Abstract
Background
A key component of many asthma management guidelines is the recommendation for patient education and regular medical review. A number of controlled trials have been conducted to measure the effectiveness of asthma education programmes. These programmes improve patient knowledge, but their impact on health outcomes is less well established. This review was conducted to examine the strength of evidence supporting Step 6 of the Australian Asthma Management Plan: "Educate and Review Regularly"; to test whether health outcomes are influenced by education and self‐management programmes.
Objectives
The objective of this review was to assess the effects of asthma self‐management programmes, when coupled with regular health practitioner review, on health outcomes in adults with asthma.
Search methods
We searched the Cochrane Airways Group trials register and reference lists of articles.
Selection criteria
Randomised trials of self‐management education in adults over 16 years of age with asthma.
Data collection and analysis
Two reviewers assessed trial quality and extracted data independently. We contacted study authors for confirmation.
Main results
We included thirty six trials, which compared self‐management education with usual care. Self‐management education reduced hospitalisations (relative risk (RR) 0.64, 95% confidence interval (CI) 0.50 to 0.82); emergency room visits (RR 0.82, 95% CI 0.73 to 0.94); unscheduled visits to the doctor (RR 0.68, 95% CI 0.56 to 0.81); days off work or school (RR 0.79, 95% CI 0.67 to 0.93); nocturnal asthma (RR 0.67, 95% CI 0.0.56 to 0.79); and quality of life (standard mean difference 0.29,CI 0.11 to 0.47). Measures of lung function were little changed.
Authors' conclusions
Education in asthma self‐management which involves self‐monitoring by either peak expiratory flow or symptoms, coupled with regular medical review and a written action plan improves health outcomes for adults with asthma. Training programmes that enable people to adjust their medication using a written action plan appear to be more effective than other forms of asthma self‐management.
Plain language summary
Self‐management education and regular practitioner review for adults with asthma
Guidelines for the treatment of asthma recommend that patients be educated about their condition, obtain regular medical review, monitor their condition at home with either peak flow or symptoms and use a written action plan. The results of trials comparing asthma self‐management education to usual care were combined. These results showed that asthma sufferers who were educated about their asthma, visited the doctor regularly and who used a written action plan had fewer visits to the emergency room; less hospital admissions; better lung function; improvement in peak expiratory flow; fewer symptoms; and used less rescue medication.
Background
The burden of illness from asthma is high and increasing (Peat 1994). There are problems with the delivery of care, which include under‐treatment with corticosteroids, limited knowledge, and poor asthma management skills amongst patients with severe asthma ( Gibson 1993a). Asthma management guidelines have been developed in many countries to assist in the application of standardised, high‐quality medical care (Woolcock 1989). These guidelines rely on expert opinion with variable reporting of their evidence base (Gibson 1993b). A key component of many asthma management guidelines, including Part 1 of the Six‐Part Asthma Management Program proposed by the International Consensus Report on diagnosis and Treatment of Asthma (Anonymous 1992), is the recommendation for patient education and regular medical review. Education is considered to be necessary "to help patients gain the motivation, skills and confidence to control their asthma" (Anonymous 1996). A narrative review of asthma education has emphasised the need for asthma education and suggested successful strategies (Clark 1993). A number of controlled trials have been conducted to identify the effectiveness of asthma education and self‐management programmes. Whilst there is general agreement that these programmes improve patient knowledge, the impact that this may have on health outcomes is less well acknowledged. For example, a review of paediatric education programmes failed to identify a positive benefit on asthma admissions, doctor visits, or school absenteeism (Bernard‐Bonnin 1995). The influence of programme characteristics on health outcomes has not been examined in adults. This review was conducted to address these issues. Specifically, it examined the strength of evidence supporting Step 6 of the Australian Asthma Management Plan, "Educate and Review Regularly" in order to identify whether health outcomes are influenced by asthma education and self‐management programmes.
A companion review has dealt with trials of limited (information only) education interventions (Gibson 1998) and concluded that education did not have a significant effect when administered without an action plan, self‐monitoring or regular review.
Objectives
This study aimed to evaluate the literature supporting Step 6 of the Australian Asthma Management Plan (AAMP), "Educate and Review Regularly." The specific questions addressed are:
Do asthma self‐management education and regular review (by doctor or nurse practitioner) lead to improved health outcomes in asthma?
What are the characteristics of those programmes which lead to measurable changes in health outcomes?
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were randomised controlled trials (RCTs) or quasi‐randomised controlled trials (CCTs) which studied the effects of asthma education and self‐management on health outcomes in adults with asthma.
Types of participants
Predominantly adults (> 16 years old) with asthma (defined by doctor's diagnosis or objective criteria or according to American Thoracic Society guidelines).
Types of interventions
We categorised the interventions according to whether or not they involved asthma education, self‐monitoring of peak expiratory flow or symptoms, regular medical review and a written action plan.
Intervention characteristics
Patient Asthma Education: a programme which transfers information about asthma in any of these forms: written, verbal, visual or audio. It may be interactive or non‐interactive, structured or unstructured. Minimal education is characterised by the provision of written material alone or the conduct of a short unstructured verbal interaction between a health provider and a patient where the primary goal is to improve patient knowledge and understanding of asthma. Maximal education is considered to be structured with the use of both interactive and non‐interactive modes of information transfer. The content of the education must be related to asthma and its management.
Self‐monitoring: consists of the regular measurement of either peak expiratory flow or symptoms. It is further characterised by the recording (or not) of those measurements in a diary.
Regular Review: consists of regular consultation with a doctor during the intervention period for the purpose of reviewing the patients' asthma status and medications. This may occur either as a formal part of the intervention or the patients may be advised to see their own doctor on a regular basis. Interventions are classified as having "regular review" either inside the programme (if the patients were seen as a part of the programme) or outside the programme (if the patients were merely advised to seek regular medical review).
Written Action Plan: an individualised written plan produced for the purpose of patient self‐management of asthma exacerbations. The action plan is characterised by being individualised to the patient's underlying asthma severity and treatment. It is also a written plan which informs participants about:
when and how to modify medications in response to worsening asthma; and
how to access the medical system in response to worsening asthma.
Types of outcome measures
Any of the following outcomes: asthma admissions, emergency room visits, doctor visits, days lost from work or school, lung function (FEV1), peak expiratory flow (PEF), use of rescue beta‐agonists, courses of oral corticosteroids, symptom scores, quality of life scores, costs.
Search methods for identification of studies
We identified studies from the following sources:
Cochrane Airways Group trial register derived from MEDLINE, EMBASE, CINAHL, handsearched respiratory journals and meeting abstracts. We searched the register using the following terms: (Asthma OR wheez*) AND (education* OR self management OR self‐management).
We obtained the articles, and handsearched their bibliographic lists for additional articles.
Data collection and analysis
Selection of studies
Two reviewers independently coded studies from the above sources into three categories based upon the abstract/key word/title:
Include: as RCT, adult, asthma, education
Possible RCT but cannot determine from abstract
Exclude: non‐RCT or CCT, paediatric age range, doctor education.
We examined full text versions of the articles or studies in category (2) in order to define if the study met the inclusion criteria.
To investigators independently categorised study eligibility, study quality and intervention type. Agreement was examined and disagreement resolved by consensus.
We included articles if they were: randomised or quasi‐randomised controlled trials; of asthma education delivered to adults (> 16 years) with asthma. We reported relevant health outcomes: hospitalisations, visits to medical practitioner, visits to emergency room, use of beta‐agonists, lung functions, quality of life, symptoms score, symptoms or peak expiratory flow diary.
Data extraction and management
Two review authors extracted data on the following variables, and entered the data into Review Manager:
Hospital admissions
Emergency room visits
Unscheduled doctor visits
Days lost from work or school
Forced Expiratory Volume in 1 second (FEV1)
Peak Expiratory Flow (PEF)
Use of 'rescue' (or reliever) medications
Quality of life, symptoms scores, symptom/peak flow diary
Economic data, cost, days lost from college/work.
The data extraction process also included collecting information on
Demographics: age, gender, ethnicity, socio‐economic level,
Type of control: several different types of control intervention were used. These included an "intervention" of low efficacy (eg. written material only), usual medical care and (waiting list control. It is likely that a true placebo has not been used in any study.
Setting of intervention: primary care vs hospital based. The severity of asthma differs in these settings and this may influence the ability to detect a change in outcome measures. For example: in a hospital based setting, the greater number of events (e.g. re‐admission) could make it easier to detect differences than in primary care.
Duration of intervention: number of sessions, hours of teaching.
Sample size
Asthma severity
Intermediate outcomes: asthma knowledge, skills.
Assessment of risk of bias in included studies
Two reviewers independently assessed the quality of the full text versions of all included papers using the Cochrane system. Study quality was assessed according to the following criteria:
Two authors assessed whether the process of concealment of allocation was adequate. This was deemed to be adequate if there was a central randomisation scheme, randomisation i.e. external person or use of coded containers/ envelopes. This was determined to be unclear if information was not available. This was deemed to be inadequate if there was alternate allocation, reference to case record number, date of birth, day of the week, or an open list of random numbers.
Additional quality variables recorded were:
Blinding of interventions
Withdrawals/ dropouts
Blinding of outcome assessment.
Dealing with missing data
We made an attempt to contact all authors for verification of methodological quality, classification of the intervention(s) and of outcomes data. Replies were received from thirteen authors who are listed in the acknowledgements section. Two were returned to sender (Snyder 1987; Huss 1992). We attempted to contact the second author if we were unsuccessful in contacting the first author.
Data synthesis
We analysed outcomes as continuous and/or dichotomous variables, using standard statistical techniques.
For continuous outcomes, the weighted mean difference (WMD) or standardised mean difference (SMD) with 95% confidence intervals (CI) were calculated as appropriate.
For dichotomous outcomes, the relative risk (RR) was calculated with 95% CI.
We examined heterogeneity using a Chi‐squared test and explored reasons for heterogeneity if appropriate.
Where appropriate, we entered data as negative values to eliminate differences in scoring scales for quality of life.
The primary comparison, based on the treatment of the intervention and control groups used was:
Self Management versus usual care.
Another review comparing different options for optimal self‐management has been published (Gibson 2002).
Subgroup analysis and investigation of heterogeneity
We further divided study groups by the intensity of their intervention into one of the following categories:
Optimal Self‐Management which involved a written action plan for self‐management of medications for exacerbations, together with self‐monitoring and regular medical review;
Self Monitoring and Regular Review without a written action plan;
Self Monitoring Only,
Regular Review Only, and
Written Action Plan but not Optimal Self‐Management: These interventions included a written action plan but did not include both self‐monitoring and regular review
Results
Description of studies
Results of the search
We identified 101 papers describing 87 potentially relevant studies of asthma education in adults. We obtained full text versions of these papers, and two reviewers independently assessed them. We agreed to include 45 papers describing 36 randomised controlled trials in this review.
Included studies
PARTICIPANTS & SETTING
6090 participants were randomised into 36 trials. Thirty four studies reported that 4593 participants completed the trial. The reported drop out rates ranged from 0% to 54%.
Participants were recruited from a variety of settings:
Hospital (n = 6)
Emergency Room (n = 3)
Hospital and Emergency Room (n = 1)
Outpatient Clinic (n = 12)
General Practice (n = 5)
Community Setting (n = 6)
Hospital and Clinic (n = 1)
Outpatients and General Practice (n = 1)
HMO (n = 1)
INTERVENTIONS
The content of the asthma self‐management interventions described in the 36 studies included:
education (n = 36, 100%)
self‐monitoring of symptoms and/or peak expiratory flow (n = 33, 92%)
regular review of treatment and asthma severity by a medical practitioner (n = 24, 67%)
written action plan (n = 18, 50%).
Some degree of patient education was provided in all of the 36 trials included in this comparison. As education has been shown not to have a significant impact on objective health outcomes when administered without an action plan, self monitoring or regular review (Gibson 1998), education was not reflected in sub‐group analysis.
COMPARISONS
Self‐management was compared with a usual care control in all 36 studies.The participants in the control groups received 'usual care' which may have included a variety of interventions. The descriptions of 'usual care' included no intervention, education, self monitoring, or regular medical review. No control group received a written action plan. In some cases, the nature of 'usual care' was not specified. The nature of the control intervention did not exclude a study from this review. Control groups received education about asthma in 12 (33%) studies. Self monitoring was performed intermittently for outcome assessment in seven (22%) studies, continuously in four (11%) and provision of a peak flow meter and encouragement of its use occurred in one (3%). Eleven (31%) of the control groups were advised to seek medical review, generally outside the programme.
These studies fell into five subgroups according to the type of self‐management intervention: (1a) Optimal self management (n = 15), (1b) Self monitoring and regular review (n = 7), (1c) Self monitoring only (n = 10), (1d) Regular Review only (n = 2) and (1e) Written action plan but not optimal self management (n = 2)
OUTCOMES: SELF MANAGEMENT VERSUS USUAL CARE
The list below describes the measurement and reporting of outcomes from the included studies. Measured and Reported (measured but not reported)
Hospitalisations 18 (6)
ER visits 20 (3)
Unscheduled Dr visits 12 (6)
Days off work or school 16 (4)
Nocturnal Asthma 7 (4)
Disrupted days 2 (6)
FEV1 8 (2)
PEFR 14 (2)
Oral corticosteroids 3 (1)
Quality of life 7(3)
Cost 4
Excluded studies
Forty seven studies were excluded for the following reasons: the participants had smoking‐related chronic obstructive airway disease and not asthma (two); the methodological criteria were not met (11); background data only reported (two); the intervention did not include education (7); or was assessing inhaler technique only (3); the outcome measured was not appropriate (two); the interventions were not patient education (two); two interventions were compared without a control group (9) or the interventions were information‐only education; and did not include elements of self‐management or behavioural change (ten). The information‐only trials were reported in a previous review and the comparisons of two interventions form the basis of a third review. Two studies are ongoing and one is waiting assessment. The results of this review are thus derived from thirty‐six RCTs of patient education and self‐management in adults with asthma.
Risk of bias in included studies
An overview of our judgements of concealment of allocation is given in Figure 1. The information we have used to as a basis for these judgements is given in Characteristics of included studies. Eight studies had adequately concealed allocation, and eight studies had inadequately concealed allocation. In the remaining studies we could not determine whether procedures to conceal allocation were adequate or inadequate due to a lack of available information.
1.
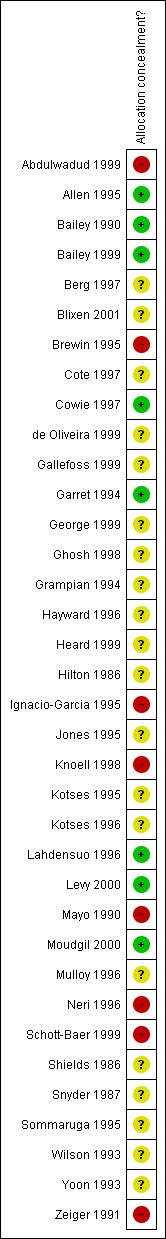
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
HOSPITALISATIONS
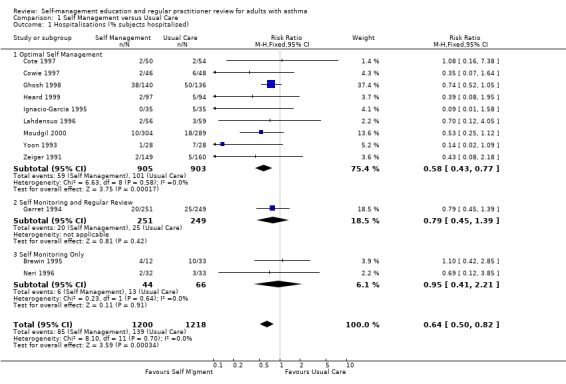
Asthma self‐management education led to a significant reduction in the proportion of participants who were hospitalised for asthma. Eighteen studies provided data of which 12 could be included in a meta analysis. Approximately 11.4% of participants in the control groups required hospitalisation for asthma exacerbations during the study periods. This was reduced to 7.1% by asthma self‐management education (RR 0.64; 95% CI 0.50 to 0.82, Figure 2). We performed a sub‐group analysis to examine the effects of different types of self‐management education on hospitalisation for asthma. Optimal self‐management involving provision of a written action plan led to a significant reduction in hospitalisations for asthma (RR 0.58; 95% CI 0.43 to 0.77), however there was insufficient power to compare the subgroups with less intensive interventions. There was no significant difference in mean hospitalisations reported in five studies and no significant difference from baseline was reported in one but data not given (Blixen 2001).
2.
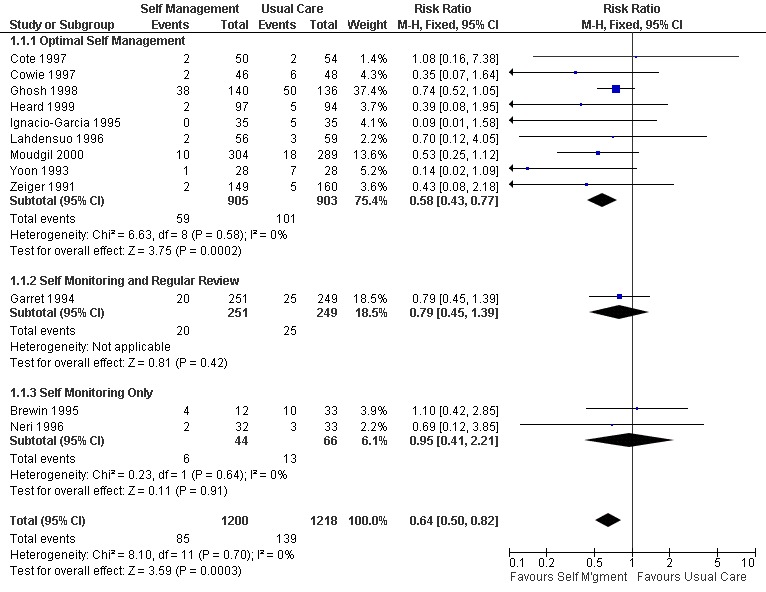
Forest plot of comparison: 1 Self Management versus Usual Care, outcome: 1.1 Hospitalisations (% subjects hospitalised).
EMERGENCY HOSPITAL VISITS
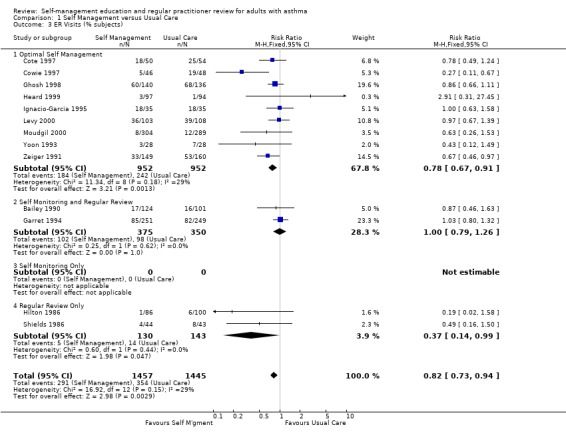
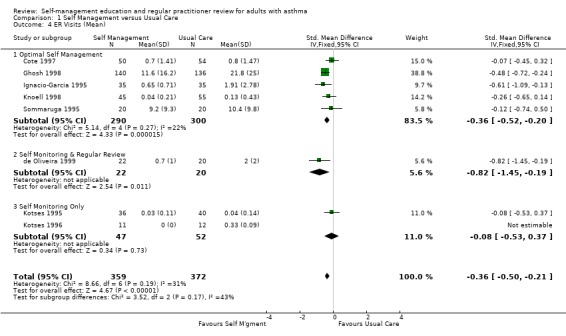
There were 20 studies that examined the effect of self‐management education on emergency room (ER) visits for asthma, thirteen providing dichotomous data, nine the mean number of visits, three reporting both and one as the number of visits. The proportion of participants who required ER visits was 24.5% in the usual care group. Overall, there was a significant effect for self‐management education to reduce the proportion of asthmatics needing ER visits (RR 0.82; 95% CI 0.73 to 0.94, Figure 3). Optimal self‐management education led to a significant reduction in ER visits (RR 0.78; 95% CI 0.67 to 0.91), as did the two interventions which included regular review of medications (RR 0.37; 0.14, 0.99). Mean ER visits were examined in eight studies suitable for meta‐analysis. There was a significant effect favouring self‐management (SMD ‐0.36; 95% CI ‐0.5 to ‐0.21), however significant heterogeneity was present (Chi Sq 8.66, p < 0.05).
3.
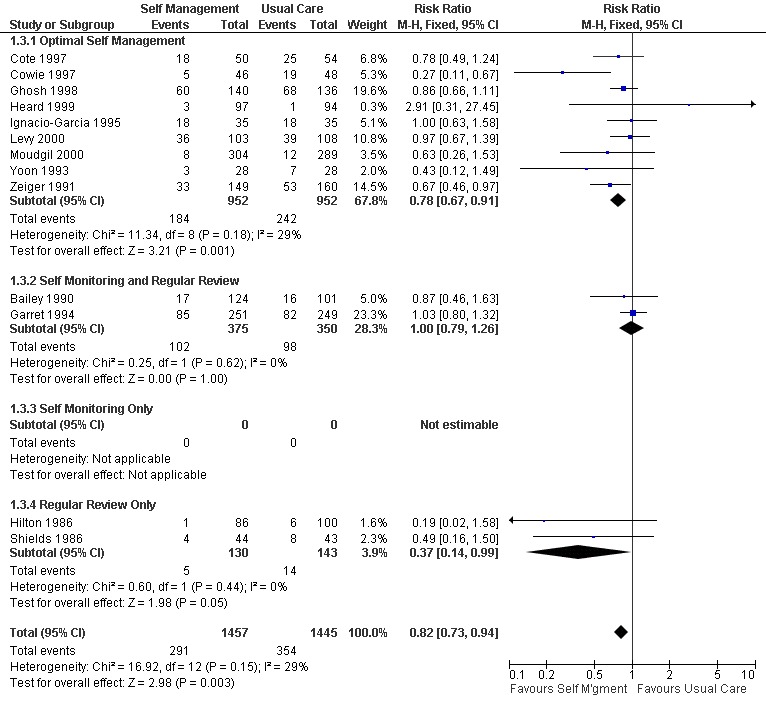
Forest plot of comparison: 1 Self Management versus Usual Care, outcome: 1.3 ER Visits (% subjects).
Two further studies measured this outcome but did not report the results and one study reported no significant difference in ER visits from baseline (Blixen 2001)
UNSCHEDULED DOCTOR VISITS
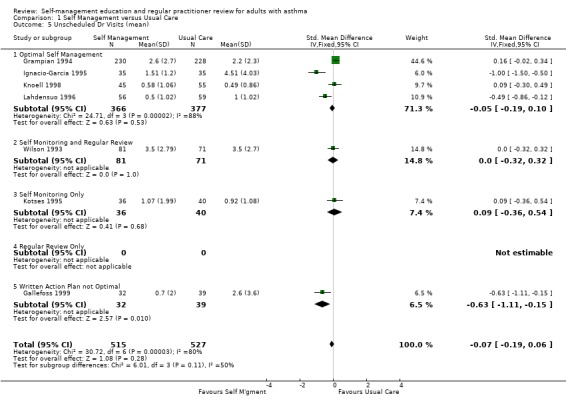
Eleven studies reported results for the effects of self management education on unscheduled doctors visits suitable for meta‐analysis; seven as the proportion of participants requiring one or more visits, and seven as the mean number of visits, with three studies reporting both. When reported as the number of participants there was a significant reduction in unscheduled visits (RR 0.68; 95% CI 0.56 to 0.81, Analysis 1.6). However there was significant heterogeneity in this group (Chi Sq 24.85, p < 0.05). In the seven studies which reported the mean number of visits, there was no significant effect and significant heterogeneity (Chi Sq 30.72, p < 0.05) which is discussed below.
1.6. Analysis.
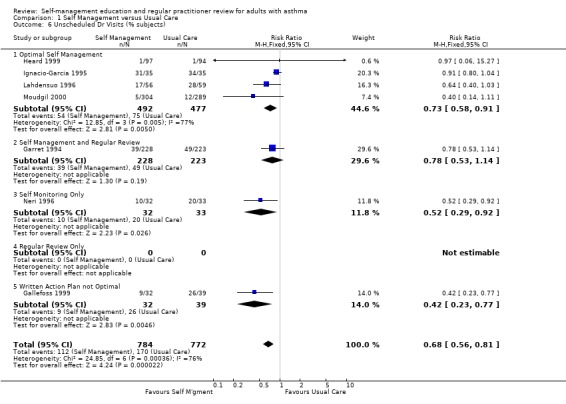
Comparison 1 Self Management versus Usual Care, Outcome 6 Unscheduled Dr Visits (% subjects).
Six further studies reported this outcome either narratively or using data not suitable for meta‐analysis. Five of these studies reported no significant effect of self‐management education between groups (Hilton 1986; Allen 1995; Cote 1997; Levy 2000; Blixen 2001). One study reported a significant decrease in number of consults for both the self‐management and control groups (Hayward 1996). Another study did not report their results (Snyder 1987).
DAYS OFF WORK
Sixteen studies reported the effects of self‐management education on days off work; seven as the number of participants who had one or more days off work or school, and thirteen as the mean number of days or absences, with five studies reporting both. Asthma self‐management education led to a significant reduction in the rest of losing days off work or school due to asthma (RR 0.79; 95% CI 0.67 to 0.93, Analysis 1.7). In the thirteen studies that reported the mean number of days off work or school or mean number of absences, there was a significant effect favouring self‐management (SMD ‐0.18; 95% CI ‐0.28 to ‐0.09, Analysis 1.8). Significant heterogeneity was present (Chi Sq 26.85, p < 0.05).
1.7. Analysis.
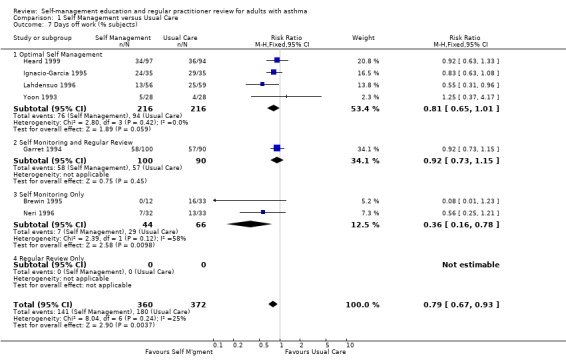
Comparison 1 Self Management versus Usual Care, Outcome 7 Days off work (% subjects).
1.8. Analysis.
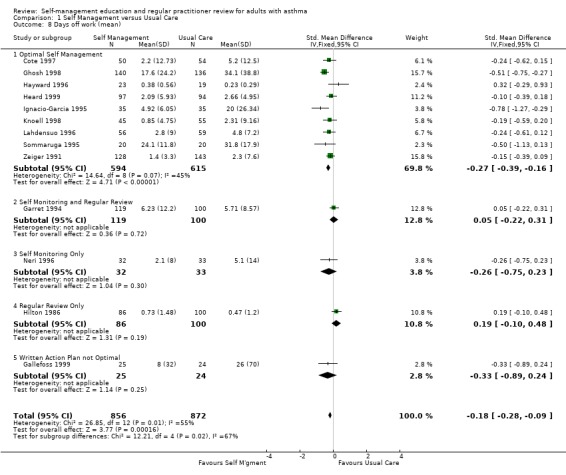
Comparison 1 Self Management versus Usual Care, Outcome 8 Days off work (mean).
NOCTURNAL ASTHMA
Nocturnal asthma was examined as an outcome in seven studies, with data from five studies contributing to a meta‐analysis. Self‐management education reduced the proportion of participants reporting nocturnal asthma (RR 0.67; 95% CI 0.56 to 0.79, Analysis 1.9). However there was significant heterogeneity within this group (Chi Sq 107.02, p < 0.05). In the four other studies which mentioned this outcome, but did not provide numerical data, an improvement was noted in three studies and no significant change in another.
1.9. Analysis.
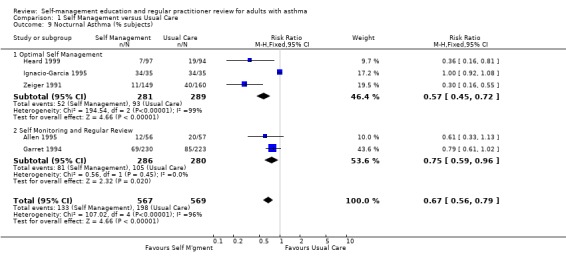
Comparison 1 Self Management versus Usual Care, Outcome 9 Nocturnal Asthma (% subjects).
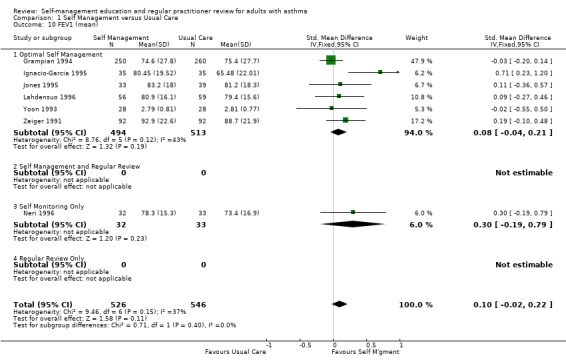
LUNG FUNCTION
Airway function was assessed as either clinic forced expiratory volume in 1 second (FEV1) (10 studies) or peak expiratory flow (PEF) (16 studies). Of the seven studies that reported data on FEV1 and were used for meta‐analysis, six were optimal self‐management interventions. No significant effect of education on this variable was found. PEF was measured in 16 studies and data from 10 studies contributed to a meta‐analysis. All of these data were available in absolute units (l/min), with one exception (Jones 1995), in which the results were presented as % predicted normal. The data tables include this trial, so the analyses used the SMD to permit aggregation. There was an overall positive effect of asthma self‐management education which led to an improvement in PEF, that achieved statistical significance at p < 0.05 (Analysis 1.11). When this study was removed and the analysis repeated using the WMD (since all the outcomes were in the same units of measurement), the overall effect remained statistically significant. The absolute improvement in PEF was small (14.5 l/min). Significant heterogeneity was present for both the SMD analysis (Chi Sq 30.13, p < 0.05) and the WMD analysis (Chi Sq 28.77, p < 0.05).
1.11. Analysis.
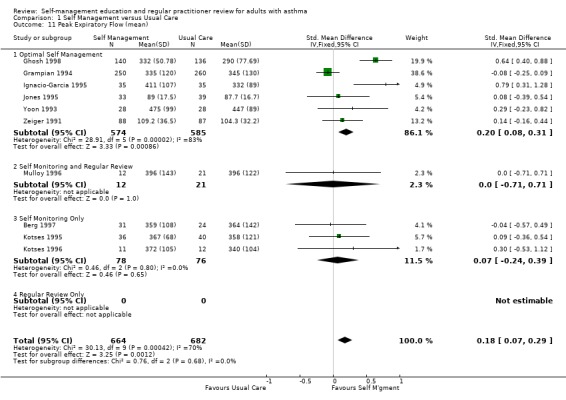
Comparison 1 Self Management versus Usual Care, Outcome 11 Peak Expiratory Flow (mean).
ORAL CORTICOSTEROIDS
Four studies assessed the use of oral corticosteroids. Grampian 1994 measured the number of courses of oral corticosteroids, reporting no between group differences. Jones 1995 reported that 47% in the treatment group and 38% in the control group used oral corticosteroids. Mayo 1990, who measured the percentage of participants using chronic daily prednisone recorded a drop from 25% to 9% in the treatment group but did not record the results of the control group. de Oliveira 1999 reported no significant difference in the percentage of participants who used oral corticosteroids continuously before and after the programme for both the self‐management and control groups.
QUALITY OF LIFE
Quality of life was assessed in ten studies, six of which provided mean total scores. Overall there was a significant improvement in total quality of life score for those receiving the self‐management intervention (SMD 0.29;95% CI 0.11 to 0.47). Significant heterogeneity was also present (Chi Sq 26.0; p < 0.05). Cote 1997 reported a significant improvement for all groups but only a clinically significant improvement (> 0.5 change in score) for the intervention group that received an action plan based on symptoms. No significant inter‐group difference was reported by Jones 1995 but a significant within group improvement in all scores was reported for the self‐management group. Knoell 1998 reported an improvement in both groups for most domains and no significant difference between the groups. Moudgil 2000 reported an improvement in score for those who received self‐management education and a decrease in score for those in the control group. These differences were significant between the two groups.
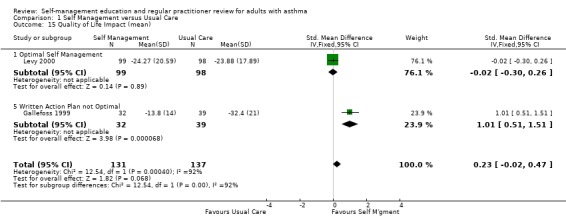
Self‐management intervention improved the impact domain of quality of life as reported in two studies (SMD 0.23; 95% CI ‐0.02 to 0.47, Analysis 1.14). However this was not statistically significant and heterogeneity was present (Chi Sq 12.54, p < 0.05). Four studies reported mean activity score, three that could be included in a meta‐analysis resulting in a non‐significant improvement in activity score. Mean symptom score for quality of life favoured the usual care group in three studies but this was not significant. Heterogeneity was present (Chi Sq 10.25, p < 0.05).
1.14. Analysis.
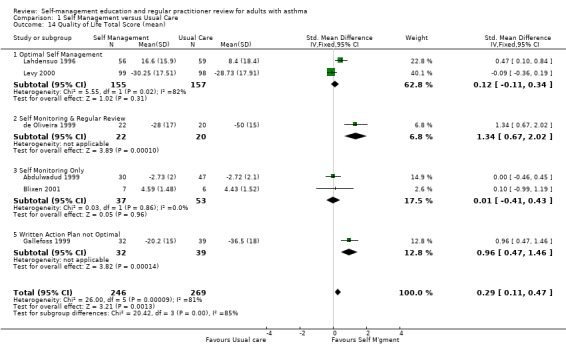
Comparison 1 Self Management versus Usual Care, Outcome 14 Quality of Life Total Score (mean).
We conducted sensitivity analyses to explore reasons for heterogeneity in the quality of life total score meta‐analysis.
Asthma Severity: two studies (Lahdensuo 1996; Gallefoss 1999) included participants with mild to moderate asthma whereas four studies included moderate to severe asthma. Removal of the mild to moderate asthma severity studies from the meta‐analysis did not eliminate heterogeneity (Chi Sq 14.8, p = 0.002).
Ethnic Minority: two studies (de Oliveira 1999; Blixen 2001) studied ethnic minority groups. Removal of these studies from the meta‐analysis did not eliminate heterogeneity (Chi‐Sq 15.9, p = 0.001).
Questionnaire: two studies (Gallefoss 1999; Levy 2000) used the St Georges Respiratory Questionnaire to measure quality of life. Removal of these studies from the meta‐analysis reduced but did not eliminate heterogeneity (Ch Sq 10.8, p = 0.013).
Intervention Type: a written action plan was part of the intervention in three studies (Lahdensuo 1996; Gallefoss 1999; Levy 2000). Heterogeneity remained in a meta‐analysis that included these studies only (Ch Sq 14.91, p = 0.006)
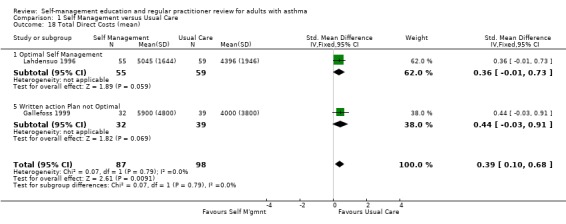
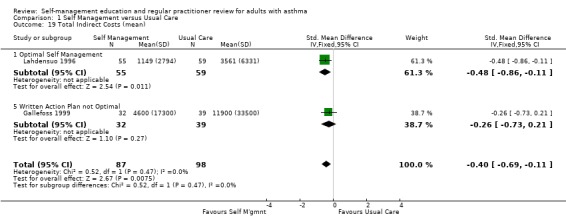
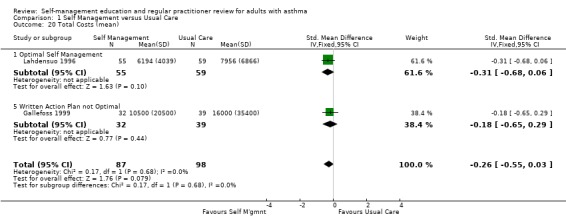
COSTS
Costs were assessed in four studies. Ghosh 1998 recruited participants from a hospital asthma and allergy clinic who had a greater than 15% reversal of FEV1 post bronchodilator and at least one ER visit or hospitalisation in the past 12 months. Participants were randomised to an optimal self‐management programme, including a written action plan, or to usual care. The intervention included four, two hour sessions, with a social scientist, commencing during the first month following the baseline interview. Economic outcomes were measured four, eight and twelve months after baseline. Direct costs were measured in Indian Rupees and included daily medication costs, hospitalisation and ER visit costs. The costs of physician visits were excluded as these were not reported consistently. Estimates of transportation, intervention and lost production costs were also provided (Ghosh 1998).
In a trial conducted by Lahdensuo 1996, mild to moderate asthmatics were recruited from outpatients and randomised to an optimal self‐management training intervention that included a written action plan or to a usual care control group that included regular medical review. Both groups were followed up every four months for one year. Direct costs, measured in Finnish marks, included counselling (instruction in self‐management for the intervention group and general information in the control group), peak flow meter, drugs, doctor visits that were not related to the study and hospital admissions. Indirect costs included absence from work (Lahdensuo 1996).
Gallefoss 1999 recruited mild to moderate asthmatics from an outpatient chest clinic and randomised them to either a self‐management intervention, which included self monitoring and a written action plan, or to a usual care control group. The intervention comprised two group sessions, each two hours long and one individual session, which was one to two hours with a physiotherapist and a nurse. All participants in the intervention group also received a booklet summarising the information received during the sessions. Participants were followed for 12 months. Costs were based on utilisation of care and unit costs (Norwegian Krone) and included patient co‐payments and reimbursement costs from the National Health Insurance fee. Direct costs were defined as costs incurred by the healthcare system, community and family. Indirect costs included productivity loss, time costs for the individual, family, society and employer. Cost‐effectiveness ratios were also estimated (Gallefoss 1999).
In an economic analysis of asthma education programmes, Neri 1996 randomised asthmatics to two different education programmes, a reduced education programme or a complete "asthma school" which involved more intense education. Both groups performed self‐monitoring and were followed for one year. Direct costs included intervention costs (personnel related costs, videotape and cost of room), "per diem" costs for hospital admissions, drug costs and medical examination costs. Indirect costs were counted as the salary per day of work lost. Cost‐effectiveness ratios were calculated by dividing the difference in the programme costs by the difference in reducing the outcome variables for the two programmes.
Ghosh 1998 reported a non‐significant reduction in direct costs and a significant reduction in indirect costs for the self‐management group when compared to the control group. Lahdensuo 1996 observed that direct costs were significantly lower for the control group, and that indirect costs were significantly lower for the self‐management group. Overall, there was a significant reduction in total costs for the self‐management group. Gallefoss 1999 reported non‐significant trends for lower direct costs and higher indirect costs for the control group. Total costs were lower for the intervention group but this was not significant when compared to the control group. Neri 1996 reported a better outcome for the intensive education programme when calculating the cost‐effectiveness ratio for asthma attacks, urgent doctor visits and days off work.
Three studies provided data on mean total, direct and indirect costs. These studies were critically appraised by a health economist (PH). The study by Ghosh 1998 excluded the costs of physician visits and was therefore not included in a meta analysis. The remaining two studies contributed to a meta analysis. As these were reported in different currencies, a standardised mean difference was used for analysis. Self‐management intervention led to a significant reduction in indirect costs (SMD ‐0.40; 95% CI ‐0.69 to ‐0.11) but increased direct costs (SMD 0.39; 95% CI 0.10 to 0.68). Overall there was a reduction in total costs (SMD ‐0.26; 95% CI ‐0.55 to 0.03) which did not quite reach significance.
Discussion
This review systematically evaluated 36 RCTs of self‐management education for adults with asthma and found that this type of intervention leads to improved health outcomes. The studies showed that with self‐management education, there was a reduction in the proportion of participants reporting hospitalisations and ER visits for asthma, unscheduled doctors visits for asthma, days lost from work due to asthma, episodes of nocturnal asthma, indirect costs and an improvement in total quality of life. The effects were large enough to be of both clinical and statistical significance. The review also identified a number of limitations to the current published literature which need to be considered. The interventions were described in varying detail, and included several differing factors. The system used to categorise the interventions in this review was based upon recommendations in current asthma management guidelines. Specifically, they were evaluated as to whether they included peak expiratory flow monitoring, regular medical review, and a written action plan. These are important aspects of asthma management which could be reliably evaluated from the papers. It could be useful in future work to extend this by looking in detail at the concordance of interventions with educational theory. This would require access to the precise details of interventions, which is generally not provided in publications because of space limitations.
There was variable contamination of the control groups with some aspects of self‐management education. For example, peak expiratory flow monitoring was used as an outcome measure in some control groups. The effect of this would be to reduce the effect size of self‐management education and hence bias against seeing an effect. Despite this, clinically meaningful effects were seen in most outcomes.
Not all papers reported outcomes in a way that could contribute to meta‐analysis. We attempted to overcome this by contacting authors, but had variable success. This limits the generalisability of the results.
In some cases, outcomes were reported as continuous measures showing no treatment effect. This is probably due to the inappropriate use of continuous measures for outcomes which are not normally distributed such as hospitalisations, ER visits, doctor visits and days off work or school. Heterogeneity was found in the latter two variables. Possible explanations may be differing definitions of what constitutes an unscheduled doctor visit or a day off work or the combination of groups of differing asthma severity. It is noted that in one study (Ignacio‐Garcia 1995) the control group was instructed to visit the emergency or doctor as a part of their management. This may have also contributed to the heterogeneity.
Authors' conclusions
Implications for practice.
Self‐management education of adults with asthma results in clinically important improvements in asthma health outcomes. This is most apparent with interventions involving a written action plan, self‐monitoring and regular medical review. These interventions result in a reduction in the proportions of participants who use health care services and who are bothered by nocturnal asthma and loss of work.
Self‐management education that involves a written action plan, self‐monitoring and regular medical review should be offered to adults with asthma.
Less intensive interventions, particularly those without a written action plan are less efficacious.
Implications for research.
Optimisation of management plan: what are the core 'actions'?
How much PEF/Symptom monitoring is optimal?
What is the duration of effect?
Is maintenance required?
What forms should it take?
How do the interventions conform to psycho‐educational theory?
What is the best form for a written action plan?
What's new
| Date | Event | Description |
|---|---|---|
| 20 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 12 March 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Airways Group who helped with database searches, obtaining studies and translations (Steve Milan, Toby Lasserson, Anna Bara, Karen Blackhall). Thanks also to Kirsty Olsen who copy edited this review.
We would like to thank the following authors for providing information about their trials: Dr M Abramson re Abdulwadad Dr R Allen, Dr I Charlton, Dr J Cote, Dr JM Ignacio‐Garcia Dr D Knoell Dr A Lahdensuo Dr K Lutteral re Yoon, Dr J Mercer re Garrett, Mr M Mullee re Jones Dr M. Neri, Dr M Sommaruga, Dr L Tougaard, Dr RS Zeiger,
We would also like to acknowledge: NSW Health Cooperative Research Centre for Asthma, Australia for financial and administrative support.
Data and analyses
Comparison 1. Self Management versus Usual Care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalisations (% subjects hospitalised) | 12 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.82] |
| 1.1 Optimal Self Management | 9 | 1808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.43, 0.77] |
| 1.2 Self Monitoring and Regular Review | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.39] |
| 1.3 Self Monitoring Only | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.41, 2.21] |
| 2 Hospitalisations (mean) | 5 | 744 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.17, 0.15] |
| 2.1 Optimal Self Management | 4 | 702 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.17, 0.15] |
| 2.2 Self Monitoring & Regular Review | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 ER Visits (% subjects) | 13 | 2902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.73, 0.94] |
| 3.1 Optimal Self Management | 9 | 1904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.67, 0.91] |
| 3.2 Self Monitoring and Regular Review | 2 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.26] |
| 3.3 Self Monitoring Only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Regular Review Only | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.99] |
| 4 ER Visits (Mean) | 8 | 731 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.50, ‐0.21] |
| 4.1 Optimal Self Management | 5 | 590 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.52, ‐0.20] |
| 4.2 Self Monitoring & Regular Review | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.45, ‐0.19] |
| 4.3 Self Monitoring Only | 2 | 99 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.53, 0.37] |
| 5 Unscheduled Dr Visits (mean) | 7 | 1042 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.19, 0.06] |
| 5.1 Optimal Self Management | 4 | 743 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.19, 0.10] |
| 5.2 Self Monitoring and Regular Review | 1 | 152 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.32, 0.32] |
| 5.3 Self Monitoring Only | 1 | 76 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.36, 0.54] |
| 5.4 Regular Review Only | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.11, ‐0.15] |
| 6 Unscheduled Dr Visits (% subjects) | 7 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.81] |
| 6.1 Optimal Self Management | 4 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.91] |
| 6.2 Self Management and Regular Review | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 6.3 Self Monitoring Only | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.92] |
| 6.4 Regular Review Only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Written Action Plan not Optimal | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.77] |
| 7 Days off work (% subjects) | 7 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.93] |
| 7.1 Optimal Self Management | 4 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.01] |
| 7.2 Self Monitoring and Regular Review | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.15] |
| 7.3 Self Monitoring Only | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.78] |
| 7.4 Regular Review Only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Days off work (mean) | 13 | 1728 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.28, ‐0.09] |
| 8.1 Optimal Self Management | 9 | 1209 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.39, ‐0.16] |
| 8.2 Self Monitoring and Regular Review | 1 | 219 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.22, 0.31] |
| 8.3 Self Monitoring Only | 1 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.75, 0.23] |
| 8.4 Regular Review Only | 1 | 186 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.10, 0.48] |
| 8.5 Written Action Plan not Optimal | 1 | 49 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.89, 0.24] |
| 9 Nocturnal Asthma (% subjects) | 5 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.79] |
| 9.1 Optimal Self Management | 3 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.72] |
| 9.2 Self Monitoring and Regular Review | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.96] |
| 10 FEV1 (mean) | 7 | 1072 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.02, 0.22] |
| 10.1 Optimal Self Management | 6 | 1007 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.04, 0.21] |
| 10.2 Self Management and Regular Review | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Self Monitoring Only | 1 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.19, 0.79] |
| 10.4 Regular Review Only | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Peak Expiratory Flow (mean) | 10 | 1346 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.07, 0.29] |
| 11.1 Optimal Self Management | 6 | 1159 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.08, 0.31] |
| 11.2 Self Monitoring and Regular Review | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.71, 0.71] |
| 11.3 Self Monitoring Only | 3 | 154 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.24, 0.39] |
| 11.4 Regular Review Only | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Hospitalisations (mean total days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Optimal Self Management | 1 | 276 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐10.47, ‐2.93] |
| 12.2 Self Monitoring & Regular Review | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Self Monitoring Only | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Regular Review Only | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Written Action Plan not Optimal | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
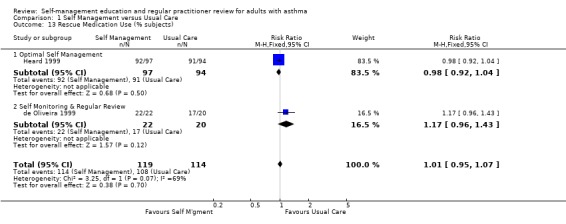
| 13 Rescue Medication Use (% subjects) | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 13.1 Optimal Self Management | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| 13.2 Self Monitoring & Regular Review | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.96, 1.43] |
| 14 Quality of Life Total Score (mean) | 6 | 515 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.11, 0.47] |
| 14.1 Optimal Self Management | 2 | 312 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.11, 0.34] |
| 14.2 Self Monitoring & Regular Review | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.34 [0.67, 2.02] |
| 14.3 Self Monitoring Only | 2 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.41, 0.43] |
| 14.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.96 [0.47, 1.46] |
| 15 Quality of Life Impact (mean) | 2 | 268 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.02, 0.47] |
| 15.1 Optimal Self Management | 1 | 197 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.30, 0.26] |
| 15.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.01 [0.51, 1.51] |
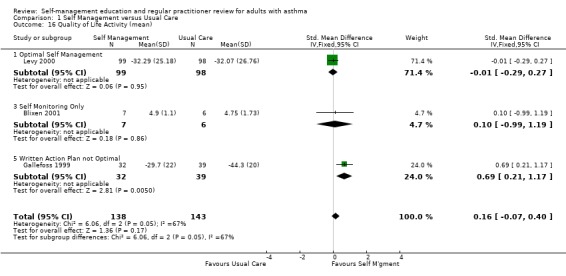
| 16 Quality of Life Activity (mean) | 3 | 281 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.07, 0.40] |
| 16.1 Optimal Self Management | 1 | 197 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.29, 0.27] |
| 16.3 Self Monitoring Only | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.99, 1.19] |
| 16.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.21, 1.17] |
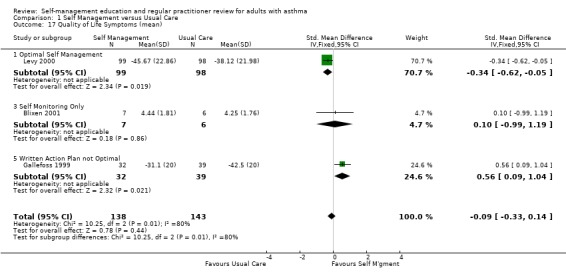
| 17 Quality of Life Symptoms (mean) | 3 | 281 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.33, 0.14] |
| 17.1 Optimal Self Management | 1 | 197 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.62, ‐0.05] |
| 17.3 Self Monitoring Only | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.99, 1.19] |
| 17.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.09, 1.04] |
| 18 Total Direct Costs (mean) | 2 | 185 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [0.10, 0.68] |
| 18.1 Optimal Self Management | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.01, 0.73] |
| 18.5 Written action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐0.03, 0.91] |
| 19 Total Indirect Costs (mean) | 2 | 185 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.69, ‐0.11] |
| 19.1 Optimal Self Management | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.86, ‐0.11] |
| 19.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.73, 0.21] |
| 20 Total Costs (mean) | 2 | 185 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.55, 0.03] |
| 20.1 Optimal Self Management | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.68, 0.06] |
| 20.5 Written Action Plan not Optimal | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.65, 0.29] |
1.1. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 1 Hospitalisations (% subjects hospitalised).
1.2. Analysis.
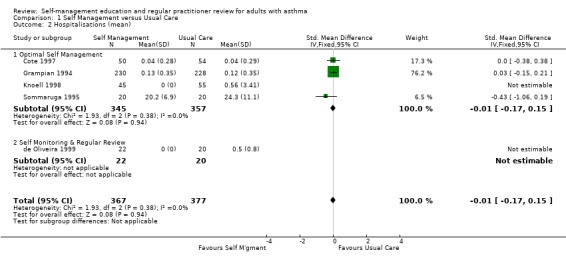
Comparison 1 Self Management versus Usual Care, Outcome 2 Hospitalisations (mean).
1.3. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 3 ER Visits (% subjects).
1.4. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 4 ER Visits (Mean).
1.5. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 5 Unscheduled Dr Visits (mean).
1.10. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 10 FEV1 (mean).
1.12. Analysis.
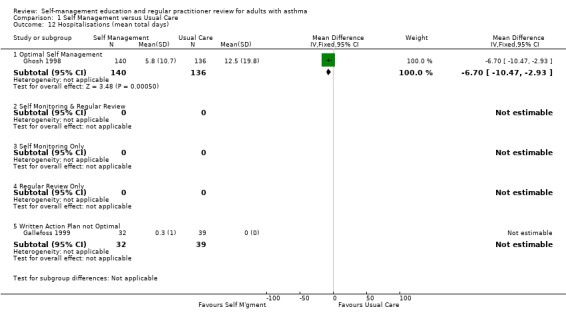
Comparison 1 Self Management versus Usual Care, Outcome 12 Hospitalisations (mean total days).
1.13. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 13 Rescue Medication Use (% subjects).
1.15. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 15 Quality of Life Impact (mean).
1.16. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 16 Quality of Life Activity (mean).
1.17. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 17 Quality of Life Symptoms (mean).
1.18. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 18 Total Direct Costs (mean).
1.19. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 19 Total Indirect Costs (mean).
1.20. Analysis.
Comparison 1 Self Management versus Usual Care, Outcome 20 Total Costs (mean).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdulwadud 1999.
| Methods | DESIGN: Randomised controlled trial METHOD OF RANDOMISATION: Simple random number table. MEANS OF ALLOCATION CONCEALMENT‐ not concealed OUTCOME ASSESSOR BLINDING‐ none WITHDRAWAL/DROPOUTS ‐ all participants accounted for. | |
| Participants | Eligible:175 Randomised: 125 (Intervention 64, Control 61) Completed: 77 (Intervention 30, Control 47) Age: Overall mean 46 yrs. Intervention 48 yrs, Control 43 yrs. Range: 16 to 82 yrs Sex: Male / Female: 50/75 Asthma Diagnosis: Doctor's diagnosis based on ATS criteria. Recruitment: Hospital asthma & allergy clinic. Diseases Included: Major Exclusions: Those with inadequate English skills, hearing or sight problems or asthma not their major illness. Baseline: 96% mod ‐ severe asthma. FEV1: Mean % predicted: Intervention 54%, Control 55%. PEF: Median PEF variability over 1 week 14.6%. Exacerbations: not stated. | |
| Interventions | Setting: Hospital outpatients Type: Basic asthma knowledge, physiology and triggers. Instruction on PEF self monitoring, and asthma action plans ‐ ? individualised. Understanding of medications and inhaler technique. Duration: Three 90 minute group sessions over 3 weeks. | |
| Outcomes | Knowledge, Skills, quality of life,attitudes and beliefs. | |
| Notes | Jadad Score = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Allen 1995.
| Methods | DESIGN: Randomised controlled trial stratified according to peak flow ownership. METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described. MEANS OF ALLOCATION CONCEALMENT‐ blinded. OUTCOME ASSESSOR BLINDING ‐ not stated. WITHDRAWAL/DROPOUTS ‐ all participants accounted for. | |
| Participants | Eligible: 116 Randomised: 58/58 Completed: 56/57 Age: Mean: 40 yrs Range: 19 to 63 Sex: Male / Female 46%/54% Asthma Diagnosis: Doctors Diagnosis Recruitment: volunteer community respondents Diseases Included: Asthma ‐ moderate to severe Major exclusions: Current smokers or quit in the past 3 months, previous asthma education programme. Baseline: FEV1: Intervention n = 56. 16% normal (> or = 80%), 77% mild/moderate (50 to 79%), 7% severe (< 50%). Control n = 57. 10% normal, 78% mild/mod, 12% severe. PEF: Intervention n = 54. median 58.4, range 35.0 to 77.0. Control n = 26. median 48.3, range 37 to 75. Exacerbations ‐ not stated. | |
| Interventions | Setting: Hospital Based community service asthma education programme Type: Education, Self Monitoring of Peak Flow and External Regular Review. (diaries of medications and symptoms were kept by members of both groups for 4 week periods at 3, 6, 9 and 12 months. If control group members owned a PF, they recorded this also. All members of the intervention group recorded PF and were taught in its use and interpretation). Duration: weekly 2.5 hour group education sessions over 4 weeks (total 10 hrs) | |
| Outcomes | Knowledge, Compliance, scheduled and unscheduled doctor and hospital visits, disrupted days (ie being confined to bed or a chair), Frequency of morning wheeze, nocturnal asthma symptoms, bronchodilator medications, pre‐bronchodilator FEV1/FVC, asthma symptoms diary, PEF. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Bailey 1990.
| Methods | DESIGN: Randomised Controlled Trial. Stratified by 11 physicians and 3 asthma severity levels. Blocked so that 2 out of 4 in a given stratum were assigned to intervention and control. METHOD OF RANDOMISATION: Separate randomisation schedule for the 33 strata were prepared in advance. Method of randomisation not stated. METHOD OF CONCEALMENT: closed envelope technique. OUTCOME ASSESSOR BLINDING: not stated. WITDHRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligible: not stated Randomised: 267 Intervention 132, Control 135 Completed: 225 Intervention 124, Control 101 Age: <20 yrs Int 1.6%, Cont 5.1%; 20‐39 yrs Int 27.4%, Cont 31.6%; 50‐59 yrs Int 37.1%, Cont 30.6 %; >/= 60 yrs Int 33.9%, Cont 32.7%. Sex: Male / Female Intervention 39/61 Control 29/71. Asthma Diagnosis: Doctor's diagnosis. Recruitment: Regular Clinic Visits Diseases Excluded: Another pulmonary or severely debilitation disease (e.g. CF, CA, severe rheumatoid arthritis. Other exclusions: Under 18 years, refusal (5 %) Baseline: FEV1: not reported PEF: not reported Exacerbations: Intervention Mild 37.1%, Mod 47.6%, Severe 16.3%; Control Mild 38.6%, Moderate 44.6%, Severe 16.8%. | |
| Interventions | Setting: Outpatient clinic Type: Education, peak flow self monitoring and regular review. Duration: one hour, one to one session ‐ Subjects were provided information about attack management but not an individualised written action plan. | |
| Outcomes | Skills, Hospitalisations, ER visits, Days off work/school, Compliance, Severity of asthma symptoms, bothered by asthma symptoms, 5 or more days coughing or dyspnoea. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Bailey 1999.
| Methods | DESIGN: Randomised controlled trial of two interventions. METHOD OF RANDOMISATION: Stratified by severity and blocked to ensure 2 of every 6 participants were assigned in each group MEANS OF ALLOCATION CONCEALMENT‐ Closed envelope technique OUTCOME ASSESSOR BLINDING‐ Blinded WITHDRAWAL/DROPOUTS‐ 7% attrition rate. All participants not accounted for. | |
| Participants | Eligible: Not stated Randomised: 236 Completed: 221 Age: Mean: not stated Range: 94 (41%) < 40 yrs, 138 (59%) >= 40 yrs Sex: Male/Female: 71 / 161 Asthma Diagnosis: Doctor diagnosis and objective lung function. Recruitment: Pulmonary clinic visit Diseases Included: Not stated. Major Exclusions: Not stated. Baseline: FEV1: not reported PEF: not reported Exacerbations: moderate to severe asthmatics | |
| Interventions | Setting: Outpatients clinic Type: Two self management interventions, one modified, and a usual care control group. (1) Counselling re use of skill‐oriented self help work book including info on asthma physiology, medications, trigger avoidance detection, response to asthma attacks and inhaler technique. No individualised action plan. (2) Instruction of use of a modified work book, inhaler technique and peak flow meter use. (3) control group received usual care and some asthma literature. Duration: (a) One 1 hour individual session and 2 asthma support group meetings. (b) One 15 minute individual session plus a 1 week follow‐up phone call and at 2 weeks a letter to encourage adherence. | |
| Outcomes | Medication & inhaler adherence, symptoms, respiratory illness, functional impairment & use of health care services. | |
| Notes | Jadad Score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Berg 1997.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: the word "random" stated ; stratified according to severity (moderate/severe) METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: not stated. WITHDRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligible: 68 Randomised: 55 Completed: 54 (Intervention 31; Control 24) Age: Overall mean: 50 yrs (sd 16) Sex: Male / Female 19/36 Asthma Diagnosis: Doctor's diagnosis Recruitment: Rural community Diseases Included: not specified ‐ but smokers were included Major exclusions: Not specified Baseline: FEV1: Patients described their asthma as moderate to severe. PEF: am intervention 360 (sd 105); control 365 (sd 137) pm intervention 347 (sd 107); control 371 (sd 140) Exacerbations: not stated | |
| Interventions | Setting: community based education program Type: Small group education and self monitoring of peak flow and symptoms. Control: No special education. Controls kept an asthma diary (symptoms and PEF) for one week at baseline and one week at the end of the study for outcome assessment. Duration: 6 x 2 hour training sessions ie 1/week over 6 weeks. | |
| Outcomes | Compliance, symptoms, am PEFR, pm PEFR, Asthma self‐efficacy, Asthma self‐management. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Blixen 2001.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Not stated MEANS OF ALLOCATION CONCEALMENT‐ Not stated OUTCOME ASSESSOR BLINDING‐ blinded WITHDRAWAL/DROPOUTS‐ all participants accounted for | |
| Participants | Eligible: 40 Randomised: 28 (14/14) Completed: 13 (Intervention 7, Control 6) Age: Mean overall: 36 yrs Range: 18 to 50 Sex: Male/Female: 7/20 Asthma Diagnosis: Doctor diagnosis Recruitment: Hospital admissions Diseases Included: Not stated Major Exclusions: not stated Baseline: 54% asthma > 10yrs FEV1: PEF: Exacerbations: In previous 2 weeks 57% had mild intermittent or persistent asthma, 32% moderate persistent & 11% severe asthma | |
| Interventions | Setting: Hospital Type: Individual sessions while an inpatient covering rationale and skills of asthma self management, explanation of "asthma self management workbook" and self monitoring techniques. Video on peak flow monitoring and written reinforcement materials sent at 3 and 6 months. Duration:Three 1hour sessions | |
| Outcomes | Hospitalisation, aer visits, unscheduled Dr visits, quality of life and depression scale | |
| Notes | Jadad Score = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Brewin 1995.
| Methods | DESIGN: not randomised ‐ all participants for intervention group were admitted to Pembury Hospital. METHOD OF ALLOCATION CONCEALMENT: Inadequate. Participants for the "control group" were allocated systematically according to a pre‐set hospital rotation. OUTCOME ASSESSOR BLINDING: implied but unclear. WITHDRAWAL/DROPOUTS: No dropouts. | |
| Participants | Eligible: ? Randomised 83 Completed: 45 Intervention 33; Control 12. Age: Overall mean: N/S Range: N/S Sex: Male / Female ‐ N/S Asthma Diagnosis: Doctor's Diagnosis Recruitment: Patients admitted to hospital for asthma Diseases Included: N/S Major exclusions: N/S Baseline: FEV1: N/S PEF: N/S Exacerbations: N/S | |
| Interventions | Setting: inpatient Type: one to one sessions with a respiratory nurse, peak flow self monitoring. Duration: at least 30 minutes | |
| Outcomes | Knowledge, symptoms, days off work, nocturnal waking, need for and frequency of bronchodilator use. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Cote 1997.
| Methods | DESIGN: Randomised controlled trial of two interventions METHOD OF RANDOMISATION: Randomised ‐ stratified randomisation MEANS OF ALLOCATION CONCEALMENT‐ not stated. OUTCOME ASSESSOR BLINDING ‐ not blinded. WITHDRAWAL/DROPOUTS ‐ all subjects accounted for. | |
| Participants | Eligible: not specified Randomised: 188 (Peak Flow 62, Symptoms Only 52, Control 74) Completed: 149 (Peak Flow 50, Symptoms Only 45, Control 54) Age: Overall mean: 36 yrs Range: Sex: Male/Female ‐ Asthma Diagnosis: Doctor's diagnosis and objective lung function Recruitment: Hospital admissions or visit to a clinic. Diseases Included: Major exclusions: current and ex‐smokers 40 yr of age or older in whom the best FEV1 after salbutamol was < 80% of predicted, patients with significant concurrent diseases, those requiring > 7.5 mg prednisone to control asthma symptoms and those who had already taken part in an asthma education program. Baseline: FEV1: not stated PEF: % predicted: Peak Flow 93+/‐3; Symptoms 91+/‐3; Control 95+/‐3. Exacerbations not stated | |
| Interventions | Setting: tertiary care setting Type: Two optimal interventions and an active control. (1) Education, peak flow self monitoring, regular review and individualised written action plan based on peak flow enabling self adjustment of medications in the event of worsening asthma. (2) Education, symptoms self monitoring, regular review and a symptoms based written action plan enabling self adjustment of medications in the event of worsening asthma. (3) Control group: Taught inhaler technique by the educator and about medication use and triggers by their pulmonologist. Their physician may have provided a verbal action plan. Duration: A minimum of 1 x one hour one to one counselling sessions for both educated groups | |
| Outcomes | Knowledge, compliance, hospitalisations, ER visits, oral corticosteroids, days lost from work or school. | |
| Notes | Jadad Score = 4 Treatment was optimised for all subjects during baseline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Cowie 1997.
| Methods | DESIGN: Randomised controlled trial of two interventions METHOD OF RANDOMISATION: Random numbers list MEANS OF ALLOCATION CONCEALMENT‐ sequentially administered identical opaque closed envelope technique OUTCOME ASSESSOR BLINDING ‐ outcome assessors blinded WITHDRAWAL/DROPOUTS ‐ all subjects accounted for. | |
| Participants | Eligible: not specified Randomised: 151 (one withdrawn: not asthma) Completed: 139 (Peak Flow 46, Symptoms Only 45, Control 48) Age: Overall mean: 36.4 yrs Standard Deviation: 15.9 yrs Sex: Male / Female ‐ (Peak Flow 17/29, Symptoms Only 20/25, Control 19/29) Asthma Diagnosis: Doctor's diagnosis and objective lung function Recruitment: Urgent emergency room treatment for asthma. Diseases Included: Not specified. Included those who already had a peak flow meter. Major exclusions: Those who already had a written action plan. Baseline: FEV1: not stated PEF: not stated Exacerbations All subjects had required urgent treatment for asthma in the previous 12 months. | |
| Interventions | Setting: individual nurse led education Type: Two optimal interventions and an active control. (1) Education (as per control), peak flow self monitoring, medication assessment within the program and advised to seek regular review outside the program and individualised written action plan based on peak flow enabling self adjustment of medications in the event of worsening asthma. (2) Education (as per control), NO symptoms or peak flow self monitoring, medication assessment within the program and advised to seek regular review outside the program and a symptoms based written action plan enabling self adjustment of medications in the event of worsening asthma. (3) Control group: 45 minutes education by the nurse educator about asthma, triggers, medication use and devices as per the interventions above. Medications assessed and if inadequate, patients physician was notified. Patients advised that their dose of corticosteroid may need to be adjusted from time to time. Duration: 45 minutes one to one counselling sessions for all groups | |
| Outcomes | Hospital admissions, ER visits. | |
| Notes | Jadad Score = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
de Oliveira 1999.
| Methods | DESIGN:Randomised Controlled Trial METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described MEANS OF ALLOCATION CONCEALMENT‐ closed envelope technique OUTCOME ASSESSOR BLINDING‐ Not stated WITHDRAWAL/DROPOUTS‐ | |
| Participants | Eligible: 80 Randomised: 53 (26/27) Completed: 42 (22/20) Age: Mean overall 39.6 yrs. intervention 41 (sd15), Control 38 (sd17) Range: not stated Sex: Male / Female: 5/37 Asthma Diagnosis: history airflow obstruction and ICRDMA criteria Recruitment: Outpatient clinic database Diseases Included: not stated Major Exclusions: not stated Baseline: FEV1: percent predicted: Intervention 70%(sd22), Control 80% (sd19) PEF: Exacerbations: | |
| Interventions | Setting: Outpatients clinic Type: concepts of asthma, asthma management, triggers, preventive measures. Video with introduction to treatment plan and inhaler technique. Symptom self monitoring. Medcially assessed at baseline and completion. Treament adjusted according to ICRDMA recommendations. Controls received routine schedule of asthma clinic. Medically assessed at baseline and completion Duration: Monthly visits for 6 months ? length of time but including 1 x individual session and 2 x 1hr group sessions at 3 and 4 months . | |
| Outcomes | Knowledge, skills, hospitalisations, ER visits, PEF, rescue medications, oral & inhaled corticosteroids, symptom frequency and quality of life. | |
| Notes | Jadad Score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Gallefoss 1999.
| Methods | DESIGN: Randomised Controlled Trial. METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described. MEANS OF ALLOCATION CONCEALMENT‐ Not stated. OUTCOME ASSESSOR BLINDING‐ for spirometry, ? for other outcomes. WITHDRAWAL/DROPOUTS‐ All subjects accounted for. | |
| Participants | Eligible: 85 Randomised: 78 (Intervention 39, Control 39) Completed: 71 (Intervention 32, Control 39). Age: mean overall: 45 yrs. Intervention 44, Control 41 Range: 18 to 70 yrs eligible. Sex: Male/Female: 23/55 Asthma Diagnosis: Objective lung function. Recruitment: Outpatients clinic. Diseases Included: Major Exclusions: Unstable CHD, heart failure, hypertension, diabetes, kidney or liver failure. Baseline: FEV1: > 80% predicted. PEF: Exacerbations: mild to moderate asthma. | |
| Interventions | Setting: Outpatients clinic Type: Basic introduction to asthma, anatomy and physiology, prevention & triggers, pharmacology of asthma drugs. Subjects received a 19 page booklet including self‐care and self‐management plan. Instructions on PEF and symptom self‐monitoring. Patients received an individual treatment plan. Controls followed by their GP. Duration: Two 2 hour group sessions on two separate days with a Doctor and pharmacist followed by 1‐2 individual sessions with a nurse and 1‐2 individual sessions with a physiotherapist. | |
| Outcomes | FEV1, quality of life, rescue medications, compliance, hospitalisations, unscheduled Dr visits, days off work, costs, patient satisfaction | |
| Notes | Jadad Score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Garret 1994.
| Methods | DESIGN: Randomised Controlled Trial. METHOD OF RANDOMISATION: randomised. METHOD OF ALLOCATION CONCEALMENT: closed envelope technique. OUTCOME ASSESSOR BLINDING: outcome assessors were blinded. WITHDRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligible: 980 Randomised: 500: Intervention 251; Control 249. Completed: 451 Age: 2‐5 years: Intervention 25% Control 26%. 6‐14 years:Intervention 19% Control 21%. 15‐29 years Intervention 30% Control 32%. 30‐55 years: Intervention 25% Control 21%. Sex: Male / Female Intervention 38/62% Control 46/54% Asthma Diagnosis: Doctor's diagnosis Recruitment: Emergency Room Attenders (whether hospitalisation was required or not) Diseases Included: not stated Major exclusions: not stated Baseline: FEV1: not stated PEF: not stated Exacerbations: not stated | |
| Interventions | Setting: Community Education Centre. Type: Education, self‐monitoring of symptoms and peak flow, regular review (advised to seek regular review from their GP). Participants were advised to obtain a written action plan from their GP which allowed self adjustment of medications in the event of worsening asthma but as this was not a part of the intervention, it was not characterised as Optimal Self Management but Self Monitoring and Regular Review. Duration: Not stated | |
| Outcomes | Hospital admissions, ER visits, Unscheduled doctor visits, days lost from work or school, nocturnal awakening, asthma status (same worse better), PEF variability, Symptoms, dyspnoea on exercise. | |
| Notes | Only about 60% of data refers to adults. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
George 1999.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: random number generation MEANS OF ALLOCATION CONCEALMENT‐ Not stated OUTCOME ASSESSOR BLINDING‐ Not stated WITHDRAWAL/DROPOUTS‐ all participants accounted for. | |
| Participants | Eligible: 88 Randomised: 77 (Intervention 44, Control 33) Completed: 77 (44/33) Age: Overall Mean: 29 yrs, Intevention 29yrs, Control 29yrs. Range: 18 to 45yrs Sex: Male/Female: 16/61 Asthma Diagnosis: Doctor diagnosis on admission Recruitment: Hospital admissions Diseases Included: N/S Major Exclusions: comorbid disease, non‐English speaking, no home telephone, pregnancy, intensive care admission. Baseline: FEV1: not reported PEF: not reported Exacerbations: enrolled on acute admission to hospital | |
| Interventions | Setting: In hospital Type: Asthma instruction, inhaler technique, early warning signs, and action plans for appropriate responses. Importance of regular follow‐up stressed. Controls received routine care. Education at "discretion of staff" Duration: repetitive sessions while in hospital and 7 day follow‐up in outpatients | |
| Outcomes | Hospitalisation, ER visits, length of hospital stay, outpatient visits. | |
| Notes | Jadad Score = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Ghosh 1998.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Not stated MEANS OF ALLOCATION CONCEALMENT‐ Not stated. OUTCOME ASSESSOR BLINDING‐ Not stated WITHDRAWAL/DROPOUTS‐ all subjects accounted for | |
| Participants | Eligible: Not stated Randomised: 303 (Intervention 153, Control 150) Completed: 276 (Intervention 140, Control 136). Age: 10 to 19 yrs Int. 27%, Cont. 33%, 20 to 29yrs Int. 23% Cont 18%, 30 to 39 yrs Int 29%, Cont 31%, 40 to 45 yrs Int 21% Cont 18%. Range: 10 to 45 yrs Sex: Male/Female: 113/163 Asthma Diagnosis: Doctor diagnosis and objective lung function. Recruitment: Asthma & Allergy Clinic Diseases Included: not stated Major Exclusions: chronic respiratory infections, bronchitis, emphysema, multisystem disorders, history of smoking. Baseline: FEV1: >15% reversibility PEF: Mean (SD) Int 281 (65) , Cont 274(67) Exacerbations: at least one admission or ER visit in past 12 months. drug therapy at least 50% of the days in a month | |
| Interventions | Setting: outpatients clinic Type: Asthma self‐management training in first month following baseline interview. Audiovisual aides used to highlight preventative measures, detailed teaching of PEFR and significance of variation. Individual written action plan. Self monitoring for 4 single months. Medically assessed and treatment adjusted at baseline. Controls kept 4 x 1 month diaries and medically assessed at baseline. No education given Duration: 4 x 2 hour sessions | |
| Outcomes | Hospitalisations, ER visits, PEF, Days off work, costs. | |
| Notes | Jadad Score = 4 Intervention 27% 10 to 19 yrs age, Control 33% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Grampian 1994.
| Methods | DESIGN: 2x2x2 block randomised trial stratified by physician. METHOD OF RANDOMISATION: the word "random" stated ; method not described. METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: not stated. WITHDRAWAL/DROPOUTS: dropouts (6%) not accounted for. | |
| Participants | Eligible: 801 consented but 232 already had peak flow meter and could not be randomised for this arm. Randomised: 569: Peak flow self monitoring 285, Conventional monitoring 284 Completed: 458: Peak flow self monitoring 230, Conventional monitoring 228. Age: Mean Intervention 51.1 yrs Control 50.5 years Range: > 16 years. Sex: Male/Female Intervention 48/52% Control 40/60% Asthma Diagnosis: Doctor's diagnosis and objective lung function Recruitment: Hospital outpatient clinics and general practices in north east Scotland. Diseases Included: N/S Major exclusions: N/S Baseline: FEV1: % of predicted: Intervention 77.3%; Control 78.1% PEF: Mean: Intervention 344.5; Control 341.6. Exacerbations: N/S | |
| Interventions | Setting: Type: education, self monitoring of peak flow, and regular review and written action plan to enable self adjustment of medications in response to worsening asthma based on peak flow. (due to the factorial design, some of the intervention group were randomised to receive enhanced education while the others had conventional education. Similarly, some were randomised to receive integrated care while others had conventional care). Control: Some had enhanced education but none had peak flow meters. Duration: not stated | |
| Outcomes | Hospitalisation, unscheduled doctor visits, FEV1 % predicted, use of rescue medication, quality of life, days off work, inhaled steroids, disrupted days, nocturnal asthma. | |
| Notes | Jadad Score = 3 Confounding due to factorial design. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Hayward 1996.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Random stated, method not described METHOD OF ALLOCATION CONCEALMENT: not stated OUTCOME ASSESSOR BLINDING: not stated. WITHDRAWAL/DROPOUTS: | |
| Participants | Eligible: 84 Randomised: 44( Intervention 23, Control 21) Completed: 42 (Intervention 23, Control 19) Age: Mean Intervention 51.1 Range: 6 to 74 yrs Sex: Male/Female Asthma Diagnosis: not stated Recruitment: GP Diseases Included: N/S Major exclusions: N/S Baseline: FEV1: PEF: Mean: Exacerbations: | |
| Interventions | Setting: ? GP clinic Type: Training from an asthma nurse specialist by telephone or attending a clinic monthly for 1 year. Education in knowledge, triggers, symptoms, reliever vs preventer medication and inhaler technique. Written support materials given. peak flow self monitoring and action plan ‐ ? written. Duration:Monthly clinic visit or telephone call for 12 months | |
| Outcomes | Knowledge, hospitalisation, unscheduled Dr visits, rescue medication, days off work or school, exacerbations, symptom score, symptoms | |
| Notes | Jadad Score = 3 Includes children |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Heard 1999.
| Methods | DESIGN: Randomised Controlled Trial. METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described MEANS OF ALLOCATION CONCEALMENT‐ not concealed‐ randomisation chart for each practice. OUTCOME ASSESSOR BLINDING‐ None WITHDRAWAL/DROPOUTS ‐ All subjects accounted for. | |
| Participants | Eligible: Not stated Randomised: 195 (Intervention 98, Control 97) Completed: 191 (Intervention 97, Control 94). Age: Mean Intervetion 27.5 yrs, Control 26.3 yrs. Range: eligible 5 to 64 yrs Sex: Male/Female: Asthma Diagnosis: Recruitment: 8 General Practices Diseases Included: Major Exclusions: Baseline: FEV1: PEF: Exacerbations: | |
| Interventions | Setting: GP Asthma Clinic Type: Nurse counselling, asthma management strategies, spirometry, peak flow and inhaler use. Explanation of diary card use for PEF self monitoring and written action plan. Assessed by GP at end of each clinic session. Controls received standard medical treatment Duration: 3 x 3 hour sessions over 6 months | |
| Outcomes | Knowledge, hospitalisation, ER visits, unscheduled Dr visits, preventer medication, days off work, productive days lost, nocturnal asthma, use of action plan, owning a peak flow meter, smoking status, morning asthma symptoms | |
| Notes | Jadad Score = 3 Includes children |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Hilton 1986.
| Methods | DESIGN: Controlled Clinical Trial METHOD OF RANDOMISATION: allocated systematically in the order in which they were recruited. METHOD OF ALLOCATION CONCEALMENT: systematically allocated ‐ not concealed. OUTCOME ASSESSOR BLINDING: unclear. WITHDRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligibility Criteria: 5 to 70 yrs, asthma diagnosis by GP, anti‐asthma treatment given on at least two occasions in the past year, no other asthma patient in the family or household recruited to the study. Eligible:415 Randomised: 339 Completed: 274 Age: mean: Not specified; Range Not specified. Sex: Male/Female ‐ not specified. Asthma Diagnosis: by General Practitioner. Recruitment: from 14 general practices in South and West London. Major exclusions: not specified Baseline FEV1 not stated; PEF not stated, Exacerbations not stated. | |
| Interventions | Setting: Type: Maximum Education Group: Education with GP, and regular review (ie 3 monthly appointments with the doctor in addition to their routine consultations for asthma). Duration: 10 to 15 minutes semi‐structured interview with their GP plus a booklet and a cassette tape. | |
| Outcomes | Knowledge, skills, ER visits, patient satisfaction, days off work, compliance, avoiding activities, Wheeze (frequency, severity), nocturnal asthma, exacerbations. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Ignacio‐Garcia 1995.
| Methods | DESIGN: Randomised Controlled trial METHOD OF RANDOMISATION: the word "random" stated; "randomly allocated in the order in which they were recruited" by alternation. METHOD OF ALLOCATION CONCEALMENT: alternation, not concealed. OUTCOME ASSESSOR BLINDING: outcome assessor of intervention group not blinded; outcome assessor of control group was "blinded with regard to registers of peak flow monitoring until the end of the study". WITHDRAWAL/DROPOUTS: all participants accounted for. | |
| Participants | Eligible: not stated. Randomised: 94 (Intervention 50, Control 44) Completed: 70 (Intervention 35, Control 35) but a further 11 in Intervention group and 5 in control group were excluded at 3 months due to poor inhalation technique, lack of PEF monitoring & non compliance with prescribed medication regimens leaving 54 (Intervention 24, Control 30). Age: Overall mean: 42 Years Range: 16 to 64 years. Sex: Male/Female 32/38 of the study population of 70. Asthma Diagnosis: Doctor's diagnosis. Recruitment: Outpatient asthma clinic Diseases Included: not specified Major exclusions: not specified Baseline: FEV1: % of predicted (Intervention 69.03 Control 65.34) PEF: not stated Exacerbations: not stated | |
| Interventions | Setting: Type: Optimal self management including education, peak flow self monitoring, regular review and a written action plan based on peak flow which enabled self adjustment of medications in the event of worsening asthma. Control group: Self monitoring of symptoms and regular review. Collected PEF as an outcome but based their treatment on physicians advice. Duration: not stated. Study period was 7 months. | |
| Outcomes | Hospitalisation, ER visits, doctor visits, lung function ‐ % predicted, use of rescue medication, days off work, antibiotic therapy, nocturnal asthma, exacerbations, PEF ‐ mean daily peak flow rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Jones 1995.
| Methods | DESIGN: Randomised Controlled Trial. Stratified by centre in blocks of six. METHOD OF RANDOMISATION: the word "random" stated ; method not described. METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: not clear ‐ "data rendered anonymous" before analysis. WITHDRAWAL/DROPOUTS: not described. | |
| Participants | Eligible: not stated Randomised: 127 Completed: 72 (Intervention self management 33; planned visits 39) Age: self managed : mean 30.4 yrs SD 11.5 yrs planned visits: mean 28.6 SD 7 yrs. Sex: Male/Female Self managed 14/19 planned visits 12/26. Asthma Diagnosis: Doctor's diagnosis Recruitment: General practices Diseases Included: Not specified Major exclusions: Those on regular oral steroids and those who were regularly conducting home PEF monitoring. Baseline: FEV1: % predicted: self management 85.1 (sd 20.8), planned visits 80.2 (sd19.9) PEF: % predicted: self management 88.2 (sd 15.4), planned visits 86.8 (sd 13.7) Exacerbations | |
| Interventions | Setting: General Practice Type: Optimal self management: education, peak flow self monitoring, regular review and an individualised written action plan based on peak flow enabling self adjustment of medications in the event of worsening asthma. Control: regular review and kept a daily diary for morbidity and bronchodilator use for outcomes. Duration: | |
| Outcomes | Lung Function ‐ % predicted, Use of rescue medication, quality of life, days off work, wheeze (frequency, severity), nocturnal asthma, oral corticosteroid use, cough, shortness of breath, disrupted days. | |
| Notes | Jadad Score = 4 Oral corticosteroids was given for 2 weeks to optimise lung function in both groups during baseline. Drop outs were more likely to be younger and male with lower initial FVC values then the completers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Knoell 1998.
| Methods | DESIGN: Non‐randomised Controlled Trial MEANS OF ALLOCATION CONCEALMENT‐ Inadequate. Subjects alternately assigned to a study group. OUTCOME ASSESSOR BLINDING‐ Yes WITHDRAWAL/DROPOUTS ‐ Not described | |
| Participants | Eligible: 188 Randomised: 100 (Intervention 45, Control 55) Completed: 100 (45,55) Age: < 25 Int 4 Cont 8, 25 to 65 Int 37, Cont 41, > 65 Int 4 Cont 6. Range: Sex: Male/Female: Not stated Asthma Diagnosis: Based on EPRII guidelines and symptoms. Recruitment: Outpatients clinic Diseases Included: All adults with asthma Major Exclusions: COPD Baseline: According to EPRII guidelines: Intermittent Int 4.4% Cont 7.3%, Mild persistent Int 13.3% Cont 16.4%, Moderate Int 77.8% Cont 56.4%, Severe Int 4.4% Cont 20.0%. FEV1: PEF: Exacerbations: | |
| Interventions | Setting: Outpatients Clinic Type: Joint pharmacist and physician consultation covering general asthma concepts, triggers, medications and written action plan. Provided with diary and instructed in use of peak flow monitoring. Assessed by physician at the clinic. Control group received routine outpatient care and were provided with a peak flow meter and diary. Duration: 1 x 30‐60minutes | |
| Outcomes | Knowledge, hospitalisation, ER visits, Dr visits, Days off work, quality of life, compliance, quality of care, drug costs, patient satisfaction | |
| Notes | Jadad Score = 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Kotses 1995.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: the word "random" stated ; method not described. METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: not stated. WITHDRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligible: 126 Randomised: 85 Completed: 76 (Intervention 36; Control 40) Age: Overall mean: 49.8 yrs (sd 12.4) Range: 27 to 70 yrs Sex: Male / Female 27/49 Asthma Diagnosis: as per American Thoracic Society Recruitment: participants whose asthma was generally under control Diseases Included: not specified Major exclusions: Fixed airways, concurrent uncontrolled medical conditions, occupational asthma, drug abuse, obesity, low weight, cognitive or intellectual deficits. Baseline: FEV1: Patients described their asthma as moderate to severe. PEF: am (intervention 331+/‐ 92; control 333 +/‐ 123.7) Exacerbations: not stated | |
| Interventions | Setting: not specified (? allergy clinic) Type: Education and self monitoring of peak flow and symptoms Control: No special education. Controls kept an asthma diary (symptoms and PEF) for 6 months on a daily basis and again for 2 weeks prior to the 12 months follow‐up. Duration: 7 x 90 minute training sessions 1/week over 7 weeks. | |
| Outcomes | Knowledge, Hospitalisation, ER visits, doctor visits, PEF ‐ evening daily average, quality of life (Beck Depression Inventory, Quality of Well‐Being Scale), Wheeze, Exacerbations, Breathing difficulty, Coughing, Chest tightness. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Kotses 1996.
| Methods | DESIGN: METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described. MEANS OF ALLOCATION CONCEALMENT‐ not stated. OUTCOME ASSESSOR BLINDING ‐ not stated. WITHDRAWAL/DROPOUTS ‐ all subjects accounted for. | |
| Participants | Eligible: not stated Randomised: 45 Completed: 34 Age: Overall mean: 42 yrs Range: Not stated Sex: Male/Female ‐ 7/27 Asthma Diagnosis: Self Reported moderate asthma for an average of 16.5 years. Recruitment: community respondents to advertisements for research participants Diseases Included: Not specified Major exclusions: Not specified Baseline: FEV1: not specified. PEF: Individual 327 +/‐91.6 group 387 +/‐ 127.7, control 310 +/‐ 105.2. Exacerbations | |
| Interventions | Setting: not stated Type: Individual: daily self monitoring of PEFR, attacks & contact with precipitants Group: Group education and daily PF monitoring Duration: Individual: 60 minute one to one session, Group: 2 x 2.5 hr education sessions | |
| Outcomes | Outcomes measured after one month: ER visits, FEV1‐(l/min evening), PEF(l/min evening), Activity Limitation, Asthma Attacks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Lahdensuo 1996.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Randomised ‐ blocks of variable sizes and stratified by centre MEANS OF ALLOCATION CONCEALMENT‐ sealed number envelopes. OUTCOME ASSESSOR BLINDING ‐ single blind. WITHDRAWAL/DROPOUTS ‐ all subjects accounted for. | |
| Participants | Eligible: 122 Randomised: 122 (Intervention 60, Control 62) Completed: 115 (Intervention 56, Control 59) Age: Overall mean: 41.7 yrs (sd 14.7) Sex: Male/Female Intervention 43/59 Asthma Diagnosis: Objective lung function Recruitment: Out patient clinics in Finland ‐ mild to moderately severe asthma. Diseases Included: Smokers Major exclusions: Not stated Baseline: FEV1: % Predicted Intervention 82.4 (sd 15.8), Control 81.7 (16.6) PEF: Exacerbations: No oral corticosteroids in the last 4 weeks before entry. | |
| Interventions | Setting: Outpatient clinics in Finland Type: Optimal self management including a written action plan allowing self adjustment of anti‐inflammatory medications according to peak flow, self monitoring of peak flow, regular review within the program and education. Duration: At the first visit, one to one education was provided which took an extra 1.5hrs longer then the control visit. | |
| Outcomes | Hosptialisations, Unscheduled doctor visits, FEV1, (%predicted ‐ pre bronchodilator), oral corticosteroids, days off work or school, quality of life, courses of antibiotics, costs. | |
| Notes | Jadad Score = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Levy 2000.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: computer generated equal blocks of 4 MEANS OF ALLOCATION CONCEALMENT‐ Random number list OUTCOME ASSESSOR BLINDING ‐ blinded WITHDRAWAL/DROPOUTS‐ all participants accounted for. | |
| Participants | Eligible: Randomised: 211 (intervention 103, Control 108) Completed: 179 (Intervention 83, Control 96). Age: Mean(SE) Intervention 43(2), Control 40(2). Range: not stated. Sex: Male/Female: 80/131 Asthma Diagnosis: not stated Recruitment: prospective rolling recruitment over 13 months through hospital admissions and ER visits. Diseases Included: Major Exclusions: COPD Baseline: severe FEV1: PEF: % predicted before A&E therapy: Int 49.1%, Cont 44.8%, after therapy Int62.8% Cont 59.5%. Exacerbations: | |
| Interventions | Setting: Outpatients clinic Type: Nurse advice and education of use of self‐management plans, recognition and self treatment of uncontrolled asthma and when to seek medical help. Instructed when to step up medication if necessary using PEF or symptoms. Use of "credit card" action plan. Control group received usual care. Both groups kept 1 week diary cards over 3 periods for data collection. Duration: 1x1hr and 2xhalf hr sessions at 6 weekly intervals. | |
| Outcomes | ER visits, unscheduled & scheduled Dr visits, PEF, rescue medication, inhaled corticosteroids, days off work, symptom score, quality of life. | |
| Notes | Jadad Score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Mayo 1990.
| Methods | DESIGN: Randomised controlled cross‐over METHOD OF RANDOMISATION: The word 'random' stated. Method of randomisation last digit on hospital chart. METHOD OF ALLOCATION CONCEALMENT: last digit on hospital chart. OUTCOME ASSESSOR BLINDING: not stated. WITHDRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligible: 212 Randomised: 104 (Intervention 47, Control 57; 19 crossed to Intervention) Completed: not stated Age: Overall mean: 44.3 yrs Range: not stated Sex: Male/Female ‐ not stated Asthma Diagnosis: American Thoriacic Society Recruitment: Outpatients with prior hospitalisation for asthma Diseases Included: Not stated Major exclusions: Severe alcoholism, overt CNS or mental illness, deaf, mute. Baseline: FEV1: Not stated PEF: Not stated Exacerbations Not stated | |
| Interventions | Setting: outpatient clinic Type: Education, peak flow self monitoring and regular medical review. Patients were encouraged to initiate self‐treatment in the event of an exacerbation based on the physician's advice. Duration: at least 2 hours one to one discussion with physician | |
| Outcomes | Skills, Hospitalisation, Mortality, Exacerbations, oral corticosteroids, inhaled corticosteroids. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Moudgil 2000.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Computer randomisation MEANS OF ALLOCATION CONCEALMENT‐ Randomised prior to initial appointment OUTCOME ASSESSOR BLINDING‐ open WITHDRAWAL/DROPOUTS‐ all subjects accounted for | |
| Participants | Eligible: 1217 Randomised: 689 (Intervention 343, Control 346) Completed: 593 (Intervention 304, Control 289). Age: Overall Mean (sd) 34.5(15) Int 33.6(15.2), Cont 35.4(15.5) Range: eligible 11 to 59 Sex: Male/Female: 337/352 Asthma Diagnosis: Doctor diagnosis and objective lung function. Recruitment: 12 inner‐city General Practices Diseases Included: not stated Major Exclusions: not stated Baseline: FEV1: Mean (sd) Int 2.4(0.89), Cont 2.42(0.88) PEF: Exacerbations: n(%) past asthma admissions: Int 115(33.5), Cont 116(33.5) | |
| Interventions | Setting: ? at GP practices Type: Emphasis on appropriate prescribing, optimising treatment, compliance, knowledge of disease severity. Provision of peak flow meters and diaries for self monitoring throughout 12 months of study. Individual written action plans. Controls received usual care. Duration: Individual 40 minute sessions reinforced at 4 and 8 months. | |
| Outcomes | Hospitalisation, ER visits, scheduled & unscheduled Dr visits, oral/inhaled corticosteroids, quality of life, antibiotic use. | |
| Notes | Jadad Score = 4 White European and Indian subcontinent ethnic groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Mulloy 1996.
| Methods | DESIGN: METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described. MEANS OF ALLOCATION CONCEALMENT‐ not stated. OUTCOME ASSESSOR BLINDING ‐ not stated. WITHDRAWAL/DROPOUTS ‐ all participants accounted for. | |
| Participants | Eligible: Not stated Randomised: 60 (Intervention 30, Control 30) Completed: 46 (after 1 month: Intervention 18, Control 28) (after 12 months: Intervention 12, Control 21) Age: Overall mean: 28.5 years Range: not stated Sex: Male/Female: Intervention 47%/53% Control 50%/50% Asthma Diagnosis: Objective lung function Recruitment: Hospital out patients Diseases Included: not stated Major exclusions: not stated Baseline: FEV1: Intervention mean 2.98 Control 2.72 PEF: mean and standard error of the mean: Intervention 394 (32) Control 361 (22) Exacerbations Not stated | |
| Interventions | Setting: Outpatient clinic program run by an asthma nurse specialist Type: Education via video and booklet, peak flow self monitoring and advised to seek regular review outside the program. Duration: one to one session of at least one hour. | |
| Outcomes | Knowledge, Inhaler technique, PEF, FEV1‐ baseline only, asthma symptom severity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Neri 1996.
| Methods | DESIGN: METHOD OF RANDOMISATION: Randomised ‐ stated. Method: alternation. MEANS OF ALLOCATION CONCEALMENT‐ Not concealed, participants alternated. OUTCOME ASSESSOR BLINDING ‐ not blinded WITHDRAWAL/DROPOUTS ‐ all participants accounted for. | |
| Participants | Eligible: not stated Randomised: 80 (Complete program 40, Reduced program 40) Completed: 65 (Complete 33, Reduced 32) Age: Overall mean: 45.5 years Range: not stated Sex: Male/Female: 25/40 Asthma Diagnosis: according to international guidelines ‐ Dr diagnosis implied. Recruitment: Outpatients department. Diseases Included: Smokers Major exclusions: not stated Baseline: FEV1: Complete 78.3 (sd 15.3), Reduced 73.4 (sd 16.9) PEF: not stated Exacerbations: not stated | |
| Interventions | Setting: Asthma School Type: Intervention: Group asthma education in an asthma school including a booklet and video. Content included self monitoring of peak flow, interpretation and use of drugs, mechanisms and triggers. Control: reduced education program involving self reading of a booklet and peak‐flow monitoring and recording. Duration: 6 x 1hr lessons in groups of 10. (2 per week for 5 lessons and the last lesson after 3 months as a reinforcer. | |
| Outcomes | FEV1 (% predicted), admission days, urgent doctor visits, rescue medication, morbidity savings, days of work or school. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Schott‐Baer 1999.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Random stated but assigned to groups according to the last digit of their hospital record number ‐even to intervention, odd to control group. MEANS OF ALLOCATION CONCEALMENT‐ not concealed OUTCOME ASSESSOR BLINDING‐ not stated WITHDRAWAL/DROPOUTS‐ | |
| Participants | Eligible: not stated Randomised: 36 (Intervention 17, Control 19) Completed: 22 (Intervention 15, Control 7) Age: Mean Int 44yrs, Cont 52 yrs Range: 24‐74 yrs Sex: Male / Female: 4 / 32 Asthma Diagnosis: Doctor diagnosis. Recently diagnosed asthma. Recruitment: Outpatient clinics Diseases Included: Major Exclusions: COPD, chronic bronchitis. Baseline: FEV1: PEF: Exacerbations: | |
| Interventions | Setting: Outpatients Clinic Type: Education on disease process, daily self monitoring, self‐ management techniques, and daily log completion including peak flow, triggers, and ratings of benefits. Controls received standard care and some information on medications and instruction on daily recording of peak flow‐not self monitoring diary. Duration: 1 x 3hr session ‐? individual or group. 3 reinforcement phone calls | |
| Outcomes | Knowledge, ER visits, Peak flow, clinic visits | |
| Notes | Jadad Score = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Shields 1986.
| Methods | DESIGN: Block randomised according to the number of ER visits or hospitalisations METHOD OF RANDOMISATION: Randomised ‐ stated. Method: not described. MEANS OF ALLOCATION CONCEALMENT‐ not described. OUTCOME ASSESSOR BLINDING ‐not described. WITHDRAWAL/DROPOUTS ‐ not described. | |
| Participants | Eligibility Criteria: > 18 years, at least 1 ER visits or hospitalisation for asthma in prior 4 years. Eligible:103 Randomised: 103 Completed: 87 Age: mean: Not specified; Range Not specified. Sex: Male/Female ‐ not specified. Asthma Diagnosis: Dr diagnosis implied as previously hospitalised or visited ER for asthma Recruitment: from prior ER visit or Hospitalisation Major exclusions: not specified Baseline FEV1 not stated; PEF not stated, Exacerbations: ER visit or hospitalisation in previous 4 years. | |
| Interventions | Setting: HMO classes Type: Group education in 4 x 1.5 hour classes OR telephone counselling. Classes or counselling were followed by telephone follow‐up according to individual patients' needs. Content: physiology of asthma, medications, respiratory infections, inflammation, use of HMO resources. Duration: Classes 4 x 1.5 hours. | |
| Outcomes | ER visits, cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Snyder 1987.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Random stated. Method not described. METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: unclear WITHDRAWAL/DROPOUTS: all subjects accounted for. | |
| Participants | Eligible: ? Randomised: 79 Completed: 75 Age: Overall mean: 27.6 Range: not stated Sex: Male/Female: 34/41 Asthma Diagnosis: Doctor Diagnosis / Objective Lung Function Recruitment: Community volunteers Diseases Included: not stated Major exclusions: smoking, fixed airway disease, other uncontrolled diseases, substance abuse, potential complicating physical disorders / obesity / little obstruction. Baseline: FEV1: PEF: Exacerbations | |
| Interventions | Setting: not stated. Type: education and probably peak flow self‐monitoring Duration: 2 x 2.5 hrs group education sessions | |
| Outcomes | Knowledge, hospital visits, ER visits, Doctor visits, quality of life (asthma attitude survey for adults), days of work, amounts and type of medications for asthma, exacerbations. | |
| Notes | Outcomes data are not provided in a form appropriate for meta‐analysis. Several attempts have been made to contact the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Sommaruga 1995.
| Methods | DESIGN: METHOD OF RANDOMISATION: Randomised ‐ stated. Method not described. MEANS OF ALLOCATION CONCEALMENT‐ not stated. OUTCOME ASSESSOR BLINDING ‐ not stated. WITHDRAWAL/DROPOUTS ‐ all participants accounted for. | |
| Participants | Eligible: not stated Randomised: 40 (Intervention 20, Control 20) Completed: 36 (Intervention 20, Control 16) Age: Overall mean: 48 Range: +/‐16 yrs Sex: Male/Female : 21/19 Asthma Diagnosis: Dr diagnosis implied: International Guidelines Recruitment: Hospital inpatients at a Respiratory Medical Centre Diseases Included: Not stated Major exclusions: Not stated Baseline: FEV1: 76+/‐ 18% predicted before and 94+/‐ 5% predicted after salbutamol. PEF: not stated Exacerbations: not stated. | |
| Interventions | Setting: Inpatient education programme Type: Education, peak flow medications and symptoms self monitoring, medical review every 2 months with the same physician, written action plan allowing the patient to alter medications in response to worsening asthma. (plus a psychological intervention). Duration: 2 lessons during admissions of unknown duration and quarterly lessons during the ensuring year. | |
| Outcomes | Hospitalisation, ER visits, Days off work, Asthma Attacks, Resp. Illness Opinion Survey, Health Locus of Control, STA1 x 2 (anxiety, AD (Depression) APF (psychophysiological disorders), Asthma Symptom Checklist. ‐ Only reported the psychological outcomes. | |
| Notes | Jadad Score = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Wilson 1993.
| Methods | DESIGN: Randomised controlled trial. METHOD OF RANDOMISATION: Random stated. Blocked according to severity. Method not described. METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: physicians who assessed asthma status were blinded as to group assignment of patients. Unclear whether the nurse who administered questionnaires and assessed MDI technique was blinded. WITHDRAWAL/DROPOUTS: participants not accounted for. | |
| Participants | Eligible: 579 Randomised: 323 (at 5 months = 271) (at 12 months = 277) Completed: not described Age: (eligibility was 18 to 50 years) Overall mean: ? Group mean ? Individual mean ?; Information Only mean ? Range: ? (p566 "no significant difference with respect to gender, age, level of education, asthma severity. Sex: Male/Female ‐ not stated ‐ see above Asthma Diagnosis: Dr diagnosis and objective lung function Recruitment: Community: patients of the Kaiser Medical Centers in California. Included: Moderate ‐ severe asthma, Dr's diagnosis. Major exclusions: Irreversible respiratory disease, emphysema, COPD. Baseline: recurrent wheeziness FEV1: > 15% change PEF: 20% variability Exacerbations: History of recurrent episodes of wheezing and/or objective evidence of airflow obstruction during episodes and improved airflow when treated with a bronchodilator. | |
| Interventions | Setting: Kaiser Permanente Patients Type: 3 Types of intervention as follows: (1) Group education, symptoms and peak flow monitoring, reviewed at 5 and 12 months. (2) Individual education, (as per intervention 1 except individual education). (3) Information Only control: patients were given a workbook to read and reviewed at 5 and 12 months. Controls were given no special education but were reviewed at 5 and 12 months. Duration: Individual ‐ 180 minutes per patient, Group 45 to 60 minutes per patient. | |
| Outcomes | Knowledge, hospitalisation, ER Visits, PEF (l/min), FEV1(% predicted), rescue medication, oral corticosteroids, inhaled corticosteroids, symptomatic days, physician evaluation of asthma status, relative bother, change in physical activity, improvement in bedroom environment, inhaler technique. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Yoon 1993.
| Methods | DESIGN: Randomised Controlled Trial METHOD OF RANDOMISATION: Random stated. Method not described. METHOD OF ALLOCATION CONCEALMENT: not described. OUTCOME ASSESSOR BLINDING: not stated. WITHDRAWAL/DROPOUTS: all participants accounted for. | |
| Participants | Eligible: not stated Randomised: 76 (Intervention 37, Control 39) Completed: 56 after 10 months Age: Overall mean: 32yrs Range: 16 to 65 years Sex: Male/Female: not stated Asthma Diagnosis: Objective Lung Function Recruitment: hospital admission for asthma Diseases Included: not stated Major exclusions: irreversible airway obstruction, significant concurrent diseases. Baseline: FEV1: reversibility of at least 15% predicted. PEF: not stated Exacerbations: not stated. | |
| Interventions | Setting: Asthma education centre connected with a tertiary teaching hospital Type: Optimal self management including education, peak flow self monitoring, regular review and a written action plan enabling self adjustment of medications based on peak flow for worsening asthma. Duration: 3 hours total | |
| Outcomes | Knowledge, Hospitalisation, ER visits, Lung function (l/min), Inhaled corticosteroids, quality of life (psychosocial disturbance du to asthma) days off work, wheeze (frequency and severity). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available (Cochrane Grade B) |
Zeiger 1991.
| Methods | DESIGN: Controlled Clinical Trial METHOD OF RANDOMISATION: Not randomised MEANS OF ALLOCATION CONCEALMENT‐ alternation / day of their ER visit, not concealed. OUTCOME ASSESSOR BLINDING ‐ "evaluated blindly" ‐ p1160. WITHDRAWAL/DROPOUTS ‐ all subjects accounted for. | |
| Participants | Eligible: not stated Randomised: 309 (Intervention 149, Control 160) Completed: 249 (Intervention 110, Control 139) Age: Overall mean: 24.4 sd: 14.5 Sex: Male/Female: 42.6%/57.4% Asthma Diagnosis: as per American Thoracic Society Recruitment: Emergency Room Diseases Included: smokers Major exclusions: previous allergy or pulmonary care. COPD Baseline: FEV1: not stated PEF: % pred. on admission: mean (sd) Intervention 46.7 (19.9), Control 52 (24.9) Exacerbations acute wheezing or dyspnea at the time of the index visit. | |
| Interventions | Setting: Allergy Clinic ‐ HMO Type: Expedited allegy assessment and education; peak flow self monitoring, regular review and written action plan enabling self adjustment of medications in response to worsening asthma Duration: one to one sessions of unknown number and duration. | |
| Outcomes | Hospitalisation, ER Visits, Inhaled Corticosteroids, Nocturnal Asthma, Perception of Asthma. | |
| Notes | Possible contamination: 21 control subjects referred to an allergist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Study investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdulwadud 1997 | Baseline data only |
| Adams 2001 | Comparison of two educational interventions |
| Aiolfi 1995 | Information only education |
| Amirav 1995 | Not patient education |
| Ayres 1996 | Comparison of two educational interventions |
| Baldwin 1997 | Comparison of two educational interventions |
| Bolton 1991 | Information only education |
| Boulet 1995 | Methodological problems |
| Charlton 1990 | Comparison of two educational interventions |
| Cote 2001 | Comparison of two educational interventions |
| Cox 1993 | Not an education intervention |
| Erickson 1998 | Sample size too small, not an RCT |
| Gergen 1995 | Non‐RCT |
| Graft 1991 | Non‐RCT |
| Grainger‐Rousseau | Not randomised. Children included. Mean age unknown |
| Grampian 1994b | Not education intervention |
| Hausen 1999 | Non‐RCT |
| Heringa 1987 | Inappropriate outcomes |
| Hindi‐Alexander 1987 | non‐RCT |
| Hoskins 1996 | Methodological problems |
| Huss 1992 | Information only education |
| Jackevicius 1999 | Inhaler technique |
| Janson‐Bjerklie 1988 | Not an education intervention |
| Jenkinson 1988 | Information only education |
| Jones 1987 | Outcomes not appropriate |
| Kauppinen 1998 | Comparison of two educational interventions |
| Kelso 1995 | Non‐RCT |
| Klein 2001 | Comparison of two educational interventions |
| LeBaron 1985 | Not an education intervention |
| Legorreta 2000 | Not an RCT |
| Lirsac 1991 | Not a education intervention |
| Lopez‐Vina 2000 | Comparison of two educational interventions |
| Maes 1988 | Not an education intervention |
| Maiman 1979 | Information only education |
| Moldofsky 1979 | Information only education |
| Muhlhauser 1991 | Non‐RCT |
| Osman 1994 | Information only education |
| Perdomo‐Ponce 1996 | Not an RCT. Focus on allergic diseases and therapeutic compliance |
| Petro 1995 | Not predominantly asthma |
| Premaratne 1999 | Nurse education |
| Ringsberg 1990 | Information only education |
| Rydman 1999 | Inhaler technique |
| Sondergaard 1992 | Information only education |
| Thapar 1994 | Information only education |
| Tougaard 1992 | Not predominantly asthma |
| Turner 1998 | Comparison of two educational interventions |
| Verver 1996 | Inhaler technique only |
| White 1989 | Not patient education |
Characteristics of ongoing studies [ordered by study ID]
Ford 1996.
| Trial name or title | An empowerment‐centered, church‐based asthma education program for African American adults |
| Methods | |
| Participants | African‐American adults with asthma |
| Interventions | General physiology of asthma, , identification of stressors, problem solving, medications, PEF monitoring |
| Outcomes | knowledge, ED visits, PEFV and inhaler technique, quality of life, perceived illness |
| Starting date | 1996 |
| Contact information | |
| Notes |
Ploska 1999.
| Trial name or title | An education based hospital nursing programme in the treatment of asthma |
| Methods | |
| Participants | Moderate asthmatics No. of participants: 80 Age group: 18‐75 |
| Interventions | |
| Outcomes | Respiratory function tests, use of corticosteroid/bronchodilator treatments, quality of life |
| Starting date | Unknown |
| Contact information | |
| Notes |
Contributions of authors
Gibson PG ‐ instigator of the review and conceptual direction ‐ inclusion/exclusion, quality assessment, data extraction, analysis and interpretation, writing and editing. Powell H ‐ responsible for review update, inclusion/exclusion, quality assessment, data extraction, analysis, interpretation and writing. Roberts JL ‐ inclusion/exclusion, quality assessment, data extraction, analysis, interpretation and writing. Wilson A ‐ inclusion/exclusion, quality assessment, data extraction and writing. Hensley MJ ‐ text of review and intellectual direction and input. Bauman A ‐ input of some guiding concepts particularly in regards to educational principles. Abramson MJ ‐ inclusion/exclusion, review of text and concepts. Walters EH ‐ academic input.
Sources of support
Internal sources
Hunter Area Health Service, Not specified.
External sources
Cooperative Research Centre for Asthma, Australia.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Abdulwadud 1999 {published data only}
- Abdulwadud O, Abramson M, Forbes A, James A, Walters EH. Evaluation of a randomised controlled trial of adult asthma education in a hospital setting. Thorax 1999;54:493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1995 {published data only}
- Allen RM, Jones MP, Oldenburg. Randomised trial of an asthma self‐management programme for adults. Thorax 1995;50:731‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 1990 {published data only}
- Bailey WC, Richards JM, Brooks CM, Soong S, Windsor RA, et al. A randomised trial to improve self‐management practice of adults with asthma. Archives of Internal Medicine 1990;150:1664‐8. [PubMed] [Google Scholar]
- Bailey WC, Richards JM, Manzella BA, Windsor RA, Brooks CM, Soong SJ. Promoting self management in adults with asthma: an overview of the UAB program. Health Education Quarterly 1987;14:345‐55. [DOI] [PubMed] [Google Scholar]
- Windsor RA, Bailey WC, Richards JM, et al. Evaluation of the efficacy and cost effectiveness of health education methods to increase medication adherence among adults with asthma. American Journal of Public Health 1990;80:1519‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 1999 {published data only}
- Bailey WC, Kohler CL, Richards JM, Windsor RA, Brooks M, Gerald LB. Asthma self‐management: do patient education programs always have an impact?. Archives of Internal Medicine 1999;159:2422‐8. [DOI] [PubMed] [Google Scholar]
Berg 1997 {published data only}
- Berg J, Dunbar‐Jacob J, Sereika SM. An evaluation of a self‐management program for adults with asthma. Clinical Nursing Research 1997;6(3):225‐38. [DOI] [PubMed] [Google Scholar]
Blixen 2001 {published data only}
- Blixen CE, Hammel JP, Murphy D, Ault V. Feasibility of a nurse‐run asthma education program for urban African‐Americans: a pilot study. Journal of Asthma 2001;38(1):23‐32. [PubMed] [Google Scholar]
Brewin 1995 {published data only}
- Brewin AM, Hughes JA. Effect of patient education on asthma management. British Journal of Nursing 1995;4:81‐101. [DOI] [PubMed] [Google Scholar]
Cote 1997 {published data only}
- Cote J, Cartier A, Robichaud P, Boutin H, Malo JI. Influence of asthma education on asthma severity, quality of life and environmental control. Canadian Respiratory Journal 2000;7(5):395‐400. [DOI] [PubMed] [Google Scholar]
- Cote J, Cartier A, Robichaud P, Poutin H, Malo J, Rouleau M, et al. Influence on Asthma Morbidity of asthma education programs based on self management plans following treatment optimization. American Journal of Respiratory & Critical Care Medicine 1997;155:1509‐14. [DOI] [PubMed] [Google Scholar]
Cowie 1997 {published data only}
- Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak flow‐based action plan in the prevention of exacerbations of asthma. Chest 1997;112:1134‐8. [DOI] [PubMed] [Google Scholar]
de Oliveira 1999 {published data only}
- Oliveira MA, Faresin SM, Bruno VF, Bittencourt AR, Fernandes ALG. Evaluation of an educational programme for socially deprived asthma patients. European Respiratory Journal 1999;14(4):908‐14. [DOI] [PubMed] [Google Scholar]
Gallefoss 1999 {published data only}
- Gallefoss F, Bakke PS. Cost‐effectiveness of self‐management in asthmatics: a 1yr follow‐up randomized, controlled trial. European Respiratory Journal 2001;17:206‐213. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. How does patient education and self‐management among asthmatics and patients with chronic obstructive pulmonary disease affect medication. American Journal of Respiratory & Critical Care Medicine 1999;160:2000‐5. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. Impact of patient education and self‐management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respiratory Medicine 2000;94:279‐87. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. Patient satisfaction with health care in asthmatics and patients with COPD before and after patient education. Respiratory Medicine 2000;94:1057‐1064. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS, Kjaersgaard P. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1999;159:812‐7. [DOI] [PubMed] [Google Scholar]
Garret 1994 {published data only}
- Garrett J, Fenwick JM, Taylor G, Mitchell E, Stewart J, Rea H. Prospective controlled evaluation of the effect of a community based asthma education centre in a multiracial working class neighbourhood. Thorax 1994;49:976‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 1999 {published data only}
- George MR, O'Dowd LC, Martin I, Lindell KO, Whitney F, Jones M, et al. A comprehensive educational progamme improves clinical outcome measures in inner‐city patients with asthma. Archives of Internal Medicine 1999;159:1710‐6. [DOI] [PubMed] [Google Scholar]
Ghosh 1998 {published data only}
- Ghosh CS, Ravindran P, Joshi M, Stearns SC. Reductions in hospital use from self management training for chronic asthmatics. Social Science & Medicine 1998;46(8):1087‐93. [DOI] [PubMed] [Google Scholar]
Grampian 1994 {published data only}
- Grampian Asthma Study of Integrated Care (GRASSIC) Effectiveness of routine self monitoring of peak flow in patients with asthma. Britich Medical Journal 1994;308:564‐7. [PMC free article] [PubMed] [Google Scholar]
Hayward 1996 {published data only}
- Hayward SA, Jordan M, Golden G, Levy M. A randomised controlled evaluation of asthma self management in general practice. Asthma in General practice 1996;4:11‐3. [Google Scholar]
Heard 1999 {published data only}
- Heard AR, Richards IJ, Alpers JH, Pilotto LS, Smith BJ, Black JA. Randomised controlled trial of general practice based asthma clinics. Medical Journal of Australia 1999;171:68‐71. [DOI] [PubMed] [Google Scholar]
Hilton 1986 {published data only}
- Hilton S, Sibbald B, Anderson HR, Freeling P. Controlled evaluation of the effects of patient education on asthma morbidity in general practice. The Lancet 1986;1:26‐9. [DOI] [PubMed] [Google Scholar]
Ignacio‐Garcia 1995 {published data only}
- Ignacio‐Garcia JM, Gonzalez‐Santos P. Asthma self‐management education program by home monitoring of peak expiratory flow. American Journal of Respiratory & Critical Care Medicine 1995;151:353‐9. [DOI] [PubMed] [Google Scholar]
Jones 1995 {published data only}
- Jones KP, Mullee MA, Middleton M, Chapman E, Holgate ST, British Society Research Committee. Peak flow based asthma self management: a randomised controlled study in general practice. Thorax 1995;50:851‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knoell 1998 {published data only}
- Knoell DL, Pierson JF, Marsh CB, Allen JN, Pathak DS. Measurement of outcomes in adults receiving pharmaceutical care in a comprehensive asthma outpatient clinic. Pharmacotherapy 1998;18(6):1365‐74. [PubMed] [Google Scholar]
Kotses 1995 {published data only}
- Kotses H, Bernstein IL, Bernstien DI, et al. A self‐management program for adult asthma. Part 1: development and evaluation. Journal of Allergy & Clinical Immunology 1995;95:529‐40. [DOI] [PubMed] [Google Scholar]
Kotses 1996 {published data only}
- Kotses H, Stout C, Mcconnaughty K, Winder JA, Creer TL. Evaluation of individualized asthma self‐management programmes. Journal of Asthma 1996;33:113‐8. [DOI] [PubMed] [Google Scholar]
Lahdensuo 1996 {published data only}
- Lahdensuo A, Haahtela T, Herrala J, et al. Randomised comparison of guided self‐management. British Medical Journal 1996;312:748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdensuo A, Haahtela T, Herrala J, Kava T, Kiviranta K, Kuusisto P, et al. Randomised comparison of cost effectiveness of guided self management and traditional treatment of asthma in Finland. British Medical Journal 1998;316:1138‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2000 {published data only}
- Levy ML, Robb M, Allen J, Doherty C, Bland JM, Winter RJD. A randomized controlled evaluation of specialist nurse education following accident and emergency attendance for acute asthma. Respiratory Medicine 2000;94:900‐8. [DOI] [PubMed] [Google Scholar]
Mayo 1990 {published data only}
- Mayo PH, Richman J, Harris HW. Results of a program to reduce admissions for adult asthma. Annals of Internal Medicine 1990;112:864‐71. [DOI] [PubMed] [Google Scholar]
Moudgil 2000 {published data only}
- Moudgil H, Marshall T, Honeybourne D. Asthma education and quality of life in the community: a randomised controlled study to evaluate the impact on white European and Indian subcontinent ethnic groups from socio‐economically deprived areas in Birmingham, UK. Thorax 2000;55:177‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulloy 1996 {published data only}
- Mulloy E, Donaghy D, Quigley C, McNicholas WT. A one‐year prospective audit of an asthma education programme in an out‐patient setting. Irish Medical Journal 1996;89:226‐8. [PubMed] [Google Scholar]
Neri 1996 {published data only}
- Neri M, Migliori GB, Spanevello A, Berra D, Nicolin E, Landoni CV, et al. Economic analysis of two structured treatment and teaching programs on asthma. Allergy 1996;51:313‐9. [PubMed] [Google Scholar]
Schott‐Baer 1999 {published data only}
- Schott‐Baer D, Christensen M. Research for practice. A pilot programme to increase self‐care of adult asthma patients. Medsurg Nursing 1999;8:178‐83. [PubMed] [Google Scholar]
Shields 1986 {published data only}
- Shields MC, Reinhard JD, Szidon JP, White PB. Effectiveness of a patient eduction program for adult asthmatics in reducing emergency room use. Clinical Research 1986;34(2). [Google Scholar]
- Shields MC, Vail MJ, Reinhard JD, Szidon JP, White PB. Counseling is better accepted than classes in patient education of adult inner city asthmatics. New Health Care Systems: HMOs & Beyond. 1986; Vol. June:289‐98.
Snyder 1987 {published data only}
- Snyder Se, Winder JA, Creer TL. Development and evaluation of an adult asthma self‐managment program: Wheezers Anonymous. Journal of Asthma 1987;11:39‐43. [DOI] [PubMed] [Google Scholar]
Sommaruga 1995 {published data only}
- Sommaruga M, Spanevello A, Migliori GB, Neri M, Callegari S, Manjani G. The effects of a cognitive behavioural intervention in asthmatic patients. Monaldi Archives for Chest Disease 1995;50:398‐402. [PubMed] [Google Scholar]
Wilson 1993 {published data only}
- Nguyen BP, Wilson SR, German DF. Patients' perceptions compared with objective ratings of asthma severity. Annals of Allergy Asthma & Immunology 1996;77:209‐15. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Scamagas P, German DF, Hughes GW, Lulla S, et al. A controlled trial of two forms of self‐managment education for adults with asthma. American Journal of Medicine 1993;94:564‐76. [DOI] [PubMed] [Google Scholar]
- Wilson‐Pessano ST, et al. An evaluation of approaches to asthma self management education for adults. The AIR/Kaiser Permanente study. Health Education Quarterly 1987;14:333‐43. [DOI] [PubMed] [Google Scholar]
Yoon 1993 {published data only}
- Yoon R, McKenzie DK, Bauman A, Miles DA. Controlled trial evaluation of an asthma education program for adults. Thorax 1993;48:1110‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeiger 1991 {published data only}
- Zeiger RS, Heller S, Mellon MH, Wald J. Falkoff R, Schatz M. Facilitated referral to asthma specialist reduces relapses in asthma emergency room visits. Journal of Allergy and Clinical Immunology 1991;87:1160‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdulwadud 1997 {published data only}
- Abdulwadud O, Abramson M, Forbes A, James A, Light L, Thien F, Walters EH. Attendance at an asthma educational intervention: characteristics of participants and non‐participants. Respiratory Medicine 1997;91:524‐9. [DOI] [PubMed] [Google Scholar]
Adams 2001 {published data only}
- Adams RJ, Boath K, Homan S, Campbell DA, Ruffin RE. A randomised trial of peak‐flow and symptom‐based action plans in adults with moderate‐to‐severe asthma. Respirology 2001;6:297‐304. [DOI] [PubMed] [Google Scholar]
Aiolfi 1995 {published data only}
- Aiolfi S, Confalonieri M, Scartabellati A, Patrini G, Ghio L, Mauri F, et al. International guidelines and educational experiences in an out‐patient clinic for asthma. Monaldi Archives for Chest Disease 1995;50:477‐81. [PubMed] [Google Scholar]
Amirav 1995 {published data only}
- Amirav I, Goren A, Kravitz RM, Pawlowski N. Physician‐tarteted program on inhaled therapy for childhood asthma. Journal of Allergy and Clinical Immunology 1995;95:818‐23. [DOI] [PubMed] [Google Scholar]
Ayres 1996 {published data only}
- Ayres JG, Campbell LM. A controlled assessment of an asthma self‐management plan involving a budesonide dose regimen. European Respiratory Journal 1996;9:886‐92. [DOI] [PubMed] [Google Scholar]
Baldwin 1997 {published data only}
- Baldwin DR, Pathak UA, King R, Vase BC, Pantin CFA. Outcome of asthmatics attending asthma clinics utilising self‐management plans in general practice. Asthma in General Practice 1997;5:31‐2. [Google Scholar]
Bolton 1991 {published data only}
- Bolton MB, Tilley BC, Kuder J, Reeeves T, Schultz LR. The cost and effectiveness of an education program for adults who have asthma. Journal of General Internal Medicine 1991;6:401‐7. [DOI] [PubMed] [Google Scholar]
- Ford ME, Havstad SL, Tilley BC, Bolton MB. Health outcomes among African and American Caucasian adults following a randomized trial of an asthma education program. Ethnicity & Health 1997;2(329‐39). [DOI] [PubMed] [Google Scholar]
Boulet 1995 {published data only}
- Boulet LP, Boutin H, Cote J, Leblanc P, Laviolettte M. Evaluation of an asthma self‐management program. Journal of Asthma 1995;32:199‐206. [DOI] [PubMed] [Google Scholar]
Charlton 1990 {published data only}
- Charlton I, Charlton G, Broomfield J, Mullee M. Evaluation of peak flow and symptoms only self management plans for control of asthma in general practice. British Medical Journal 1990;301:1355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cote 2001 {published data only}
- Cote J, Bowie DM, Robichaud P, Parent J‐G, Battiti L, Boulet L‐P. Evaluation of two different educational interventions for adult patients consulting with an acute asthma exacerbation. American Journal of Respiratory and Critical Care Medicine 2001;163:1415‐9. [DOI] [PubMed] [Google Scholar]
Cox 1993 {published data only}
- Cox NJM, Hendricks JC, Binkhorst Ra, Herwaarden CLA. A pulmonary rehabilitation program for patients with asthma and mild chronic obstructive pulmonary diseases (COPD). Lung 1993;171:235‐44. [DOI] [PubMed] [Google Scholar]
Erickson 1998 {published data only}
- Erickson SR, Ascione FJ, Kirking DM, Johnson CE. Use of a paging system to improve medication self‐management in patients with asthma. Journal of the American Pharmaceutical Association 1998;38:767‐9. [DOI] [PubMed] [Google Scholar]
Gergen 1995 {published data only}
- Gergen PJ, Goldstein RA. Does asthma education equal asthma intervention?. International Archives of Allergy & Immunology 1995;107:166‐8. [DOI] [PubMed] [Google Scholar]
Graft 1991 {published data only}
- Graft DF. A randomised trial to improve self‐management practices of adults with asthma: reviewers comments. Journal of Asthma 1991;28:228‐9. [Google Scholar]
Grainger‐Rousseau {published data only}
- Grainger‐Rousseau T‐J, McElnay JC. A model for community pharmacist involvement with general practitioners in the management of asthma patients. Journal of Applied Therapeutics 1996;1:145‐61. [Google Scholar]
Grampian 1994b {published data only}
- Grampian Asthma Study of Integrated Care (GRASSIC) Integrated care for asthma: a clinical, social, economic evaluation. British Medical Journal 1994;308:559‐64. [PMC free article] [PubMed] [Google Scholar]
Hausen 1999 {published data only}
- Hausen T. Patient education‐how can the long‐term effect be analyzed and how long does it last? (German). Pneumologie 1999;53:289‐95. [PubMed] [Google Scholar]
Heringa 1987 {published data only}
- Heringa P, Lawson L, Reda D. The effects of a structured education program on knowledge and psychomotor skills of patients using Beclomethasone Dipropionate Aerosol for Steroid Dependent Asthma. Health Education Quarterly 1987;14:309‐17. [DOI] [PubMed] [Google Scholar]
Hindi‐Alexander 1987 {published data only}
- Hindi‐Alexander MC. Asthma education programs: their role in asthma morbidity and mortality. Journal of Allergy and Clinical Immunology 1986;80:492‐4. [DOI] [PubMed] [Google Scholar]
Hoskins 1996 {published data only}
- Hoskins G, Neville RG, Smith B, Clark RA. Do self‐management plans reduce morbidity in patients with asthma?. British Journal of General Practice 1996;46:169‐71. [PMC free article] [PubMed] [Google Scholar]
Huss 1992 {published data only}
- Huss K, Huss RW, Squire EN, et al. Computer education for asthmatics: What effects?. Journal of Nursing Care Quality 1992;6:57‐66. [DOI] [PubMed] [Google Scholar]
Jackevicius 1999 {published data only}
- Jackevicius CA, Chapman KR. Inhaler education do hospital based pharmacists: How much is required?. Canadian Respiratory Journal 1999;6:237‐44. [DOI] [PubMed] [Google Scholar]
Janson‐Bjerklie 1988 {published data only}
- Janson‐Bjerklie S, Shnell S. Effect of peak flow information on patterns of self‐care in adult asthma. Heart & Lung 1988;17:543‐9. [PubMed] [Google Scholar]
Jenkinson 1988 {published data only}
- Jenkinson D, Davison J, Jones S, Hawtin P. Comaparison of effects of a self management booklet and audio cassette for patients with asthma. British Medical Journal 1988;297:267‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1987 {published data only}
- Jones PK, Jones SL, Katz J. Improving compliance for asthmatic patients visiting the emergency department using a health belief model. Journal of Asthma 1987;24:199‐206. [DOI] [PubMed] [Google Scholar]
Kauppinen 1998 {published data only}
- Kauppinen R, Sintonen H, Tukiainen H. One‐year economic evaluation of intensive versus conventional patient education and supervision for self‐management of new asthmatic patients. Respiratory Medicine 1998;92:300‐7. [DOI] [PubMed] [Google Scholar]
- Kauppinen R, Sintonen H, Vilkka V, Tukianen H. Long‐term (3‐year) economic evaluation of intensive patient education for self‐management during the first year in new asthmatics. Respiratory Medicine 1999;93:283‐9. [DOI] [PubMed] [Google Scholar]
- Kauppinen R, Vilkka V, Sintonen H, Klaukka T, Tukianen H. Long term economic evaluation of intensive patient education during the first treatment year in newly diagnosed asthma. Respiratory Medicine 2001;95:56‐63. [DOI] [PubMed] [Google Scholar]
Kelso 1995 {published data only}
- Kelso TM, Abou‐Shala N, Neilker GM, Arheart KL, Portner TS, Self TH. Comprehensive long‐term management program for asthma: effect on outcomes in adult African‐Americans. American Journal of the Medical Sciences 1996;311:272‐80. [DOI] [PubMed] [Google Scholar]
- Kelso TM, Self TH, Rumbak MJ, Stephens MA, Garrett W, Arheart KL. Educational and Long‐Term Therapeutic Intervention in the ED: Effect on Outcomes in Adult Indigent Minority Asthmatics. American Journal of Emergency Medicine 1995;13:632‐8. [DOI] [PubMed] [Google Scholar]
Klein 2001 {published data only}
- Klein JJ, Palen J, Uil SM, Zielhaus GA, Seydel ER, Herwaarden CLA. Benefit from the inclusion of self‐treatment guidelines to a self‐management programme for adults with asthma. European Respiratory Journal 2001;17:386‐394. [DOI] [PubMed] [Google Scholar]
LeBaron 1985 {published data only}
- LeBaron S, Zeltzer LK, Patner P, Kniker WT. A controlled study of education for improving compliance with Cromolyn Sodium (Intal): the importance of Physician‐patient communication. Annals of Allergy 1985;55:811‐8. [PubMed] [Google Scholar]
Legorreta 2000 {published data only}
- Legorreta AP, Leung K‐M, Berkbigler D, Evans R, Liu X. Outcomes of a population‐based asthma management program: quality of life, absenteeism, and utilization. Annals of Allergy Asthma & Immunology 2000;85:28‐34. [DOI] [PubMed] [Google Scholar]
Lirsac 1991 {published data only}
- Lirsac B, Braunstein G. Randomised evaluation of two teaching methods using aerosol inhalers. Revue des Maladies Respiratories 1991;8:559‐65. [PubMed] [Google Scholar]
Lopez‐Vina 2000 {published data only}
- Lopez‐Vina A, Castillo‐Arevalo F. Influence of peak expiratory flow monitoring on an asthma self‐management education programme. Respiratory Medicine 2000;94:760‐6. [DOI] [PubMed] [Google Scholar]
Maes 1988 {published data only}
- Maes S, Schlosser M. Changing health behaviour outcomes in asthmatic patients: A pilot intervention study. Social Science & Medicine 1988;26:359‐64. [DOI] [PubMed] [Google Scholar]
Maiman 1979 {published data only}
- Maiman LA, Green LW, Gibson G, MacKenzie EJ. Education for self‐treatment by adult asthmatics. The Journal of the American Medical Association 1979;241:1919‐22. [PubMed] [Google Scholar]
Moldofsky 1979 {published data only}
- Moldofsky H, Broder I, Davies G, Leznoff A. Videotape educational program for people with asthma. Canadian Medical Association Journal 1979;120:669‐72. [PMC free article] [PubMed] [Google Scholar]
Muhlhauser 1991 {published data only}
- Muhlhauser I, Richter B, Kraut D, Weske G, Worth H, Berger M. Evaluation of a structured treatment and teaching programme of asthma. Journal of Internal Medicine 1991;230:157‐64. [DOI] [PubMed] [Google Scholar]
Osman 1994 {published data only}
- Osman LM, Abdalla MI, Beattie JAG, Ross SJ, Russell IT, Friend JA, et al. Reducing hospital admission through computer supported education for asthma patients. British Medical Journal 1994;308:568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perdomo‐Ponce 1996 {published data only}
- Perdomo‐Ponce D, Benarroch L, Gonzalez‐Cerrutti R, Barroso R, Carneiro F, Meijomil P. Family education, a model for allergy prevention [Spanish]. Investigacion Clinica 1996;37:221‐45. [PubMed] [Google Scholar]
Petro 1995 {published data only}
- Petro W, Hollander P, Hamann B, Lauber B, Mzyk C, Prittwitz M. Patientenschulung in der pneumologischen Rehabilitation steigert den terapeutischen Erfolg. Atemwegs und Lungerkrankheiten 1995 Suppl;49‐58. [Google Scholar]
Premaratne 1999 {published data only}
- Premaratne UN, Sterne JAC, Marks GB, Webb R, Azima H, Burney PGJ. Clustered randomised trial of an intervention to improve the management of asthma: Greenwich asthma study. British Medical Journal 1999;318:1251‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringsberg 1990 {published data only}
- Ringsberg KC, Wiklund I, Wilhelmsen L. Education of adult patients at an "asthma school": effects on quality of life, knowledge and need for nursing. European Respiratory Journal 1990;3:33‐7. [PubMed] [Google Scholar]
Rydman 1999 {published data only}
- Rydman RJ, Sonenthal K, Laksminarayana T, Butki N, McDermott, MF. Evaluating the outcome of two teaching methods of breath actuated inhaler in an inner city asthma clinic. Journal of Medical Systems 1999;23:349‐56. [DOI] [PubMed] [Google Scholar]
Sondergaard 1992 {published data only}
- Sondergaard B, Davidsen F, Kirkeby B, Rasmussen M, Hey H. The economics of an intensive educational programme for asthmatic patients. A prospective controlled trial. Pharmacoeconomics 1992;3:207‐12. [DOI] [PubMed] [Google Scholar]
Thapar 1994 {published data only}
- Thapar A. Educating asthmatic patients in primary care: a pilot study of small group education. Family Practice 1994;11:39‐43. [DOI] [PubMed] [Google Scholar]
Tougaard 1992 {published data only}
- Tougaard L, Krone T, Sorknaes, Ellegaard H, PASTMA group. Economic benefits of teaching patients with chronic obstructive pulmonary disease about their asthma. Lancet 1992;339:1517‐20. [DOI] [PubMed] [Google Scholar]
Turner 1998 {published data only}
- Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial comparing peak expiratory flow and symptom self‐management plans for patients with asthma attending a primary care clinic. American Journal of Respiratory and Critical Care Medicine 1998;157:540‐6. [DOI] [PubMed] [Google Scholar]
Verver 1996 {published data only}
- Verver S, Poelman M, Bogels A, Chisholm SL, Dekker FW. Effects of instruction by practice assistance on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Family Practice 1996;13:35‐40. [DOI] [PubMed] [Google Scholar]
White 1989 {published data only}
- White PT, Pharoah CA, Anderson HR, Freeling P. Randomised controlled trial of small group education on the outcome of chronic asthma in general practice. Journal of the Royal College of General Practitioners 1989;39:182‐6. [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Im 1993 {published data only}
- Im J. Evaluation of effectiveness of an asthma clinic managed by an ambulatory care pharmacist. California Journal of Hospital Pharmacy 1993;5:5‐6. [Google Scholar]
References to ongoing studies
Ford 1996 {published data only}
- Ford ME, Edwards G, Rodrigues JL, Gibson RC, Tilley BC. An empowerment‐centered, church‐based asthma education program for African American adults. Health and Social Work 1996;21:70‐5. [DOI] [PubMed] [Google Scholar]
Ploska 1999 {published data only}
- Ploska JF. Asthma education nurses in the hospital (French) [Infirmiere educatrice de l'asthme en secteur hospitalier]. Revue de l Infirmiere 1999;50:35‐40. [PubMed] [Google Scholar]
Additional references
Anonymous 1992
- Anonymous. International Consensus Report on Diagnosis and Treatment of Asthma. National Heart, Lung, and Blood Institute Bethesda, Maryland, 1992.
Anonymous 1996
- Annonymous. Asthma Management Handbook 1996. [Google Scholar]
Bernard‐Bonnin 1995
- Bernard‐Bonnin A, et al. Self‐management teaching programs and morbidity of pediatric asthma: a meta‐analysis. Journal of Allergy & Clinical Immunology 1995;95:23‐41. [DOI] [PubMed] [Google Scholar]
Clark 1993
- Clark NM, Gotsch A, Rosenstoc IR. Patient , professional and public education on behavioural aspects of asthma: a review of strategies for change and needed research. Journal of Asthma 1993;30:241‐55. [DOI] [PubMed] [Google Scholar]
Gibson 1993a
- Gibson PG, et al. A prospective audit of asthma management following emergency asthma treatment at a teaching hospital. Medical Journal of Australia 1993;158:775‐8. [DOI] [PubMed] [Google Scholar]
Gibson 1993b
- Gibson PG. Asthma guidelines and evidenced‐based medicine. Lancet 1993;342:1305. [DOI] [PubMed] [Google Scholar]
Gibson 1998
- Gibson PG, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, Bauman A, et al. The effects of limited (information‐only) asthma education on health outcomes of adults with asthma (Cochrane Review). The Cochrane Library 1998, Issue 1. [CD001005] [Google Scholar]
Peat 1994
- Peat JK, et al. Changing prevalence of asthma in Australian Children. British Medical Journal 1994;308:1591‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolcock 1989
- Woolcock A, et al. Asthma Managment Plan. Medical Journal of Australia 1989;151:650‐3. [PubMed] [Google Scholar]


