Abstract
Background
Quetiapine is an atypical antipsychotic with, theoretically, a low propensity for movement disorder adverse effects. It is used for the treatment of schizophrenia and other psychoses.
Objectives
To determine the effects of quetiapine for schizophrenia in comparison to placebo, and other antipsychotics.
Search methods
Electronic searches of the Cochrane Schizophrenia Group's Register of Trials (Febuary 2003), Biological Abstracts (1982‐2000), CINAHL (1982‐2000), the Cochrane Library (2000, Issue 1),EMBASE (1980‐2000), MEDLINE (1966‐2000), PsycLIT (1974‐2000), SIGLE on CD (1980‐1997), SocioFile (1974‐1997) and many conference proceedings and hand searches of specific journals were undertaken. We contacted AstraZeneca Pharmaceuticals for information regarding unpublished trials. The review was updated in February 2003.
Selection criteria
All randomised controlled trials where adults with schizophrenia or similar illnesses were assigned to quetiapine, placebo or other neuroleptic drugs and where clinically relevant outcomes were reported.
Data collection and analysis
Citations and, where possible, abstracts were inspected independently by reviewers, papers ordered, re‐inspected and quality assessed. We independently extracted data. We analysed data using fixed effects relative risk (RR) and estimated the 95% confidence interval (CI). Only homogeneous data were interpreted as favouring treatment or control. Where possible we calculated the number needed to treat (NNT) or number needed to harm statistics (NNH). We calculated relative risk (RR) for dichotomous data, and weighted mean differences (WMD) for continuous data.
Main results
Despite the fact that 3443 people were randomised in 12 quetiapine studies, there are almost no data on service utilisation, economic outcomes, social functioning and quality of life. Over half of those within the quetiapine versus placebo comparison were lost to follow up (53% quetiapine vs 61% placebo, n=716, 4RCTs, RR 0.84 CI 0.7 to 0.9, NNT 11 CI 7 to 55) so it is impossible to interpret any ratings of global or mental state within this comparison with confidence. People allocated quetiapine, however, did not have more movement disorders than those given placebo (n=395, 2 RCTs, RR needing medication for EPSE 0.62 CI 0.3 to 1.2). The same applies to the comparison of >/= 250 mg/day quetiapine with < 250 mg/day quetiapine (49% dropout >/= 250 mg/day vs 58% < 250 mg/day, n=1066, 3 RCTs, RR 0.84 CI 0.8 to 0.9, NNT 11 CI 7 to 29). It should be noted that two deaths occurred in the higher dose group (n=618, 1 RCT, RR 0.1 CI 0.0 to 2.1). When quetiapine was compared with typical antipsychotics, about 36% of both groups failed to complete the short‐term studies (n=1624, 6 RCTs, RR 0.87 CI 0.8 to 1.0). Average change in global state was heterogeneous and equivocal (n=762, 3 RCTs, WMD in short term 0.19 CI 0.00 to 0.38, I squared 76%). Mental state measures were also equivocal (n=1247, RR not improved 0.97 CI 0.9 to 1.1) including specific measures of negative symptoms (n=305, 1 RCT, MD change in SANS short term 0.94 CI ‐0.2 to 2.0). Movement disorders were less prevalent for those allocated quetiapine (n=1117, 4 RCTs, RR needing medication for extrapyramidal adverse effects 0.47 CI 0.4 to 0.6, NNT 4 CI 4 to 5, I squared 88%). Dry mouth (n=649, 2 RCTs, RR short term 2.85 CI 1.5 to 5.6, NNH 17 CI 7 to 65) and sleepiness (n=959, 3 RCTs, RR 1.51 CI 1.1 to 2.2, NNH 18 CI 8 to 181) may also be more prevalent for people given quetiapine compared with the older drugs. In the quetiapine versus risperidone comparison, over 30% of people left the study before completion (n=728, 1 RCT, RR 0.94 CI 0.7 to 1.2). Four people, all treated with quetiapine, died during the study (n=728, 1 RCT, RR 2.86 CI 0.2 to 52.8). Continuous mental state measures did not show clear differences between the two drugs (n=637, 1 RCT, MD PANSS 1.2 CI ‐2.0 to 4.4). However, considerably fewer people given quetiapine needed medication for extrapyramidal side effects compared with those allocated to risperidone (n=712, 1 RCT, RR 0.27 CI 0.2 to 0.5, NNT 11 CI 10 to 16). Quetiapine caused more dizziness (n=728, 1 RCT, RR 1.85 CI 1.0 to 3.3, NNH 18 CI 7 to 487), more dry mouth (n=728, 1 RCT, RR 2.11 CI 1.2 to 3.8, NNH 14 CI 6 to 82) and more sleepiness than risperidone (n=728, 1 RCT, RR 2.03 CI 1.4 to 2.9, NNH 7 CI 4 to 17).
Authors' conclusions
Quetiapine is effective for the treatment of schizophrenia, but it is not much different from first‐generation antipsychotics and risperidone with respect to treatment withdrawal and efficacy. In comparison to first‐generation antipsychotics and risperidone, quetiapine has a lower risk of movement disorders but higher risks of dizziness, dry mouth and sleepiness. More clearly reported pragmatic randomised controlled trials should be carried out to determine its position in everyday clinical practice. Studies of medium and long‐term effects, including cost‐effectiveness, quality of life, social functioning and service utilisation, in comparison with the effects of typical and atypical antipsychotics should be priority areas.
Keywords: Adult, Humans, Antipsychotic Agents, Antipsychotic Agents/therapeutic use, Dibenzothiazepines, Dibenzothiazepines/therapeutic use, Randomized Controlled Trials as Topic, Schizophrenia, Schizophrenia/drug therapy
Plain language summary
Quetiapine for schizophrenia
We looked for randomised controlled trials to determine the effects of quetiapine for schizophrenia in comparison to placebo and other antipsychotics. We included results of ten short‐term trials and two medium‐term studies. Quetiapine is effective for the treatment of schizophrenia but not much different from first‐generation antipsychotics and risperidone in the respects of treatment withdrawal and efficacy. In comparison to typical antipsychotics and risperidone, it has a lower risk of movement disorders but higher risks of dizziness, dry mouth and sleepiness.
Background
Choosing the most appropriate, effective and tolerable antipsychotic/neuroleptic medication for people with schizophrenia is key to maximising treatment outcomes. First‐generation (typical) antipsychotics such as chlorpromazine and haloperidol have been used to treat schizophrenia since the 1950's. The older generation of drugs tended to cause movement disorders such as tremor, stiffness, slow movement, restlessness and abnormal involuntary movements. Also, about one‐third of people with schizophrenia do not respond to these drugs. Since 1988 a newer generation of antipsychotic drugs has become available. These 'atypical' antipsychotics are defined as antipsychotic drugs with low propensity to induce extrapyramidal side effects and to increase serum prolactin levels (Kerwin 1994) Atypical antipsychotics such as clozapine, risperidone and olanzapine are said to have some advantages over first‐generation antipsychotics. Clozapine is convincingly more effective than typical antipsychotics (Kane 1988, Wahlbeck 2000). Olanzapine, quetiapine and risperidone are considered to be first‐line treatments for newly diagnosed people with schizophrenia, those experiencing unacceptable side effects and those previously experiencing unsatisfactory management with first‐generation antipsychotics (NICE 2002).
The efficacy and safety of quetiapine were promising in early (phase II) clinical trials (Hirsch 1996). It has been suggested that it is effective for treating both positive and negative symptoms of schizophrenia and that it is generally well tolerated. In addition, its propensity to induce movement disorders is reputedly no different to that of placebo.
Technical background of quetiapine Behavioural, electrophysiological and biochemical studies have indicated that quetiapine is a clozapine‐like atypical antipsychotic (Migler 1993, Goldstein 1993, Saller 1993). While olanzapine, risperidone, sertindole and ziprasidone have high affinities (<50 nM) to both D2 and 5‐HT2A receptors, quetiapine is similar to clozapine in having only moderate affinities (<500 nM) to these sites (Goldstein 1995). Quetiapine has a high affinity for histamine receptors (<50 nM).
Objectives
To determine the clinical effects of quetiapine for schizophrenia and other schizophrenia‐like illnesses in comparison to placebo, typical and other atypical antipsychotics.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. Quasi‐randomised studies, such as those allocating by using alternate days of the week, were excluded.
Types of participants
Adults aged 16‐65 with schizophrenia, schizoaffective disorder or schizophreniform disorders, diagnosed by any criteria.
Types of interventions
1. Quetiapine: any dose. Since people with schizophrenia may have dose‐related response to quetiapine, the doses of quetiapine were categorised into two dose‐ranges: (i) < 250 mg/d and (ii) >/= 250 mg/d. 2. Placebo. 3. Typical antipsychotics: any drugs, any dose. 4. Other atypical antipsychotics: any drugs, any dose.
Types of outcome measures
The outcomes of interest were:
1. Leaving the study early.
2. Death.
3. Clinical response 3.1 No clinically significant response in global state ‐ as defined by each of the studies* 3.2 Average score/change in global state 3.3 No clinically significant response on psychotic symptoms ‐ as defined by each of the studies 3.4 Average score/change on psychotic symptoms 3.5 No clinically significant response on positive symptoms ‐ as defined by each of the studies 3.6 Average score/change in positive symptoms 3.7 No clinically significant response on negative symptoms ‐ as defined by each of the studies 3.8 Average score/change in negative symptoms
4. Extrapyramidal side effects (EPS) 4.1 Incidence of use of antiparkinson drugs 4.2 Clinically significant EPS ‐ as defined by each of the studies 4.3 Average score/change in EPS
5. Other adverse effects, general and specific
6. Service utilisation outcomes: 6.1 Hospital admission 6.2 Days in hospital
7. Economic outcomes
8. Quality of life/satisfaction with care for either recipients of care or carers 8.1 No significant change in quality of life/satisfaction ‐ as defined by each of the studies 8.2 Average score/change in quality of life/satisfaction
All outcomes were reported for the short term (up to 12 weeks), medium term (13‐26 weeks), and long term (more than 26 weeks).
* Primary outcomes. In previous versions of this review primary outcomes were not stipulated. It is now the policy of the Cochrane Schizophrenia Group to choose primary outcomes, but this is usually undertaken before becoming familiar with the data, as post hoc stipulation of the emphasis of the review is prone to be influenced by the knowledge of the results. In the case of this review this was impossible, but choosing the primary outcomes still remains good practice. In order to minimise the inclusion of bias, the reviewers did not choose the primary outcomes themselves but copied them from very similar reviews investigating the effects of other atypical drugs. In this way researchers interested in clinically important outcomes, but not familiar with the quetiapine data, chose primary outcomes for this review.
Search methods for identification of studies
1. Electronic searches for update 2003
1.1 The Cochrane Schizophrenia Group's Register (February 15, 2003) was searched with the phrase:
[(quetiapine* or serequel* or ICI‐204636* or (ICI and 204636) or ICI204636* in title or *quetiapine* or *serequel* or *ICI‐204636* or (ICI and 204636) or *ICI204636* in title, abstract or index terms of REFERENCE) or (quetiapine in interventions of STUDY)]
The Schizophrenia Groups trials register is based on regular searches of BIOSIS Inside; CENTRAL; CINAHL; EMBASE; MEDLINE and PsycINFO; the hand searching of relevant journals and conference proceedings, and searches of several key grey literature sources. A full description is given in the groups module.
2. Details of previous electronic searches
2.1 Cochrane Schizophrenia Group's Register (Issue 1, 2000) Searched using the phrase: [QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636 or #42=428].
2.2 Cochrane Library (Issue 1, 2000) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
2.3 Biological Abstracts on CD (1980‐2000) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
2.4 CINAHL on CD (1982‐2000) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
2.5 EMBASE on CD (1980‐2000) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636 or explode "2‐2‐4‐(DIBENZOB,F1,4THIAZEPIN‐11‐YL)‐1‐PIPERAZINYLETHOXYETHANOL"/ all subheadings].
2.6 MEDLINE EXPRESS on CD (1966‐2000) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
2.7 PsycLIT Journal Articles on CD (1974‐2000) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
2.8 SIGLE on CD (1980‐1997) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
2.9 Sociofile on CD (1974‐1997) Searched according to the suggested Cochrane Schizophrenia Group's search strategy for controlled studies and schizophrenia (see Group search strategy) in combination with the phrase: [and QUETIAPINE or SEROQUEL or ICI‐204636 or (ICI and 204636) or ICI204636].
3. Hand searches The following journals were searched: Biological Psychiatry (1993‐present) Journal of Clinical Psychiatry (1993‐present) Journal of Nervous and Mental Diseases (1993‐present) Journal of Psychiatric Research (1993‐present) Psychiatric Services (1993‐present) Psychopharmacology Berlin (1993‐present)
4. Pharmaceutical company contact We contacted AstraZeneca Pharmaceuticals in the UK for information regarding unpublished trials.
5. Authors of studies We contacted the first author of studies if we had any doubts about the data in them.
Data collection and analysis
1. Selection of trials For the 2003 update, MS and NM independently inspected all citations identified by the search strategy. Full reports of relevant studies were obtained. Where disputes arose the full report was acquired for more detailed scrutiny. Similarly, all reports identified by the hand search or obtained from pharmaceutical companies were independently inspected by the reviewers (MS and NM), and the studies of agreed relevance identified. In each case, where disputes arose, BM acted as an arbitrator. Where this was not possible BM attempted to contact authors in order to acquire further details. If this was impossible the study was added to the list of those awaiting assessment.
2. Quality assessment The methodological quality of the included studies was independently rated by (MS and NM) using the Cochrane Collaboration Handbook (Clarke 2002). The rating of trial quality was based on the evidence of a strong relationship between the potential for inclusion of bias in the results and the concealment of allocation (Schulz 1995) and was defined as below: A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment)
Trials were included if they met criteria A or B. When disputes arose as to which category a trial should be allocated to, resolution was attempted by discussion. If more information was needed, BM attempted to contact the author of the paper. When this was not possible and further information was necessary to clarify the situation, data were not entered and the trial was allocated to the list of those awaiting assessment.
3. Data management Data were extracted independently by MS and NM. Again, if disputes arose these were resolved either by discussion between the two reviewers, the third member of the team (BM) or, as a last resort, the first author of the paper.
4. Data synthesis 4.1 Leaving the study early In this review, pooled data were considered to have high dropout rates and be prone to bias if pooled short‐term data of a treatment or control group had a dropout rate of 30% or more, or pooled medium or long‐term data of a treatment or control group had a dropout rate of 45% or more. The findings derived from these data should subsequently be viewed with caution. This decision was based on a quality assessment criterion which suggests that a dropout rate of 10% or more for a study lasting three months or less, or a dropout rate of 15% or more for a study lasting more than three months introduces bias (Hardon 1996).
4.2 Dichotomous data Relative Risk (RR) with the 95% confidence interval (CI) was used. A fixed effect model was used for all data sets, but only the ones with non‐significant heterogeneity (see section 6) were interpreted as favouring treatment or control. In addition, the number needed to treat (NNT) was also calculated for any significant difference in the respects of dropout rate and treatment improvement. NNT was calculated taking into account baseline risk using Visual Rx (http://www.nntonline.net/).
A reviewer, blind to the individual trial results, was asked to choose the important side effects to present in this review. This was undertaken because each study might report a large variety of general (non‐movement disorders) side effects. Those chosen included constipation, dizziness, dry mouth, low blood pressure and sleepiness. Where the studies presented dichotomous data, the various dose regimes of quetiapine were combined.
4.3 Intention‐to‐treat The reviewers applied the following guidelines to analyse data from included studies: (i) the analysis included all those who entered the trial; and (ii) the analysis maintained the study groups according to the original randomisation procedure. The reviewers assigned people lost to follow‐up to the worst outcome. In earlier versions of the review, before seeing the data, we stated that should attrition be greater than 50% for a given outcome we would not report the data. We were surprised by the degree of loss to follow up. We have not kept to this rule as that would leave few data to present (see Results 2. Loss to follow up, and Discussion).
4.4 Continuous data 4.4.1 Normal data: to be included in a parametric test, the data had to fulfil the following criteria: (i) standard deviations and means must be reported in the paper or obtainable from authors; (ii) when a score starts from a finite number (such as 0), the standard deviation, when multiplied by 2, must be less than the mean (Altman 1996). Otherwise such data are skewed and not appropriate to be presented in graphical form within RevMan. Skewed data of this sort were entered into the 'other data' tables.
4.4.2 Multiple doses: As a study might investigate a number of fixed doses of quetiapine, the combining of continuous data from those different groups was not possible. So, the scores from the highest dose group were used.
4.4.3 Summary statistic: a weighted mean difference (WMD) between groups was estimated for continuous outcomes. A fixed effect model was used for all data sets, but only the ones with non‐significant heterogeneity (see section 6) were interpreted in the respects of favouring treatment or control.
4.4.4 Valid scales: continuous data from rating scales were included only if the measuring instrument had been described in a peer‐reviewed journal and the instrument was either a self report or completed by an independent rater or relative (not the therapist). Whenever possible we took the opportunity to make a direct comparison between trials that used the same instrument of measurement to quantify specific outcomes. With the exception of 'positive' and 'negative' symptom scales, only total score data were presented and not subgroups.
4.4.5 Change versus endpoint data: where possible, change data were presented. If both change and endpoint data were available for the same outcomes only the former were reported in this review. Change data (where the rule concerning skew (4.2.1) did not apply) were presented graphically if they were from published scales and hadnt been analysed within the trial with non‐parametric tests.
5. Sensitivity analysis Because unpublished data are prone to bias, the inclusion of unpublished data in systematic reviews remains controversial. Cook 1993 suggested that sensitivity analyses should be undertaken for results in which data are included from both published and unpublished studies. We have done this for primary outcomes. If both analyses pointed to the same conclusion with regard to significant heterogeneity, the meta‐analyses conducted by the inclusion of the unpublished data were taken into consideration. Otherwise, the meta‐analyses conducted by the exclusion of the published data were considered.
6. Test for inconsistency Firstly, consideration of all the included studies within any comparison was undertaken to estimate clinical heterogeneity. Then visual inspection of graphs was used to investigate the possibility of statistical heterogeneity. This was supplemented by the use of the I‐squared statistic. This provides an estimate of the percentage of inconsistency thought to be due to chance. Where the I‐squared estimate was 75% or more, this was interpreted as evidence of high levels of heterogeneity (Higgins 2003). Data were then re‐analysed using a random effects model to see if this made a substantial difference. If it did, and results became more consistent, falling below 75% in the estimate, the studies were added to the main body of trials. If using the random effects model did not make a difference and inconsistency remained high, data were not summated, but were presented separately and reasons for heterogeneity investigated. (This paragraph has been reworded for the update of 2003 taking into account new developments in software and statistics.)
7. Addressing publication bias Data from all identified and selected trials were entered into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. 8. General Where possible, data were entered into RevMan in such a way that the area to the left of the line of no effect indicates a favourable outcome for quetiapine. The reviewers' effort to comply with this convention sometimes leads to clumsily worded outcomes such as 'no important clinical improvement'.
Results
Description of studies
1. Excluded studies For the study tag of excluded studies we used the name of the first author listed in the primary reference of each study as well as the year of publication.
We excluded seven studies. Five were not randomised controlled trials, one involved healthy volunteers as participants and not people with schizophrenia (Shimada 1994) and one reported no usable data (Kufferle 1997). This last study may also not have been randomised but we have been unable to get any further clarification.
2. Awaiting assessment Seven quetiapine trials were presented in scientific meetings but the results have not yet been published (Germany 2002, Italy 2002, Japan 2002, Netherlands 2002, Turkey 2001, USA 1999, USA 2001). According to abstracts, these studies compared the effects of quetiapine with those of typical or atypical antipsychotics. Because we could not obtain the posters of these trials and the abstracts provided very limited data, they were all classified as studies awaiting assessment.
3. Ongoing studies For ongoing and included studies, the reviewers used the country or continent names in the study tag. 'Multi‐country' was used for studies carried out in more than three continents. The first year that the study results were presented was also added to the study tag.
Three studies are ongoing. USA 1998 proposes to compare quetiapine using dosages up to 800 mg/day with standard care. USA 2000b is potentially a large study that will compare several antipsychotic drugs: quetiapine, clozapine, fluphenazine decanoate, olanzapine, perphenazine, risperidone and ziprasidone. We do not know when these studies are due to report. UK 2003 compares quetiapine with risperidone and is thought to be still recruiting. It is hoped that the final sample size will be over 200. This important study is eagerly awaited.
4. Included studies In total, 12 studies were included in this review. All studies were supported by grants from AstraZeneca Pharmaceuticals.
4.1 Allocation and blinding All included studies were stated to be randomised. All except USA 2000a used double‐blind techniques. Only Europe‐USA 1994 explicit stated how the sequence of allocation was concealed and no study tested blinding (see Methodological quality of included studies below).
4.2 Length of trials Medium‐term outcomes were available in two trials (Canada 2000, USA 2000a), the other ten trials were short‐term studies (<12 weeks). Among these short‐term trials, one trial lasted for only 21days (USA 1990). The study durations of the other nine trials were between 6 and 8 weeks, preceded by 1‐10 day washout periods.
4.3 Participants We were able to extract short‐term data on 2,725 people from 11 studies. We obtained medium‐term data on 753 patients from two trials (Canada 2000, USA 2000a). All the studies, apart from (USA 2000a) included people diagnosed with schizophrenia according to operational (DSM‐III‐R, DSM‐IV or ICD‐10) criteria. Multi‐country 1995 included people who had either not responded or partially responded to four weeks of treatment with fluphenazine. In USA 2000a, over 60% of participants had schizophrenia or schizoaffective disorders, the others having diagnoses of delusional disorders, psychotic mood disorders or dementias with psychotic features.
4.4 Study size The numbers of participants in all trials were between 109 and 728, except USA 1990 (n=12) and Canada 2000 (n=25). In all but USA 2000a, the number of men was higher than that of women with ratios of between 1.8:1 (Africa‐Europe 1994) and 8.9:1 (USA 1994). For USA 2000a the numbers of men and women were almost equal.
4.5 Interventions Quetiapine was compared with placebo in four studies (USA 1990, USA 1994, Europe‐USA 1994, North America 1996). Seven trials compared the effects of quetiapine with those of typical antipsychotics ‐ haloperidol, chlorpromazine and mosapramine (Africa‐Europe 1994, Multi‐country 1996, North America 1996, Japan 1999a, Japan 1999b, Multi‐country 1999, Canada 2000). The risks and benefits of quetiapine at different doses were compared in three studies (Europe‐USA 1994, Multi‐country 1995, North America 1996). Finally USA 2000a compared quetiapine with risperidone. For doses see Table 1.
1. Dose of control drug for quetiapine versus typical antipsychotic comparisons.
| Study tag | Control drug | Dose |
| Africa‐Europe 1994 | Chlorpromazine | mean ˜ 400 mg/day |
| Canada 2000 | Haloperidol | modal ˜ 15.5 mg |
| Japan 1999a | Mosapramine | mean ˜ 103 mg/day |
| Multi‐country 1996 | Haloperidol | mean ˜ 8mg/day |
| Multi‐country 1999 | Haloperidol | 20mg/day |
| North America 1996 | Haloperidol | 12mg/day |
4.6 Outcomes All studies, except Canada 2000 reported only short‐term outcomes. Canada 2000 was small (n=25) and compared quetiapine with haloperidol and reported both short and medium‐term outcomes. Some data were presented solely as graphs, p‐values or statements of significant or non‐significant differences. Presenting outcomes in such a way made it impossible to acquire raw data for synthesis. Some continuous outcomes could not be extracted because the number of participants was missing, or because means, standard deviations or standard errors were not reported. All included studies except Japan 1999a and Japan 1999b used last observation carried forward (LOCF) strategy for the intention‐to‐treat analysis of continuous data.
4.6.1 Outcome scales 4.6.1.1 Global state scales 4.6.1.1.1 Clinical Global Impression Scale ‐ CGI Scale (Guy 1976) This scale has been used to assess the overall condition of a mentally ill person ‐ both severity of illness and clinical improvement ‐ by comparing those conditions of investigated patients with those of other patients with the same diagnosis. An eight‐point (0‐7) scoring system is used with low scores indicating decreased severity and/or overall improvement.
4.6.1.2 Mental state scales 4.6.1.2.1 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has sixteen items, but a revised eighteen‐item scale is commonly used. Scores can range from 0‐126. Each item is rated on a seven‐point scale, with high scores indicating more severe symptoms.
4.6.1.2.2 Positive and negative syndrome scale ‐ PANSS (Kay 1986) The Positive and Negative Symptom Scale was developed from the BPRS and the Psychopathology Rating Scale. It is used as a method for evaluating positive, negative and other symptom dimensions in schizophrenia. The scale has 30 items, and each item can be defined on a seven‐point scoring system varying from one (absent) to seven (extreme). This scale can be divided into three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P) and negative symptoms (PANSS‐N). A low score indicates low levels of symptoms.
4.6.1.2.3 Scale for the assessment of negative symptoms ‐ SANS (Andreasen 1984) A six‐point (0‐5) scoring system can be used for each global rating of alogia, affective blunting, avolition‐apathy, anhedonia‐asociality, and attention impairment. A low score indicates low levels of psychotic symptoms.
4.6.1.3 Adverse effects scales 4.6.1.3.1 Abnormal involuntary movement scale ‐ AIMS (Guy 1976) The Abnormal Involuntary Movement Scale has been used to assess abnormal involuntary movements associated with antipsychotic drugs, such as tardive dyskinesia and chronic akathisia, as well as 'spontaneous' motor disturbance related to the illness itself. Tardive dyskinesia is a long‐term, drug‐induced movement disorder. However, using this scale in short‐term trials may also be helpful to assess some rapidly occurring abnormal movement disorders such as tremor. Scoring consists of rating movement severity in the anatomical areas (facial/oral, extremities, and trunk) on a five point scale (0‐4). A low score indicates low levels of dyskinetic movements.
4.6.1.3.2 Barnes Akathisia Scale (Barnes 1989) Akathisia is a short‐term, drug‐induced movement disorder. The scale comprises items rating the observable, restless movements that characterise akathisia, the subjective awareness of restlessness, and any distress associated with the condition. These items are rated from zero ‐ normal to three ‐ severe. In addition, there is an item for rating global severity (from zero ‐ absent to five ‐ severe). A low score indicates low levels of akathisia.
4.6.1.3.3 Simpson Angus Scale ‐SAS (Simpson 1970) This SAS is a 10‐item scale, used to evaluate the presence and severity of drug‐induced parkinsonian symptoms. The ten items focus on rigidity rather than bradykinesia, and do not assess subjective rigidity or slowness. Items are rated for severity on a 0‐4 scale, with a scoring system of 0‐4 for each item. A low score indicates low levels of parkinsonism.
Risk of bias in included studies
1. Randomisation With the exception of Europe‐USA 1994, no study provided details about its method of randomisation. All allocation concealment was therefore, rated as 'unclear' or quality 'B'. AstraZeneca Pharmaceuticals informed us that the randomisation in all studies were performed by the use of randomisation schedule. We are nevertheless unclear as regards concealment of allocation. The ratios of quetiapine to controlled treatment were 1:1 in most studies, except USA 1990 (2:1) and USA 2000a (3:1). 2. Blinding to interventions All studies, except one (USA 2000a), were conducted on a double‐blind basis, but none of them explicitly described how this was undertaken or tested the blindness of raters, clinicians and trial participants.
3. Loss to follow up The numbers leaving the study early were high but often reasons were given.
4. Overall As concealment of allocation is crucial if biases are to be minimised, all studies had to be rated as 'moderate risk of bias'. There was an emphasis on blinding within the studies yet no reassurance that this had been successful.
Effects of interventions
1. The search In total, we identified 60 citations for inclusion in this review. This includes 18 reports of studies added during the 2003 review update. Seven studies presented at scientific meetings, in abstract form and not yet published, were classified as awaiting assessment as no data could be extracted.
2. Loss to follow up The reviewers examined the overall rates of people leaving before study completion in the four comparisons. These rates were very high indeed (for example placebo 61%, typical antipsychotics ‐ short‐term data 36%, typical antipsychotics ‐ medium‐term data 75%, low dose quetiapine 58% and risperidone 34%). Normally with such high rates of attrition we would not consider presenting the data. Other reviews produced by the Cochrane Schizophrenia Group state that if over 50% of data for a particular outcome are lost by a designated period of time, those data will not be presented (Hunter 2003, Leucht 2003, Rummel 2003). If we had applied this rule within this review very few data would have been available and we thought it a disservice to the reader to be so exclusive. Outcomes with high attrition rate (30+% for short‐term comparisons and 45+% for medium‐ and long‐term comparisons) were labelled as 'prone to bias' and should be viewed with great caution.
3. Missing outcomes Dichotomous data on positive or negative symptoms were not reported within the published papers. Service utilisation data, data on economic outcomes and data on quality‐adjusted life years within USA 2000a were only presented in posters and we could not extract data for presentation here.
4. COMPARISON 1: QUETIAPINE (any dose) versus PLACEBO (all short‐term data)
4.1 Leaving the study early The overall dropout rate due to any cause was 53% for the quetiapine group and 61% for people allocated to placebo (n=716, 4RCTs, RR 0.84 CI 0.7 to 0.97, NNT 11 CI 7 to 55). While the overall dropout rates due to adverse events in both groups are not significantly different (n=716, 4 RCTs, RR 1.37 CI 0.6 to 3.1, I‐squared 63%), attrition due to treatment failure is lower in quetiapine group (n=716, 4 RCTs, RR 0.68 CI 0.6 to 0.8, NNT 7 CI 5 to 12).
Due to this high dropout rate all the following findings should be viewed with great caution. 4.2 Global state Although CGI was used to assess the patients' global state in all studies, only USA 1994 reported this as a dichotomous result (improved/not improved). While the dichotomous data show no significant difference (n=109, RR 0.95 CI 0.8 to 1.2), the continuous data show a significant difference in favour of quetiapine treatment (n=704, 4 RCTs, WMD ‐0.53 CI ‐0.7 to ‐0.3). These data have very considerable loss to follow up and are likely to be skewed. They have been analysed here using tests for parametric data and carry a moderate degree of heterogeneity (I‐squared 33%).
4.3 Mental state Dichotomous data relating to no improvement in psychotic symptoms (measured by BPRS or PANSS) significantly favoured quetiapine treatment (n=607, 3 RCTs, RR 0.79 CI 0.7 to 0.9, NNT 8 CI 5 to 20). All studies used BPRS for measuring the changes of psychotic symptoms. In comparison to placebo, the quetiapine group showed a significant improvement (n=607, 4 RCTs, WMD overall BPRS change ‐7.04 CI ‐9.9 to ‐ 4.2).
Positive and negative symptom sub‐scores are available from a variety of studies. Average change in BPRS positive sub‐score favoured quetiapine over placebo (n=701, 4 RCTs, WMD ‐0.69 CI ‐0.9 to ‐0.5, I‐squared 79%)) but these data, which contain considerable assumptions about those who were lost to follow up, are also heterogeneous. BPRS negative subscale data were equivocal (n=11, 1 RCT, WMD ‐4.3 CI ‐9.0 to 0.4), as were PANSS negative subscale data (n=113, 1 RCT, WMD ‐1.75 CI ‐4.5 to 1.0). SANS data, again attempting to measure negative symptoms, favoured quetiapine (n=574, 3 RCTs, WMD ‐1.23, CI ‐1.9 to ‐0.5).
4.4 Adverse effects People allocated quetiapine did not have more movement disorders than those given placebo (n=395, 2 RCTs, RR needing medication for EPSE 0.62 CI 0.3 to 1.1). Other results were not so clear. Constipation was prevalent for those on quetiapine but not significantly more than in people allocated to placebo (n=704, 3 RCTs, RR 1.88 CI 1.0 to 3.7). Dizziness was more common for those given quetiapine (n=716, 4 RCTs, RR 2.23 CI 1.1 to 4.4, NNH 19 CI 7 to 191, I‐square 42%) and low blood pressure (n=418, 2 RCTs, RR 1.92 CI 0.8 to 4.7, I‐squared 54%). Dry mouth may be a problem with quetiapine (n=395, 2 RCTs, RR 3.67 CI 1.3 to 10.8, NNH 10 CI 3 to 106) as well as sleepiness (n=716, 4 RCTs, RR 2.00 CI 1.3 to 3.0, NNH 10 CI 5 to 30, I‐squared 54%).
5. COMPARISON 2: >/= 250 mg/d QUETIAPINE versus < 250 mg/d QUETIAPINE (all short‐term data)
5.1 Leaving the study early The overall dropout rate in the >/= 250 mg/d quetiapine group (49%) is significantly lower than that of the < 250 mg/d quetiapine group (58%) (n=1066, 3 RCTs, RR 0.84 CI 0.8 to 0.9, NNT 11 CI 7 to 29). While the overall dropout rates due to adverse events are not significantly different between groups (n=1066, 3 RCTs, RR 1.24 CI 0.66 to 2.33) leaving the study early because of treatment failure was significantly lower in the low dose group (n=1066, 3 RCTs, RR 0.81 CI 0.7 to 0.96, NNT 14 CI 9 to 66).
Due to the high overall dropout rates in treatment and control groups all of the following findings should be viewed with great caution.
5.2 Death There were two deaths occurring in the Multi‐country 1995 study, both participants were receiving < 250 mg/d quetiapine (n=618, RR 0.1 CI 00.0 to 2.12).
5.3 Global state The overall improvement of global state (dichotomous data) as measured by the use of CGI shows a marginal but significant difference favouring the lower dose of quetiapine (n=618, 1 RCT, RR no important improvement 0.85 CI 0.7 to 0.99, NNT 12 CI 7 to 170). This concurs with the CGI continuous data (global improvement) also showing a significant improvement in favour of the low dose group (n=442, 2 RCTs, WMD ‐0.26 CI ‐0.5 to ‐0.04).
5.4 Mental state Mental state, as measured by the BPRS and PANSS (dichotomous data ‐ not improved), shows no difference between the low and higher dose groups (n=1066, 3 RCTs, RR 0.93 CI 0.8 to 1.1). However, the BPRS change scores are significantly lower in the lower dose group (n=1059, 3 RCTs, WMD ‐3.46 CI ‐5.6 to ‐1.4). The change scores of BPRS positive subscale were not significantly different between groups (n=441, 2 RCTs, WMD ‐0.23 CI ‐0.5 to 0.01). The PANSS negative subscale was also equivocal (n=76, 1 RCT, WMD ‐1.50 CI ‐4.7 to 1.7). SANS scores were, however, marginally positive in favour of the low dose group (n=361, 2 RCTs, WMD ‐0.88 CI ‐1.65 to ‐0.12) although data are heterogeneous (I‐square 76%).
5.5 Adverse effects Movement disorders were uncommon in each group (n=808, 2 RCTs, RR receiving medications for extrapyramidal side effects 1.22 CI 0.7 to 2.1). About 10% of both groups were constipated (n=448, 2 RCTs, RR 1.33 CI 0.7 to 2.5). Dizziness tended to be more common in the lower dose group (n=1066, 3 RCTs, RR 1.81 CI 1.1 to 3.1, NNH 27 CI 11 to 304) as was sleepiness (n=1066, 3 RCTs, RR 1.44 CI 1.0 to 2.0, NNH 22 CI 10 to 470).
6. COMPARISON 3: QUETIAPINE (any dose) versus TYPICAL ANTIPSYCHOTICS ‐ short & medium‐term data
6.1 Leaving the study early For short‐term data, about 36% of both groups failed to complete the study (n=1624, 6 RCTs, RR leaving the study early for any cause 0.87 CI 0.8 to 1.0). When reasons were specified, fewer people allocated quetiapine left because of adverse effects compared with those given typical antipsychotics (n=1624, 6 RCTs, RR 0.55 CI 0.39 to 0.76, NNT 20 CI 13 to 15, I‐squared 71%). Short‐term dropout due to treatment failure was not different between groups (n=1046, 3 RCTs, RR 1.27 CI 1.0 to 1.7). Only Canada 2000 (n=25) reports medium‐term loss to follow up. Fourteen out of 25 people (56%) left before study completion (6 months) and confidence intervals are wide with no clear differences between people allocated quetiapine and those given haloperidol (n=25, 1 RCT, RR leaving for any cause 0.51 CI 0.2 to 1.1). With this level of attrition all medium term data in this comparison must be viewed with great caution.
6.2 Global state Most data are short term (n=762, 3 RCTs) and record average change. There was not a large difference between quetiapine and typical antipsychotics in these heterogeneous data (WMD 0.19 CI 0.0 to 0.4, I squared 76%). In the medium term there was also no clear difference (n=25, 1 RCT, WMD ‐0.31 CI ‐1.4 to 0.8).
6.3 Mental state BPRS and PANSS were used in four studies (total n=1247) to rate 'not improved' by pre‐defined criteria. These short‐term data are not significantly different between groups (RR 0.97 CI 0.9 to 1.1). The BPRS and PANSS were also used to score overall change. Total scores from these scales did not point to differences between quetiapine and the older drugs (n=305, 1 RCT, MD change in BPRS ‐ short term 0.92 CI ‐3.6 to 5.4; n=455, 2 RCTs, WMD change in PANSS ‐ short term 1.92 CI ‐2.4 to 6.3, I squared 83%). When positive subscores were reported, again no differences were apparent (n=305, 1 RCT, MD change in BPRS positive subscore ‐ short term 0.08 CI ‐0.3 to 0.5; n=18, 1 RCT, MD change in PANSS positive subscore ‐ short term ‐4.96 CI ‐10.5 to 0.6). Medium term data (n=25, 1 RCT) are similarly equivocal. Negative symptoms were scored using the SANS (n=305, 1 RCT, MD change in the short term 0.94 CI ‐0.2 to 2.0), the PANSS negative subscale (n=18, 1 RCT, MD short term change ‐6.07 CI ‐10.9 to ‐1.3; n=25, 1 RCT, MD medium term data ‐3.15 CI ‐7.9 to 1.6).
6.4 Adverse effects Movement disorders were clearly less prevalent for those allocated quetiapine. Needing medication for extrapyramidal adverse effects was less prevalent, but data were heterogeneous (n=1117, 4 RCTs, RR 0.47 CI 0.4 to 0.6, NNT 4 CI 4 to 5, I squared 88%). People taking quetiapine did have less parkinsonism (n=758, 2 RCTs, RR 0.22 CI 0.2 to 0.3, NNT 4 CI 4 to 5) akathisia (n=1247, 4 RCTs, RR 0.29 CI 0.2 to 0.4, NNT 10 CI 8 to 12) and dystonia (n=959, 3 RCTs, RR 0.14 CI 0.0 to 0.5, NNT 24 CI 21 to 40).
In the short term quetiapine is not clearly either more or less constipating than the older drugs (n=959, 3 RCTs, RR 1.39 CI 0.8 to 2.5), nor does it cause either more or less dizziness (n=959, 3 RCTs, RR 1.21 CI 0.7 to 2.1) or postural hypotension (n=959, 3 RCTs, RR 1.00 CI 0.6 to 1.7, I‐squared 82%). Dry mouth may be more prevalent for those taking quetiapine compared with chlorpromazine or haloperidol (n=649, 2 RCTs, RR short term 2.85 CI 1.5 to 5.6, NNH 17 CI 7 to 65, I‐squared 70%). Sleepiness may also be more prevalent for people given quetiapine, at least in the short term (n=959, 3 RCTs, RR 1.51 CI 1.1 to 2.2, NNH 18 CI 8 to 181).
7. COMPARISON 4: QUETIAPINE (any dose) versus RISPERIDONE (any dose) (all short‐term data)
People in USA 2000a suffered from a variety of psychotic symptoms, but about two‐thirds were diagnosed with schizophrenia or schizoaffective disorder. Although the authors of this study performed a sub‐group analysis of the data obtained from patients with schizophrenia, we did not take those results into account, as the presentation of subgroup analysis might distort randomisation procedure. This decision was supported by the fact that the ratio of quetiapine:risperidone allocation is different for this subgroup (202: 62 or 3.25:1) compared with the 3:1 ratio in the overall study design.
7.1 Leaving the study early A little over 30% of people left the study before completion (USA 2000a). Rates were similar for each group (n=728, RR any cause 0.94 CI 0.7 to 1.2). While there is no significant difference in respect of leaving due to adverse events (n=728, RR 1.69 CI 0.9 to 3.4), when the reason for attrition was thought to be due to treatment failure, the results favoured people given quetiapine (n=728, RR 0.56 CI 0.3 to 0.1, NNT 23 CI 15 to 487). 7.2 Death Four people, all treated with quetiapine, died during the study (n=728, RR 2.86 CI 0.2 to 52.9). 7.3 Mental state All measures of mental state are continuous and are likely to contain considerable skew. In no result was quetiapine statistically different to risperidone. The average change in PANSS scores was not much different (n=637, 1 RCT, MD 1.2 CI ‐2.0 to 4.4), nor were changes in the PANSS positive subscale (n=639, 1 RCT, MD 0.70 CI ‐0.2 to 1.6) and PANSS negative subscale (n=641, 1 RCT, MD 0.30 CI ‐0.8 to 1.4).
7.4 Adverse effects Considerably fewer people given quetiapine needed medication for extrapyramidal side effects compared with those allocated to risperidone (n=712, 1 RCT, RR 0.27 CI 0.2 to 0.5, NNT 11 CI 10 to 16). Fewer people allocated to the quetiapine group were thought to have clinically significant extrapyramidal problems compared with risperidone (n=712, 1 RCT, RR 0.73 CI 0.6 to 0.9, NNT 10 CI 6 to 28).
Quetiapine caused more dizziness than risperidone (n=728, 1 RCT, RR 1.85 CI 1.0 to 3.3, NNH 18 CI 7 to 487), more dry mouth than risperidone (n=728, 1 RCT, RR 2.11 CI 1.2 to 3.8, NNH 14 CI 6 to 82) and more sleepiness than risperidone (n=728, 1 RCT, RR 2.03 CI 1.4 to 2.9, NNH 7 CI 4 to 17).
Discussion
1. The update This update (2003) includes considerably more data than previous versions of this review. There are still a number of studies which we cannot include, perhaps as many as seven, as they do not seem to have been fully published. These conference presentations suggest that there are many more data to consider but that these data are as yet, inaccessible. In addition, ongoing studies (USA 2000b, UK 2003) present the hope for better reported, more clinically useful data in the future. The extensive reformatting of the review in this version means the data are presented more clearly.
2. Limitation of evidence 2.1 Missing outcomes Quetiapine is a well studied drug. Thousands of people have been randomised in studies, yet we still do not have enough data that are easy to interpret on service utilisation, economic outcomes and quality of life.
2.2 Loss to follow up The rate of attrition in quetiapine trials is, on average, greater than for studies of other atypical compounds (Duggan 1999, Gilbody 2000, Hunter 2003). Loss to follow up is less than 50% only for short‐term data when quetiapine is compared with typical antipsychotics or with risperidone. In the comparisons of standard dose quetiapine with placebo, medium term typical antipsychotics, and low dose quetiapine, over half the data have to be assumed which makes the results impossible to interpret with confidence. Whatever the reason for study attrition, a high dropout rate affects the validity of the results. Although last observation carried forward (LOCF) is common analytic practice to account for missing observations, this technique could introduce bias (Peduzzi 2002).
2.3 Applicability of findings In all studies, except (USA 2000a), participants met DSM‐III‐R, DSM‐IV or ICD‐10 diagnostic criteria for schizophrenia. Schizophrenic patients with other psychiatric or medical problems concurrent with schizophrenia (about 50% of people with schizophrenia in everyday practice, Green 2003) were excluded from most studies. This limits the generalisability of the data for everyday practice.
3. COMPARISON 1: QUETIAPINE versus PLACEBO 3.1 Leaving the study early Fifty three percent of people allocated to quetiapine left before study completion compared with 61% given placebo. Although this favoured quetiapine (NNT 11 CI 7 to 55), such enormous attrition could reflect on the particular participants in the trial, the unacceptability of both interventions, or, and probably most likely, study design. The four randomised studies in this comparison were complex studies that involved asking thousands of questions to people with schizophrenia. Perhaps this design was not acceptable and resulted in a great haemorrhage of people and hence data. As a result assumptions were made concerning what people's outcomes would have been had they continued to the end of the study. This practice is common but nevertheless leaves the reader with the discomfort that over half the data carry considerable assumptions about outcome. 3.2 Global state Most data for the measure of global state were reported as continuous data (n=704). The change data showed a decline of half a point on the measure used (CGI) and although this was statistically different, it is difficult to know what it means clinically. The picture is further confused by the fact that over half the data are based on an assumption of stability once the person left, and on the assumption that the data are parametric and homogeneous. The binary data show no difference between placebo and quetiapine. These are fewer (n=111) and easier to understand, but carry similar considerable assumptions. From global measures it is hard to know if quetiapine has any advantage over placebo.
3.3 Mental state Binary measures of mental state tend to favour treatment with quetiapine. However, even if the confusion resulting from assumptions within these data is ignored, the number of people needed to be treated with quetiapine compared with placebo in order to improve a single person is still ten (CI 6 to 25).
The continuous measures of positive and negative symptoms tended to favour quetiapine but are difficult to interpret from the clinical perspective and are laden with assumptions. It is hard to tell if quetiapine has any advantage over placebo from mental state measures.
3.4 Adverse effects Quetiapine did not clearly cause the troublesome movement disorders seen with older antipsychotics. It may, however, cause constipation and low blood pressure and seems to cause dizziness (NNH 19 CI 7 to 191), dry mouth (NNH 10 CI 3 to 106) and sleepiness (NNH 10 CI 5 to 30).
4. COMPARISON 2: >/= 250 mg/d QUETIAPINE versus < 250 mg/d QUETIAPINE (all short‐term data)
4.1 Leaving the study early Less quetiapine resulted in less attrition (NNT 11 CI 7 to 29) but still, over half the data were lost because people did not want to stay in these trials (n=1066, 3 RCTs). All subsequent data and claims subsequently have to be viewed with great caution.
4.2 Death That the only two deaths in these studies occurred in the higher dose group, and that the short duration and relatively small size of most randomised studies preclude finding this rare adverse outcome, should serve as a warning. The mortality data from post‐market surveillance of quetiapine are awaited.
4.3 Global state The lower dose of quetiapine is favoured for both dichotomous and continuous measures of global state, although the NNT is considerable and imprecise (NNT 12 CI 7 to 170). Assumptions carried in these data render them impossible to interpret with confidence.
4.4 Mental state Although most binary and continuous measures of mental state were largely equivocal, low dose showed disadvantage over higher dose in reducing BPRS scores. The heterogeneity of data relevant to the average change of SANS scores may be explained by the comparison doses assigned in the studies. While the mean doses of quetiapine in North America 1996 were 552 and 110 mg/d, those in Europe‐USA 1994 were 360 and 209 mg/d. 4.5 Adverse effects Quetiapine does not seem to cause movement disorders, either at higher or lower doses. However, lower doses appear to cause less dizziness (NNH 27 CI 11 to 304) and sleepiness (NNH 22 CI 10 to 470).
5. COMPARISON 3: QUETIAPINE versus TYPICAL ANTIPSYCHOTICS
5.1 Leaving the study early For short‐term data about 36% of both groups failed to complete the study. Although this rate is better than for the other comparisons, it still does not reflect well on study design. Fewer people allocated to quetiapine left because of adverse effects compared with those given typical antipsychotics, although the number needed to treat is large (20). Short‐term dropout due to treatment failure is not different between groups (n=1046, 3 RCTs). In the medium term data are far too few to meaningfully compare dropout rates of quetiapine and typical antipsychotics.
5.2 Global state The results were presented as average change and are therefore difficult to interpret. Quetiapine is, however, not clearly different to the older generation of drugs on global measures.
5.3 Mental state Mental state measures, binary or continuous, did not highlight any significant differences between quetiapine and typical antipsychotics. This applies not only to the total scores, but also to measures of positive and negative symptoms. There is no evidence that quetiapine is particularly effective for negative symptoms of schizophrenia.
5.4 Adverse effects Movement disorders were clearly less prevalent for those allocated quetiapine. In at least one study, quetiapine was compared to a reasonably high dose of control drugs (20 mg/day of haloperidol) (Multi‐country 1999) so some extrapyramidal effects could be expected. Nevertheless quetiapine does seem to have less such effects than the older drugs and more of the profile expected with atypical drugs.
Quetiapine may cause constipation but not more so than older generation of drugs. It might have been hoped that it would cause less. This also applies to dizziness. Dry mouth may well be a problem with quetiapine, even more so than with chlorpromazine or haloperidol, although the number needed to harm is quite high (NNH 17 CI 7 to 65). Sleepiness may also be more prevalent for people given this new drug (NNH 18 CI 8 to 181). This adverse effect can be welcome to clinicians, but rarely to people taking the drug.
5.5 Heterogeneity The heterogeneity found in six data sets may be caused by the results obtained from three studies (Africa‐Europe 1994, Multi‐country 1999, Canada 2000). The study of Africa‐Europe 1994 is the only study that used chlorpromazine (mean ˜ 400mg/day) as a comparator to quetiapine and results were particularly favourable for the experimental drug for adverse effects. People in Multi‐country 1999 were those who had not responded or only partially responded to fluphenazine. This may cause the heterogeneity of data relevant to leaving the study early due to adverse events. Canada 2000 has a small sample size (n=25), which may lead to random error and heterogeneity relevant to short‐term changes of CGI and PANSS scores.
6. COMPARISON 4: QUETIAPINE (any dose) versus RISPERIDONE (any dose) (all short‐term data)
We found one large study (USA 2000a, n=728), although results from others are expected (UK 2003).
6.1 Leaving the study early Quetiapine and risperidone have similar rates of attrition in the short term, and this rate of a little over 30% is identical to results for all studies involving risperidone (Hunter 2003). 6.2 Death Four people, all treated with quetiapine, died before the study finished at 16 weeks (n=728, RR 2.86 CI 0.15 to 52.84). This, along with the findings reported above, all linking quetiapine with death, should be considered cause for concern. Results of post‐marketing surveys are urgently needed. 6.3 Mental state The continuous mental state scores are impossible to interpret and, as for many other studies in this area, represent a considerable waste of opportunity. On these measures quetiapine was not better or worse than risperidone. There is no evidence that quetiapine has a clear effect on negative symptoms.
6.4 Adverse effects Quetiapine causes less extrapyramidal side effects than risperidone (NNT 11 CI 10 to 16).
Within this comparison with risperidone, quetiapine causes more dizziness (NNH 18 CI 7 to 487), dry mouth (NNH 14 CI 6 to 82) and sleepiness (NNH 7 CI 4 to 17).
Authors' conclusions
Implications for practice.
1. For people with schizophrenia Quetiapine is not clearly different from first‐generation antipsychotics and risperidone in terms of global outcomes or ratings of mental state. It seems to cause less movement disorders but more sedation and dry mouth, and similar rates of constipation and dizziness. It is not significantly associated with mortality, although all deaths in these studies have occurred in the quetiapine groups.
2. For clinicians Quetiapine is as effective as first‐generation antipsychotics and risperidone for schizophrenia. It may carry lower risks of movement disorders but does carry risks of other adverse effects, eg, dizziness and sleepiness. There is no indication that it benefits negative symptoms. More studies are needed to replicate and validate these findings.
3. For managers/policy makers There is currently insufficient clinical evidence to support the claim that quetiapine is superior to typical antipsychotics or risperidone in respect of treatment withdrawal, efficacy and tolerability. Thousands of people have been randomised to take quetiapine or a control, but we still need more data on mental state from pragmatic randomised‐controlled trials with low dropout rates, as well as data on cost‐effectiveness, service utilisation, social functioning and quality of life/satisfaction. There is a particular lack of medium or long term data.
Implications for research.
1. General As with all similar studies, public registration of a study before anyone is randomised would ensure that participants could be confident that people would know that the study had at least taken place. Use of unique study identifiers would help researchers to identify single studies from multiple publications and reduce the risk of double counting of data. Compliance with CONSORT (Moher 2001), both on the part of authors and editors, would help to clarify methodology and many outcomes. Failure to comply results in both loss of data and confusion in the results, neither of which help clinicians and patients.
Intention‐to‐treat analysis should be performed on both dichotomous and continuous outcomes and all trial data should be made easily accessible. A minimal requirement should be that all data should, at least, be presented as numbers. In addition, continuous data should be presented with means, standard deviations (or standard errors) and the number of participants. Data from graphs, 'p' values of differences and statements of significant or non‐significant differences are of little value.
2. Specific As an antipsychotic agent with very low risk of movement disorders, quetiapine is an interesting compound, but pragmatic (real world) randomised controlled trials should be carried out to determine its position in everyday clinical practice. Studies of medium and long‐term risks, benefits and cost‐effectiveness in comparison to typical and atypical antipsychotics are a priority.
Studies so far have used people with schizophrenia without medical or psychiatric co‐morbidity. To enable results to be applied to a wide range of people with schizophrenia, the inclusion criteria used in future trials should be broadened to include people with schizophrenia who have other medical or psychiatric co‐morbidities. Pragmatic entry criteria and non‐blinding with hard endpoints may be helpful in decreasing the study attrition (Roland 1998).
Feedback
General comments from Zeneca Pharmaceuticals
Summary
1. General issues The review appears to criticise the design of clinical trials evaluating antipsychotics and the high drop‐out rate observed with quetiapine. It would have been reasonable to point out that high drop‐out rates have occurred in other trials involving both standard and atypical antipsychotics (eg in the "landmark" study M93‐113 involving sertindole (Zimbroff 1997) the overall study drop‐out rate was 51% and in the recently published study by Tran (1997) which compared olanzapine with risperidone, drop out rates in the two groups were 42.4% and 52.7%respectively). The quetiapine studies mentioned in this review were designed after extensive consultations with opinion leaders in the field, including statisticians and other specialists. They reflect, we believe, the best practice at that time.
We are concerned that an unnecessarily pessimistic view is given of the efficacy of quetiapine and that the advantages of the compound are not given due prominence (see 'Conclusions'). The clinical data have been scrutinised by the FDA, the MCA and other regulatory bodies who have found it satisfactory and awarded a product licence.
2. Textual issues There are numerous errors and inconsistencies throughout the text and it seems that further editing is required. Some examples: 'Results' heading number 3 ‐ "No important improvement on global and mental state" is a conclusion, rather than a heading. The comment in the 'Discussion' section that "people in less affluent countries, that is, the majority of people in the world with schizophrenia, will not have access to quetiapine at current prices" applies to all the atypical antipsychotics and many other drugs. We do not consider this a helpful statement, unless it forms part of a comprehensive health economic review. Again in the 'Discussion' section, the statement under the "Heterogeneity" heading that "there is quite a bit of heterogeneity" appears inappropriate in a scientific document.
3. Specific issues
3.1 Abstract, Main Results, paragraph 2 ‐ Although the author states that there is no difference between quetiapine and placebo with regard to extrapyramidal side effects (EPS), he goes on to state that "Other than the high incidences of dry mouth and sleepiness in the quetiapine group this drug is not much different from classical antipsychotics in its effects". We submit that one of the crucial differences between standard antipsychotics and quetiapine is the relative lack of EPS seen with quetiapine across the entire dose range. This issue influences outcome, compliance and patient satisfaction. In contrast, dry mouth and sleepiness are of minor concern, and were as common with quetiapine as with chlorpromazine in the comparative study of the two compounds. In the last sentence, the statement that there are no clear differences between high and low dose quetiapine with regard to EPS is correct, but the implication in this context is that there is an EPS problem with quetiapine.
3.2 Background, Technical background of quetiapine. Quetiapine has a very high affinity for histamine receptors (IC50 30 nM), not as stated "very little affinity".
3.3 Results Quetiapine vs classic antipsychotics
4. Change scores, line 4. Haloperidol was the comparator antipsychotic in Studies 013 and 014. Quetiapine (high dose) vs quetiapine (low dose)
5. Side effects: movement disorders. The implication here seems to be that high quetiapine doses(750 mg/day) caused akathisia. The incidence of akathisia at this dose in Trial 013 was 2% (and 8% in the placebo group). The incidence of akathisia was 4% at a mean dose of 360 mg/day in Trial 008, and there was no trend for a greater incidence at higher doses (see also Heterogeneity in the Discussion section).
3.4 Discussion Generalisability (sic) of findings. Lines 5‐7, the implied criticism of use of DSM‐III‐R criteria is unhelpful. All pivotal studies of novel antipsychotics have used DSM/ICD criteria. The relevance of such a selection of patients to the population encountered in practice is an academic question. What criteria does the author suggest we adopt when investigating anti‐schizophrenic compounds? Loss to follow up. The author is extremely conservative about data interpretation in general, but in this section speculates that "it is unlikely that those who left early remained stable". Quetiapine vs placebo. The author speculates that the lower drop out rate with quetiapine could be due to sedation and movement disorders which erode the ability of patients to make a decision to leave the study. This is no evidence for this, especially as motor system disturbance with quetiapine is acknowledged elsewhere in the article to be not significantly different to placebo.
3.5 Conclusions For clinicians. It would be helpful to include lack of elevation of serum prolactin (and the clinical consequences of this, such as reduced disturbance of sexual function). This advantage is not discussed elsewhere in the review. Data on patient satisfaction have been published (Hellewell1998). For managers/policy makers. The statement "other than the weak suggestion that the extrapyramidal side effects are comparable to those of placebo "is mis‐representative. If it means that the evidence is weak this is not so (see elsewhere in the article). If it means that this is not an important issue we would disagree strongly; EPS has long been recognised as a major disadvantage of standard antipsychotics and may worsen symptomatology and have adverse effects on patient's quality of life, compliance with treatment and other outcomes.
4. Statistical issues These are the comments of the Zenaca statistician: From a statistical perspective, I would question the inclusion of chlorpromazine and haloperidol in the same analysis. In the discussion, the author mentions that some of the heterogeneity may be due to this. In this case, I would have thought a logical approach would be to make two separate comparisons. In the discussion, they also mention some heterogeneity between the haloperidol studies, but there is no mention of whether these data were analysed separately, or is this a conclusion based solely on consideration of the forest plots.
In general, the issue of heterogeneity has been used with little thought. In some cases, the presence of heterogeneity has meant that the results should be disregarded, whilst in others that the results should be treated with caution. The former case has usually been taken when the data tend to favour quetiapine e.g. EPS profile. The presence of heterogeneity should invite closer examination, but it does not categorically rule out a meaningful conclusion. If you examine the parkinsonism data versus classical antipsychotics, the paper does not mention the result due to the heterogeneity. However, if you look at the forest plot, both the individual studies clearly favour quetiapine. The heterogeneity may indicate different methods of data collection or some other artefact of the studies, but the pattern of results consistently favour one drug. Hence a conclusion can be made. It is perhaps for such reasons that the EPS differences do not have the prominence they deserve.
Confidence intervals should be reported within the text for non‐significant differences, as well as for significant e.g. the need for EPS medication versus placebo. This would help the reader to evaluate the difference and decide whether the lack of difference indicates equivalence or a lack of data.
5. References Hellewell JSE, Kalali AH, Langham SJ, McKellar J. Patients' satisfaction and acceptability of long‐term treatment with 'Seroquel': Results of an international study. European Neuropsychopharmacology 1998;8(supp 2):248.
Tran PV, Hamilton SH, Kuntz AJ Potvin JH, Andersen SW, Beasley C Jr, Tollefson GD. Double‐blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. Journal of Clinical Psychopharmacology 1997;17:407‐18.
Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Wozniak PJ, Sebree TB, Wallin BA, Kashkin KB. Controlled, dose‐response study of sertindole and haloperidol in the treatment of schizophrenia. American Journal of Psychiatry 1997;154:782‐91.
Reply
1. General issues High dropout rates: The dropout rates in the quetiapine studies are around 50%. This poses two questions: (i) how does a high dropout rate affect the validity of a study and (ii) are the dropout rates of quetiapine studies higher than other studies of typical or atypical antipsychotics? It is widely accepted that a high dropout rate affects the validity of a study. The Agency for Health Care Policy and Research (AHCPR) proposed criteria for assessing a randomised controlled trial's quality that included dropout rates. They suggest that any study of less than three months' duration with a dropout rate exceeding 10% should be considered as flawed to a major degree (Hadorn 1996). In placebo controlled studies, the pooled dropout rate of 20% for chlorpromazine studies (Thornley 1999) is much lower than that of 53% for quetiapine studies. In atypical antipsychotic controlled studies, the pooled dropout rates of 15% for clozapine (Wahlbeck 1999) and 26% for risperidone (Kennedy 1999) are much lower than those for quetiapine. The pooled dropout data for olanzapine, however, is comparable at 42% at six weeks (Duggan 1999). For both reasons, we cautioned readers on the interpretation of data. Most other antipsychotics are better than quetiapine with respect to the outcome of completion of study.
2. Textual issues 'Results' heading number 3 "No important improvement on global and mental state": This is an appropriate heading. When we talk about odd ratios, risk reduction, numbers needed to treat, we are dealing with adverse outcomes(e.g. death, morbidity). In this case, we chose a simple phrase that is easily understood. For academic wording, it might be described as "Non response rates in the respects of global and mental state". The comment that "people in less affluent countries, that is, the majority of people in the world with schizophrenia, will not have access to quetiapine at current prices": Not only quetiapine but also other atypical antipsychotics are now too expensive for most people with schizophrenia in developing countries. In clinical practice, this is an issue of great concern for patients, clinicians and policy makers ‐ perhaps not Zeneca Pharmaceuticals. The "Heterogeneity" heading that "…there is quite a bit of heterogeneity": The reviewers are grateful for having had their attention drawn to this and it has been amended.
3. Specific Issues 3.1 Abstract, main results, paragraph 2: "Other than the high incidences of dry mouth and sleepiness in the quetiapine group this drug is not much different from classical antipsychotics in its effects". Due to the lack of statistical significance relevant to most respects of EPS, we could not support the commentator's assertion that "the incidence of quetiapine‐induced EPS is lower than that of typical antipsychotics." Dry mouth and sleepiness are of minor concern. In a recent survey, sponsored by Zeneca Pharmaceuticals, sleepiness was found to be of very major concern to those who contacted a UK‐based consumer help line for people with serious mental health problems (Fakhouri 1999). The incidence of dry mouth and sleepiness is significantly higher for those taking quetiapine compared to typical antipsychotics.
3.2 Background, technical background of quetiapine: "very little affinity for histamine receptors". We have amended our mistake.
3.3 Results Quetiapine vs classical antipsychotics. 4. Change score, line 4 "Haloperidol was the comparator antipsychotic in Studies 013 and 014. This is correct. Quetiapine (high dose) vs quetiapine (low dose). 5. Side effects: movement disorders. Our review found no difference in this respect. We declined to state that both doses of quetiapine did not induce EPS since quetiapine‐induced EPS could be found in some studies. For example, in the study 012, the Peto Odds Ratio for the incidence of akathisia is significantly higher in the "high dose" group.
3.4 Discussion Generalisability of findings. Lines 5‐7: The problematic statement seems to be that "Although this results in a reasonably homogenous group of people (participants meeting the DSM‐III‐R diagnosis criteria of schizophrenia), how representative they are of the wider population of those with schizophrenia is debatable". It should not be implied that the use of DSM‐III‐R criteria is unhelpful. The statement was written to communicate that the results of this review should be applied with caution for people with schizophrenia whose clinical features are different from the DSM‐III‐R diagnostic criteria. With different clinical features, the responses to quetiapine of people with ICD‐10 or DSM‐IV schizophrenia may be different from those of the people with DSM‐III‐R schizophrenia included in the review. Those with DSM‐III‐R schizophrenia may well be systematically different from the very many people with schizophrenia that does not readily fall into such well defined categories or those with organic and drug problems compounding their illness. Loss to follow up and dropout rates: There are many reasons behind the problem of non‐compliance. After dropping out the participants' conditions were unknown. We chose a pessimistic view. If Zeneca have data to the contrary we shall include it in the review.
3.5 Conclusions For clinicians: We agree that quetiapine is an atypical antipsychotic with lack of elevation of serum prolactin. However, for two reasons we declined to include this issue in our review: (i) this issue is not an outcome of interest we specified in the review protocol; and (ii) our review concerns itself with clinical outcomes, not biochemical data. We have scrutinised the included trials to investigate whether lack of prolactin elevation relates to reducing sexual dysfunction and found no benefit. Theoretically, quetiapine may be associated with a lower incidence of sexual dysfunction, but this was not supported by data presented in the included trials. Regarding satisfaction with quetiapine (Kalali 1996), we excluded this study from our review because it was not randomised. We are grateful for having Hellewell (1998) drawn to our attention. For managers/policy makers "the weak suggestion that the extrapyramidal side effects are comparable to those of placebo…". We agree that EPS are major drawbacks of antipsychotic treatment, and an antipsychotic with fewer propensities to induce EPS is of help to our patients with schizophrenia. Although we found that quetiapine‐induced EPS are comparable to those of placebo, the evidence was not strong because the high dropout rates may affect the validity of study results. Nevertheless we have reworded this statement. Regarding typical antipsychotics, heterogeneous data do not reveal significant differences in EPS other than for dystonia which was lower in the quetiapine‐treated group (OR 0.18 CI 0.07‐0.48).
4. Statistical aspects Dealing with the heterogeneous results We have largely re‐written the results and present the heterogeneous findings in a more informative way. Presentation of non‐significant data It is a dilemma how to present the data informatively and not to crowd the test with numbers that are readily available within the graphs. Many more of the equivocal data have been presented in the text and the anticipated hyperlinks between text and graphs in future versions of the Cochrane Library will solve this problem once and for all.
5. References Duggan L, Fenton M, Dardennes RM, El‐Dosoky A, Indran S. Olanzapine for schizophrenia (Cochrane Review). In: The Cochrane Library, Issue 1, 1999. Oxford: Update Software.
Fakhouri W. A survey of patients satisfaction with medication. Stratford‐upon‐Avon Schizophrenia Trials Meeting 5th‐7th May 1999.
Hadorn DC, Baker D, Hodges JS, Hicks N. Rating the quality of evidence of clinical practice guidelines. Journal of Clinical Epidemiology 1996;49(7):749‐54. Kalali AH, Potkin SG. Patients' satisfaction with and acceptability of seroquel (ICI 204,636). European Neuropsychopharmacology 1996;6(supp 3):249.
Kennedy E, Song F, Hunter R, Clark A, Gilbody S. Risperidone versus typical antipsychotic medication for schizophrenia (Cochrane Review). In: The Cochrane Library, Issue 1, 1999. Oxford: Update Software.
Thornley B, Adams CE, Awad G. Chlorpromazine versus placebo for schizophrenia (Cochrane Review). In: The Cochrane Library, Issue 1, 1999.Oxford: Update Software.
Wahlbeck K, Cheine M, Essali MA. Clozapine versus typical neuroleptic medication for schizophrenia (Cochrane Review). In: The Cochrane Library, Issue 1, 1999. Oxford: Update Software.
Contributors
Comment received from Stephen Litherland, Alderley Park, Macclesfield, July 1998 [Mr Litherland, Senior Product Information Manager, is an employee of Zeneca Pharmaceuticals and works in Medical and Marketing Communications.] Reply received from Manit Srisurapanont, Chaing Mai, Thailand, April 1999
What's new
| Date | Event | Description |
|---|---|---|
| 12 December 2012 | Amended | Response to comment added, see Published notes |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 3 May 2012 | Amended | Additional table linked to text. |
| 25 April 2008 | Amended | Converted to new review format. |
| 21 January 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
It has been drawn to our attention that there are parts of the data of this review that may need correction (1).
All comments relate to the placebo comparison and, so far in our investigations, we have found no substantive issue that changes conclusions. Amendments to this review will be made shortly. If the reader is interested in this comparison specifically a new review has been underway for some time covering this specific topic and will be published shortly.
(1) Hutton P, Morrison AP, Yung AR, Taylor PJ, French P, Dunn G. Effects of drop‐out on efficacy estimates in five Cochrane reviews of popular antipsychotics for schizophrenia. Acta Psychiatr Scand. 2012 Jul;126(1):1–11.
Acknowledgements
For the first version of this review: Chamlong Disayavanish contributed to the study selection, data extraction, analysis and writing of final report. Kittiwan Taimkaew was responsible for dispute resolution and also contributed to the writing of the final report. The Zeneca Bibliography supplied at the American Psychiatric Association Meeting (San Diego, California, May 17‐22, 1997) was a valuable source of citations. Those in the Zeneca Medical Information Department in the UK, especially Ms Su Webber and Dr Richard Owen, were genuinely collaborative in supplying data on file for the study Multi‐country 1996.
For the first and second updates of this review: The Astrazeneca Medical Information Department in the UK were collaborative in supplying a collection of posters presented between 1998‐2003.
We wish to thank the staff at the editorial base of Cochrane Schizophrenia Group, in particular, Clive Adams, Leanne Roberts, Nicola Howson, Nancy Owens, Gillian Rizzello and Mark Fenton who provided much support for this review.
Data and analyses
Comparison 1. QUETIAPINE (any dose) versus PLACEBO (all short‐term data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 any cause | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.97] |
| 1.2 due to adverse events | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.60, 3.12] |
| 1.3 due to treatment failure | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.82] |
| 2 Global state: 1. No important improvement (CGI) | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 3 Global state: 2. Average change (CGI, low score = good) | 4 | 704 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| 4 Mental state: 1. No clinically important change (BPRS or PANSS) | 3 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.92] |
| 5 Mental state: 2. Average change (BPRS, low score = good) | 4 | 607 | Mean Difference (IV, Fixed, 95% CI) | ‐7.04 [‐9.85, ‐4.24] |
| 6 Mental state: 3. Average change in positive symptoms (BPRS positive subscale, low score = good) | 4 | 701 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐0.91, ‐0.47] |
| 7 Mental state: 4. Average change in negative symptoms (low score = good) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 BPRS negative subscale | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐4.3 [‐9.03, 0.43] |
| 7.2 PANSS negative subscale | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐4.47, 0.97] |
| 7.3 SANS | 3 | 574 | Mean Difference (IV, Fixed, 95% CI) | ‐1.23 [‐1.94, ‐0.52] |
| 8 Adverse effects: 1. Movement disorders | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 patients receiving medications for extrapyramidal adverse effects | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.15] |
| 8.2 parkinsonism | 3 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.19] |
| 8.3 akathisia | 2 | 595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.06] |
| 8.4 dystonia | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.04, 4.28] |
| 9 Adverse effects: 2. General | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 constipation | 3 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.95, 3.74] |
| 9.2 dizziness | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.12, 4.42] |
| 9.3 dry mouth | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [1.25, 10.83] |
| 9.4 low blood pressure (postural) | 2 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.79, 4.70] |
| 9.5 sleepiness | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.32, 3.04] |
1.1. Analysis.
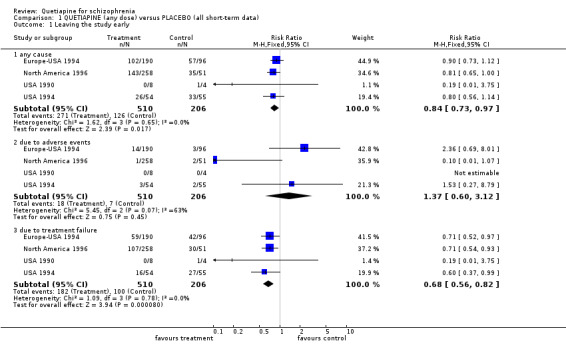
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 1 Leaving the study early.
1.2. Analysis.
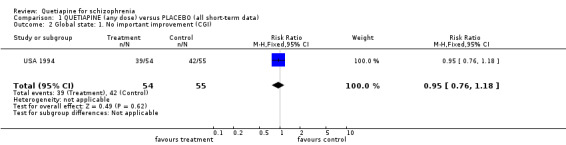
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 2 Global state: 1. No important improvement (CGI).
1.3. Analysis.
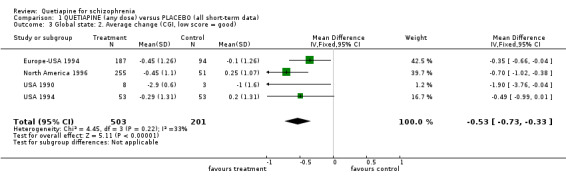
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 3 Global state: 2. Average change (CGI, low score = good).
1.4. Analysis.
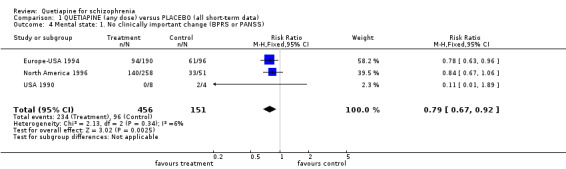
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 4 Mental state: 1. No clinically important change (BPRS or PANSS).
1.5. Analysis.
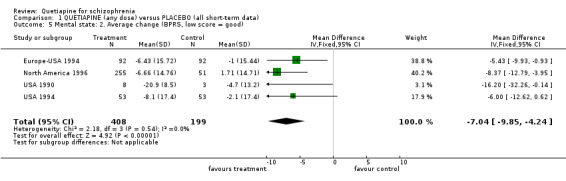
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 5 Mental state: 2. Average change (BPRS, low score = good).
1.6. Analysis.
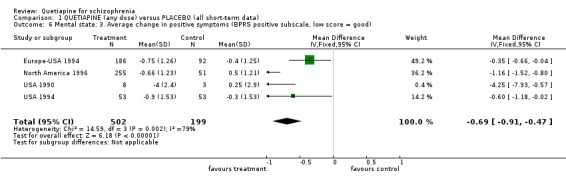
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 6 Mental state: 3. Average change in positive symptoms (BPRS positive subscale, low score = good).
1.7. Analysis.
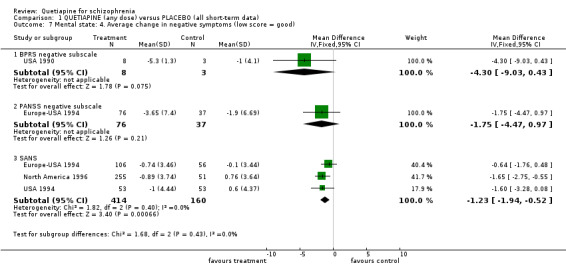
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 7 Mental state: 4. Average change in negative symptoms (low score = good).
1.8. Analysis.
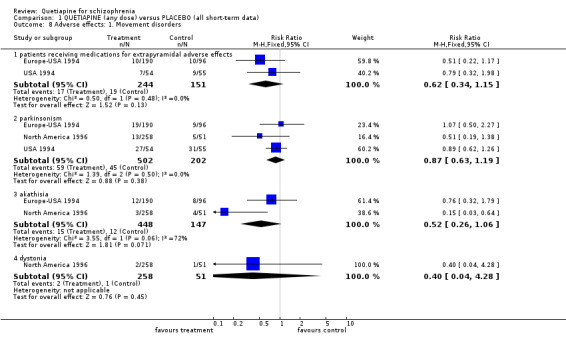
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 8 Adverse effects: 1. Movement disorders.
1.9. Analysis.
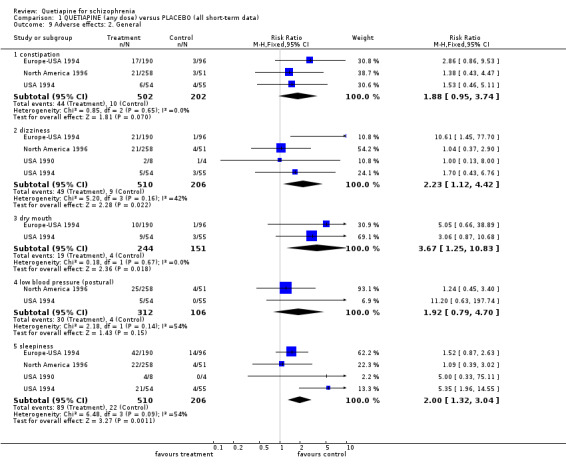
Comparison 1 QUETIAPINE (any dose) versus PLACEBO (all short‐term data), Outcome 9 Adverse effects: 2. General.
Comparison 2. >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 any cause | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 1.2 due to adverse event | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.66, 2.33] |
| 1.3 due to treatment failure | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.96] |
| 2 Death (any cause, any dose) | 1 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 2.12] |
| 3 Global state: 1. No important improvement (CGI) | 1 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 0.99] |
| 4 Global state: 2. Average change (CGI, low score = good) | 2 | 442 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.47, ‐0.04] |
| 5 Mental state: 1. No important improvement (BPRS or PANSS) | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.82, 1.05] |
| 6 Mental state: 2. Average change (BPRS, low score = good) | 3 | 1059 | Mean Difference (IV, Fixed, 95% CI) | ‐3.46 [‐5.57, ‐1.35] |
| 7 Mental state: 3. Average change in positive symptoms (BPRS positive subscale, low score = good) | 2 | 441 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.46, 0.01] |
| 8 Mental state: 4. Average change in negative symptoms (low score = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 PANSS negative subscale (low score = good) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.69, 1.69] |
| 8.2 SANS (low score = good) | 2 | 361 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.65, ‐0.12] |
| 9 Adverse effects: 1. Movement disorders | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 patients receiving medications for extrapyramidal adverse effects | 2 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.71, 2.08] |
| 9.2 parkinsonism | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.20] |
| 9.3 akathisia | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.33] |
| 9.4 dystonia | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.08, 4.04] |
| 10 Adverse effects: 2. General | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 constipation | 2 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.71, 2.49] |
| 10.2 dizziness | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.07, 3.08] |
| 10.3 dry mouth | 2 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.96, 4.00] |
| 10.4 low blood pressure (postural) | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.85, 2.67] |
| 10.5 sleepiness | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.02, 2.03] |
2.1. Analysis.
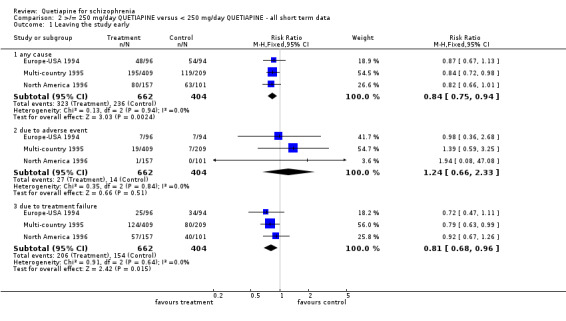
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 1 Leaving the study early.
2.2. Analysis.
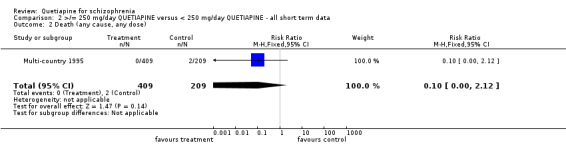
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 2 Death (any cause, any dose).
2.3. Analysis.
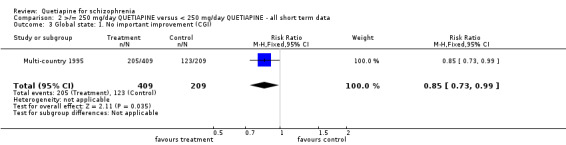
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 3 Global state: 1. No important improvement (CGI).
2.4. Analysis.
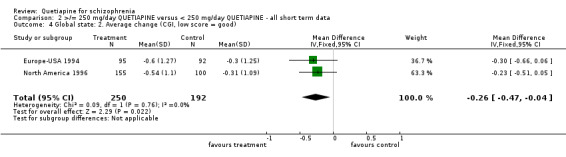
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 4 Global state: 2. Average change (CGI, low score = good).
2.5. Analysis.
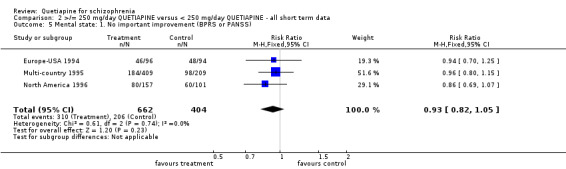
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 5 Mental state: 1. No important improvement (BPRS or PANSS).
2.6. Analysis.
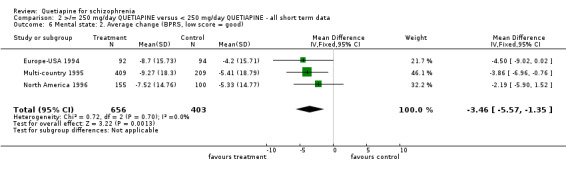
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 6 Mental state: 2. Average change (BPRS, low score = good).
2.7. Analysis.
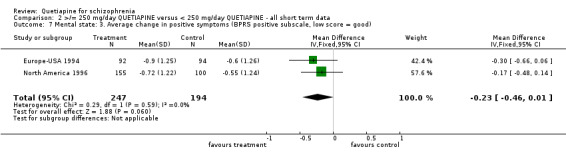
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 7 Mental state: 3. Average change in positive symptoms (BPRS positive subscale, low score = good).
2.8. Analysis.
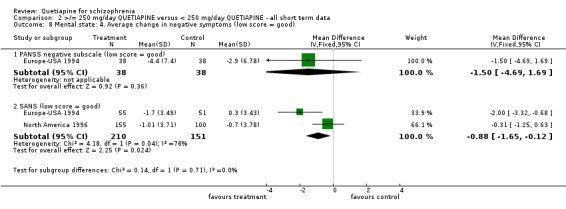
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 8 Mental state: 4. Average change in negative symptoms (low score = good).
2.9. Analysis.
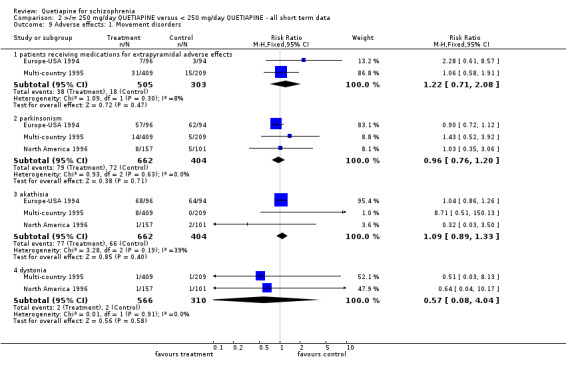
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 9 Adverse effects: 1. Movement disorders.
2.10. Analysis.
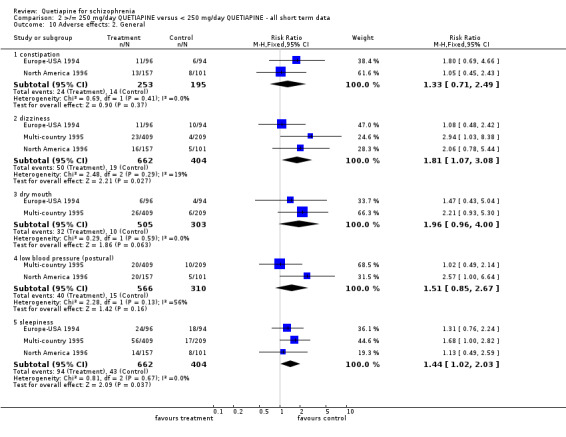
Comparison 2 >/= 250 mg/day QUETIAPINE versus < 250 mg/day QUETIAPINE ‐ all short term data, Outcome 10 Adverse effects: 2. General.
Comparison 3. QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: 1. Short‐term | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 any cause | 6 | 1624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 1.2 due to adverse events | 6 | 1624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.39, 0.76] |
| 1.3 due to treatment failure | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.71] |
| 2 Leaving the study early: 2. Medium‐term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 any cause | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.10] |
| 2.2 due to adverse events | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.15, 5.56] |
| 2.3 due to treatment failure | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.33] |
| 3 Global state: Average change (CGI, low score = good) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 short‐term | 3 | 762 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.00, 0.38] |
| 3.2 medium‐term | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.39, 0.77] |
| 4 Mental state: 1. No important improvement (BPRS or PANSS) ‐ short term only | 4 | 1247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| 5 Mental state: 2. Average change (low score = good) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 BPRS score ‐ short‐term | 1 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [‐3.58, 5.42] |
| 5.2 PANSS score ‐ short‐term | 2 | 455 | Mean Difference (IV, Fixed, 95% CI) | 1.92 [‐2.43, 6.28] |
| 5.3 PANSS score ‐ medium‐term | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐6.89 [‐27.27, 13.49] |
| 6 Mental state: 3. Average change in positive symptoms (low score = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 BPRS positive subscale ‐ short‐term | 1 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.30, 0.46] |
| 6.2 PANSS positive subscale ‐ short‐term | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐4.96 [‐10.53, 0.61] |
| 6.3 PANSS positive subscale ‐ medium‐term | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐7.05, 7.47] |
| 7 Mental state: 4. Average change in negative symptoms (low score = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 SANS ‐ short‐term | 1 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐0.16, 2.04] |
| 7.2 PANSS negative subscale ‐ short‐term | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐6.07 [‐10.85, ‐1.29] |
| 7.3 PANSS negative subscale ‐ medium‐term | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐7.85, 1.55] |
| 8 Adverse effects: 1. Movement disorders | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 patients receiving medications for extrapyramidal adverse effects | 4 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.39, 0.55] |
| 8.2 parkinsonism | 2 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.15, 0.33] |
| 8.3 akathisia | 4 | 1247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.19, 0.44] |
| 8.4 dystonia | 3 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.49] |
| 9 Adverse effects: 2. General | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 constipation ‐ short ‐term data | 3 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.77, 2.49] |
| 9.2 dizziness ‐ short‐term data | 3 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.68, 2.14] |
| 9.3 low blood pressure (postural) ‐ short‐term data | 3 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.60, 1.67] |
| 9.4 dry mouth ‐ short‐term data | 2 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.46, 5.57] |
| 9.5 dry mouth ‐ medium‐term data | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.12, 62.48] |
| 9.6 sleepiness ‐ short‐term data | 3 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.05, 2.16] |
| 9.7 sleepiness ‐ medium‐term data | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.06, 13.18] |
3.1. Analysis.
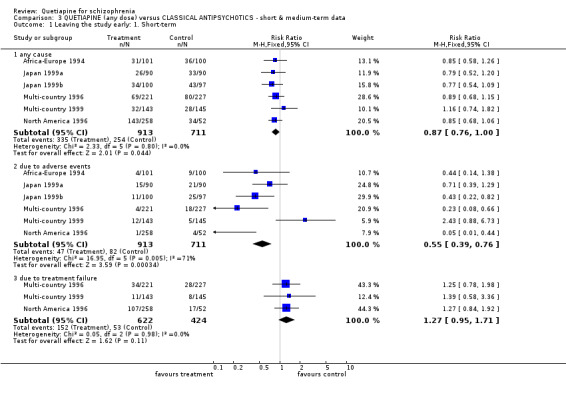
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 1 Leaving the study early: 1. Short‐term.
3.2. Analysis.
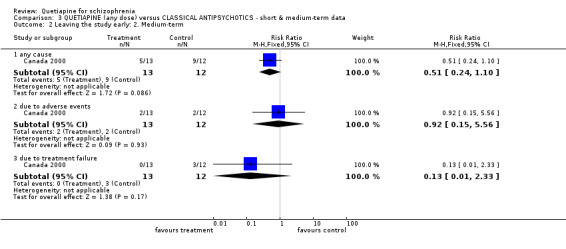
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 2 Leaving the study early: 2. Medium‐term.
3.3. Analysis.
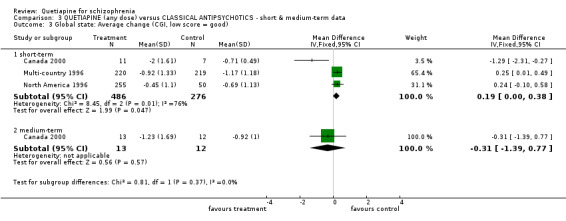
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 3 Global state: Average change (CGI, low score = good).
3.4. Analysis.
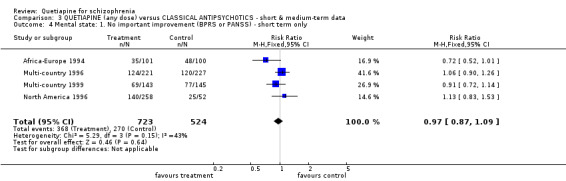
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 4 Mental state: 1. No important improvement (BPRS or PANSS) ‐ short term only.
3.5. Analysis.
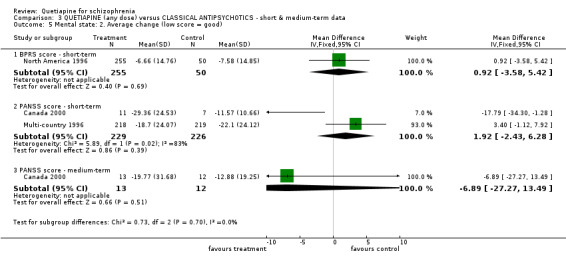
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 5 Mental state: 2. Average change (low score = good).
3.6. Analysis.
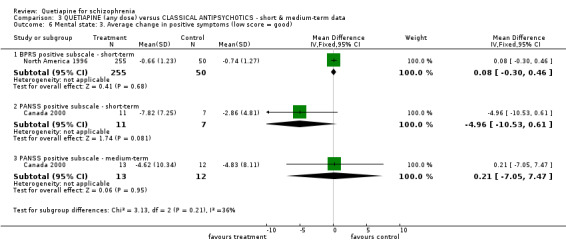
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 6 Mental state: 3. Average change in positive symptoms (low score = good).
3.7. Analysis.
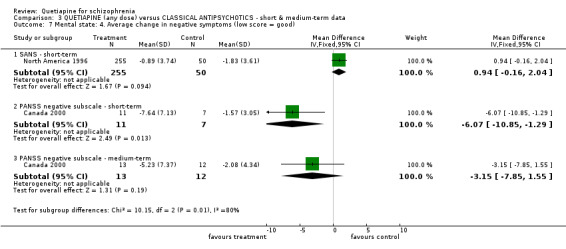
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 7 Mental state: 4. Average change in negative symptoms (low score = good).
3.8. Analysis.
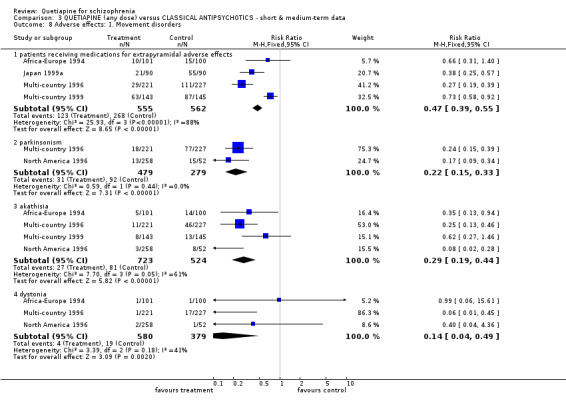
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 8 Adverse effects: 1. Movement disorders.
3.9. Analysis.
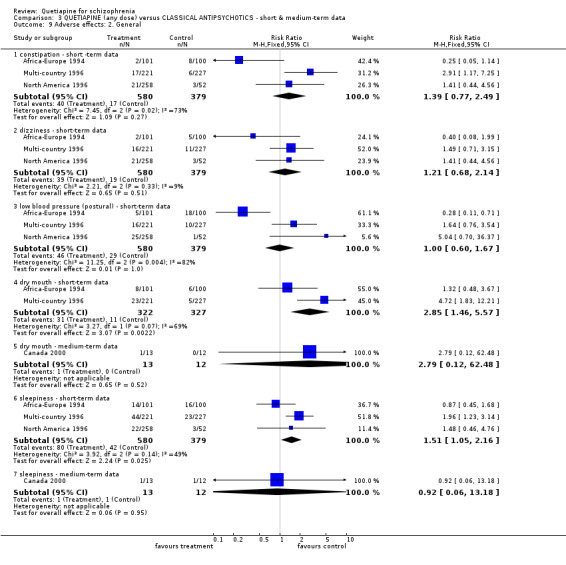
Comparison 3 QUETIAPINE (any dose) versus CLASSICAL ANTIPSYCHOTICS ‐ short & medium‐term data, Outcome 9 Adverse effects: 2. General.
Comparison 4. QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 any cause | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.20] |
| 1.2 due to adverse events | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.85, 3.37] |
| 1.3 due to treatment failure | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 2 Death | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.15, 52.84] |
| 3 Mental state: 1. Average change (PANSS, low score = good) | 1 | 637 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐1.95, 4.35] |
| 4 Mental state: 2. Average change in positive symptoms (PANSS positive subscale, low score = good)) | 1 | 639 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.18, 1.58] |
| 5 Mental state: 3. Average change in negative symptoms (PANSS negative subscale, low score = good) | 1 | 641 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.77, 1.37] |
| 6 Adverse effects: 1. Movement disorders | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 patients receiving medications for extrapyramidal adverse effects | 1 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.15, 0.49] |
| 6.2 patients with clinically significant extrapyramidal adverse effects | 1 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.91] |
| 7 Adverse effects: 2. General | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 dizziness | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.03, 3.32] |
| 7.2 dry mouth | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.18, 3.78] |
| 7.3 sleepiness | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.40, 2.93] |
4.1. Analysis.
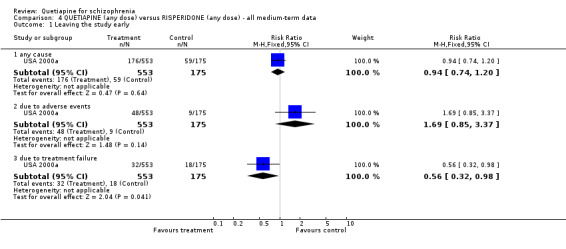
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 1 Leaving the study early.
4.2. Analysis.
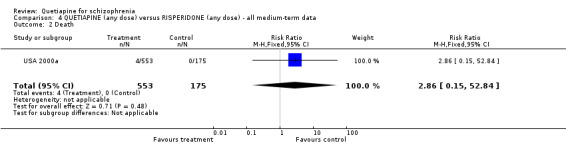
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 2 Death.
4.3. Analysis.
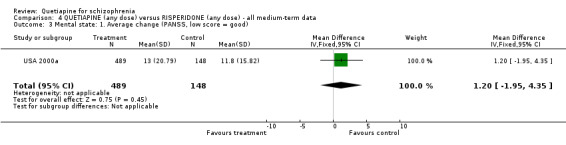
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 3 Mental state: 1. Average change (PANSS, low score = good).
4.4. Analysis.
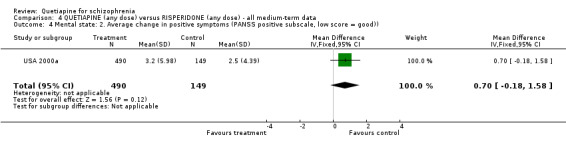
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 4 Mental state: 2. Average change in positive symptoms (PANSS positive subscale, low score = good)).
4.5. Analysis.
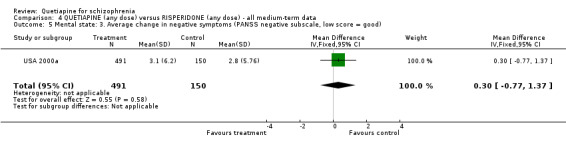
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 5 Mental state: 3. Average change in negative symptoms (PANSS negative subscale, low score = good).
4.6. Analysis.
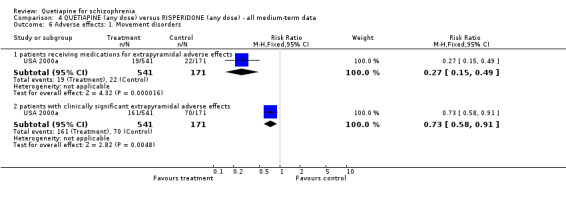
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 6 Adverse effects: 1. Movement disorders.
4.7. Analysis.
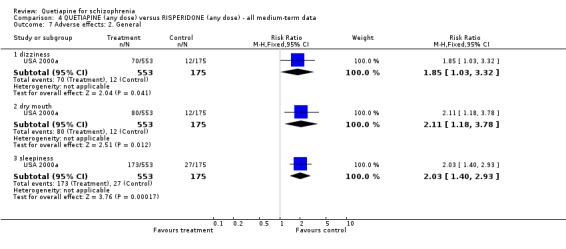
Comparison 4 QUETIAPINE (any dose) versus RISPERIDONE (any dose) ‐ all medium‐term data, Outcome 7 Adverse effects: 2. General.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Africa‐Europe 1994.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 6 weeks (1 day washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: BPRS* >/= 27, CGI >/= 4. N=201. Age: mean ˜33 years. Sex: M 129, F 72. | |
| Interventions | 1. Quetiapine: mean dose 407 mg/day. N=101. 2. Chlorpromazine: mean dose 384 mg/day. N=100. | |
| Outcomes | Global state: CGI. Mental state: BPRS, PANSS‐N. Adverse effects: AIMS, Barnes Akathesia Scale, SAS and specific symptom list. Leaving the study early. | |
| Notes | * 0‐6 scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Canada 2000.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 6 months (2 days washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). Inclusion criteria: no details. N=25. Age: mean ˜34 years. Sex: M 20, F 5. | |
| Interventions | 1. Quetiapine: modal dose 468 mg/day. N=13. 2. Haloperidol: modal dose 15.5 mg/day. N=12. | |
| Outcomes | Global state: CGI. Mental state: PANSS, PANSS‐P, PANSS‐N. Adverse effects: AIMS, SAS. Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Europe‐USA 1994.
| Methods | Allocation: randomised ‐ computer generated. Blindness: double‐blind ‐ no further details. Duration: 6 weeks (> 1 day washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: BPRS* >/=27, CGI >/=4. N=286. Age: mean ˜37 years. Sex: M 203, F 83. | |
| Interventions | 1. Quetiapine: mean dose 360 mg/day. N=96. 2. Quetiapine: mean dose 209 mg/day. N=94. 3. Placebo: N=96. | |
| Outcomes | Global state: CGI. Mental state: BPRS, BPRS positive, SANS, PANSS‐N. Adverse effects: AIMS, Barnes Akathisia Scale, SAS, specific side‐effects list. Leaving the study early. | |
| Notes | * 0‐6 scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Japan 1999a.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 8 weeks (no details of washout period). | |
| Participants | Diagnosis: schizophrenia (DSM‐IV or ICD‐10). Inclusion criteria: no details. N=180. Age: mean ˜45 years. Sex: M 112, F 68. | |
| Interventions | 1. Quetiapine: mean dose 215 mg/day. N=90. 2. Mosapramine: mean dose 103 mg/day. N=90. | |
| Outcomes | Mental state: BPRS*, PANSS, PANSS‐P, PANSS‐N. Adverse effects: extrapyramidal. | |
| Notes | * no details of scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Japan 1999b.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 8 weeks (no details of washout period). | |
| Participants | Diagnosis: schizophrenia (DSM‐IV or ICD‐10). Inclusion criteria: no details. N=197. Age: mean ˜45 years. Sex: M 129, F 68. | |
| Interventions | 1. Quetiapine: mean dose 226 mg/day. N=100. 2. Haloperidol: mean dose 6.7 mg/day. N=97. | |
| Outcomes | Mental state: BPRS*, PANSS, PANSS‐P, PANSS‐N. Adverse effects: EPS‐related events. Leaving the study early. | |
| Notes | * no details of scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Multi‐country 1995.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 6 weeks (> 2 days washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: BPRS* >/= 27, CGI >/= 4. N=618. Age: mean ˜ 26 years. Sex: M 409, F 209. History: mean age at 1st treatment ˜25 years. | |
| Interventions | 1. Quetiapine: dose 225 mg twice daily. N=200. 2. Quetiapine: dose 150 mg 3 times daily. N=209. 3. Quetiapine: dose 25 mg twice daily. N=209. | |
| Outcomes | Death. Global state: CGI. Mental state: BPRS, BPRS ‐ positive, SANS. Adverse effects: AIMS, SAS, specific side‐effects list. Leaving the study early. | |
| Notes | * 0‐6 scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Multi‐country 1996.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 6 weeks (2 days washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: PANSS >/= 60, CGI >/= 4. N=448. Age: mean ˜ 37 years. Sex: M 305, F 143. | |
| Interventions | 1. Quetiapine: mean dose 455 mg/day. N=221. 2. Haloperidol: mean dose 8 mg/day. N=227. | |
| Outcomes | Global state: CGI. Mental state: PANSS. Adverse effects: AIMS, SAS. Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Multi‐country 1999.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 8 weeks (no details of washout period). | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). Inclusion criteria: persistent positive symptoms whilst previously taking 20 mg/day of fluphenazine for 4 weeks, a reduction of PANSS total score of < 30% and PANSS‐P >/=15, CGI >/=3. N=288. Age: mean ˜39 years. Sex: M 204, F 84. | |
| Interventions | 1. Quetiapine: 600 mg/day. N=143. 2. Haloperidol: 20 mg/day. N=145. | |
| Outcomes | Global state: CGI. Mental state: BPRS, PANSS, PANSS‐P, PANSS‐N. Adverse effects: EPS‐related adverse events, SAS. Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
North America 1996.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 6 weeks (7 days washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: BPRS* >/=27; CGI >/=4. N=361. Age: mean ˜ 37 years. Sex: M 274, F 87. | |
| Interventions | 1. Quetiapine: fixed dose 75 mg/day. N=53. 2. Quetiapine: fixed dose 150 mg/day. N=48. 3. Quetiapine: fixed dose 300 mg/day. N=52. 4. Quetiapine: fixed dose 600 mg/day.N=51. 5. Quetiapine: fixed dose 750 mg/day. N=54. 6. Haloperidol: fixed dose 12 mg/day. N=52. 7. Placebo: N=51. | |
| Outcomes | Global state: CGI. Mental state: BPRS, modified SANS. Adverse effects: AIMS, modified SAS, specific side‐effects list. Leaving the study early. | |
| Notes | * 0‐6 scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
USA 1990.
| Methods | Allocation: randomised, ratio of 2:1 ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 3 weeks (2 days washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: BPRS** >/=30, CGI >/=3. N=12. Age: mean ˜34 years. Sex: unknown. History: mean age at 1st treatment ˜22‐23 years. | |
| Interventions | 1. Quetiapine: mean dose 250 mg/day. N=8. 2. Placebo: N=4. | |
| Outcomes | Global state: CGI. Mental state: BPRS, BPRS ‐ positive, BPRS ‐ negative. Adverse effects: specific side‐effects list. Leaving the study. | |
| Notes | **1‐7 scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
USA 1994.
| Methods | Allocation: randomised ‐ no further details. Blindness: double‐blind ‐ no further details. Duration: 6 weeks (2‐10 days washout). | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Inclusion criteria: BPRS**>/=45, CGI >/=4. N=109. Age: mean ˜ 36 years. Sex: M 98, F11. History: mean age at 1st treatment 21 years. | |
| Interventions | 1. Quetiapine: mean dose 307 mg/day. N=54. 2. Placebo: N=55. | |
| Outcomes | Death. Global state: CGI. Mental state: BPRS, modified SANS. Adverse effects: AIMS, modified SAS, specific side‐effects list. Leaving the study early. | |
| Notes | **1‐7 scoring system. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
USA 2000a.
| Methods | Allocation: randomised at the ratio of 3:1 ‐ no further details. Blindness: no (open‐label). Duration: 16 weeks (previous antipsychotics discontinued within 1 month after trial entry). | |
| Participants | Diagnosis: 63.1% of quetiapine and 66.9% of risperidone groups (out‐ patients only) diagnosed as schizophrenia or schizoaffective disorder. N= 728 Age: 18 years or more. Sex: M 372, F 356. | |
| Interventions | 1. Quetiapine: mean dose 253.9 mg/day. N=553. 2. Risperidone: mean dose 4.4 mg/day. N=175. | |
| Outcomes | Death. Global state: CGI. Mental state: PANSS, PANSS positive , PANSS negative. Adverse effects: specific side‐effects list. Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
AIMS = Abnormal Involuntary Movement Scale BPRS = Brief Psychiatric Rating Scale CGI = Clinical Global Improvement DSM‐III‐R = Diagnostic and Statistical Manual, 3‐rd revised edition F= female M = male N= total number PANSS = Positive and negative syndrome scale SANS = Scale for the Assessment of Negative Symptoms SAS = Simpson and Angus Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Faustman 1996 | Allocation: not randomised, case series. |
| Gefvert 1995 (029) | Allocation: not randomised, case series. |
| Kufferle 1997 | Allocation: unclear, but analysed as case‐control. Participants: people with schizophrenia. Interventions: quetiapine versus clozapine versus haloperidol. Outcomes: no usable data. |
| Shimada 1994 | Allocation: not described. Participants: healthy male volunteers. |
| Tauscher 1997 | Allocation: not randomised, case series. |
| Thyrum 1996 (017) | Allocation: not randomised, controlled study. |
| Wetzel 1995 (005) | Allocation: not randomised, case series. |
Characteristics of studies awaiting assessment [ordered by study ID]
Germany 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
Italy 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
Japan 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
Netherlands 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
Turkey 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
USA 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
USA 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting assessment |
Characteristics of ongoing studies [ordered by study ID]
UK 2003.
| Trial name or title | Unknown. |
| Methods | |
| Participants | Diagnosis: people with schizophrenia. N projected ˜ 240, current recruitment ˜120. |
| Interventions | 1. Quetiapine: dose unknown. 2. Risperidone: dose unknown. |
| Outcomes | Unknown. |
| Starting date | Not known. |
| Contact information | Unknown. |
| Notes | Allocation: randomised. Supported by a grant from AstraZeneca Pharmaceuticals. Unclear if recruitment will continue. |
USA 1998.
| Trial name or title | Unknown. |
| Methods | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). Inclusion criteria: hospitalised 2+ during past 2 years; hospitalised 1+ and admitted 1+ to crisis shelter; or admitted 3+ times to crisis shelter within past year. |
| Interventions | 1. Quetiapine: up to 800 mg/day. N ˜182. 2. Usual care. N ˜182. |
| Outcomes | Service use. Quality of life. Psychiatric assessment. Safety assessment. Neurologic assessment. Medication compliance. Medication satisfaction. |
| Starting date | Not yet known. |
| Contact information | Dr IW Rak. AstraZeneca Pharmaceuticals 1800 Concord Pike PO Box L15437 Wilmington, DE 19850‐5437, USA. |
| Notes | Allocation: randomised. Supported by a grant from AstraZeneca Pharmaceuticals, Wilmington, DE 19850, USA. |
USA 2000b.
| Trial name or title | Comparative effectiveness of antipsychotic medications in patients with schizophrenia. |
| Methods | |
| Participants | Diagnosis: schizophrenia. |
| Interventions | 1. Clozapine. 2. Fluphenazine decanoate. 3. Olanzapine. 4. Perphenazine. 5. Quetiapine. 6. Risperidone. 7. Ziprasidone. |
| Outcomes | General: CGI. Mental state: PANSS. Adverse effects: AIMS, BAS, SAS. Economic/financial outcomes: cost‐effectiveness. Quality of life. Compliance: medication adherence. |
| Starting date | Not yet known. |
| Contact information | Dr. Jeffrey Lieberman |
| Notes |
Contributions of authors
Manit Srisurapanont ‐ protocol development, searching, study selection, data extraction, analysis, writing of final report and review maintenance.
Narong Maneeton ‐ study selection, data extraction, analysis, writing of final report.
Benjaruk Maneeton ‐ dispute resolution and writing of final report.
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Schizophrenia Group General Fund, UK.
Health Systems Research Institute, Thailand.
Declarations of interest
In the past five years Manit Srisurapanont has received honoraria and support for attending national and international scientific meetings from AstraZeneca (Thailand), Eli Lilly Asia, Inc. (Thailand), GlaxoSmithKline (Thailand), Janssen‐Cilag (Thailand), Servier (Thailand) and Solvay Pharmaceuticals (Thailand). Narong Maneeton and Benjaluk Maneeton have received similar support for attending national scientific meetings from GlaxoSmithKline (Thailand) and Janssen‐Cilag (Thailand).
Edited (no change to conclusions)
References
References to studies included in this review
Africa‐Europe 1994 {published data only}
- Link C, Smith A, Miller B, Ryan J. A multicentre, double‐blind, controlled comparison of 'Seroquel' and chlorpromazine in acute exacerbation of schizophrenia. 1994 International Conference on Schizophrenia, Vancouver, BC, Canada. Aug 21‐24, 1994.
- Link C, Smith A, Miller B, Ryan J, Study Group. A multicentre, double‐blind, controlled comparison of 'Seroquel' and chlorpromazine in the treatment of hospitalised patients with acute exacerbation of subchronic and chronic schizophrenia. European Neuropsychopharmacology. 1994; Vol. 4:385.
- Peuskens J, Link CGG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatrica Scandinavica 1997;96:265‐73. [DOI] [PubMed] [Google Scholar]
Canada 2000 {unpublished data only}
- Purdon S, Malla A, Labelle A, Litt W. Long‐term treatment of quetiapine improves cognitive function in schizophrenia: a double‐blind study. Schizophrenia Winter Workshop, Davos, Switzerland 2000.
- Purdon SE, Malla A, Labelle A, Lit W. Neuropsychological change in patients with schizophrenia after treatment with quetiapine or haloperidol. Journal of Psychiatry and Neuroscience 2001;26(2):137‐49. [PMC free article] [PubMed] [Google Scholar]
Europe‐USA 1994 {published data only}
- Hirsch S, Arvanitis L, Miller B, Smith A, Study Group. A multicentre, double‐blind, placebo‐controlled comparison of low and high dosage regimens of 'Seroquel' in the treatment of hospitalised patients with acute exacerbation of subchronic or chronic schizophrenia. European Neuropsychopharmacology 1994;4:384‐5. [Google Scholar]
- Link CGG, Arvanitis L, Study Group. 'Seroquel' treatment of hospitalised patients with acute exacerbation of subchronic or chronic schizophrenia. A multicentre, placebo‐controlled, double‐blind comparison of low and high dosage regimens. European Neuropsychopharmacology 1995;5:346‐7. [Google Scholar]
- Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CGG, the Seroquel Study Group. Quetiapine in patients with schizophrenia. A high‐ and low‐dose double‐blind comparison with placebo. Archives of General Psychiatry 1997;54:549‐57. [DOI] [PubMed] [Google Scholar]
Japan 1999a {unpublished data only}
- Kudo Y, Nomura J, Ikawa G, Nakajima T, Saito M, Sakai T, Yoshimasu F, Hanada M, Kuroda S, Watanabe M, Yamawaki S, Tashiro N, Nakane Y, Tanaka M. Clinical evaluation of quetiapine fumarate for the treatment of schizophrenia: a double‐blind controlled study using mosapramine hydrochloride as a control. Rinsyo Iyaku 2000;16(12):1807‐42. [Google Scholar]
- Kudo Y, Nomura J, Ikawa G, Nakajima T, Saito M, Sakai T, Yoshimasu F, Hanada M, Kuroda S, Watanabe S, Yamawaki S, Tahiro N, Nakame Y, Tanaka M, on behalf of the ICI 204 636 clinical evaluation group. Clinical trial of quetiapine in schizophrenia ‐ efficacy and tolerability of quetiapine: a comparative double‐blind study with masopramine in schizophrenic patients. 1999 Annual Meeting of the World Psychiatric Association, Hamburg, Germany 1999.
Japan 1999b {unpublished data only}
- Inada T, Marasaki M. The drug‐induced extrapyramidal symptoms scale: differentiation of extrapyramidal symptom profiles and identification of favourable extrapyramidal symptom profile of quetiapine in Japanese patients. European Neuropsychopharmacology. 2001; Vol. 11 (Suppl 3):S265.
- Murasaki M, Koyama T, Yamauchi T, Yagi MG, Ushijima S, Kamijima K. Clinical evaluation of quetiapine in schizophrenia ‐ efficacy and tolerability of quetiapine compared with haloperidol in patients with schizophrenia. 1999 Annual Meeting of the World Psychiatric Association 1999.
Multi‐country 1995 {published data only (unpublished sought but not used)}
- Barzega G, Bogetto F, Maina G, Ravizza L. [Efficacia e tollerabilita dell' antipsychotico atipico quetiapina: uno studio in doppio cieco]. Minerva Psichiatrica 1999;40:297‐305. [Google Scholar]
- Barzega G, Bogetto F, Maina G, Ravizza L. Quetiapine in schizophrenic patients: a high‐ and low‐dose double‐blind comparison. European Journal of Psychiatry 2000;14(4):221‐32. [Google Scholar]
- Fleischhacker WW, Link CCG, Horne B. A multicentre, double‐blind, randomised comparison of dose and dose regimen of 'Seroquel' in the treatment of patients with schizophrenia. 34th Annual Meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico. Dec 11‐15, 1995.
- King DJ, Link CGC. 'Seroquel' (ICI 204636): an atypical antipsychotic ‐ results from Phase III. European Neuropsychopharmacology 1996;6(Suppl 3):202. [Google Scholar]
- King DJ, Link CGG. Seroquel (ICI 204636): an atypical antipsychotic results from Phase III. XXth Collegium Internationale Neuro‐psychopharmacologicum, Melbourne, Australia. June 23‐27, 1996.
- King DJ, Link CGG, Kowalcyk B. A comparison of bid and tid dose regimens of quetiapine (Seroquel) in the treatment of schizophrenia. Psychopharmacology (Berl) 1998;137(2):139‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reuther E, Degner D. Results of a multicenter study comparing the dosage and the dosage regimen of ICI 204,636 ('Seroquel'). Nervenarzt 1996;67(Suppl 1):S192. [Google Scholar]
Multi‐country 1996 {unpublished data only}
- Copolov DL, Link CGG, Kowalcyk B. A multicentre, double‐blind, randomized comparison of quetiapine (ICI 204,636, 'Seroquel') and haloperidol in schizophrenia. Psychological Medicine 2000;30(1):95‐106. [DOI] [PubMed] [Google Scholar]
- Fleishhacker W, Link C, Hurst B. ICI 204,636 (Seroquel) ‐ a putative new antipsychotic: results from Phase III trials. Schizophrenia Research 1996;18:32. [Google Scholar]
- Hellewell JSE, Hurst BC, Link CGG. Switching from a conventional antipsychotic (haloperidol) to an atypical antipsychotic ('Seroquel'‐ICI 204,636). European Neuropsychopharmacology 1996;6(Suppl 4):123. [Google Scholar]
- Link C, Farrow L. 'Seroquel'™ (ICI 204, 636) EPS and prolactin ‐ comparison with haloperidol. 10th World Congress of Psychiatry, Madrid, Spain. Aug 23‐28, 1996.
- Zeneca Pharmaceuticals. Data on file supporting the claims: study 0014 (quetiapine vs haloperidol).
Multi‐country 1999 {unpublished data only}
- Buckley PF, Goldstein JM, Emsley RA. Comparison of quetiapine and haloperidol in treatment‐resistant schizophrenia. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. 2001.
- Emsley RA, Bailey P, Jones AM, Raniwalla JTI. Efficacy of quetiapine fumarate in partial responders. 1999 Annual Meeting of the American Psychiatry Association, Washington, DC, USA. 1999.
- Emsley RA, Jones AM. Treatment of depressive symptoms in partially refractory schizophrenia: efficacy of quetiapine versus haloperidol. European Neuropsychopharmacology 2001;11(3):S264. [Google Scholar]
- Emsley RA, Raniwalla J, Bailey P, Jones AM. [Efficacy and tolerability of 'Seroquel' compared with haloperidol in schizophrenic patients partially responsive to conventional antipsychotic treatment]. European Neuropsychopharmacology. 1999; Vol. 9, issue Suppl 5:S267.
- Emsley RA, Raniwalla J, Bailey P, Jones AM. Efficacy and tolerability of 'Seroquel' compared with haloperidol in schizophrenic patients partially responsive to conventional antipsychotic treatment. 1999 Congress of the European College of Neuropsychopharmacology, London, UK. 1999.
- Emsley RA, Raniwalla J, Bailey P, Jones AM, on behalf of the PRIZE Study Group. Efficacy and tolerability of 'Seroquel' compared with haloperidol in schizophrenic patients partially responsive to conventional antipsychotic treatment. 1999 World Psychiatric Association Annual Meeting, Hamburg, Germany 1999.
- Emsley RA, Raniwalla J, Bailey PJ, Jones AM, on behalf of the PRIZE Study Group. A comparison of the effects of quetiapine ('Seroquel') and haloperidol in schizophrenic patients with a history of and a demonstrated, partial response to conventional antipsychotic treatment. International Clinical Psychopharmacology 2000;15(3):121‐31. [DOI] [PubMed] [Google Scholar]
- Emsley RA, Raniwalla J, Jones AM on behalf of the PRIZE Study Group. Efficacy of 'Seroquel' in treating all of the positive and negative symptoms of schizophrenia. 2000 Schizophrenia Winter Workshop, Davos, Switzerland 2000.
- Goldstein JM, Buckley P. Comparison of the effects of quetiapine and haloperidol in a cohort of patients with treatment‐resistant schizophrenia. 39th Annual Meeting of the American College of Neuropsychopharmacology, 2000 Dec 10‐14; San Juan, Puerto Rico. 2000.
- Nasrallah HA, Rennie P, Altman C. Patterns of response in patients with schizophrenia partially responsive to fluphenazine and switched to quetiapine or haloperidol. European Neuropsychopharmacology. 2001; Vol. 11 (Suppl 3):S262.
- Weiden PJ, Ashworth P. Switching from fluphenazine to quetiapine ameliorates extrapyramidal symptoms and neuroendocrine side effects in patients with schizophrenia. European Neuropsychopharmacology. 2001; Vol. 11 (Suppl 3):S262.
North America 1996 {published data only}
- Addington DE, Arvanitis LA. 'Seroquel' (quetiapine): an atypical antipsychotic ‐ results from a multiple fixed dose, placebo‐controlled study. Schizophrenia 1996 ‐ Breaking Down the Barriers, 4th International Conference, Vancouver, BC, Canada. Oct 6‐9, 1996.
- Arvanitis LA, Miller BG. 'Seroquel' (ICI 204,636): an atypical antipsychotic: results from a multiple fixed dose, placebo‐controlled study. European Neuropsychopharmacology 1996;6(Suppl 3):148. [Google Scholar]
- Arvanitis LA, Miller BG. Quetiapine, an atypical antipsychotic ‐ results from a multiple fixed dose, placebo‐controlled study. 149th Annual Meeting of American Psychiatric Association, New York, NY, USA. May 4‐9, 1996.
- Arvanitis LA, Miller BG and Study Group. ICI 204,636, an atypical antipsychotic: results from a multiple fixed‐dose, placebo‐controlled trial. Pyschopharmacology Bulletin 1966;32:391. [Google Scholar]
- Arvanitis LA, Miller BG, Kowalcyk BB. Efficacy of 'Seroquel' (Quetiapine Fumarate) in affective symptoms of schizophrenia. 36th Annual Meeting of the American College of Neuropsychopharmacology. 1997.
- Arvanitis LA, Miller BG, the Seroquel Trial 13 Study Group. Multiple fixed doses of 'Seroquel' (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biological Psychiatry 1997;42:233‐46. [DOI] [PubMed] [Google Scholar]
- Borison RL, Arvanitis LA, Miller BG. A comparison of five fixed doses of 'Seroquel' (ICI 204,636) with haloperidol and placebo in patients with schizophrenia. Schizophrenia Research 1996;18:132. [Google Scholar]
- Borison RL, Arvanitis LA, Miller BG. A multiple fixed‐dose, placebo‐controlled trial with 'Seroquel' ‐ an atypical antipsychotic. Biological Psychiatry 1996;39:597‐8. [Google Scholar]
- Cantillon M. Quetiapine fumarate reduces aggression. 11th Annual Meeting of the American Association for Geriatric Psychiatry, San Diego, California, USA. 1998.
- Cantillon M, Goldstein JM. Efficacy of quetiapine fumarate in affective symptoms of schizophrenia. 1998 Annual Meeting of the American Psychiatric Association, Toronto, ON, Canada. May 30‐June 4, 1998.
- Cantillon M, Goldstein JM. Quetiapine fumarate reduces aggression and hostility in patients with schizophrenia. 1998 Annual Meeting of the American Psychiatric Association, Toronto, ON, Canada. May 30‐June 4, 1998.
- Hong WW, Arvanitis LA, Miller BG. 'Seroquel' (ICI 204,636): not different from placebo for EPS or prolactin. Biological Psychiatry 1996;39:598. [Google Scholar]
- Hong WW, Arvanitis LA, Miller BG. 'Seroquel' (ICI 204‐636) does not differ from placebo in the incidence of EPS or effect on plasma prolactin. European Neuropsychopharmacology 1996;6(Suppl 3):171. [Google Scholar]
- Hong WW, Arvanitis LA, Miller BG. Quetiapine does not differ from placebo in the incidence of extrapyramidal syndrome or effect on plasma prolactin. 149th Annual Meeting of the American Psychiatric Association, New York, NY, USA. May 4‐9, 1996.
- Hong WW, Miller BG, Arvanitis LA. The atypical profile of 'Seroquel' (ICI 204,636) is supported by the lack of sustained elevation of plasma prolactin in schizophrenic patients. 34th Annual Meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico. Dec 11‐15, 1995.
USA 1990 {published data only}
- Fabre L, Slotnick V, Jones V, Murray G, Malick J. ICI 204,636, a novel atypical antipsychotic: early indication for safety and efficacy in man. 17th Congress of Collegium International Neuro‐Psychopharmacologicum, Kyoto, Japan. Sep 10‐14, 1990.
- Fabre LF, Arvanitis L, Pultz J, Jones VM, Malick JB, Slotnick VB. ICI 204,636, a novel, atypical antipsychotic ‐ early indication of safety and efficacy in patients with chronic and subchronic schizophrenia. Clinical Therapeutics 1995;17:366‐78. [DOI] [PubMed] [Google Scholar]
USA 1994 {published and unpublished data}
- Borison RL, Arvanitis LA, Miller BG, Study Group. ICI 204,636, an atypical antipsychotic ‐ efficacy and safety in a multicenter, placebo‐controlled trial in patients with schizophrenia. Journal of Clinical Psychopharmacology 1996;16:158‐69. [DOI] [PubMed] [Google Scholar]
- Link C, Arvanitis L, Miller B, Fennimore J, Study Group. A multicentre, placebo‐controlled double‐blind evaluation of 'Seroquel' in hospitalised patients with acute exacerbation of chronic and subchronic schizophrenia. European Neuropsychopharmacology 1994;4:385‐6. [Google Scholar]
- Link CGG, Arvanitis L, Study Group. 'Seroquel' treatment of hospitalised patients with acute exacerbation of chronic and subchronic schizophrenia. A multicentre, placebo‐controlled, double‐blind study. European Neuropsychopharmacology 1995;5:346. [Google Scholar]
USA 2000a {published and unpublished data}
- Mullen J. A health economic evaluation of quetiapine compared with risperidone: a supplement analysis of the QUEST Trial. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000 Dec 10‐14; San Juan; Puerto Rico. 2000.
- Mullen J. Health economic evaluation of quetiapine and risperidone in the QUEST Trial. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. 2001.
- Mullen J, Jibson JD, Sweitzer D, for the QUEST Study Group. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizohrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clinical Therapeutics 2001;23:1839‐54. [DOI] [PubMed] [Google Scholar]
- Tandon R. Quetiapine and risperidone in outpatients with schizophrenia: subanalysis of the QUEST Trial. 7th World Congress of Biological Psychiatry; 2001 Jul 1‐6; Berlin, Germany. 2001.
- Tandon R. Quetiapine and risperidone in outpatients with schizophrenia: subanalysis of the QUEST trial. Annuan Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. 2001.
References to studies excluded from this review
Faustman 1996 {published and unpublished data}
- Faustman WO, Ringo DL, Lauriello J, Lim KO, Pfefferbaum A. Effects of 'Seroquel' (quetiapine) on platelet serotonin‐2 binding in schizophrenia [letter]. Journal of Clinical Psychopharmacology 1996;16:464‐6. [DOI] [PubMed] [Google Scholar]
- Faustman WO, Ringo DL, Lauriello J, Lim KO, Pfefferbaum A, Bardgett ME, et al. Effects of 'Seroquel' (ICI 204,636) on platelet serotonin‐2 binding in schizophrenia. Schizophrenia Research 1995;15:149. [DOI] [PubMed] [Google Scholar]
Gefvert 1995 (029) {published data only}
- Gefvert O, Lindström LH, Langström B, Bergström M, Lundberg T, Yates RA. Time course for dopamine and serotonin receptor occupancy in the brain of schizophrenic patients following dosing with 150 mg 'Seroquel' tid. European Neuropsychopharmacology 1996;6(Suppl 3):74. [Google Scholar]
- Gefvert O, Lindström LH, Langström B, Bergström M, Lundberg T, Yates RA. Time course for dopamine and serotonin receptor occupancy in the brain of schizophrenic patients following dosing with 150 mg 'Seroquel' tid. Schizophrenia Research 1996;18:139‐40. [Google Scholar]
- Gefvert O, Lindström LH, Langström B, Bergström M, Lundberg T, Yates RA, Larsson SD, Tuersley MD. Time course for dopamine and serotonin receptor occupancy in the brain of schizophrenic patients following dosing with 150 mg 'Seroquel' (ICI 204,636) tid. 34th Annual Meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico. Dec 11‐15, 1995.
- Gefvert O, Lindström LH, Langström B, Bergström M, Lundberg T, Yates RA, Larsson SD, Tuersley MD. Time course of dopamine and serotonin receptor occupancy in the brain of schizophrenic patients following dosing with 150 mg 'Seroquel' tid. European Neuropsychopharmacology 1995;5:347. [Google Scholar]
Kufferle 1997 {published data only}
- Kufferle B, Tauscher J, Asenbaum S, Vesely C, Podreka I, Bruke T, Kasper S. IBZM SPECT imaging of striatal dopamine‐2 receptors in psychotic patients treated with the novel antipsychotic substance quetiapine in comparison to clozapine and haloperidol. Psychopharmacology 1997;133:323‐8. [DOI] [PubMed] [Google Scholar]
Shimada 1994 {published data only (unpublished sought but not used)}
- Shimada E, Murasaki M, Miura S, Sadakuni F, Yoshimoto W. A Phase I study in healthy volunteers of ICI 204,636, a novel neuroleptic agent. Canadian Journal of Physiology and Pharmacology 1994;72(Suppl 1):445. [Google Scholar]
Tauscher 1997 {published data only}
- Tauscher J, Kufferle B, Asenbaum S, Brucke T, Kasper S. Previous treatment as a confounding variable in studies with novel antipsychotics: two cases of high dopamine‐2 receptor occupancy with quetiapine. Psychopharmacology 1997;133:102‐5. [DOI] [PubMed] [Google Scholar]
Thyrum 1996 (017) {published data only (unpublished sought but not used)}
- Thyrum PT, Jaskiw G, Fuller M, Wongs JYW, Ewing BJ, Yeh C. Multiple‐dose pharmacokinetics of ICI 204,636 in elderly patients with selected psychotic disorders. Psychopharmacology Bulletin 1996;32:524. [Google Scholar]
Wetzel 1995 (005) {published data only}
- Benkert O, Wetzel H, Hillert A. Does 5‐HT2 blockade contribute to the effect of antipsychotic drugs? Focus on risperidone and 'Seroquel'. European Psychiatry 1994;9(Suppl1):53S. [Google Scholar]
- Fabre LF. A multicenter, open trial of ICI 204,636 in hospitalized patients with acute psychotic symptomatology. Schizophrenia Research 1993;9:273. [Google Scholar]
- Hain C, Schlegel S, Szegedi A, Wetzel H. Topographic EEG analysis in schizophrenic patients treated with ICI 204636, a new dibenzothiazepine with atypical antipsychotic properties. Pharmacopsychiatry 1993;26:156. [Google Scholar]
- Szegedi A, Wiesner J, Hillert A, Hammes E, Wetzel H, Benkert O. ICI 204636, a putative "atypical" antipsychotic, in the treatment of schizophrenia with positive symptomatology: an open clinical trial. Pharmacopsychiatry 1993;26:197. [Google Scholar]
- Wetzel H, Szegedi A, Hain C, Wiesner J, Schlegel S, Benkert O. Seroquel (ICI 204 636), a putative "atypical" antipsychotic, in schizophrenia with positive symptomatology: results of an open clinical trial and changes of neuroendocrinological and EEG parameters. Psychopharmacology 1995;119:231‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Germany 2002 {unpublished data only}
- Lambert M, Mortiz S, Karow A, Krausz M, Naber D. Quality of life in the treatment of schizophrenia ‐ a randomized, open‐label comparison trial of amisulpride, olanzapine, quetiapine, risperidone and zotepine. Schizophrenia Research 2002;53(3 Suppl 1):176. [Google Scholar]
Italy 2002 {unpublished data only}
- Mauri MC, Steinhilber CPC, Laini V, Pavone F, Barale F. Olanzapine and quetiapine in the treatment of acute schizophrenia: a descriptive analysis. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S119. [Google Scholar]
Japan 2002 {unpublished data only}
- Mori K, Nagao M, Yamashita H, Yamawaki S. Effects of switching to atypical antipsychotics on memory in chronic schizophrenic patients. International Journal of Neuropsychopharmacology. 2002; Vol. 5, issue Suppl 1:S80.
- Nagao M, Mori K, Yamashita H, Yamawaki S. Switching to typical antipsychotics in chronic schizophrenic patients: prediction factors of efficacy. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S123. [Google Scholar]
Netherlands 2002 {unpublished data only}
- Bruggeman R, Knegtering H, Castelein S, Linde J, Bous J. Risperidone versus quetiapine: preliminary results of a comparative study on sexual dysfunctioning and prolactin elevation. Nordic Journal of Psychiatry 2002;56(2):14‐5. [Google Scholar]
Turkey 2001 {unpublished data only}
- Atmaca M, Kuloglu M, Tezcan E, Gecici O, Unal A, Firidin B. The comparison of quetiapine and haloperidol effects on serum prolactin in patients with schizophrenia. European Neuropsychopharmacology 2001;11(Suppl 3):S240. [Google Scholar]
USA 1999 {unpublished data only}
- Velligan DI. Neurocognitive advantages of quetiapine. Annual Meeting of the American Psychiatric Association, LA, USA. 2001.
- Velligan DI, Pultz J, Csernansky JG, Hoff AL, Mahurin R, Miller AL, Newcommer JW. Changes in cognitive function in quetiapine fumarate versus haloperidol. 1999 American Psychiatric Association Annual Meeting, Washington, DC, USA. 1999.
USA 2001 {unpublished data only}
- Lee M, Meltzer HY. Quetiapine is significantly superior to haloperidol and placebo in improving mood in patients with schizophrenia. 7th World Congress of Biological Psychiatry; 2001 Jul 1‐6; Berlin, Germany. 2001.
References to ongoing studies
UK 2003 {unpublished data only}
- Personal communication. Anonymous 2003.
USA 1998 {published data only}
- Hong WW, Rak IW, Ciuryla VT, Wilson AM, Kylstra JW, Meltzer HY, Carpenter Jr WT, Lehman A, Arvanitis LA. Medical‐claims databases in the design of a health‐outcomes comparison of quetiapine ('Seroquel') and usual‐care antipsychotic medication. Schizophrenia Research 1998;32:51‐8. [DOI] [PubMed] [Google Scholar]
USA 2000b {unpublished data only}
- Lieberman J, McEnvoy J, Stroup S. Protocol: comparative effectiveness of antipsychotic medications in patients with schizophrenia: revised in response to DSMB comments. National Institute of Mental Health 2000.
- Lieberman J, McEvoy J, Stroup S. Trial design summary: comparative effectiveness of antipsychotic medications in patients with schizophrenia: Draft. National Institute of Mental Health 2002.
- Lieberman JA, Schneider LS, McEvoy J, Pariot P, Stroup S, Adiao J, Lebowitz BD. Effectiveness trials of antipsychotic drugs. Annual Meeting of the American Psychiatry Association; 2001 May 5‐10; LA, USA.. 2001.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [QUE020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1984
- Andreasen NC. Scale for the assessment of negative symptoms (SANS). Iowa City: University of Iowa, 1984. [Google Scholar]
Barnes 1989
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD. The Cochrane Reviewers' Handbook. The Cochrane Library. Oxford: Update Software, 2002, issue 2.
Cook 1993
- Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, McIlroy W, Oxman AO. Should unpublished data be included in meta‐analyses?. JAMA 1993;269:2749‐53. [PubMed] [Google Scholar]
Duggan 1999
- Duggan L, Fenton M, Dardennes RM, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001359] [DOI] [Google Scholar]
Gilbody 2000
- Gilbody SM, Bagnall AM, Duggan L, Tuunainen A. Risperidone versus other atypical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002306.pub4] [DOI] [PubMed] [Google Scholar]
Goldstein 1993
- Goldstein JM, Litwin LC, Sutton EM, Malick JB. Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology 1993;112:293‐8. [DOI] [PubMed] [Google Scholar]
Goldstein 1995
- Goldstein JM. Preclinical tests that predict clozapine‐like atypical antipsychotic actions. In: Brunello N, Racagni G, Langer SZ, Mendlewicz J editor(s). Critical issues in the treatment of schizophrenia. International Academy of Biomedical Drug Research. Vol. 10, Basel: Darger, 1995:95‐101. [Google Scholar]
Green 2003
- Green AI, Canuso CM, Brenner MJ, Wojcik JD. Detection and management of comorbidity in patients with schizophrenia. Psychiatric Clinic of North America 2003;26:115‐39. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy Q. ECDEU assessment manual for psychopharmacology. Publication ADM 76‐338. Rockville, MD: US Department of Health, Education and Welfare, 1976. [Google Scholar]
Hardon 1996
- Hardon DC, Baker D, Hodges JS, Hicks N. Rating the quality of evidence for clinical practice guidelines. Journal of Clinical Epidemiology 1996;49:749‐54. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch 1996
- Hirsch SR, Link CGG, Goldstein JM, Arvanitis LA. ICI 204,636: a new atypical antipsychotic drug. British Journal of Psychiatry 1996;168(Suppl 29):45‐56. [PubMed] [Google Scholar]
Hunter 2003
- Hunter RH, Joy CB, Kennedy E, Gilbody SM, Song F. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000440] [DOI] [PubMed] [Google Scholar]
Kane 1988
- Kane J, Honigfeld G, Singer J, Meltzer HY. Clozapine for the treatment‐resistant schizophrenia: a double‐blind comparison with chlorpromazine/benztropine. Archives of General Psychiatry 1988;45:789‐96. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kerwin 1994
- Kerwin RW. The new atypical antipsychotics. British Journal of Psychiatry 1994;164:141‐8. [DOI] [PubMed] [Google Scholar]
Leucht 2003
- Leucht S, McGrath J, Kissling W. Lithium for schizophrenia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003834.pub2] [DOI] [PubMed] [Google Scholar]
Migler 1993
- Migler BM, Warawa EJ, Malick JB. Seroquel: behavioral profile of a potential atypical antipsychotic. Psychopharmacology 1993;112:299‐307. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
NICE 2002
- National Institute for Clinical Excellence. Guidance on the use of newer (atypical) antipsychotic drugs for the treatment of schizophrenia. NICE Technology Appraisal Guidance 2002; Vol. 43:1‐21.
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Peduzzi 2002
- Peduzzi P, Henderson W, Hartigan P, Lavori P. Analysis of randomized controlled trial. Epidemiological reviews 2002;24:26‐38. [DOI] [PubMed] [Google Scholar]
Roland 1998
- Roland M, Torgerson DJ. What are pragmatic trials?. BMJ 1998;316(7127):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rummel 2003
- Rummel C, Hamann J, Kissling W, Leucht S. New generation antipsychotics for first episode schizophrenia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004410.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saller 1993
- Saller CF, Salama AI. Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology 1993;112:285‐92. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;Suppl 212:11‐9. [DOI] [PubMed] [Google Scholar]
Wahlbeck 2000
- Wahlbeck K, Cheine M, Essali MA, Rezk E. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000059.pub2] [DOI] [PubMed] [Google Scholar]


