Abstract
Background
There is a wide geographical variation in the prevalence of asthma and observational studies have suggested that dietary sodium may play a role.
Objectives
To assess the effect of dietary sodium manipulation on asthma control.
Search methods
We carried out a search using the Cochrane Airways Group asthma register. We searched the bibliographies of included randomised controlled trials (RCTs) for additional studies. We carried out the most recent search in November 2010.
Selection criteria
We considered only RCTs that involved dietary sodium reduction or increased sodium intake in patients with asthma.
Data collection and analysis
Both review authors assessed study and extracted data. We conducted data analyses in RevMan 5 using mean differences and random effects.
Main results
We identified a total of nine studies in relation to sodium manipulation and asthma, of which five were in people with asthma (318 participants), and four in people with exercise‐induced asthma (63 participants). There were no significant benefits of salt restriction on the control of asthma. There was some evidence from the exercise‐induced asthma studies that a low sodium diet may improve lung function after exercise and possibly baseline lung function, but this is based on findings from a very small numbers of participants.
Authors' conclusions
This review did not find any evidence that dietary sodium reduction significantly improves asthma control. Although dietary sodium reduction may result in improvements in lung function in exercise‐induced asthma, the clinical significance of this effect is unclear.
Plain language summary
Does reducing the amount of salt in a diet improve asthma symptoms?
A review of the current literature suggests that reduction in the amount of dietary sodium consumed has no significant effect on the symptoms of asthma but may be associated with improvements in some lung function measurements in exercise‐induced asthma.
Background
Asthma prevalence
Asthma is a respiratory disease which is characterised by increased airway responsiveness to a wide variety of stimuli and causes variable airflow obstruction (Tattersfield 2002). Asthma prevalence has been increasing over recent decades, with 300 million individuals globally reporting asthma symptoms and worldwide. Reports indicate that 255,000 individuals died of asthma in 2005 (Masoli 2004; WHO, 2006). The prevalence of asthma and atopy (a tendency to experience allergic reactions) is higher in developed countries (Anderson 1994; Peat 1994; Shaw 1990) than in developing or less affluent countries (Keeley 1991; Van Niekerk 1979; Yemaneberhan 1997). Some of this difference may be a consequence of differential methods of diagnosing asthma, but there is evidence that asthma appears to be associated with the economic development of a country. One suggestion that might explain the difference in asthma prevalence between countries is that diet is important in the aetiology of asthma (Fogarty 2000; McKeever 2004), and one of the characteristics of developed countries is a higher level of dietary sodium intake (Gleibermann 1973; Page 1974). This has led to the hypothesis that sodium has a role in the aetiology of asthma (Burney 1987).
Epidemiological and cross‐sectional studies
The first studies of the relationship of dietary sodium and asthma were by Peter Burney in the late 1980s (Burney 1986; Burney 1987a). An ecological study (Burney 1987a) investigated the relationship between the standardised mortality ratio for each area of England and Wales and the amount of table salt purchased. The table salt purchases were estimated by a Ministry of Agriculture, Fisheries and Food survey. This showed that table salt purchases were strongly and significantly related to asthma mortality. However, this relationship was observed in men (15 to 64 years old, r = 0.80, P < 0.05 ) and children (5 to 14 years old, r = 0.82. P < 0.05 ), but not in women (r = 0.40, P > 0.05 ). In addition, Burney 1986 investigated the relationship between sodium intake and bronchial reactivity. Questionnaires were sent to adults enquiring about symptoms of asthma and bronchial reactivity. Individuals with symptoms and 20% of responders without symptoms had a bronchial histamine challenge and skin prick test performed. Participants also provided 24‐hour urinary sodium excretion samples. There was a significant increase in bronchial reactivity (log10PD20 ‐histamine) with an increase in 24‐hour urinary sodium concentration. There was on average a 10‐fold difference in reactivity over the 95% range of sodium excretion recorded in the study. However, a large study (Britton 1994) failed to demonstrate any relationship between sodium and bronchial reactivity in 1,702 adults who were randomly selected from a population‐based sample. Participants provided data on a methacholine challenge and a 24‐hour urinary sodium sample collection was performed. There was no relationship found between 24‐hour urinary sodium excretion and methacholine challenge after adjustment for age, smoking and gender.
There have been several cross‐sectional studies in different populations throughout the world. These studies have used different definitions of asthma such as patient questionnaires, physicians' diagnosis or bronchial reactivity. The measurement of dietary sodium consumption varies between studies. Some studies used food frequency questionnaires, others used three‐day food recall and some used 24‐hour urinary sodium which is the most accurate for daily intake. Some of the studies suggest a relationship between dietary sodium and asthma or bronchial reactivity (Demissie 1996; Mohamed 1995; Pistelli 1993; Schwartz 1990; Tribe 1994), whereas other studies have not demonstrated any relationship (Devereux 1995; Sparrow 1991; Sausenthaler 2005; Zoia 1995;). Therefore, it is unclear at a population level what the role of dietary sodium is on asthma control. Interventional randomised controlled trials are a more robust method to assess the role of dietary sodium on asthma.
Objectives
To assess the effect of dietary sodium manipulation on asthma control.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised, placebo‐controlled trials (RCTs). We included double blind, single blind and open studies.
Types of participants
We included trials that involved adults or children with asthma as defined by the American, British Thoracic Society criteria, physician diagnosis or by objective measurements such as bronchial reactivity. Exercise‐induced asthma was defined as a drop greater than 10% in forced expiratory volume (FEV1) after exercise.
Types of interventions
We included studies that involved modification of dietary sodium with either an increase or decrease in dietary sodium.
Types of outcome measures
Primary outcomes
The primary outcomes for people with asthma were bronchial hyper‐responsiveness (Lewis 2001) and asthma quality of life. For people with exercise‐induced asthma, the primary outcomes were baseline and five‐minute post‐exercise FEV1 (ATS 2000) and asthma quality of life score. We chose these outcomes as they best reflect the severity of, and patients' experience of, the disease.
Secondary outcomes
In subjects with asthma, we examined the following secondary outcomes:
forced expiratory volume in one second (FEV1);
ratio of forced expiratory volume in one second divided by forced volume capacity (FEV1/FVC);
peak flow (PEFR);
bronchodilator use (puffs/day);
24‐hour sodium secretion (mmol/24 hours).
In subjects with exercised induced asthma, we examined the following outcomes:
baseline forced vital capacity (FVC) (pre‐exercise challenge);
baseline FEV1/FVC (pre‐exercise challenge);
five‐minute post‐exercise challenge FVC;
five‐minute post‐exercise challenge FEV1/FVC;
24‐hour sodium secretion (mmol/24 hours).
Search methods for identification of studies
Electronic searches
We conducted a search using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group search methods for further details). We searched all records in the Specialised Register coded as 'asthma' using the following terms:
salt* or nacl OR (sodium* and (chloride or diet* or intake or restriction*))
We carried out the search on 11 November 2010.
Searching other resources
We searched bibliographies of all selected RCTs for additional studies that might have contained further RCTs. We contacted authors of identified RCTs where necessary to clarify any data which were unclear.
Data collection and analysis
Selection of studies
Both review authors independently examined the results of the search, selected trials for inclusion in the review and assessed the full text of all trials that appeared potentially relevant. We reached complete agreement on the inclusion and exclusion of all studies. The authors of the previous version of this review contacted the study authors for further information. However, to date only one author (Gotshall 2000) has replied with additional information. In addition, we obtained information on randomisation and blinding from one author during the editorial process of the review update (Mickleborough 2000; Mickleborough 2001; Mickleborough 2005). We had access to data from Pogson 2008 and we re‐analysed the data to allow for comparison with data from the other studies.
Data extraction and management
We designed a data collection form for the review and we independently collected the following items:
publication details;
patient population, inclusion/exclusion criteria;
randomisation/allocation concealment;
details of blinding measures;
description of the intervention;
results;
potential source of bias;
funding/conflict of interest.
We resolved all disagreements by discussion until we reached a consensus.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We assessed the risk of bias according to the following domains.
Allocation sequence generation.
Concealment of allocation.
Blinding of participants and investigators.
Incomplete outcome data.
Selective outcome reporting.
We graded each potential source of bias as yes, no or unclear, relating to whether the potential for bias was low, high or unknown respectively.
Measures of treatment effect
We entered data as mean differences and pooled results using a random‐effects model. We entered data for high versus low dose sodium diets.
Data synthesis
All but one of the trials was designed as a cross‐over trial. For the parallel group study, the data in the analysis was the difference in the change in baseline in the two groups. For the cross‐over trials, where possible we extracted and used the paired mean difference with the 95% confidence intervals (CI) and/or the exact P values. If only summary measure were presented (mean, standard error (SE) or standard deviation (SD)) for the two groups, we entered the data into Review Manager (RevMan 5) as if the results were a parallel group study and the mean difference (MD) and SE were estimated. We adjusted the SE to match the significance in the research paper, where this was reported. We entered the actual or derived mean difference and SE for each of the studies in RevMan 5 using the generic inverse variance method. We pooled the data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analysis to investigate heterogeneity.
Results
Description of studies
Results of the search
We identified 263 references from the electronic search. Both review authors screened the references and we excluded 250 on the basis of the title and abstract. We retrieved 13 full‐text papers relating to 11 studies for further scrutiny. We included nine studies and excluded two studies.
Included studies
We included nine studies with a combined total of 381 participants. We found five studies with a total of 318 patients with asthma and four studies with 63 participants with exercise‐induced asthma. The studies that included people with asthma ranged in size from 17 to 220 participants and the size of the studies with participants who had exercise‐induced asthma ranged from 8 to 24. All the studies were cross‐over trials which ranged from two to five weeks in each arm, except for the study by Pogson 2008 which was a parallel group study of six weeks. The majority of studies placed individuals on a low sodium diet and then intervened with either sodium or placebo tablets. The amount of additional sodium differed between the studies, with supplementation being aimed at producing a sodium consumption similar to the average consumption in the UK (Burney 1989; Lieberman 1992; Medici 1993; Pogson 2008) or a high supplementation (Carey 1993; Gotshall 2004; Mickleborough 2000; Mickleborough 2001; Mickleborough 2005). We have provided a full description of all the studies in Table 1.
1. Details of included studies.
| Study | Number of Participants | Initial Diet Requirements | Interventions | Intervention period | |
| Low sodium diet | High sodium diet | ||||
| Asthma studies | |||||
| Burney 1989 | 36 | Low sodium diet | Low sodium diet and placebo tablets | Low sodium diet and 80 mmol of sodium a day | Cross‐over 2 weeks each limb |
| Lieberman 1992 | 17 | Low sodium diet used for hypertension | Low sodium diet | Add sodium to diet and consuming 34 mmol of sodium a day | Cross‐over 2 weeks each limb |
| Carey 1993 | 27 | Low sodium diet aiming for 80 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 200 mmol of sodium a day | Cross‐over 5 weeks each limb |
| Medici 1993 | 18 | Low sodium diet aiming for 86 to 103 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 154 mmol of sodium a day | Cross‐over 3 weeks each limb |
| Pogson 2008 | 220 | Low sodium diet aiming for 80 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 80 mmol of sodium a day | Parallel group 6 weeks intervention period |
| Exercise‐ induced asthma studies | |||||
| Mickleborough 2000 | 15 | Meal plan aiming for 65 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 174 mmol of sodium a day | Cross‐over 2 weeks each limb |
| Mickleborough 2001b | 16 | Meal plan aiming for 65 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 174 mmol of sodium a day (sodium bicarbonate) | Cross‐over 2 weeks each limb |
| Gotshall 2000 | 8 | Meal plan aiming for 65 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 174 mmol of sodium a day | Cross‐over 2 weeks each limb |
| Mickleborough 2005 | 24 | Meal plan aiming for 65 mmol of sodium a day | Low sodium diet and placebo tablets | Low sodium diet and 174 mmol of sodium a day | Cross‐over 2 weeks each limb |
A number of other outcomes were presented in the asthma papers including PD10, forced vital capacity, skin prick tests, blood pressure, asthma symptom scores, PEFR (morning and evening) and the number of asthma attacks. Other outcomes presented for exercise‐induced asthma included pre‐exercise PEFR, pre‐exercise forced expiratory flow (FEF) 25% to 50%, post‐exercise lung function measurements at 1, 10, 15, 20, 45, 75, 90, 105, and 120 minutes, diffusing capacity of the lung for carbon monoxide (DLco), carbon monoxide transfer coefficient (Kco), alveolar volume (Va), intrinsic diffusing capacity of the alveolar capillary membrane (DMco), pulmonary capillary blood volume (Vc), and ratio of Va/Vc. In addition, Mickleborough 2005 collected induced sputum for a total cell count, eosinophils, neutrophils, lymphocytes, macrophages, bronchial epithelial cells, interleukin 8, mean leukotriene b4, cysteinyl leukotriene, and PGD2‐methoxine. These samples were collected pre‐exercise and 1, 6 and 24 hours post‐exercise.
One study only recruited men (Carey 1993) and one study presented separate results for men and women (Burney 1989). Three studies recruited the participants from a university population (Gotshall 2000; Mickleborough 2000; Mickleborough 2001). Patients in two studies were told to stop their regular medications and take their medications on an as‐needed basis and this could limit the applicability of the results, as it would have potentially affected the patients' asthma control during the trial (Medici 1993; Mickleborough 2005).
Excluded studies
We excluded two studies at the full text stage as neither were RCTs (Gotshall 2004; Javaid 1988).
Risk of bias in included studies
We have provided full details of the risk of bias for each study in Characteristics of included studies. See Figure 1 for a summary of the risk of bias.
1.
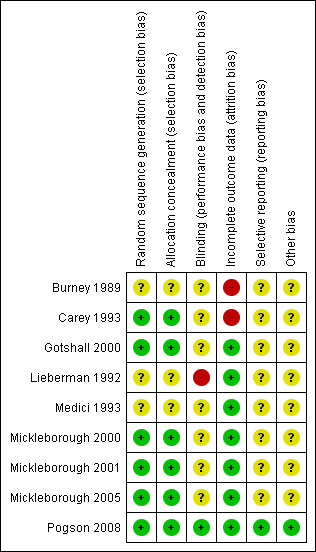
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were described as randomised. Pogson 2008 randomised subjects in blocks of eight; Carey 1993 used random numbers and Gotshall 2000 drew lots and therefore we judged all three to have a low risk of bias for sequence generation. In Mickleborough 2000, Mickleborough 2001 and Mickleborough 2005, an independent investigator having no contact with the subjects and no involvement in data collection or analysis used a computerised random number generator to create the randomisation sequence. We judged these studies to have a low risk of bias for sequence generation. Carey 1993 and Pogson 2008 both used sealed envelopes, therefore we considered these studies to be at a low risk of bias for allocation concealment, but the rest of the studies gave no detail of allocation concealment and were therefore at unclear risk of bias.
Blinding
Lieberman 1992 was the only study which was not blinded and we judged it to have a high risk of bias. Pogson 2008 states the clinicians and subjects were blinded and the code was not broken until primary analysis had been completed and therefore had the lowest risk of bias. The rest of studies were described as blinded but gave no information on blinding; however in one study, Medici 1993, the amount of salt was altered due to side effects of salt loading. In addition, salt does have a distinctive and known taste so it would be possible for participants to become aware of their treatment.
Incomplete outcome data
We judged six studies to be at a low risk of bias for incomplete outcome data as there were no drop‐outs (Gotshall 2000; Lieberman 1992; Medici 1993; Mickleborough 2000; Mickleborough 2001; Mickleborough 2005). Pogson 2008 used intention‐to‐treat analysis to address incomplete outcome data and we therefore considered this study to have a low risk of bias. Burney 1989 and Carey 1993 excluded drop‐outs from their analysis and therefore did not address incomplete data. We considered both of these studies to be at a high risk of bias, although the number of drop‐outs in these studies was small.
Selective reporting
Pogson 2008 stated all the outcomes at the beginning of study so was at low risk of bias, but the rest of the studies did not and so it was not clear if they were free from selective reporting. Although this does not seem likely, we judged these studies to be at unclear risk of bias.
Other potential sources of bias
In most of the studies there were areas where bias might have affected the results. Only Pogson 2008 reported a power calculation and clear primary and secondary outcomes. Mickleborough 2005 had a power calculation but it was not clearly or fully described as the primary outcomes of the study were not stated. All the studies except Pogson 2008 were cross‐over in design. Only Gotshall 2000; Mickleborough 2005 and Mickleborough 2001 examined for cross‐over effects and there was no wash‐out period in four studies (Burney 1989; Carey 1993; Lieberman 1992; Medici 1993). In Medici 1993, participants experienced heartburn (the number who experienced it was not stated in the paper) when taking sodium tablets, which may indicate that the study was unblinded.
Effects of interventions
Asthma
For the primary objective outcome, bronchial hyper‐reactivity, none of the data from the three included studies could be combined due to different substances being used for provocation and differences in the presentation of the data (Burney 1989; Medici 1993; Pogson 2008).The first of these studies found a significant mean change in the provoking dose in men (n = 11) but not for women (n = 20) (Burney 1989). The second study, in 14 individuals, found no differences between a low sodium and higher sodium diet and PD20 (Medici 1993). Finally, in the parallel group study there was no difference in mean change from baseline of doubling doses of PD20 between individuals on a low sodium and higher sodium diet (n = 220) (Pogson 2008).
Only one study reported data on change in asthma quality of life and it found no difference in asthma quality of life between the low sodium and higher sodium intake (P = 0.49) (Pogson 2008).
None of the secondary outcomes FEV1, FEV1/FVC, PEFR or bronchodilator usage demonstrated any significant differences between lower sodium and higher sodium diets (Analysis 1.1, (n = 265); Analysis 1.2 (n = 238); Analysis 1.3 (n = 255); Analysis 1.4, (n = 255) respectively) despite significant changes in urinary sodium between different diets. The differences in urinary sodium concentration indicates that diet manipulation and the different interventions did impact sodium intake (Analysis 1.5, (n = 282)).
1.1. Analysis.
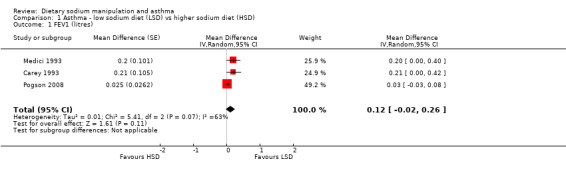
Comparison 1 Asthma ‐ low sodium diet (LSD) vs higher sodium diet (HSD), Outcome 1 FEV1 (litres).
1.2. Analysis.
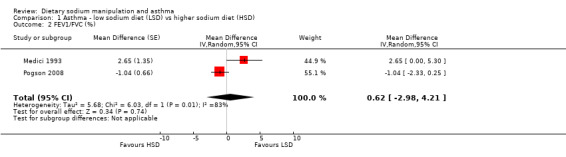
Comparison 1 Asthma ‐ low sodium diet (LSD) vs higher sodium diet (HSD), Outcome 2 FEV1/FVC (%).
1.3. Analysis.
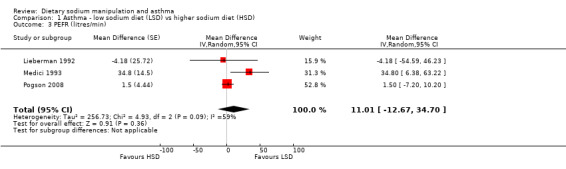
Comparison 1 Asthma ‐ low sodium diet (LSD) vs higher sodium diet (HSD), Outcome 3 PEFR (litres/min).
1.4. Analysis.
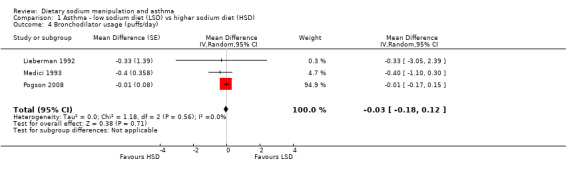
Comparison 1 Asthma ‐ low sodium diet (LSD) vs higher sodium diet (HSD), Outcome 4 Bronchodilator usage (puffs/day).
1.5. Analysis.
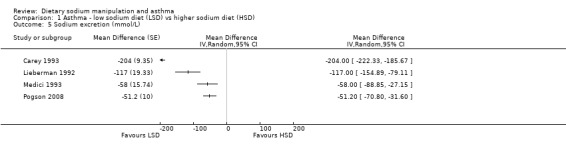
Comparison 1 Asthma ‐ low sodium diet (LSD) vs higher sodium diet (HSD), Outcome 5 Sodium excretion (mmol/L).
Exercise‐induced Asthma
The primary outcome of five‐minute post‐exercise FEV1 (MD 0.46; 95% CI 0.01 to 0.91; (n = 23); Figure 2) was significantly better in the low sodium diet as compared with the high sodium diet, although the difference was borderline. None of the studies reported on asthma quality of life. There were borderline significant results (P = 0.05) for baseline FEV1 (MD 0.15; 95% CI ‐0.00 to 0.30; (n = 47); Figure 3) and baseline FVC (MD 0.13 L; 95% CI ‐0.00 to 0.26, (n = 47); Figure 4). A low sodium diet was associated with significantly better five‐minute post‐exercise FVC (MD 0.86; 95% CI 0.04 to 1.68, (n = 23); Figure 5). However differences in diet did not effect FEV1/FVC at baseline (Figure 6, n = 47) or five minutes post‐exercise (Figure 7, n = 23). There were large significant changes in urinary sodium (MD ‐236.00; 95% CI ‐281.56 to ‐190.44; (n = 23); Analysis 2.7).
2.
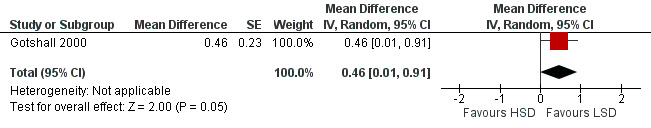
Forest plot of comparison: 2 Exercise induced asthma ‐ low sodium diet vs high sodium diet, outcome: 2.4 5‐minute post‐exercise FEV1 (litres).
3.
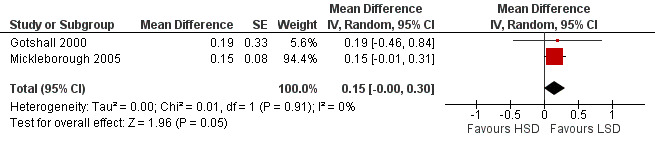
Forest plot of comparison: 2 Exercise induced asthma ‐ low sodium diet vs high sodium diet, outcome: 2.1 Baseline FEV1 (litres).
4.
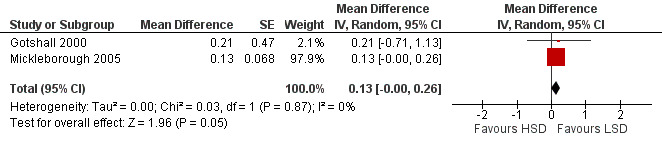
Forest plot of comparison: 2 Exercise induced asthma ‐ low sodium diet vs high sodium diet, outcome: 2.2 Baseline FVC (litres).
5.
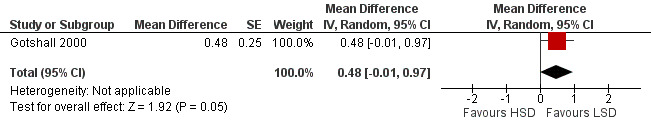
Forest plot of comparison: 2 Exercise induced asthma ‐ low sodium diet vs high sodium diet, outcome: 2.5 5‐minute post‐exercise FVC (litres).
6.
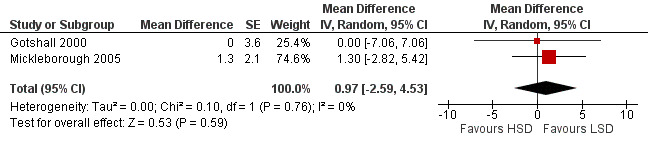
Forest plot of comparison: 2 Exercise induced asthma ‐ low sodium diet vs high sodium diet, outcome: 2.3 Baseline FEV1/FVC (%).
7.

Forest plot of comparison: 2 Exercise induced asthma ‐ low sodium diet vs high sodium diet, outcome: 2.6 5‐minute post‐exercise FEV1/FVC (%).
2.7. Analysis.
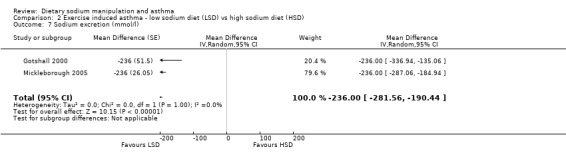
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 7 Sodium excretion (mmol/l).
Discussion
This review investigated the effect of sodium manipulation in people with either asthma or exercise‐induced asthma. Most of the lung function outcomes improved for people with exercise‐induced asthma who were consuming a low sodium diet. We were able to pool data from only four studies in patients with asthma and these were mostly studies on small numbers of participants. One included study was larger and involved 220 participants. This large study also demonstrated the lowest risk of potential bias in the design and conduct of the study. The analyses used six different measures of asthma control and the number of participants in each analysis varied from 14 to 239. The only outcome affected by sodium manipulation was the FEV1/FVC ratio. All other measures were negative despite a significant change in urinary sodium. However, it must be recognised that there was a wide difference in the amount of change in urinary sodium between the different studies depending on the intervention. In addition, patients did not benefit from an improvement in quality of life, however only one study reported on this outcome. The four studies in exercise‐induced asthma had a small number of participants with between 23 and 59 participants in each analysis. This review found that some lung function parameters for exercise‐induced asthma were improved by reducing sodium intake. There were improvements in FEV1 and FVC at baseline for individuals when a low sodium diet was compared with a high sodium diet; however these differences in lung function were not demonstrated in the asthma population. The five‐minute post‐exercise lung function suggests that a low sodium diet may be beneficial for people with exercise‐induced asthma, with a large reduction of FEV1 of 630 mL and FVC of 860 mL. It should be noted that the changes of dietary sodium intake within the exercise‐induced asthmatic population were extreme. In 2004 the UK consumption of sodium was estimated as 165 mmol (Dietary and Nutritional Survey 2004) but the high sodium diet group were consuming at least 230 mmol and had changes in urinary sodium of 270 mmol/L between comparison groups. Therefore these changes might only be possible in a RCT environment. Another limitation of the analyses of the exercise‐induced asthma is that the mean difference (MD) and standard error (SE) were all derived from the summary data given and therefore did not take account of the paired data. It is also possible that the people with exercise‐induced asthma who were predominantly recruited from university students may not represent the general population of people with exercise‐induced asthma and therefore the results may not be generalisable to the wider population of people with exercise‐induced asthma.
Authors' conclusions
Implications for practice.
This review suggests that people with asthma may not benefit significantly from altering their dietary sodium intake in order to improve their asthma control. People with exercise‐induced asthma might benefit from a reduction of dietary sodium; however the change in dietary sodium needed is extremely large and the clinical significance of this effect is unclear. The effects seen were unique to the exercise‐induced asthma population, possibly due to having a different phenotype of asthma and possibly because of the large changes in the sodium consumption they achieved. Therefore these findings could be limited to a small number of people with exercise‐induced asthma and the change in sodium may be difficult to achieve and sustain outside a clinical trial environment.
Implications for research.
This review demonstrates that dietary sodium reduction did not have a significant effect on asthma control within a population of people with asthma. Therefore, no further research in this area is recommended. There appears to be an effect of dietary sodium manipulation on some outcomes of lung function in people with exercise‐induced asthma. This could potentially be further investigated in larger studies in order to determine the clinical relevancy of these changes as the changes were inconsistent across different markers of lung functions. In addition, it is not known if these changes persist longer than two weeks.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2014 | Amended | PLS title amended |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 13 January 2011 | New search has been performed | New literature search carried out, review updated with three new studies. |
| 13 January 2011 | New citation required but conclusions have not changed | The author team has changed. The review has been rewritten from a new protocol and the title has changed. |
| 18 February 2004 | New citation required and conclusions have changed | Updated March 2004 by FR with the inclusion of another RCT (Mickleborough 2001) however, this did not alter the conclusion of the review. |
Acknowledgements
The authors would like to thank the Members of the Cochrane Airways Group for their help and support. We would like to thank previous authors Kate Ardern and Felix Ram. We would like to thank Jo Leonard‐Bee for her invaluable advice.
Data and analyses
Comparison 1. Asthma ‐ low sodium diet (LSD) vs higher sodium diet (HSD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 3 | Mean Difference (Random, 95% CI) | 0.12 [‐0.02, 0.26] | |
| 2 FEV1/FVC (%) | 2 | Mean Difference (Random, 95% CI) | 0.62 [‐2.98, 4.21] | |
| 3 PEFR (litres/min) | 3 | Mean Difference (Random, 95% CI) | 11.01 [‐12.67, 34.70] | |
| 4 Bronchodilator usage (puffs/day) | 3 | Mean Difference (Random, 95% CI) | ‐0.03 [‐0.18, 0.12] | |
| 5 Sodium excretion (mmol/L) | 4 | Mean Difference (Random, 95% CI) | Totals not selected |
Comparison 2. Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Baseline FEV1 (litres) | 2 | Mean Difference (Random, 95% CI) | 0.15 [‐0.00, 0.30] | |
| 2 Baseline FVC (litres) | 2 | Mean Difference (Random, 95% CI) | 0.13 [‐0.00, 0.26] | |
| 3 Baseline FEV1/FVC (%) | 2 | Mean Difference (Random, 95% CI) | 0.97 [‐2.59, 4.53] | |
| 4 5‐minute post‐exercise FEV1 (litres) | 1 | Mean Difference (Random, 95% CI) | 0.46 [0.01, 0.91] | |
| 5 5‐minute post‐exercise FVC (litres) | 1 | Mean Difference (Random, 95% CI) | 0.48 [‐0.01, 0.97] | |
| 6 5‐minute post‐exercise FEV1/FVC (%) | 1 | Mean Difference (Random, 95% CI) | 3.0 [‐6.80, 12.80] | |
| 7 Sodium excretion (mmol/l) | 2 | Mean Difference (Random, 95% CI) | ‐236.0 [‐281.56, ‐190.44] |
2.1. Analysis.
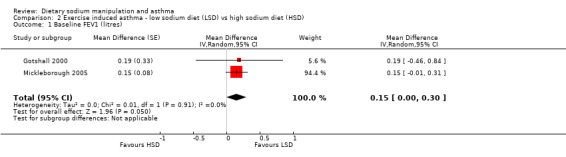
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 1 Baseline FEV1 (litres).
2.2. Analysis.
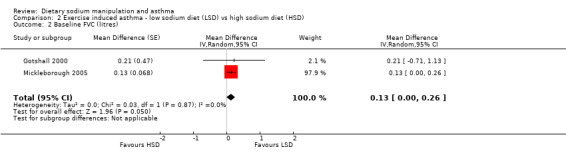
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 2 Baseline FVC (litres).
2.3. Analysis.
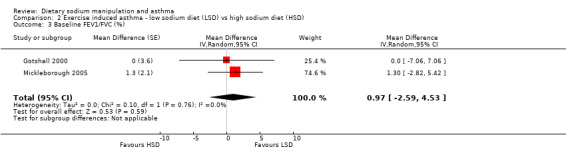
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 3 Baseline FEV1/FVC (%).
2.4. Analysis.
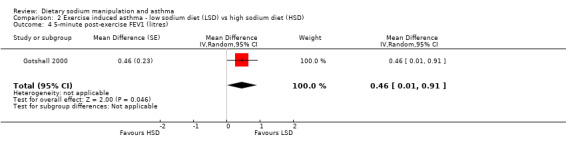
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 4 5‐minute post‐exercise FEV1 (litres).
2.5. Analysis.
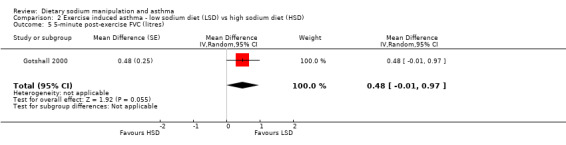
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 5 5‐minute post‐exercise FVC (litres).
2.6. Analysis.
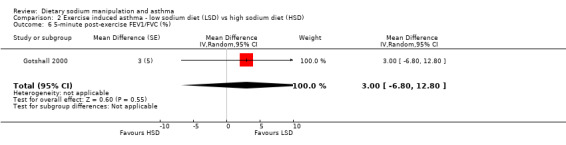
Comparison 2 Exercise induced asthma ‐ low sodium diet (LSD) vs high sodium diet (HSD), Outcome 6 5‐minute post‐exercise FEV1/FVC (%).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burney 1989.
| Methods | Randomised placebo‐controlled cross‐over study. . | |
| Participants | 36 participants (14 men, 22 women) with moderately severe asthma were included in the study. Age range 18 to 53 years. 9 current smokers, 10 ex‐smokers and 17 never smoked. 35 subjects on inhaled bronchodilators and 12 using inhaled steroids. | |
| Interventions | After one week run‐in, subjects were put on a low sodium diet and asked to take either slow sodium tablets (80 mmol/day) or placebo. After a 2‐week period the subjects were crossed over (no wash‐out period). Study measurements were performed after the run‐in period, 2 weeks after first intervention and 2 weeks after the secondary intervention. Exclusion criteria: none stated in the paper. |
|
| Outcomes | FEV1, FVC, PD20, PD10, skin prick tests and blood pressure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given in paper. |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised; no other information available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded; no other information available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 subjects were excluded but not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not clear from the information presented. |
| Other bias | Unclear risk | The significant results are only present in men and has no calculation of power described. There was no wash‐out period. |
Carey 1993.
| Methods | Randomised placebo‐controlled cross‐over study. | |
| Participants | 27 male patients (5 dropped out ‐ 1 due to exacerbation and 4 due to poor compliance), age range 12 to 68 years with stable asthma. All had been previously given a diagnosis of asthma and were currently on medication (all on beta 2 agonists, 12 inhaled corticosteroids, 4 inhaled cromoglycate, 3 oral theophyllines). No subjects were using oral beta agonists, antihistamines or steroids at the time of the study. None were current smokers, only one had previously smoked. Exclusion criteria: history, clinical or laboratory evidence of renal, hepatic, cardiovascular disease, hypertension or electrolyte imbalance. In addition participants could not take diuretic therapy. |
|
| Interventions | All participants placed on low sodium diet (80 mmol daily) and then randomised to receive either slow sodium (200 mmol daily) or placebo (placebo tablets) for 5 weeks and then crossed over (no wash‐out period). | |
| Outcomes | PD20, PEFR (morning and evening), symptom score, bronchodilator requirements and FEV1. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coded random numbers used for treatment allocation. |
| Allocation concealment (selection bias) | Low risk | Treatment given in sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blind; no other information given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Subjects who dropped out were not included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not clear from the information presented. |
| Other bias | Unclear risk | 5 drop‐out not included in analysis. No washout period. Only men included in this paper. No calculation of power described. |
Gotshall 2000.
| Methods | Randomised placebo‐controlled cross‐over study. | |
| Participants | 8 (1 male and 7 female) participants with objectively diagnosed exercise‐induced asthma (> 10% drop in FEV1 after exercise). Mean age 23 years. All subjects used short‐acting rescue medications and none were on maintenance medications. Control group were 8 (4 male and 4 female) non‐asthmatics. Exclusion criteria: no participants had atopic asthma. |
|
| Interventions | All participants entered the study on their normal sodium diet for 1 week. Participants then consumed a low sodium diet (65 mmol of sodium a day by means of a meal plan) and randomly assigned to either high sodium limb or low sodium limb for 2 weeks. Thereafter, a 1‐week wash‐out period on a normal sodium diet followed, then all patients followed alternative diet for 2 weeks (crossover). In the low sodium limb participants consumed a low sodium diet and placebo tablets and participants in the high sodium limb consumed a low sodium diet (174 mmol of sodium/day). | |
| Outcomes | FEV1, FVC, FEV1/FVC and PEFR pre‐exercise test and 1,5,10,15 minutes post‐exercise tests. | |
| Notes | Author reply received (03/02/01) further information provided on allocation (drawing lots) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation of treatment was done by drawing lots. |
| Allocation concealment (selection bias) | Low risk | Treatments were drawn by lots so both participants and investigators blinded to the randomisation sequence. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded; no other information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not clear from information presented. |
| Other bias | Unclear risk | Population recruited from an university population only. No calculation of power described. |
Lieberman 1992.
| Methods | Randomised open placebo‐controlled cross‐over study. | |
| Participants | 17 patients with mild asthma (9 men, 8 women) recruited from pulmonary outpatient clinic. Mean age 43 (range 27 to 62) years. All patients had a history of intermittent wheezing and greater than 15% change in FEV1. All used beta 2 agonists, 5 used inhaled corticosteroids, 10 used oral theophyllines. Exclusion criteria: hypertensive patients and smokers. |
|
| Interventions | 3 regimens of diet were tested for 2 weeks each with no wash‐out period in‐between: normal diet (regular diet with no deliberate change in sodium intake), low sodium (used for hypertensive patients) and high sodium (eat as much sodium as possible and consuming 34 mmol of sodium/day) | |
| Outcomes | PEFR (3 times daily), daily asthma medication requirements. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised; no other information available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not clear from the information given. |
| Other bias | Unclear risk | No wash‐out period and no calculation of power described. |
Medici 1993.
| Methods | Randomised placebo‐controlled cross‐over study. . | |
| Participants | 18 patients recruited (4 excluded during run‐in due to poor compliance). Study group 14(9 men, 5 women). Age range 20 to 65 years. They had stable atopic asthma and fulfilled the ATS criteria for the diagnosis of asthma. Exclusion criteria: instability of asthma, therapy with oral steroids, cromoglycan or diuretics, cardiac insufficiency, arrhythmias, liver diseases, kidney diseases, diabetes mellitus, smoking and pregnancy. |
|
| Interventions | After 2 weeks on a low sodium diet (86 to 103 mmol/day), given 157 mmol of sodium daily (in the form of sodium chloride) or placebo for 3 weeks. Then the 2 groups were crossed over for a second 3‐week treatment period (sodium citrate with 154 sodium mmol). There was no washout period. | |
| Outcomes | PD20, FEV1, FVC, PEFR x 3 a day, inhaler bronchodilator and corticosteroids sprays, number of asthma attacks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised; no information given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blind, however the papers states that due to side effects when on the salt tablets, less than intended amount was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not clear from information given. |
| Other bias | Unclear risk | Subjects had heartburn on the sodium tablets and so could have been unblinded. Subjects changed medication from regular to prn and there was no wash‐out period. No calculation of power described. |
Mickleborough 2000.
| Methods | Randomised placebo‐controlled cross‐over study. | |
| Participants | 15 participants (age range 18 to 36 years) with exercise‐induced asthma (10% drop of FEV1 with exercise). Participants were recruited from university population. Exclusion criteria: none stated. |
|
| Interventions | All participants had a 1‐week run‐in on normal sodium diet. All participants then started a low sodium diet (65 mmol of sodium a day by a meal plan) and then randomised to high sodium limb (174 mmol of sodium tablets) or low sodium limb (placebo tablets). After 2 weeks a 1‐week washout period took place on a normal sodium diet and then subjects consumed the other limb for 2 weeks. | |
| Outcomes | Pre‐exercise, 1 minute and 5 minutes post‐exercise FEV1, FVC, FEV1/FVC, PEFR, FEF 25‐75%. VE and VO2 at 1 minute. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent investigator had no contact with the subjects and no involvement in data collection or analysis used a computerized random number generator to create the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Randomised study with independent investigator generating the sequence. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded; no other information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not clear from information given. |
| Other bias | Unclear risk | Subjects were from a university population. |
Mickleborough 2001.
| Methods | Randomised placebo‐controlled cross‐over study. | |
| Participants | 16 participants. 8 participants with exercise‐induced asthma (mean age 22 years and all using short beta agonists) and 8 controls (mean age 23 years). The population was recruited from a university population. Exclusion criteria: none of the participants had atopic asthma. |
|
| Interventions | All participants entered the study on a normal sodium diet and were started a low sodium diet (65 mmol of sodium using a meal plan). All participants were randomised to a low sodium limb (placebo tablets) or high sodium low chloride limb (174 mmol of sodium a day as sodium bicarbonate) for 2 weeks. After this a 1‐week wash‐out period followed and then participants switched to the alternative diet. | |
| Outcomes | FEV1,FVC, FEV1/FVC pre‐exercise and post‐exercise measurement at 1, 5, 10 and 15 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent investigator had no contact with the subjects and no involvement in data collection or analysis used a computerised random number generator to create the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Randomised study with independent investigator generating the sequence. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded; no other information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not clear from information given. |
| Other bias | Unclear risk | Participants recruited from a university population. No calculation of power. |
Mickleborough 2005.
| Methods | Randomised placebo‐controlled cross‐over study. | |
| Participants | 24 participants (mean age 24 years) were recruited from a university population and local community. 14 participants were on inhaled short beta 2 agonists and 12 participants on inhaled corticosteroids. All had documented exercise‐induced asthma ‐ wheezing, shortness of breath and chest tightness after exercise and atopic asthma. Exclusion criteria: pregnancy, hypertension, hyperlipidaemia, diabetes, bleeding disorders, delayed clotting time or taking aspirin. |
|
| Interventions | All participants had a run‐in period of 1 week on a normal sodium diet; after this all participants consumed a low sodium diet (65 mmol of sodium a day as meal plan). Participants were then randomised to either high sodium limb (sodium tablets 174 mmol of sodium a day) or low sodium limb (placebo tablets). After 2 weeks there was a wash‐out period on a normal sodium diet for one week. Finally participants had two weeks on the different limb. | |
| Outcomes | Pre‐ and post‐exercise FEV1, FVC, FEV1/FVC, FEF25‐50%, DLCO, KCO, Va, DMC0, VL, VC/VA. Post‐exercise measures were 1, 5, 20, 45, 75, 90, 105, 120 minutes. In addition inhaled sputum was collected for total cell count, neutrophils, lymphocytes, macrophages, epithelial cells, interleukin 8, mean leukotriene b4, cysteinyl leukotriene, PGD2‐methoxine. This was collected pre‐exercise, 1 hour, 6 hours and 24 hours post‐exercise. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent investigator had no contact with the subjects and no involvement in data collection or analysis used a computerised random number generator to create the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Randomised study with independent investigator generating the sequence. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as blinded; no other information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not clear from information given. |
| Other bias | Unclear risk | Subjects had to stop maintenance medication during the study. The study was controlled for cross‐over effect. Power calculation was not fully described. |
Pogson 2008.
| Methods | Randomised double‐blind placebo‐controlled parallel group study. | |
| Participants | 220 participants (mean age 44 years) with GP diagnosis of asthma and bronchial reactivity. Population recruited from general practice. All participants used short acting beta 2 agonists, 74% used inhaled corticosteroids and 38% used long acting beta 2 agonists. Exclusion criteria: oral steroids or change in asthma medication in the last 4 weeks, smoking history over 10 pack year, use of diuretics or angiotension converting enzyme inhibitors, pregnancy or planned pregnancy. |
|
| Interventions | All participants consumed a low sodium diet for six weeks and half of participants were randomised to placebo tablets and half of participants were randomised to sodium chloride tablets (80 mmol of sodium). | |
| Outcomes | PD20, FEV1,FVC, PEFR‐morning and evening, twice daily symptoms score, twice daily bronchodilator use, atopic status and Juniper asthma quality of life questionnaire. | |
| Notes | Data was re‐analysed in order for it to be combined with other data, as it is presented in the paper as mean change from baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in permuted block of 8 and stratified by the presence or absence of inhaler corticosteroid use. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes (author stated). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, clinicians and outcome assessor blind and code not broken until end of the study and primary analyses completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated at the beginning of the study. |
| Other bias | Low risk | |
DLCO:diffusing capacity of the lung for carbon monoxide DMCO: intrinsic diffusing capacity of the alveolar capillary membrane FEF: forced expiratory flow FEV1: forced expiratory volume FVC: forced volume capacity KCO: carbon monoxide transfer coefficient PEFR: peak flow PD10: provocative dose of ventilation causing a 10% fall in FEV1 PD20: provocative dose of ventilation causing a 20% fall in FEV1 PGD2: prostaglandin D2 Va: alveolar volume VC/VA: Alveolar volume / Pulmonary capillary blood volume VC: Pulmonary capillary blood volume vo2: volume of oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gotshall 2004 | This is not a randomised controlled trial. |
| Javaid 1988 | This is not a randomised controlled trial. |
Differences between protocol and review
This was an update of a previous review. This previous review did not have a protocol and a review of the data only was performed.
Contributions of authors
ZP and TM conducted all of the work for this review with help from various people as listed in the acknowledgment section.
Sources of support
Internal sources
University of Nottingham, UK.
External sources
No external support, Not specified.
Declarations of interest
ZP and TM were both involved with the largest study reported in this review (Pogson 2008).
Edited (no change to conclusions)
References
References to studies included in this review
Burney 1989 {published data only}
- Burney PG, Neild JE, Twort CH, Chinn S, Jones TD, Mitchell WD, et al. Effect of changing dietary sodium on the airway response to histamine. Thorax 1989;44:36‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carey 1993 {published data only}
- Carey OJ, Locke C, Cookson JB. Effect of alterations of dietary sodium on the severity of asthma in men. Thorax 1993;48:714‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gotshall 2000 {published and unpublished data}
- Gotshall RW, Mickleborough TD, Cordain L. Dietary salt restriction improves pulmonary function in exercise‐induced asthma. Medicine and Science in Sports and Exercise 2000;32(11):1815‐9. [MEDLINE: ; UI:20529892] [DOI] [PubMed] [Google Scholar]
Lieberman 1992 {published data only}
- Lieberman D, Heimer D. Effect of dietary sodium on the severity of bronchial asthma. Thorax 1992;47:360‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medici 1993 {published data only}
- Medici TC, Vetter W. Bronchial asthma and kitchen salt. Schweizerische Medizinische Wochenschrift 1991;121(14):501‐8. [MEDLINE: ; UI: 91240257] [PubMed] [Google Scholar]
- Medici TC, Zumstein Schmid A, Hacki M, Vetter W. Are asthmatics salt‐sensitive? A preliminary controlled study. Chest 1993;104(4):1138‐43. [DOI] [PubMed] [Google Scholar]
Mickleborough 2000 {published and unpublished data}
- Mickleborough TD, Cordain L, Gotshall RW, Tucker A. A low sodium diet improves indices of pulmonary function in exercise‐induced asthma. Journal of Exercise Physiology Online 2000;3:46‐54. [Google Scholar]
- Mickleborough TD, Gotshall RW, Cordain L, Lindley M. Dietary salt alters pulmonary function during exercise in exercise‐induced asthmatics. Journal of Sports Sciences 2001;19(11):865‐73. [PUBMED: Other: PMID: 11695508] [DOI] [PubMed] [Google Scholar]
Mickleborough 2001 {published and unpublished data}
- Mickleborough TD, Gotshall RW, Kluka EM, Miller CW, Cordain L. Dietary chloride as a possible determinant of the severity of exercise‐induced asthma. European Journal of Applied Physiology 2001;85:450‐6. [DOI] [PubMed] [Google Scholar]
Mickleborough 2005 {published and unpublished data}
- Mickleborough TD, Lindley MR, Ray S. Dietary salt, airway inflammation and diffusion capacity in exercise‐induced asthma. Medicine and Science in Sports and Exercise 2005;37(6):904‐4. [PubMed] [Google Scholar]
Pogson 2008 {published and unpublished data}
- Pogson ZEK, Antoniak MD, Pacey SJ, Lewis SA, Britton JR, Fogarty AW. Does a low sodium diet improve asthma control?. American Journal of Respiratory & Critical Care Medicine 2008;178:132‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gotshall 2004 {published data only}
- Gotshall RW, Rasmussen JJ, Fedorczak LJ. Effect of one week versus two weeks of dietary NaCl restriction on severity of exercise‐induced bronchoconstriction. Journal of Exercise Physiology Online 2004;7:1‐7. [Google Scholar]
Javaid 1988 {published data only}
- Javaid A, Cushley MJ, Bone MF. Effect of dietary salt on bronchial reactivity to histamine in asthma. British Medical Journal 1988;297:454. [MEDLINE: ; UI: 89001930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Anderson 1994
- Anderson HR, Butland BK, Strachan DP. Trends in prevalence and severity of childhood asthma. BMJ 1994;308:1600‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATS 2000
- American Thoracic Society. Guidelines for Methacholine and Exercise Challenge Testing. American Journal of Respiratory and Critical Care Medicine 200;161:309‐29. [DOI] [PubMed] [Google Scholar]
Britton 1994
- Britton J, Pavord I, Richards K, Knox A, Wisniewski A, Weiss S, et al. Dietary sodium intake and the risk of airway hyperreactivity in a random adult population. Thorax 1994;49:875‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burney 1986
- Burney PGJ, Britton JR, Chinn S, Tattersfield AE, Platt HS, Papacosta AO, et al. Response to inhaled histamine and 24 hours sodium excretion. BMJ 1986;292:1483‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burney 1987
- Burney PGJ. The causes of asthma‐does salt potentiate bronchial reactivity?. Journal of the Royal Society of Medicine 1987;80:364‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burney 1987a
- Burney P. A diet rich in sodium may potentiate asthma. Chest 1987;91(6):S143‐8. [DOI] [PubMed] [Google Scholar]
Demissie 1996
- Demissie K, Ernst P, Gray Donald K, Joseph L. Usual dietary salt intake and asthma in children: a case‐control study. Thorax 1996;51:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devereux 1995
- Devereux G, Beach J R, Bromly C, Avery A J, Taghi Ayatollahi SM, Williams SM, et al. Effect of dietary sodium on airways responsiveness and its importance in the epidemiology of asthma: an evaluation in three areas of northern England. Thorax 1995;50:941‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietary and Nutritional Survey 2004
- Hoare J, Henderson L, Bates CJ, Prentice A, Birch M, Swan G, et al. The National Diet & Nutrition Survey:adults aged 19 to 64 years Summary Report. Vol. 5, London: TSO, 2004. [Google Scholar]
Fogarty 2000
- Fogarty A, Britton J. The role of diet in the aetiology of asthma. Clinical and Experimental Allergy 2000;30(5):615‐27. [DOI] [PubMed] [Google Scholar]
Gleibermann 1973
- Gleibermann L. Blood pressure and dietary salt in human populations. Ecology of food and nutrition 1973;2:143‐56. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, Available from www.cochrane‐handbook.org, 2008. [Google Scholar]
Keeley 1991
- Keeley DJ, Neill P, Gallivan S. Comparison of the prevalence of reversible airways obstruction in rural and urban Zimbabwean children. Thorax 1991;46:549‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2001
- Lewis SA, Weiss ST, Britton JR. Airway responsiveness and peak flow variability in the diagnosis of asthma for epidemiological studies. European Respiratory Journal 2001;18:921‐7. [DOI] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 2004;59:469‐78. [DOI] [PubMed] [Google Scholar]
McKeever 2004
- McKeever TM, Britton J. Diet and asthma. American Journal of Respiratory and Critical Care Medicine 2004;170(7):725‐9. [DOI] [PubMed] [Google Scholar]
Mohamed 1995
- Mohamed N, Ng'ang'a L, Odhiambo J, Nyamwaya J, Menzies R. Home environment and asthma in Kenyan schoolchildren: a case‐control study. Thorax 1995;50:74‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 1974
- Page LB, Damon A, Moellering Jr RC. Antecedents of cardiovascular disease in six Solomon Islands societies. Circulation 1974;49:1132‐46. [DOI] [PubMed] [Google Scholar]
Peat 1994
- Peat JK, Berg RH, Green WF, Mellis CM, Leeder SR, Woolcock AJ. Changing prevalence of asthma in Australian children. BMJ 1994;308:1591‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pistelli 1993
- Pistelli R, Forastiere F, Corbo GM, Dell'Orco V, Ancato G, Abiti N, et al. Respiratory symptoms and bronchial responsiveness are related to dietary salt intake and urinary potassium excretion in male children. European Respiratory Journal 1993;6:517‐22. [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Sausenthaler 2005
- Sausenthaler S, Kompauer I, Brasche S, Linseisen J, Heinrich J. Sodium intake and bronchial hyperresponsiveness in adults. Respiratory Medicine 2005;99:864‐70. [DOI] [PubMed] [Google Scholar]
Schwartz 1990
- Schwartz J, Weiss S. Dietary factors and their relation to respiratory symptoms. The second national health and nutrition examination survey. American Journal of Epidemiology 1990;132(1):67‐76. [DOI] [PubMed] [Google Scholar]
Shaw 1990
- Shaw RA, Crane J, O'Donnell TV, Porteous LE, Coleman ED. Increasing asthma prevalence in a rural New Zealand adolescent population: 1975‐89. Archives of Disease in Childhood 1990;65:1319‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparrow 1991
- Sparrow D, O'Connor GT, Rosner B, Weiss S. Methacholine and airway responsiveness and 24‐hour urine excretion of sodium and potassium. American Review of Respiratory Disease 1991;144:722‐5. [DOI] [PubMed] [Google Scholar]
Tattersfield 2002
- Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet 2002;360:1313‐22. [DOI] [PubMed] [Google Scholar]
Tribe 1994
- Tribe RM, Barton JR, Poston L, Burney PGJ. Dietary sodium intake, airway responsiveness, and cellular sodium transport. American Journal of Respiratory and Critical Care Medicine 1994;149:1426‐33. [DOI] [PubMed] [Google Scholar]
Van Niekerk 1979
- Niekerk CH, Weinberg EG, Shore SC, Heese H, Schalkwyk DJ. Prevalence of asthma: a comparative study of urban and rural Xhosa children. Clinical Allergy 1979;9:319‐24. [DOI] [PubMed] [Google Scholar]
WHO, 2006
- WHO, 2006. Asthma‐Factsheet No. 307. WHO 2006.
Yemaneberhan 1997
- Yemaneberhan H, Bekele Z, Venn A, Lewis S, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet 1997;350:85‐90. [DOI] [PubMed] [Google Scholar]
Zoia 1995
- Zoia MC, Fanfulla F, Bruschi C, Basso O, Marco R, Cascali L, et al. Chronic respiratory symptoms, bronchial responsiveness and dietary sodium and potassium: a population‐based study. Monaldi Archives of Chest Disease 1995;50(2):104‐8. [PubMed] [Google Scholar]
References to other published versions of this review
Ardern 2004
- Ardern K. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000436.pub2] [DOI] [PubMed] [Google Scholar]
Ram 2001
- Ram FSF, Ardern KD. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000436] [DOI] [PubMed] [Google Scholar]


