Abstract
Background
Shigella causes an estimated 500 000 enteric illnesses in the United States annually, but the association with socioeconomic factors is unclear.
Methods
We examined possible epidemiologic associations between shigellosis and poverty using 2004–2014 Foodborne Diseases Active Surveillance Network (FoodNet) data. Shigella cases (n = 21 246) were geocoded, linked to Census tract data from the American Community Survey, and categorized into 4 poverty and 4 crowding strata. For each stratum, we calculated incidence by sex, age, race/ethnicity, and FoodNet site. Using negative binomial regression, we estimated incidence rate ratios (IRRs) comparing the highest to lowest stratum.
Results
Annual FoodNet Shigella incidence per 100 000 population was higher among children <5 years old (19.0), blacks (7.2), and Hispanics (5.6) and was associated with Census tract poverty (incidence rate ratio [IRR], 3.6; 95% confidence interval [CI], 3.5–3.8) and household crowding (IRR, 1.8; 95% CI, 1.7–1.9). The association with poverty was strongest among children and persisted regardless of sex, race/ethnicity, or geographic location. After controlling for demographic variables, the association between shigellosis and poverty remained significant (IRR, 2.3; 95% CI, 2.0–2.6).
Conclusions
In the United States, Shigella infections are epidemiologically associated with poverty, and increased incidence rates are observed among young children, blacks, and Hispanics.
Keywords: Census tract, diarrheal disease, FoodNet, poverty, Shigella
Shigellosis is an acute diarrheal illness caused by bacteria of the genus Shigella. Shigella causes an estimated 500 000 enteric illnesses in the United States annually [1, 2]. Transmission occurs via the fecal–oral route, and the low infectious dose (as few as 10 organisms) allows for efficient spread from person to person and through contaminated food and water [2]. Outbreaks have been seen in childcare and congregate living settings, among persons experiencing homelessness, and among men who have sex with men (MSM) [3–5]. There are currently 4 known Shigella species: Shigella sonnei accounts for the majority (72%–80%) of human infections in the United States, followed by Shigella flexneri (12%–16%) [6]. Both typically cause mild diarrheal illness; severe symptoms, including bloody diarrhea, are more common among S. flexneri (34%) compared with S. sonnei (25%) [7]. S. boydii and S. dysenteriae are rarely seen in the United States, but the latter can cause epidemic dysentery [2, 8]. An estimated 25% of US shigellosis infections are associated with international travel [9].
Among Shigella infections reported to the Foodborne Disease Active Surveillance Network (FoodNet), the incidence is greatest among blacks and Hispanics compared with whites [6, 10]. Among adults with laboratory-diagnosed shigellosis, the rate of severe disease is higher among blacks compared with other racial groups [7]. The reasons for these disparities remain unclear, though recent studies have suggested that socioeconomic factors, such as income and education, may be associated [11, 12]. Two limited studies found that the incidence of shigellosis increases with Census tract poverty: This was seen among residents of New York City and among children <15 years of age in California [13, 14]. A better understanding of the extent to which the racial/ethnic disparities in shigellosis are related to socioeconomic inequalities could help guide prevention efforts.
US disease surveillance systems do not commonly collect individual socioeconomic information. However, by geocoding the addresses of patient residences to their Census tracts, existing neighborhood-level socioeconomic data can be used to identify and monitor socioeconomic disparities [15]. Using FoodNet surveillance data, we examined the association between shigellosis incidence and Census tract–level poverty and household crowding. We include household crowding because the incidence of Shigella (and its potential association with poverty) may be related to crowding, given that infection is easily spread from person to person. We sought to determine whether potential associations varied by sex, age group, race/ethnicity, FoodNet site, and Shigella species. Additionally, we explored the extent to which poverty and crowding may explain racial disparities in shigellosis incidence in the United States.
METHODS
Surveillance Data
FoodNet is a collaboration among the US Centers for Disease Control and Prevention’s (CDC’s) Emerging Infections Program (EIP), 10 state health departments, the US Department of Agriculture’s Food Safety and Inspection Service, and the Food and Drug Administration. FoodNet conducts active, population-based surveillance for laboratory-diagnosed cases of shigellosis in the states of Connecticut, Georgia, Maryland, Minnesota, New Mexico, Oregon, and Tennessee and selected counties in California, Colorado, and New York. This surveillance area covers ~15% of the US population [16]. In 2011, FoodNet expanded surveillance to capture cases diagnosed with a culture-independent diagnostic test, such as a multiplex polymerase chain reaction (PCR) panel, in addition to those diagnosed by culture.
For each laboratory-diagnosed case, FoodNet personnel at each site collect clinical, laboratory, epidemiologic, and demographic data, including address of residence, race, and ethnicity. These data are abstracted from medical charts and patient interviews. We created a composite race/ethnicity variable for analysis because race was often missing among persons of Hispanic ethnicity, and <3% of Hispanics reported nonwhite race, similar to the proportion in the 2010 US Census [17]. Composite race/ethnicity variable categories included Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, and “other.” Due to small case numbers, the “other” category combined American Indian/Native Alaskan, Pacific Islander or Native Hawaiian, and multiracial.
Data Collection
Laboratory-diagnosed shigellosis cases with a specimen collection date between January 1, 2004, and December 31, 2014, were included in analyses. Cases among persons who reported traveling internationally ≤7 days before illness onset (incubation period for shigellosis) were excluded from analyses.
Case patient residences were geocoded by each FoodNet site and linked to a Census tract using 2010 US Census boundaries. Census tract data were aggregated by CDC staff and linked to Census tract poverty and crowding data from the American Community Survey (ACS) [18]. The 2006–2010 ACS 5-Year estimates were used for surveillance years 2004–2009, and the 2010–2014 ACS 5-Year estimates were used for surveillance years 2010–2014. Data were unavailable for case patients with a residential address that could not be geocoded to the Census tract level (eg, homeless, PO Box). Following methodology developed by the Harvard Public Health Disparities Geocoding Project [19], our primary socioeconomic measure was Census tract poverty level. Shigella cases were categorized into 1 of 4 poverty strata based on the percentage of households in the Census tract living below the federal poverty line (<5%, 5%–<10%, 10%–<20%, or ≥20%). Breakpoints were chosen to align with similar studies analyzing all-site EIP data [20–23]. Cases were also assigned a Census tract crowding level based on the percentage of households in the Census tract with >1 person per room (<1%, 1%–<3%, 3%–<5%, ≥5%), the national standard for measuring household overcrowding [24]. The first 3 categories were aggregated in our multivariable models.
Statistical Analyses
Within each poverty or crowding stratum, the annual incidence rate (IR) of shigellosis per 100 000 population was calculated overall and for each FoodNet site, sex, age group, and each racial/ethnic category. Census tract–level population denominators by sex, age, and race/ethnicity were obtained from the 2010 US Census. Census tract denominators were aggregated across the poverty and crowding strata separately for cases reported from 2004–2009 and from 2010–2014 using the respective ACS poverty and crowding data. Rates were age-adjusted using the 2000 US standard population to account for age-dependent differences in disease risk and probability of diagnosis. We assessed for a gradient relationship between incidence and each socioeconomic measure using the Cochran-Armitage trend test; P values <.05 were considered statistically significant. Age-adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were used to compare the highest stratum with the lowest stratum of each measure [19, 25].
Adjusted shigellosis IRRs were also estimated using multivariable logistic regression with a population denominator offset (log [person-years exposed]). Including a denominator offset, which has a regression coefficient of 1, allows for modeling rates rather than counts. Explanatory variables included sex, age group, race/ethnicity, FoodNet site, poverty, and crowding. We examined all 2-way interactions among sex, age group, race/ethnicity, poverty, and crowding. Cases of unknown race/ethnicity were excluded from regression models.
Our initial Poisson regression model had a poor fit, as measured by deviance and a Pearson chi-square statistic much greater than 1. We fit a negative binomial model, which allows the variance to exceed the mean, and better accounts for overdispersion and excess 0s in the surveillance data [26–28]. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Of 25 795 laboratory-diagnosed shigellosis cases reported to FoodNet during 2004–2014, 89% (23 028) were successfully geocoded to a Census tract, ranging from 70% in New Mexico to 97% in the New York FoodNet site. We further excluded 1684 patients who reported international travel ≤7 days before illness onset, 6 who were assigned a Census tract without poverty and crowding data available, and 92 for whom sex, age, or both were missing. Characteristics of the remaining 21 246 cases and average annual shigellosis incidence are shown in Table 1.
Table 1.
Characteristics and Annual Incidence of Geocoded Shigella Cases, FoodNet, 2004–2014
| Characteristic | No. of Cases | % | Incidence per 100 000 Population |
|---|---|---|---|
| All cases | 21 246 | 100.0 | 4.1 |
| Sex | |||
| Male | 10 778 | 50.7 | 4.3 |
| Female | 10 468 | 49.3 | 4.0 |
| Age group, y | |||
| 0–4 | 6329 | 29.8 | 19.0 |
| 5–17 | 6968 | 32.8 | 7.8 |
| 18–49 | 6008 | 28.3 | 2.6 |
| ≥50 | 1941 | 9.1 | 1.2 |
| Race/ethnicitya | |||
| White, non-Hispanic | 8736 | 47.6 | 2.6 |
| Black, non-Hispanic | 5604 | 30.6 | 7.2 |
| Hispanic | 3250 | 17.7 | 5.6 |
| Asian, non-Hispanic | 273 | 1.5 | 1.1 |
| Other | 479 | 2.6 | 3.0 |
| Census tract povertyb | |||
| <5% | 2244 | 10.6 | 2.0 |
| 5%–<10% | 3817 | 18.0 | 3.1 |
| 10%–<20% | 6684 | 31.5 | 4.3 |
| ≥20% | 8501 | 40.0 | 7.0 |
| Census tract crowdingc | |||
| <1% | 6785 | 31.9 | 3.1 |
| 1%–<3% | 6504 | 30.6 | 4.1 |
| 3%–<5% | 3542 | 16.7 | 5.3 |
| ≥5% | 4415 | 20.8 | 6.3 |
aA total of 2904 cases were missing race/ethnicity.
bDefined as the percentage of persons in the Census tract living below the federal poverty line.
cDefined as the percentage of households in the Census tract with >1 person per room.
During 2004–2014, the annual FoodNet Shigella IR was 4.1 per 100 000 population and was higher among males (4.3), children aged <5 years (19.0), blacks (7.2), and Hispanics (5.6) (Table 1). Crude shigellosis IR increased with Census tract poverty and crowding (Table 1). Total numbers of reported cases and annual IR per 100 000 population were highest in Georgia (8.1) and lowest in Oregon (1.1). Most infections were caused by S. sonnei (79%) and S. flexneri (14%). Species was unknown for 1379 (6%) infections. Overall, 50.7% of cases were male, but this varied by Shigella species and FoodNet site: Among S. flexneri cases, 70.6% were male (ranging from 49.7% in NM to 83.3% in GA), compared with 46.9% of S. sonnei cases (ranging from 43% in NM to 64.9% in CA).
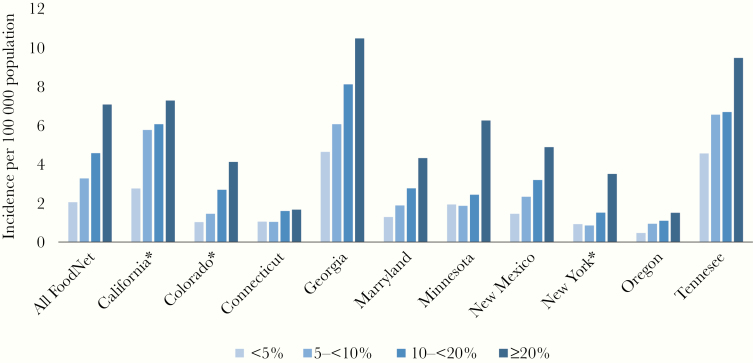
When adjusted for age, persons living in the highest poverty Census tracts (with ≥20% of households below poverty) had 3.5 times the IR of persons in the lowest poverty Census tracts (7.1 vs 2.1) (Table 2). The trend of increasing IR with poverty was statistically significant among all sex, age, and racial/ethnic groups, with the strongest association occurring among children aged <5 years (IRR, 4.8) and among whites (IRR, 3.5). Blacks had the highest overall IR at all levels of Census tract poverty. Among children aged <5 years, the IR among blacks in the most impoverished Census tracts was 10.7 times that of whites in the least impoverished Census tracts (50.3 vs 4.7 per 100 000 population). When stratified by Shigella species, the association with poverty was stronger for S. sonnei (IRR, 3.6; 95% CI, 3.4–3.8) than for S. flexneri (IRR, 2.5; 95% CI, 2.2–2.8) (Table 2) but did not vary by sex. The gradient relationship with increasing poverty was statistically significant in all FoodNet sites (Figure 1) and for all years.
Table 2.
Age-Adjusted Shigellosis Incidence and Incidence Rate Ratios by Census Tract Poverty Level, FoodNet, 2004–2014
| Age-Adjusted Incidence per 100 000 Population by Poverty Levela | ||||||
|---|---|---|---|---|---|---|
| Characteristic | <5% | 5%–<10% | 10%–<20% | ≥20% | IRR (≥20% vs <5%) | 95% CI |
| All cases | 2.1 | 3.3 | 4.6 | 7.1 | 3.5 | 3.3–3.6 |
| Sex | ||||||
| Male | 2.0 | 3.5 | 4.6 | 7.0 | 3.5 | 3.2–3.7 |
| Female | 2.1 | 3.1 | 4.6 | 7.2 | 3.4 | 3.2–3.7 |
| Age group, y | ||||||
| 0–4 | 7.1 | 11.6 | 19.0 | 34.3 | 4.8 | 4.4–5.3 |
| 5–17 | 3.1 | 5.6 | 8.7 | 14.0 | 4.5 | 4.2–4.9 |
| 18–49 | 1.6 | 2.4 | 2.8 | 3.5 | 2.2 | 2.1–2.4 |
| ≥50 | 0.9 | 1.1 | 1.2 | 1.5 | 1.8 | 1.6–2.1 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 1.5 | 2.5 | 3.6 | 5.2 | 3.5 | 3.2–3.7 |
| Black, non-Hispanic | 3.1 | 4.3 | 6.1 | 9.1 | 2.9 | 2.6–3.3 |
| Hispanic | 2.7 | 3.9 | 4.2 | 5.7 | 2.1 | 1.8–2.4 |
| Asian, non-Hispanic | 0.9 | 1.0 | 1.1 | 2.1 | 2.4 | 1.7–3.4 |
| Other | 1.3 | 2.1 | 2.5 | 3.3 | 2.7 | 1.9–3.8 |
| Shigella species | ||||||
| S. sonnei | 1.6 | 2.6 | 3.7 | 5.7 | 3.6 | 3.4–3.8 |
| S. flexneri | 0.3 | 0.5 | 0.6 | 0.9 | 2.5 | 2.2–2.8 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
aDefined as the percentage of households in the Census tract living below the federal poverty line.
Figure 1.
Age-adjusted shigellosis incidence by Census tract poverty level and FoodNet site, 2004–2014. Poverty level was determined by the percentage of households in each Census tract living below the federal poverty line. *In California, Colorado, and New York, the FoodNet surveillance catchment area includes only selected counties [16].
The age-adjusted IR of shigellosis also increased with increasing Census tract household crowding (IRR, 1.8; 95% CI, 1.7–1.9) (Supplementary Table 1). This association did not differ significantly by sex or age group, was most pronounced among whites, and was seen for all years and in all FoodNet sites except among males in California (data not shown). S. flexneri was more strongly associated with crowding (IRR, 2.9; 95% CI, 2.7–3.2) than S. sonnei (IRR, 1.6; 95% CI, 1.6–1.7) (Supplementary Table 1), particularly among females and children (data not shown).
In multivariable models adjusting for sex, age group, race/ethnicity, and FoodNet site, the IR was 2.3 times higher among persons living in the highest (≥20%) vs lowest (<5%) poverty Census tracts (95% CI, 2.0–2.6) (Table 3); the IR was 1.7 times higher among persons in the highest (≥5%) vs lowest (<5%) crowding Census tracts (95% CI, 1.5–1.9) (data not shown). Racial/ethnic differences in the incidence of shigellosis remained significant after controlling for poverty or crowding; the IR among blacks and Hispanics remained >1.5 times that of whites.
Table 3.
Incidence Rate Ratios of Shigellosis by Age, Race/Ethnicity, and Census Tract Poverty, Unadjusted and Adjusted by Negative Binomial Regression, FoodNet, 2004–2014
| Unadjusted | Adjusted | |
|---|---|---|
| Characteristic | IRR (95% CI) | IRR (95% CI) |
| Sex (male vs female) | 1.0 (0.9–1.2) | 1.1 (1.0–1.2) |
| Age group, y | ||
| 0‒4 | 16.1 (15.3–17.0) | 9.5 (8.3–10.8) |
| 5–17 | 6.6 (6.3–7.0) | 4.2 (3.7–4.8) |
| 18‒49 | 2.2 (2.1–2.4) | 1.8 (1.6–2.0) |
| ≥50 (ref) | 1.0 | 1.0 |
| Race/ethnicity | ||
| White (ref) | 1.0 | 1.0 |
| Black | 2.8 (2.7–2.9) | 1.6 (1.4–1.7) |
| Hispanic | 2.2 (2.1–2.3) | 1.8 (1.6–2.0) |
| Asian | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) |
| Other | 1.2 (1.1–1.3) | 0.8 (0.7–0.9) |
| Povertya | ||
| <5% (ref) | 1.0 | 1.0 |
| 5%‒<10% | 1.6 (1.5–1.7) | 1.3 (1.2–1.5) |
| 10%–<20% | 2.3 (2.2–2.5) | 1.7 (1.5–1.9) |
| ≥20% | 3.9 (3.7–4.1) | 2.3 (2.0–2.6) |
IRRs compare the rate in each category with the reference group; they were adjusted for sex, age category, race/ethnicity category, and FoodNet surveillance site. Cases missing these variables were excluded.
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; ref, reference group for IRR.
aDefined as the percentage of households in the Census tract living below the federal poverty line.
In the model including age, race/ethnicity, FoodNet site, and poverty, there was significant statistical interaction between age group and Census tract poverty, and between age group and race/ethnicity. Differences in shigellosis IRs across poverty levels and between racial/ethnic groups were greatest among children. The IRR comparing the highest and lowest poverty Census tracts was 3.2 among children aged 5–17 years (Supplementary Table 2). The IR among black and Hispanic children aged <5 years was 2.6 times the IR among white children (Supplementary Table 3).
Regression results were similar when restricted to S. sonnei, though sex was not a statistically significant explanatory variable. Among S. flexneri, male sex was significantly associated with illness (IRR, 1.9; 95% CI, 1.7–2.3), and there was significant interaction between age and sex. Among adults aged ≥18 years, the IR of S. flexneri infection among males was 3.7 times that among females. There was also a significant interaction between age and race; among children aged <5 years, the IR of S. flexneri infection among Hispanics was 7 times that of whites (95% CI, 5.0–10.6).
DISCUSSION
Our analysis of 2004–2014 FoodNet data documents higher incidence of shigellosis among children, blacks, and Hispanics compared with the general population and reveals a strong association between shigellosis incidence in the United States and Census tract poverty and crowding. This was seen at each FoodNet site and for both S. sonnei and S. flexneri. The incidence of shigellosis increased with Census tract–level poverty and household crowding among all racial/ethnic groups examined. When controlling for sex, race/ethnicity, and FoodNet site, the association with poverty was most pronounced among children. Racial/ethnic disparities in the incidence of shigellosis remained significant after controlling for poverty and crowding.
This was the first multisite analysis of population-based shigellosis surveillance data in the United States that included Census tract–level socioeconomic measures. The identified association between shigellosis and Census tract poverty is consistent with findings from 2 previous studies, which were limited in scope to New York City residents and children in California [13, 14]. Our analysis demonstrates that this association exists in all FoodNet sites, for all age groups, and was consistent across an 11-year time period. Shigella infection could be associated with poverty due to less awareness of prevention measures, lack of access to basic sanitation supplies and diapers, and differences in food safety knowledge, food handling practices, and diet [9, 10, 29, 30].
The observed association with poverty could also reflect differential probability of diagnosis by socioeconomic status. An estimated 20% of persons with symptoms of gastroenteritis seek medical care [31]. Whereas severity of illness is the strongest predictor for care-seeking and subsequent stool testing, seeking care was also found to be associated with age <5 or ≥65 years, lower income, less education, and having medical insurance [31, 32]. Higher-income individuals may be more likely to have private health insurance, but they may be less likely to see a medical provider for mild diarrheal illness due to high co-payments or deductibles, lost work time, or simply because they may have more financial and social resources for dealing with a bout of shigellosis.
Few studies have looked at the association between poverty and shigellosis in other high-income countries [12]. A 1993–2004 study in Denmark found shigellosis incidence to be positively associated with higher individual income and higher education, which the authors attributed to differences in international travel and diet [33]. Our findings may differ for several reasons, including the exclusion of international travel–associated cases and geographic, socioeconomic, and temporal differences in our study population. Additionally, Denmark has a public medical care system that is free to all, which might reduce diagnostic reporting bias.
We also identified an association with Census tract–level household crowding, particularly for S. flexneri. Overcrowded housing, which reflects demographic and socioeconomic conditions, has been linked to an increased risk of infection from communicable diseases and greater vulnerability to homelessness among the poor [34]. Household crowding measured at the level of Census tract may also be a proxy for overall population density in some communities. Shigella outbreaks can easily spread from their initial source (eg, homeless shelter, restaurant, daycare) to the wider population, and transmission may be enhanced in those urban settings where the population has more frequent contact with public facilities (eg, public restrooms, public transportation).
Observed differences by Shigella species, sex, and age group likely reflect differences in primary mode of transmission. Our findings support the literature documenting male predominance among S. flexneri infections, which is largely attributed to increased transmission among MSM [5, 35]. We found S. flexneri infection to be strongly associated with poverty among children and adult females, but not among adult males, which supports the hypothesis that for adult males, other risk factors (eg, oral–anal sexual contact) play a more important role than poverty. Although patient exposures are not collected as part of standard FoodNet surveillance, a FoodNet study found that among domestically acquired shigellosis cases, S. sonnei patients were more likely than S. flexneri patients to report contact with a child or nonsexual contact with a household member with diarrhea (55% vs 17%) [9]. Lack of access to basic sanitation supplies and diapers among low-income families could lead to higher rates of shigellosis [30, 36]. The facts that the association between S. sonnei and crowding did not differ by age group and that the association with poverty was highest among children aged 5–17 years, suggests that shigellosis prevention messaging should target household transmission risks (eg, diaper reuse, handwashing) in addition to daycare exposures.
We observed some variation by FoodNet site, particularly among adults and regarding Census tract crowding. This is not surprising considering the demographic diversity of the underlying populations in each FoodNet site. In some communities, including within the California FoodNet surveillance area, the incidence of shigellosis among adults is increasingly driven by outbreaks and sustained transmission among MSM and persons experiencing homelessness [4, 5]. Geographic variation may also be due to differences in rates or reporting of international travel–associated shigellosis. Although in-depth site-level analyses are beyond the scope of this study, they may shed light on the nuances of risk factors among adults.
As has been seen in similar studies of other communicable diseases [22], racial/ethnic disparities in the incidence of shigellosis remained significant after controlling for socioeconomic explanatory variables. This could be due to racial differences in unmeasured factors, such as education, dietary patterns, and access to and use of health care services [37]. Our results emphasize the need to focus prevention efforts among black and Hispanic households, particularly those with children.
This study has limitations beyond those previously discussed. The ecological nature of this analysis prevents drawing inferences about the effect of individual-level poverty or household crowding on the incidence of shigellosis. Additionally, we were unable to identify infections among persons experiencing homelessness in our geocoded data set. Exclusion of nongeocoded cases (including those with PO Box addresses) might have resulted in differential underestimation of Shigella incidence, particularly in more impoverished and rural Census tracts. Therefore, the reported measures of association are likely conservative estimates.
Incomplete race and ethnicity information in our data set (~14% of cases) might have resulted in an underestimation of shigellosis incidence for some racial/ethnic groups, and therefore could have biased regression results if the race/ethnicity information was not missing at random. Combining American Indian/Alaska Native, Native Hawaiian/other Pacific Islander, and multiracial into a single “other” category prevented assessment of disparities among these groups. Race and ethnicity information abstracted from medical charts may differ from self-described race in the 2010 US Census.
Lastly, we used population denominators from the 2010 US Census for all incidence calculations. Although the population changed from 2004 through 2014, these are the most reliable population estimates at the Census tract level. To account for changes in the geographic distribution of poverty and crowding across Census tracts during this period, all numerators and denominators were aggregated using ACS data obtained during the most relevant time period (2006–2010 or 2010–2014).
In summary, this study estimates the epidemiologic association between shigellosis incidence and poverty and crowding by linking Census tract information to geocoded surveillance data from a national population-based surveillance system. The multivariable analysis revealed that incidence of shigellosis was associated with increasing Census tract poverty and crowding after adjusting for demographic factors and surveillance site. The association with poverty was more pronounced among children than among adults. Shigellosis prevention efforts in the United States should target households that are poor, black, or Hispanic, particularly those with young children.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the many staff at each FoodNet site for collecting and geocoding the data used in this paper, Jennifer Huang and Logan Ray for their assistance in collating the data from the 10 FoodNet sites, and Dr. James Hadler and Cary Parker for comments on the manuscript.
Financial support. This work was supported by the Centers for Disease Control and Prevention (5 NU50CK000482-03-00).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Potential conflicts of interest. None of the authors has a commercial or other association that might pose a conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. This work was presented in part at the International Conference on Emerging Infectious Diseases; August 26–29, 2018; Atlanta, GA (poster board number 339).
References
- 1. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011; 17(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention Shigella - shigellosis. Available at: https://www.cdc.gov/shigella/index.html. Accessed 30 January 2019.
- 3. Garrett V, Bornschlegel K, Lange D, et al. A recurring outbreak of Shigella sonnei among traditionally observant Jewish children in New York City: the risks of daycare and household transmission. Epidemiol Infect 2006; 134:1231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hines JZ, Pinsent T, Rees K, et al. Notes from the field: shigellosis outbreak among men who have sex with men and homeless persons—Oregon, 2015–2016. Morb Mortal Wkly Rep 2016; 65(31):812–3. [DOI] [PubMed] [Google Scholar]
- 5. Aragón TJ, Vugia DJ, Shallow S, et al. Case-control study of shigellosis in San Francisco: the role of sexual transmission and HIV infection. Clin Infect Dis 2007; 44:327–34. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention FoodNet Fast. Available at: https://www.cdc.gov/foodnet/foodnet-fast.html. Accessed 19 February 2019.
- 7. McCrickard LS, Crim SM, Kim S, Bowen A. Disparities in severe shigellosis among adults—Foodborne Diseases Active Surveillance Network, 2002–2014. BMC Public Health 2018; 18:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Yellow book - shigellosis. 2019. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/shigellosis. Accessed 12 February 2019.
- 9. Haley CC, Ong KL, Hedberg K, et al. Risk factors for sporadic shigellosis, FoodNet 2005. Foodborne Pathog Dis 2010; 7(7):741–7. [DOI] [PubMed] [Google Scholar]
- 10. Quinlan JJ. Foodborne illness incidence rates and food safety risks for populations of low socioeconomic status and minority race/ethnicity: a review of the literature. Int J Environ Res Public Health 2013; 10:3634–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang M, Groseclose SL, Zaidi AA, Braden CR. An ecological analysis of sociodemographic factors associated with the incidence of salmonellosis, shigellosis, and E. coli O157:H7 infections in US counties. Epidemiol Infect 2009; 137:810–20. [DOI] [PubMed] [Google Scholar]
- 12. Newman KL, Leon JS, Rebolledo PA, Scallan E. The impact of socioeconomic status on foodborne illness in high-income countries: a systematic review. Epidemiol Infect 2015; 143:2473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greene SK, Levin-Rector A, Hadler JL, Fine AD. Disparities in reportable communicable disease incidence by Census tract-level poverty, New York City, 2006–2013. Am J Public Health 2015; 105(9):e27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson R, Smith D, Tabnak F, Vugia D. Disparities of shigellosis rates among California children by race/ethnicity and Census tract poverty level, 2000–2010. Pediatr Infect Dis J 2015; 34(8):843–7. [DOI] [PubMed] [Google Scholar]
- 15. Krieger N, Chen JT, Waterman PD, et al. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health 2005; 95:312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Foodborne Diseases Active Surveillance Network (FoodNet). Available at: https://www.cdc.gov/foodnet/about.html. Accessed 30 January 2019.
- 17. Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. United States Census Bureau. 2011 Available at: https://www.Census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed 16 February 2019.
- 18. US Census Bureau. American Fact Finder Advanced. Available at: https://factfinder.Census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t. Accessed 11 February 2018.
- 19. Public Health Disparities Geocoding Project Available at: https://www.hsph.harvard.edu/thegeocodingproject/. Accessed 14 February 2019.
- 20. Hadler JL, Yousey-Hindes K, Pérez A, et al. Influenza-related hospitalizations and poverty levels—United States, 2010–2012. MMWR Morb Mortal Wkly Rep 2016; 65(05):101–5. [DOI] [PubMed] [Google Scholar]
- 21. Hadler JL, Clogher P, Huang J, et al. The relationship between Census tract poverty and shiga toxin–producing E. coli risk, analysis of FoodNet data, 2010–2014. Open Forum Infect Dis 2018; 5(7):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burton DC, Flannery B, Bennett NM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am J Public Health 2010; 100:1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chandrasekhar R, Sloan C, Mitchel E, et al. Social determinants of influenza hospitalization in the United States. Influenza Other Respir Viruses 2017; 11:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Census.gov. American Housing Survey (AHS). Available at: https://www.Census.gov/programs-surveys/ahs.html. Accessed 20 May 2019.
- 25. Rothman KJ, Greenland S, Lash TL.. Modern Epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health, Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 26. Byers AL, Allore H, Gill TM, Peduzzi PN. Application of negative binomial modeling for discrete outcomes: a case study in aging research. J Clin Epidemiol 2003; 56:559–64. [DOI] [PubMed] [Google Scholar]
- 27. Kim H, Kriebel D. Regression models for public health surveillance data: a simulation study. Occup Environ Med 2009; 66:733–9. [DOI] [PubMed] [Google Scholar]
- 28. Henao OL, Scallan E, Mahon B, Hoekstra RM. Methods for monitoring trends in the incidence of foodborne diseases: Foodborne Diseases Active Surveillance Network 1996–2008. Foodborne Pathog Dis 2010; 7(11):1421–6. [DOI] [PubMed] [Google Scholar]
- 29. Patil SR, Cates S, Morales R. Consumer food safety knowledge, practices, and demographic differences: findings from a meta-analysis. J Food Prot 2005; 68:1884–94. [DOI] [PubMed] [Google Scholar]
- 30. Smith MV, Kruse A, Weir A, Goldblum J. Diaper need and its impact on child health. Pediatrics 2013; 132:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scallan E, Jones TF, Cronquist A, et al. ; FoodNet Working Group Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog Dis 2006; 3:432–8. [DOI] [PubMed] [Google Scholar]
- 32. Tam CC, Rodrigues LC, O’Brien SJ. The study of infectious intestinal disease in England: what risk factors for presentation to general practice tell us about potential for selection bias in case-control studies of reported cases of diarrhoea. Int J Epidemiol 2003; 32:99–105. [DOI] [PubMed] [Google Scholar]
- 33. Simonsen J, Frisch M, Ethelberg S. Socioeconomic risk factors for bacterial gastrointestinal infections. Epidemiology 2008; 19:282–90. [DOI] [PubMed] [Google Scholar]
- 34. California Health and Human Services Agency. Percent of household overcrowding. Available at: https://data.chhs.ca.gov/dataset/housing-crowding. Accessed 10 February 2019.
- 35. Baker KS, Dallman TJ, Ashton PM, et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis 2015; 15:913–21. [DOI] [PubMed] [Google Scholar]
- 36. Lange BCL, Dáu ALBT, Goldblum J, et al. A mixed methods investigation of the experience of poverty among a population of low-income parenting women. Community Ment Health J 2017; 53:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weinick RM, Zuvekas SH, Cohen JW. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev 2000; 57(Suppl 1):36–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.