Abstract
Cardiovascular disease (CVD) is the leading cause of death among women in the United States. As compared to men, women are less likely to be diagnosed appropriately, receive preventive care, or be treated aggressively for CVD. Sex differences between men and women have allowed for the identification of CVD risk factors and risk markers that are unique to women. The 2018 AHA/ACC-Multi-society cholesterol guideline and 2019 ACC/AHA guideline on the primary prevention of CVD introduced the concept of risk- enhancing factors that are specific to women and are associated with an increased risk of incident atherosclerotic cardiovascular disease (ASCVD) in women. These factors, if present, would favor more intensified lifestyle interventions and consideration of initiation or intensification of statin therapy for primary prevention to mitigate the increased risk. In this primer, we highlight sex-specific CVD risk factors in women, stress the importance of eliciting a thorough obstetrical and gynecologic history during cardiovascular risk assessment, and provide a framework for how to initiate appropriate preventive measures when sex-specific risk factors are present.
Keywords: Risk-enhancing factors, premature menopause, premature ovarian failure, pre-eclampsia, gestational hypertension, gestational diabetes, preterm birth, small for gestational age infants
Introduction
Cardiovascular disease (CVD) is the leading cause of death among women in the United States. Women are less likely to be diagnosed appropriately, receive preventive care, or be treated aggressively for CVD1, 2. This may be due to a lower perceived risk in women by patients and clinicians, even when traditional risk factors are present1, 3.
In addition to screening for and managing traditional risk factors, sex-specific differences between men and women allow for the identification of CVD risk factors unique to women. A woman’s lifespan offers several opportunities to identify additional CVD risk factors beyond traditional risk factors. We have learned that a one-size fits-all model of cardiovascular risk stratification is no longer an acceptable way to deliver health care to 51% of the population4. We must capitalize on sex-specific differences to deliver optimal preventive medical care.
The 2011 American Heart Association (AHA) guideline for the prevention of CVD in women considered the presence of preeclampsia, gestational diabetes (GDM), gestational hypertension, or systemic autoimmune collagen-vascular disease (i.e. lupus or rheumatoid arthritis) as factors associated with an increased risk of CVD5. The 2018 AHA/ American College of Cardiology (ACC) Multi-Society cholesterol guideline and the 2019 ACC/AHA guideline on the primary prevention of CVD introduced the concept of “risk-enhancing factors” that can be applied to patients who are at borderline- and intermediate-risk, after estimating their 10-year atherosclerotic cardiovascular disease (ASCVD) risk using the pooled cohort equations6, 7. The presence of one or more of these factors could elevate patients to a higher risk category and may favor initiation or intensification of statin therapy6, 8. Notably, these guidelines have raised awareness of risk-enhancing factors specific to women that may increase a woman’s risk of CVD6, 7. The risk-enhancing factors mentioned in these guidelines were premature menopause and pre-eclampsia. However, several additional factors are also associated with increased CVD risk in women: adverse pregnancy outcomes (APOs) such as gestational hypertension, GDM, pre-term delivery (PTD), and delivery of small for gestational age (SGA) infants5.
This primer highlights features that may increase a woman’s CVD risk, discusses the evidence and rationale behind them, and provides a framework for how to incorporate these factors when making lifestyle and treatment recommendations.
Adverse Pregnancy Outcomes
The gestational window is a natural stress test on a woman’s body and a time that is ripe for gathering data on cardiovascular risk. APOs such as pre-eclampsia, hypertensive disorders of pregnancy, and GDM afflict between 3–20% of pregnancies. Each of these conditions has been associated with an increased CVD risk9–14.
Pre-eclampsia and Gestational Hypertension:
The hallmark of pre-eclampsia is the combination of elevated blood pressure and proteinuria after 20 weeks gestation. Gestational hypertension is characterized by the development of hypertension after 20 weeks gestation in the absence of proteinuria or pre-eclampsia. It is defined by a blood pressure >140/90 mmHg on two separate occasions. Hypertensive disorders of pregnancy are thought to confer not only an elevated risk for the subsequent development of hypertension and diabetes, but notably for incident CVD and heart failure (HF)14. A meta-analysis of >6.4 million women (258,000 with pre-eclampsia) demonstrated that pre-eclampsia was independently associated with a 4-fold increased risk of incident HF [relative risk (RR) 4.19, (95% CI 2.09–8.38)] and a 2-fold increased risk of coronary heart disease (CHD) RR, 2.50 (1.43–4.37)14. A study that included 1,033,559 women without pre-existing CVD prior to their first pregnancy showed that women who experienced a placentally-mediated condition during pregnancy (7% of the cohort) such as gestational hypertension, pre-eclampsia, placental abruption, or placental infarction were twice as likely to develop CVD [hazards ratio (HR) 2.0 (1.7–2.2)] after a median follow up of 8.7 years. Adjusted HRs for incident CVD for women with gestational hypertension and pre-eclampsia were 1.8 (1.4–2.2) and 2.1 (1.8–2.4), respectively15. The composite outcome of CVD was highest in women with maternal placental syndrome and either poor fetal growth or intrauterine fetal death concurrently15. Another meta-analysis evaluated 146,748 women during their first pregnancy for hypertensive disorders and the development of CVD and hypertension later in life. Women with hypertensive disorders of pregnancy had higher rates of CVD, HR 2.2 (1.7–2.7) and hypertension, HR 5.6 (5.1–6.3)11. Hypertensive disorders of pregnancy manifest via multiple phenotypes that are thought to arise from an interplay of underlying contributing factors including endothelial dysfunction, insulin resistance, and thrombophilia16. Studies are ongoing to understand the molecular mechanisms that determine the phenotypic heterogeneity in these disorders17–19.
Gestational diabetes is the development of elevated blood sugars during pregnancy, typically secondary to insulin resistance. Women with GDM have an 8-fold increased risk of developing type 2 diabetes mellitus (T2DM) after pregnancy compared to normoglycemic women20. GDM is also associated with future risk of CVD21, 22. According to one study, GDM confers 63% higher odds (1.02–2.62) of developing incident CVD and an absolute risk increase of 2.8%23. A recent meta-analysis demonstrated that women with GDM have a 2-fold greater risk for future cardiovascular events compared to those without GDM, RR 1.98 (1.57–2.50)21. After restricting the sensitivity analysis to women who did not subsequently develop T2DM, the relative risk of future CVD was attenuated, but remained significant, RR 1.56 (1.04, 2.32). These findings suggest an independent association between the development of GDM and future CVD risk. It is thought that women who develop dysglycemia have an underlying cardiometabolic phenotype that predisposes them to GDM and CVD. Glucose screening during pregnancy could identify women at risk for CVD.
Pre-term delivery is the birth of a baby at fewer than 37 weeks gestational age. In the Nurses’ Health Study (NHS), a history of PTD, particularly one that occurred very prematurely (<32 weeks), was independently associated with increased maternal CVD risk24, 25. The NHS II demonstrated that PTD during the first pregnancy was associated with an increased CVD risk compared to a term delivery, HR 1.42 (1.16–1.72)24. Women with a history of PTD are at an increased risk of developing chronic hypertension, T2DM, and hypercholesterolemia, particularly within the first decade after the pregnancy and are more likely to have subclinical atherosclerosis26, 27. These factors account for about 25% of the association between PTD and CVD. Though other pathways are thought to be involved, our understanding of the mechanism behind this is currently limited.
Small for gestational age:
Infants who are SGA are characterized as being below the 10th percentile for their gestational age. Delivery of a SGA infant is associated with future maternal CVD in a dose-response fashion related to severity of SGA and number of SGA deliveries28. It is thought that women who are at higher risk for CVD may be less likely to accommodate the hemodynamic changes during pregnancy and this may manifest as fetal growth restriction. While SGA infants and future maternal CVD appear to be associated, it is unclear as to whether this association is independent of maternal placental syndromes including hypertensive disorders of pregnancy as many of these factors are interrelated28, 29. Women who deliver SGA infants should be monitored carefully for the development of hypertension.
Hypertensive disorders of pregnancy portend a greater risk of incident hypertension while GDM is associated with a substantially increased risk of T2DM. PTD is associated with an array of downstream effects including hypertension, hyperlipidemia, and T2DM. When a history of APOs is elicited during risk stratification, aggressive lifestyle interventions should be implemented early on along with careful screening to prevent or mitigate the development of downstream intermediate phenotypes associated with an increased CVD risk.
Ovarian Failure
Premature ovarian failure (POF) and early onset menopause are characterized by loss of ovarian function prior to the age of 40 or 45, cessation of menstruation, hypergonadotropism, and hypoestrogenism and an increased CVD risk30–32. The average age for the onset of menopause is 51 years. Women who experienced menopause at an age younger than 45 years, compared to those ≥45 years, had an increased risk of CHD, RR 1.50 (1.28–1.76) according to one meta-analysis30. Women who experienced menopause between the ages of 50 and 54 years had a decreased risk of fatal CHD, RR 0.87 (0.80–0.96) but not stroke, compared to those who experienced it at younger than 50 years30, 32. Another meta-analysis assessed the relationship between POF and CHD among 190,588 women with 9,440 cardiovascular events. Women with POF had an increased risk of CVD mortality, HR 1.69 (1.29–2.21) and of overall incident CVD, HR 1.61 (1.22–2.12)30, 32. A prospective cohort from the NHS including 73,814 participants showed that a reproductive lifespan of <30 years compared with that of ≥42 years was associated with an increased risk for incident CVD, RR 1.32 (1.16–1.49). Age at menopause <40 years compared with 50 to <55 years was also associated with an increased CVD risk, RR 1.32 (1.16–1.51)31. Age at menopause is thought to be a marker for reproductive aging and general health, and a shorter reproductive lifespan mediated by early loss of estrogens has been associated with a higher CVD risk.
Regardless of the etiology, whether spontaneous or iatrogenic, women with estrogen deprivation secondary to POF are at higher risk of cardiovascular morbidity and mortality33. Estrogens help regulate blood flow and assist in the relaxation of blood vessels. Early loss of ovarian function with associated changes in sex hormones is also associated with long-term activation of the renin-angiotensin-aldosterone system. These effects lead to endothelial dysfunction, inflammation, and immune dysfunction, all of which contribute to vascular damage34. Loss of estrogens also leads to a loss of their beneficial effects on cholesterol metabolism and atherosclerotic plaque formation and thereby contribute to the increased CVD risk. A higher testosterone to estradiol ratio in post-menopausal women is associated with a greater risk for incident CVD, CHD, and HF35. While it is currently unknown whether estrogen therapy is beneficial in women with POF, women with this condition should be monitored closely and treated with aggressive lifestyle modifications to prevent the development of CVD.
Additional CVD risk factors in women outside of pregnancy and menopause
Several factors beyond pregnancy and menopause are thought to confer an elevated CVD risk and should be considered during risk stratification: premature menarche, polycystic ovarian syndrome (PCOS), hormone-based contraceptive use, multiple spontaneous miscarriages, and breast cancer.
Premature menarche is associated with an increased CHD and CVD risk. The average age of menarche is 13 years, and menarche is considered premature when it occurs at age 10 years or younger. A meta-analysis showed a 3% reduction in the relative risk of all-cause mortality for every 1-year increase in age at menarche, pooled HR 0.97 (0.96–0.98). Women who experienced menarche at age <12 versus ≥12 years were at an increased risk of all-cause mortality, HR 1.23 (1.10–1.38)36. Another study suggests that both early and late menarche increase the relative risk of CHD; the RR in women with menarche age ≤10 years was 1.27 (1.22–1.31) and for women with menarche age ≥17 years was 1.23 (1.16–1.30)37. Premature menarche is associated with the development of hypertension and components of metabolic disease including T2DM and hypercholesterolemia, all of which increase CVD risk. Adjusting for these factors slightly attenuated CVD risk estimates, however, the risk still remained significant, suggesting the possibility of additional shared risk factors between premature menarche and CVD37.
Polycystic ovarian syndrome is the most common cause of infertility in women and frequently manifests during adolescence. Key features include excess androgens, ovulatory dysfunction, and polycystic ovaries on imaging. There is an associated higher prevalence of hypertension, central adiposity, insulin resistance, dyslipidemia, and metabolic syndrome. PCOS has notable shared risk factors with CVD. Women with PCOS have a higher degree of subclinical atherosclerosis noted by a greater carotid intima-media thickness and greater coronary artery calcium38. In some studies, PCOS was associated with future risk of CVD; however there is heterogeneity of risk based on PCOS phenotype39, 40. Higher body-mass indices, waist circumferences, blood pressure, glucose levels, and hyperlipidemia at the time of PCOS diagnosis are associated with greater CVD risk. Understandably, these features associated with PCOS are also independent risk factors for CVD.
Pregnancy loss, particularly recurrent miscarriages, is associated with an increased CVD risk. Women with a history of two or more miscarriages, regardless of whether they were consecutive or not, are at a higher risk for CHD. In one study, women who experienced two miscarriages or three or more miscarriages were at higher risk for CHD as compared to women who did not experience miscarriage, HR 1.75 (1.22–2.52) and HR 3.18, (1.49–6.80) respectively41. While the emotional aspects of pregnancy loss and concerns regarding fertility are understandably the immediate focus after miscarriage, the cardiovascular burden of recurrent miscarriages also merits careful consideration.
Premature menarche, PCOS, and recurrent pregnancy loss are all associated with an underlying higher risk cardiometabolic phenotype. Women with this medical history should be closely monitored for signs of metabolic dysregulation and preventive lifestyle measures ought to be implemented early on.
Hormone-based contraceptive methods can affect the lipid profile and thereby cardiovascular risk. Estrogen-based contraceptives typically elevate triglycerides and high-density lipoprotein cholesterol (HDL-C) levels while decreasing low-density lipoprotein cholesterol (LDL-C) levels and should be used with caution, particularly among women with elevated triglycerides. Some contraceptive methods contain norgestrel or levonorgestrel, both of which have androgenic components that elevate LDL-C levels and decrease HDL-C levels. Progesterone- based contraceptive methods tend to have neutral effects on the lipid profile. Obtaining information on the type and duration of hormone-based contraceptive use as well as identifying potential contraindications to their use is of clinical value when implementing preventive strategies.
Breast cancer history and associated treatments may portend an increased risk of incident CVD. Radiation to the left breast is associated with an increased risk of CHD and treatment with chemotherapeutic agents such as anthracyclines and trastuzumab increase the risk of cardiomyopathy and HF42. Given several shared risk factors between cancer and CVD such as smoking, obesity, hypertension, and oxidative stress to name a few, there are opportunities for shared preventive strategies to reduce the risk for and morbidity and mortality from both conditions43. These include education and awareness on shared risk factors for cancer and CVD using public health campaigns, collaborative screening initiatives, and emphasis on lifestyle modifications.
Inflammatory disorders such as rheumatoid arthritis, psoriasis, and systemic erythematous lupus have robust associations with CVD44–46. These conditions are considered risk-enhancing factors in the 2018 Cholesterol Guideline and their presence in intermediate or select borderline risk patients should yield consideration of initiation or intensification of statin therapy6. Given their substantially higher prevalence in women, careful screening and preventive measures should be utilized in women with a history of these conditions.
Lactation
Lactation is an important factor in the reproductive continuum and has been shown to be associated with lower maternal cardiovascular risk. Breastfeeding is thought to aid in reversing the metabolic changes of insulin resistance, dyslipidemia, and accumulation of fat mass that take place during pregnancy47. Lactation has been associated with a lower risk of hypertension and metabolic dysfunction, both of which are risk factors for CVD. In a cohort of roughly 63,000 Danish women followed up to 7 years postpartum, breastfeeding ≥4 months was associated with a 30% and 20% lower risk of hypertension and CVD respectively compared to breastfeeding <4 months48. The protective effects of breastfeeding were noted both in women who were normal/underweight and overweight/obese prior to pregnancy. While further studies are needed to determine how the interplay of lactation with traditional and sex-specific factors affect CVD risk, clinicians should highlight the protective effects of breastfeeding with regard to maternal CVD risk.
Putting it all together: Comprehensive cardiovascular risk stratification in women
As we have reviewed, factors unique to women can have a substantial impact on a woman’s cardiovascular health (Figure 1). We assert that cardiovascular risk stratification in women is incomplete without a thorough obstetrical and gynecologic history. Table 1 outlines the elements that comprise a comprehensive history for cardiovascular risk stratification in women.
Figure 1: Sex-specific CVD risk factors in women.
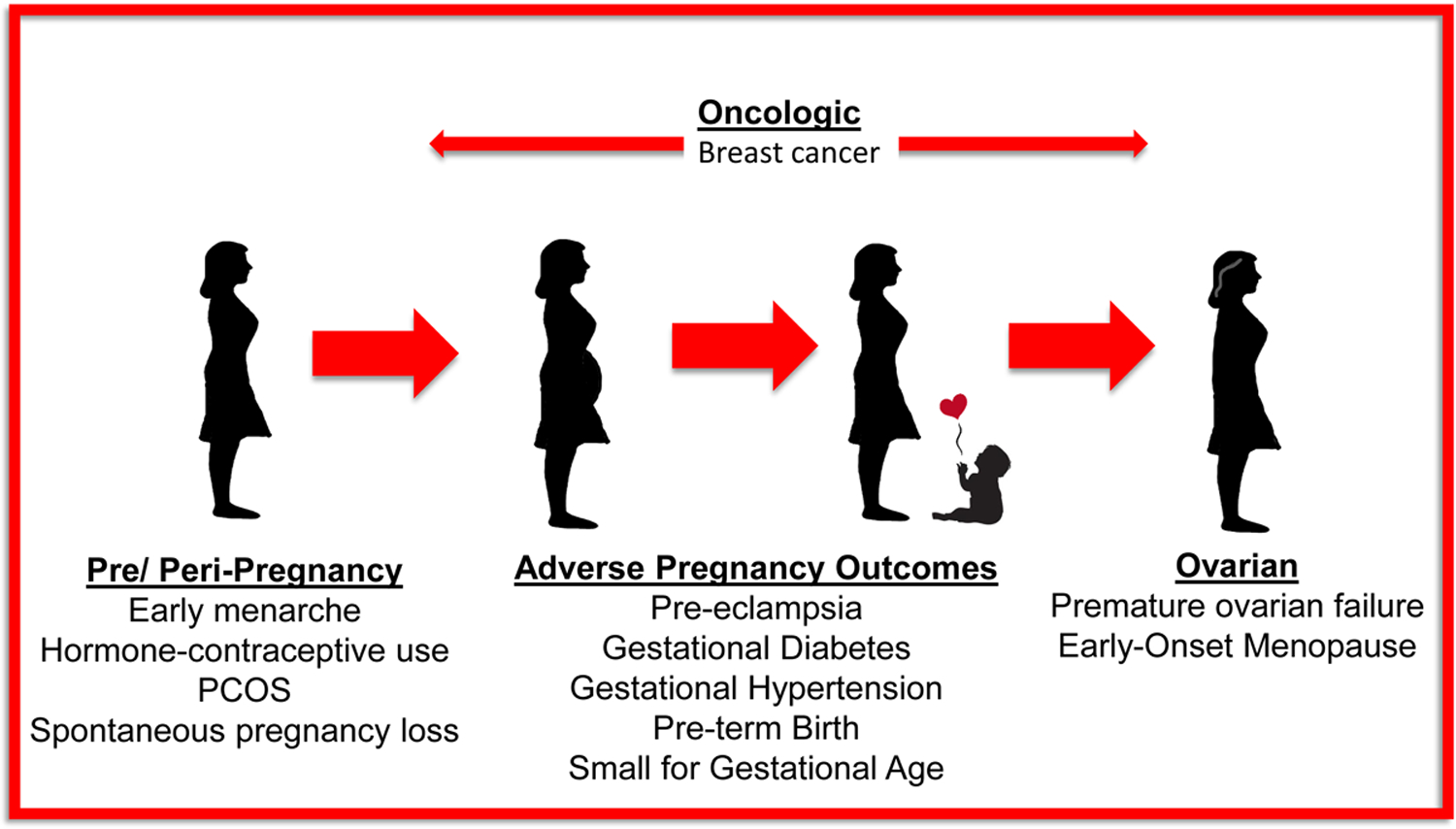
PCOS = Polycystic Ovarian Syndrome
Table 1:
Elements of a comprehensive cardiovascular history in women
| Birth | Prematurity/ gestational age |
| Birth weight/ born small for gestational age | |
| Puberty/ Adolescence | Age at menarche |
| Diagnosis of PCOS* | |
| Contraception | Use of contraception, type, duration, and inclusion of hormonal components |
| Childbearing peripartum period | Miscarriage(s) |
| Gestational diabetes | |
| Gestation hypertension | |
| Pre-eclampsia | |
| Pre-Term Delivery | |
| Delivery of a small for gestational age infant | |
| Lactation | |
| Co-morbidities | Autoimmune disorders (i.e. rheumatoid arthritis, lupus, Crohn’s) |
| Cancer history including history of radiation and/or chemotherapy (i.e anthracycline or trastuzumab) | |
| Menopause | Age at menopause/ premature ovarian failure |
| Use of hormone therapy |
PCOS- Polycystic Ovarian Syndrome
We recommend a four-fold approach to cardiovascular risk stratification in women (Figure 2). First and most importantly is eliciting a thorough history on CVD risk factors that includes a comprehensive obstetrical and gynecologic history. Second, if sex- specific risk factors are present, early and frequent screening for traditional CVD risk factors is crucial in addition to screening for, preventing, and treating intermediate phenotypes related to the sex-specific risk factors (i.e. hypertension, diabetes, hyperlipidemia, and metabolic syndrome). Many reproductive conditions including GDM, early menarche, PTD, PCOS, and recurrent pregnancy loss, are associated with intermediate phenotypes of T2DM and metabolic syndrome. Additionally, maternal placental syndromes and hypertensive disorders of pregnancy portend a higher risk of hypertension. Vigilant screening and early detection and treatment of these intermediate phenotypes can substantially reduce future CVD risk. Third, implementation of aggressive lifestyle changes is key. Lifestyle modifications can be approached using the AHA’s Life’s Simple 7 (LS7) to approach ideal cardiovascular health. The seven metrics include: managing blood pressure, controlling cholesterol, reducing blood sugar, becoming and remaining active, healthy eating, weight loss, and smoking cessation. These 7 steps, when implemented early on, can have lasting effects on CVD risk reduction49, 50. Finally, for patients between the ages of 40 and 75, the 10-year ASCVD risk calculator can be used to estimate risk; for those aged 20–59, lifetime risk can be assessed. Individuals with low 10-year risk, but high lifetime risk may be treated with aggressive lifestyle modifications as recommended by the 2018 AHA/ACC Multi-society cholesterol management guideline and the 2019 ACC/AHA Prevention of CVD guideline6. In individuals at borderline or intermediate risk with the presence of these risk markers, early statin initiation or intensification could be considered to reduce atherosclerotic risk in addition to lifestyle modifications. For some sex-specific phenotypes, clinicians could follow these patients vigilantly for the development of heart failure and institute therapies to further reduce this risk.
Figure 2: Stepping to Success: Reducing CVD Risk in Women when Sex- Specific Risk Factors are Encountered.
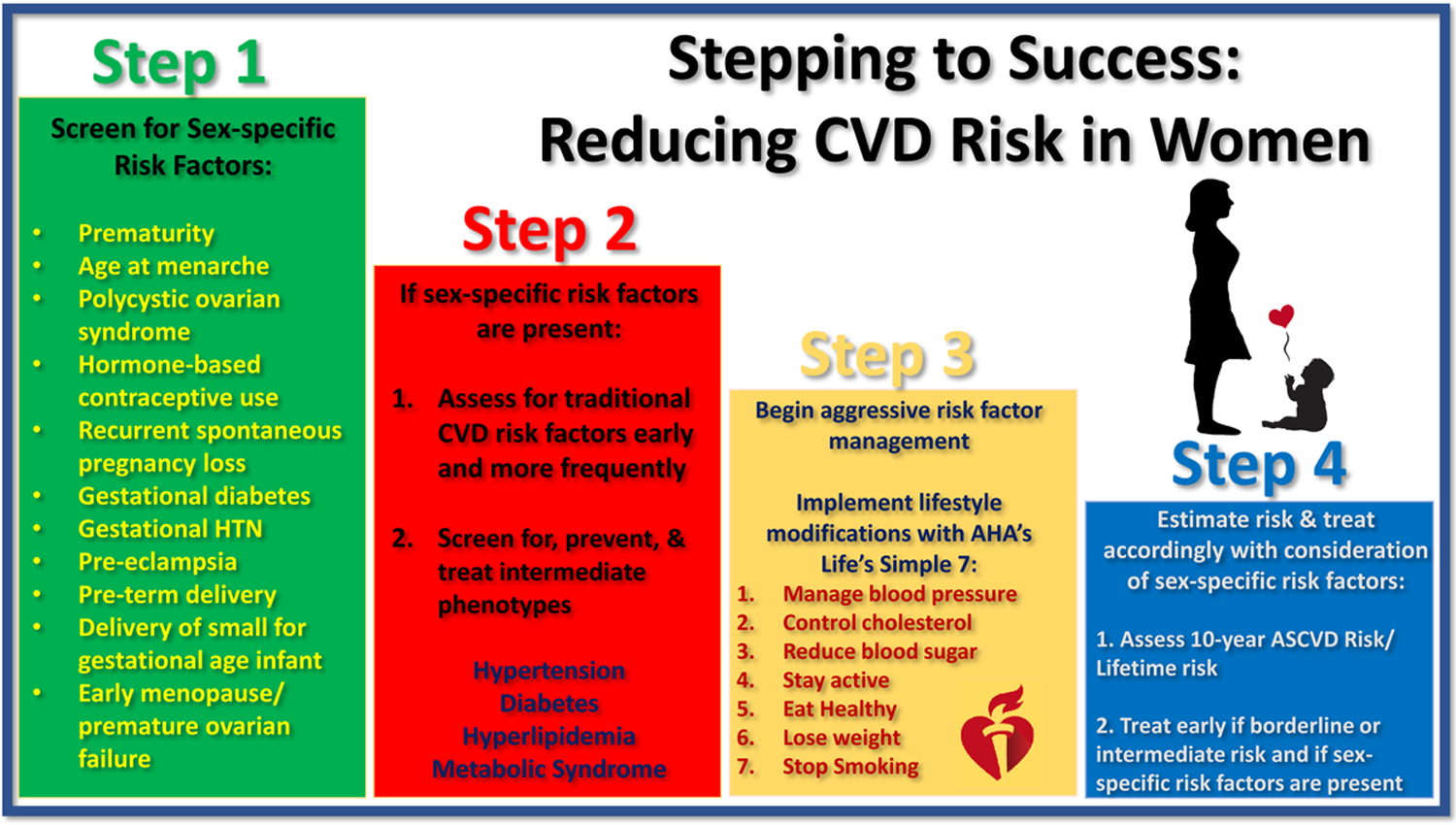
AHA= American Heart Association, ASCVD= Atherosclerotic Cardiovascular Disease
Future studies are needed to understand whether sex-specific risk factors allow risk reclassification beyond traditional risk scoring algorithms and how best to assess high-risk phenotypes. As we await a more formalized framework of how to incorporate sex-specific risk factors into current risk stratification algorithms, clinical judgement with regard to the number of risk factors and phenotype severity must be incorporated during risk stratification and shared decision making with regard to treatment. Collaborative efforts among primary care clinicians, obstetricians and gynecologists, and cardiologists are key to streamlining early identification of sex-specific risk factors and institution of preventive care for women.
Conclusion
The current state of cardiovascular care for women is far from ideal. It is our duty as the cardiovascular community to change the paradigm of underdiagnosing and undertreating CVD in women and doing so will require a concerted effort. Together, we can optimize cardiovascular care for women.
Funding:
Department of Veterans Affairs Health Services Research & Development Service Investigator Initiated Grant, World Heart Federation, Jooma and Tahir Family.
Non-standard Abbreviations and Acronyms:
- CVD
Cardiovascular Disease
- ASCVD
Atherosclerotic Cardiovascular Disease
- AHA
American Heart Association
- ACC
American College of Cardiology
- APO
Adverse Pregnancy Outcome
- PTD
Pre-term Delivery
- SGA
Small for Gestational Age
- GDM
Gestational Diabetes Mellitus
- NHS
Nurses’ Health Study
- T2DM
Type II Diabetes Mellitus
- POF
Premature Ovarian Failure
- CHD
Coronary Heart Disease
- PCOS
Polycystic Ovarian Syndrome
- HDL-C
High-density Lipoprotein Cholesterol
- LDL-C
Low-density Lipoprotein Cholesterol
- LS7
Life’s Simple 7
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Disclosures:
Anandita Agarwala MD: None
Erin D. Michos MD MHS: None
Zainab Samad MBBS MHS: None
Christie M. Ballantyne, MD: All significant. (All paid to institution, not individual): Abbott Diagnostic, Akcea, Amgen, Esperion, Novartis, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, NIH, AHA, ADA. Consultant- Abbott Diagnostics, Akcea, Amarin, Amgen, Astra Zeneca*, Boehringer Ingelheim, Denka Seiken, Esperion, Intercept, Janssen, Matinas BioPharma Inc, Merck*, Novartis, Novo Nordisk, Regeneron, Roche Diagnostic, Sanofi-Synthelabo*
*Significant where noted (>$10,000); remainder modest (<$10,000).
Salim S. Virani, MD, PhD: Steering Committee for the Patient and Provider Assessment of Lipid Management (PALM) registry at the Duke Clinical Research Institute (no financial remuneration). Honorarium: American College of Cardiology (Associate Editor, Innovations, acc.org).
References
- 1.Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, Fabunmi RP, Kwan J, Mills T and Simpson SL. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. [DOI] [PubMed] [Google Scholar]
- 2.Garcia M, Mulvagh SL, Merz CN, Buring JE and Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res. 2016;118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leifheit-Limson EC, D’Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, Krumholz HM and Lichtman JH. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients With Acute Myocardial Infarction: The VIRGO Study. J Am Coll Cardiol. 2015;66:1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howden LMJ. Age and Sex Composition: 2010. 2011;2019. [Google Scholar]
- 5.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. Circulation 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. Epub 2018 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019: 140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwala A, Liu J, Ballantyne CM and SS V. The Use of Risk Enhancing Factors to Personalize ASCVD Risk Assessment: Evidence and Recommendations from the 2018 AHA/ACC Multi-Society Cholesterol Guidelines. 2019. doi: 10.1007/s12170-019-0616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed R, Dunford J, Mehran R, Robson S and Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63:1815–1822. [DOI] [PubMed] [Google Scholar]
- 10.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC and Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 11.Grandi SM, Vallée-Pouliot K, Reynier P, Eberg M, Platt RW, Arel R, Basso O and Filion KB. Hypertensive Disorders in Pregnancy and the Risk of Subsequent Cardiovascular Disease. Paediatr Perinat Epidemiol. 2017;31:412–421. [DOI] [PubMed] [Google Scholar]
- 12.Hutcheon JA, Lisonkova S and Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 13.Casagrande SS, Linder B and Cowie CC. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res Clin Pract. 2018;141:200–208. [DOI] [PubMed] [Google Scholar]
- 14.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 15.Ray JG, Vermeulen MJ, Schull MJ and Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 16.Wikström AK, Haglund B, Olovsson M and Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112:1486–1491. [DOI] [PubMed] [Google Scholar]
- 17.Benton SJ, Leavey K, Grynspan D, Cox BJ and Bainbridge SA. The clinical heterogeneity of preeclampsia is related to both placental gene expression and placental histopathology. Am J Obstet Gynecol. 2018;219:604.e601–604.e625. [DOI] [PubMed] [Google Scholar]
- 18.Leavey K, Bainbridge SA and Cox BJ. Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia. PLoS One. 2015;10:e0116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leavey K, Benton SJ, Grynspan D, Kingdom JC, Bainbridge SA and Cox BJ. Unsupervised Placental Gene Expression Profiling Identifies Clinically Relevant Subclasses of Human Preeclampsia. Hypertension. 2016;68:137–147. [DOI] [PubMed] [Google Scholar]
- 20.Bellamy L, Casas JP, Hingorani AD and Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet (London, England). 2009;373:1773–1779. [DOI] [PubMed] [Google Scholar]
- 21.Kramer CK, Campbell S and Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. [DOI] [PubMed] [Google Scholar]
- 22.Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U and Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG. 2014;121:1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shostrom DCV, Sun Y, Oleson JJ, Snetselaar LG and Bao W. History of Gestational Diabetes Mellitus in Relation to Cardiovascular Disease and Cardiovascular Risk Factors in US Women. Front Endocrinol (Lausanne). 2017;8:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Mukamal KJ and Rich-Edwards JW. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation. 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minissian MB, Kilpatrick S, Eastwood JA, Robbins WA, Accortt EE, Wei J, Shufelt CL, Doering LV and Merz CNB. Association of Spontaneous Preterm Delivery and Future Maternal Cardiovascular Disease. Circulation. 2018;137:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanz LJ, Stuart JJ, Williams PL, Missmer SA, Rimm EB, James-Todd TM and Rich-Edwards JW. Preterm Delivery and Maternal Cardiovascular Disease Risk Factors: The Nurses’ Health Study II. J Womens Health (Larchmt). 2019;28:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catov JM, Snyder GG, Bullen BL, Barinas-Mitchell EJM and Holzman C. Women with Preterm Birth Have Evidence of Subclinical Atherosclerosis a Decade After Delivery. J Womens Health (Larchmt). 2019;28:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngo AD, Roberts CL, Chen JS and Figtree G. Delivery of a Small-For-Gestational-Age Infant and Risk of Maternal Cardiovascular Disease--A Population-Based Record Linkage Study. Heart Lung Circ. 2015;24:696–704. [DOI] [PubMed] [Google Scholar]
- 29.Hooijschuur MC, Ghossein-Doha C, Al-Nasiry S and Spaanderman ME. Maternal metabolic syndrome, preeclampsia, and small for gestational age infancy. Am J Obstet Gynecol. 2015;213:370.e371–377. [DOI] [PubMed] [Google Scholar]
- 30.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M and Franco OH. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- 31.Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB and Rexrode KM. Duration of Reproductive Life Span, Age at Menarche, and Age at Menopause Are Associated With Risk of Cardiovascular Disease in Women. J Am Heart Assoc. 2017;6 pii: e006713. doi: 10.1161/JAHA.117.006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A and Disorders cotDMGDGoCRMaR. Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:178–186. [DOI] [PubMed] [Google Scholar]
- 33.Torrealday S, Kodaman P and Pal L. Premature Ovarian Insufficiency - an update on recent advances in understanding and management. F1000Res. 2017;6:2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89:3907–3913. [DOI] [PubMed] [Google Scholar]
- 35.Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Lima JA, Allison MA, Shah SJ, Bertoni AG, et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J Am Coll Cardiol. 2018;71:2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charalampopoulos D, McLoughlin A, Elks CE and Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol. 2014;180:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, Green J, Cairns BJ and Collaborators* MWS. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131:237–244. [DOI] [PubMed] [Google Scholar]
- 38.Guzick DS. Cardiovascular risk in PCOS. J Clin Endocrinol Metab. 2004;89:3694–3695. [DOI] [PubMed] [Google Scholar]
- 39.Glintborg D, Rubin KH, Nybo M, Abrahamsen B and Andersen M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc Diabetol. 2018;17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osibogun O, Ogunmoroti O and Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2019. 10.1016/j.tcm.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 41.Wagner MM, Bhattacharya S, Visser J, Hannaford PC and Bloemenkamp KW. Association between miscarriage and cardiovascular disease in a Scottish cohort. Heart. 2015;101:1954–1960. [DOI] [PubMed] [Google Scholar]
- 42.Guenancia C, Lefebvre A, Cardinale D, Yu AF, Ladoire S, Ghiringhelli F, Zeller M, Rochette L, Cottin Y and Vergely C. Obesity As a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2016;34:3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handy CE, Quispe R, Pinto X, Blaha MJ, Blumenthal RS, Michos ED, Lima JAC, Guallar E, Ryu S, Cho J, et al. Synergistic Opportunities in the Interplay Between Cancer Screening and Cardiovascular Disease Risk Assessment. Circulation. 2018;138:727–734. [DOI] [PubMed] [Google Scholar]
- 44.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM and Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. [DOI] [PubMed] [Google Scholar]
- 45.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB and Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westerweel PE, Luyten RK, Koomans HA, Derksen RH and Verhaar MC. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1384–1396. [DOI] [PubMed] [Google Scholar]
- 47.Stuebe AM and Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkegaard H, Bliddal M, Støvring H, Rasmussen KM, Gunderson EP, Køber L, Sørensen TIA and Nohr EA. Breastfeeding and later maternal risk of hypertension and cardiovascular disease - The role of overall and abdominal obesity. Prev Med. 2018;114:140–148. [DOI] [PubMed] [Google Scholar]
- 49.Oyenuga AO, Folsom AR, Cheng S, Tanaka H and Meyer ML. Greater Adherence to Life’s Simple 7 Is Associated With Less Arterial Stiffness: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2019;32:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Association AH. My Life Check | Life’s Simple 7. https://www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-simple-7 Accessed 1/13/2019


