Abstract
Background
Motor Complications are an important issue in the management of patients with Parkinson's disease and dopamine agonists have been introduced to ameliorate this problem.
Objectives
To assess the efficacy and safety of adjunct bromocriptine (BR) therapy compared to placebo in the treatment of Parkinson's disease (PD) patients with motor complications.
Search methods
Sources including the Cochrane Library, a MEDLINE search‐strategy, reference lists of the reviews found by the MEDLINE search‐strategy, Sandoz (producer of BR), symposia reports, PD handbooks, SCISEARCH, contacts with colleagues who had co‐ordinated trials on BR and reference lists of all included studies were used to identify randomized controlled trials (RCTs) of interest.
Selection criteria
Randomized trials were eligible for inclusion if they evaluated the efficacy of BR as adjunctive to LD‐therapy compared to placebo in PD patients with motor complications. Outcome measures that were evaluated, included occurrence and severity of motor complications, scores on impairment and disability, and the occurrence of side effects.
Data collection and analysis
Three reviewers independently reviewed the quality of identified trials. To determine the feasibility of a quantitative systematic review each eligible study was evaluated concerning the methodological quality.
Main results
This review identified important shortcomings regarding the methodological quality of eight trials. All studies failed to describe adequately their randomization procedure. Consultation with the trialists revealed that three trials adequately randomized their patients. Contrary to the information of the published report, one placebo‐controlled trial appeared to be carried out as an open study and was therefore excluded. The remaining seven trials were reported to be carried out according to a double‐blind design, although one was unblinded after five weeks. There was a conspicuous variability in the duration of trials: four to forty weeks (mean 14 weeks). None of the included trials was performed according to the intention‐to‐treat principle. With regard to the inclusion criteria, it frequently remained unclear if PD patients actually suffered from motor complications. Prominent differences between studies regarding the baseline characteristics and the rate by which BR was introduced during the titration phase were found. Major differences between studies emerged concerning the applied outcomes. The various methods used to evaluate the occurrence and/or severity of motor complications lacked a sound clinimetric basis. A great diversity of scales to evaluate impairment and disability was applied. None of the included trials reported whether scores on impairment and disability level referred to the "on"‐ or "off"‐phase.
Authors' conclusions
This review highlights major methodological problems and sources of heterogeneity that not only hamper the comparability of trials but also preclude a conclusion on the efficacy of BR in the adjunct treatment of PD patients with motor complications.
Background
Parkinson's disease (PD) is a progressive neurological disorder that produces a slowly increasing disability in movement. The main features of PD are trembling (tremor), increased muscle tone (rigidity), slowness of movement (bradykinesia) and disturbance of posture and balance. These features are caused by a depletion of the neurotransmitter dopamine due to progressive loss of the nigral neurons in the brain (Hornykiewicz 1966, Bernheimer 1973). The general approach to the treatment of patients with PD is the administration of drugs to alleviate symptoms. This may be achieved by the restoration of dopamine by administering its precursor levodopa (LD). LD provides immediate and satisfactory control of most symptoms. However, after 2 to 5 years of stable response to LD treatment, approximately half of the patients develop motor complications (Marsden 1982). Some of these motor complications are felt to be highly correlated with prolonged LD exposure (Marsden 1976, Shaw 1980, and Lees 1989). The motor complications that may appear in a predictable or unpredictable relation to the timing of LD are: ‐ Wearing‐off (end‐of‐dose) is a predictable motor complication in which the perception of loss of mobility or dexterity occurs gradually over minutes (up to an hour) as the effect of the last dose of LD is waning. ‐ On‐off motor fluctuations are generally sudden (seconds to minutes) shifts between "on" (mobility) and "off" (immobility or worsening of parkinsonian features) that are not apparently related and therefore unpredictable to the timing of LD. "Off"‐periods may last minutes to hours. ‐ Dyskinesias are predictable abnormal involuntary movements that occur shortly after or before a single LD dose (diphasic) or in between two LD administrations (peak‐dose). ‐ Dystonia is a movement disorder characterized by sustained or intermittent muscle activity, leading to altered voluntary movement or abnormal postures. Dystonia is one of the most complex motor complications that can occur in PD. On the one hand, dystonia may manifest itself in a predictable relation with the timing of LD, and occur both during the "off"‐ and "on"‐periods (Luquin 1992). On the other hand, dystonia may also appear in untreated patients or as a result of the intrinsic advancement of PD (Poewe 1988). Because of the aforementioned long‐term complications of LD‐therapy new therapeutic approaches were explored. In the 1970's bromocriptine (BR), a dopamine agonist, was introduced as an adjunct to conventional LD therapy in PD patients with motor complications (Calne 1974). It was hoped that this treatment strategy would allow a lower dose of LD, thus potentially alleviating the severity of LD‐related motor complications.
Objectives
To determine the effectiveness and safety of adjunct BR in PD‐patients with motor complications associated with LD‐therapy, the following aspects were evaluated: 1) Does adjunct BR therapy show a reduction in the severity of motor complications compared to monotherapy LD? 2) Does adjunct BR therapy show an improvement in impairment and disability scores compared to monotherapy LD? 3) Do side effects and dropouts occur more frequently in patients receiving adjunct BR therapy than in patients receiving LD and placebo?
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled double‐blind trials relevant to this study. Crossover trials were eligible if the first phase of the study fulfilled these criteria.
Types of participants
Studies with PD patients (diagnosed by the enrolling investigators) that suffered from motor complications associated with LD‐therapy were included. Anticholinergic co‐medication, peripheral decarboxylase inhibitors or the use of Amantadine was permitted.
Types of interventions
Oral BR or placebo.
Types of outcome measures
The following outcome measures were used:
1) Motor complications: the occurrence and severity of "off"‐period related motor fluctuations (wearing‐off and on‐off motor fluctuations, including "off"‐period related dystonia) and dyskinesias (chorea, including "on"‐period related dystonia).
2) Symptomatic efficacy: scores of scales that evaluated impairment (Webster, Columbia University Rating Scale and modified versions of the two former scales), and/or disability (Northwestern University Disability Scale, Webster (1 item)). Impairment‐based items provide a better reflection of the independent elementary features (bradykinesia, tremor) of PD. Disability‐based items depend upon overlapping sets of elementary PD features and are more relevant from the patient's point of view.
3) The occurrence of side effects.
Search methods for identification of studies
Nine sources were consulted in order to locate relevant articles: 1) The Cochrane Controlled Trials Register. 2) The SilverPlatter 3.10 version of MEDLINE. The search strategy for identifying randomized controlled trials (RCTs) was based on the method devised by Dickersin (Dickersin 1994) for the SilverPlatter 3.10 version. This method was slightly adjusted in order to locate publications regarding PD, LD and BR from 1974 onwards. The following search‐strategy was used:
1. RANDOMIZED‐CONTROLLED‐TRIAL in PT 2 RANDOMIZED‐CONTROLLED‐TRIALS 3. RANDOM‐ALLOCATION 4. DOUBLE‐BLIND‐METHOD 5 SINGLE‐BLIND‐METHOD 6. #1 or #2 or #3 or #4 or #5 7. CLINICAL‐TRIAL in PT 8. explode CLINICAL TRIALS/ all subheadings 9. CLIN* near TRIAL* 10. (#9 in TI) or (#9 in AB) 11. (SINGL* or DOUBL* or TREBL* or TRIPL*) near (BLIND* or MASK*) 12. (#11 in TI) or (#11 in AB) 13. RANDOM* 14. (#13 in TI) or (#13 in AB) 15. RESEARCH‐DESIGN 16. #7 or #8 or #10 or #12 or #14 or #15 17. TG=COMPARATIVE‐STUDY 18. explode EVALUATION STUDIES/ all subheadings 19. FOLLOW‐UP‐STUDIES 20. PROSPECTIVE‐STUDIES 21. CONTROL* or PROSPECTIV* or VOLUNTEER* 22. (#21 in TI) or (#21 in AB) 23. #17 or #18 or #19 or #20 or #22 24. #6 or #16 or #23 25. TG=HUMAN 26. #24 and #25 27. explode PARKINSON'S DISEASE/ all subheadings 28. PARKINSON* 29. (#28 in TI) or (#28 in AB) 30. explode BROMOCRIPTINE/ all subheadings 31. (BROMOCR* in TI) or (BROMOCR* in AB) 32. (PARLODEL* in TI) or (PARLODEL* in AB) 33. (CB‐154 in TI) or (CB‐154 in AB) 34. explode LEVODOPA/ all subheadings 35. (LEVODOPA* in TI) or (LEVODOPA* in AB) 36. (L‐DOPA in TI) or (L‐DOPA in AB) 37. #27 or #29 38. #30 or #31 or #32 or #33 39. #34 or #35 or #36 40. #26 and #37 and #38 and #39 41. #40 and (PY>"1974")
3) Reference lists of the reviews found by running the above‐mentioned MEDLINE search‐strategy. 4) Sandoz (producer of Parlodel). 5) Symposia‐reports. 6) PD Handbooks. 7) SCISEARCH. 8) Colleagues who had co‐ordinated trials on BR were contacted for information on unpublished studies. 9) Reference lists of all included studies.
Data collection and analysis
Three reviewers independently reviewed the identified trials according to a two‐step review process. First, the abstracts were reviewed for eligibility. Thereafter, eligible reports of studies were reviewed. Discrepancies were registered and resolved by consensus with a fourth reviewer.
To determine the feasibility to perform a quantitative systematic review on adjunct BR therapy in PD patients with motor complications, the following issues for each of the eligible studies were first addressed: 1) Application of general principles of trial methodology. 2) Patient baseline characteristics. 3) BR titration schedules. 4) Assessment procedures and outcome measures.
If the information on the aforementioned issues (1‐4) was insufficiently reported, we attempted to contact the trialists to obtain additional clarifying data.
Results
Description of studies
‐See table: Characteristics of included studies.‐
* Number of trials identified
During the period of 1974 to January 1998, seven eligible RCTs that evaluated BR versus placebo in PD patients with motor complications were identified. These studies randomized almost 400 patients to either a BR or a placebo regimen.
* Patient characteristics.
Not all of the included trials adequately described the included patient population. Reasons for inclusion sometimes suggested a possible role of motor complications. Some listed the number of patients that suffered from motor complications per type of complication (Hoehn, Schneider, Temlett, and Toyokura). Bateman included a patient with the Shy‐Drager syndrome and Toyokura included ten patients with symptomatic parkinsonism: possibly, these patients had an initial diagnose of idiopathic PD, which was revised in a later stage. The mean age of all the participants was 62.9 years (range 30 ‐ 81 years). Only Toyokura reported age at onset. The mean disease duration ranged from 8.5 (Gron, Jansen) to more than 13 years (Temlett). There were substantial differences between the studies concerning the mean pre‐trial daily dosages of LD and the reported ratios of LD/decarboxylase inhibitor. The mean pre‐trial LD treatment duration ranged from at least one year (Toyokura) to seven years (Hoehn).
* Titration schedule design characteristics.
Five trials introduced BR at dosage of 2,5 mg daily; the remaining two studies started with 1,25 mg BR every day (Hoehn and Toyokura). Maximum BR dosages at the end of the titration phase were reported by all trials and ranged from 20 (Hoehn, Temlett) to 100 mg daily (Jansen). Gron, Bateman, and Jansen reported the diurnal distribution of the BR dosages at the end of the titration phase: three or four times daily. Dose increment of BR varied between the trials, ranging from 1,25 mg each two weeks (Hoehn) to 2,5 mg every second day (Bateman, Gron). Only Schneider allowed a LD reduction during the trial.
* Assessment procedures and outcome measures.
Occurrences of the various types of motor complications were reported by all included trials. Changes in wearing‐off were reported by two (Hoehn, Toyokura) and on‐off by five studies (Bateman, Jansen, Schneider, Temlett, and Toyokura). All trials reported the occurrence of dyskinesias. Additionally, Hoehn and Temlett reported the occurrence of dystonia. Five included trials recorded the severity of motor complications but used different and non‐standardized methods for this purpose. Toyokura and Temlett used four categories ("none", "mild", "moderate" or "severe"), Bateman reported global severity of on‐off fluctuations with a visual analogue scale. In the trial performed by Schneider the duration of "on"‐ and "off"‐hours during the day was reported, while it remained unclear how the severity of dyskinesias was assessed. Hoehn only reported severity of motor complications without specifying any categories. All trials used impairment and six implemented disability scales (all, except Bateman). None of the included RCTs reported if impairment and disability scores referred to the "on"‐ or "off"‐phase. Bateman used a visual analogue scale to assess items as writing, tremor, walking, speech and global severity of motor fluctuations. Toyokura evaluated parkinsonian features, i.e. akinesia, tremor, rigidity and gait disturbance, and activities of daily life using a semi‐quantitative scale ranging from 0 to 4, assessing respectively impairment and disability. Occurrences of side effects were reported by Toyokura, Jansen and Hoehn, and partly by Schneider. The remaining three trials reported only the side effects that resulted in withdrawal of patients.
Risk of bias in included studies
All studies showed shortcomings of the reported information on relevant methodological issues of the trial. Therefore, we attempted to approach all trialists to obtain additional clarifying data. Of seven trialists, four responded (Bateman, Jansen, Temlett, and Toyokura). One trialist was deceased and therefore the requested information could not be retrieved (Gron).
* Trial design Five trials used a parallel placebo group. Bateman's and Gron's study had a crossover design. In one trial all patients started with placebo during the first four weeks (Hoehn). Subsequently the patients were allocated to BR or placebo for 32 weeks. Hereafter, both groups continued on placebo for 4 weeks. Temlett broke the randomization code after a five weeks' duration and patients allocated to placebo were transferred to BR; therefore only these first five weeks were evaluated for this review.
* Study duration There was a highly variable trial duration: four (Schneider) to thirty‐two weeks (Hoehn). The mean duration of the included trials was 14.2 weeks.
* Assessors and centers Most trials were unclear about the number of assessors that participated in a study. The patients were evaluated by one assessor in the trials performed by Bateman and Jansen, and by at least two assessors in Temlett's study. For all other studies, this remained unclear. Toyokura reported the numbers of centers, that is fifty‐nine.
* Randomization Only Bateman partly described the allocation procedure. Additionally obtained information showed that four trials (Bateman, Jansen, Temlett, and Toyokura) adequately randomized their patients by means of a computer randomization procedure. Hoehn apparently randomized 27 patients to the BR branch, while nine patients were allocated to the placebo group. None of the studies reported if treatment assignment was concealed until the assessment of the outcomes. Additionally obtained information showed that the treatment assignment of three trials (Jansen, Temlett, and Toyokura) was adequately concealed until outcome assessment.
* Trial performance All studies were performed according to a double‐blind design. Temlett's study was unblinded before the follow‐up period was accomplished; only the data of the double‐blind period was evaluated for this review.
* Attrition characteristics All trials provided detailed information on the reasons for patients leaving the trials. Toyokura did not specify all side effects that caused the withdrawal of patients from the study.
* Data analysis None of the trials provided intention‐to‐treat data.
Effects of interventions
* Motor complications All trials reported outcomes in motor complications, but focused on different aspects. Three trials (Jansen, Temlett, and Toyokura) reported an increase of the occurrence of dyskinesias in the group using BR. Only Toyokura reported this difference to be statistically significant. Schneider reported no difference with respect to the occurrence of dyskinesias, but this study was the only one allowing a LD reduction during the trial. Hoehn reported no difference in the occurrence of dyskinesias between the two groups, although no statistical evaluation was mentioned. For wearing‐off no (or marginal) difference between the two tiers was found in two studies (Hoehn, Toyokura). Schneider and Toyokura reported improvement of patients in the BR tier with respect to on‐off fluctuations. This difference was statistically significant in the former, and non‐significant in the latter. Two trials reported no change in "on" and "off" time (Jansen, Temlett). Compared to placebo, the patients on BR improved with respect to duration and severity of dystonia in the trial performed by Hoehn. Temlett reported no differences in dystonia between the two groups. Unfortunately, two trialists did not provide data on motor complications at the end of the first phase of their trials and therefore, the results could not be evaluated (Bateman and Gron).
* Impairment All trials reported outcomes at the impairment level. Compared to placebo, BR reduced impairment scores, which were statistically significant in two studies (Jansen, Schneider). Hoehn reported the results on impairment for different subgroups of the BR tier, which were based on the disease severity at baseline. Statistically significant improvement was reported only for the patients with baseline scores less than 50 (modified Columbia scale, the maximal score being 96) (Hoehn). Toyokura found a statistically non‐significant improvement at the impairment level. Temlett did not provide outcomes at the moment patients using placebo switched to BR. Likewise, both Gron and Bateman did not provide data at the moment of crossover.
* Disability Six trials reported outcomes at the disability level (all, except Bateman). Jansen and Schneider found a statistically significant improvement for disability in the BR tier, whereas Toyokura reported a statistically non‐significant improvement. Temlett, Bateman, and Gron did not provide data on disability at the end of the first phase of their trials and therefore the results could not be evaluated.
* LD dose reduction Only the trial performed by Schneider allowed a LD dosage reduction. This study reported a statistically non‐significant reduction in LD dose for both groups.
* Side effects Occurrences of side effects were reported by two trials (Hoehn and Toyokura) and partly by two others (Jansen and Schneider). Compared to placebo, Hoehn found only transient nausea, vomiting, and episodic sweating slightly more frequent in those receiving BR (statistical significance not available). Toyokura reported no statistically significant differences in incidences of side effects between the two groups. Three trialists revealed only the side effects responsible for withdrawal of patients.
* Number of withdrawals ‐Metaview. Tables and Figures.‐ Dropout rates of Gron's study reflect the first part of the crossover study. With respect to dropout rates due to orthostatic hypotension, mental disturbances, poor compliance, deterioration of PD, and all causes, no differences were found between BR and placebo. In Bateman's study, the patient with the Shy‐Drager syndrome was not among the dropouts. Regarding other rare causes of withdrawal, no major differences were found between both tiers.
Discussion
This review identified important shortcomings regarding general and PD‐related trial methodology in seven eligible trials that evaluated the efficacy and safety of adjunct BR therapy in PD patients with LD‐induced motor complications.
With respect to general trial methodology, all trials failed to describe adequately the randomization procedure in their published report. However, additionally obtained information revealed that four trials applied an adequate randomization procedure. All trials were reported to be carried out according to a double‐blind design, although one was actually unblinded after five weeks. One trial, reported as being carried‐out according to a double‐blind design, finally turned out to be an open trial after additional information from the trialist and was excluded for this reason (Mackenzie). None of the included trials was performed according to the intention‐to‐treat principle. Intention‐to‐treat analysis reflects clinical practice and failure to analyze by this principle can give misleading interpretations.
We found a conspicuous variability in the duration of trials: one to nine months. There is no consensus on the minimum duration of a trial that evaluates the influence of a drug in this phase of PD. It is not unusual for patients in this phase of Parkinson's disease to develop further worsening after a previous short‐lasting (weeks) good control of motor complications. Hence, the clinical validity of short‐term results in this phase of PD is questionable.
With regard to the inclusion criteria, it frequently remained unclear if PD patients actually suffered from LD‐induced motor complications. Trials included patients that initially did not respond to LD or cases other than PD (Parkinson‐plus syndromes). Although randomization would equally distribute these patients over both tiers of the trial, they are likely to influence the overall trial outcome. Moreover, between studies prominent differences regarding the baseline characteristics, including disease duration, LD pre‐trial duration and dosage emerged. These differences are likely to influence the performance of trials. Prominent differences regarding the rate by which BR was introduced during the titration phase were found. Large differences between trials concerning the execution of the titration phase may influence the occurrence of adverse events and consequently the dropout rate. Major differences between studies emerged regarding the evaluated outcomes. Although all studies assessed the occurrence of different aspects of LD‐induced motor complications, different and non standardized methods were applied. Additionally, many different impairment and disability scales were used, and none of the included trials reported whether scores on impairment and disability level referred to the "on"‐ or "off"‐phase.
The conspicuous differences in applied general principles of trial conduct and PD‐related methodology between the trials hamper the comparability of trials and preclude a final quantitative synthesis. Nevertheless, some limited qualitative conclusions can be drawn. Three trials (that did not allow a LD dose reduction) reported an increased occurrence of dyskinesias in the BR tier. The largest of these trials reported this difference to be statistically significant. One trial allowed a LD dosage reduction and found no difference with respect to the occurrence of dyskinesias. This underscores the influence of trial performance on the occurrence of dyskinesias. Two studies that evaluated wearing‐off reported no clinically relevant difference between both tiers. Four studies evaluated the occurrence of on‐off fluctuations. These fluctuations showed a trend towards improvement in one, statistically significant improvement in one, and no change in two studies.
Four trials reported an improvement of impairment, which was statistically significant in two. Three trials reported improvement of disability. In only two of these trials, the results were statistically significant.
The incidences of side effects were fully reported by two, and incompletely by two other studies. From these trials, no clear pattern of BR‐related side effects emerged. With respect to the adverse events that resulted in withdrawal of patients, a trend was found for orthostatic hypotension. This occurred more frequently in the group using BR.
Although several reviews on the role of BR in the management of PD patients with motor complications have appeared, this issue has remained controversial (Stern 1978, Hardie 1985, Lieberman 1985, Calne 1986). These reviews summarized and/or evaluated the results of mostly uncontrolled or non‐randomized trials. It should be noted that four RCTs on this issue have appeared before 1985. Hardie on the other hand emphasized several methodological issues that hampered the interpretation of available trials in PD patients with motor complications treated with BR.
This review highlights major methodological problems and sources of heterogeneity that not only hamper the comparability of trials but also preclude a conclusion on the efficacy of BR in the adjunct treatment of PD patients with motor complications.
Authors' conclusions
Implications for practice.
The treatment of PD, lacking a cure, aims to limit the gradually increasing amount of disability. In this regard, the most effective strategy is the treatment with LD, which improves some of the PD features. However, for every year of LD treatment the number of patients that will develop motor complications increases. These complications contribute to an additional disease burden and become a source of increased medical care. In the 1970's, BR was introduced as an adjunct to conventional LD therapy in PD patients with motor complications (Calne 1974). This treatment strategy aimed to alleviate the severity of LD‐related motor complications. During the period of 1974 to January 1998, eight RCTs were identified that evaluated the efficacy of adjunct BR therapy in PD patients suffering from motor complications. However, during our review process major methodological problems that hamper the comparability of these BR trials were found. Consequently, our findings preclude a conclusion on the efficacy of BR as an adjunct to LD in PD patients with motor complications.
Implications for research.
This review emphasizes many methodological shortcomings of seven adjunct BR trials in PD patients suffering from motor complications. The issues arising from this review have a significant bearing on the conduct of future dopamine agonist trials in this type of patient.
There is a clear requirement to apply a uniform general and PD‐related trial methodology. With respect to the former we underscore the application of the guidelines suggested by the Consolidated Standards of Reporting Trials (CONSORT, 1996).
In addition to the CONSORT statement, trials should encompass a clear description of relevant aspects of PD‐related methodology: ‐ Inclusion and exclusion criteria: trials should aim to enrol uniform cohorts of PD patients. ‐ Diagnostic criteria: application of the UK brain bank criteria for all enrolled patients. ‐ Titration phase: titration schedules are strongly dependent on the drug that will be evaluated. Nevertheless, there is a need of standardization of titration schedules for trials that deal with the same drug. ‐ Assessments: motor complications in PD contribute to an additional disease burden. Hence, the evaluation of the efficacy of dopamine agonists in this phase of PD should not only focus on motor complications, but also encompass the assessment of disability. Trials should record similar types of motor complications. This raises the need for more information on the assessor reliability on evaluations of dyskinesias. Additionally, scales that assess motor complications should be standardized and based on a sound clinimetric methodology. Regardless of the scale used, trials should report whether scores on impairment and disability refer to the "on"‐ or "off"‐phase. A standardized scale that evaluates impairment and disability is strongly desired in order to generate comparable scores. However, it is emphasized that the development of new scales is an ongoing process that will probably hinder the choice for one standard rating scale. We suggest the use of outcomes that can be used parallel with the endpoints that are selected by a trialist. These co‐endpoints would facilitate the international comparability of trials that deal with the same drug and population. This suggest the need for a taskforce that will develop a proposal for these co‐endpoints.
What's new
| Date | Event | Description |
|---|---|---|
| 14 November 2008 | Amended | Converted to new review format. |
History
Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 22 May 1998 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful for the information provided by Dr.D.N.Bateman, Dr.E.N.H.Jansen, Dr.R.A.Mackenzie, Prof.Y.Mizuno, Prof.Dr.Pakkenberg, Prof.J.A.Temlett, Drs.E.v.d.Walt.
Data and analyses
Comparison 1. Bromocriptine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5 Withdrawal rate: all causes | 6 | 385 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.68, 1.95] |
| 7 Withdrawal rate: orthostatic hypotension | 5 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.32 [0.83, 47.86] |
| 10 Withdrawal rate: mental disturbances | 5 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.36, 5.47] |
| 11 Withdrawal rate: deterioration PD | 6 | 385 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.12, 2.04] |
| 12 Withdrawal rate: poor compliance | 6 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.28, 3.35] |
| 14 Withdrawal rate: other reasons | 6 | 389 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.11, 1.57] |
1.5. Analysis.
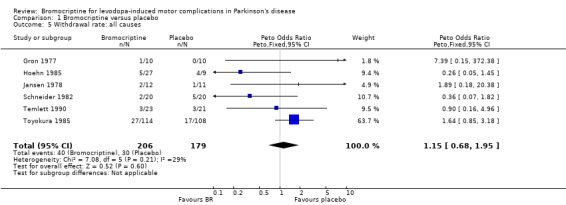
Comparison 1 Bromocriptine versus placebo, Outcome 5 Withdrawal rate: all causes.
1.7. Analysis.
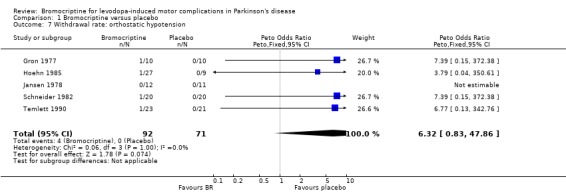
Comparison 1 Bromocriptine versus placebo, Outcome 7 Withdrawal rate: orthostatic hypotension.
1.10. Analysis.
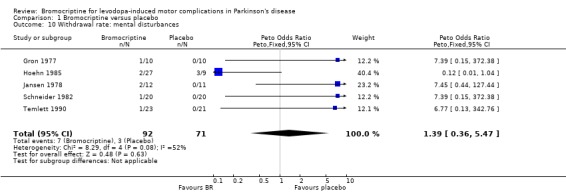
Comparison 1 Bromocriptine versus placebo, Outcome 10 Withdrawal rate: mental disturbances.
1.11. Analysis.
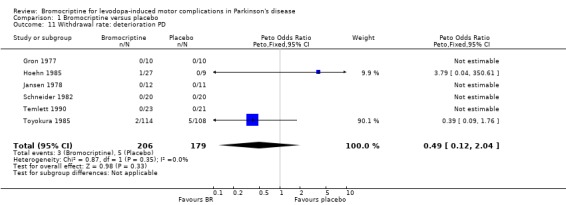
Comparison 1 Bromocriptine versus placebo, Outcome 11 Withdrawal rate: deterioration PD.
1.12. Analysis.
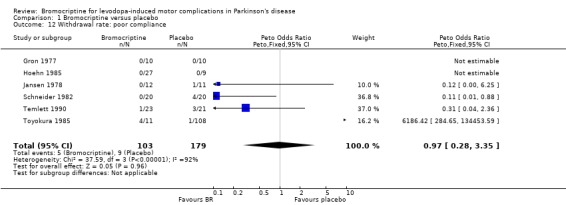
Comparison 1 Bromocriptine versus placebo, Outcome 12 Withdrawal rate: poor compliance.
1.14. Analysis.
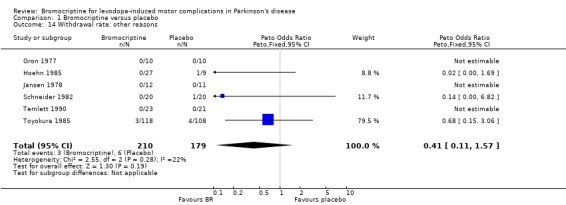
Comparison 1 Bromocriptine versus placebo, Outcome 14 Withdrawal rate: other reasons.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bateman 1978.
| Methods | Randomized: Random number tables. 'Double‐blind'. Design: two period cross‐over (no wash‐out). Duration: 6 weeks in each period (preceeded by 4 weeks dose‐titration). | |
| Participants | Country: UK. Diagnose: Parkinsonism with the "on‐off" syndrome. No.randomized: 11. Mean age: 58.1 (36‐72) years. Mean disease duration: not available. Mean LD treatment duration: over 2 years. Mean daily LD dosage: 357.5 mg Sinemet˜1[4], 1191,7 mg Sinemet˜2 [3], drop‐outs [4]: na. | |
| Interventions | 1. BR (start: 2,5 mg/day, increased with 2,5 mg/ 2 days to a max. of 30 mg in a 4 weeks period). 2. placebo. Used scales:visual analogue scales. | |
| Outcomes | On‐off fluctuations: not available#. Dyskinesias: not available#. Impairment: not available#. Disability: not available#. | |
| Notes | LD:DDI ratio = ‐Sinemet˜1: 10:1. ‐Sinemet˜2: 4:1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gron 1977.
| Methods | Randomized: method not described. 'Double‐blind'. Design: two period cross‐over. Trial duration: 12 weeks in each period. | |
| Participants | Country: Denmark. Diagnoses: Idiopathic parkinsonism with a decreasing LD effect or increasing side effect. No.randomized: 20. Mean age:64 (50‐81)years. Mean disease duration: 8.5 years. Mean LD treatment duration: 4 years. Mean daily LD dosage: 2663 mg LD [4], 554 mg Madopar [7], 653 mg Sinemet [9]. | |
| Interventions | 1. BR (start: 2,5 mg/day, increased with 2,5 mg/2 days to a max. of 30 mg in a 30 days period) [10]. 2. placebo [10]. Used scales: Webster and NUDS. | |
| Outcomes | On‐off fluctuations: not available#. Dyskinesias: not available#. Impairment: not available#. Disability: not available#. | |
| Notes | LD:DDI ratio = ‐Madopar: 4:1. ‐Sinemet: 4:1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hoehn 1985.
| Methods | Randomized: method not described (placebo:BR‐ratio = 1:3). 'Double blind'. Duration: 32 weeks (preceeded and followed by 4 weeks placebo for both groups). | |
| Participants | Country: US. Diagnoses: PD with peak‐dose dyskinesias, wearing‐off effects and/ or off‐dose movements. No. randomized: 36. Mean age: 62.9 (41‐78) years. Mean disease duration: 9.9 (1‐25) years. Mean LD treatment duration: 7 (1‐13) years. Mean LD dosage: 3188 mg LD [2], 677.6 mg Sinemet [34]. | |
| Interventions | 1. BR (start: 1.25 mg/day first increased with 1.25/ 2 weeks, later with 2.5 mg/ 4 weeks, to a max. of 20 mg in a 36 weeks period [27]. 2. placebo [9]. Used scales: modified CURS. | |
| Outcomes | Wearing‐off: no change. Dyskinesias: no change. Dystonia:decreased (stat.s.na). Impairment: reduced (stat.S. for baseline<50 see text). Disability:not available (stat.s.na). | |
| Notes | LD:DDI ratio = ‐Sinemet: not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jansen 1978.
| Methods | Randomized: by means of a computer. 'Double‐blind'. Duration: 10 weeks dose titration, followed by 10 weeks at maximally effective dose. | |
| Participants | Country: Holland. Diagnoses: Advanced PD with deteriorating respons, insufficient LD response and/or initial LD failure. No. randomized: 23. Mean age: 58.5 years (BR), 59 years (placebo). Mean disease duration: 8.8 years (BR), 8.5 years (placebo). Mean LD treatment duration: 4.6 years (BR), 4.3 years (placebo). Mean LD dosage: 2930 mg LD (BR), 2237 mg LD (placebo). | |
| Interventions | 1. BR (start: 2.5 mg/day, increased to a max. of 100 mg in a 10 weeks period [12]. 2. placebo [11]. Used scales: Webster and NUDS. | |
| Outcomes | On‐off fluctuations: no change. Dyskinesias: increased (stat.s.na). Impairment: reduced (stat.S.). Disability: reduced (stat.S.). | |
| Notes | Only plain LD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schneider 1982.
| Methods | Randomized: method not described. 'Double‐blind'. Duration: 4 weeks. | |
| Participants | Country: Germany. Diagnoses: PD with decreasing LD effect, dyskinesias and/or on‐off syndrome. No.randomized: 40. Mean age: 64.8 years (BR), 64.5 years (placebo). Mean disease duration: 8.6 years (BR), 9.6 years (placebo). Mean LD treatment duration: 68.5 months (BR), 77.0 months (placebo). Mean daily LD dosage: 700 mg LD+DDI (BR), 710 mg LD+DDI (placebo). | |
| Interventions | 1. BR (start: 2.5 mg/day, increased to a max. of 30‐40 mg) [20]. 2. placebo [20]. LD reduction was permitted. Used scales: modified Webster. | |
| Outcomes | On‐off fluctuations: improved (stat.S.). Dyskinesias: no change. Impairment: reduced (stat.S.). Disability: reduced (stat.S.). LD reduction: slightly for both groups (stat.n.s.). | |
| Notes | LD:DDI ratio = not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Temlett 1990.
| Methods | Randomized: computer generated random numbers. 'Double‐blind'. Duration: 5 weeks followed by 6 weeks open phase. | |
| Participants | Country: South Africa. Diagnoses: PD with dyskinisias, freezing, on‐off phenonema and/or dystonia. No. randomized: 44. Mean age: 64.3 years (BR), 65.3 years (placebo). Mean disease duration: 13.4 years (BR), 13.3 years (placebo). Mean LD treatment duration: not available. Mean LD dosage: 669.3 mg LD (BR), 622.2 mg LD (placebo). | |
| Interventions | 1. BR (start: 2.5 mg/day, increased with 5 mg/ week to a max. of 20 mg in a 5 weeks period [23]. 2. placebo [21]. Used scales: Webster, CURS, NUDS and self‐made scale. | |
| Outcomes | On‐off fluctuations: no change. Dyskinesias: increased (stat.s.na). Dystonia: no change. Impairment: not available#. Disability: not available#. | |
| Notes | LD:DDI ratio = not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Toyokura 1985.
| Methods | Randomized: by means of a computer. 'Double blind'. Duration: 8 weeks. | |
| Participants | Country: Japan. Diagnoses: PD with declining efficacy of LD, wearing‐off phenomena, frozen gait and/or on‐off phenomena. No.randomized: 222. Mean age: 62.5 years (BR), 63.2 years (placebo). Mean disease duration: see text. Mean LD treatment duration: over one year. Mean LD dosage: 418.3 mg LD+DI (BR), 465 mg LD+DDI (placebo) see text. | |
| Interventions | 1. BR (start 1.25 mg/day, increased to a max. of 22.5 mg in a 6 weeks period [114]. 2. placebo [108]. Used scales: self‐made scales. | |
| Outcomes | Wearing‐off phenonema: marginally decreased. On‐off fluctuations: improved (stat.n.s.) Dyskinesias: increased (stat.S.). Impairment: reduced (stat.n.s.). Disability: reduced (stat.n.s.). | |
| Notes | LD:DDI ratio = 4:1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
_______________________________________________________
‐(stat. S.) = statistically significant ‐(stat.n.s.) = statistically non significant ‐(stat.s.na) = statistical significancy not available
[X] = number of patients
# = data not available at moment of crossover/switch to open phase.
‐NUDS = Northwestern University Disability Scale ‐CURS = Columbia University Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mackenzie 1978 | Although the study was reported to be a double‐blind trial, additionally obtained information from the trialist revealed that this was a nonrandomized open study. |
Sources of support
Internal sources
Prinses Beatrix Fonds, Netherlands.
External sources
No sources of support supplied
Declarations of interest
<None>
Edited (no change to conclusions)
References
References to studies included in this review
Bateman 1978 {published and unpublished data}
- Bateman DN, Coxon A, Legg NJ, Reid JL. Treatment of the on‐off syndrome in Parkinsonism with low‐dose bromocriptine in combination with levodopa. J Neurol Neurosurg Psychiatry. 1978; Vol. 41, issue 12:1109‐1113. [DOI] [PMC free article] [PubMed]
Gron 1977 {published data only}
- Gron U. Bromocriptine versus placebo in levodopa treated patients with Parkinson's disease. Acta Neurol Scand. 1977; Vol. 56, issue 3:269‐273. [PubMed]
Hoehn 1985 {published data only}
- Hoehn MM, Elton RL. Low dosages of bromocriptine added to levodopa in Parkinson's disease. Neurol 1985;35:199‐206. [DOI] [PubMed] [Google Scholar]
Jansen 1978 {published and unpublished data}
- Jansen ENH. Bromocrytine in levodopa response‐losing parkinsonism. Eur Neurol 1978;17:92‐99. [DOI] [PubMed] [Google Scholar]
Schneider 1982 {published data only}
- Schneider E, Fischer P‐A. Bromocriptin in der Behandlung der fortgeschrittenen Stadien des Parkinson‐Syndroms. Dtsch Med Wschr 1982;107:175‐179. [DOI] [PubMed] [Google Scholar]
Temlett 1990 {published and unpublished data}
- Temlett JA, Ming A, Saling M, Fritz VU, Blumenfeld A, Bilchik TR, Becker AL, Fourie PB, Reef HE. Adjunctive therapy with bromocriptine in Parkinson's disease. S Afr Med J 1990;78:680‐685. [PubMed] [Google Scholar]
Toyokura 1985 {published and unpublished data}
- Toyokura Y, Mizuno Y, Kase M, Sobue I, Kuroiwa Y, Narabayashi J, Uono M, Nakanishi T, Kameyama M, Ito H, Shimada Y, Iwata M. Effects of bromocriptine in parkinsonism: a nation‐wide collaborative double‐blind study. Acta Neurol Scand 1985;72:157‐170. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mackenzie 1978 {published and unpublished data}
Additional references
Begg
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schilz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials; the CONSORT statement. JAMA 1996;276(8):637‐639. [DOI] [PubMed] [Google Scholar]
Bernheimer
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington: clinical, morphological and neurochemical correlations. J Neurol Sci 1973;20:415‐455. [DOI] [PubMed] [Google Scholar]
Calne
- Calne DB, Teychenne PF, Leigh PN, Bamji AN, Greenacre JK. Treatment of Parkinsonism with bromocriptine. Lancet 1974 Dec;1355‐1356. [DOI] [PubMed] [Google Scholar]
Calne‐2
- Calne DB. Dopaminergic agonists in the treatment of Parkinsonism. In: Clinical Neuropharmacology, edited by HL Klawans. Raven Press, New York 1978;3:153‐166. [Google Scholar]
Dickersin
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardie
- Hardie RJ, et al. The controversial role of bromocriptine in Parkinson's disease. Clin Neuropharmacol 1985;8(2):150‐155. [DOI] [PubMed] [Google Scholar]
Hoehn
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurol (Minneapolis) 1967;17:427‐442. [DOI] [PubMed] [Google Scholar]
Hornykiewicz
- Hornykiewicz O. Dopamine (3‐hydroxytyramine) and brain functions. Pharm Rev 1966;18:925‐964. [PubMed] [Google Scholar]
Lees
- Lees AJ. The on‐off phenomenon. J Neurol Neurosurg Psych 1989; suppl. Vol. suppl.:29‐37. [DOI] [PMC free article] [PubMed]
Lieberman
- Lieberman AN, Goldstein M. Bromocriptine in Parkinson Disease. In: The American Society for Pharmacology and Experimental Therapeutics. Pharmacological Reviews 1985;37(2):217‐227. [PubMed] [Google Scholar]
Luquin
- Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa‐induced dyskinesias in Parkinson's disease: clinical and pharmacological classification. Mov Disord 1992;7(2):117‐124. [DOI] [PubMed] [Google Scholar]
Marsden
- Marsden CD, Parkes JD, Quinn N. Fluctuations of disability in Parkinson's disease ‐ clinical aspects. In: Marsden CD, Fahn S, eds. Movement disorders. London: Butterworths Scientific 1982:Butterworths Scientific 1982; 96‐122. [Google Scholar]
Marsden‐2
- Marsden CD, Parkes JD. On‐off effects in patients with Parkinson's disease on chronic levodopa therapy. Lancet 1976;1:292‐296. [DOI] [PubMed] [Google Scholar]
Poewe
- Poewe WH, Lees AJ, Stern GM. Dystonia in Parkinson's disease: clinical and pharmacological features. Ann Neurol 1988;23(1):73‐78. [DOI] [PubMed] [Google Scholar]
Rijk de
- Rijk MC de, Breteler MMB, Graveland GA, Ott A, Grobbee DE, Meché FGA van der, Hofman A. Prevalence of Parkinson's disease in the elderly: the Rotterdam study. Neurology 1995;45:2143‐2146. [DOI] [PubMed] [Google Scholar]
Schneider
- Schneider E, Fischer P‐A. Bromocriptin in der Behandlung der fortgeschrittenen Stadien des Parkinson‐Syndroms. Dtsch Med Wschr 1983;107:175‐179. [DOI] [PubMed] [Google Scholar]
Shaw
- Shaw K. The impact of treatment with L‐dopa on Parkinson's disease. Q J Med 1980;49:283‐293. [PubMed] [Google Scholar]
Stern
- Stern G, LeesA. Bromocriptine in Parkinson's disease. Britisch Journal of Hospital Medicine 1978;20(6):666‐670. [PubMed] [Google Scholar]
Zhang
- Zhang Z, Roman GC. Worldwide occurrence of Parkinson's disease: an updated review. Neuroepidemiology 1993;12:195‐208. [DOI] [PubMed] [Google Scholar]


