Abstract
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most common cancer worldwide and epidermal growth factor receptor (EGFR) is overexpressed in greater than 90% of patient tumors. Cetuximab is a monoclonal antibody that binds to EGFR and can activate immune cells, such as natural killer (NK) cells, that express receptors for the Fc (constant region) of immunoglobulin G. IL-15 (interleukin-15) is a critical factor for the development, proliferation and activation of effector NK cells. A novel IL-15 compound known as ALT-803 that consists of genetically modified IL-15 plus the IL-15 receptor alpha protein (IL15Rα) fused to the Fc portion of IgG1 has recently been developed. We hypothesized that treatment with ALT-803 would increase NK cell-mediated cytotoxicity of cetuximab-coated head and neck squamous cells. CD56+ NK cells from normal healthy donors were treated overnight with ALT-803 and tested for their ability to lyse cetuximab-coated tumor cells. Cytotoxicity was greater following NK cell ALT-803 activation, as compared to controls. ALT-803-treated NK cells secreted significantly higher levels of IFN-γ than control conditions. Additionally, NK cells showed increased levels of phospho-ERK and phospho-STAT5 when co-cultured with cetuximab-coated tumors and ALT-803. Administration of both cetuximab and ALT-803 to mice harboring Cal27 SCCHN tumors resulted in significantly decreased tumor volume when compared to controls and compared to single-agent treatment alone. Overall, the present data suggest that cetuximab treatment in combination with ALT-803 in patients with EGFR-positive SCCHN may result in significant NK cell activation and have important anti-tumor activity.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02372-2) contains supplementary material, which is available to authorized users.
Keywords: IL-15, ALT-803, Squamous cell carcinoma of the head and neck, Antibody-dependent cellular cytotoxicity, Natural killer cells, Cetuximab
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most common cancer worldwide, and its global incidence has been steadily climbing for the last decade [2]. Epidermal growth factor receptor (EGFR) is frequently overexpressed in epithelial malignancies including squamous cell carcinoma of the head and neck. Studies have demonstrated that EGFR is overexpressed on the surface of these cells irrespective of their HPV status [3]. This information provides a suitable target for monoclonal antibody-based immunotherapy [3]. The EGFR monoclonal antibody (mAb) cetuximab has been incorporated into treatment regimens for head and neck cancers; however, as a single agent, it has been shown to have beneficial clinical responses in only 10–15% of patients with advanced, metastatic disease.
Current management for advanced head and neck cancers in the United States includes surgery if possible, followed by 5-fluorouracil with a platinum-based chemotherapeutic agent such as cisplatin and cetuximab. In the metastatic setting, cetuximab has shown benefit when used as a first-line treatment and when used in combination with chemotherapy [4]. The addition of cetuximab to standard chemotherapy regimens improved overall survival to 10.1 months compared to 7.4 months with chemotherapy alone. Progression-free survival with combination therapy was 5.6 months as compared to 3.3 months with chemotherapy alone. Additionally, the response rate was significantly improved from 20% to 36% with the addition of cetuximab [4].
Natural killer (NK) cells originate in the bone marrow. They are large granular lymphocytes that are capable of killing transformed cells without prior sensitization. NK cells are unique in their constitutive expression of receptors for numerous cytokines (i.e., IL-12, -15, -18, and -21) and their expression of an activating receptor for the Fc region of IgG (FcγRIIIa) [5–7]. NK cells have specialized receptors for the constant or “Fc” region of immunoglobulin G that permits them to bind to antibody-coated target cells and lyse these cells via the induction of antibody-dependent, cell-mediated cytotoxicity (ADCC) and the secretion of cytokines and chemokines (i.e., IFN-γ, TNF-α, IL-8). Receptors expressed by the NK cell interact with antibodies bound to ligands or antigens expressed by the tumor cell. This interaction typically results in the release of cytotoxic factors leading to the death of the target cell. In addition to mediating ADCC, NK cells can also secrete factors such as IFN-γ, TNF-α and other cytokines and chemokines responsible for inhibition of tumor cell proliferation, enhanced antigen presentation and stimulation of T cell chemotaxis [8, 9]. Thus, cytokine administration could enhance NK cell activation thereby increasing their ability to recognize and kill tumor cells coated with cetuximab [10, 11].
Interleukin-15 (IL-15) is a pleiotropic cytokine that has been shown to be a critical factor for the development, proliferation and activation of effector NK cells and CD8+ T cells [5, 12]. In a first-in-human clinical trial, recombinant human IL-15 administration was shown to expand NK cells tenfold and significantly increase the proliferation of γδT cells as well as CD8+ T cells. Despite promising anti-tumor immune activity, treatment with IL-15 resulted in some clinical toxicity and was shown to induce limited anti-tumor responses in patients, potentially due in part to a short half-life [13, 14]. A novel IL-15 compound, ALT-803, that consists of genetically modified IL-15 plus the IL-15 receptor alpha protein (IL15Rα) fused to the Fc portion of IgG1 has been developed to address the limitations of IL-15-based therapies. When compared to native IL-15, ALT-803 was shown to exhibit a longer serum half-life, retention in lymphoid organs, and improved in vivo biological activity by 5–25-fold. ALT-803 has been shown to be effective in several experimental animal models of cancer, namely multiple myeloma, bladder cancer [15], glioblastoma, ovarian cancer [16], breast and colon carcinomas. ALT-803 is currently being evaluated in the settings of human hematological and solid cancers in multiple clinical trials. Given the known half-life profile of ALT-803 when compared to IL-15, it is likely that treatment with this compound can avoid the potential limitations seen with IL-15 therapy [17].
The results in this report provide evidence that the addition of ALT-803 could enhance the anti-tumor activity of NK cells against cetuximab-coated target cells and allow for increased cytotoxicity, release of IFN-gamma and T cell chemotaxis [1].
Materials and methods
Cell lines, NK cells, T cells, and reagents
SCCHN cell lines (Cal27, SCC47, and SCC2) were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Human healthy donor NK cells and T cells were isolated from fresh peripheral blood leukopacks (American Red Cross, Columbus, OH) by negative selection RosetteSep (Stem Cell Technologies, Waltham, MA) via incubation with NK cell or T cell enrichment antibody cocktails followed by Ficoll Hypaque (Sigma, St. Louis, MO) density gradient centrifugation. Purity of NK cell isolation and T cell isolation was confirmed by flow cytometry to be greater than 90% (data not shown). Cells were then harvested, counted and cultured in RPMI media supplemented with 10% heat-inactivated pooled human antibody (HAB) serum as previously described [18]. NK cells were stimulated for 48 h with 10 ng/mL human interleukin-12 (IL-12) or with 500 pmol/L (222.5 ng/mL) of human interleukin-2 (IL-2) as positive controls. Recombinant human interleukin-15 (IL-15) was obtained from R&D Systems (Minneapolis, MN) and IL-15SA/IL-15RαSu-Fc (ALT-803) was kindly supplied by Altor BioScience Corporation (Miramar, FL). Human T cells were isolated via negative selection from fresh peripheral blood leukopacks by 20-min incubation with RosetteSep cocktail (Stem Cell Technologies, Waltham, MA) before Ficoll Hypaque (Sigma, St Louis, MO) density gradient centrifugation and greater than 90% purity was confirmed by flow cytometry.
Antibody-dependent cellular cytotoxicity (ADCC) assays
Purified NK cells from normal healthy donors were treated with IL-15 (10 ng/ml) or ALT-803 (10 ng/ml) overnight in RPMI-1640 media supplemented with 10% HAB serum at 37 °C. As previously described for immobilized antibody experiments, wells of a 96-well flat-bottom plate were coated with 100 μg/ml cetuximab or 100 μg/ml polyclonal human IgG overnight at 37 °C. [19] Eighteen hours later, cetuximab- or IgG-coated 51Cr-labeled tumor cells were incubated with NK cells at various effector:target (E:T) ratios (50:1, 25:1, 12.5:1, and 6.25:1). Following a 4-h incubation, supernatants were harvested, and the percentage of lysis was calculated as previously described [20]. Similar ADCC assays were performed with Cal27 cells pretreated with cisplatin and then coated with cetuximab for 24 h. NK cells were then co-cultured with 51Cr labeled tumor cells and ADCC was measured as described above.
HER1/EGFR analysis
Expression of EGFR in human squamous cell carcinoma of the head and neck (SCCHN) cell lines was validated by immunoblot and flow cytometry analysis as previously described [18]. SCCHN cell lysates were immunoblotted for EGFR (Cell Signaling, Danvers, MA) and analyzed using a LiCOR imager (Lincoln, NE). SCCHN cell lines were stained with an EGFR-FITC antibody (Santa Cruz, Dallas, TX) for 30 min at 4 °C and fixed with 1% formalin. Stained cells were analyzed using an Attune flow cytometer (Thermo Fisher, Waltham MA).
NK cell cytokine secretion
SCCHN tumor cells were first treated with 100 μg/ml of cetuximab or with an isotype control IgG for 1 h at 37 °C. Purified healthy donor NK cells in RPMI-1640 media supplemented with 10% HAB serum were stimulated with or without IL-15 or ALT-803 and added at a concentration of (2 × 105 cells/well) to tumor and cetuximab-containing cultures. Cell-free supernatants were collected at 48 h and the cytokine (IFN-γ) and chemokine (RANTES, IL-8) levels were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
T cell chemotaxis
Healthy human donor T cells were isolated by negative selection and activated with anti-CD3/CD28 beads for 6 days. Purified healthy donor NK cells were stimulated with IL-15 or ALT-803 and co-incubated with Cal27 tumor cells coated with isotype or cetuximab antibodies. After 48 h of incubation, NK cells were removed by centrifugation and the supernatants were collected. NK cell co-culture supernatants were placed in the lower chambers of a 24-well flat-bottom plate. Migration experiments were conducted by placing 2 × 105 activated T cells in 100 μl of 10% RPMI medium in the upper chambers of 5-μm pore size Transwell inserts (Corning Inc, Corning, NY). The plates were incubated for 4 h at 37 °C followed by a 10-min incubation at 4 °C. The number of migrated T cells was determined using flow cytometric tracking beads (Invitrogen).
Intracellular flow cytometry
Levels of intracellular phospho-ERK and phospho-STAT5 of CD56+ NK cells were measured and detected using a pERK-FITC mAb (BD Biosciences) or a pSTAT5-FITC mAb (BD Biosciences). The percentage of positively staining cells and mean MFI compared to isotype controls was calculated for the specified cell population.
Murine tumor model
Age-matched female athymic nude mice were injected subcutaneously in the right flank with 3 × 106 Cal27 tumor cells in 100 μl of PBS. Tumors became palpable approximately 7–10 days later. ALT-803 was administered by intraperitoneal (i.p.) injections at a dose of 0.25 mg/kg and cetuximab was administered at a dose of 5 mg/kg. All treatments were administered i.p. thrice weekly in 100 μl of PBS and continued until tumors were greater than 1.6 cm in maximum dimensions. Subcutaneous tumor volumes were calculated 3×/week as follows: tumor volume = 0.5 × [(large diameter) × (small diameter)2].
Statistics
ANOVA models and t tests were used for cell line data. Linear mixed models were used for mouse tumor data. Multiple comparisons were adjusted by Tukey’s method. Significance was determined if P value < 0.05.
Results
ALT-803 enhances cetuximab-mediated ADCC of SCCHN tumor cells
IL-15 treatment of NK cells will enhance their ability to lyse antibody-coated target cells [5]. We, therefore, tested whether stimulation of NK cells with a genetically modified IL-15 plus the IL15Rα fused to the Fc portion of IgG1 (ALT-803) would enhance NK cell ADCC activity against cetuximab-coated EGFR-expressing squamous cell carcinoma of the head and neck (SCCHN). Expression of EGFR (also known as HER1) in the Cal27, SCC2 and SCC47 cell lines was measured by flow cytometry and confirmed by immunoblot analysis. All three cell lines had > 85% expression of EGFR which was confirmed by immunoblot (Fig. 1a) and flow cytometry (Fig. 1b). ADCC assays were performed using normal donor NK cells as effector cells and EGFR-positive SCCHN cells as targets. There was a statistically significant enhancement of NK cell-mediated ADCC against cetuximab-coated targets following overnight ALT-803 activation as compared to control conditions (Fig. 2a–c, P < 0.001). In each instance, lysis of uncoated tumor cells by untreated NK cells was low, including at the 50:1 E:T ratio. Stimulation of NK cells with ALT-803 or treatment of tumor cells with cetuximab led to modest gains in tumor cell killing. However, the combination of ALT-803 and cetuximab treatment led to marked levels of ADCC as compared to controls. A graphical representation of this cytotoxicity data of three healthy donors at the 50:1 E:T ratio is presented in Fig. 2d (*P < 0.05 compared to IgG; #P < 0.05 compared to cetuximab alone). Notably, the lysis of HPV-negative cell lines (Cal27) was on the same order as that of HPV-positive cell lines (SCC2, SCC47).
Fig. 1.
Squamous cell carcinoma of the head and neck (SCCHN) cell lines express EGFR. Human head and neck cancer (SCCHN) cell lines (Cal27, SCC2, and SCC47) were analyzed for expression of EGFR (HER1) by a immunoblot and b flow cytometry. Grey bars in flow cytometry histograms represent isotype controls and open histograms represent EGFR expression
Fig. 2.
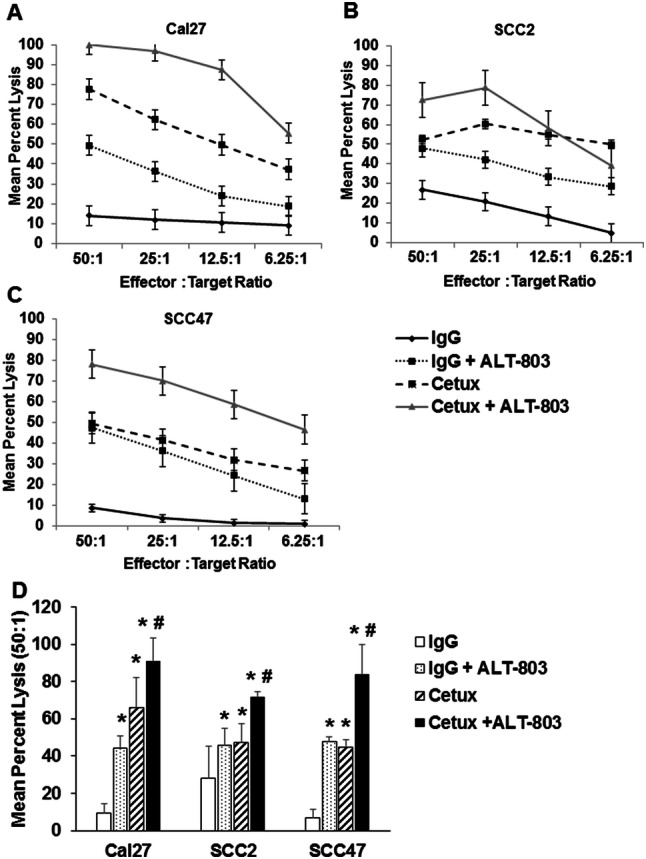
ALT-803 stimulation of human NK cells enhances lytic activity against cetuximab-coated SCCHN cell lines. Purified human CD56+ NK cells were incubated overnight in medium alone or stimulated with ALT-803 (10 ng/ml). The lytic activity of NK cells against cetuximab-coated squamous cell carcinoma of the head and neck (SCCHN) cell lines a Cal27, b SCC2, c SCC47 was assessed via a standard 4-h 51Cr release assay. Each graph depicts results from one representative donor. N = 3 for Cal27 and SCC47 and n = 2 for the SCC2 cell line. d Graphical representation of cytotoxicity data for each cell line at the 50:1 E:T ratio. Means + STD lysis of three different healthy donors, *P < 0.05 compared to IgG; #P < 0.05 compared to cetuximab alone
ALT-803 enhances NK cell ADCC against SCCHN cell lines compared to IL-15 cytokine stimulation
A comparison of IL-15 and ALT-803 was conducted to determine their relative ability to stimulate NK cell ADCC against cetuximab-coated SCCHN cells. NK cells were incubated overnight with molar equivalent doses of recombinant human IL-15 or ALT-803 and then tested against control IgG-treated tumor cells in an ADCC assay. As seen in Fig. 3a, there was minimal target cell lysis when unstimulated NK cells were used as effectors and a marked improvement in NK lysis with IL-15 or ALT-803 treatment. ALT-803 and IL-15 both enhanced NK lysis of cetuximab-coated targets. In the context of this assay, significantly greater lysis was observed for ALT-803-stimulated NK cells compared to those treated with IL-15 (Fig. 3a). In general, ALT-803 was superior to IL-15 in this assay, but not to a significant degree. Next, cytokine-treated NK cells were tested against cetuximab-coated SCCHN target cells (Fig. 3b). Unstimulated NK cells mediated between 20 and 40% lysis of Ab-coated targets, whereas overnight stimulation of NK cells led to greatly increased target cell lysis according to the cell line being examined. For example, NK cell lysis of the cetuximab-coated SCC47 cell line was approximately 40% in the absence of cytokine stimulation but increased to 80 or 100% lysis with IL-15 or ALT-803 activation, respectively. These results indicate that ALT-803 is capable of enhancing NK cell lysis of Ab-coated tumor targets in a manner similar to that of native IL-15. Additionally, Cal27 cells were incubated with cisplatin for 24 h and then coated with cetuximab. ALT-803- or IL-15-treated NK cells were then used as effector cells against these targets. This experiment demonstrated that NK cells were still able to kill cisplatin-treated SCCHN target cells and this cytotoxicity was increased when NK cells were cultured with cetuximab plus IL-15 or ALT-803 (Supplemental Figure 1).
Fig. 3.
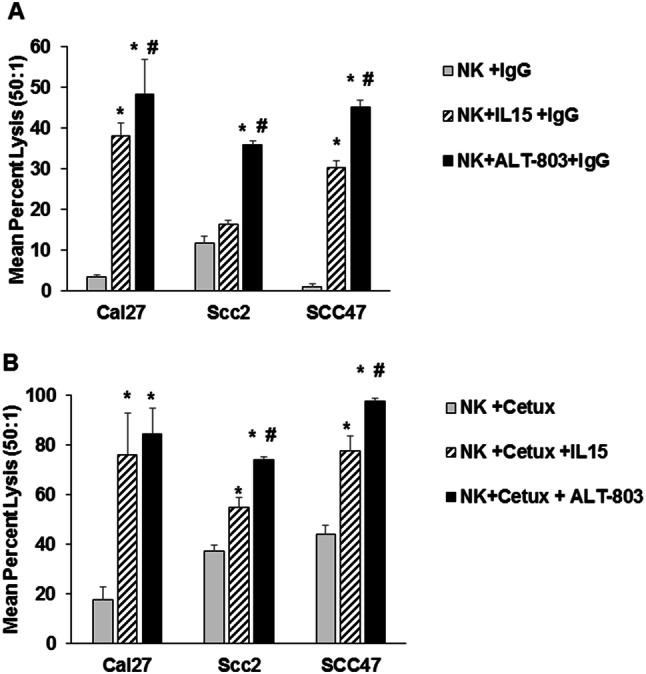
ALT-803 enhances NK cell cytotoxicity against SCCHN and ADCC against SCCHN cell lines compared to IL-15 cytokine stimulation. Purified human CD56+ NK cells were stimulated overnight in medium alone, ALT-803, or IL-15 (10 ng/ml). The cytolytic activity of NK cells was assessed against a IgG or b cetuximab-coated SCCHN cell lines via a standard 4-h 51Cr release assay. Data represent NK cell cytotoxic activity at the 50:1 effector to target cell ratio. Means + STD of 3 donors was tested per cell line, *P < 0.05 compared to NK + IgG; #P < 0.05 compared to IL-15 group
NK cells secrete immune stimulatory cytokines upon co-stimulation with ALT-803 and antibody-coated tumor cells
NK cells are finely tuned to recognize and lyse target tumor cells, however, NK cells can also initiate and promote the adaptive immune response by providing an early source of IFN-γ [21]. To test the ability of ALT-803 to stimulate NK cell-mediated cytokine secretion, healthy donor NK cells were cultured for 48 h in the presence of ALT-803 or IL-15 and cetuximab-coated SCCHN tumor cells. Cultures were monitored for their ability to secrete IFN-γ into the cell culture supernatants. Control conditions consisted of NK cells cultured with tumor cells in the presence of cytokine alone or cetuximab alone. NK cells co-cultured with tumor cells for 48 h in the presence of IL-2 and IL-12 served as a positive control. As seen in Fig. 4, NK cell release of IFN-γ in response to SCCHN tumor lines was enhanced following treatment of target cells with cetuximab and either ALT-803 or IL-15 (Fig. 4a, b; *P < 0.05 compared to NK + tumor cell co-culture; #P < 0.05 compared to NK + IgG + co-culture). Likewise, cytokine stimulation of NK cells led to an increase in IFN-γ production in response to uncoated target cells. However, maximal IFN-γ production was observed when NK cells were stimulated via the IL-15R in the presence of cetuximab-treated SCCHN cells. A similar pattern of NK cell IFN-γ production was observed for all three cell lines; however, the effects of dual stimulation were greatest for the SCC2 cell line.
Fig. 4.
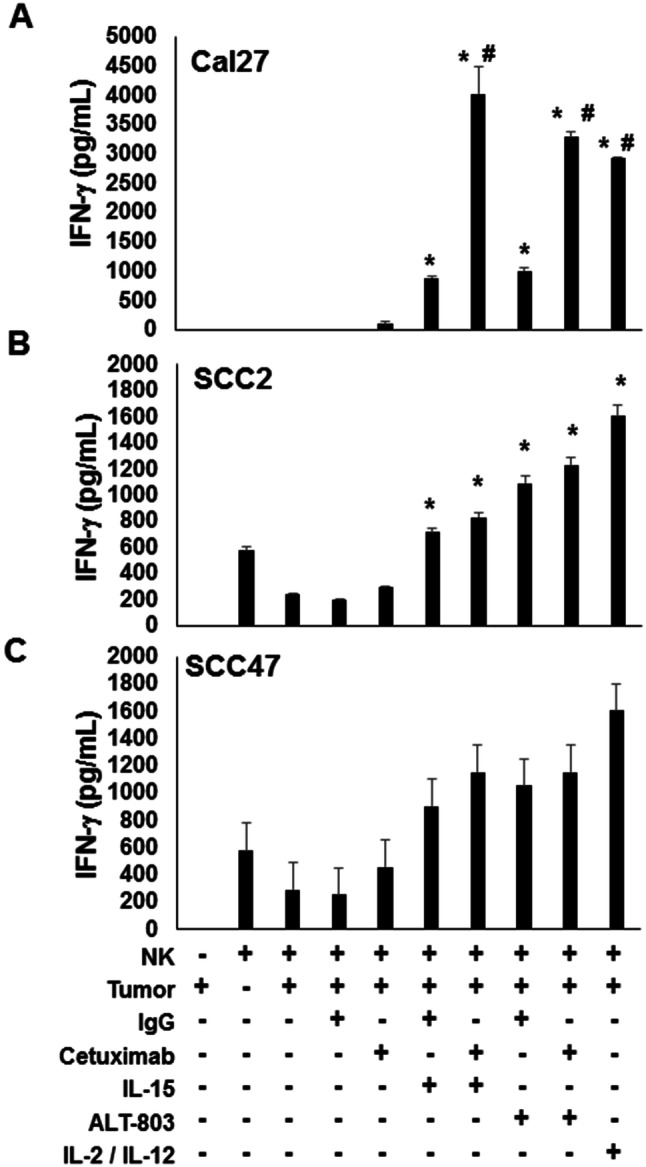
ALT-803 stimulation of NK cells enhances secretion of IFN-γ. a SCC2, b Cal27, and c SCC47 SCCHN tumor cells were treated with 100 μg/ml of cetuximab or control IgG for 1 h at 37 °C. Purified healthy donor CD56+ human NK cells incubated with cytokines (IL-12, IL-2, IL-15, or ALT-803) were co-cultured with tumor cells (cetuximab or control IgG) for 48 h and supernatants assayed for IFN-γ by ELISA. A minimum of 3 donors was tested per cell line, *P < 0.05 compared to NK + tumor cell co-culture; #P < 0.05 compared to NK + IgG + co-culture
ALT-803-stimulated NK cells produce the chemokines RANTES and IL-8 in vitro
Activated NK cells secrete several chemokines that can promote the chemotaxis of T cells [22]. Healthy donor NK cells and Cal27 SCCHN cells were cultured for 48 h in the presence of IL-15 or ALT-803 plus cetuximab. At the end of the culture, supernatants were harvested and tested via ELISA assay to determine levels of IL-8 and RANTES (CCL5). Dual stimulation of NK cells resulted in the production of high levels of IL-8 as compared to control conditions (Fig. 5a). However, the production of RANTES was noted to be increased upon NK cell stimulation with ALT-803 or IL-15 alone, and there was little increase upon the addition of Ab-coated targets (Fig. 5b).
Fig. 5.
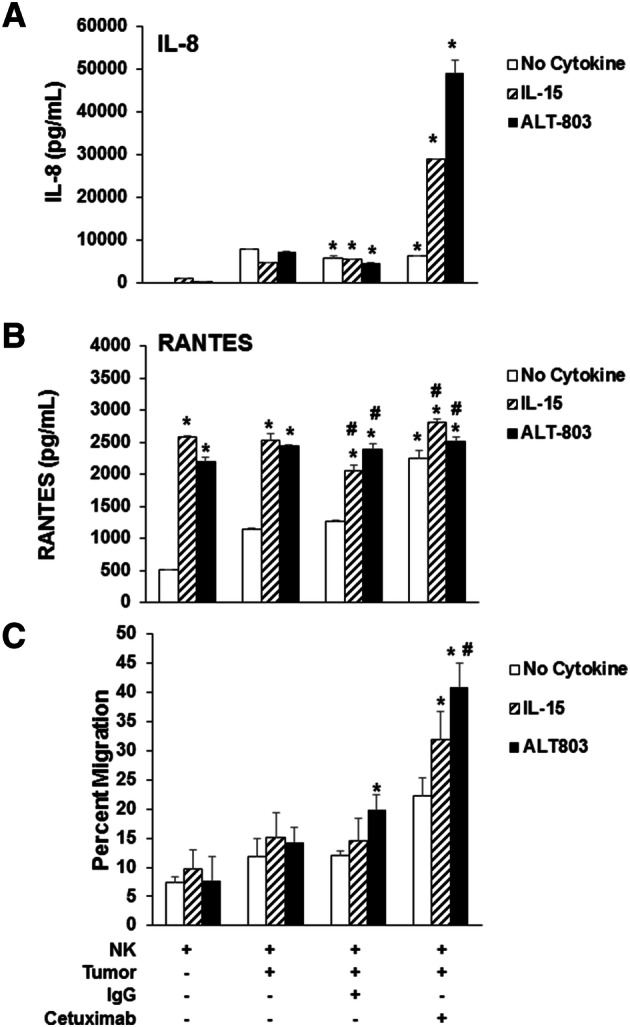
Cetuximab-coated tumor cells with ALT-803-stimulated NK cells enhance T cell chemotaxis. Cal27 tumor cells were treated with 100 μg/ml of cetuximab or control IgG and the indicated stimulus (IL-15 or ALT-803) for 1 h at 37 °C. Purified healthy donor CD56+ human NK cells were co-cultured with tumor cells (cetuximab or control IgG) for 48 h and supernatants assayed for a RANTES and b IL-8 by ELISA. Representative of 3 donors; *P < 0.05 compared to NK cells; #P < 0.05 compared to NK cells + tumor cells. c Supernatants from NK cells and tumor cell co-cultures from media control, tumor cells alone, IgG-coated tumor cells, and cetuximab-coated tumor cells were plated in the bottom of a transwell and a filter was placed above which contained autologous isolated healthy donor CD3+ T cells. After 4 h, the number of T cells migrating to bottom well was quantified by flow cytometric analysis. Means + STD of 3 donors; *P < 0.05 compared to NK cells; #P < 0.05 compared to NK cells + tumor cells
ALT-803-stimulated NK cells produce chemokines that promote T cell chemotaxis
Stimulation of NK cells via the IL-15R can lead to the production of chemokines that promote T cell migration and can potentially enhance the anti-tumor effects of immune therapy [24]. To determine whether ALT-803 can affect the ability of NK cell-derived chemokines to promote T cell migration, a chemotaxis migration was performed in which culture supernatants from activated NK cells were used as the stimulus for migration of normal donor T cells. The migration of T cells was significantly enhanced when culture supernatants derived from IL-15 or ALT-803-stimulated NK cells were co-cultured with cetuximab-coated SCCHN cells. The migration of T cells was increased when exposed to supernatants from dually stimulated NK cells (cytokine plus cetuximab) as compared to culture supernatants from the control conditions (Fig. 5c). These results indicate that in the presence of ALT-803, cetuximab-coated tumor cells have the ability to stimulate NK cells to release cytokines that trigger T cell migration.
ALT-803 and cetuximab activate JAK/STAT- and MAPK-signaling pathways in NK cells
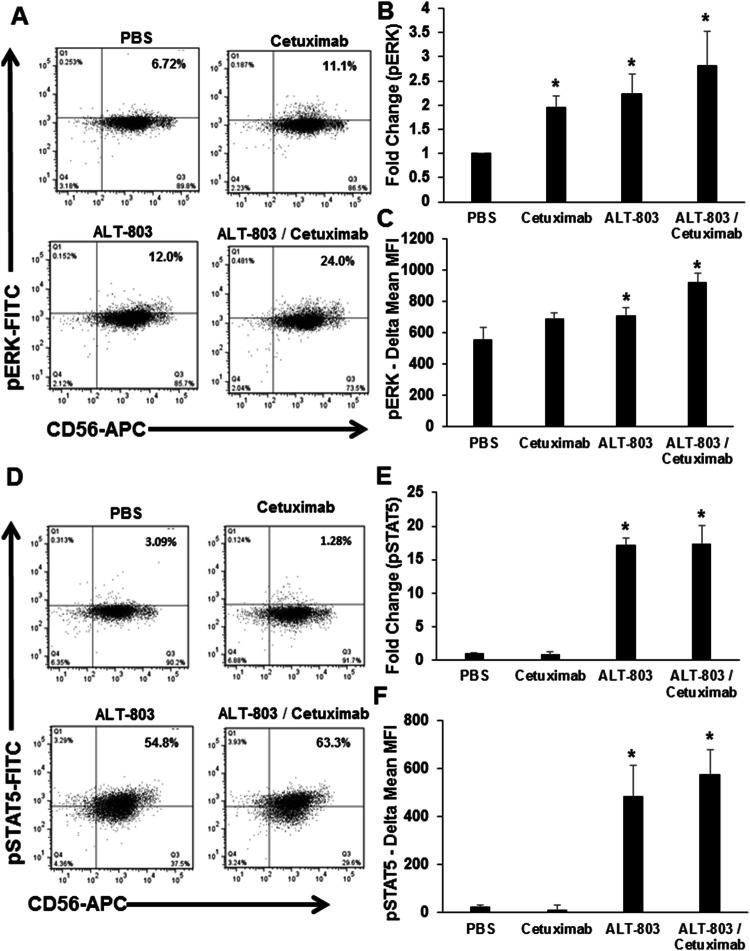
We hypothesized that the interaction of the human NK cell FcR with cetuximab-coated SCCHN tumor cells and the IL-15 receptor in the presence of ALT-803 would activate the JAK/STAT- and MAPK-signaling pathways and result in enhanced phosphorylation of ERK and STAT5 within NK cells, respectively. Human NK cells were co-cultured with cetuximab-coated SCCHN cancer cells lines (Cal27, SCC47, and SCC2) in the presence or absence of ALT-803. Following this culture, and after a 30-min incubation period, cells were harvested and assayed for expression of CD56 as well as activated phosphorylated-ERK (pERK) or activated phosphorylated-STAT5 (pSTAT5). NK cells co-cultured with cetuximab-coated SCCHN cancer cells or stimulated with ALT-803 alone exhibited 11.1% and 12% staining, respectively, while only 6.72% of resting NK cells demonstrated activation of ERK (Fig. 6a). As predicted, NK cells stimulated with both Ab-coated SCCHN tumor cells and ALT-803 demonstrated the highest level of ERK activation with 24% of cells staining positively for pERK (Fig. 6a) and a significant increase in fold expression of pERK compared to PBS (Fig. 6b) and isotype controls (Fig. 6c). Thus, cetuximab-coated tumor cells provide a strong stimulus to NK cells that result in significant induction of MAPK pathway signaling. Likewise, NK cells stimulated with Ab-coated tumor cells and ALT-803 demonstrated the highest level of STAT5 activation with 63.3% of cells staining for pSTAT5 (Fig. 6d). NK cells co-cultured with cetuximab-coated SCCHN cancer cells or stimulated with ALT-803 alone exhibited 1.28% and 54.8% staining, respectively, while only 3.09% of resting NK cells demonstrated activation of STAT5 (Fig. 6d). ALT-803 treatment provides a strong stimulus that results in a 15-fold change in the induction of the JAK/STAT signaling pathway compared to PBS (Fig. 6e) and isotype controls (Fig. 6f). Similar results were obtained for SCC2, and SCC47 (data not shown). Thus, the combination treatment led to concurrent activation of NK cells via the MAPK and JAK/STAT pathways that was not present in the control conditions.
Fig. 6.
ALT-803 induces pSTAT5 and pERK in NK cells. Healthy donor CD56+ NK cells were treated overnight with ALT-803, cetuximab, combination of ALT-803 with cetuximab, or vehicle control and stained for pERK and pSTAT5 via intracellular flow cytometry. Percentage of CD56+ NK cells positive for a pSTAT5 and quantified for b fold change in expression and c delta mean MFI (pERK-isotype control expression). Percentage of CD56+ NK cells positive for d pSTAT5 quantified for e fold change in expression and f delta mean MFI (pSTAT5-isotype control expression). Means + STD of 3 donors. *P < 0.05 compared to PBS/vehicle control-treated cells
ALT-803 enhances the anti-tumor effect of cetuximab therapy in a murine model of SCCHN
The ability of cetuximab to bind to tumor-expressed EGFR in murine models in vivo has been previously demonstrated [23–26]. A murine subcutaneous tumor model of EGFR-positive SCCHN was employed to determine the effect of combining ALT-803 with cetuximab to treat squamous cell carcinoma in vivo. It was confirmed that the murine SCCHN tumor cell line Cal27 expresses the human EGFR protein (Fig. 1). Tumors were generated in athymic nude mice by subcutaneous injection of 3 × 106 Cal27 tumor cells. At 7 days, mice bearing Cal27 tumors were randomized to treatment with PBS, ALT-803 (0.25 mg/kg), cetuximab or ALT-803 plus cetuximab i.p. three times per week until the study endpoint. Single-agent ALT-803 or cetuximab alone significantly limited tumor growth compared to the PBS control (Fig. 7; P < 0.001). Additionally, a significant inhibition in tumor growth was observed in response to combination therapy with no detectable tumor burden in any of the mice treated with ALT-803 and cetuximab (P < 0.001). Cetuximab alone and combination-treated mice were observed for 3 weeks following the completion of treatment. 4 out of 5 mice in the cetuximab group still had small palpable tumors 3 weeks after stopping treatment, whereas 0 of the 5 mice in the combination group had detectable tumors. Combination treatment with ALT-803 and cetuximab completely cured the mice of their tumors with no palpable or visible tumors present, whereas palpable, slowly growing tumors were still present in the mice treated with cetuximab alone.
Fig. 7.
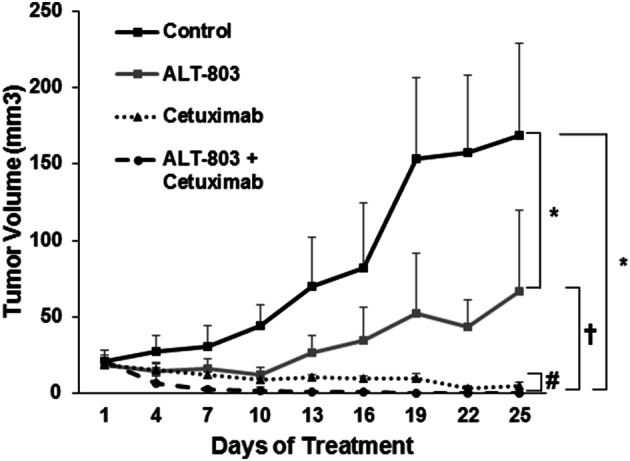
Combination ALT-803 therapy with cetuximab reduces SCCHN tumor burden in nude mice. Nude mice were injected with 3 × 106 CAL27 cells and treatment was started once tumors were palpable. Mice were treated i.p. every 3 days with PBS control, cetuximab (0.25 mg/kg), ALT-803 (5 mg/kg), or the combination of ALT-803 and cetuximab. Mice were monitored for tumor growth over the course of treatment. n = 5 mice per group, bars = SE; *P < 0.001 compared to control; †P < 0.01 compared to ALT-803-alone group; #P < 0.01 combination ALT-803 and cetuximab compared to cetuximab-alone group
Discussion
In this study, we have demonstrated that ALT-803, an IL-15 superagonist, can enhance NK cell-mediated effector functions against cetuximab-coated EGFR-positive head and neck tumor cell lines irrespective of HPV status. Combination NK cell stimulation with ALT-803 or IL-15 against cetuximab-coated SCCHN cancer cells elicited greater NK cell-mediated ADCC compared to control conditions in healthy donors. Notably, the addition of ALT-803 to a regimen of cetuximab and cisplatin further enhanced ADCC indicating that cytokines added to standard chemotherapy-antibody regimens could have potential beneficial effects. Dual stimulation of NK cells in this fashion also induced the secretion of IFN-γ, RANTES and IL-8 which had the ability to direct the chemotaxis of activated T cells. An increase in pSTAT5 levels was found in NK cells following ALT-803 stimulation. In most cases, ALT-803 performed either more effectively or similarly to IL-15. Additionally, co-administration of ALT-803 and cetuximab to tumor-bearing mice resulted in reduced tumor burden and complete regression compared to vehicle control or single-agent-treated mice. These data demonstrate the ability of ALT-803 to enhance NK cell activation in response to cetuximab-coated SCCHN cancer cells.
Clinical responses to cetuximab in combination with standard chemotherapy in SCCHN patients are observed in only a subset of patients (10–20%). Alternative strategies to improve the efficacy of cetuximab therapy in patients are necessary. One such approach would be to utilize the ability of NK cells to kill and clear cetuximab-coated SCCHN tumor cells. Further, activation of NK cells via cytokine stimulation could further enhance ADCC against SCCHN tumor cells. IL-15 can stimulate NK cell activation and is an ideal candidate for clinical immunotherapy combinations; however, IL-15 requires IL-15 receptor α-chain-binding prior to activating target cells which limits the therapeutic applications of free IL-15. ALT-803 consists of human IgG1 Fc fused to two IL-15Ralpha subunits bound to an IL-15 superagonist, resulting in higher biological activity and longer serum half-life compared with free IL-15 [27]. ALT-803 has been shown to expand and activate NK cells in a fashion similar to IL-15 [16, 28, 29].
Early-phase clinical trials using ALT-803 have exhibited promising activity and efficacy. In a phase I clinical trial, patients with hematologic malignancies who relapsed after allogenic hematopoietic cell transplantation were treated with ALT-803 and exhibited increased expansion and function of CD56+ NK and CD8+ T cells when compared to baseline levels [30]. In another clinical phase I trial combining ALT-803 with nivolumab in patients with metastatic non-small cell lung cancer, three out of seven patients observed a partial response that correlated with increased IFN-γ serum levels. Notably, this regimen was well tolerated [31]. In reported clinical trials using ALT-803, adverse events were low and it was generally well tolerated in patients which is in contrast to other cytokine regimens such as IL-2 where side effects can be problematic. In another elegant study, the combination of rituximab (anti-CD20 monoclonal antibody) and ALT-803 enhanced the cytotoxicity of NK cells against B cell lymphoma targets [32]. These findings are similar to our findings when using the ALT-803 in conjunction with the mAb cetuximab in SCCHN. These early-phase I clinical findings and our pre-clinical results support the exploration of ALT-803 to enhance cetuximab therapy in SCCHN cancers.
Clinical trials have provided evidence that cetuximab added to first-line chemotherapy (cisplatin) provided a significant response rate and increased survival in patients with SCCHN cancer [4, 33]. In the platinum-refractory disease setting, cetuximab only has modest activity as a monotherapy with an overall response rate of around 13% [34]. As responses are only observed in a subset of SCCHN patients, development of novel combinations with cetuximab are needed. To overcome antibody resistance in EGFR-positive SCCHN tumors, we propose that stimulation of the innate immune system with cytokines may promote ADCC-mediated killing of cetuximab-coated target cells. Previous work from our group has shown that IL-12 stimulation of NK cells can promote ADCC against cetuximab-coated tumors [3]. Additionally, recent data provide pre-clinical evidence that IL-21 enhances NK cell responses to cetuximab-coated pancreatic tumor cells [35]. Thus, we propose that treatment of SCCHN cancer with the IL-15 superagonist ALT-803 in combination with cetuximab may be a potential therapeutic option for overcoming resistance in patients.
In the present article, it has been demonstrated that the administration of ALT-803 is able to activate NK cells and enhance their ability to recognize and eliminate cetuximab-coated tumor cells via the induction of ADCC and the release of cytokines with anti-tumor activity. In addition, ALT-803 can result in NK cell secretion of chemokines that can promote the migration of T lymphocytes. Treatment of SCCHN tumor-bearing mice with ALT-803 in combination with cetuximab can result in tumor regression. This work demonstrates in vitro and in vivo that NK cell activation combined with antibody-based therapy can lead to improved anti-tumor effects in SCCHN cell lines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- Ab
Antibody
- ADCC
Antibody-dependent cellular cytotoxicity
- ATCC
American Type Culture Collection
- Cetux
Cetuximab
- EGFR
Epidermal growth factor receptor
- ERK
Extracellular signal-regulated kinase
- E:T
Effector to target ratio
- FcγRIIIa
Activating receptor for the Fc region of IgG
- HAB
Human AB serum
- HPV
Human papillomavirus
- IFN-γ
Interferon-gamma
- IL
Interleukin
- mAb
Monoclonal antibody
- MAPK
Mitogen-activated protein kinases
- MFI
Mean fluorescent intensity
- NK
Natural killer
- p-ERK
Phosphorylated-ERK
- p-STAT5
Phosphorylated-STAT5
- RANTES
Regulated on activation, normal T cell expressed and secreted
- SCCHN
Squamous cell carcinoma of the head and neck
- STD
Standard deviation
- TNF-α
Tumor necrosis factor-alpha
Author contributions
AP, EM, NBC, FC, DA, and TAM performed experiments. MD, and BNB assisted with experiments. AP, EM, LY, TAM analyzed the data. AP, EM, and WEC conceived and designed the project. AP, TAM, and WEC wrote the manuscript.
Funding
This work was supported by NIH Grants P01 CA095426 (M. Caligiuri), P30 CA016058 (M. Caligiuri), CA84402, K24 CA93670 (W.E. Carson, III), and T32 CA009338.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethical standards
All animal protocols were approved by the Ohio State University Animal Care and Use Committee at The Ohio State University (Approved IACUC protocol 2009A0179-R3) and mice were treated in accordance with institutional guidelines for animal care. The Ohio State University Laboratory Animal Shared Resource is an Association for Assessment and Accreditation of Laboratory Animal Care International accredited program that follows Public Health Service policy and guidelines. All other experiments were completed under the research protocols (2006R0042) approved by the Ohio State University Institutional Biosafety Committee.
Informed consent
Fresh peripheral blood leukopacks were purchased from the American Red Cross, Columbus, OH. Donors agreed to the use of their blood for research.
Cell line authentication
Cal27 cells were obtained from ATCC (Manassas, VA, USA). SCC47 cells were provided by Dr. Theodoros Teknos (Case Western Reserve University, Cleveland, OH, USA) and SCC2 cells were provided by Dr. Henning Bier (Heinrich-Heine University, Düsseldorf, Germany). The Cal27 cell line was authenticated by the ATCC. Re-authentication of the SCC47 and SCC2 cell lines has not been performed since being provided from respective laboratories. All cell lines were cultured for no more than 2–3 weeks after thawing, routinely checked for mycoplasma infection when cultured, and showed consistent phenotypes by microscopy prior to in vitro and in vivo experiments.
Animal source
Female athymic nude mice were purchased from Charles River (Wilmington, MA).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thomas A. Mace and William E. Carson III are the co-senior authors.
References
- 1.Society of Surgical Oncology 70th Annual Cancer Symposium. (2017) Ann Surg Oncol 24(1):1–202, Abstract PT326
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Luedke E, et al. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery. 2012;152(3):431–440. doi: 10.1016/j.surg.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermorken JB, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 5.Carson WE, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180(4):1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson WE, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85(12):3577–3585. [PubMed] [Google Scholar]
- 7.Fehniger TA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162(8):4511–4520. [PubMed] [Google Scholar]
- 8.Bluman EM, et al. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J Clin Invest. 1996;97(12):2722–2727. doi: 10.1172/JCI118726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somersalo K, Carpen O, Saksela E. Stimulated natural killer cells secrete factors with chemotactic activity, including NAP-1/IL-8, which supports VLA-4- and VLA-5-mediated migration of T lymphocytes. Eur J Immunol. 1994;24(12):2957–2965. doi: 10.1002/eji.1830241206. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Larrea C, et al. The NKG2D receptor: sensing stressed cells. Trends Mol Med. 2008;14(4):179–189. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6(9):928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 12.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 13.Conlon KC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, et al. Differences of biodistribution, pharmacokinetics, and tumor targeting between interleukins 2 and 15. Cancer Res. 2000;60(13):3577–3583. [PubMed] [Google Scholar]
- 15.Gomes-Giacoia E, et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS One. 2014;9(6):e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felices M, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017;145(3):453–461. doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhode PR, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016;4(1):49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondadasula SV, et al. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood. 2008;111(8):4173–4183. doi: 10.1182/blood-2007-01-068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roda JM, et al. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13(21):6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 20.Carson WE, et al. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31(10):3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5(10):996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 22.Roda JM, et al. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66(1):517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 23.Ciardiello F, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5(4):909–916. [PubMed] [Google Scholar]
- 24.Overholser JP, et al. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89(1):74–82. doi: 10.1002/1097-0142(20000701)89:1<74::AID-CNCR11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Prewett M, et al. The biologic effects of C225, a chimeric monoclonal antibody to the EGFR, on human prostate carcinoma. J Immunother Emphasis Tumor Immunol. 1996;19(6):419–427. doi: 10.1097/00002371-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Prewett M, et al. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinoma xenografts in nude mice. Clin Cancer Res. 1998;4(12):2957–2966. [PubMed] [Google Scholar]
- 27.Xu W, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73(10):3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg TK, et al. The IL15 superagonist complex ALT-803 potently enhances the proliferative capacity and activity of in vitro expanded natural killer cells. Blood. 2017;130(Suppl 1):5452. [Google Scholar]
- 29.Liu B, et al. A novel fusion of ALT-803 (interleukin (IL)-15 superagonist) with an antibody demonstrates antigen-specific antitumor responses. J Biol Chem. 2016;291(46):23869–23881. doi: 10.1074/jbc.M116.733600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romee R, et al. First-in-human phase 1 clinical study of the Il-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrangle JM, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19(5):694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosario M, et al. The IL-15-based ALT-803 complex enhances FcγRIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin Cancer Res. 2016;22(3):596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burtness B, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 34.Van den Wyngaert T, et al. Fluorodeoxyglucose-positron emission tomography/computed tomography after concurrent chemoradiotherapy in locally advanced head-and-neck squamous cell cancer: the ECLYPS study. J Clin Oncol. 2017;35(30):3458–3464. doi: 10.1200/JCO.2017.73.5845. [DOI] [PubMed] [Google Scholar]
- 35.McMichael EL, et al. Activation of the FcgammaReceptorIIIa on human natural killer cells leads to increased expression of functional interleukin-21 receptor. Oncoimmunology. 2017;6(5):e1312045. doi: 10.1080/2162402X.2017.1312045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.