Abstract
Background
There is a clear link between stopping antipsychotic medications and a relapse of psychotic symptoms. A series of long‐acting intra‐muscular preparations has been developed since the 1960s in the hope of reducing the frequency of relapse and, hence, overall disability. These depot preparations, active for weeks at a time, are frequently used for those who find taking oral medication on a regular basis difficult or unacceptable. It has, however, been a consistent concern that any reduction in relapse rate afforded by depot preparations may be offset by an increase in adverse effects such as drug‐induced movement disorders.
Objectives
To compare zuclopenthixol decanoate to oral zuclopenthixol and other antipsychotic preparations for the treatment of schizophrenia and similar serious mental illness.
Search methods
Electronic searches of Biological Abstracts (1982‐1998), CINAHL (1982‐1998), The Cochrane Library (Issue 2, 1998), The Cochrane Schizophrenia Group's Register (April 1998), EMBASE (1980‐1998), MEDLINE (1966‐1998), and PsycLIT (1974‐1998) were searched. References of all eligible studies were searched for further trials. The manufacturer of zuclopenthixol was contacted.
Selection criteria
Inclusion criteria were that the clinical study should be randomised, focus on people with schizophrenia or other serious mental illness with psychotic symptoms, and compare the use of zuclopenthixol decanoate to oral zuclopenthixol or other antipsychotic preparations.
Data collection and analysis
Data was extracted independently by two reviewers (EC, MF). Authors of trials were contacted for additional and missing data. Odds ratios (ORs) and 95% confidence intervals (CIs) of homogenous dichotomous data were calculated with the Peto method. Where possible the number needed to treat (NNT) and its 95% confidence interval was also calculated.
Main results
Four studies relating to zuclopenthixol decanoate were included. All compared zuclopenthixol decanoate with other depot preparations. Zuclopenthixol decanoate prevented or postponed relapses when compared to other depots (NNT 8, CI 5‐53). However, zuclopenthixol decanoate may induce more adverse effects (NNH 5, CI 3‐31) although it decreases need for anticholinergic medication when compared to a group of other depot preparations (NNT 9, CI 5‐38). For the risk of leaving the study early, there was also a trend for benefit to those allocated to zuclopenthixol decanoate. None of the studies reported outcomes on service utilisation, costs, or quality of life.
Authors' conclusions
Choice of which depot to use must always take into account clinical judgement and the preferences of the recipients of care and their carers. Limited trial data suggests, however, that there are real differences between zuclopenthixol decanoate and other depots and these differences largely favour the former.
This review highlights the need for good controlled clinical trials to fully address the effects of zuclopenthixol decanoate for those with schizophrenia. Future studies should report service utilisation data, as well as satisfaction with care and economic outcomes. Duration of such trials should be of a longer duration than the included studies (12 months or more).
Plain language summary
Zuclopenthixol decanoate for schizophrenia and other serious mental illnesses
Synopsis pending.
Background
In the years after the discovery of oral antipsychotic medications, such as chlorpromazine or haloperidol, it became clear that there was a link between stopping medication and a relapse of psychotic symptoms (Davis 1990). A series of long‐acting intra‐muscular preparations has been developed since the 1960s in the hope of reducing the frequency of relapse and, hence, overall disability. These depot preparations, active for weeks at a time, are frequently used for those who find taking oral medication on a regular basis difficult or unacceptable. They are now a cornerstone of the treatment of those with schizophrenia and are often used to assist the transition from hospital to community care (Johnson 1990). The main properties of these depot preparations that may contribute to controlling the illness and disability of those with chronic schizophrenia are ease of compliance, stable drug plasma levels and, perhaps, an efficacy for people refractory to oral medications. It has, however, been a consistent concern that any reduction in relapse rate afforded by depot preparations may be offset by an increase in adverse effects such as drug‐induced movement disorders (Davis 1990).
Zuclopenthixol is the cis(Z)‐isomer of clopenthixol, a neuroleptic of the thioxanthene group, used for treating people with psychotic symptoms. There is one oral preparation (marketing names Cisordinol, Clopixol) and two depot forms: zuclopenthixol acetate (Cisordinol‐Acutard, Clopixol‐Acuphase, Clopixol‐Acutard) and zuclopenthixol decanoate (Cisordinol depot, Clopixol depot, Clopixol Inj.). The acetate version does not stay in the body for very long (a single dose persists for only 72 hours) whereas the decanoate form lasts for at least 2‐4 weeks (Baastrup 1993). It is also said to be especially suitable for people who are agitated or aggressive (DSC 1998) and is the subject of a separate Cochrane review (Fenton 1999). This review focuses on the effects of zuclopenthixol decanoate.
Objectives
To compare zuclopenthixol decanoate with oral zuclopenthixol and other antipsychotic preparations for treating schizophrenia or other serious mental illness with psychotic symptoms.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials.
Types of participants
Those with schizophrenia, however diagnosed and those with other serious psychotic mental illnesses were also included. People with dementing illness, depressive disorder and problems associated with substance misuse were, where possible, excluded.
Types of interventions
1. Depot zuclopenthixol: any dose or frequency of administration. 2. Oral zuclopenthixol: any dose or frequency of administration. 3. Other depot antipsychotic preparations: any dose or frequency of administration. 4. Other oral antipsychotics: any dose or frequency of administration. 5. Placebo.
Types of outcome measures
The outcomes of interest were:
1. Death, suicide or natural causes.
2. Leaving the study early.
3. Clinical response 3.1 Relapse* 3.2 Clinically significant response in global state ‐ as defined by each of the studies* 3.3 Average score/change in global state 3.4 Clinically significant response on psychotic symptoms ‐ as defined by each of the studies 3.5 Average score/change on psychotic symptoms 3.6 Clinically significant response on positive symptoms ‐ as defined by each of the studies 3.7 Average score/change in positive symptoms 3.8 Clinically significant response on negative symptoms ‐ as defined by each of the studies 3.9 Average score/change in negative symptoms
4. Extrapyramidal side effects 4.1 Incidence of use of antiparkinson drugs 4.2 Clinically significant extrapyramidal side effects ‐ as defined by each of the studies 4.3 Average score/change in extrapyramidal side effects
5. Other adverse effects, general and specific
6. Service utilisation outcomes 6.1 Hospital admission* 6.2 Days in hospital
7. Economic outcomes
8. Quality of life / satisfaction with care for either recipients of care or carers 8.1. Significant change in quality of life / satisfaction ‐ as defined by each of the studies 8.2 Average score / change in quality of life / satisfaction
Outcomes were grouped into immediate (0‐5 weeks), short term (6 weeks‐5 months), medium term (6 months‐1 year) and longer term (over 12 months).
* Primary outcomes.
Search methods for identification of studies
1. Electronic searching a. BIOLOGICAL ABSTRACTS/RRM (January 1982 to April 1998) was searched using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase:
[and zuclopenthixol or ciatyl or cisordinol* or clopenthixol or clopixol* or sordinol]
b. CINAHL (January 1982 to April 1998) was searched using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase:
[and zuclopenthixol or ciatyl or cisordinol* or clopenthixol or clopixol* or sordinol]
c. COCHRANE LIBRARY (Issue 2, 1998) was searched using the phrase:
[zuclopenthix* or ciatyl or cisordinol* or clopenthix* or clopixol* or sordinol]
d. COCHRANE SCHIZOPHRENIA GROUP'S REGISTER (April 1998) was searched using the phrase:
[zuclopenthix* or (cis and ?‐clopenthixol) or 0‐108 or cisordinol* or clopenthix* or clopixol* or #42 = 545 or #42 = 556 or #42 = 128 or #42 = 281 or #42 = 352 or #42 = 550 or #42 = 546]
(#42 is the intervention code field of the Register.)
e. EMBASE (January 1980 to May 1998) was searched using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase:
[and zuclopenthixol or zuclopenthixol/ explode all subheadings or ciatyl or cisordinol* or clopenthixol or clopixol* or sordinol]
f. MEDLINE (January 1966 to May 1998) was searched using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase: [and zuclopenthixol or explode clopenthixol(MeSH)/all subheadings or ciatyl or cisordinol* or clopixol* or sordinol]
g. PsycLIT (January 1974 to May 1998) was searched using the Cochrane Schizophrenia Group's phrase for both randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase: [and zuclopenthixol or explode zuclopenthixol or ciatyl or cisordinol* or clopixol* or clopenthixol or sordinol]
2. Reference searching The references of all identified studies were also inspected for more studies. 3. Industry UK subsidiary of H. Lundbeck A/S, the company producing zuclopenthixol, was contacted and asked for data on relevant published and unpublished trials.
Data collection and analysis
Methods of the review [For definitions of terms used in this, and other sections, please refer to The Cochrane Library Glossary.]
1. Selection of trials Two reviewers (EC, MF) inspected study citations identified by the electronic searches. Reviewers were not blinded to the names of the authors, institutions, journal of publication and results when they applied the inclusion criteria. Full reports of the studies of agreed relevance were obtained. Where disputes arose the full report was also acquired for more detailed scrutiny. Both reviewers then independently inspected all these full study reports. Where disagreement about the relevance of a given study occurred this was resolved by discussion. When it was not possible to agree without further information, these studies were added to the list of those awaiting assessment, and the principal author contacted.
2. Assessment of methodological quality Trials were allocated to three quality categories, as described in the Cochrane Collaboration Handbook (Mulrow 1997). Again, when dispute arose as to which category a trial was to be allocated, agreement was attempted by discussion. When this was not possible and further information was necessary to clarify into which category to allocate the trial, data were not entered and the trial was allocated to the list of those awaiting assessment. Only trials in Category A or B were included in the review.
As this categorisation only takes into account the quality of the allocation of intervention, a sensitivity analysis was undertaken. In this analysis, the result of including only trials in which double‐blinding was stated to have taken place was compared to that gained when data from all trials were used.
3. Addressing publication bias Data from trials identified in the way described above were entered into a funnel graph in an attempt to investigate the likelihood of potential systematic publication bias (Egger 1997).
4. Data extraction Each reviewer independently extracted data. Disputes were resolved by discussion, and where data were not possible to extract or further information was needed from authors these trials were added to the list of those awaiting assessment.
5. Data synthesis The reviewers applied the following guidelines to analyse data from the studies selected: (a) the analysis included all people who entered the trial; (b) the analysis maintained the study groups according to the original randomisation procedure; and (c) suicide was treated as relapse in all groups. Where possible, the reviewers gave people lost to follow‐up the worst outcome, with the exception of death. For example those lost to follow up for the outcome of relapse were treated in the analysis as having relapsed. People who were 'never discharged' (those randomised during admission and not discharged during follow‐up) were considered to have relapsed in both groups. The reviewers agreed upon these rules before knowing the studies selected. However, this assumption was employed only to the point where less than 50% of the individuals were lost to follow up. If more than this proportion were lost to final inclusion in the presented analysis, the outcome was not used.
Comparison of different outcome measures required different strategies for dichotomous and continuous data. For dichotomous outcomes, Peto odds ratio (OR) and a 95% confidence interval (CI) was used. In addition, as a measure of efficiency, the number needed to treat (NNT) was also calculated. For continuous data, whenever possible we took the opportunity to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. Comparison of continuous data outcomes measured by different outcome scales is problematic. Mental health continuous data is often not normally distributed. To avoid the pitfall of applying parametric tests to non‐normally distributed data the following standards were applied to all data before inclusion: (i) standard deviations and means were reported in the paper or were obtained from the authors; (ii) if the data were scale‐derived, or finite measures from, for example 0‐100, the standard deviation was multiplied by two. If the result was less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution (Altman 1996)) data were presented in graphical form. Non‐normally distributed data were reported in the 'Other data types' tables.
6. Sensitivity analysis We undertook three sensitivity analyses. The first investigated whether trials using rigorous diagnostic criteria systematically differed in their results from trials using more pragmatic entry criteria. The second examined whether our decision to perform an intention‐to‐treat analysis affected the final results of the review compared to an analysis that was based on only those who completed the studies. The third compared data only from trials stating that double‐blinding had taken place.
7. Test for heterogeneity The reviewers checked whether the differences among the results of trials were greater than could be expected by chance alone (statistical heterogeneity). This was done by looking at the graphical display of the results but also by using chi tests of heterogeneity. As those tests usually show small statistical power, clinical heterogeneity was also investigated looking for differences in treatment regimen, selection criteria, outcome measures and other methodological aspects.
Results
Description of studies
Please see Excluded and Included Studies Table.
Excluded studies Most of the excluded studies used oral zuclopenthixol rather than the decanoate preparation. Gravem 1990 and Viala 1988 were excluded because, although randomised, they did not measure clinical outcomes but primarily physiological measures such as plasma levels or the effect of injecting the acetate and decanoate forms simultaneously as opposed to separately. Walker 1983 was randomised and had relevant participants, interventions, and outcomes. The study was excluded due to loss in the analysis of six people whose original group of allocation was unclear.
Awaiting assessment Three studies are awaiting assessment needing further information from their authors (Saxena 1996, Svestka 1986, Tegler 1985).
Included studies Duration This ranged from 12 weeks (Wistedt 1991) to 1 year (Dencker 1980) with the other two being 24‐26 weeks.
Participants The participant group was homogeneous with all studies including those with a diagnosis of schizophrenia or similar psychotic disorder although using differing diagnostic criteria (Bleuer, Schneiderian, and DSM III). People of both sexes, with ages ranging from 20 to 67 years old were included. Participants frequently had long histories of illness (>two years) and were considered stable and to have chronic symptoms. One study included those who had an acute exacerbation of illness and were drug free (Martyns‐Yellowe 1993).
Setting The trials were both community and hospital based in the developed world. One study was based in a prison hospital in Africa (Martyns‐Yellowe 1993).
Interventions All trials used depot comparitors. One trial compared zuclopenthixol decanoate with haloperidol decanoate (Wistedt 1991), two flupenthixol decanoate (Dencker 1980, Martyns‐Yellowe 1993) and the remaining study with perphenazine enathate (Ahlfors 1980).
Outcome measures Apart from leaving the study early and use of additional medication, most outcomes, even those later dichotomised, were measured on rating scales which are listed below.
Many trials presented findings in graphs, in percentiles or by p‐values alone. Graphical presentation made it impossible to acquire data for synthesis. 'p'‐values were commonly used as a measure of association between intervention and outcomes instead of showing the strength of the association. Many did not provide standard deviations or did not give any information. At present requests for further information from authors have failed.
Global functioning 1. Clinical Global Impression ‐ CGI (Guy 1976) A rating instrument commonly used in studies of schizophrenia that enables clinicians to quantify severity of illness and overall clinical improvement during therapy. A seven‐point scoring system is usually used with low scores indicating decreased severity and/or greater recovery.
Side‐Effects 1. Extrapyramidal Symptom Rating Scale ‐ EPS (Chouinard 1980) This consists of a questionnaire relating to parkinsonian symptoms (nine items), a physician's examination for parkinsonism and dyskinetic movements (eight items), and a clinical global impression of tardive dyskinesia. High scores indicate severe levels of movement disorder.
2. UKU Side Effects Rating Scale ‐ UKU‐SERS (Lingjærde 1987). The UKU rates four major topics: psychological side effects (10 items), neurological side effects (eight items), autonomic side effects (11 items) and other side effects (19 items). Each item is defined by means of a four‐point scale where zero means not or doubtfully present. Scoring range 0‐144.
Missing outcomes Not one study evaluated hospital/service outcomes, satisfaction with care and economic outcomes.
Risk of bias in included studies
Randomisation No trialists reported the methods used for randomisation and all allocation concealment has been rated as 'unclear' or quality 'B'. As poor reporting of randomisation has consistently been associated with an overestimate of effect, the results in these trials could be a 30‐40% overestimate of effect (Schulz 1994, Moher 1998).
Blinding at outcome All studies described themselves as 'double‐blind' but there is no report of this being tested. All described method for blinding. All four studies used nurses who either did or did not know the codes to give the injections but were not involved in the study in any other way. No studies reported testing the integrity of blinding. Trialists asked hundreds of questions of those in the studies or their carers. The two questions, one to the participant ‐ "what do you think you have been given?" and one to the rater ‐ "what drug do you think this person was allocated?" would have clarified the situation. Scale data, as was often measured in the included studies, may be prone to bias when poor blinding has taken place.
Follow‐up Overall, losses to follow up were well described.
Data reporting No study presented continuous data in useable format. Either no data were presented at all or, if presented, no variance for mean estimates were reported.
Effects of interventions
The search One hundred and fifty one citations were found using the search strategy. Ten studies were related to zuclopenthixol decanoate and four are included in this review. Three are awaiting assessment due to no useable data being reported, two were excluded as they did not measure clinical outcomes, and one because people were randomised but neither were their data or original group of allocation reported. The study authors are being contacted for further information.
1. Death, suicide or natural causes. Three studies reported deaths of trial participants. One person each in the zuclopenthixol decanoate groups of Ahlfors 1980 and Wistedt 1991 committed suicide (n=233, OR 6.5 CI 0.4‐105). One person from the control group in Dencker 1980 died of natural causes.
2. Global clinical effect Three studies gave information on important clinical change (Ahlfors 1980, Dencker 1980, Wistedt 1991), whilst data from Martyns‐Yellowe provides information on those discharged (n=332, homogeneous OR 0.8 CI 0.5‐1.2).
3. Mental state 3.1 Relapse Using relapse of illness as a proxy measure of mental state, fewer participants on zuclopenthixol decanoate relapsed than those taking a comparitor drug (n=296, OR 0.54 CI 0.3‐0.9, NNT 8.3 CI 5‐53).
3.2 Needing additional medication 3.2.1 Antipsychotics: Only one trial reported on the use of additional antipsychotic medication (Ahlfors 1980). The result favouring zuclopenthixol decanoate was not statistically significant (OR 0.6 CI 0.3‐1.1). 3.2.2 Sedative medication: No statistical significance was found in the one study (Dencker 1980) that reported the use of additional sedative medication, although four studies allowed the use of sedatives or hypnotic drugs in their protocol. 3.2.3 Antidepressants: Fewer people taking zuclopenthixol decanoate needed antidepressant medication (n=296; OR 0.6 CI 0.3‐0.8, NNT 7 CI 4‐32).
4. Leaving the study early 4.1 Any reason Fewer people taking zuclopenthixol decanoate left the study early (n=332, OR 0.59 CI 0.3‐1.0, NNT 11 CI 5‐¥).
4.2 Due to lack of efficacy There was no difference in the rates of study attrition (14%) when the reason cited was due to lack of efficacy.
5. Adverse effects 5.1. Adverse effect ‐ general Fewer people receiving comparitor drugs experienced adverse effects in general than those taking zuclopenthixol decanoate (n=100, OR 2.9 CI 1‐7, NNH 5 CI 3‐31).
5.2 Parkinsonism and movement disorders There were no clear differences between zuclopenthixol decanoate and haloperidol decanoate (Wistedt 1991), flupenthixol decanoate (Dencker 1980, Martyns‐Yellowe 1993) and perphenazine enathate (Ahlfors 1980) on any of the outcomes reported (dyskinesia, dystonia, hypersalivation, oculogyric crisis, protrusion of the tongue rigidity, and tremor). Fewer people on zuclopenthixol received anticholinergic medication than those on the comparitor drugs (n=296, OR 0.4 CI 0.2‐0.8, NNT 9 CI 5‐38).
Missing outcomes None of the studies reported on service utilisation, economic or quality of life outcomes. The three proposed sensitivity analyses (see Methods) were not undertaken due to lack of data.
Publication bias There are too few studies to enter a funnel graph for assessing presence of possible publication bias.
Discussion
Generalisability Two studies were multi‐centre, most were within the developed world, and studies were randomising those with well defined and recognisable disorders. The varied diagnostic criteria used in the studies suggest that the diagnoses could be heterogeneous, as is the situation in routine practice. One study took place in an African prison (Martyns‐Yellowe 1993), on mentally ill, drug free vagrants. On average the duration of illness was long with the minimum duration of illness being 2 years in the studies that gave this information. Only one study lasted longer than 28 weeks, which gives some cause for concern regarding generalisability of the results to a life long course of illness. On average, however, the trial participants seem more generalisable than are usually seen in trials that are more recent.
Death This was reported in three studies, where two people who were taking zuclopenthixol decanoate committed suicide and one person taking a comparitor drug died of natural causes. No inference can be drawn form this outcome. If there were further trials on depot zuclopenthixol, it would be a useful outcome to record. This would allow pooled data to be more informative. Death is rarely and poorly reported, despite there being a 10% increase in mortality in those with schizophrenia (Clare Harris 1998). Global change Using the dichotomised CGI (Guy 1976) as a measure of global change, no significant difference between zuclopenthixol decanoate and other depot antipsychotics was seen. About 50% of participants in both groups showed some important global changes for the better. Although zuclopenthixol decanoate does not stand out from other depots, it is heartening to note the overall high percentage of improvement in this very ill group of people. It would, of course, have been more informative to see if this improvement was greater than that afforded by oral antipsychotic drugs or even placebo.
Mental state Fewer people in the zuclopenthixol decanoate group relapsed than those allocated to other depots. There was, however, no difference between zuclopenthixol decanoate and the other drugs in use of additional antipsychotics or sedative medication, which may imply equal short term clinical efficacy. Zuclopenthixol patients used less antidepressant drugs than people using comparitor drugs, which may indicate that zuclopenthixol depot has antidepressant properties or less propensity to induce depressive symptoms. The findings relating to relapse and use of antidepressants, broadly favourable to zuclopenthixol decanoate, are based on data from about 300 people. Although all findings should be replicated, these are rare homogeneous findings suggesting that one depot does have advantages over others. The studies used several scale‐derived measures of mental state, but failed to report them at all or in a useable format.
Leaving the study early About 28% of the people in the four studies included left the study early, the maximum duration of the studies being one year (Dencker 1980) the minimum 12 weeks (Wistedt 1991). This is a much better rate than those seen in trials of the atypical antipsychotics where up to 60% of participants have been lost at six weeks (Thornley 1998). How 28% attrition relates to clinical practice remains unknown, as the reviewers' anecdotal clinical experience of losing patients is not as high as 28% per six months. These findings may cast some doubt as to the generalisability of the findings.
Adverse effects From the two studies that provide useable information of those 'general' experiencing adverse events as against those not, results favour the comparitor drugs. It is unclear what 'general' side effects really means in the clinical situation, but, as with the data on mental state, there may be a real difference between zuclopenthixol decanoate and other depots. Zuclopenthixol decanoate‐treated people needed less anticholinergic drugs (OR 0.43, CI 0.2‐0.8). This may indicate that they experienced less extrapyramidal symptoms (movement disorder symptoms) or cholinergic problems such as dry mouth, blurred vision, constipation and low blood pressure. Despite this imbalance of adjunctive treatment, there are no data to show differences in rates of encountering parkinsonism or movement disorders. Perhaps the greater use of anticholinergic drugs in the comparitor groups may have masked greater rates of movement disorders.
Authors' conclusions
Implications for practice.
Those with schizophrenia Data from trials of zuclopenthixol decanoate suggests that in some ways it is different from other depot antipsychotic drugs. It does seem to prevent people from experiencing a relapse in illness and may be more acceptable (using dropout as a proxy measure of acceptability) when compared to other depot antipsychotics. Zuclopenthixol decanoate‐treated people may also experience less depressive symptoms as fewer people in the reported studies required antidepressant medications. However, people may have more effects that are 'generally' adverse. This is difficult to assess, however, as less adjunctive medication to ward off adverse effects was used by the people using zuclopenthixol decanoate than by those who took the control medications.
Managers or policy makers Data relating to service utilisation, satisfaction with care and economic outcomes were not reported. Zuclopenthixol decanoate may be a depot antipsychotic that has some advantages over others in the field. Further trials to replicate the important findings in this review should record outcomes that are of use to managers and policy makers. Until then the proxy measures reported in this review are likely to remain the best trial‐based evidence.
Clinicians People with schizophrenia can be assured that zuclopenthixol decanoate does seem as effective as comparitor depot medications. Using zuclopenthixol decanoate instead of comparitor depots prevents/delays one relapse if eight people with schizophrenia are treated (CI 5‐53). However, zuclopenthixol decanoate may induce more adverse effects (NNH 5, CI 3‐31) although it decreases need for anticholinergic medication when compared to a group of other depot preparations (NNT 9, CI 5‐38).
Implications for research.
General If the recommendations of the CONSORT statement (Begg 1996) had been followed by trialists much more data would have been available to inform practice. Clear descriptions of randomisation would have reassured the user of the trials that selection bias had been minimised. Well described and tested blinding could have encouraged confidence in the control of performance and detection bias. It is also important to know how many ‐ and from which groups ‐ people were withdrawn, in order to evaluate exclusion bias.
It would have been helpful if authors had presented data in a useful manner which reflects association between intervention and outcome, for example, relative risk, odds‐ratio, risk or mean differences, as well as raw numbers. Binary outcomes should be calculated in preference to continuous results as they are easier to interpret. If p‐values are used, the exact value should be reported.
Specific This review highlights the need for good controlled clinical trials to address the effectiveness and clinical outcome of using zuclopenthixol decanoate for those with schizophrenia. Well designed, conducted and reported studies should compare zuclopenthixol decanoate to oral medications and other depots. These studies should report service utilisation data, as well as satisfaction with care and economic outcomes. Duration of such trials should be greater than 12 months as schizophrenia is a life long illness and trials over this length of time are have not yet been completed.
What's new
| Date | Event | Description |
|---|---|---|
| 21 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 25 May 1999 | New citation required and conclusions have changed | Substantive amendment |
Notes
Cochrane Schizophrenia Group internal peer review complete (see Module). External peer review scheduled.
Acknowledgements
Other acknowledgements The reviewers would like to thank Lesley Jones, Medical Information Officer at Lundbeck Ltd. (UK) for supplying a published report that had not been identified in the search strategy, and the staff of the Cochrane Schizophrenia Group editorial base for assistance.
Data and analyses
Comparison 1. ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 suicide | 2 | 236 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.54 [0.40, 105.74] |
| 1.2 physical illness | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 2 Global functioning: No clinically important change | 4 | 332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 3 Mental state: 1. Relapse | 3 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| 4 Mental state: 2. Needing additional medication | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 antidepressants | 3 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.34, 0.89] |
| 4.2 antipsychotics | 1 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.33, 1.09] |
| 4.3 sedation ‐ medication unspecified | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.41, 3.17] |
| 5 Leaving the study early | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 any reason | 4 | 332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.35, 1.00] |
| 5.2 due to lack of effect | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.44, 1.90] |
| 6 Adverse effects: 1. General | 2 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.87 [1.13, 7.27] |
| 7 Adverse effects: 2. Parkinsonism and movement disorders | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 adjunctive anticholinergic drugs used | 3 | 296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.23, 0.83] |
| 7.2 akathisia | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 dyskinesia | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [0.20, 20.86] |
| 7.4 dystonia | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.16, 2.18] |
| 7.5 hypersalivation | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.24, 10.12] |
| 7.6 oculogyric crisis | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 7.7 protusion of tongue | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [0.41, 6.38] |
| 7.8 rigidity | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.24, 10.12] |
| 7.9 tremor | 2 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.14, 2.36] |
1.1. Analysis.
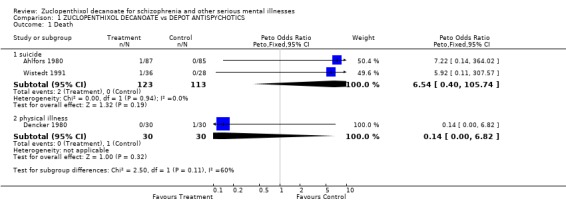
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 1 Death.
1.2. Analysis.
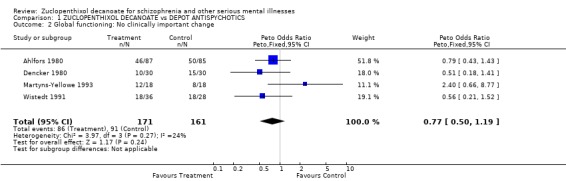
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 2 Global functioning: No clinically important change.
1.3. Analysis.
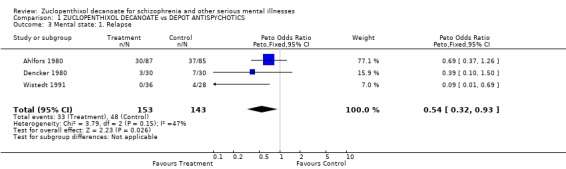
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 3 Mental state: 1. Relapse.
1.4. Analysis.
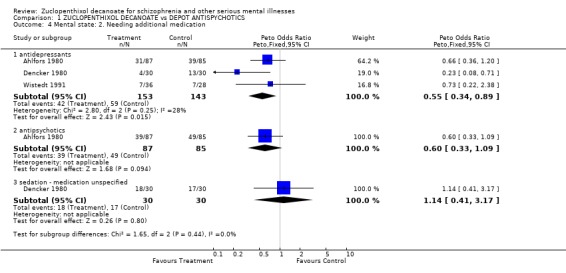
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 4 Mental state: 2. Needing additional medication.
1.5. Analysis.
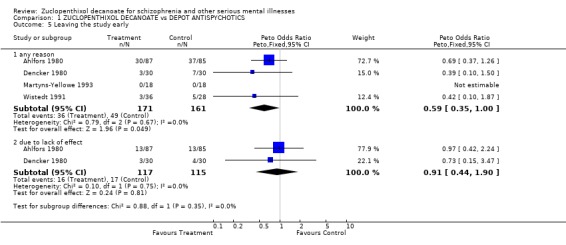
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 5 Leaving the study early.
1.6. Analysis.
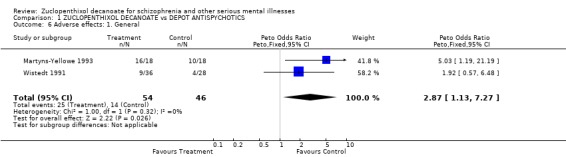
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 6 Adverse effects: 1. General.
1.7. Analysis.
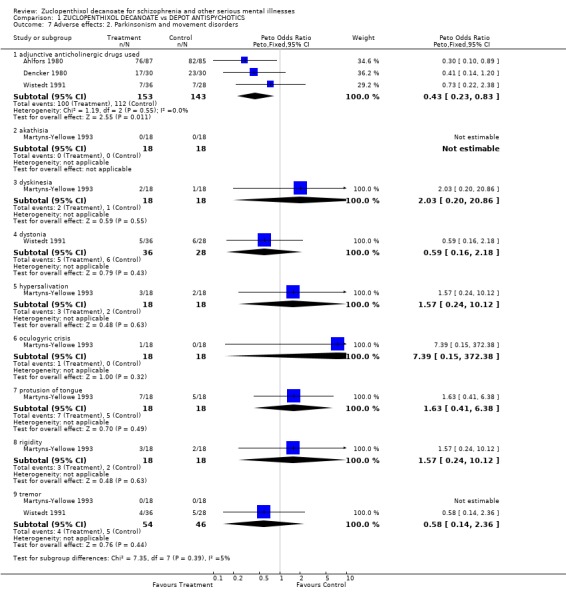
Comparison 1 ZUCLOPENTHIXOL DECANOATE vs DEPOT ANTISPYCHOTICS, Outcome 7 Adverse effects: 2. Parkinsonism and movement disorders.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahlfors 1980.
| Methods | Allocation: randomised, no further information. Blindness: double ‐ drugs given by a nurse not involved in trial. Setting: hospital, multi‐centre. Rating: reliablity study undertaken. Duration: 6 months ‐ preceeded by 1‐4 week wash‐out period. | |
| Participants | Diagnosis: schizophrenia (Bleuler criteria). N=172. Sex: 114 M, 58 F. Age: 20‐65 years. Inclusion: 2+ years ill & poor response to present drugs or admitted for exacerbation of illness. Exclusion: somatic disease, pregnancy, drug or alcohol misuse, >65yrs. | |
| Interventions | 1. Zuclopenthixol decanoate: dose mean 280mg/IM, range 50‐800 mg/IM. N=87. 2. Perphenazine enanthate: dose mean 141 mg/IM, range 20‐600 mg/IM. N=85. Dose interval: 2/52. Other medications allowed: nitrazepam or chloral hydrate, amitryptiline, diazepam, biperiden. | |
| Outcomes | Death.
Global Impression (CGI).
Relapse.
Leaving the study early.
Additional medication.
Side effects (EPS). Unable to use ‐ Mental state (BPRS ‐ no SD, scores given as residual %). Behaviour (NOSIE‐36 ‐ no SD). Lab tests (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dencker 1980.
| Methods | Allocation: randomised, no further detials. Blindness: double, injections given nurses who knew codes but did not participate in study. Duration: 1year ‐ preceeded by pretrial period of 3 months. Setting: outpatients. | |
| Participants | Diagnosis: schizophrenia (NIMH ‐ Schneiderian). History: chronic, stable, all previously received depot medication. Inclusion: 3+ years ill. Exclusion: if had > protocol doses of test medications during lead in. N=60*. Age: mean ˜40 years, range 20‐65. Sex: 47 M, 13 F. | |
| Interventions | 1. Clopenthixol decanoate: dose range 50‐600mg/IM. N=30.
2. Flupenthixol palmitate: dose range 25‐300mg/IM. N=30.
Dose interval: 4/52. Additional drugs: benztropine or biperiden, nitrszepam or chloral hydrate, diazepam, amitriptyline. |
|
| Outcomes | Death.
Global Impression (Global Rating).
Relapse.
Leaving the study early.
Additional medication.
Side effects (EPS). Unable to use ‐ Mental state (BPRS, CPRS, Hamiliton ‐ no SD). Side effects (CSE ‐ no usuable data). Social (ADL, Katz), ego functioning (non‐clinical outcomes, data not usable). |
|
| Notes | *7 people were possibly withdrawn from analysis and 60 reported on. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Martyns‐Yellowe 1993.
| Methods | Allocation: randomised, no further details. Blindness: double ‐ drugs given by a nurse not involved in trial. Duration: 24 weeks*. Setting: prison hospital. | |
| Participants | Diagnosis: schizophrenia (DSM III). History: chronic, stable, drug free. N=36. Age: 21‐40 years. Sex: all male. | |
| Interventions | 1. Clopenthixol decanoate: dose 40 mg/IM. N=18.
2. Flupenthixol decanoate: dose 200 mg/IM. N=18.
Dosing regime: 4 fortnightly, 3 three‐weekly, 2 monthly = 20 weeks. Additional medication: 2mg benztropine. |
|
| Outcomes | Discharge.
Side effects. Unable to use ‐ Global Impression (no data). Mental state (BPRS ‐ no SD, residual % of symptoms from baseline ‐ endpoint). |
|
| Notes | *Last measurement at 24 weeks, although last dose was at 20 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wistedt 1991.
| Methods | Allocation: randomised, no further details. Blindness: triple ‐ drugs given by a nurse not involved in trial. Duration: 12 weeks ‐ preceeded by 3 months. Setting: multi‐centre. Ethics: committee approved. Rating: reliablity study undertaken. | |
| Participants | Diagnosis: schizophrenia (DSM III). History: stable BPRS score of 3‐26. N= 64*. Age: mean ˜39 years, range 20‐61 years. | |
| Interventions | 1. Zuclooenthixol decanoate: dose mean 284 mg/IM, range 100‐600 mg/IM. N=36. 2. Haloperidol decanoate: dose mean 92 mg/IM, range 39‐200 mg/IM. N=28. Dose interval:4/52. Additional meds: levopromazine, oxazepam, orphenadrine, biperiden, choral hydrate, amitryptiline. | |
| Outcomes | Relapse.
Leaving the study early.
Additional medication.
Side effects (UKU). Unable to use ‐ Clinical Global Impression (CGI ‐ no SD). Mental state (BPRS, MADRS ‐ no SD). SAS & Lab tests (non‐clinical outcomes, data not usable). |
|
| Notes | * Results are given for 61, but information is availabe on which groups from which they were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Diagnostic tool DSMIII‐R and DSM‐IV ‐ Diagnostic Statistical Manual version 3 Revised and version 4
Global rating scales CGI ‐ Clinical Global Impression
Mental state BPRS ‐ Brief Psychiatric Rating Scale MADRS ‐ Montgomery‐Asberg Depression Rating Scale PANSS ‐ Positive and Negative Syndrome Scale SANS ‐ Scale for the Assessment of Negative Symptoms
Side effects AIMS ‐ Abnormal Involuntary Movement Scale AMDP‐5 ‐ Association for Methodology and Documentation in Psychiatry adverse event questionnaire BMI ‐ Body mass index SAS ‐ Simpson‐Angus Index ‐ for neurological side effects
Quality of Life QOL ‐ Quality of Life Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaes‐Jorgensen 1981 | Allocation: random, no further information. Participants: people with paranoid schizophrenia. Interventions: oral cis(z)‐clopenthixol versus oral cis(z)/trans(E)‐clopenthixol, not depot zuclopenthixol. |
| Gravem 1990 | Allocation: random, no further details. Participants: people with schizophrenia or mania. Interventions: combined or separate injections of zuclopenthixol acetate and zuclopenthixol decanoate. |
| Heikkilä 1981 | Allocation: random, no further information. Participants: people with schizophrenia. Interventions: oral zuclopenthixol versus clopenthixol, not depot zuclopenthixol. |
| Heikkilä 1982 | Allocation: random, no further information. Participants: people with schizophrenia. Interventions: oral haloperidol versus zuclopenthixol, not depot zuclopenthixol. |
| Heikkilä 1992 | Allocation: random, no further information. Participants: people with schizophrenia. Interventions: oral haloperidol verus zuclopenthixol, not depot zuclopenthixol. |
| Kabes 1981 | Allocation: not randomised, case series. |
| Kingstone 1970 | Allocation: random, on admission assigned to a number allocated to a bottle. Participants: those with schizophrenia. Interventions: oral clopenthixol versus chlorpromazine, not depot zuclopenthixol. |
| Lublin 1991 | Allocation: randomised, crossover. Participants: those with schizophrenia and tardive dyskinesia. Interventions: oral haloperidol versus zuclopenthixol, not depot zuclopenthixol. |
| Peacock 1996 | Allocation: consecutively on admission. Participants: those with schizophrenia. Interventions: clozapine, zuclopenthixol or flupenthixol, not depot zuclopenthixol. |
| Remvig 1987 | Allocation: random, no further information. Participants: people with schizophrenia. Interventions: oral zuclopenthixol versus perphenazine, not depot zuclopenthixol. |
| Serafetinides 1972 | Allocation: random, no further information. Participants: those with schizophrenia. Interventions: oral clopenthixol, chlorpromazine, haloperidol or placebo, not depot zuclopenthixol. |
| Simpson 1972 | Allocation: unclear. Participants: people with schizophrenia. Interventions: oral clopenthixol, not depot zuclopenthixol.. |
| Viala 1988 | Allocation: unclear. Participants: people with schizophrenia. Interventions: zuclopenthixol decanoate versus fluphenazine decanoate Outcomes: plasma concentration levels, no clinical outcomes. |
| Vinar 1967 | Allocation: unclear. Participants: two groups of people with schizophrenia. Interventions: oral zuclopenthixol, unclear if different doses, not depot zuclopenthixol. |
| Walker 1983 | Allocation: random ‐ no further details. Participants: those with schizophrenia. Interventions: zuclopenthixol decanoate versus fluphenazine decanoate. Outcomes: six people excluded from analysis and it is unclear from which groups they were excluded. Further information is being sought from trialists. |
Contributions of authors
Evandro Coutinho ‐ protocol development, study selection, data extraction and assimilation, report writing.
Mark Fenton ‐ protocol development, searching, study selection, data extraction and assimilation, report writing.
Seema Quraishi ‐ searching, study selection, data entry.
Sources of support
Internal sources
Cochrane Schizophrenia Group, UK.
Oswaldo Cruz Foundation, Brazil.
University of the State of Rio de Janeiro, Brazil.
External sources
CNPq (Brazilian National Council of Research), Brazil.
NHS‐R&D Health Technology Assessment Programme., UK.
Declarations of interest
Evandro Countinho ‐ no known conflicts of interest
Mark Fenton ‐ has led Eli Lilly and Zeneca sponsored workshops for clinicians.
Seema Quraishi ‐ no known conflicts of interest.
The Cochrane Schizophrenia Group editorial base in Oxford has received general support funding from Eli Lilly during the years 1996‐8 (see Group Module). This, along with some funds from other pharmaceutical companies, is used to support any ongoing work of the editorial base and is not linked to any particular review (annual report available on request).
Edited (no change to conclusions)
References
References to studies included in this review
Ahlfors 1980 {published data only}
- Ahlfors UG, Dencker SJ, Gravem A, Remvig J. Clopenthixol decanoate and perphenazine enanthate in schizophrenic patients. A double‐blind Nordic multicentre trial. Acta Psychiatrica Scandinavica Supplementum 1980;279:77‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dencker 1980 {published data only}
- *Dencker SJ, Lepp M, Malm U. Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. I. A one year double‐blind study of clopenthixol decanoate and flupenthixol palmitate. Acta Psychiatrica Scandinavica Supplementum 1980;279:10‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dencker SJ, Elgen K. Aspects of clinical psychiatric research on depot neuroleptics. The presentation of two double‐blind trials with cis(z)‐clopenthixol decanoate and a withdrawal study in schizophrenics. Acta Psychiatrica Scandinavica Supplementum 1980;279:5‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dencker SJ, Lepp M, Malm U. Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. II. Factor analysis of the CPRS sub‐scale for schizophrenia. Acta Psychiatrica Scandinavica Supplementum 1980;279:29‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dencker SJ, Malm U, Jorgensen A, Overo KF. Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. IV. Serum levels and clinical outcome. Acta Psychiatrica Scandinavica Supplementum 1980;279:55‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martyns‐Yellowe 1993 {published data only}
- *Martyns‐Yellowe IS. The decanoates of flupenthixol and clopenthixol in the treatment of chronic schizophrenic in‐patients. Implications for community psychiatry. West African Journal of Medicine 1993;12(2):110‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Martyns‐Yellowe IS. The positive and negative symptoms of schizophrenia: patterns of response to depot neuroleptic treatment. West African Journal of Medicine 1994;13:4:200‐3. [PubMed] [Google Scholar]
Wistedt 1991 {published data only}
- Wistedt B, Koskinen T, Thelander S, Nerdrum T, Pedersen V, Molbjerg C. Zuclopenthixol decanoate and haloperidol decanoate in chronic schizophrenia: a double‐blind multicentre study. Acta Psychiatrica Scandinavica 1991;84(1):14‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaes‐Jorgensen 1981 {published data only}
- Aaes‐Jorgensen T, Gravem A, Jorgensen A. Serum levels of the isomers of clopenthixol in patients given cis(Z)‐clopenthixol or cis(Z)/trans(E)‐clopenthixol. Acta Psychiatrica Scandinavica Supplementum 1981;294:70‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Gravem 1990 {published data only}
- Gravem A, Aaes Jorgensen T. Co‐injection of zuclopenthixol acetate and zuclopenthixol decanoate. Nordisk Psykiatrisk Tidsskrift 1990;44:403‐5. [Google Scholar]
Heikkilä 1981 {published data only}
- Heikkilä L, Karsten D, Valli K. A double‐blind clinical investigation of cis(Z)‐clopenthixol and clopenthixol in chronic schizophrenic patients. Acta Psychiatrica Scandinavica Supplementum 1981;294:25‐9. [PubMed] [Google Scholar]
Heikkilä 1982 {published data only}
- Heikkilä L, Laitinen J, Vartiainen H. Cis(Z)‐clopenthixol and haloperidol in chronic schizophrenic patients: a double‐blind clinical multicentre investigation. Acta Psychiatrica Scandinavica Supplementum 1981;294:30‐8. [PubMed] [Google Scholar]
Heikkilä 1992 {published data only}
- Heikkilä L, Eliander H, Vartiainen H, Turunen M, Pedersen V. Zuclopenthixol and haloperidol in patients with acute psychotic states. A double‐blind, multi‐centre study. Current Medical Research and Opinion 1992;12(9):594‐603. [DOI] [PubMed] [Google Scholar]
Kabes 1981 {published data only}
- Kabes J, Balon R, Papezova H. Clinical trial with clopenthixol decanoate. Activitas Nervosa Super Praha 1981;23(3):255‐6. [Google Scholar]
Kingstone 1970 {published data only}
- Kingstone E, Kolivakis T, Kossatz I. Double blind study of clopenthixol and chlorpromazine in acute hospitalized schizophrenics. Internationale Zeitschrift fur Klinische Pharmakologie Therapie und Toxikologie 1970;3(1):41‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Lublin 1991 {published data only}
- Lublin H, Gerlach J, Hagert U, Meidahl B, Molbjerg C, Pedersen V, et al. Zuclopenthixol, a combined dopamine D1/D2 antagonist, versus haloperidol, a dopamine D2 antagonist, in tardive dyskinesia. European Neuropsychopharmacology 1991;1(4):541‐8. [DOI] [PubMed] [Google Scholar]
Peacock 1996 {published data only}
- Peacock L, Solgaard T, Lublin H, Gerlach J. Clozapine versus typical antipsychotics. A retro‐ and prospective study of extrapyramidal side effects. Psychopharmacology 1996;124(1‐2):188‐96. [DOI] [PubMed] [Google Scholar]
Remvig 1987 {published data only}
- Remvig J, Larsen H, Rask P, Skausig OB, Skov S, Stromgren LS. Zuclopenthixol and perphenazine in patients with acute psychotic states. A double‐blind multicentre study. Pharmacopsychiatry 1987;20(4):147‐54. [DOI] [PubMed] [Google Scholar]
Serafetinides 1972 {published data only}
- Serafetinides EA, Willis D, Clark ML. Haloperidol, clopenthixol, and chlorpromazine in chronic schizophrenia. II. The electroencephalographic effects of chemically unrelated antipsychotics.. Journal of Nervous and Mental Disease 1972;155:366‐9. [DOI] [PubMed] [Google Scholar]
Simpson 1972 {published data only}
- Simpson GM, Arengo AD, Angus JW, Beckles ED, Rochlin D. A one‐year trial of clopenthixol in chronic schizophrenia. Canadian Psychiatric Association Journal 1972;17:321‐4. [DOI] [PubMed] [Google Scholar]
Viala 1988 {published data only}
- Viala A, Ba B, Durand A, Gouezo F, Hou N, Jorgensen A. Comparative study of the pharmacokinetics of zuclopenthixol decanoate and fluphenazine decanoate. Psychopharmacology (Berlin) 1988;94(3):293‐7. [DOI] [PubMed] [Google Scholar]
Vinar 1967 {published data only}
- Vinar O, Bastecky J, Taussigova D, Formankova M, Ruzicka S. Clopenthixol in schizophrenia. Comparison of two groups of patients treated by the same drug. Activitas Nervosa Super Praha 1967;9(4):401‐4. [PubMed] [Google Scholar]
Walker 1983 {published data only}
- Walker CA. A double‐blind comparative trial of the decanoates of clopenthixol and fluphenazine in the treatment of chronic schizophrenic out‐patients. Pharmatherapeutica 1983;3(5):289‐93. [PubMed] [Google Scholar]
References to studies awaiting assessment
Saxena 1996 {published data only}
- Saxena B. The value of depot neuroleptic injections in the treatment of chronic schizophrenia. Schizophrenia 1996: Breaking down the Barriers. 4th International Conference. Vancouver, BC, Canada. Oct 6‐9 1996.
Svestka 1986 {published data only}
- Svestka J, Nahunek K, Ceskova E. Controlled cross‐over comparison of oxyprothepine and clopenthixol decanoate in the prophylaxis of schizophrenic psychoses. Activitas Nervosa Superior 1986;28:35‐6. [Google Scholar]
Tegler 1985 {published data only}
- Tegeler J. A comparative trial of Cis(Z)‐clopenthixol decanoate and fluphenazine decanoate. Pharmacopsychiatry 1985;18:78‐9. [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland MJ. Detecting skewness from summary information. BMJ 1996;313:1200. [ZD020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asberg 1978
- Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall GA. A comprehensive psychopathological rating scale. Acta Psychiatrica Scandinavica Supplementum 1978;271:5‐27. [DOI] [PubMed] [Google Scholar]
Baastrup 1993
- Baastrup PC, Ahlfors UG, Bjerkenstedt L, Dencker SJ, Fensbo C, Gravem A, et al. A controlled Nordic multicentre study of zuclopenthixol acetate in oil solution, haloperidol and zuclopenthixol in the treatment of acute psychosis. Acta Psychiatrica Scandinavica 1993;87:48‐58. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
Chouinard 1980
- Chouinard G, Ross‐Chouinard A, Annable L. Extrapyramidal symptom rating scale. Canadian Journal of Neurological Science 1980;7:233. [Google Scholar]
Clare Harris 1998
- Clare Harris E, Barraclough B. Excess of mortality of mental disorder. British Journal of Psychiatry 1998;173:11‐53. [DOI] [PubMed] [Google Scholar]
Davis 1990
- Davis JM. Drug treatment of schizophrenia. Current Opinion in Psychiatry 1990;3:9‐34. [Google Scholar]
DSC 1998
- Data Sheet Compendium 1997‐98. London: Datapharm Publications. [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenton 1999
- Fenton M, Coutinho E, Campbell C. Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses (Cochrane Review). The Cochrane Library 1999, Issue 1. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. National Institute of Mental Health Publication 76‐338. Washington, DC: US Government Printing Office, 1976. [Google Scholar]
Honigfeld 1962
- Honigfeld G, Gillis RD, Klett CJ. NOSIE‐30: A treatment sensitive ward behavior scale. Psychological Reports 1962;10:799‐812. [DOI] [PubMed] [Google Scholar]
Johnson 1990
- Johnson DAW, Wright NF. Drug prescribing for schizophrenia out‐patients on depot injections. British Journal of Psychiatry 1990;156:827‐34. [DOI] [PubMed] [Google Scholar]
Krawiecka 1977
- Krawiecka M, Goldberg D, Vaughan M. A standardised psychiatric assessment scale for rating psychotic patients. Acta Psychiatrica Scandinavica 1977;55:299‐308. [DOI] [PubMed] [Google Scholar]
Lingjærde 1987
- Lingjærde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatrica Scandinavica Supplementum 1987;334:1‐100. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD (eds). Cochrane Collaboration Handbook [updated September 1997]. The Cochrane Library [database on disk and CDROM]. The Cochrane Collaboration. [Google Scholar]
NIMH 1976
- DOTES ‐ Dosage Record and Treatment Emergent Symptom Scale. In: Guy W editor(s). ECDEU Assessment Manual for Psychopharmacology ‐ Revised Edition. Rockville, MD: National Institute of Mental Health, 1976. [Google Scholar]
Schulz 1994
- Schulz KF, Chalmers I, Grimes DA, Altman DG. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA 1994;272:125‐8. [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:s11‐9. [DOI] [PubMed] [Google Scholar]
Thornley 1998
- Thornley B, Adams CE. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ 1998;317:1181‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]


