue Q, Yu Y, Shi Z, et al. Prediction of BRAF mutation status of craniopharyngioma using magnetic resonance imaging features. J Neurosurg. 2018;129: 27–34. [DOI] [PubMed] [Google Scholar]