Abstract
Background
Clinical relapse in acute myeloid leukemia (AML) is associated with the reduced treatment response of leukemia stem cells (LSCs). This study aimed to investigate the effects of the ginseng derivative, ginsenoside Rg1 (Rg1), on CD34+CD38− LSCs derived from KG1α human acute myeloid leukemia cells.
Material/Methods
CD34+CD38− LSCs were isolated from KG1α human acute myeloid leukemia cells by cell sorting. CD34+CD38− KG1α LSCs were divided into the control group and the Rg1 group (treated with Rg1). The cell counting kit-8 (CCK-8) assay evaluated the proliferation of CD34+CD38− KG1α LSCs and flow cytometry studied the cell cycle. The mixed colony-forming unit (CFU-Mix) assay and staining for senescence-associated beta-galactosidase (SA-β-Gal) evaluated cell senescence. Expression of sirtuin 1 (SIRT1) and tuberous sclerosis complex 2 (TSC2) were evaluated using Western blot and quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Results
CD34+CD38− KG1α LSCs were isolated at 98.72%. Rg1 significantly reduced the proliferation of CD34+CD38− KG1α LSCs compared with the control group (p<0.05). Cells in the G0/G1 phase were significantly increased, and cells in the G2/M and S phase were significantly reduced compared with the control group (p<0.05). Rg1 significantly increased SA-β-Gal and reduced CFU-Mix formation compared with the control group (p<0.05), significantly down-regulated SIRT1 expression in CD34+CD38− KG1α LSCs compared with the control group (p<0.05), and significantly reduced TSC2 expression in CD34+CD38− KG1α LSCs compared with the control group (p<0.05).
Conclusions
Rg1 inhibited cell proliferation and induced cell senescence markers in CD34+CD38− KG1α LSCs by activating the SIRT1/TSC2 signaling pathway.
MeSH Keywords: Adult Stem Cells; Ginsenosides; Leukemia, Myeloid, Acute
Background
Acute myeloid leukemia (AML) arises from progenitor hematopoietic cells and is associated with molecular changes that affect cell proliferation and differentiation [1,2]. Clinical relapse in AML is associated with the reduced treatment response of leukemia stem cells (LSCs) [1,2]. Clinically, adult patients with AML have a poor clinical outcome with a 5-year overall survival (OS) rate of 50% for younger adults, and less than one-year OS for older patients [3]. Although chemotherapy may reduce the symptoms of AML, relapse is common due to chemo-resistance [4]. Therefore, novel treatment strategies continue to be investigated.
In AML, LSCs are associated with disease occurrence and disease persistence [5,6]. The LSC phenotype was originally identified as CD34+ CD38− [7]. The identification of this LSC phenotype has resulted in the ability to label and select LSCs by cell sorting techniques, which may lead to the removal of this cell population as a novel therapeutic approach in patients with AML [8,9]. Cell aging, or senescence, limits cell survival and the proliferative capabilities of cells by inducing the dysfunction of cellular metabolism [10]. Although cancer is a disease of aging and the incidence increases with age [11], cancer cells show properties of cell immortalization, and inducing the senescence of malignant cells may suppress tumor growth, including in leukemias such as AML.
Ginsenoside Rg1 (Rg1), derived Panax ginseng and Panax japonicus, has previously been reported to have antitumor effects by inhibiting radiation-resistance and oxidation [12]. Previous studies have shown that Rg1 could affect cell senescence and cell proliferation of normal hematopoietic progenitor cells (HPCs) and hematopoietic stem cells (HSCs) [13,14]. Rg1 has been shown to inhibit the proliferation of cells of the AML cell line, K562, and to accelerate cell senescence [15]. However, the effects of Rg1 on the senescence of LSCs remain poorly understood.
Sirtuin 1 (SIRT1) participates in many biological functions, including cell senescence, and is involved in the pathogenesis of many age-related disorders [16]. The tuberous sclerosis complex 2 (TSC2) molecule and gene are involved in both cell autophagy and senescence [17].
Therefore, this study aimed to investigate the effects of the ginseng derivative, ginsenoside Rg1 (Rg1), on CD34+CD38− LSCs derived from KG1α acute myeloid leukemia cells in vitro. Cell senescence was studied using a mixed colony-forming unit (CFU-Mix) assay and detection of senescence-associated beta-galactosidase (SA-β-Gal).
Material and Methods
Cell culture of KG1α human acute myeloid leukemia (AML) cells and cell sorting to isolate CD34+CD38− leukemia stem cells (LSCs)
The human acute myeloid leukemia (AML) cell line, KG1α, was purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). The KG1α cells were cultured in the Iscove’s Modified Dulbecco’s Medium (IMDM) (Gibco BRL Co. Ltd., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL Co. Ltd., Grand Island, NY, USA), 100 μg/ml of streptomycin, and 100 units/ml of penicillin (Beyotime Biotech., Shanghai, China) at 37°C and 5% CO2.
Cell sorting for CD34+CD38− KG1α cell was performed by immunostaining with an antibody to CD38 conjugated to allophycocyanin (CD38–APC) (eBioscience, Thermo Fisher Scientific, San Diego, CA, USA) and an antibody to CD34 conjugated with fluorescein isothiocyanate (CD-34-FITC) (BD Bioscience, Oxford, UK) at 4°C for 30 min, according to the previously published method [18]. The stained cells were washed using phosphate-buffered saline (PBS) (Beyotime Biotech., Shanghai, China) and sorted using a FACS Aria III flow cytometer (BD Bioscience, Oxford, UK).
Study groups
Ginsenoside Rg1 (Rg1) was purchased from Hongjiu BioTech (Dalian, China) (Cat. No. 060427), and had a purity >95%. In this study, the sorted CD34+CD38− LSCs were divided into the control group and the Rg1 group. The CD34+CD38− LSCs in the control group were cultured in IMDM for 48 h. The CD34+CD38− LSCs in the Rg1 group were cultured in IMDM for 48 h and treated with Rg1 dissolved in dimethyl sulfoxide (DMSO), at a final concentration of 40 μmol/l. The control group consisted of CD34+CD38− LSCs treating with DMSO alone.
Cell counting kit 8 (CCK-8) assay
The cytotoxic effect and cell proliferative activity of CD34+CD38− LSCs were studied using the CCK-8 kit (Cat. No. C0037) (Beyotime Biotech. Shanghai, China), according to the manufacturer’s instructions. Briefly, the cells were seeded onto the 96-well plates at a density of 1×108 cells/well and cultured at 37°C and 5% CO2 for 48 h. The CCK-8 reagents were added to the culture plates at a dose of 20 μl/well and incubated for another 2 h. The optical density (OD) of cells was examined using an ELx808 microplate reader (Bio-Tek Inc., Winooski, VT, USA) at a wavelength of 450 nm. The inhibition rate (%)=(the OD of the control group–the OD of the Rg1 group)/(the OD of the control–the OD of the blank group)×100%.
Flow cytometry assay measurement for cell cycle
CD34+CD38− LSCs were harvested and washed twice in PBS. CD34+CD38− LSCs were fixed overnight using 70% ice-cold ethanol (Beyotime Biotech., Shanghai, China), and washed twice using PBS. Then, the CD34+CD38− LSCs were treated using bovine pancreatic ribonuclease (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 1 mg/ml for 30 min at 37°C. The cells were stained with the propidium iodide (PI) at a concentration of 50 μg/ml in the dark for 30 min. The stained CD34+CD38− LSCs were viewed and imaged using the FACS Aria IIU flow cytometer (BD Bioscience, Oxford, UK) and the percentages of cells in each phase of the cell cycle were analyzed using MultiCycle software (Phoenix Flow Systems, Tokyo, Japan).
Colony formation assay
The colony formation assay was performed, as previously described, but with some modifications [19]. Briefly, the CD34+CD38− LSCs were adjusted to the density of 5×103 cells/well in 96-well plates containing IMDM, and incubated with 1×10−4 mol/l 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA), 3% L-glutamine (Sigma-Aldrich, St. Louis, MO, USA), interleukin 3 (IL-3) (Sigma-Aldrich), 0.8% methylcellulose (Sigma-Aldrich, St. Louis, MO, USA), recombinant human granulocyte macrophage colony-stimulating factor (rhGM-CSF), and human erythropoietin (rhEPO) (Beijing Kylin Culture Co. Ltd. Shanghai, China). The treated CD34+CD38− LSCs were cultured at 37°C and 5% CO2 for two weeks. The colony formation units (CFU) consisting of 50 or more CD34+CD38− LSCs were assigned to the mixed colony forming unit (CFU-Mix) assay, which were counted using a BX51 light microscope (Olympus, Tokyo, Japan).
Senescence-associated beta-galactosidase (SA-β-Gal) staining
CD34+CD38− LSCs were stained with the SA-β-Gal detection kit (Cell Signaling Technology Inc., Beverly, MA, USA), according to the manufacturer’s instructions. Briefly, CD34+CD38− LSCs were washed twice with PBS and stained with the staining solution for 12 h at 37°C. The CD34+CD38− LSCs were centrifuged, and cytospin slides were prepared. The cytospin preparations were sealed with the 70% glycerol (Sigma-Aldrich, St. Louis, MO, USA). A total of 400 CD34+CD38− LSCs on the slides were randomly selected, and the positively-stained cells were counted.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The total RNAs of CD34+CD38− LSCs were extracted with TRIzol regent (Beyotime Biotech., Shanghai, China). The complementary DNA (cDNA) was synthesized using a Reverse Transcription kit (Cat. No. 4366596) (Pierce Thermo Scientific, Rockford, IL, USA) and amplified using SYBR green I (Takara, Dalian, China). The qRT-PCR assay was conducted using 7900HT real-time PCR equipment (ABI, Foster City, CA, USA). The PCR conditions were as follows: initiation for 4 min at 94°C; amplification for 35 cycles at 94°C for 20 s; amplification at 60°C for 30 s; amplification at 72°C for 30 s; and termination at 72°C for 10 min. The qRT-PCR primers for SIRT1, TSC2, and β-actin are shown in Table 1. The amplified products of qRT-PCR were loaded onto 1.5% agarose gels and analyzed with the Quantity-One 1D gel imaging software (Bio-Rad Laboratories, Hercules, CA, USA). Relative gene expression was normalized to the expression of the β-actin gene using the 2−ΔΔCT method [20].
Table 1.
Primers used in this study.
| Genes | Sequences | Length (bp) | |
|---|---|---|---|
| SIRT1 | Forward | CAAGGGATGGTATTTATGCTCG | 173 |
| Reverse | CAAGGCTATGAATTTGTGACAGAG | ||
| TSC2 | Forward | TCTGTCTGCTGTGCGTCCG | 172 |
| Reverse | AGCTTCCAGCAAGGCTCG | ||
| β-actin | Forward | TGACGTGGACATCCGCAAAG | 205 |
| Reverse | CTGGAAGGTGGACAGCGAGG |
Western blot
The CD34+CD38− LSCs cells were lysed using protein lysis buffer (Applygen Tech. Inc., Beijing, China). The protein concentration of the lysates was evaluated using the bicinchoninic acid (BCA) protein concentration detection kit (Cat. No. P0012) (Beyotime Biotech., Shanghai, China). The lysates were separated with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Sigma-Aldrich, St. Louis, MO, USA) and transferred onto polyvinylidene fluoride (PVDF) membranes (Beyotime Biotech., Shanghai, China). The PVDF membranes were incubated overnight at 4°C in the primary antibodies, which were all purchased from Abcam Biotech (Cambridge, MA, USA). The primary antibodies included rabbit anti-human SIRT1 monoclonal antibody (Cat. No. ab32441) (1: 2000), rabbit anti-human TSC2 monoclonal antibody (Cat. No: ab190349) (1: 2000), rabbit anti-human polyclonal antibody (Cat. No. ab8227) (1: 2000). The membranes then incubated in the secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Cat. No. ab6721) (1: 1000) (Abcam Biotech., Cambridge, MA, USA) at 37°C for 1 h. Western blot images were visualized using the electrochemiluminescence (ECL) kit (Pierce Thermo Scientific, Rockford, IL, USA).
Statistical analysis
Data were expressed as the mean±standard deviation (SD) and analyzed using SPSS version 11.0 software (SPSS Inc., Chicago, Ill, USA). Experiments were performed in triplicate. One-way analysis of variance (ANOVA) was validated by Tukey’s post hoc test to compare data between groups. A P-value <0.05 was considered to be statistically significant.
Results
Isolation and survival rate of cell sorting for CD34+CD38− leukemia stem cells (LSCs)
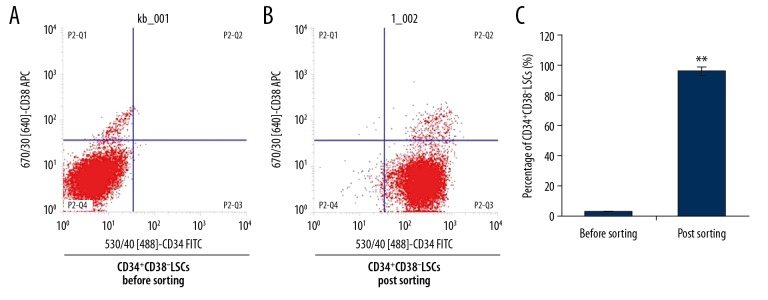
The flow cytometry results showed that following cell sorting, the purity of CD34+CD38− leukemia stem cells (LSCs) was significantly greater compared with the cells before cell sorting (95.86% vs. 2.36%) (Figure 1) (p<0.05). The survival rate of the sorted CD34+CD38− LSCs was 98.72%. The findings demonstrated the successful isolation of CD34+CD38− LSCs.
Figure 1.
Cell sorting of the CD34+CD38− leukemia stem cells (LSCs) derived from KG1α human acute myeloid leukemia (AML) cells. (A) Flow cytometry of CD34+CD38− LSCs derived from KG1α human acute myeloid leukemia cells before cell sorting. (B) Flow cytometry of CD34+CD38− LSCs following cell sorting. (C) Statistical analysis of the sorted CD34+CD38− LSCs. * p<0.05 vs. the control group.
Ginsenoside Rg1 (Rg1) reduced the proliferation rate of CD34+CD38− LSCs
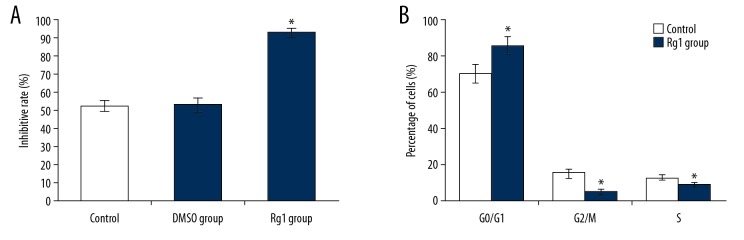
The cell-counting kit-8 (CCK-8) assay was performed to determine the effects of Rg1 on the proliferation of CD34+CD38− LSCs. Rg1 treatment significantly inhibited the CD34+CD38− LSC proliferation compared with the control group (Figure 2A) (p<0.05). There were no significant differences in the proliferation rates of CD34+CD38− LSCs between the control group and the DMSO group, which indicated that DMSO was safe and had no significant cell toxicity.
Figure 2.
Evaluation for the proliferation and cell cycle of CD34+CD38− leukemia stem cells (LSCs) derived from KG1α human acute myeloid leukemia (AML) cells. (A) Statistical analysis of the rate of inhibition of cell proliferation of the CD34+CD38− LSCs derived from KG1α human acute myeloid leukemia cells treated with ginsenoside Rg1 (Rg1). (B) Statistical analysis for the cell cycle of CD34+CD38− LSCs treated with Rg1. * p<0.05 vs. the control group.
Rg1 modulated the phases of the cell cycle in CD34+CD38− LSCs
Cell cycle analysis showed that CD34+CD38− LSCs in the G0/G1 phase of the cell cycle were significantly increased, and cells in the G2/M and S phases were significantly reduced compared with that of the control group (Figure 2B) (p<0.05).
Rg1 increased the expression of senescence-associated beta-galactosidase (SA-β-Gal) in CD34+CD38− LSCs
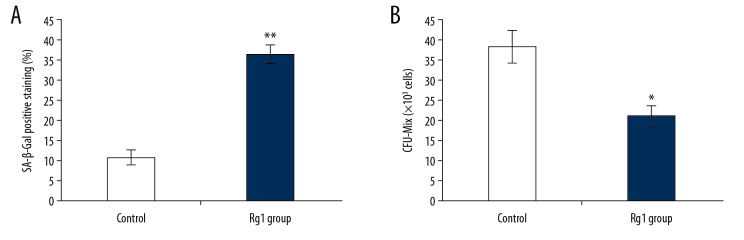
Previous studies have shown that measurement of the expression of SA-β-Gal and the mixed colony-forming unit (CFU-Mix) assay are biomarkers of cell senescence [21,22]. Therefore, in this study, the levels of SA-β-Gal and CFU-Mix in CD34+CD38− LSCs were evaluated. Rg1 treatment significantly increased the levels of SA-β-Gal compared with the control group (Figure 3A) (p<0.05). Also, the CFU-Mix formation was significantly lower in the Rg1 group compared with the control group (Figure 3B) (p<0.05).
Figure 3.
(A, B) The effects of ginsenoside Rg1 (Rg1) on senescence-associated beta-galactosidase (SA-β-Gal) expression and the mixed colony-forming unit (CFU-Mix) assay of CD34+CD38− leukemia stem cells (LSCs) derived from KG1α human acute myeloid leukemia (AML) cells. * p<0.05, ** p<0.01 vs. the control group.
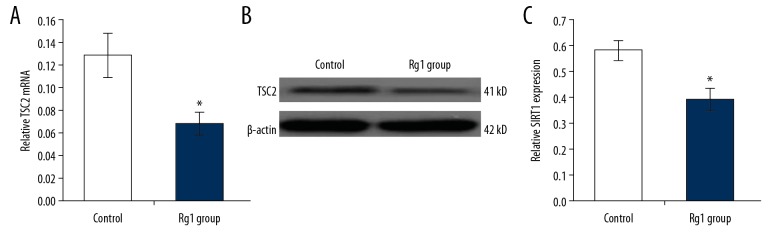
Rg1 down-regulated expression of sirtuin 1 (SIRT1) in CD34+CD38− LSCs
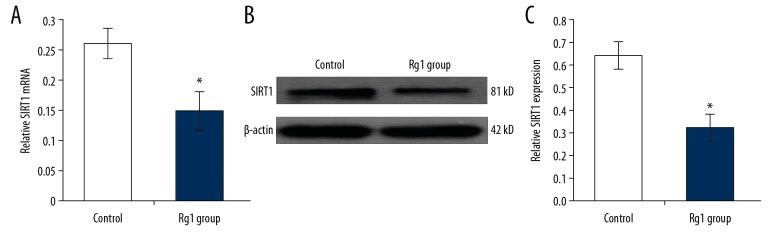
In this study, the expression of SIRT1 mRNA and protein were determined using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot, respectively. The qRT-PCR findings showed that the SIRT1 mRNA levels in the Rg1 group were significantly lower compared with the control group (Figure 4A) (p<0.05). Western blot showed that Rg1 treatment significantly down-regulated SIRT1 expression compared with the control group (Figure 4B, 4C) (p<0.05).
Figure 4.
The effects of ginsenoside Rg1 (Rg1) on SIRT1 expression in CD34+CD38− leukemia stem cells (LSCs) derived from KG1α human acute myeloid (AML) leukemia cells. (A) The evaluation for mRNA expression of SIRT1 following treatment with ginsenoside Rg1 (Rg1) using the quantitative reverse transcription-polymerase chain reaction (qRT-PCR). (B) The evaluation of sirtuin 1 (SIRT1) protein expression following treatment with Rg1 using Western blot. (C) Statistical analysis of SIRT1 expression. * p<0.05 vs. the control group.
Rg1 down-regulated the expression of tuberous sclerosis complex 2 (TSC2) in CD34+CD38− LSCs
TSC2 is a downstream molecule in the SIRT1 pathway. The expression of TSC2 mRNA and protein were determined using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot, respectively. The qRT-PCR findings showed that the TSC2 mRNA levels in the Rg1 group were significantly lower compared with the control group (Figure 5A) (p<0.05). Western blot showed that Rg1 treatment significantly down-regulated TSC2 expression compared with the control group (Figure 5B, 5C) (p<0.05).
Figure 5.
The effects of ginsenoside Rg1 (Rg1) on TSC2 expression in CD34+CD38− leukemia stem cells (LSCs) derived from KG1α human acute myeloid (AML) leukemia cells. (A) The evaluation for mRNA expression of TSC2 following treatment with ginsenoside Rg1 (Rg1) using the quantitative reverse transcription-polymerase chain reaction (qRT-PCR). (B) The evaluation of tuberous sclerosis complex 2 (TSC2) protein expression following treatment with Rg1 using Western blot. (C) Statistical analysis of TSC2 expression. * p<0.05 vs. the control group.
The downstream molecule of SIRT1, TSC2, in the CD34+CD38− LSCs was also examined using qRT-PCR (Figure 5A) and Western blot (Figure 5B). The results showed that the Rg1 treatment significantly decreased the mRNA (Figure 5A) and protein expression (Figure 5B, 5C) of TSC2 compared with that in the control group (p<0.05).
Discussion
Leukemia stem cells (LSCs) play crucial roles in initiating the occurrence and development of acute myeloid leukemia (AML), and are characterized by insensitivity to chemotherapy [24]. Therefore, LSCs have an important role in multidrug resistance and clinical relapse of AML [25]. LSCs also play critical roles in metastasis and treatment failure in leukemia [26,27]. Therefore, traditional chemotherapy may not treat AML effectively. Studies have shown that for patients who have refractory AML, allogeneic stem cell transplantation may be the only treatment option that is effective [26,27]. Therefore, targeted control of LSCs may be a future treatment strategy in patients with AML.
Cell senescence is inevitable, and LSCs also undergo senescence [28]. Recent studies investigated the role of increasing the rate of senescence of cancer cells or cancer stem cells. The traditional Chinese medicine theory has recommended the use of ginseng for its anti-aging, anti-apoptosis, anti-oxidant, and immune-enhancing properties [15,29]. Previous studies have reported that the ginsenoside Rg1 (Rg1) promoted the proliferation of hematopoietic progenitor cells (HPCs) and hematopoietic stem cells (HSCs) and reduced the longevity of mice by reducing DNA damage and down-regulating the expression of p16INK4a and p21CIP1/WAF1 [30,31]. Rg1 was previously shown to inhibit the proliferation of the leukemia cell, K562, by inducing cell senescence [32]. The findings from these previous studies indicated that Rg1 had dual regulatory effects on senescence in different cells.
In the present study, the Rg1 was administrated to CD34+CD38− LSCs, with the typical LSC phenotype [18,19,33]. The findings showed that Rg1 reduced the proliferation rate of CD34+CD38− LSCs, which was consistent with the findings from a previous study [34]. Also, the results of the evaluation of the cell cycle showed that Rg1 triggered G1 phase cell cycle arrest, which suggests that the effects of Rg1 on inhibition of cell proliferation of CD34+CD38− LSCs were induced by G1 phase arrest.
A previous study reported that the senescence of tumor cells was associated with suppressed cell proliferation [35]. Therefore, the effects of Rg1 administration on the expression of senescence-associated beta-galactosidase (SA-β-Gal) by CD34+CD38− LSCs were studied [21,22]. The findings showed that Rg1 treatment significantly increased SA-β-Gal levels and significantly reduced the formation of the mixed colony-forming unit (CFU-Mix), which suggests that Rg1 was associated with changes of cell senescence of CD34+CD38− LSCs by reducing the renewal and proliferative ability of cells. These results support the previous findings on the effects of Rg1 on the K562 human leukemia cell line [36,37], but to our knowledge, these findings are the first on the effects of Rg1 on senescence of CD34+CD38− LSCs.
Sirtuin 1 (SIRT1) has a critical role in several molecular processes, including cell senescence, cell proliferation, and inflammation [38,39]. A previous study reported that the SIRT1 over-expresses in patients with AML was associated with treatment-resistance [40]. The effects of SIRT1 occur by its interaction with tuberous sclerosis complex 2 (TSC2) in the leukemia cells [41]. Therefore, in the present study, the expression of both SIRT1 and TSC2 in the CD34+CD38− LSCs treated with Rg1 was investigated. The findings showed that Rg1 down-regulated the expression of both SIRT1 and TSC2 in the CD34+CD38− LSCs. These results suggest that the Rg1 mediated senescence of CD34+CD38− LSCs is triggered through the down-regulation of the SIRT1/TSC2 signaling pathway.
Conclusions
This study aimed to investigate the effects of the ginseng derivative, ginsenoside Rg1 (Rg1), on CD34+CD38− leukemia stem cells (LSCs) derived from KG1α human acute myeloid leukemia (AML) cells. The findings showed that Rg1 inhibited cell proliferation and induced the G1 cell cycle arrest of the CD34+CD38− LSCs. Rg1 significantly enhanced the expression of senescence-associated beta-galactosidase (SA-β-Gal) and reduced the mixed colony-forming unit (CFU-Mix), which were indicators of cell senescence, by activating the SIRT1/TSC2 signaling pathway. Future studies should be undertaken to investigate the effects of Rg1 on LSCs both in vitro and in vivo.
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China (Grant Numbers: 81860038, 81660731, and 81873103)
Conflict of interest
None.
References
- 1.Siveen KS, Uddin S, Mohammad RM. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol Cancer. 2017;16:13. doi: 10.1186/s12943-016-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin YZ, Zhao T, Zhu HH, et al. High EVI1 expression predicts poor outcomes in adult acute myeloid leukemia patients with intermediate cytogenetic risk receiving chemotherapy. Med Sci Monit. 2018;24:758–67. doi: 10.12659/MSM.905903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24:711–19. doi: 10.1097/CCO.0b013e328358f62d. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Stiehl T, Raffel S, et al. Reduced hematopoietic stem cell frequency predicts outcome in acute myeloid leukemia. Haematologica. 2017;102:1567–77. doi: 10.3324/haematol.2016.163584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 6.Tan BT, Park CY, Ailles LE, et al. The cancer stem cell hypothesis: A work in progress. Lab Invest. 2006;86:1203–7. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–48. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Wong TN, Miller CA, Klco JM, et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood. 2016;127:893–97. doi: 10.1182/blood-2015-10-677021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strickland SA, Mohan SR, Savona MR. Unfavorable-risk acute myeloid leukemia dissected. Curr Opin Hematol. 2016;23:144–49. doi: 10.1097/MOH.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 10.Xian F, Hu X, Hu XS, et al. DUSP facilitates RPMI8226 myeloma cell aging and inhibited TLR4 expression. Eur Rev Med Pharmacol Sci. 2018;22:6030–34. doi: 10.26355/eurrev_201809_15939. [DOI] [PubMed] [Google Scholar]
- 11.Navarrete-Reyes AP, Soto-Perez-de-Celis E, Hurria A. Cancer and aging: A complex biological association. Rev Invest Clin. 2016;68:17–24. [PubMed] [Google Scholar]
- 12.Chu SF, Zhang JT. New achievements in ginseng research and its future prospects. Chin J Integr Med. 2009;15:403–8. doi: 10.1007/s11655-009-0403-6. [DOI] [PubMed] [Google Scholar]
- 13.Xu FT, Li HM, Yin QS, et al. Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro. Can J Physiol Pharmacol. 2014;92:467–75. doi: 10.1139/cjpp-2013-0377. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Mu XY, Zhou Y, et al. Ginsenoside Rg1 enhances the resistance of hematopoietic stem/progenitor cells to radiation-induced aging in mice. Acta Pharmacol Sin. 2014;35:143–50. doi: 10.1038/aps.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zhang J, Fang YM, et al. Ginsenoside Rg1 delays tert-butyl hydroperoxide-induced premature senescence in human WI-38 diploid fibroblast cells. J Biol Sci Med Sci. 2008;63:253–64. doi: 10.1093/gerona/63.3.253. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Cruzat VF, Newsholme P, et al. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech Aging Dev. 2016;155:10–21. doi: 10.1016/j.mad.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Miki Y, Tanji K, Mori F, et al. Autophagy mediators (FOXO1, SESN3 and TSC2) in Lewy body disease and aging. Neurosci Lett. 2018;25:35–41. doi: 10.1016/j.neulet.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi S, Ghaffari SH, Shaiegan M, et al. Curcumin veto the effects of osteopontin (OPN) specific inhibitor on leukemic stem cell colony-forming potential via promotion of OPN overexpression. Int J Hematol Oncol Stem Cell Res. 2016;10:120–29. [PMC free article] [PubMed] [Google Scholar]
- 19.Xu B, Wang S, Li R, et al. Disulfiram/copper selectively eradicates AML leukemia stem cells in vitro and in vivo by simultaneous induction of ROS-JNK and inhibition of NF-κB and Nrf2. Cell Death Dis. 2017;8:e2797. doi: 10.1038/cddis.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–67. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K, Hou BX. The regulation of DLTA gene in bacterial growth and biofilm formation by Parvimonas micra. Eur Rev Med Pharmacol Sci. 2018;22:4033–44. doi: 10.26355/eurrev_201807_15390. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Zhang S, Geng Y, et al. Transient receptor potential ankyrin 1 protects against sepsis-induced kidney injury by modulating mitochondrial biogenesis and mitophagy. Am J Transl Res. 2018;10:4163–72. [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Guo L, Jie S, et al. Berberine inhibits SDF-1-induced AML cells and leukemic stem cells migration via regulation of SDF-1 level in bone marrow stromal cells. Biomed Pharmacother. 2008;62:573–78. doi: 10.1016/j.biopha.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang Y, Zhou Y, et al. Cooperative effect of chidamide and chemotherapeutic drugs induce apoptosis by DNA damage accumulation and repair defects in acute myeloid leukemia stem and progenitor cells. Clin Epigenetics. 2017;9:83. doi: 10.1186/s13148-017-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmati PG, Terwey TH, Na IK, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia: A single center analysis of long-term outcome. Eur J Haematol. 2015;6:498–506. doi: 10.1111/ejh.12522. [DOI] [PubMed] [Google Scholar]
- 27.Bodet-Milin C, Kraeber-Bodere F, Eugene T, et al. Radioimmunotherapy for treatment of acute leukemia. Semin Nucl Med. 2016;46:135–46. doi: 10.1053/j.semnuclmed.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Armanios M, De Cabo R, Mannick J, et al. Translational strategies in aging and age-related disease. Nat Med. 2015;21:1395–99. doi: 10.1038/nm.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asadullina NR, Usacheva AM, Gudkov SV. Protection of mice against X-ray injuries by the post-irradiation administration of inosine-5′-monophosphate. J Radiat Res. 2012;53:211–16. doi: 10.1269/jrr.11050. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZL, Chen LB, Qiu Z, et al. Ginsenoside Rg1 ameliorates testicular senescence changes in D gal induced aging mice via anti inflammatory and antioxidative mechanisms. Mol Med Rep. 2018;17:6269–76. doi: 10.3892/mmr.2018.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai SZ, Zhou Y, Liu J, et al. Alleviation of ginsenoside Rg1 on hematopoietic homeostasis defects caused by lead-acetate. Biomed Pharmacother. 2018;97:1204–11. doi: 10.1016/j.biopha.2017.10.148. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Liu J, Cai S, et al. Protective effects of ginsenoside Rg1 on aging Sca-1+ hematopoietic cells. Mol Med Rep. 2015;12:3621–28. doi: 10.3892/mmr.2015.3884. [DOI] [PubMed] [Google Scholar]
- 33.Pollyea D, Gutman JA, Gore L, et al. Targeting acute myeloid leukemia stem cells: A review and principles for the development of clinical trials. Haematologica. 2014;99:1277–84. doi: 10.3324/haematol.2013.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahiani MH, Eassa S, Parnell C, et al. Carbon nanotubes as carriers of Panax ginseng metabolites and enhancers of ginsenosides Rb1 and Rg1 anti-cancer activity. Nanotechnology. 2017;28:015101. doi: 10.1088/0957-4484/28/1/015101. [DOI] [PubMed] [Google Scholar]
- 35.Yuan F, Zhang Y, Ma L, et al. Enhanced NOLC1 promotes cell senescence and represses hepatocellular carcinoma cell proliferation by disturbing the organization of nucleolus. Aging Cell. 2017;16:726–37. doi: 10.1111/acel.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Cai SZ, Zhou Y, et al. Senescence as a consequence of ginsenoside Rg1 response on K562 human leukemia cell line. Asia Pac J Cancer Prev. 2012;13:6191–96. doi: 10.7314/apjcp.2012.13.12.6191. [DOI] [PubMed] [Google Scholar]
- 37.Cai S, Zhou Y, Liu J, et al. [Experimental study on human leukemia cell line K562 senescence induced by ginsenoside Rg1]. Zhongguo Zhong Yao Za Zhi. 2012;37:2424–28. [in Chinese] [PubMed] [Google Scholar]
- 38.Zeng Y, Yang K. Sirtuin 1 participates in the process of age-related retinal degeneration. Biochem Biophys Res Commun. 2015;468:167–72. doi: 10.1016/j.bbrc.2015.10.139. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Kageyama S, Ke B, et al. Sirtuin 1 attenuates inflammation and hepatocellular damage in liver transplant ischemia/reperfusion: From mouse to human. Liver Transpl. 2017;23:1282–93. doi: 10.1002/lt.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasca D, Hahnel PS, Szybinski J, et al. SIRT1 prevents genotoxic stress-induced p53 activation in acute myeloid leukemia. Blood. 2014;124:121–33. doi: 10.1182/blood-2013-11-538819. [DOI] [PubMed] [Google Scholar]
- 41.Maiese K. Moving to the Rhythm with clock (circadian) genes, autophagy, mTOR, and SITR1 in degenerative disease and cancer. Curr Neurovasc Res. 2017;14:299–304. doi: 10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]