Abstract
Background
Glioma-associated microglia/macrophages (GAMs) comprise macrophages of peripheral origin and brain-intrinsic microglia, which support tumor progression. Chemokine C-C ligand 5 (CCL5) is an inflammatory mediator produced by immune cells and is involved in tumor growth and migration in several cancers, including glioma. However, the mechanisms detailing how CCL5 facilitates glioma invasion remain largely unresolved.
Methods
Glioma migration and invasion were determined by wound healing, transwell assay, and 3D µ-slide chemotaxis assay. The expression levels of CCL5, CD68, matrix metalloproteinase 2 (MMP2), phosphorylated Ca2+/calmodulin-dependent protein kinase II (p-CaMKII), p-Akt, and phosphorylated proline-rich tyrosine kinase 2 were determined by cytokine array, quantitative PCR, western blot, or immunohistochemistry. Zymography and intracellular calcium assays were used to analyze MMP2 activity and intracellular calcium levels, respectively.
Results
CCL5 modulated the migratory and invasive activities of human glioma cells in association with MMP2 expression. In response to CCL5, glioma cells underwent a synchronized increase in intracellular calcium levels and p-CaMKII and p-Akt expression levels. CCL5-directed glioma invasion and increases in MMP2 were suppressed after inhibition of p-CaMKII. Glioma cells tended to migrate toward GAM-conditioned media activated by granulocyte-macrophage colony-stimulating factor (GM-CSF) in which CCL5 was abundant. This homing effect was associated with MMP2 upregulation, and could be ameliorated either by controlling intracellular and extracellular calcium levels or by CCL5 antagonism. Clinical results also revealed the associations between CCL5 and GAM activation.
Conclusion
Our results suggest that modulation of glioma CaMKII may restrict the effect of CCL5 on glioma invasion and could be a potential therapeutic target for alleviating glioma growth.
Keywords: CCL5, glioma, invasion, microglia, MMP2
Key Points.
1. Glioma migration and invasion increased by CCL5 were associated with CaMKII-dependent MMP2 expression.
2. CCL5 of GM-CSF–activated GAMs may increase glioma invasion as the disease progresses.
Importance of the Study.
GAMs are the largest portion of infiltrating immune cells and act to reciprocate the various factors in the tumor mass supporting glioma growth. We identified the GM-CSF–activated GAMs (which may enrich the level of CCL5 in the glioma microenvironment) that human glioma tended to home toward, in which CCL5 was abundantly expressed, and the cellular and molecular events that underlie CCL5-directed glioma invasion. In addition, we showed that intracellular calcium level and p-CaMKII expression were mediators of MMP2 expression. Moreover, we demonstrated that chelation of extracellular calcium and inhibition of p-CaMKII could eliminate CCL5-directed glioma invasion. Collectively, our findings suggest that the CCL5/CC receptor 5 axis may be an important antitumor drug target.
Glioblastoma (GBM) is the most lethal type of primary malignant brain tumor and is characterized by diffusely infiltrative growth.1 Despite current optimal therapies, including surgery, radiation, and chemotherapy, the prognosis of patients with GBM remains poor, with a median survival of about 1.5 years due to therapeutic resistance and tumor recurrence.2 Recurrent glioma arising from residual invasive cells may undergo a multistep reactivation process that facilitates adhesion to the extracellular matrix, secretion of enzymes that degrade the surrounding matrix, and migration through the glioma microenvironment prior to tumorigenesis.3,4 This process involves cross-talk between neoplastic and nonneoplastic cells residing in the tumor microenvironment and reciprocation of the autocrine and paracrine chemotactic factors that give rise to tumor recurrence.5,6 Recently, tumor-associated immune cells including lymphocytes and macrophages/microglia identified in the tumor mass have been shown to induce intrinsic inflammation, thereby contributing to a pro-tumorigenic microenvironment.7,8 As critical immune cells of the central nervous system, microglia/macrophages demonstrably represent the major regulators of pro-angiogenic factors and facilitate glioma development.9 In glioma, chronic inflammation always accompanies tumor development as part of the organism’s self-defense, but this ultimately involves feedback mechanisms that tend to benefit tumor growth.10 Various chemotactic factors, including CC chemokines and colony-stimulating factors, have been identified in the human glioma microenvironment and are associated with immune cell development and migration along with glioma progression.11
Chemokine C-C ligand 5 (CCL5), also known as RANTES (regulated on activation, normal T cell expressed and secreted), is identified as a pro-inflammatory mediator secreted by multiple cell types, including cancer cells and immune cells.12 CCL5 elicits a strong chemotactic activity toward T lymphocytes and monocytes, which are the primary origin of CCL5 associated with chronic inflammatory diseases, including cancers.13 The expression of CCL5 has been shown to positively regulate tumor growth and angiogenesis via the mammalian target of rapamycin/Akt pathway in breast cancer,14 and phosphorylated (p-)Akt activation in glioma.15 Recently, CCL5 from tumor-associated microglia/macrophages was demonstrated to promote tumor progression via CC receptor 5 (CCR5) in gastric cancer and glioma.13,16 Glioma patients with high CCR5 levels have poor survival. Glioma-associated microglia/macrophages (GAMs) have been postulated to play a role in glioma development.15,17
The CCL5/CCR5 axis has been demonstrated to enhance tumor local invasiveness via the induction of intracellular calcium cascades and matrix metalloproteinases (MMPs).18–20 Cell migration depends on adhesion to the extracellular matrix mediated by calcium-induced integrin activation21,22 and proline-rich tyrosine kinase 2 (PYK2).23,24 As a family of zinc-dependent extracellular endopeptidases, MMPs facilitate tissue remodeling, tissue repair, and tumorigenesis via the degradation of extracellular matrix components.25,26 Most MMPs are secreted as inactive pro-MMPs and become activated when cleaved by extracellular proteinases such as membrane type 1 (MT1)-MMP.27,28 Several studies have indicated that MMP2 is highly expressed in human GBM tissues29,30 and that this can regulate tumor invasion.28,31 The expression of MMPs has been associated with the phosphorylation of Akt and Ca2+/calmodulin-dependent protein kinase II (CaMKII) and to be synchronized with calcium influx in neurons and glioma.28,32 Downregulation of phosphatidylinositol-3 kinase/Akt signaling has been previously reported to diminish MMP2 gelatinolytic activity,28 and silencing of MT1-MMP has been shown to hinder both MMP2 expression and intracellular calcium mobility during glioma invasion, indicating the involvement of calcium signaling in MMP-regulated cell invasiveness.21,27,33 In addition, intracellular calcium influx has been demonstrated to produce a synergistic effect during the tumor invasive process as tumor microtubes emerge to consolidate intercellular connections.3
Recently, GAMs have been identified as a critical source of CCL5, which serves to establish a potent paracrine regulatory circuit for glioma growth.16,34,35 As CD8+ tumor-infiltrating lymphocytes (TILs) are also associated with glioma prognosis,36 the involvement of GAMs and CD8+ TILs and the underlying mechanism of CCL5-regulated glioma invasion await validation. In the present study, we sought to determine the regulatory effect of CCL5 produced by GAMs and TILs on glioma migration and invasion. We found that CCL5 enhances the invasiveness of glioma cells through a novel calcium-dependent MMP2 signaling pathway, which could be suppressed via CaMKII small interfering (si)RNA-mediated knockdown. GAMs appeared to provide a more critical source of CCL5 relative to CD8+ TILs, and glioma displayed an increased homing effect along with MMP2 expression toward activated GAM-conditioned media (GAM-CM) in which CCL5 was enriched by granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation. This MMP2 upregulation was downregulated after depletion of intracellular calcium or blockage of CCL5. Collectively, these findings broaden the concept that the production of CCL5 by GAMs is reinforced by growth factors such as GM-CSF residing in the glioma microenvironment, and that this regulates glioma migration and invasion and facilitates intracellular calcium mobilization.
Materials and Methods
Glioma Patient Sample Collection and Usage
This study was approved by the Chang Gung Medical Foundation institutional review board (no. 104-9839B). Records of all patients with a diagnosis of GBM over a 10-year period were retrospectively reviewed. Whole blood samples were collected from glioma patients recruited from the Department of Neurosurgery at Chang Gung Memorial Hospital, Taiwan, between 2015 and 2017. All eligible patients were informed about the details of the study, and written consents regarding utilization of patient samples were obtained prior to sample collection. Patient criteria and characteristics are outlined in Supplementary Tables 1 and 2).
Cell Culture
Human A172 and U87 GBM cells were obtained from the American Type Culture Collection and were maintained in Dulbecco’s modified Eagle’s medium or Minimum Essential Medium. The patient-derived GBM cell line, W802, was obtained from Dr Kuo-Chen Wei at Chang Gung Memorial Hospital. The identity of W802 was validated via short tandem repeat analysis.
Preparation of GAMs from Patient Samples
Tumor biosamples and blood were collected from patients with primary glioma diagnosed histopathologically according to current World Health Organization criteria (Supplementary Figure 1). Tumor tissues were freshly collected during surgery into Roswell Park Memorial Institute (RPMI) medium and separated under sterile conditions as described previously.37,38 Briefly, tissues were washed, digested, and filtered extensively to obtain a single-cell suspension. Cells were labeled with CD11b MicroBeads and isolated using magnetic activated cell sorting separation (Miltenyi Biotec). Isolated GAMs were confirmed to have approximately 90% purity, and day 3 post-culture viability was confirmed with an average of 67% of the cells characterized with Ki-67+ CD11b+ (detailed in Supplementary Figure 2C–F).
Immunohistochemistry
Paraffin sections of glioma tissues were deparaffinized and dehydrated in a graded ethanol series. Antigens were retrieved in boiling citric acid buffer following blockage of nonspecific binding and permeabilization by incubating sections in 5% bovine serum albumin and 0.01% Triton X-100. The sections were stained overnight in polyclonal and monoclonal primary antibodies against human p-CaMKII, CCL5 (Santa Cruz), or cluster of differentiation (CD)68 (Abcam) with 1:500 dilution at 4°C overnight. The samples were then washed and stained with Alexa Fluor–tagged secondary antibody (Thermo Fisher) for 2 h at room temperature. The samples were then counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) and mounted in fluorescent mounting medium (Dako), and images were captured on an upright microscope (DM2500, Leica).
GAM-Conditioned Medium
GAMs collected from tumors of glioma patients were cultured in RPMI-1640 and seeded after reaching confluence. They were then treated with the indicated stimulators, and the medium was collected after 72 h and immediately applied to cultured A172 and U87 cells for analysis (see the Supplementary Material).
Flow Cytometry Analysis
GAMs and lymphocytes were stained with multiple cell surface markers to determine the cell types and percentages of GAM and CD8+ T cells in the tumor tissues and peripheral blood mononuclear cells (see the Supplementary Material).
Cytokine Protein Array
Human glioma tissue lysates were analyzed using a Proteome Profiler human XL cytokine array kit (R&D Systems) according to the manufacturer’s instructions.
Quantitative Real-Time PCR
Quantitative RT-PCR was performed as described in the Supplementary Materials and Methods. The oligonucleotide primers used for RT-PCR are shown in Supplementary Table 3.
Migration and Invasion Assays
Cell invasion was assessed using Matrigel-coated transwell inserts (8.0-µm pore size; Falcon). Cells were seeded to Matrigel-coated inserts (Corning), and the invading cells on the underside of the inserts were fixed and stained with crystal violet. The number of invading cells was counted. A 3D µ-slide chemotaxis assay (Ibidi) was performed to determine the chemotactic responses of glioma cells. Cells were mixed with Matrigel and suspended in a polymerizable 3D gel and treated with GAM-CM to induce chemotactic invasion. Images of cell-free areas covered by invasive cells were measured at 24 h and quantified using ImageJ software v1.47 (NIH; see the Supplementary Material).
Western Blotting
Cells were treated with CCL5, the CaMKII inhibitor KN-93 (KN), the calcium chelator BAPTA-AM (1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid–tetra(acetoxymethyl) ester) (BAP) (Sigma-Aldrich), Met-CCL5 (MET), or anti–GM-CSF (R&D Systems) for the indicated time periods. Protein samples were prepared and transferred to polyvinylidene difluoride membranes, blocked, and probed with primary antibodies against human species. After extensive washing and incubation with secondary antibodies, the blots were visualized using Amersham Hyperfilm electrochemiluminescence (GE Healthcare; see the Supplementary Material).
Zymography Analysis
The culture media of A172 and U87 cells were collected after the indicated treatment to evaluate MMP activity. The culture medium was mixed with non-reducing sample buffer and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis containing 1% bovine type B gelatin (Sigma-Aldrich) followed by washing with 2.5% Triton X-100 to remove sodium dodecyl sulfate and rinsing with 50 mM Tris-HCl (pH 7.5). The gel was incubated in developing buffer for at least 24 h at 37°C for gelatin digestion by MMPs. The enzyme activities of MMPs were determined by staining the gels with 2% Coomassie blue.
Intracellular Calcium Assay
Intracellular calcium was detected using a calcium detection kit according to the manufacturer’s instructions (Abcam).
Small Interfering RNA Transfection
Cells were grown to confluence and transfected with Dharmacon On-Target siRNA (GE Healthcare) against CaMKII or a scramble siRNA according to the manufacturer’s protocol. The siRNA target sequences are outlined in Supplementary Table 4.
Enzyme-Linked Immunosorbent Assay
The CM of GAMs was used to determine CCL5 and GM-CSF levels using a CCL5 and GM-CSF enzyme-linked immunosorbent assay kit (R&D Systems) according to the manufacturer’s instructions.
Statistical Analysis
All results are presented as means ± SE from at least 3 independent experiments unless otherwise stated. Statistical analyses were performed using 2-tailed unpaired Student’s t-tests to determine statistical significance between the groups using SPSS v19, and P-values of <0.05 were considered significant.
Results
CCL5 Level, Tumor Volume, and Survival in Glioma Patients
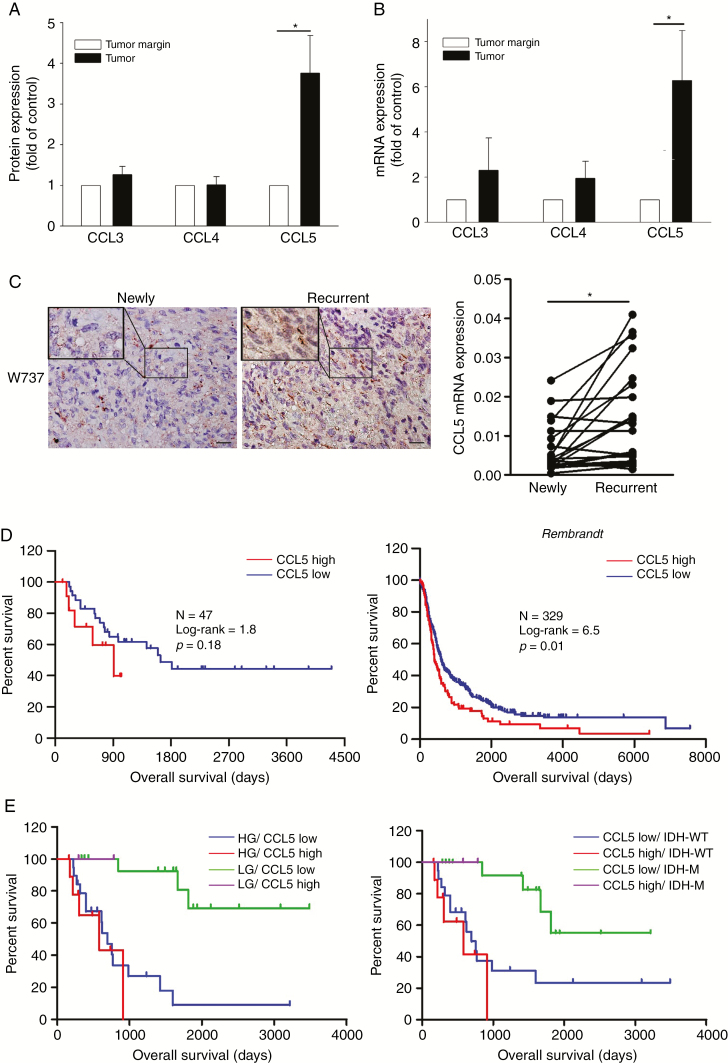
We analyzed the CCR5 ligands in tumor biopsies of 4 newly diagnosed GBMs using protein cytokine arrays. As shown in Figure 1A, the level of CCL5 in comparison to CCL3 and CCL4 was higher in the tumor biopsies than in tumor margin (Supplementary Figure 3A). We confirmed this elevated CCL5 expression using a larger sample size including patients with15 newly diagnosed GBMs (Figure 1B), and the results showed that this elevation of the CCL5 mRNA was even higher in the paired-recurrent gliomas (Figure 1C; Supplementary Figure 3B). The clinical data verified a significant negative correlation between CCL5 and overall survival (OS) of the newly diagnosed cases (Supplementary Figure 3C). Kaplan–Meier survival plots validated that the patients with a low CCL5 level had a substantially longer OS than those with a high CCL5 level (median OS = 1637 vs 909 days; Figure 1D). The database of the National Cancer Institute’s Repository of Molecular Brain Neoplasia Data (REMBRANDT) confirmed a significant survival advantage for patients with a low CCL5 level (median OS = 406 vs 598 days; Figure 1D). Progression-free survival was weakly associated with CCL5 (Supplementary Figure 3D and E). To further investigate the influence of CCL5, we stratified glioma patients based on grading, and isocitrate dehydrogenase (IDH) status. Low-grade glioma patients with low CCL5 levels had longer OS than high-grade glioma patients with high CCL5 levels (hazard ratio [HR] = 0.02, P = 0.001; Figure 1E). While including IDH status, IDH wildtype (WT) was associated with shorter OS (HR = 4.3, P = 0.001; Supplementary Figure 3F). Patients with mutant IDH/low CCL5 were associated with better outcome than those with WT IDH/high CCL5 (HR = 49.46, P = 0.001; Figure 1E). When adjusting for tumor size, age, sex, grade, IDH, and CCL5 status in the multivariate analysis, CCL5 showed minor prognostic value (HR = 1.08, P = 0.90), whereas the prognostic values of grade (HR = 8.30, P < 0.01) and tumor size (HR = 4.06, P = 0.02; Supplementary Table 5) were statistically significant. Further analysis of linear regression revealed that tumor volume was a significant predictor of CCL5 expression level, indicating associations between increased tumor volume and increased CCL5 expression (beta = 0.42, P < 0.01; Supplementary Table 6).
Fig. 1.
Survival analysis results for CCL5 in newly diagnosed glioma. (A) CCL5 expression quantified from 4 newly diagnosed GBM tissues. (B) The CCL5 mRNA expression of newly diagnosed gliomas (n = 15) relative to the tumor margin was determined. (C) IHC staining of CCL5 (brown) in paired newly diagnosed and recurrent GBM tumor sections. CCL5 mRNA identified in paired newly diagnosed (n = 20) and recurrent GBM (n = 20) tumors. (D) Kaplan–Meier survival plots for glioma patients with a low CCL5 level (low = 12, high = 35) showed a longer OS. Using the REMBRANDT database, Kaplan–Meier survival plots confirmed a survival advantage in the patients with a low CCL5 level (low = 83, high = 246). (E) Glioma patients (n = 47) were stratified based on CCL5, grading, and IDH status. LG: low-grade; HG: high-grade; IDH-M: mutant IDH. Bar: 100 µm. All data are presented as means ± SE. *P < 0.05, #P < 0.01.
CCL5 Promotes Heterogeneous Glioma Migration and Invasion
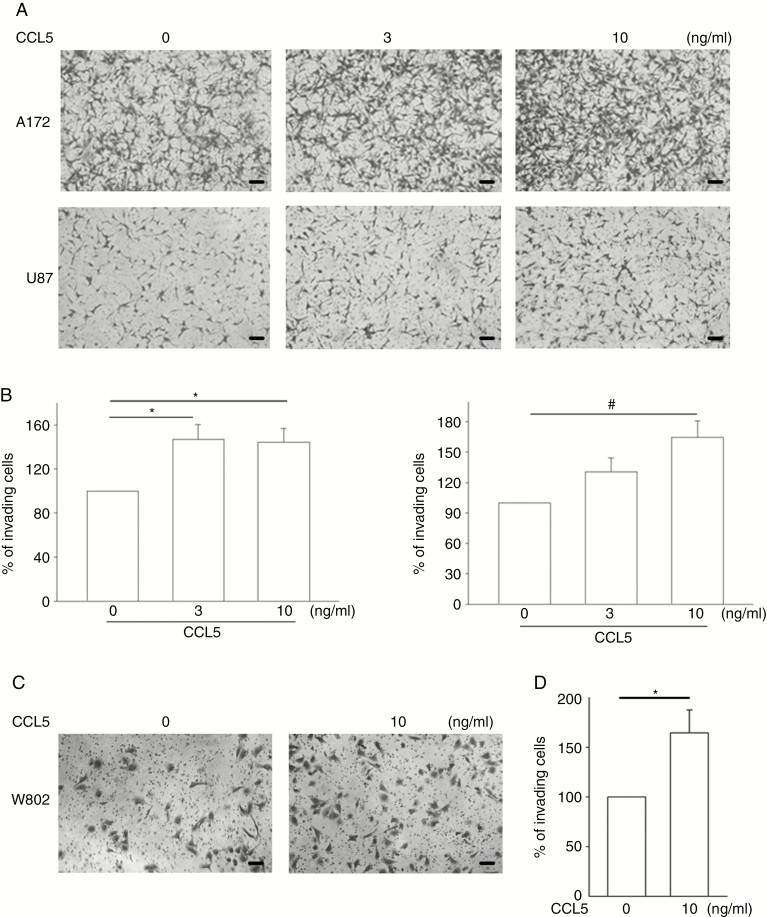
To clarify the direct effect of CCL5 on glioma infiltration, wound-healing and transwell invasion assays were performed on A172 and U87 cells in the presence of CCL5 at different concentrations (0–10 ng/mL). There was no significant reduction in cell-free gaps without the addition of CCL5 after 12 h. However, in the presence of CCL5, the gaps were significantly covered by migrating cells in both the A172 and U87 groups, and this was associated with an increase in gap reduction rate in a CCL5 dose-dependent manner (Supplementary Figure 4A–D). In addition, the transwell invasion assays showed that CCL5 enhanced cell invasiveness, with an approximate 1.5-fold increase in 10 ng/mL CCL5-treated A172 and U87 cells (Figure 2A) and W802 (Figure 2C) cells. Cell invasion ability was quantified as percent of invading cells compared with the groups without CCL5 stimulation (Figure 2B and D).
Fig. 2.
CCL5 promotes heterogeneous glioma invasive activity. (A, C) The effect of CCL5 on glioma invasion was analyzed. The numbers of invading cells were increased in CCL5-treated A172, U87, and W802 cells after 24 h. (B, D) The number of invading cells are presented as percent of invading cells. Bar: 100 µm. All data are presented as means ± SE. *P < 0.05, #P < 0.01.
CCL5 Induces PYK2 Phosphorylation and MMP2 Activation
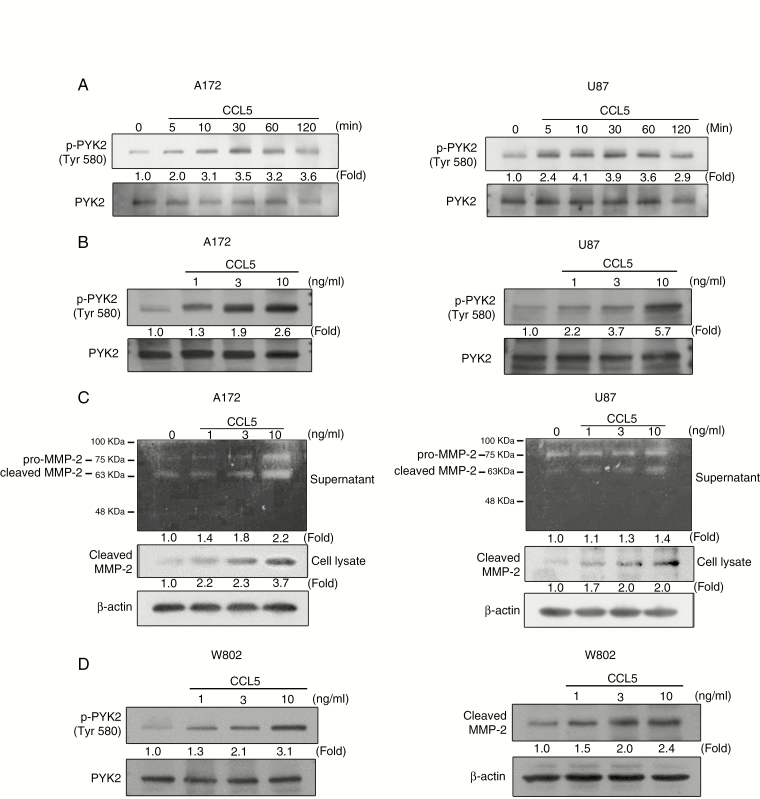
As CCL5 could significantly enhance cell migration and invasion initiated by MMP2 upregulation, we examined the involvement of PYK2 and MMP2 in the CCL5 signaling pathway. As shown in Figure 3A and B, CCL5 induced time- and concentration-dependent phosphorylation of PYK2 in both A172 and U87 cells. The amounts of cleaved MMP2 in gelatin zymography and western blot using CM from CCL5-treated glioma cells were increased in a CCL5 dose-dependent manner in A172 and U87 cells (Figure 3C). W802 cells also showed CCL5-directed upregulation of p-PYK2 and cleaved MMP2 protein expression levels (Figure 3D).
Fig. 3.
CCL5 induces PYK2 phosphorylation and MMP2 activation in glioma cells. (A) PYK2 phosphorylation in A172 and U87 cells was induced by CCL5 (10 ng/mL) in a time-dependent manner. (B) CCL5 (0–10 ng/mL) induced a concentration-dependent increase in PYK2 phosphorylation in A172 and U87 cells. (C) MMP2 protease activity and protein expression were induced by 24 h of CCL5 treatment (10 ng/mL) in both A172 and U87 cells. (D) CCL5 (0–10 ng/mL) induced an increase in PYK2 phosphorylation and cleaved MMP2 expression in W802 cells. Blots are quantified as fold of control.
Controlling Calcium Levels Eliminates CCL5-Regulated Calcium-Dependent Protein Kinases in Glioma
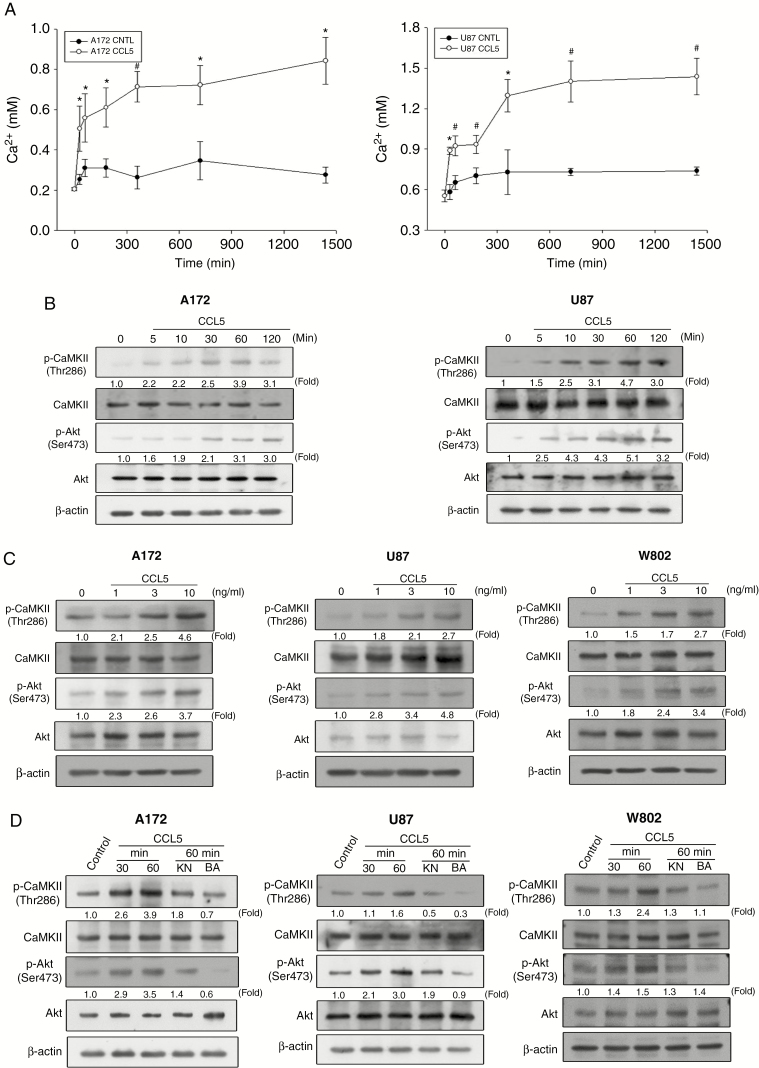
We next investigated whether CCL5 stimulation was correlated with intracellular calcium elevation and the phosphorylation of Akt and CaMKII. The intracellular calcium concentration significantly increased after the addition of CCL5 in both A172 and U87 cells compared with the control groups (Figure 4A). The phosphorylation of CaMKII and Akt was induced after the addition of CCL5 for 5 min, and gradually increased within 60 min of treatment (Figure 4B). Treatment of glioma cells with different concentrations of CCL5 induced concentration-dependent phosphorylation of Akt and CaMKII (Figure 4C). Immunocytochemistry results validated the CCL5-induced phosphorylation of CaMKII in A172, U87, and W802 cells (Supplementary Figure 5A–C), which was consistent with the p-CaMKII expression identified in newly diagnosed and recurrent GBM (Supplementary Figure 5D). Inhibition of CaMKII phosphorylation by KN or blocking of calcium influx via BAP significantly reduced Akt and CaMKII phosphorylation in response to CCL5 stimulation (Figure 4D).
Fig. 4.
CCL5 induces a calcium-dependent signaling pathway in a concentration- and time-dependent manner in glioma cells. (A) CCL5 (10 ng/mL) induced an increase in intracellular calcium from 60 to 1440 min in A172 and U87 cells. (B) CCL5 (10 ng/mL) induced CaMKII and Akt phosphorylation over the indicated time periods. (C) CaMKII and Akt phosphorylation was increased in a concentration-dependent manner by CCL5 (0–10 ng/mL). (D) CaMKII and Akt phosphorylation was determined after cells pretreated with KN (20 µM) or BAP (20 µM) followed by CCL5 (10 ng/mL) treatment. The data are presented as means ± SE. *P < 0.05, #P < 0.01 compared with control. Blots are quantified as fold of control.
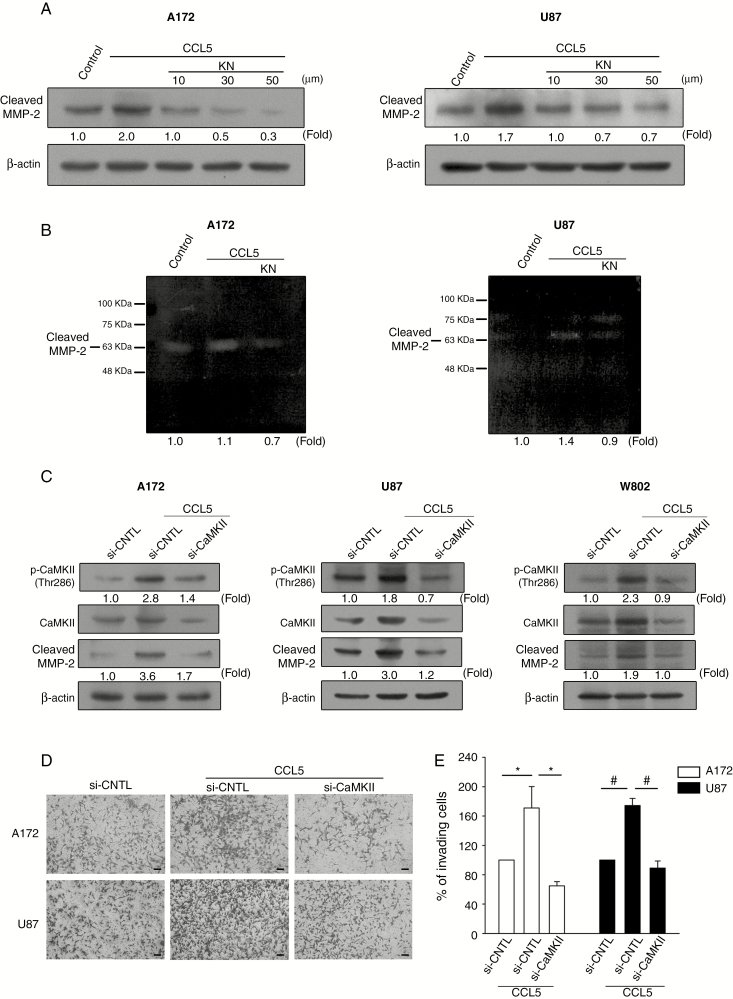
Inhibition of CaMKII Phosphorylation Suppresses CCL5-Induced MMP2 Expression
To elucidate the relationship between CaMKII phosphorylation and MMP2 expression levels, KN was applied to glioma cells at different concentrations (10–50 µM) for 1 h prior to CCL5 stimulation. The protein expression and activity of cleaved MMP2 were downregulated with increasing concentrations of KN (Figure 5A and B, Supplementary Figure 6A). Furthermore, knockdown of CaMKII via siRNA (si-CaMKII) in the glioma cells suppressed the expression levels of CaMKII protein and CCL5-induced cleaved MMP2 (Figure 5C). The transwell invasion assays revealed that si-CaMKII suppressed CCL5-stimulated cell invasion. The number of invading cells was increased in the presence of CCL5 and was significantly reduced following si-CaMKII treatment (Figure 5D), which was quantified as percent of invading cells compared with control siRNA (Figure 5E).
Fig. 5.
Inhibition of CaMKII phosphorylation downregulates CCL5-induced MMP2 expression. (A, B) Cells pretreated with KN (10–50 µM) followed by CCL5 treatment showed inhibition of cleaved MMP2 expression. (C) Glioma cells transfected with CaMKII siRNA followed by CCL5 (10 ng/mL) treatment showed inhibited CaMKII phosphorylation and cleaved MMP2 protein expression. (D) Invasive activity was inhibited in the glioma cells with si-CaMKII followed by CCL5 (10 ng/mL) treatment. (E) The invading cells in (D) were quantified and presented as percent of invading cells. Bar: 100 µm. The data are presented as means ± SE. *P < 0.05, #P < 0.01. Blots are quantified as fold of control.
Inhibition of Calcium-Dependent Signaling Reduces GAM-CM with GM-CSF–Induced MMP2 Expression in Glioma Cells
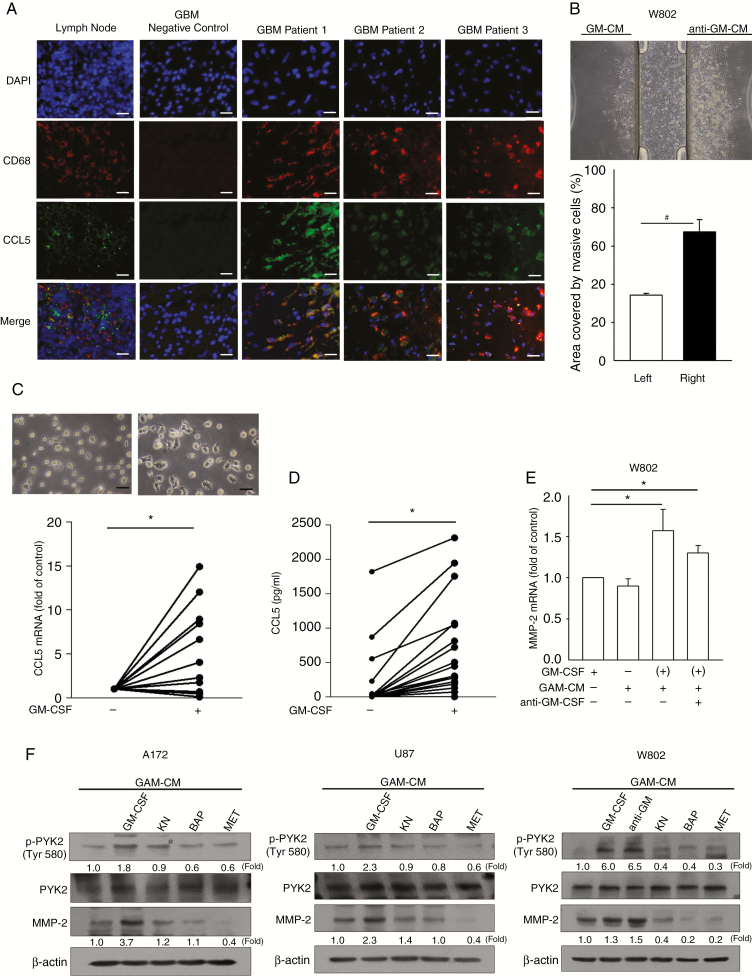
As GAMs represent a crucial source of CCL5 regulating gliomagenesis, we speculated that the GAMs of newly diagnosed cases would be pivotal for CCL5 production. Immunohistochemistry (IHC) results of newly diagnosed gliomas revealed the co-localization of microglia/macrophages (CD68) with CCL5 (Figure 6A) in approximately 30% of the glioma microenvironment (Supplementary Figure 6B, Supplementary Figure 2A), with a higher GAM percentage in high-grade glioma (40% vs 13%; Supplementary Figure 2B). We then investigated whether activated GAMs contributed to CCL5 production and glioma invasion. A 3D chemotaxis assay was performed to validate the chemotactic invasion of glioma toward GAM-CM with GM-CSF (GM-CM) or without GM-CSF (CM) stimulation, since GM-CSF is a critical growth factor for GAM activation and is known to be involved in glioma progression.39 We observed that glioma cells tended to preferentially invade toward GM-CM relative to CM after 24 h of incubation (Supplementary Figure 6C). This homing effect of glioma was unlikely to be mediated by activated-CD8+ TILs after anti-CD3/CD28 stimulation, as they resulted in the downregulation of CCL5 production (Supplementary Figure 7). Furthermore, GM-CSF was neutralized in GM-CM (anti–GM-CM; Supplementary Figure 6D), and this appeared to elicit similar invasive activity in W802 cells (Figure 6B). CCL5 mRNA expression in GAMs and protein secretion in GAM-CM were both increased after GM-CSF stimulation compared with those without stimulation (Figure 6C and D). To elucidate the invasive ability of GM-CM–treated glioma cells, MMP2 mRNA expression was also assessed and was found to be elevated in glioma cells treated with anti–GM-CM compared with cells treated with GM-CSF only (Figure 6E and Supplementary Figure 6E and F). Blockade of calcium influx by BAP, inhibition of CaMKII phosphorylation by KN, or antagonism of CCL5 by MET significantly reduced PYK2 phosphorylation and cleaved MMP2 expression levels induced by GM-CM treatment (Figure 6F).
Fig. 6.
The increased MMP2 expression by GM-CM is suppressed by inhibition of calcium-related signaling pathways. (A) IHC staining of CD68 (red), CCL5 (green), and DAPI (blue) in newly diagnosed GBM tumor sections (n = 3). (B) W802 cells invaded toward the reservoir containing anti–GM-CM. Percentages of areas covered by invading W802 cells are shown. (C) Representative images of GAMs stimulated with or without GM-CSF, which showed that the CCL5 mRNA level was increased in GM-CSF–stimulated GAMs (n = 21). (D) The amounts of CCL5 in CM and GM-CM were Figure 6 Continued.determined (n = 21). (E) MMP2 mRNA expression in glioma cells was determined after 24 h of treatment with CM or GM-CM. (F) Glioma cells pretreated with KN, BAP, or MET prior to GM-CM or CM treatment showed downregulation of PYK2 phosphorylation and cleaved MMP2 protein expression. “(+)” represents CM collected from GM-CSF–activated GAMs. Bar: 100 µm. All data are presented as means ± SE. *P < 0.05, #P < 0.01. Blots are quantified as fold of control.
Discussion
Glioma is relatively rare compared with other human cancers, and the aggressiveness of glioma is due to its high migratory potential and recurrence rate.40,41 The secretion of matrix metalloproteinases is an important step for cancer metastasis and facilitates cancer invasion. Emerging evidence has demonstrated that autoregulation of CCL5 in GAMs can influence glioma growth and that it is associated with a poor prognosis in patients with glioma.5,16,34,35 The influence of GAMs and TILs on glioma progression is not fully understood as there are fundamental differences between GAMs and TILs, and their effects on glioma development remain to be elucidated. Our results highlight the clinical value of CCL5 production and its association with GAM activation. We analyzed the involvement of the CCL5/CCR5 axis on glioma invasion with regard to the different CCR5 ligands, including CCL3, CCL4, and CCL5, of which CCL5 is critical in newly diagnosed GBM (Figure 1A and B). We demonstrated that the newly diagnosed cases with a high CCL5 level were associated with increased tumor volume (Supplementary Table 6). GAMs of GBM were involved in the production of CCL5, whereas the impact on CCL5 production by CD8+ TILs was negligible. CCL5 is known to mediate its biological activities via activation of G-protein coupled receptors or binding to glycosaminoglycans.42,43 Previous clinical results have suggested that CCR5 appears to be involved in tumorigenesis, with a stronger expression in high-grade glioma.44 This upregulation of CCR5 in the glioma microenvironment is presumably due to the production of CCL5 from GAMs leading to direct glioma growth and metastasis,34,35 and it has also been associated with intracellular calcium mobilization.45 Our results are partially consistent with previous reports, since we observed that high-grade glioma contained a higher level of GAM infiltration, as characterized by CD11b+CD45−/CD11b+CD45+, of which CD11b+CD45− cells were predominant (Supplementary Figure 2A and B).
GM-CSF has been demonstrated to act as an autocrine growth factor in astrocytic cell lines isolated from human glioma, and its expression has been significantly correlated with higher-grade tumors.39 Animal models have shown that GM-CSF–secreting glioma/glioma stem cells can promote the differentiation of monocytes into macrophages characterized by CD11chigh, and this has been shown to promote tumorigenesis.7 Similarly, mice with GL261-induced glioma have been shown to display higher GM-CSF expression along with microglia/macrophage infiltration.46 In addition, GM-CSF is known to promote the production of GAMs.7 This prompted us to investigate whether exposing GAMs to GM-CSF would promote stronger glioma invasion. We determined that GM-CSF–activated GAMs from human glioma secreted higher levels of CCL5. The invasive ability of glioma toward GAM-CM was verified, and glioma cells elicited a more robust homing response toward GM-CSF–activated GAM-CM (Figure 6B). Importantly, the elevation of CCL5 in the glioma microenvironment did not appear to be critically mediated by activated-CD8+ TILs, since CCL5 downregulation was observed after anti-CD3/CD38 stimulation (Supplementary Figure 7A and B). In addition, GAMs accounted for the largest portion (~30–50%) of infiltrating immune cells, of which the propagation of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor alpha are crucial in modulating glioma GM-CSF production and relative functional proteins that aid tumorigenesis, including MMPs.39,47 The burst of CCL5 production by GAMs after contact with GM-CSF from glioma may have a considerable impact on glioma progression. As such, the connection between glioma and GAMs in relation to GM-CSF stimulation and CCL5 production might be a potential starting point for new therapeutic approaches.
The expression of MMPs, including MMP2, has been shown to coordinate calcium mobilization and regulate glioma invasion.21,33 In this study, we demonstrated that CCL5-regulated glioma migration and invasion were associated with the expression levels of p-PYK2 and MMP2. Consistently, the addition of CCL5 increased the level of intracellular calcium in glioma, which was dependent on the time of exposure to CCL5. Importantly, the intracellular calcium levels did not rise above physiological concentrations (1.2–2.5 mM) following 24 h of CCL5 stimulation (Figure 4A), suggesting that the time of exposure to CCL5 with respect to alterations in calcium level may be critical for glioma invasion.48 Increased levels of calcium-dependent p-CaMKII and p-Akt were observed in response to CCL5 stimulation in both time- and concentration-dependent manners. Moreover, alterations in calcium levels following treatment with KN-93 or BAPTA-AM resulted in downregulation of p-PYK2 and MMP2 (Figure 6F). These results indicated that CCL5-induced glioma invasion requires the calcium-dependent regulation of p-PYK2 and MMP2. Moreover, restriction of intracellular and extracellular calcium flux or CCL5 level eliminated the effect of GAMs in relation to glioma invasion (Supplementary Figure 8). Taken together, our results indicate that the chemokine axis in the glioma microenvironment is subject to CCL5-mediated invasion, and such regulation is facilitated by GAM activation. Moreover, restriction of calcium-dependent pathways may be pivotal for eliminating CCL5/GAM-regulated glioma invasion.
Supplementary Material
Funding
This work was supported by grants from the Ministry of Science and Technology, Taiwan (107-2314-B-182A-168, 105-2314-B-182-007 and 106-2119-M-182-001), the National Health Research Institutes, Taiwan (NHRI-EX108-10502NI) and Chang Gung Memorial Hospital (CMRPG3F1732).
Conflict of interest statement. The authors have no conflicts to disclose.
Authorship statement. C.Y-J.W., C-Y.L., C-Y.F., and P-Y.C. were involved in the conception and design of the study; C.Y-J.W., and L-Y.F. performed the experiments; C-Y.L., L-Y.F., and C-H.C. provided technical support; C.Y-J.W., L-Y.F., C-H.C., C-Y.L., Y-C.L., C-Y.H., and P-Y.C. analyzed the data; K-C.W. and P-Y.C. provided important materials and critical revision of the manuscript; C.Y-J.W., P-Y.C., and C-Y.F. wrote the manuscript.
References
- 1. Hsieh WT, Yeh WL, Cheng RY, et al. . Exogenous endothelin-1 induces cell migration and matrix metalloproteinase expression in U251 human glioblastoma multiforme. J Neurooncol. 2014;118(2):257–269. [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Li Y, Yu TS, et al. . A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osswald M, Jung E, Sahm F, et al. . Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–98. [DOI] [PubMed] [Google Scholar]
- 4. Galvao RP, Kasina A, McNeill RS, et al. . Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc Natl Acad Sci U S A. 2014;111(40):E4214–E4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eder K, Kalman B. The dynamics of interactions among immune and glioblastoma cells. Neuromolecular Med. 2015;17(4):335–352. [DOI] [PubMed] [Google Scholar]
- 7. Kokubu Y, Tabu K, Muramatsu N, et al. . Induction of protumoral CD11c(high) macrophages by glioma cancer stem cells through GM-CSF. Genes Cells. 2016;21(3):241–251. [DOI] [PubMed] [Google Scholar]
- 8. Liang H, Yi L, Wang X, Zhou C, Xu L. Interleukin-17 facilitates the immune suppressor capacity of high-grade glioma-derived CD4 (+) CD25 (+) Foxp3 (+) T cells via releasing transforming growth factor beta. Scand J Immunol. 2014;80(2):144–150. [DOI] [PubMed] [Google Scholar]
- 9. Brandenburg S, Müller A, Turkowski K, et al. . Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016;131(3):365–378. [DOI] [PubMed] [Google Scholar]
- 10. Hao C, Parney IF, Roa WH, Turner J, Petruk KC, Ramsay DA. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathol. 2002;103(2):171–178. [DOI] [PubMed] [Google Scholar]
- 11. Mostofa AG, Punganuru SR, Madala HR, Al-Obaide M, Srivenugopal KS. The process and regulatory components of inflammation in brain oncogenesis. Biomolecules. 2017;7(2):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lapteva N, Huang XF. CCL5 as an adjuvant for cancer immunotherapy. Expert Opin Biol Ther. 2010;10(5):725–733. [DOI] [PubMed] [Google Scholar]
- 13. Ding H, Zhao L, Dai S, Li L, Wang F, Shan B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomed Pharmacother. 2016;77:142–149. [DOI] [PubMed] [Google Scholar]
- 14. Sax MJ, Gasch C, Athota VR, et al. . Cancer cell CCL5 mediates bone marrow independent angiogenesis in breast cancer. Oncotarget. 2016;7(51):85437–85449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao L, Wang Y, Xue Y, Lv W, Zhang Y, He S. Critical roles of chemokine receptor CCR5 in regulating glioblastoma proliferation and invasion. Acta Biochim Biophys Sin (Shanghai). 2015;47(11):890–898. [DOI] [PubMed] [Google Scholar]
- 16. Pan Y, Smithson LJ, Ma Y, Hambardzumyan D, Gutmann DH. Ccl5 establishes an autocrine high-grade glioma growth regulatory circuit critical for mesenchymal glioblastoma survival. Oncotarget. 2017;8(20):32977–32989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pham K, Luo D, Liu C, Harrison JK. CCL5, CCR1 and CCR5 in murine glioblastoma: immune cell infiltration and survival rates are not dependent on individual expression of either CCR1 or CCR5. J Neuroimmunol. 2012;246(1–2):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang CH, Yamamoto A, Lin YT, Fong YC, Tan TW. Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem Pharmacol. 2010;79(2):209–217. [DOI] [PubMed] [Google Scholar]
- 20. Shen Z, Li T, Chen D, et al. . The CCL5/CCR5 axis contributes to the perineural invasion of human salivary adenoid cystic carcinoma. Oncol. Rep. 2014;31(2):800–806. [DOI] [PubMed] [Google Scholar]
- 21. Fortier S, Labelle D, Sina A, Moreau R, Annabi B. Silencing of the MT1-MMP/ G6PT axis suppresses calcium mobilization by sphingosine-1-phosphate in glioblastoma cells. FEBS Lett. 2008;582(5):799–804. [DOI] [PubMed] [Google Scholar]
- 22. Brzdak P, Wlodarczyk J, Mozrzymas JW, Wojtowicz T. Matrix metalloprotease 3 activity supports hippocampal EPSP-to-spike plasticity following patterned neuronal activity via the regulation of NMDAR function and calcium flux. Mol Neurobiol. 2017;54(1):804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14(9):598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsin H, Kim MJ, Wang CF, Sheng M. Proline-rich tyrosine kinase 2 regulates hippocampal long-term depression. J Neurosci. 2010;30(36):11983–11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freitas-Rodriguez S, Folgueras AR, Lopez-Otin C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim Biophys Acta. 2017;1864(11):2015–2025. [DOI] [PubMed] [Google Scholar]
- 26. Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122(Pt 17):3015–3024. [DOI] [PubMed] [Google Scholar]
- 27. Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115(Pt 4):839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwiatkowska A, Kijewska M, Lipko M, Hibner U, Kaminska B. Downregulation of Akt and FAK phosphorylation reduces invasion of glioblastoma cells by impairment of MT1-MMP shuttling to lamellipodia and downregulates MMPs expression. Biochim Biophys Acta. 2011;1813(5):655–667. [DOI] [PubMed] [Google Scholar]
- 29. Tabouret E, Boudouresque F, Farina P, et al. . MMP2 and MMP9 as candidate biomarkers to monitor bevacizumab therapy in high-grade glioma. Neuro-Oncol. 2015;17(8):1174–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du R, Petritsch C, Lu K, et al. . Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008;10(3):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang L, Zhao D, Liu HB, et al. . Activation of sonic hedgehog signaling enhances cell migration and invasion by induction of matrix metalloproteinase-2 and -9 via the phosphoinositide-3 kinase/AKT signaling pathway in glioblastoma. Mol Med Rep. 2015;12(5):6702–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu J, Kurup P, Bartos JA, Patriarchi T, Hell JW, Lombroso PJ. Striatal-enriched protein-tyrosine phosphatase (STEP) regulates Pyk2 kinase activity. J Biol Chem. 2012;287(25):20942–20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han KY, Dugas-Ford J, Seiki M, Chang JH, Azar DT. Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal fibroblasts. Invest Ophthalmol Vis Sci. 2015;56(9):5323–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solga AC, Pong WW, Kim KY, et al. . RNA sequencing of tumor-associated microglia reveals Ccl5 as a stromal chemokine critical for neurofibromatosis-1 glioma growth. Neoplasia. 2015;17(10):776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toonen JA, Anastasaki C, Smithson LJ, et al. . NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet. 2016;25(9):1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han S, Zhang C, Li Q, et al. . Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110(10):2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olah M, Raj D, Brouwer N, et al. . An optimized protocol for the acute isolation of human microglia from autopsy brain samples. Glia. 2012;60(1):96–111. [DOI] [PubMed] [Google Scholar]
- 38. Menck K, Behme D, Pantke M, et al. . Isolation of human monocytes by double gradient centrifugation and their differentiation to macrophages in teflon-coated cell culture bags. J Vis Exp. 2014(91):e51554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Revoltella RP, Menicagli M, Campani D. Granulocyte-macrophage colony-stimulating factor as an autocrine survival-growth factor in human gliomas. Cytokine. 2012;57(3):347–359. [DOI] [PubMed] [Google Scholar]
- 40. Ostrom QT, Gittleman H, Xu J, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl_5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin YJ, Chiu HY, Chiou MJ, et al. . Trends in the incidence of primary malignant brain tumors in Taiwan and correlation with comorbidities: a population-based study. Clin Neurol Neurosurg. 2017;159:72–82. [DOI] [PubMed] [Google Scholar]
- 42. Wu L, LaRosa G, Kassam N, et al. . Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186(8):1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin L, Blanpain C, Garnier P, Wittamer V, Parmentier M, Vita C. Structural and functional analysis of the RANTES-glycosaminoglycans interactions. Biochemistry. 2001;40(21):6303–6318. [DOI] [PubMed] [Google Scholar]
- 44. Kouno J, Nagai H, Nagahata T, et al. . Up-regulation of CC chemokine, CCL3L1, and receptors, CCR3, CCR5 in human glioblastoma that promotes cell growth. J Neuro Oncol. 2004;70(3):301–307. [DOI] [PubMed] [Google Scholar]
- 45. Chernova I, Lai JP, Li H, et al. . Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R). J leukoc Biol. 2009;85(1):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6(8):e23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sielska M, Przanowski P, Wylot B, et al. . Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230(3):310–321. [DOI] [PubMed] [Google Scholar]
- 48. Bronner F. Extracellular and intracellular regulation of calcium homeostasis. Sci World J. 2001;1:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.