Mucin O-glycans are conjugated carbohydrate chains that contain at the reducing terminus N-acetylgalactosamine linked covalently to serine/threonine in the peptide backbone. They are found in mucins and many nonmucin glycoproteins. The presence of a tandem repeat peptide heavily glycosylated with mucin O-glycans distinguishes mucins from other glycoproteins that contain mucin O-glycans. Muc14, −15, and −18, which do not contain a tandem repeat peptide, are the exceptions. To date, about six secreted and 14 membrane-tethered mucins have been reported based on cloned complementary DNA (cDNA) sequences [1,2]. The functions of mucin O-glycans vary according to where they reside. For secreted mucins, O-glycans can retain water, maintain the viscoelastic properties of mucus secretion, and bind and clear inhaled and ingested pathogens, such as mycoplasma [3,4], viruses [5], and bacteria [6]. This function depends primarily on heterogeneous carbohydrates, while the other two functions are determined by high carbohydrate content. High carbohydrate content and very heterogeneous carbohydrate structures found in secreted mucins enable them to perform the first line of innate immune defense at the epithelial surface of many mucus-secretory tissues, such as airways and the gastrointestinal tract. Under pathological conditions, overproduction of secreted mucins coupled with poor clearance of mucus causes obstructive lung diseases [7]. On the other hand, loss of secreted mucins as shown in Muc2-gene–knockout mice can result in the loss of mucus protective function and lead to the development of colitis [8] and colorectal cancer [9]. Mucin-type O-glycans in membrane-tethered mucins protect the epithelium by trapping water at the cell surface, transmitting signals from the extracellular environment to the cells, and serving as selectin ligands [10,11]. Expression or elevated expression of these mucins tends to be associated with malignancy, despite the fact that the significance is not fully understood [12]. The best characterized function of mucin-type glycans comes from membrane-bound nonmucin glycoproteins, including the P-selectin glycoprotein ligand (PSGL)-1 and peripheral node addressin. These glycoproteins contain sialyl Lewis X (sLex) or 6-sulfo-sLex located at the nonreducing termini of mucin O-glycans. These glycotopes can guide the migration of leukocytes to the site of injury and lymphoid organs [13]. These two processes depend primarily on interactions of sLeX, sLeX plus sulfated tyrosine, and sulfated sLeX with E-, P-, and L-selectins, respectively [14]. Such interactions may also play a key role in cancer metastasis [15]. The above-mentioned functions of mucin O-glycans are controlled by β6GlcNAc branch structures, which include core 2, Galβ1–3(GlcNAcβ1–6)GalNAcαSer/Thr; core 4, GlcNAcβ1–3(GlcNAcβ1–6)GalNAcαSer/Thr; and blood group I antigen, GlcNAcβ1–3(GlcNAcβ1–6)Gal. Core 2 is the branch structure present in membrane-tethered mucins and other nonmucin glycoproteins, while all three branch structures are found in secreted mucins. The differences in these mucin glycan branch structures contribute to the differences in function of these glycoproteins.
The enzyme activity responsible for the synthesis of the core-2 structure was first reported in 1980 [16]. The enzyme activity responsible for the core-4 structure was identified in 1985 [17] and for the blood group I structure in 1986 [18]. In 1991, our group employed a purified enzyme to demonstrate that this enzyme can make all three β6GlcNAc structures found in secreted mucins [19]. cDNA coding C2GnT-1/L was first cloned in 1992 [20], IGnT in 1993 [21], C2GnT-2/M in 1999 [22,23], and C2GnT-3/T in 2000 [24]. Enzymatic characterization of the recombinant protein of C2GnT-M [22,23] has confirmed its multiple substrate specificity as previously reported [19]. These β6GlcNAc transferases contain nine conserved cysteines [20–24]. Recently, the disulfide bond distribution in C2GnT-L [25] and C2GnT-M [26] has been reported. Also, the genomic structures of the IGnT [27], C2GnT-L [28], and C2GnT-M [29] genes have been elucidated. Furthermore, the 3–D structure of C2GnT-L has been determined [30]. Since many biological functions of mucins reside in the carbohydrates extended from the β6GlcNAc branch structures, alterations of the activities of these enzymes and their gene expressions can have a profound effect on mucin functions. The current review is an update of an article published previously [34].
Role of β6GlcNAc Transferases in Mucin O-Glycan Biosynthesis
The three mucin O-glycan β6GlcNAc structures [34,35], including core 2, core 4, and I antigen, are shown in Fig. 1. Mucin O-glycan synthesis is initiated by the formation of the GalNAcαSer/Thr structure as catalyzed by peptidyl GalNAc transferases (ppGalNAc-T) [36]. At least 20 ppGalNAc-Ts have been identified to date. These enzymes can be grouped into three categories based on acceptor specificities: one works on naked peptides, one works on partially glycosylated peptides, and one works on both types of acceptors [37–39]. To extend the glycan structure, GalNAc can be decorated with either β3Gal or β3GlcNAc to form core-1 and core-3 structures, respectively. Each of these two enzymes is encoded by a single gene. Core-1 (or T) synthase requires a chaperone protein for its function [40]. Loss of this chaperone protein can result in the loss of core-1 synthase activity [40]. The core-1 structure can be extended by either β3GlcNAcT-3 or C2GnTs, which produce linear or β1–6 branch structures, respectively. There are three types of C2GnTs: C2GnT-1/L, C2GnT-2/M, and C2GnT-3/T. These enzymes can catalyze the transfer of GlcNAc from UDP-GlcNAc to GalNAc in the core-1 acceptor. The core-1 structure (or T antigen) is required for C2GnT activity. To generate L-selectin ligand, C-6 of GlcNAc is sulfated by ST6GlcNAcT-1/2 [41,42] before galactosylation by β4GalT [43], as shown in Fig. 2. To generate P- and E-selectin ligands, GlcNAc on core 2 and extended core 1 is galactosylated without sulfation. If a polylactosamine structure is to be generated, the terminal β4Gal will be extended by N-acetyllactosamine unit(s), as catalyzed sequentially by β3GlcNAcT-3 [44] and β4GalT. To generate an sLex structure, the terminal Gal will be further decorated with α2–3 sialic acid as catalyzed by ST3Gal-III, -IV, or -VI [45] followed by fucosylation as catalyzed by FUT-III, -IV, or -VII [45].
Fig. 1.
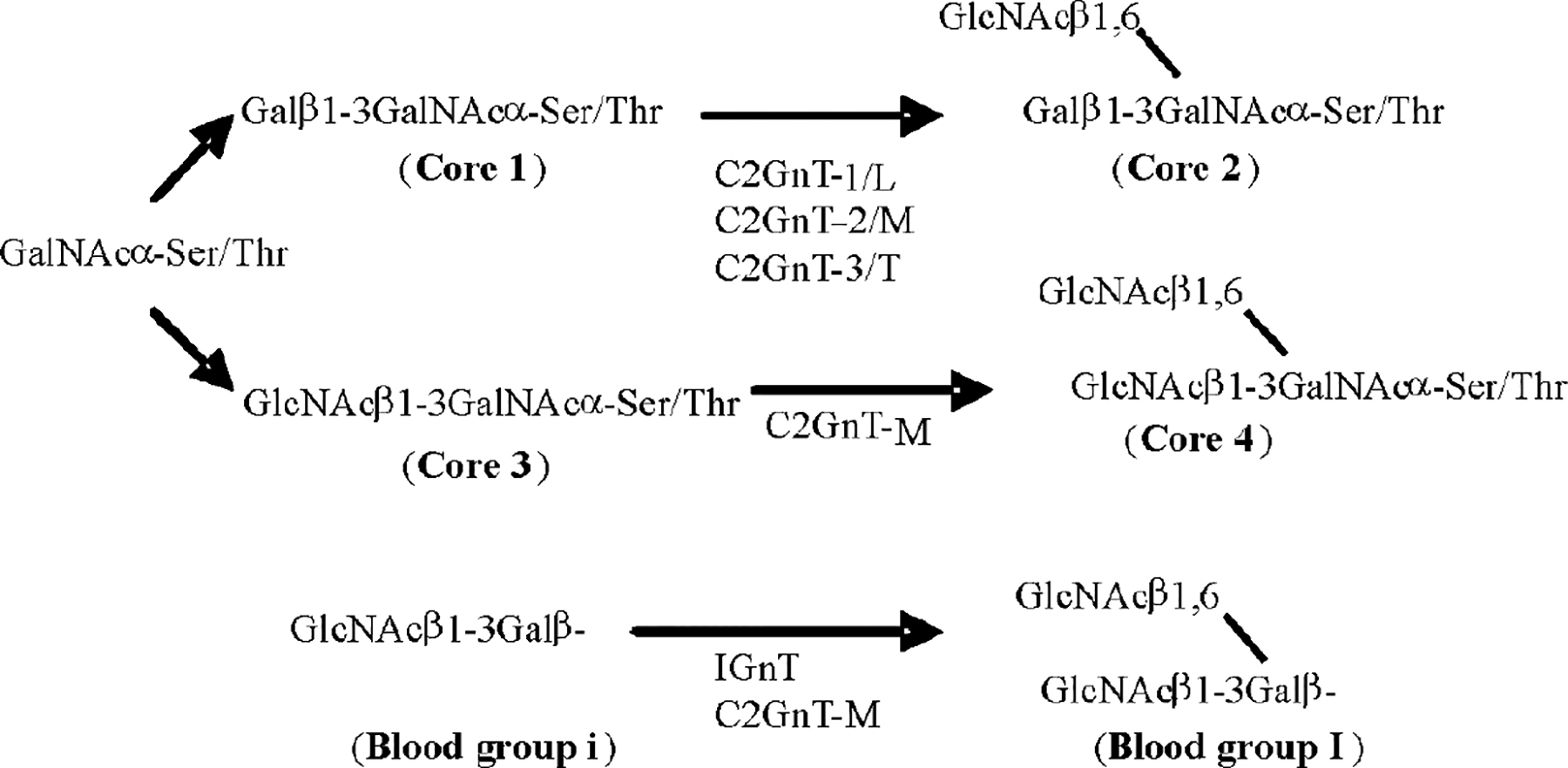
Biosynthesis of mucin O-glycan core 2, core 4, and blood group I antigen as catalyzed by β6GlcNAc transferases. Core 2 can be synthesized by C2GnT-1/L, C2GnT-2/M, and C2GnT-3/T; core 4 can be synthesized only by C2GnT-M; blood group I antigen can be synthesized by IGnT and C2GnT-M.
Fig. 2.
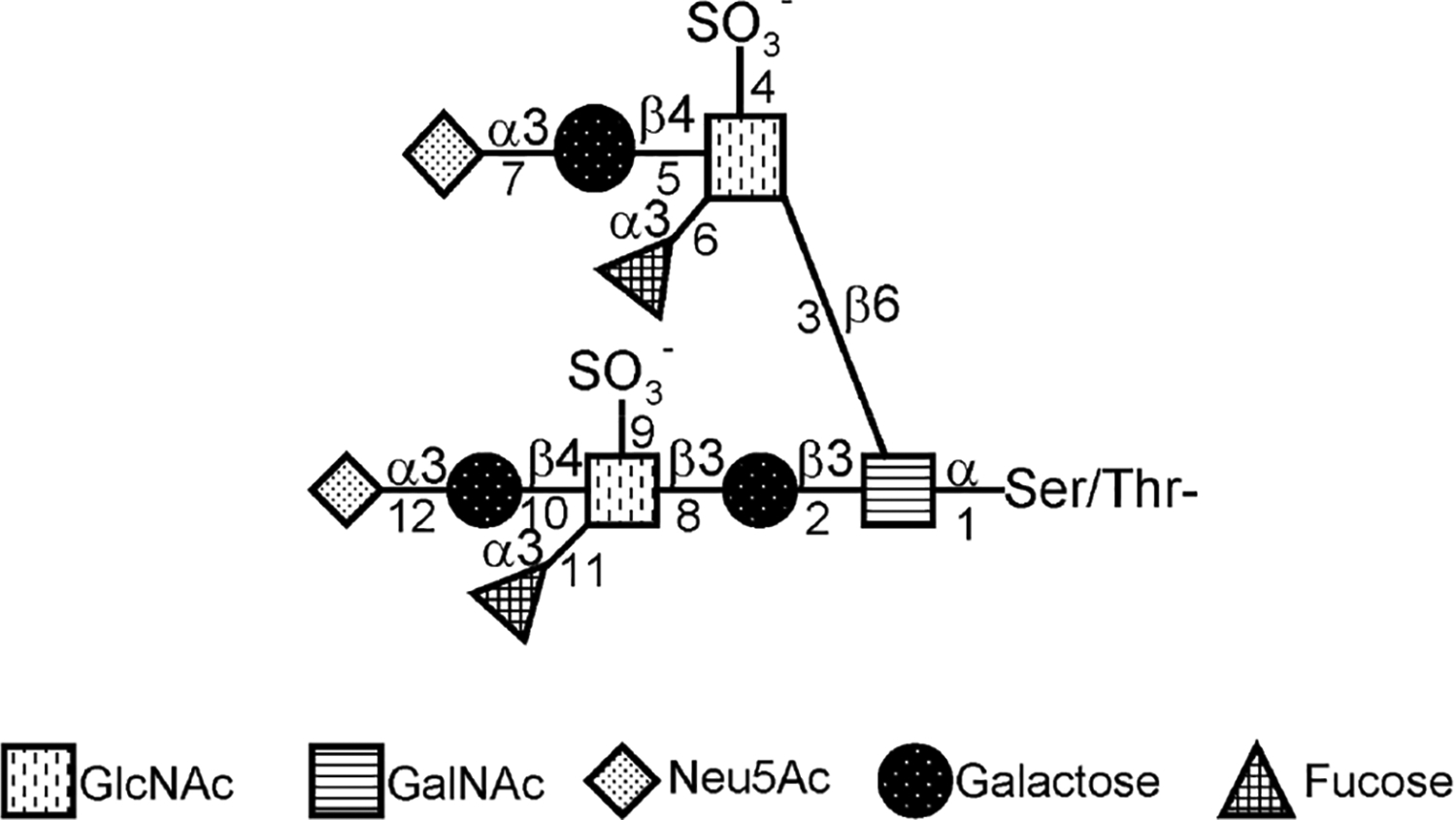
Structure and biosynthesis of core-1– and core-2–associated sLex and key enzymes involved in the synthesis. 1, ppGalNAcT; 2, β3Gal-T1 (T synthase); 3, C2GnT-1/2/3; 4 and 9, GlcNAc6ST1/3; 5, β4GalT-4; 6 and 11, FUT-III/IV/VII; 7 and 12, ST3Gal-III/VI; 8, β3GlcNAcT-3; and 10, β4GalT
Core 4 is the second β6GlcNAc branch structure found in secreted mucins. It is synthesized by C2GnT-M after the core-3 structure has been generated by core-3 synthase [46]. A core-3 structure is required for C4GnT activity. There is only one gene that can produce core-3 synthase. Further, C2GnT-M is the only enzyme that exhibits C4GnT activity. Core-3 and core-4 structures can be further extended by galactosyltransferases, N-acetylglucosaminyltransferases, sialyltransferases, and fucosyltransferases to generate biologically active glycotopes, such as sLex, as described above.
The blood group I antigen is the third β6GlcNAc branch structure found in polylactosamine chains of the glycans in secreted mucins and N-linked glycans. The structures can be generated by two different enzymes, IGnT [21] and C2GnT-M [22,23], which act centrally and distally, respectively. C2GnT-M transfers GlcNAc from UDP-GlcNAc to Gal in GlcNAcβ1–3Gal, while IGnT transfers GlcNAc to Gal at the reducing end of Galβ1–3/4GlcNAcβ1–3Gal.
It is clear that carbohydrate content and complexity of mucin O-glycan structures are controlled to a large extent by these mucin O-glycan branching enzymes. Therefore, modulation of the activities of these branching enzymes is crucial for regulating mucin functions. The next two sections will focus on the protein and genomic structures of these mucin O-glycan branching enzymes.
Protein Structures of β6GlcNAc Transferases
As described above, mucin core-2 structure can be synthesized by three different β6GlcNAc transferases; the core-4 structure by C2GnT-M; and I antigen by two different β6GlcNAc transferases. cDNAs of these β6GlcNAc transferases have been cloned from several different species, including bovine, bovine herpesvirus, dog, chimpanzee, human, mouse, rat, and zebra fish.
The amino acid sequences deduced from these cDNAs show that these branching enzymes are single-pass type II membrane proteins. They are similar in size (399 to 454 amino acids) and exhibit domain structures typical of most glycosyltransferases. They contain a short cytoplasmic tail at the N-terminus followed by a short transmembrane region, a short stem, and a long catalytic domain. The transmembrane domain plus cytoplasmic tail is the primary signal for targeting C2GnT-L to the cis-medial Golgi compartment [47].
The alignment of the amino acid sequences deduced from the cDNAs of these β6GlcNAc branching enzymes shows several distinct features (Fig. 3). First, all β6GlcNAc transferases contain nine conserved cysteines located beyond the stem region. C2GnT-T contains an additional cysteine located at the cytoplasmic tail. C2GnT-M contains four additional cysteines located at the N-terminal region, including the cytoplasmic tail, transmembrane region, and stem. These four cysteines are not required for enzyme activity. The designation of the various regions of these branching enzymes, such as cytoplasmic tail, transmembrane domain, stem, and catalytic domain, is to indicate the general domain structures. The exact lengths of the cytoplasmic tail and the transmembrane domain of each branching enzyme as predicted by different software may vary and need to be determined individually because cDNA devoid of this region still retain all three activities [26]. The function of these four cysteines is not known, although they may be involved in disulfide bond formation to help retain C2GnT-M in specific Golgi compartments according to the oligomerization/kin recognition model of the Golgi retention theory [48]. Second, all β6GlcNAc transferases, except h- and mIGnT, contain one conserved N-glycosylation site: b- and bhvC2GnT-M (N-72); dogC2GnT-M (N-71); c-, h-, m-, and rC2GnT-M (N-69); c- and hC2GnT-T (N-72); dogC2GnT-T (N-73); dC2GnT-T (N-59); and all C2GnT-L (N-58). The corresponding N-glycosylation site for h- and mIGnT is located at N-37 instead. While there is no additional N-glycosylation site for zebra fish C2GnT-T, the number of additional N-glycosylation sites found in other β6GlcNAc transferases ranges from one to four. The locations of these N-glycosylation sites are b- and bhvC2GnT-M (N-108); dogC2GnT-M (N-107); c- and hC2GnT-M (N-289); m- and rC2GnT-M (N-288); c- and hC2GnT-T (N-286, N-317, and N-448); dog- (N-318) and mC2GnT-T (N-318 and N-381); hIGnT (N-212, N-255, N-314, and N-388); mIGnT (N-212, N-255, N-315, and N-389); and all C2GnT-L (N-95). Third, amino acid sequence identity analysis of these β6GlcNAc transferases reveals that human is closest to chimpanzee, mouse to rat, and bovine to bovine herpesvirus (Fig. 4).
Fig. 3.
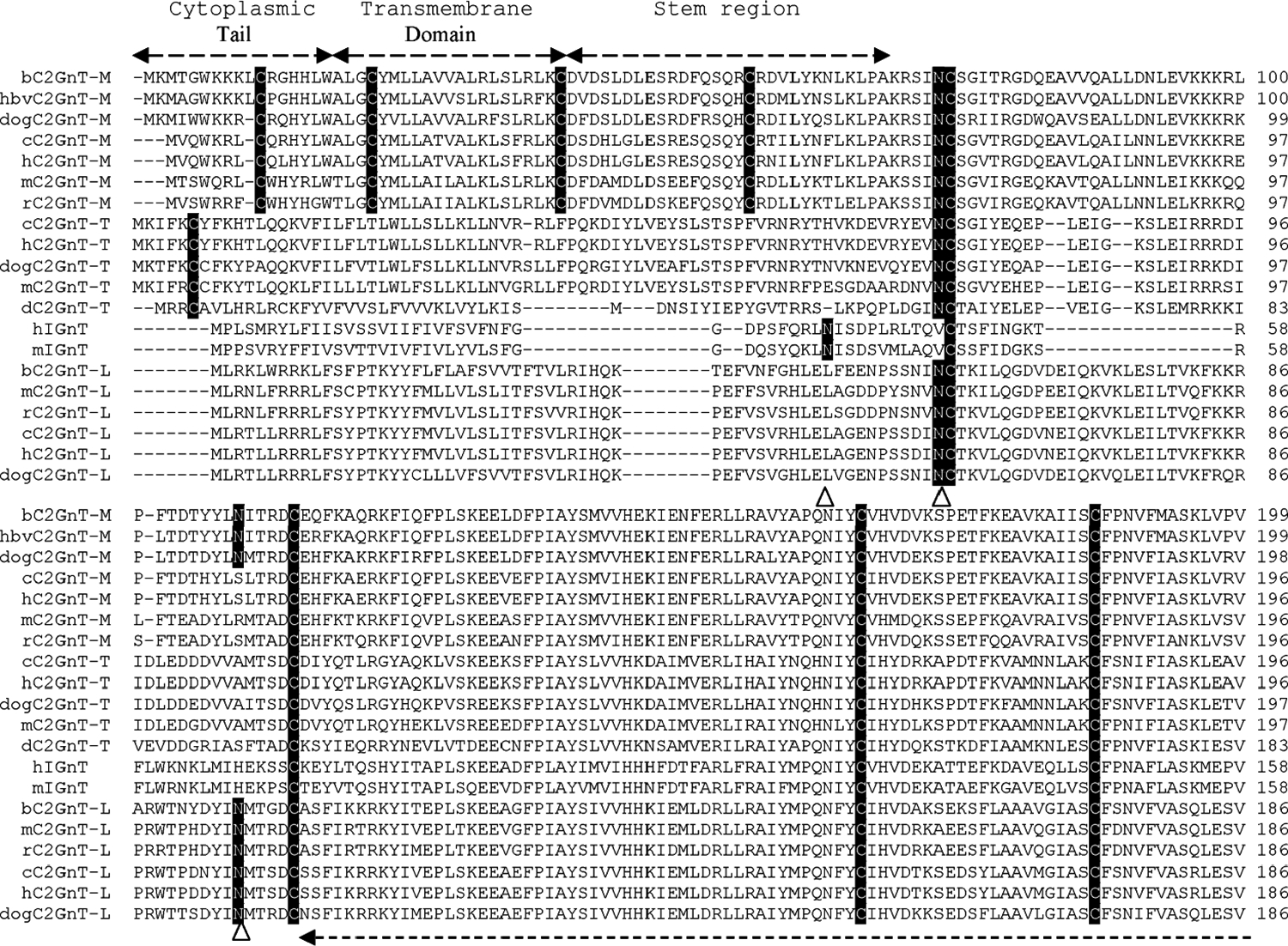
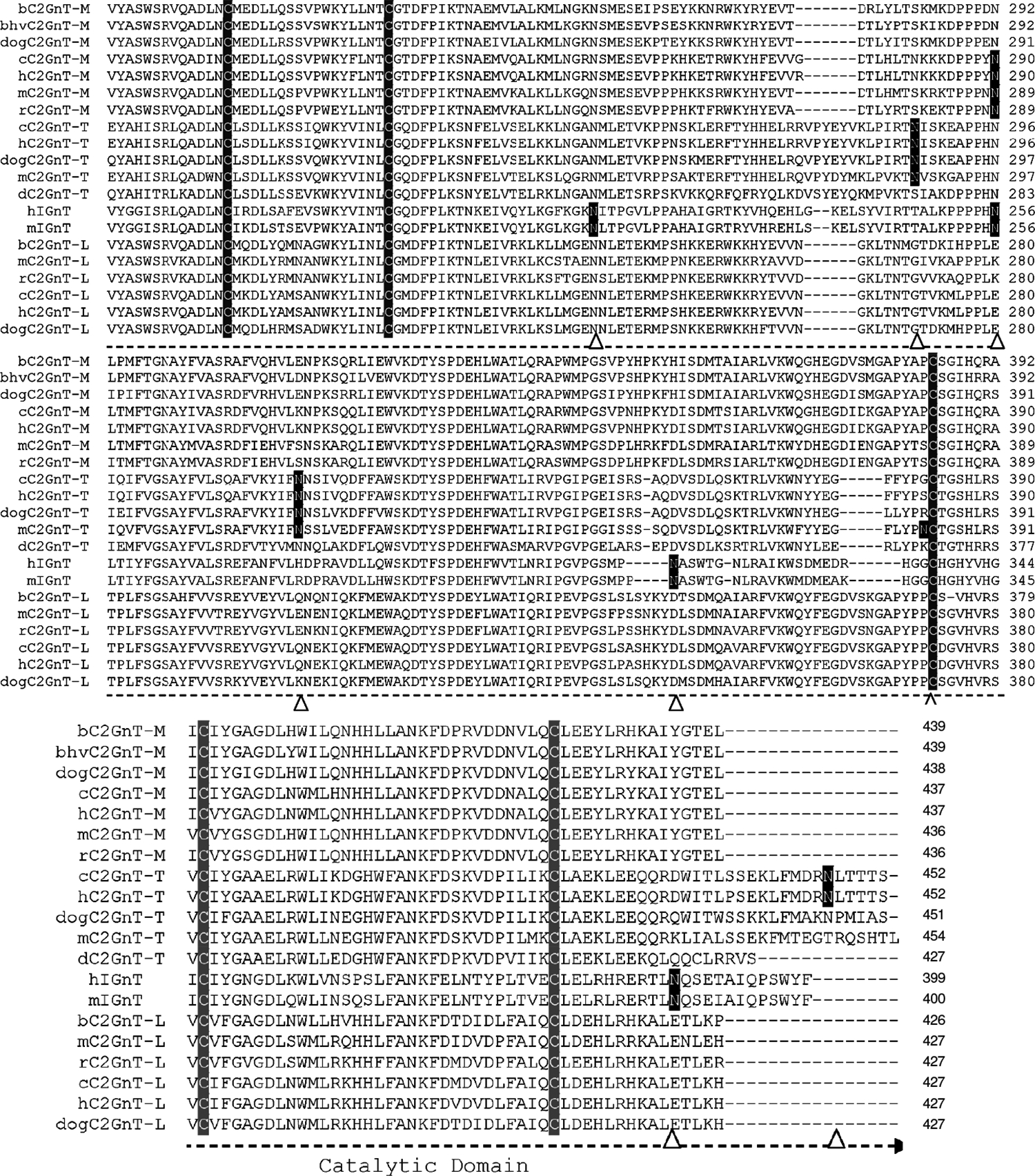
Alignment of deduced amino acid sequences of four β6GlcNAc transferases from human (Homo sapiens) (h), chimpanzee (Pan troglodytes) (c), rat (Rattus norvegicus) (r), mouse (Mus musculus) (m), bovine (Bos taurus) (b), bovine herpesvirus (bhv), dog (Canis familiaris) (dog), and zebra fish (Danio rerio) (d). Multiple sequence alignment was performed with Invitrogen Vector NTI 10 software. The amino acid sequences of dog C2GnT-M and C2GnT-T, and mC2GnT-T used for alignment, include only those that are comparable to those of other β6GlcNAc transferases. The N-terminal amino acid sequences that are excluded are 96 amino acid residues of dog C2GnT-M, 137 residues of dog C2GnT-T, and 133 residues of mC2GnT-T. The N-glycosylation sites (N) are highlighted dark, and the nine cysteines conserved among all β6GlcNAc transferases, the four cysteines conserved among C2GnT-Ms, and the one cysteine conserved among C2GnT-Ts are highlighted light. The potential N-glycosyltaion sites in each sequence are indicated by arrow heads. The accession numbers for these proteins are C2GnT-2/M: bovine, NP_991378.1; bovine herpesvirus, Q80RC7; dog, XP_544703; chimpanzee, XP_510451.2; human, NP_004742.1; mouse, NP_082363.2; rat, NP_775434.1. C2GnT-3/T: chimpanzee, XP_517702.2; human, NP_057675.1; dog, XP_546063.2; mouse, XP_980153.1; zebra fish, NP_963877.1. IGnT: human, Q06430–1; mouse, NP_032131. C2GnT-1/L: bovine, NP_803476.1; mouse, NP_034395.1; rat, NP_071612.1; chimpanzee, XP_001145936.1; human, NP_001091103.1; dog, XP_541274.2. The accession numbers of the corresponding cDNAs are C2GnT-2/M: bovine, NM_205809.1; bovine herpesvirus, NC_002665; dog, XM_544703; chimpanzee, XM_510451.2; human, NM_004751.1; mouse, NM_028087.2; rat, NM_173312.1. C2GnT-3/T: human, NM_016591.1; chimpanzee, XM_517702.2; dog, XM_546063.2; mouse, XM_975059.1; zebra fish, NM_201583.1. IGnT: human, Z19550; mouse, NM_008105. C2GnT-1/L: bovine, NM_177510.2; mouse, NM_010265.2; rat, NM_022276.1; chimpanzee, XM_001145936.1; human, NM_001097634.1; dog, XM_541274.2
Fig. 4.
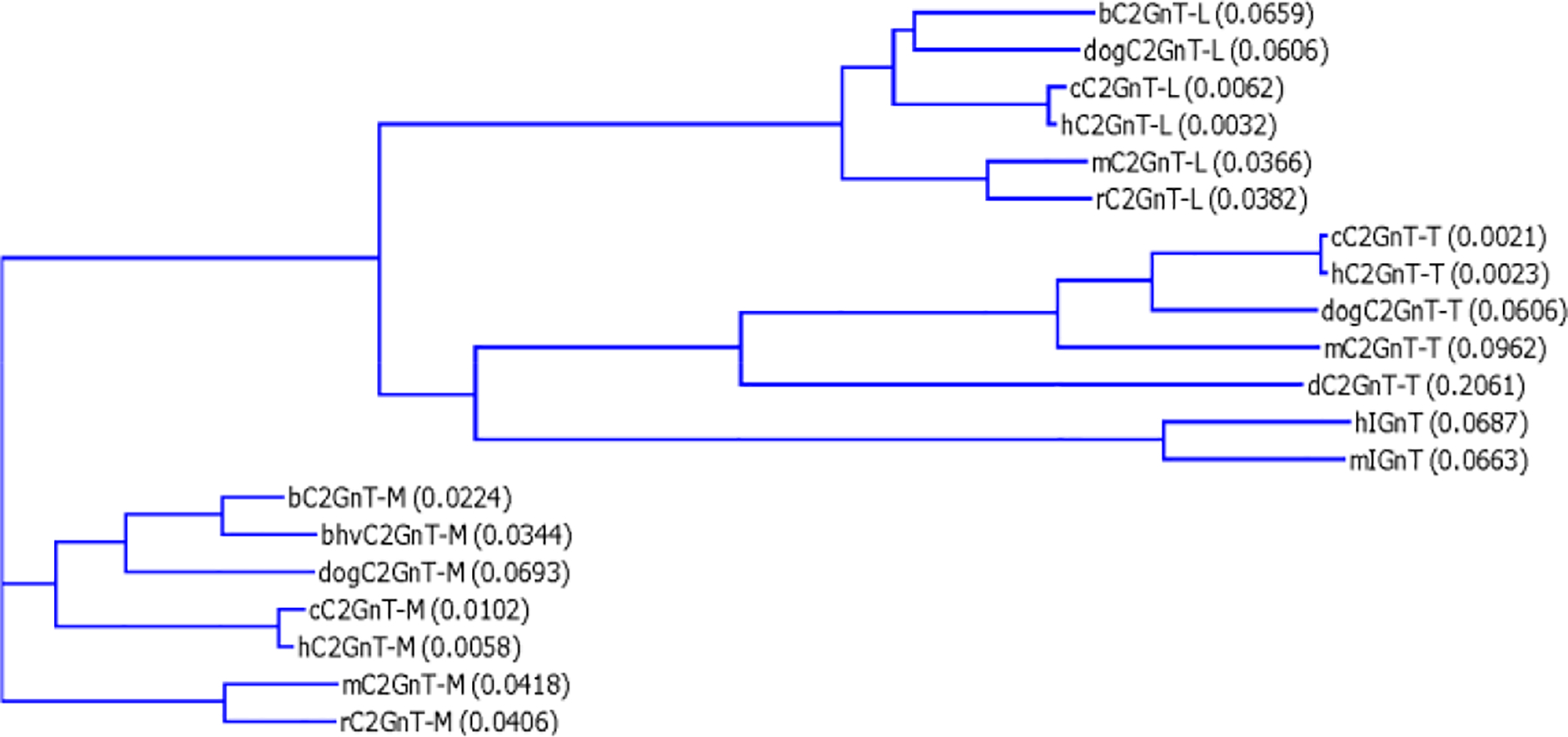
Cladogram of four β6GlcNAc transferases from different species. Amino acid sequences of the same isozyme are closest between human and chimpanzee, mouse and rat, and bovine and bovine herpesvirus. Multiple sequence alignment was performed with European Molecular Biology Laboratory–European Bioinformatics Institute (EMBL–EBI) clustalW. The number in parenthesis represents the degree of divergence in a pair of sequences.
The pattern of distribution of the disulfide bonds derived from these nine cysteines conserved among all β6GlcNAc transferases has been elucidated for mC2GnT-L [25] and bC2GnT-M [26]. For both isozymes, eight cysteines are engaged in the formation of disulfide bonds, although different cysteines are involved. In mC2GnT-L [25], the sulfhydryl group of the sixth cysteine (Cys217) is not conjugated, and the cysteine pairs involved in the formation of disulfide bonds are first and ninth (Cys59-Cys413), second and fourth (Cys100-Cys172), third and fifth (Cys151-Cys199), and seventh and eighth (Cys372-Cys381). Enzyme activity is retained when Cys217 is at the reduced state [25]. In addition to these nine conserved cysteines, mC2GnT-L contains an extra cysteine (Cys235), which is responsible for the formation of a dimer through formation of an intermolecular disulfide bond. In the case of bC2GnT-M, the sulfhydryl group of the second conserved cysteine (Cys113) is not conjugated. The disulfide bonds are formed between the following cysteine pairs: first and ninth (Cys73-Cys425), third and seventh (Cys164-Cys381), fourth and fifth (Cys185-Cys212), and sixth and eighth (Cys230-Cys393). Except for the disulfide bond formed between the first and ninth cysteines, bC2GnT-M and mC2GnT-L do not share the same pattern of distribution of free cysteine and disulfide bonds among the remaining seven conserved cysteines, which may contribute to the difference in substrate specificity between these two enzymes [26].
Recently, Pak et al. elucidated the 3–D structure of mC2GnT-L by X-ray crystallography [30]. This enzyme exhibits a GT-A fold but lacks the characteristics of a metal ion-binding DXD motif. The amino acids involved in the binding to UDP-GlcNAc include Arg-378, Lys-401, Asp-155, Val-128, Val-354, Val-380, Glu-320, and Cys-217. Arg-378 and Lys-401, which are conserved among all GT-14 family members and take the place of a metal ion in all other GT-A structures, stabilize the β-phosphate of UDP-GlcNAc. The use of basic amino acid side chains in this way is strikingly similar to that observed in a number of metal ion-independent GT-B-fold glycosyltransferases and suggests a convergence of catalytic mechanisms shared by both GT-A- and GT-B-fold glycosyltransferases [30]. The existence of Lys-401 in the cis-peptide conformation suggests an important functional role. Asp-155 is an amino acid well-conserved among many GT-A-fold glycosyltransferases. It forms a key hydrogen bond with N-3 of the uracil moiety. The backbone carbonyl group accepts a hydrogen bond from O-3’ of the ribose moiety. Val-354 and Val-380 form an apolar pocket that interacts with the GlcNAc N-acetyl methyl group. Further, Glu-320 is closely aligned in the three–dimensional space in the catalytic site. It is positioned for an inline attack on the C-1 of GlcNAc in UDP-GlcNAc. It activates the C-6 hydroxyl group of GalNAc in the core-1 acceptor to make it more nucleophilic to attack C-1 of the donor substrate. Cys-217 is located in the UDP-GlcNAc binding pocket near the ribose moiety and involved in catalysis. C2GnT-L activity is activated by β-mercaptoethanol and dithiothreitol, indicating requirement of maintaining the sulfhydryl group in the reduced state for enzyme activity [31]. The enzyme activity is partially, i.e., 40%, retained when Cys-217 is converted to serine, indicating that the serine OH group can partially substitute the SH group in this cysteine [31]. Treatment of this enzyme with iodoacetamide, 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) or N-ethylmaleimide inactivates the enzyme by covalent blocking of the sulfhydryl group in Cys-217. The enzyme activity is protected from inactivation by iodoacetamide or DTNB in the presence of UDP-GlcNAc but not the disaccharide acceptor. The result supports the X-ray crystallography result that this cysteine is in close contact with UDP-GlcNAc and not the acceptor. The amino acids involved in binding to the disaccharide acceptor through formation of hydrogen bonds include Glu-320, Arg-254, Glu-243, Tyr-358, Lys-251, and Try-356. Glu-320, which is a critical amino acid for the catalytic activity of GT-A-fold glycosyltransferases [32,33] and conserved among all β6GlcNAc transferases, forms a bidentate with O-4 and O-6 of GalNAc by accepting hydrogen bonds from O-4 and the nucleophilic O-6. Arg-254 donates a hydrogen bond to O-4 of GalNAc in the acceptor. Glu-243 forms a bidentate with O-4 and O-6 of Gal by accepting hydrogen bonds from both oxygens. Tyr-358 bridges the two monosaccharides in the acceptor by simultaneously accepting a hydrogen bond from GalNAc NH and donating a hydrogen bond to Gal O-2. Lys-251 forms a hydrogen bond with the glycosidic oxygen of the acceptor disaccharide. The acceptor binding is further stabilized by a stacking interaction between Try-356 and both Gal and GalNAc moieties. It is of interest to note that the amino acid Y358, which was identified to be the amino acid involved in the binding of mC2GnT-L with core-1 disaccharide acceptor, was proposed to be unique to C2GnT-L because a different amino acid was found in the same location of both human (G458) and bovine (G460) C2GnT-M. Since tyrosine (Y460) instead of glycine is found at the same location in bhvC2GnT-M, tyrosine cannot be the amino acid unique to C2GnT-L. Therefore, the difference in this amino acid between mC2GnT-L and h-/bC2GnT-M cannot explain the difference in acceptor specificity between these two isozymes, suggesting that amino acids other than Y460 are involved in determining the multiacceptor specificity of C2GnT-M.
Genomic Organization of C2GnT Genes
To date, the complete genomic structures of C2GnT1/-L [49], C2GnT2/-M [50], and IGnT [21] and a partial genomic structure of C2GnT3/-T [32] have been reported (Fig. 5). It is worth noting that the open reading frame (ORF) of IGnT is distributed over three exons [23], while the entire ORF of the three C2GnT genes is located in a single exon [24,34]. As shown in Table 1, these four human β6GnT genes are located at different chromosomes. Since the hIGnT and mC2GnT-1/L gene structures have been reviewed previously [34], the current article will concentrate on the structures of the hC2GnT-1/L; b-, m-, and hC2GnT-2/M; and hC2GnT-3/T genes.
Fig. 5.
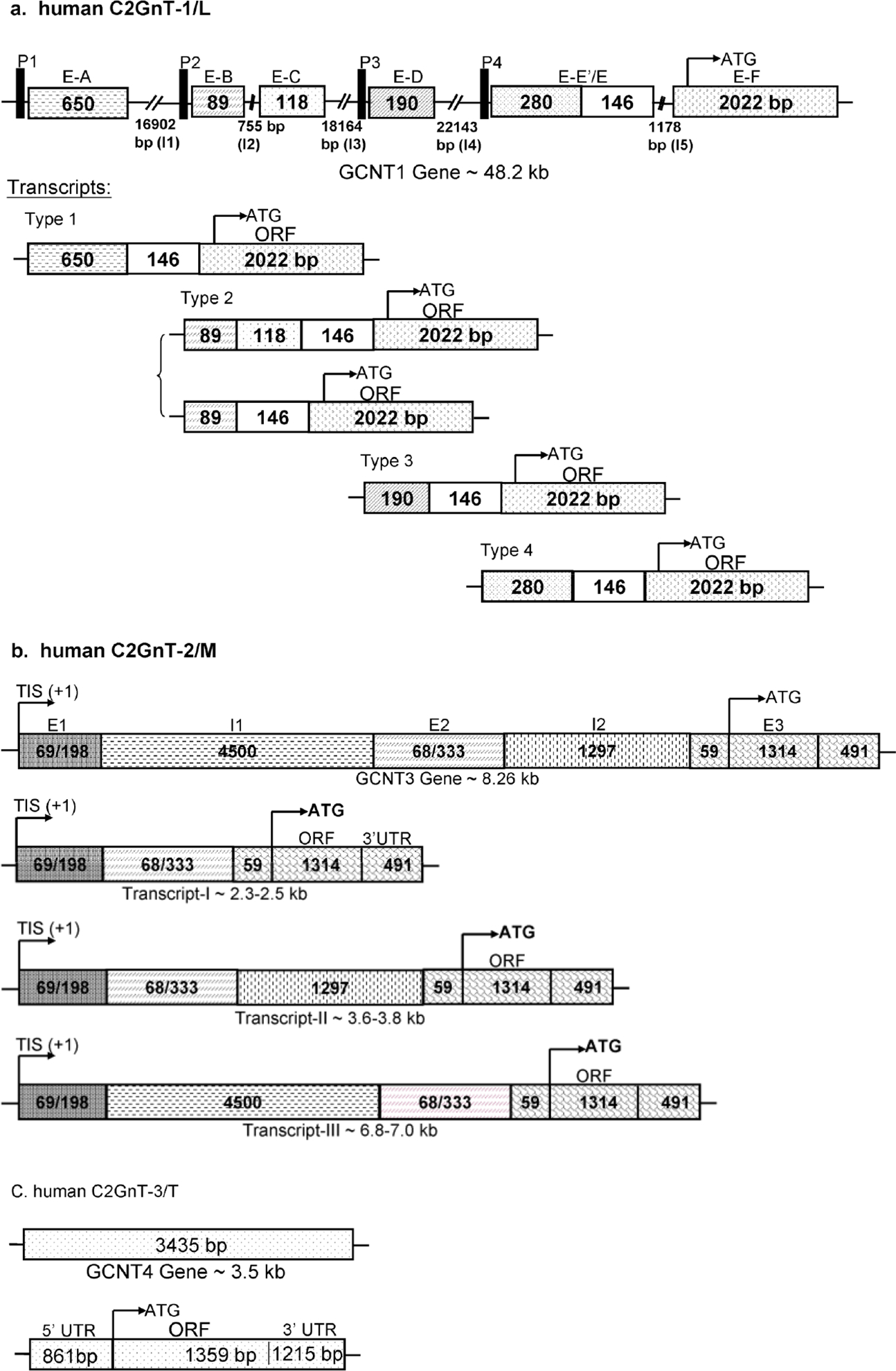
Genomic structures and expression of human (a) C2GnT-1/L (GCNT1), (b) C2GnT-2/M (GCNT3), and C2GnT-3/T (GCNT4) genes. ORF of all three C2GnT isozymes is located in one exon.
Table 1.
Chromosomal localization of human β6GlcNAc transferase genes and tissue distribution
| Enzyme | Chromosomal location | Tissue specificity |
|---|---|---|
| C2GnT-L | 9q13 | Ubiquitously expressed in all tissues and highly expressed in activated T lymphocytes and myeloid cells |
| C2GnT-M | 15q21.3 | Primarily expressed in mucus-secreting tissues, including . colon, testis, stomach, small intestine, kidney, trachea, adrenal gland, thyroid gland, uterus, ovary, and pancreas |
| C2GnT-T | 5q12 | Predominantly expressed in the thymus. Weakly expressed in pancreas, peripheral blood leukocytes, placenta, small intestine, and stomach. Barely detectable in liver, spleen, lung, and lymph node |
| IGnT | 9q21 | Erythroid cells, lymphocytes, monocytes, granulocytes, platelets, lens epithelium, and other tissues. Differential expression of specific transcripts in different tissues |
Mouse [51–53] and human [49] C2GnT-L genes contain six exons distributed over 60 and 48 Kb, respectively. Human C2GnT-L gene (48.2 kb) is made of six exons—A (650 bp), B (89 bp), C (118 bp), D (190 bp), E (426 bp), and F (2,022 bp)—and five introns—I1 (16,902 bp), I2 (755 bp), I3 (18,164 bp), I4 (22,143 bp), and I5 (1,178 bp) (Fig. 5a). Like the mC2GnT-L gene, the hC2GnT-L gene is expressed in a tissue-specific manner employing multiple transcription-initiation and alternative splicing mechanisms. The four promoters identified are located at the regions upstream of exons A, B, D, and E. The major promoter is promoter 2, which lacks a TATA box and is very GC rich. In addition, exons B, C, D, and part of exon E are alternately spaced out [63].
The structures of bovine [29], mouse [54], and human [50] C2GnT-M genes have recently been characterized. The C2GnT-M genes from these three species are made of three exons and two introns. The bovine gene exhibits a tissue-specific expression not observed in the other two species [50,54]. The human C2GnT-M gene is located on chromosome 15 in the region of q21.3, oriented from centromere to telomere. The human C2GnT-M gene (~8.26 kb) is made of 3 exons—E1 (69–198 bp), E2 (333–401 bp), and E3 (1,864 bp)—and 2 introns—I1 (4.5 kb) and I2 (1.3 kb). It produces three transcripts of 2.3–2.5 kb, 3.6–3.8 kb, and 6.8–7.0 kb (Fig. 5b). The shortest transcript (~2.3–2.5 kb) is made of three exons: 69–198 bp (exon 1); 333–401 bp (exon 2); and 1,864 bp (exon 3). This exon includes 56-bp 5’-UTR; 1,317-bp ORF; and 491-bp 3’-UTR. It does not contain any intron and is the most abundant transcript. The intermediate size transcript (3.6–3.8 kb) contains intron 2, and the longest transcript (6.8–7.0 kb) contains intron 1 in addition to all three exons. The bovine C2GnT-M gene (5.3 kb) is made of a 128-bp exon 1; 1,427-bp intron 1; 186-bp exon 2; 1,771-bp intron 2; and 1,796-bp exon 3. Exon 3 contains 51-bp 5’-UTR; 1,323-bp ORF; and 424-bp 3’-UTR. The smaller-size transcripts are the most abundant transcripts. There are two northern transcript bands, one at 2.1 kb and the other one at 3.7 kb. Each transcript band contains one transcript with and one transcript without exon 1. The transcripts that contain exon 1 are detected only in trachea and testis, indicating tissue-specific expression of these exon-1-containing transcripts. Mouse C2GnT-M gene (6.6 kb) is made of 116-bp exon 1; 998-bp intron 1; 342-bp exon 2; 1,294-bp intron 2; and 1,794/3,843-bp exon 3. The mC2GnT-M transcripts do not contain any intron, and the difference in the transcript size, i.e., 2.25 kb versus 4.3 kb, lies in a difference in the length of 3’-UTR of exon 3; the short transcript contains 561 bp while the long transcript has 2.6-kb 3’-UTR. In addition, both transcripts contain 54-bp 5’-UTR and 1,311-bp ORF.
The human C2GnT-T gene characterized to date is 3,435 kb. It contains 861-bp 5’-UTR; 1,359-bp ORF; and 1,215-bp 3’-UTR (Fig. 5c). The expression of this enzyme is highly restricted to the thymus tissue [24].
Human IGnT belongs to the β6GlcNAc transferase gene family and forms branched poly-N-acetyllactosamine from linear poly-N-acetyllactosamine [55]. This enzyme is mainly involved in red-cell differentiation and maturation during embryonic development. It has three transcripts: IGnTA, IGnTB, and IGnTC. The last transcript is mainly involved in red-cell development. Expression of IGnTC is regulated by transcription factor C/EBP alpha [56]. Also, a nonsense mutation in this gene is associated with autosomal recessive congenital cataracts in four distantly related Arab families from Israel [57]. During tumor development, this IGnT expression is upregulated. The structure and function of the IGnT enzyme have previously been described in detail [34].
Role of Human C2GnTs in Health and Disease
Core-2 branching is an important step in the biosynthesis of mucin O-glycans. Core-2–associated glycotopes, such as sLex, can serve as ligands for selectin- and galectin-mediated cell–cell interactions involved in T-cell development [34,58–64] and cancer metastasis [15,65–68]. The roles of C2GnT in cell development [58,59], differentiation [69–71], and diseases, such as Wiskott-Aldrich syndrome [72–78], acquired immune deficiency syndrome (AIDS), multiple sclerosis [79–83], and diabetes [81,72], have been reviewed previously [34]. Therefore, they will not be dealt with in the current review. Instead, we will focus on the role of C2GnT in immune functions, cancer progression, and the estrous cycle.
Role of C2GnT in Immune Function
Because many important functions of the immune cells reside in core-2–associated glycotopes, changes in C2GnT activity can greatly affect the functions of these cells. For example, a dramatic increase of C2GnT enzyme activity has been reported in activated T cells [84,85]. Stimulation of T lymphocytes with anti-CD3 antibodies or interleukin (IL)–2 increases the apparent size, as shown by decreased electrophoretic mobility, of CD43 expressed on the cell surface from 115 kDa to 130 kDa [86,87]. The mobility shift of CD43 is due to changes in the CD43 mucin O-glycan structures, which include conversion of these glycans from core-1– to core-2–based structures and decoration of core 2 with new glycotopes, such as sLex. This transition resulted from a tenfold increase of C2GnT enzyme activity after T-cell activation coupled with a moderate decrease of α2–3sialyltransferase activity. This enzyme competes with C2GnT for core 1. Since C2GnT-L is localized at cis-and medial-Golgi [88] and α2–3sialyltransferase is localized at medial- and trans-Golgi [88], increase of C2GnT-L activity ensures the synthesis of predominantly core-2–associated sLex, which helps recruit circulating neutrophils to the inflamed peritoneum [89]. α2–3Sialyltransferase I is highly expressed during Th2, but not Th1, differentiation [90]. Lack of expression of this glycosyltransferase in Th1 allows the formation of more core-2 glycans to help Th1 cells bind to selectins on the endothelial cells. Similarly, transfection of the EL-4 T-lymphoma cell line with C2GnT-L cDNA upregulates not only the highly glycosylated form of CD43 (130 kDa) but also the sizes of RPTPα, CD44, and CD45 by 3–5 kDa [91]. Since CD44 and CD45 along with CD43 are involved in cell–cell interactions, changes in their glycoforms after activation of T cells can have a significant impact on T-cell functions. Further, Kikuchi et al. [92] found that C2GnT-L is essential for differentiation of human precursor B cells.
The connection between C2GnT activity and polylactosamine came from the finding that mucin glycans from granulocytic cells [93] contain core-2–based polylactosamine structure. Such core-2–based polylactosamines along with sLex and sLea epitopes were detected in HL-60 cells, which expressed C2GnT, but not in K562 cells, which did not express C2GnT [94]. The polylactosamine chains are often terminated with sLea, sLex, and 6-sulfo sLex, which serve as selectin ligands. These selectin–ligand interactions are involved in directing leukocyte trafficking under inflamed conditions. P-selectin is found on the surface of activated endothelial cells and platelets, and E-selectin on the endothelial cells and L-selectin at the surface of leukocytes [95–98]. Circulating leukocytes also provide carbohydrate ligands for P- and E-selectins, and endothelial cells at the high endothelial venues provide sulfated carbohydrate ligands for L-selectin. Activated endothelial cells at the injured site mobilize prestored P-selectin to the cell surface to capture circulating leukocytes, which contain PSGL-1 [99], a molecule containing sulfated tyrosine and sLex on mucin the core-2 chain at the N-terminus. The synthesis of selectin ligands on PSGL-1 in CD4+ T cells is enhanced by IL–12, which upregulated C2GnT gene expression via the STAT4 pathway [100]. E-selectin recognizes sLex located at either mucin-type or N-linked glycan, while L-selectin recognizes sulfated sLex on mucin-type glycan [101,102]. These lectin–glycan interactions facilitate initial rolling of leukocytes on endothelial cells and subsequent firm binding prior to extravasation. In this case, endothelial cells at the high endothelial venues provide sulfated sLex located on the mucin core-2 branch of membrane-bound glycoproteins [103], such as GlyCAM-1, CD34, podocalyxin-like protein, sgp200, endomucin, and MAdCAM-1. These results strongly demonstrate the involvement of mucin-type core-2 glycans in leukocyte trafficking during inflammation. It is of interest to note that eosinophil and neutrophil, which express core-2 glycans, are mobilized to the peritoneum and not the lungs [104]. Dimerization of PSGL-1 coupled with formation of core-2 and associated glycans increases tethering rate under flow, while C2GnT-L levels influence tether bond strength [105]. Such selectin–ligand interactions were verified by binding assay between immobilized selectins and recombinant Chinese hamster ovary (CHO) cells that had expressed various combinations of PSGL-1, C2GnT, and α1–3fucosyltransferase, key players involved in the formation of PSGL-1–associated sLex, which is absent in wild-type CHO cells. P- and E-selectins were found to bind recombinant CHO cells that expressed PSGL-1 only when both C2GnT and α1–3fucosyltransferase were coexpressed [106,107]. Further, L-selectin bound to the CHO cells that coexpressed C2GnT-L, FUT-VII, and L-selectin ligand sulfotransferase [108]. Although core-2–associated glycan is the preferred ligand for L-selectin, L-selectin also can bind to similar structures located on the core-1 chain but with lesser efficiency [61]. In oral cavity carcinomas, both C2GnT and α1–3fucosyltransferase contribute to the formation of sLex and binding to E-selectin but not to other selectins [66]. These reports indicate that cell-specific expression of these two enzymes exhibits different specificity for different selectins. The L-selectin ligands also include sulfated core-2–based O-glycans, unlike the glycan ligands for P- and E-selectins. The selectin ligands on mucin O-glycans are contributed mainly by C2GnT-L and FUT-VII, although C2GnT-L may play a more significant role than FUT-VII [109]. This report is consistent with the finding that transfection of antisense oligonucleotides against C2GnT-L suppresses the formation of selectin ligands [110].
C2GnT activity is also implicated in T-cell maturation [58]. The immature thymocytes express higher level of core-2–based O-glycans than matured thymocytes. Increased core-2–associated glycan is correlated with increased binding of galectin-1 containing epithelial cells to T cells. Galectin 1 is known to induce cell death in immature thymocytes and activated T cells by binding to the cell surface glycoproteins CD45, CD43, and CD7. Cells that lack C2GnT are resistant to galectin-1–induced cell death [111]. This concept has been confirmed by Nguyen et al. [112], who showed that transfection of CD45+ BW5147 T cells with C2GnT cDNA rendered these cells susceptible to galectin-1–induced cell death. The results suggest that core-2–based O-glycans play an important role in T-cell development. Further, this concept was supported by decreased core-2 O-glycans (130 kDa) on CD43 in thymic-positive selection. These results clearly demonstrate the biological significance of C2GnT in immune system development.
Mucin core-2 structure can be generated by three isozymes: C2GnT1, C2GnT2, and C2GnT3. C2GnT1 and C2GnT3 are involved in the synthesis of core 2 found in the mucin-type glycans of membrane-bound glycoproteins, while C2GnT3 is involved in the synthesis of core 2 found in secreted mucins. C2GnT1–deficient mice exhibited compromised functions of not only innate but also acquired immunities [63]. C2GnT3–deficient mice did not show any noticeable abnormal phenotype in thymus or immune functions, although a slight neutrophilia was observed. However, it is rather intriguing that these mice exhibited a significant increase in social dominance behavior, which was linked to a reduced level of circulating thyroxin T4 [129]. C2GnT2–deficient mice displayed reduced levels of immunoglobulins, including IgG1, IgG2a, IgG2b, and mucosal IgA. Although the mechanism for this phenomenon is not clear, it may explain the increased susceptibility of these animals to developing colitis [129] and possibly colorectal cancer [126]. Further, mice deficient in multiple combinations of these three C2GnTs did not show any apparent abnormalities in viability, development, and fertility [129].
Role of Mucin Glycan Branch Structure in Cancer Development, Progression, and Metastasis
It has been well documented that the development and progression of cancer are accompanied by alterations in glycoconjugates. However, the significance of these changes has only begun to emerge recently. It has been noted that most of the reported tumor-associated antigens belong to mucin-type glycans. Since the functions of mucin-type glycans vary according to where they reside, i.e., membrane-bound glycoproteins or secreted mucins, the functions of these two tumor-associated mucin-type glycans will be reviewed accordingly.
Membrane-associated mucin-type O-glycans
Generation of core 2 and its associated glycotopes on membrane-bound glycoproteins in advanced cancer can alter tumor-associated antigens and help promote tumor metastasis. We previously showed that the tandem repeat peptide of MUC1 ectopically expressed in pancreatic cancer (Panc 1) cells was easily detected with an antibody specific for this peptide [67]. However, this peptide epitope was no longer recognized by the same antibody after forced expression of C2GnT-L cDNA [67]. Removal of sialic acid did not uncover the antigen, thus excluding the role of sialic acid in masking the antigen. In addition, ectopic expression of the core-2 branch generated sLex on the core-2 branch at the expense of sialyl-T antigen, sialic acidα2–3Galβ1–3GalNAcαSer/Thr, in this cell. This example illustrates how mucin glycan branching enzyme can alter tumor-associated antigens and enhance tumorigenicity.
As described above, sLex is a key glycotope that can help target circulating leukocytes to distant sites, including lymphoid tissues [15]. Expression of sLex in advanced cancer is a common finding, which could provide a rational explanation for how these tumors metastasize [113,114]. Several studies have confirmed the involvement of mucin-associated sLea and sLex glycotopes [115,116] in lymphatic and venous invasion [15]. In mucin-type glycans, the core-2 branch is frequently decorated with sLex and sLea [117] (Fig. 2). Core-2 branches can be synthesized by C2GnTs, and expression of C2GnT directly correlates with the formation of those epitopes on mucin O-glycan. A recent study shows enhanced adhesion and aggressive tumor formation [118] after transfection of human prostate cancer cells (LNCaP) with C2GnT-L cDNA. Therefore, C2GnT-L can be considered as an oncogene and serves as a progression marker for prostate cancer. Expression of C2GnT-L also correlates with tumor progression of other types of cancers, including colorectal and lung [30,65,119]. These observations suggest that the expressions of C2GnT and resultant core-2–associated glycans play an important role in the progression of numerous tumors. Changes in C2GnT activity have also been documented in cells from leukemia patients, in particular, leukocytes of two patients, one with chronic myelogenous leukemia and one with acute myelogenous leukemia. A four- and 18-fold increase of C2GnT activity was found in the leukocytes of these two patients, respectively, as compared to normal donors [65]. Similar to what was described above (D-1), expression of the core-2 branch can be regulated by relative expression of C2GnT-L and α2–3sialyltransferase I genes. As compared to normal breast tissues, breast cancer cells have reduced C2GnT-L gene expression by up to 50% but increased expression of α2–3sialyltransferase I gene, which culminates in the production of sialyl T antigen [120].
The involvement of P-, E-, and L-selectins in promoting cancer metastasis also has been demonstrated. P-selectin deficiency attenuates tumor growth and metastasis. Tumors are significantly smaller in size when mice are treated with receptor antagonists [121]. Lung colonization of B-16 melanoma cells that express sLex is significantly reduced in E- and P-selectin–deficient mice [121]. Furthermore, expression of L-selectin on tumor cells can enhance cancer metastasis to lymph nodes [122]. It is clear that there is a strong link between selectin–ligand interactions in blood-borne metastasis of various cancers. Figure 6 shows how the selectin–ligand interactions help promote cancer metastasis [121].
Fig. 6.
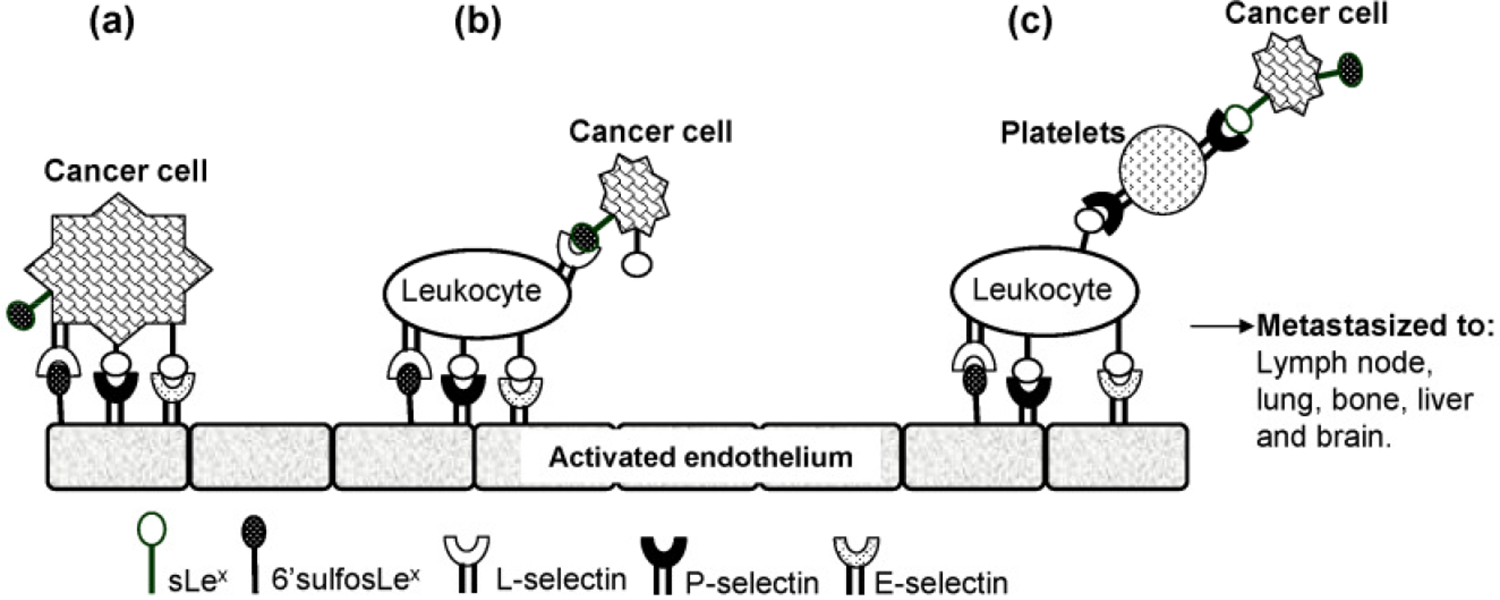
Proposed mechanisms of cancer metastasis. Following detachment from the primary site, the cancer cells in circulation utilize three possible routes to metastasize: (a) direct binding to selectins on activated endothelium at distant sites using sLex and/or selectin expressed on the surface of cancer cells; (b) enlisting the help of circulating leukocytes via interaction of sLex with L-selectin present on the surface of leukocytes; (c) utilizing the help of platelets and leukocytes [121].
It is important to point out that upregulation of C2GnT activity is not always associated with poor prognosis of cancer. In certain cancers, such as cutaneous T-cell lymphoma, downregulation/deletion of C2GnT-L renders these cells resistant to galectin-1–induced cell death [123]. Although upregulation of C2GnT-L is associated with poor prognosis for a large number of cancers, downregulation of C2GnT-L can benefit the progression of some types of cancer. Therefore, understanding the function of core 2–associated glycans in each cancer is the key to understanding the mechanism of tumorigenesis.
Branch structures in secreted mucins
As described above, the primary function of secreted mucins is to protect the underlying mucus-secretory tissues. Some of these tissues are constantly exposed to very harsh conditions, such as noxious gas in the lungs from polluted air, strong acid in the stomach, proteases throughout the gastrointestinal tract, and heavy loads of microorganisms in the colon. To protect the peptide backbones of these mucins from being degraded, the tandem repeat peptides rich in ser and thr are decorated with clusters of mucin-type O-glycans. To accomplish this goal, secreted mucins are equipped with high carbohydrate content, which can be up to 90% of the molecule by weight, and an extremely heterogeneous carbohydrate structure, which can be >300–400 oligosaccharide structures [124]. In addition, secreted mucins contain core 3 and core 4, which are unique to these mucins. As described above, core 3 is synthesized by core-3 synthase and core 4 by C2GnT-M. Downregulation of core-3 synthase or the C2GnT-M enzyme has been reported in colorectal cancers [125,126]. Loss of core-3 or core-4 structure can lead to loss of integrity in these mucins, resulting in prolonged residency of bacteria, irritation of the epithelium, induction of chronic inflammation, and development of pathological conditions, such as colitis and cancer. This condition is similar to that found in Muc2 (−/−) mice. In this case, loss of secreted mucins resulted in loss of mucus protection, which led to the development of colorectal cancer [9] and colitis [8].
Core 3 is the obligatory precursor for core 4 [24,127] in a reaction catalyzed by C2GnT-M, the only enzyme that can synthesize core 4 [22,23]. Therefore, loss of the core-3 enzyme would result in the loss of not only core 3 but also core 4. Interestingly, when core-3 synthase cDNA was introduced to a colonic cancer cell line devoid of core-3 synthase gene, in vitro migration and in vivo lung metastasis were suppressed in an athymic mouse tumor xenograft model [125]. This result was confirmed in core-3-synthase-gene knockout mice [128]. In this mouse model, both core-3 and core-4 O-glycans in secreted mucins were missing, which resulted in increased susceptibility to development of colitis and colorectal tumors [128]. This report also showed that mice devoid of this gene had increased degradation of Muc2, intestinal permeability, and retention of bacteria. Further, this animal showed increased colonic infiltration of T lymphocytes and monocytes/macrophages after dextran sulfate sodium challenge. These results show that animals devoid of the core-3 synthase gene lose the integrity of colorectal epithelium, as well as the ability to efficiently clear pathogens, and have increased inflammation and incidence of colorectal cancer. Since core 3 is the precursor of core 4, these results suggest that core-3– and core-4–derived glycans are important for the epithelium-protective function of secreted mucins.
Loss of all three mucin glycan branch structures as a result of downregulation of the C2GnT-M gene has also been observed in human colorectal cancers [126]. Loss of more than 50% of C2GnT-M gene expression was found in about 61–66% of human colorectal tumors. Forced expression of C2GnT-M cDNA in a colonic cancer cell line devoid of this enzyme suppresses cell spreading, attachment to extracellular matrix, colony formation, and invasion. In addition, introduction of C2GnT-M to this cell line induces apoptosis and suppresses cell growth in vitro and in vivo. These results support the idea that core-4 glycans are important for inhibiting the above-mentioned tumorigenic properties. As described previously, ablation of C2GnT2 gene leads to reduced levels of immunoglobulins and development of colitis [129], a likely prelude to development of colorectal cancer.
In summary, except in rare cases as described above, elevated expression of C2GnT activity is associated with poor prognosis of cancer. Enhanced metastasis through formation of sLex on the core-2 branch in membrane-associated glycoproteins is the most likely explanation. However, loss of C4GnT or core-3 synthase activity in mucus secretory tissues results in loss of the protective function of secreted mucins, which can lead to development of colorectal cancer. In this regard, the C2GnT-L gene functions like an oncogene, and core-3 synthase and C2GnT-M function like tumor suppressors.
Role of C2GnT in the Estrous Cycle
C2GnT enzymes are also implicated in the reproductive system of golden hamster (Mesocricetus auratus) [130]. Glycosylation of hamster oviductin, a member of the mucin glycoprotein family, is regulated during the estrous cycle. The glycosylation process of oviductal glycoproteins is mainly involved in the synthesis of mucin O-glycans in the hamster oviduct. Hamster oviduct has high activities of glycosyltransferases that synthesize O-glycans that contain core-1, −2, −3, and −4 branched structures. During the estrous cycle, C2GnT-M enzyme activity is elevated at the stages of proestrus and estrus, but reduced at diestrus 1. Further, regulation of the activities of these enzymes is correlated with messenger RNA levels of C2GnT-M in the estrous cycle stages. Increase of C2GnT-M activity in the hamster oviduct at the time of ovulation suggests that glycosylation of oviductal glycoproteins may be essential for the function of these proteins during fertilization.
Regulation of C2GnT Gene Expression
Given the important role C2GnTs play in health and disease as described above, changes in the activities of these enzymes would have a significant impact on the overall health of an individual. To date, C2GnT-L and C2GnT-M are the only two mucin glycan branching enzymes of which detailed genomic structure and some characterization of gene regulation have been reported.
C2GnT-L gene expression has been shown to be activated by IL–12 in T cells [100], butyrate in CHO cells [70], and Th2 cytokines (IL–4 and IL–13) in NCI-H292 lung carcinoma cells [131]; moderately downregulated by epidermal growth factor (EGF) [132]; and not affected by retinoic acid [131]. SP1 is the transcription factor involved in the activation of this gene [49]. It remains to be established whether this transcription factor plays a role in the activation of this gene in metastatic cancer. IL–12 induces STAT4 expression in CD4+ T cells. STAT4 acts either directly or indirectly through the transcription factor T-bet to influence the expression of C2GnT-L and thus the function of PSGL-1 [133]. Sp1 transcription factor is essential for transcription of this gene in Jurkat cells, which are T cells derived from mesoderm, and NCI-H292 cells, which are derived from lung carcinomas [49]. Dose dependency of Sp1 is found in Jurkat cells, and only the Sp1s located at the proximal region are required for the expression of this gene.
C2GnT-M gene expression can be upregulated by retinoic acid and Th2 cytokines [50,131] and tumor necrosis factor (TNF)α [134], but downregulated by EGF in H292 cells [132,134]. EGF-mediated inhibition is through suppression of the EGF receptor (EGFR)-Ras-MEK-ERK signaling pathway. The EGFR-phosphatidylinositol-phospholipase C pathway is involved in TNFα-mediated activation of the C2GnT-M gene. Retinoic acid acts through retinoic acid receptor-α, while Th2 cytokines act through the JAK3-mediated signaling pathway. The promoter of the C2GnT-M gene is responsive to retinoic acid and Th2 cytokine [50]. However, the transcription factors involved in the activation of this gene remain to be identified.
In addition, introduction of N-glycan branching N-acetylglucosaminyltransferase V (GnTV) cDNA into H7721 hepatocellular carcinoma cells results in decreased expression of the sLex epitope. This is attributed to decreased expression of O-glycan branching enzymes such as C2GnT-L/-M and FUT-III, -VI, and -VII [135]. The mechanism remains unclear. Table 2 summarizes the regulation of C2GnTs under different growth conditions.
Table 2.
Regulation of human C2GnT gene expression under various conditions
| hC2GnT | Agents | Effects | Cells | Mechanism | References |
|---|---|---|---|---|---|
| C2GnT-L | butyrate, Th2 cytokines, LPS, | increased | pancreas, airway | transcription enzyme activity | [131], [136], [137], [132] |
| EGF | decreased | airway | |||
| C2GnT-M | butyrate, all-trans retinoic acid, Th2 cytokines, LPS | increased | pancreas, airway | transcription enzyme activity | [131], [136], [137] |
| EGF | decreased | airway | [132], [138] |
Summary and Future Directions
β6GlcNAc branching through formation of core 2, core 4, and I antigen is an important event in the synthesis of mucin O-glycans. These branch structures allow further elaboration of mucin-type glycans to increase not only content but also heterogeneous structure of mucin-type glycans. This is particularly significant for secreted mucins in view of their essential role in protecting the mucus secretory epithelium. Because many biologically important glycotopes are built on these branch structures, regulation of the synthesis of these branch structures is critically important for the regulation of many biological events, such as inflammation and cancer metastasis. With rare exceptions, increased expression of the C2GnT gene leads to decoration of the core-2 branch with selectin ligands, which correlates with poor prognosis of cancer. On the other hand, downregulation of the C2GnT-M gene could result in loss of epithelium-protective function of secreted mucins, which can lead to development of colitis and colorectal cancers. Thus, understanding the regulation of the expression of these genes could help elucidate the pathogenesis of cancer. Hence, future emphasis should be placed on understanding the mechanisms of coordinated gene expression of mucins, mucin glycan branching enzymes, and other glycosyltransferases involved in the synthesis of biologically important glycotopes, such as selectin ligands.
Acknowledgements
The authors wish to acknowledge the support of the following funding agencies: NIH RO1 HL48282, R21 HL097238, Cystic Fibrosis Foundation, and the State of Nebraska-NRI Cancer Glycobiology Program, LB 595 and LB506.
References
- 1.Rose MC, Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86:245–278 [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer 4:45–60 [DOI] [PubMed] [Google Scholar]
- 3.Loomes LM, Uemura K, Childs RA, Paulson JC, Rogers GN, Scudder PR, Michalski JC, Hounsell EF, Taylor-Robinson D, Feizi T (1984) Erythrocyte receptors for mycoplasma pneumoniae are sialylated oligosaccharides of ii antigen type. Nature 307:560–563 [DOI] [PubMed] [Google Scholar]
- 4.Loveless RW, Feizi T (1989) Sialo-oligosaccharide receptors for mycoplasma pneumoniae and related oligosaccharides of poly-N-acetyllactosamine series are polarized at the cilia and apical-microvillar domains of the ciliated cells in human bronchial epithelium. Infect Immun 57:1285–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki Y, Suzuki T, Matsumoto M (1983) Isolation and characterization of receptor sialoglycoprotein for hemagglutinating virus of japan (sendai virus) from bovine erythrocyte membrane. J Biochem 93:1621–1633 [DOI] [PubMed] [Google Scholar]
- 6.Andersson B, Dahmen J, Frejd T, Leffler H, Magnusson G, Noori G, Eden CS (1983) Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med 158:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose MC, Nickola TJ, Voynow JA (2001) Airway mucus obstruction: Mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol 25:533–537 [DOI] [PubMed] [Google Scholar]
- 8.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW (2006) Muc2-deficient mice spon-taneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131:117–129 [DOI] [PubMed] [Google Scholar]
- 9.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L (2002) Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295:1726–1729 [DOI] [PubMed] [Google Scholar]
- 10.Singh PK, Hollingsworth MA (2006) Cell surface-associated mucins in signal transduction. Trends Cell Biol 16:467–476 [DOI] [PubMed] [Google Scholar]
- 11.Carson DD, Julian J, Lessey BA, Prakobphol A, Fisher SJ (2006) MUC1 is a scaffold for selectin ligands in the human uterus. Front Biosci 11:2903–2908 [DOI] [PubMed] [Google Scholar]
- 12.Schroeder JA, Masri AA, Adriance MC, Tessier JC, Kotlarczyk KL, Thompson MC, Gendler SJ (2004) MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 23:5739–5747 [DOI] [PubMed] [Google Scholar]
- 13.Tsuboi S, Fukuda M (2001) Roles of O-linked oligosaccharides in immune responses. Bioessays 23:46–53 [DOI] [PubMed] [Google Scholar]
- 14.McEver RP, Moore KL, Cummings RD (1995) Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem 270:11025–11028 [DOI] [PubMed] [Google Scholar]
- 15.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M (1997) Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res 57:5201–5206 [PubMed] [Google Scholar]
- 16.Williams D, Longmore G, Matta KL, Schachter H (1980) Mucin synthesis. II. substrate specificity and product identification studies on canine submaxillary gland UDP-GlcNAc:Gal beta 1–3GalNAc(GlcNAc leads to GalNAc) beta 6-N-acetylglucosaminyltransferase. J Biol Chem 255:11253–11261 [PubMed] [Google Scholar]
- 17.Brockhausen I, Matta KL, Orr J, Schachter H (1985) Mucin synthesis, UDP-GlcNAc:GalNAc-R beta 3-N-acetylglucosaminyltransferase and UDP-GlcNAc:GlcNAc beta 1–3GalNAc-R (GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase from pig and rat colon mucosa. Biochemistry 24:1866–1874 [DOI] [PubMed] [Google Scholar]
- 18.Brockhausen I, Matta KL, Orr J, Schachter H, Koenderman AH, van den Eijnden DH (1986) Mucin synthesis, conversion of R1-beta 1–3Gal-R2 to R1-beta 1–3(GlcNAc beta 1–6)gal-R2 and of R1-beta 1–3GalNAc-R2 to R1-beta 1–3(GlcNAc beta 1–6)GalNAc-R2 by a beta 6-N-acetylglucosaminyltransferase in pig gastric mucosa. Eur J Biochem 157:463–474 [DOI] [PubMed] [Google Scholar]
- 19.Ropp PA, Little MR, Cheng PW (1991) Mucin biosynthesis: Purification and characterization of a mucin beta 6N-acetylglucosaminyltransferase. J Biol Chem 266:23863–23871 [PubMed] [Google Scholar]
- 20.Bierhuizen MF, Fukuda M (1992) Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1–3-GalNAc-R (GlcNAc to GalNAc) beta 1–6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci U S A 89:9326–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierhuizen MF, Mattei MG, Fukuda M (1993) Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes Dev 7:468–478 [DOI] [PubMed] [Google Scholar]
- 22.Schwientek T, Nomoto M, Levery SB, Merkx G, van Kessel AG, Bennett EP, Hollingsworth MA, Clausen H (1999) Control of O-glycan branch formation. molecular cloning of human cDNA encoding a novel beta1,6-N-acetylglucosaminyltransferase forming core 2 and core 4. J Biol Chem 274:4504–4512 [DOI] [PubMed] [Google Scholar]
- 23.Yeh JC, Ong E, Fukuda M (1999) Molecular cloning and expression of a novel beta-1, 6-N-acetylglu-cosaminyltransferase that forms core 2, core 4, and I branches. J Biol Chem 274:3215–3221 [DOI] [PubMed] [Google Scholar]
- 24.Schwientek T, Yeh JC, Levery SB, Keck B, Merkx G, van Kessel AG, Fukuda M, Clausen H (2000) Control of O-glycan branch formation. molecular cloning and characterization of a novel thymus-associated core 2 beta1, 6-n-acetylglucosaminyltransferase. J Biol Chem 275:11106–11113 [DOI] [PubMed] [Google Scholar]
- 25.Yen TY, Macher BA, Bryson S, Chang X, Tvaroska I, Tse R, Takeshita S, Lew AM, Datti A (2003) Highly conserved cysteines of mouse core 2 beta1,6-N-acetylglucosaminyltransferase I form a network of disulfide bonds and include a thiol that affects enzyme activity. J Biol Chem 278:45864–45881 [DOI] [PubMed] [Google Scholar]
- 26.Singh J, Khan GA, Kinarsky L, Cheng H, Wilken J, Choi KH, Bedows E, Sherman S, Cheng PW (2004) Identification of disulfide bonds among the nine core 2 N-acetylglucosaminyltransferase-M cysteines conserved in the mucin beta6-N-acetylglucosaminyltransferase family. J Biol Chem 279:38969–38977 [DOI] [PubMed] [Google Scholar]
- 27.Bierhuizen MF, Maemura K, Kudo S, Fukuda M (1995) Genomic organization of core 2 and I branching beta-1,6-N-acetylglucosaminyltransferases. implication for evolution of the beta-1,6-N-acetylglucosaminyl-trans-ferase gene family. Glycobiology 5:417–425 [DOI] [PubMed] [Google Scholar]
- 28.Sekine M, Nara K, Suzuki A (1997) Tissue-specific regulation of mouse core 2 beta-1,6-N-acetylglucosaminyltransferase. J Biol Chem 272:27246–27252 [DOI] [PubMed] [Google Scholar]
- 29.Choi KH, Osorio FA, Cheng PW (2004) Mucin biosynthesis: Bovine C2GnT-M gene, tissue-specific expression, and herpes virus-4 homologue. Am J Respir Cell Mol Biol 30:710–719 [DOI] [PubMed] [Google Scholar]
- 30.Pak JE, Arnoux P, Zhou S, Sivarajah P, Satkunarajah M, Xing X, Rini JM (2006) X-ray crystal structure of leukocyte type core 2 beta1,6-N-acetylglucosaminyltransferase. evidence for a convergence of metal ion-independent glycosyltransferase mechanism. J Biol Chem 281:26693–26701 [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Qin W, Lehotay M, Toki D, Dennis P, Schutzbach JS, Brockhausen I (2003) Soluble human core 2 beta6-N-acetylglucosaminyltransferase C2GnT1 requires its conserved cysteine residues for full activity. Biochim Biophys Acta 1648:62–74 [DOI] [PubMed] [Google Scholar]
- 32.Tarbouriech N, Charnock SJ, Davies GJ (2001) Three-dimensional structures of the Mn and Mg dTDP complexes of the family GT-2 glycosyltransferase SpsA: a comparison with related NDP-sugar glycosyltrans-ferases. J Mol Biol 314:655–661 [DOI] [PubMed] [Google Scholar]
- 33.Garinot-Schneider C, Lellouch AC, Geremia RA (2000) Identification of essential amino acid residues in the Sinorhizobium meliloti glucosyltransferase Exo. J Biol Chem 275:31407–31413 [DOI] [PubMed] [Google Scholar]
- 34.Beum PV, Cheng PW (2001) Biosynthesis and function of beta 1,6 branched mucin-type glycans In: Wu AM () The molecular immunology of complex carbohydrates-2. Kluwer Acedemic/Plenum Publishers, New York, 271–312 [Google Scholar]
- 35.Brockhausen I (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep 7:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clausen H, Bennett EP (1996) A family of UDP-GalNAc: Polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology 6:635–646 [DOI] [PubMed] [Google Scholar]
- 37.Elhammer AP, Poorman RA, Brown E, Maggiora LL, Hoogerheide JG, Kezdy FJ (1993) The specificity of UDP-GalNAc:Polypeptide N-acetylgalactosaminyltransferase as inferred from a database of in vivo substrates and from the in vitro glycosylation of proteins and peptides. J Biol Chem 268:10029–10038 [PubMed] [Google Scholar]
- 38.Wang Y, Agrwal N, Eckhardt AE, Stevens RD, Hill RL (1993) The acceptor substrate specificity of porcine submaxillary UDP-GalNAc:Polypeptide N-acetylgalactosaminyltransferase is dependent on the amino acid sequences adjacent to serine and threonine residues. J Biol Chem 268:22979–22983 [PubMed] [Google Scholar]
- 39.Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, Nielsen PA, Hollingsworth MA, Burchell J, Taylor-Papadimitriou J, Clausen H (1997) Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J Biol Chem 272:23503–23514 [DOI] [PubMed] [Google Scholar]
- 40.Ju T, Aryal RP, Stowell CJ, Cummings RD (2008) Regulation of protein O-glycosylation by the endo-plasmic reticulum-localized molecular chaperone cosmc. J Cell Biol 182:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, Muramatsu T, von Andrian UH, Rosen SD (2005) A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol 6:1105–1113 [DOI] [PubMed] [Google Scholar]
- 42.Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, Uchimura K, Kadomatsu K, Muramatsu T, Lowe JB, Fukuda M (2005) N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol 6:1096–1104 [DOI] [PubMed] [Google Scholar]
- 43.Ujita M, McAuliffe J, Schwientek T, Almeida R, Hindsgaul O, Clausen H, Fukuda M (1998) Synthesis of poly-N-acetyllactosamine in core 2 branched O-glycans. the requirement of novel beta-1,4-galactosyl-trans-ferase IV and beta-1,3-n-acetylglucosaminyltransferase. J Biol Chem 273:34843–34849 [DOI] [PubMed] [Google Scholar]
- 44.Sasaki K, Kurata-Miura K, Ujita M, Angata K, Nakagawa S, Sekine S, Nishi T, Fukuda M (1997) Expression cloning of cDNA encoding a human beta-1,3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc Natl Acad Sci U S A 94:14294–14299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins WM, Clarke JL (2001) The genetic regulation of fucosylated and sialylated antigens on developing myeloid cells : Wu AM () The molecular immunology of complex carbohydrates-2. Kluwer Acedemic/Plenum Publishers, New York, 231–265 [DOI] [PubMed] [Google Scholar]
- 46.Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, Kudo T, Togayachi A, Ishizuka Y, Nakanishi H, Narimatsu H (2002) Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem 277:12802–12809 [DOI] [PubMed] [Google Scholar]
- 47.Zerfaoui M, Fukuda M, Langlet C, Mathieu S, Suzuki M, Lombardo D, El-Battari A (2002) The cytosolic and transmembrane domains of the beta 1,6 N-acetylglucosaminyltransferase (C2GnT) function as a cis to medial/Golgi-targeting determinant. Glycobiology 12:15–24 [DOI] [PubMed] [Google Scholar]
- 48.Colley KJ (1997) Golgi localization of glycosyltransferases: more questions than answers. Glycobiology 7:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falkenberg VR, Fregien N (2007) Control of core 2 beta1,6 N-acetylglucosaminyltransferase-I transcription by Sp1 in lymphocytes and epithelial cells. Glycoconj J 24:511–519 [DOI] [PubMed] [Google Scholar]
- 50.Tan S, Cheng PW (2007) Mucin biosynthesis: identification of the cis-regulatory elements of human C2GnT-M gene. Am J Respir Cell Mol Biol 36:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekine M, Taya C, Shitara H, Kikkawa Y, Akamatsu N, Kotani M, Miyazaki M, Suzuki A, Yonekawa H (2006) The cis-regulatory element Gsl5 is indispensable for proximal straight tubule cell-specific transcription of core 2 beta-1,6-N-acetylglucosaminyltransferase in the mouse kidney. J Biol Chem 281:1008–1015 [DOI] [PubMed] [Google Scholar]
- 52.Sekine M, Kikkawa Y, Takahama S, Tsuda K, Yonekawa H, Suzuki A (2002) Phylogenetic development of a regulatory gene for the core 2 GlcNAc transferase in mus musculus. J Biochem 132:387–393 [DOI] [PubMed] [Google Scholar]
- 53.Sekine M, Taya C, Kikkawa Y, Yonekawa H, Takenaka M, Matsuoka Y, Imai E, Izawa M, Kannagi R, Suzuki A (2001) Regulation of mouse kidney tubular epithelial cell-specific expression of core 2 GlcNAc transferase. Eur J Biochem 268:1129–1135 [DOI] [PubMed] [Google Scholar]
- 54.Falkenberg VR, Alvarez K, Roman C, Fregien N (2003) Multiple transcription initiation and alternative splicing in the 5′ untranslated region of the core 2 beta1–6 N-acetylglucosaminyltransferase I gene. Glycobiology 13:411–418 [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto M, Tan S, Mori N, Cheng H, Cheng PW (2007) Mucin biosynthesis: Molecular cloning and expression of mouse mucus-type core 2 beta1,6 N-acetylglucosaminyltransferase. Glycobiology 17:994–1006 [DOI] [PubMed] [Google Scholar]
- 56.Inaba N, Hiruma T, Togayachi A, Iwasaki H, Wang XH, Furukawa Y, Sumi R, Kudo T, Fujimura K, Iwai T, Gotoh M, Nakamura M, Narimatsu H (2003) A novel I-branching beta-1,6- N-acetylglucosaminyl-transferase involved in human blood group I antigen expression. Blood 101:2870–2876 [DOI] [PubMed] [Google Scholar]
- 57.Twu YC, Chen CP, Hsieh CY, Tzeng CH, Sun CF, Wang SH, Chang MS, Yu LC (2007) I branching formation in erythroid differentiation is regulated by transcription factor C/EBPalpha. Blood 110:4526–4534 [DOI] [PubMed] [Google Scholar]
- 58.Pras E, Raz J, Yahalom V, Frydman M, Garzozi HJ, Pras E, Hejtmancik JF (2004) A nonsense mutation in the glucosaminyl (N-acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci 45:1940–1945 [DOI] [PubMed] [Google Scholar]
- 59.Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart CH, Fukuda M, Seilhamer JJ (1995) Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 O-glycans on thymocytes and T lymphoblastoid cells. J Exp Med 181:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellies LG, Tao W, Fellinger W, The HS, Ziltener HJ (1996) The CD43 130-kD peripheral T-cell activation antigen is downregulated in thymic positive selection. Blood 88:1725–1732 [PubMed] [Google Scholar]
- 61.Granovsky M, Fode C, Warren CE, Campbell RM, Marth JD, Pierce M, Fregien N, Dennis JW (1995) GlcNAc-transferase V and core 2 GlcNAc-transferase expression in the developing mouse embryo. Glycobiology 5:797–806 [DOI] [PubMed] [Google Scholar]
- 62.Mitoma J, Petryniak B, Hiraoka N, Yeh JC, Lowe JB, Fukuda M (2003) Extended core 1 and core 2 branched O-glycans differentially modulate sialyl lewis X-type L-selectin ligand activity. J Biol Chem 278:9953–9961 [DOI] [PubMed] [Google Scholar]
- 63.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD (1998) Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity 9:881–890 [DOI] [PubMed] [Google Scholar]
- 64.Lowe JB (2002) Glycosylation in the control of selectin counter-receptor structure and function. Immunol Rev 186:19–36 [DOI] [PubMed] [Google Scholar]
- 65.Machida E, Nakayama J, Amano J, Fukuda M (2001) Clinicopathological significance of core 2 beta1,6-N-acetylglucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res 61:2226–2231 [PubMed] [Google Scholar]
- 66.Renkonen J, Rabina J, Mattila P, Grenman R, Renkonen R (2001) Core 2 beta1,6-N-acetylglucosaminyl-transferases and alpha1,3-fucosyltransferases regulate the synthesis of O-glycans on selectin ligands on oral cavity carcinoma cells. APMIS 109:500–506 [DOI] [PubMed] [Google Scholar]
- 67.Beum PV, Singh J, Burdick M, Hollingsworth MA, Cheng PW (1999) Expression of core 2 beta-1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes. J Biol Chem 274:24641–24648 [DOI] [PubMed] [Google Scholar]
- 68.Brockhausen I, Kuhns W, Schachter H, Matta KL, Sutherland DR, Baker MA (1991) Biosynthesis of O-glycans in leukocytes from normal donors and from patients with leukemia: increase in O-glycan core 2 UDP-GlcNAc:Gal beta 3 GalNAc alpha-R (GlcNAc to GalNAc) beta(1–6)-N-acetylglucosaminyltransferase in leukemic cells. Cancer Res 51:1257–1263 [PubMed] [Google Scholar]
- 69.Heffernan M, Lotan R, Amos B, Palcic M, Takano R, Dennis JW (1993) Branching beta 1–6N-acetylglucosaminetransferases and polylactosamine expression in mouse F9 teratocarcinoma cells and differentiated counterparts. J Biol Chem 268:1242–1251 [PubMed] [Google Scholar]
- 70.Datti A, Dennis JW (1993) Regulation of UDP-GlcNAc:Gal beta 1–3GalNAc-R beta 1–6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc) in chinese hamster ovary cells. J Biol Chem 268:5409–5416 [PubMed] [Google Scholar]
- 71.Nakamura M, Kudo T, Narimatsu H, Furukawa Y, Kikuchi J, Asakura S, Yang W, Iwase S, Hatake K, Miura Y (1998) Single glycosyltransferase, core 2 beta1-->6-N-acetylglucosaminyltransferase, regulates cell surface sialyl-lex expression level in human pre-B lymphocytic leukemia cell line KM3 treated with phorbolester. J Biol Chem 273:26779–26789 [DOI] [PubMed] [Google Scholar]
- 72.Kenney D, Cairns L, Remold-O’Donnell E, Peterson J, Rosen FS, Parkman R (1986) Morphological abnormalities in the lymphocytes of patients with the wiskott-aldrich syndrome. Blood 68:1329–1332 [PubMed] [Google Scholar]
- 73.Perry GS 3rd, Spector BD, Schuman LM, Mandel JS, Anderson VE, McHugh RB, Hanson MR, Fahlstrom SM, Krivit W, Kersey JH (1980) The Wiskott-Aldrich syndrome in the United States and Canada (1892–1979). J Pediatr 97:72–78 [DOI] [PubMed] [Google Scholar]
- 74.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ (1980) The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood 55:243–252 [PubMed] [Google Scholar]
- 75.Parkman R, Remold-O’Donnell E, Kenney DM, Perrine S, Rosen FS (1981) Surface protein abnormalities in lymphocytes and platelets from patients with Wiskott-Aldrich syndrome. Lancet 2:1387–1389 [DOI] [PubMed] [Google Scholar]
- 76.Remold-O’Donnell E, Kenney DM, Parkman R, Cairns L, Savage B, Rosen FS (1984) Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med 159:1705–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park JK, Rosenstein YJ, Remold-O’Donnell E, Bierer BE, Rosen FS, Burakoff SJ (1991) Enhancement of T-cell activation by the CD43 molecule whose expression is defective in Wiskott-Aldrich syndrome. Nature 350:706–709 [DOI] [PubMed] [Google Scholar]
- 78.Manjunath N, Johnson RS, Staunton DE, Pasqualini R, Ardman B (1993) Targeted disruption of CD43 gene enhances T lymphocyte adhesion. J Immunol 151:1528–1534 [PubMed] [Google Scholar]
- 79.Lefebvre JC, Giordanengo V, Limouse M, Doglio A, Cucchiarini M, Monpoux F, Mariani R, Peyron JF (1994) Altered glycosylation of leukosialin, CD43, in HIV-1-infected cells of the CEM line. J Exp Med 180:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orlacchio A, Sarchielli P, Gallai V, Datti A, Saccardi C, Palmerini CA (1997) Activity levels of a beta1,6 N-acetylglucosaminyltransferase in lymphomonocytes from multiple sclerosis patients. J Neurol Sci 151:177–183 [DOI] [PubMed] [Google Scholar]
- 81.Nishio Y, Warren CE, Buczek-Thomas JA, Rulfs J, Koya D, Aiello LP, Feener EP, Miller TB Jr, Dennis JW, King GL (1995) Identification and characterization of a gene regulating enzymatic glycosylation which is induced by diabetes and hyperglycemia specifically in rat cardiac tissue. J Clin Invest 96:1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koya D, Dennis JW, Warren CE, Takahara N, Schoen FJ, Nishio Y, Nakajima T, Lipes MA, King GL (1999) Overexpression of core 2 N-acetylglycosaminyltransferase enhances cytokine actions and induces hypertrophic myocardium in transgenic mice. FASEB J 13:2329–2337 [DOI] [PubMed] [Google Scholar]
- 83.Panicot L, Mas E, Thivolet C, Lombardo D (1999) Circulating antibodies against an exocrine pancreatic enzyme in type 1 diabetes. Diabetes 48:2316–2323 [DOI] [PubMed] [Google Scholar]
- 84.Fukuda M (2006) Roles of mucin-type O-glycans synthesized by core2beta1,6-N-acetylglucosaminyltransferase. Methods Enzymol 416:332–346 [DOI] [PubMed] [Google Scholar]
- 85.Sperandio M, Thatte A, Foy D, Ellies LG, Marth JD, Ley K (2001) Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood 97:3812–3819 [DOI] [PubMed] [Google Scholar]
- 86.Kimura AK, Wigzell H (1978) Cell surface glycoproteins of murine cytotoxic T lymphocytes. I. T 145, a new cell surface glycoprotein selectively expressed on ly 1–2+ cytotoxic T lymphocytes. J Exp Med 147:1418–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andersson LC, Gahmberg CG, Kimura AK, Wigzell H (1978) Activated human T lymphocytes display new surface glycoproteins. Proc Natl Acad Sci U S A 75:3455–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dalziel M, Whitehouse C, McFarlane I, Brockhausen I, Gschmeissner S, Schwientek T, Clausen H, Burchell JM, Taylor-Papadimitriou J (2001) The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J Biol Chem 276:11007–11015 [DOI] [PubMed] [Google Scholar]
- 89.Carlow DA, Ziltener HJ (2006) CD43 deficiency has no impact in competitive in vivo assays of neutrophil or activated T cell recruitment efficiency. J Immunol 177:6450–6459 [DOI] [PubMed] [Google Scholar]
- 90.Grabie N, Delfs MW, Lim YC, Westrich JR, Luscinskas FW, Lichtman AH (2002) Beta-galactoside alpha2,3-sialyltransferase-I gene expression during Th2 but not Th1 differentiation: implications for core2-glycan formation on cell surface proteins. Eur J Immunol 32:2766–2772 [DOI] [PubMed] [Google Scholar]
- 91.Barran P, Fellinger W, Warren CE, Dennis JW, Ziltener HJ (1997) Modification of CD43 and other lymphocyte O-glycoproteins by core 2 N-acetylglucosaminyltransferase. Glycobiology 7:129–136 [DOI] [PubMed] [Google Scholar]
- 92.Kikuchi J, Shinohara H, Nonomura C, Ando H, Takaku S, Nojiri H, Nakamura M (2005) Not core 2 beta 1,6-N-acetylglucosaminyltransferase-2 or −3 but −1 regulates sialyl-lewis x expression in human precursor B cells. Glycobiology 15:271–280 [DOI] [PubMed] [Google Scholar]
- 93.Fukuda M, Carlsson SR, Klock JC, Dell A (1986) Structures of O-linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. J Biol Chem 261:12796–12806 [PubMed] [Google Scholar]
- 94.Maemura K, Fukuda M (1992) Poly-N-acetyllactosaminyl O-glycans attached to leukosialin. the presence of sialyl le(x) structures in O-glycans. J Biol Chem 267:24379–24386 [PubMed] [Google Scholar]
- 95.Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B (1999) Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med 190:1769–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimizu Y, Shaw S, Graber N, Gopal TV, Horgan KJ, Van Seventer GA, Newman W (1991) Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature 349:799–802 [DOI] [PubMed] [Google Scholar]
- 97.Von Andrian UH, Hansell P, Chambers JD, Berger EM, Torres Filho I, Butcher EC, Arfors KE (1992) L-selectin function is required for beta 2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol 263:H1034–1044 [DOI] [PubMed] [Google Scholar]
- 98.Lowe JB (2003) Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol 15:531–538 [DOI] [PubMed] [Google Scholar]
- 99.Lenter M, Levinovitz A, Isenmann S, Vestweber D (1994) Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol 125:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lim YC, Xie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW (2001) IL-12, STAT4-dependent up-regulation of CD4(+) T cell core 2 beta-1,6-n-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J Immunol 167:4476–4484 [DOI] [PubMed] [Google Scholar]
- 101.Honn KV, Tang DG, Crissman JD (1992) Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev 11:325–351 [DOI] [PubMed] [Google Scholar]
- 102.Karpatkin S, Pearlstein E (1981) Role of platelets in tumor cell metastases. Ann Intern Med 95:636–641 [DOI] [PubMed] [Google Scholar]
- 103.Hiraoka N, Kawashima H, Petryniak B, Nakayama J, Mitoma J, Marth JD, Lowe JB, Fukuda M (2004) Core 2 branching beta1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J Biol Chem 279:3058–3067 [DOI] [PubMed] [Google Scholar]
- 104.Broide DH, Miller M, Castaneda D, Nayar J, Cho JY, Roman M, Ellies LG, Sriramarao P (2002) Core 2 oligosaccharides mediate eosinophil and neutrophil peritoneal but not lung recruitment. Am J Physiol Lung Cell Mol Physiol 282:L259–L266 [DOI] [PubMed] [Google Scholar]
- 105.Smith MJ, Smith BR, Lawrence MB, Snapp KR (2004) Functional analysis of the combined role of the O-linked branching enzyme core 2 beta1–6-N-glucosaminyltransferase and dimerization of P-selectin glycoprotein ligand-1 in rolling on P-selectin. J Biol Chem 279:21984–21991 [DOI] [PubMed] [Google Scholar]
- 106.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP (1996) Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem 271:3255–3264 [PubMed] [Google Scholar]
- 107.Kumar R, Camphausen RT, Sullivan FX, Cumming DA (1996) Core2 beta-1,6-N-acetylglucosaminyl-transferase enzyme activity is critical for P-selectin glycoprotein ligand-1 binding to P-selectin. Blood 88:3872–3879 [PubMed] [Google Scholar]
- 108.Kanda H, Tanaka T, Matsumoto M, Umemoto E, Ebisuno Y, Kinoshita M, Noda M, Kannagi R, Hirata T, Murai T, Fukuda M, Miyasaka M (2004) Endomucin, a sialomucin expressed in high endothelial venules, supports L-selectin-mediated rolling. Int Immunol 16:1265–1274 [DOI] [PubMed] [Google Scholar]
- 109.Prorok-Hamon M, Notel F, Mathieu S, Langlet C, Fukuda M, El-Battari A (2005) N-glycans of core2 beta(1,6)-N-acetylglucosaminyltransferase-I (C2GnT-I) but not those of alpha(1,3)-fucosyltransferase-VII (FucT-VII) are required for the synthesis of functional P-selectin glycoprotein ligand-1 (PSGL-1): effects on P-, L- and E-selectin binding. Biochem J 391:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kikuchi J, Ozaki H, Nonomura C, Shinohara H, Iguchi S, Nojiri H, Hamada H, Kiuchi A, Nakamura M (2005) Transfection of antisense core 2 beta1,6-N-acetylglucosaminyltransferase-1 cDNA suppresses selectin ligand expression and tissue infiltration of B-cell precursor leukemia cells. Leukemia 19:1934–1940 [DOI] [PubMed] [Google Scholar]
- 111.Valenzuela HF, Pace KE, Cabrera PV, White R, Porvari K, Kaija H, Vihko P, Baum LG (2007) O-glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res 67:6155–6162 [DOI] [PubMed] [Google Scholar]
- 112.Nguyen JT, Evans DP, Galvan M, Pace KE, Leitenberg D, Bui TN, Baum LG (2001) CD45 modulates galectin-1-induced T cell death: Regulation by expression of core 2 O-glycans. J Immunol 167:5697–5707 [DOI] [PubMed] [Google Scholar]
- 113.Brockhausen I (2006) Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep 7:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ohyama C, Tsuboi S, Fukuda M (1999) Dual roles of sialyl lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J 18:1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL (1991) A carbohydrate domain common to both sialyl le(a) and sialyl le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem 266:14869–14872 [PubMed] [Google Scholar]
- 116.Takada A, Ohmori K, Takahashi N, Tsuyuoka K, Yago A, Zenita K, Hasegawa A, Kannagi R (1991) Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl lewis A. Biochem Biophys Res Commun 179:713–719 [DOI] [PubMed] [Google Scholar]
- 117.Kannagi R (1997) Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J 14:577–584 [DOI] [PubMed] [Google Scholar]
- 118.Hagisawa S, Ohyama C, Takahashi T, Endoh M, Moriya T, Nakayama J, Arai Y, Fukuda M (2005) Expression of core 2 beta1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology 15:1016–1024 [DOI] [PubMed] [Google Scholar]
- 119.Nakayama J, Shimizu F, Katsuyama T (2002) Glycosyltransferase genes as tumor marker. Rinsho Byori Suppl 123:142–148 [PubMed] [Google Scholar]
- 120.Dalziel M, Whitehouse C, McFarlane I, Brockhausen I, Gschmeissner S, Schwientek T, Clausen H, Burchell JM, Taylor-Papadimitriou J (2001) The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J Biol Chem 276:11007–11015 [DOI] [PubMed] [Google Scholar]
- 121.Kim YJ, Borsig L, Varki NM, Varki A (1998) P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A 95:9325–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qian F, Hanahan D, Weissman IL (2001) L-selectin can facilitate metastasis to lymph nodes in a transgenic mouse model of carcinogenesis Proc Natl Acad Sci U S A 98:3976–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cabrera PV, Amano M, Mitoma J, Chan J, Said J, Fukuda M, Baum LG (2006) Haploinsufficiency of C2GnT-I glycosyltransferase renders T lymphoma cells resistant to cell death. Blood 108:2399–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xia B, Royall JA, Damera G, Sachdev GP, Cummings RD (2005) Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology 15:747–775 [DOI] [PubMed] [Google Scholar]
- 125.Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H (2005) Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci U S A 102:4572–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang MC, Chen HY, Huang HC, Huang J, Liang JT, Shen TL, Lin NY, Ho CC, Cho IM, Hsu SM (2006) C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene 25:3267–3276 [DOI] [PubMed] [Google Scholar]
- 127.Hounsell EF, Fukuda M, Powell ME, Feizi T, Hakomori S (1980) A new O-glycosidically linked tri-hexosamine core structure in sheep gastric mucin: a preliminary note. Biochem Biophys Res Commun 92:1143–1150 [DOI] [PubMed] [Google Scholar]
- 128.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L (2007) Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204:1417–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, Ho SB, Dell A, Fukuda MF, Marth JD (2009) Glycosyltransferase function in core 2-type protein O gycosylation. Mol Cell Biol 29:3770–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stone EL, Marth JD (2006) In vivo and genetic analyses of mammalian core 2 O-glycan function : Annual conference of the society for glycobiology in vivo and genetic analyses of mammalian core 2 O-glycan function. Oxford University Press, Cary, NC, 1157 [Google Scholar]
- 131.McBride DS, Brockhausen I, Kan FW (2005) Detection of glycosyltransferases in the golden hamster (mesocricetus auratus) oviduct and evidence for the regulation of O-glycan biosynthesis during the estrous cycle. Biochim Biophys Acta 1721:107–115 [DOI] [PubMed] [Google Scholar]
- 132.Beum PV, Basma H, Bastola DR, Cheng PW (2005) Mucin biosynthesis: upregulation of core 2 beta 1,6 N-acetylglucosaminyltransferase by retinoic acid and Th2 cytokines in a human airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 288:L116–L124 [DOI] [PubMed] [Google Scholar]
- 133.Beum PV, Bastola DR, Cheng PW (2003) Mucin biosynthesis: Epidermal growth factor downregulates core 2 enzymes in a human airway adenocarcinoma cell line. Am J Respir Cell Mol Biol 29:48–56 [DOI] [PubMed] [Google Scholar]
- 134.White SJ, Underhill GH, Kaplan MH, Kansas GS (2001) Cutting edge: differential requirements for Stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J Immunol 167:628–631 [DOI] [PubMed] [Google Scholar]
- 135.Ishibashi Y, Inouye Y, Okano T, Taniguchi A (2005) Regulation of sialyl-lewis x epitope expression by TNF-alpha and EGF in an airway carcinoma cell line. Glycoconj J 22:53–62 [DOI] [PubMed] [Google Scholar]
- 136.Guo P, Zhang Y, Shen ZH, Zhang XY, Chen HL (2004) Effect of N-acetylglucosaminyltransferase V on the expressions of other glycosyltransferases. FEBS Lett 562:93–98 [DOI] [PubMed] [Google Scholar]
- 137.Radhakrishnan P, Beum PV, Tan S, Cheng PW (2007) Butyrate induces sLex synthesis by stimulation of selective glycosyltransferase genes. Biochem Biophys Res Commun 359:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yanagihara K, Seki M, Cheng PW (2001) Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am J Respir Cell Mol Biol 24:66–73 [DOI] [PubMed] [Google Scholar]
- 139.Ishibashi Y, Inouye Y, Okano T, Taniguchi A (2005) Regulation of sialyl-lewis x epitope expression by TNF-alpha and EGF in an airway carcinoma cell line. Glycoconj J 22:53–62 [DOI] [PubMed] [Google Scholar]


