Abstract
Many natural structures out-perform the conventional synthetic counterparts due to the specially evolved reinforcement architectures. Here we report an electrically assisted additive manufacturing approach that bio-mimic the Bouligand structure in natural creatures to create highly impact resistant architectures. The alignment of surface modified Multi-walled Carbon Nanotubes (MWCNT-S) was controlled by rotating electric field during printing. Besides, the composite shows anisotropic mechanical properties with the highest tensile modulus parallel to the alignment, 6 times higher than the modulus in perpendicular direction. The Bouligand-type MWCNT-S with controllable rotating angle leads to 3 times enhanced impact resistance compared with random distribution due to the energy dissipation by the rotating anisotropic layers. This enables us to create complex bioinspired reinforcement architectures possessing enhanced performance. Furthermore, this approach is used to mimic the Collagen fiber alignment in human meniscus to create reinforced artificial meniscus with circumferential and radial aligned MWCNT-S. The printed meniscus shows enhanced tensile modulus and fracture energy compared with native menisci, which shows a potential application as a replica for tissue constructs to circumvent meniscus tear. The electrically assisted three-dimensional (3D) printing technology enables us to design and evolve reinforced architectures with arbitrary geometries, which shows promising applications in aerospace, armor, mechanical and tissue engineering.
1. Introduction
Biological architectures offer inspiration for the design of next-generation structural materials due to their low density, high strength and toughness through specially evolved structures 1–8. One of the inspiration originates from superior mechanical properties of naturally evolved composites featured with different orientations of reinforcing fibers or particles (known as Bouligand or twisted plywood structure). For example, the dactyl clubs of peacock mantis shrimp and gigas fish scales, Beetle wings, the claws of crab and lobster 9–18. The Bouligand structure with ordered collagen or chitin fibers in one layer, yet heterogeneous between different layers is widely studied and proven to make a great contribution to the reinforcement of crack stopping 19–21. The crack cannot follow a straight path, thereby increasing energy dissipation and impact resistance. The biological systems grow these reinforcement architectures with best performance through a long-term evolution. However, the complicated architectures in natural materials far exceed the design technology, which hinders the progress of study in reinforcement architectures. Bioinspired reinforced architecture with fibers or ceramic platelets aligned by shear force, magnetic field or electric field were fabricated by conventional technology and 3D printing2, 22–26 (Table S1, supporting information). But the study of the relationship between the mechanical properties and the rotating angle between aligning direction was not found. Here we present an electrically assisted additive manufacturing/three-dimensional (3D) printing technology for the fabrication of reinforcement architecture with anisotropic layers of aligned surface modified Multi-walled Carbon Nanotubes (MWCNT-S). The aligning direction of MWCNT-S in each layer is accurately controlled by a rotation stage and how to use such control to induce improved mechanical properties is studied. Carbon nanotubes (CNTs) have shown great potential as multifunctional nanofillers for polymer-based nanocomposites due to their unique structure and excellent mechanical properties27. Controlled alignment of CNTs in polymer matrix by electric field will be studied for further improving the multifunctional properties by using anisotropic properties arising from the extremely high aspect ratio of CNTs.
Additive manufacturing is one of the effective ways to fabricate customized parts with complicated architecture and has a wide application in industry, academia and daily usages 28, 29. The bioinspired architectures with Bouligand MWCNT-S were designed to enhance the impact resistance as the same for lobster claw and fish scale. The relationship between mechanical property and rotation angles between adjacent layers was studied. Furthermore, this approach is used to mimic the fiber alignment in human meniscus to create reinforced artificial meniscus replica. The meniscus tear is the most common disease that affects more than 1.5 million people through United States and Europe 30. The surgery of meniscectomy will increase the risk of osteoarthritis while the meniscus transplantation is restricted by the lack of shortages of donors and tissue mismatch31. As a result, artificial substitute for the natural menisci has been proposed as the best solution and attracted much attention31. Many kinds of meniscal scaffolds were made from polymers (such as polyurethane etc.), silks and even aligned scaffolds by electrospinning32, 33. But the biomechanical properties of these scaffolds still need to be improved and the aligned scaffold only partially mimic the circumferential architecture34, 35. Thus, an optimized design should be developed to fully imitating the wedge-shaped, circumferential and radial aligned collagen fibrous architecture. Carbon nanotubes are widely used to enhance the mechanical properties in the medical devices attributed to its high modulus and bio-compatibility36. In our study, artificial meniscus was successfully fabricated with radial and circumferential aligned MWCNT-S by electrically assisted nanocomposite 3D printing. The printed meniscus shows enhanced mechanical properties compared with native menisci, which serves as a promising replica for the tissue constructs to solve the problem of meniscus tear.
2. Results
Bioinspired reinforcement architectures with Bouligand-type MWCNT-S
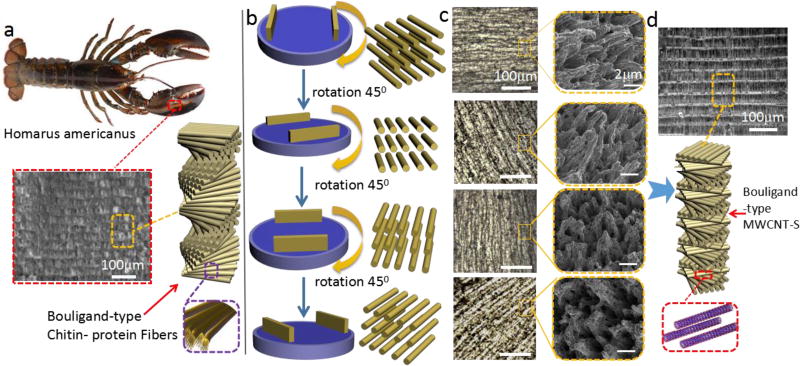
The multifunctionality combined with their robust mechanical properties of Bouligand arranged structures in biological organisms make them a rich source of inspiration for the design of new materials. The particular examples of Bouligand arranged structures in natural materials are found in the scales of a Arapaima gigas fish and the claws of crab and Homarus americanus, which is used either to protect themselves from predating or specifically adapted for close-range combat (Figure 1a) 37–39. In the claws of Homarus americanus, the Bouligand structure is formed by the helicoidal stacking sequence of the fibrous chitin–protein layers (Figure 1a). The presence of the Bouligand arrangement fibers enhance the impact resistance by increasing energy dissipation and fracture toughness40. Here we present a method termed as electrically assisted nanocomposite 3D printing that can dynamically align MWCNT-S by controlling a rotating electrical field for the fabrication of bioinspired reinforcement architecture. Instead of pure MWCNT, MWCNT-S were used to promote the homogeneous distribution in polymer matrix (Figure S1, Supporting information). Schematic diagrams (Figure 1b), optical microscopy images and SEM images (Figure 1c) show the rotating alignment of carbon nanotubes bundles to the electric field. Tensile tests show that alignment of MWCNT-S gives rise to an anisotropic elastic modulus compared with random distribution (Figure S2, Supporting information). The samples possess the highest modulus in the direction that is parallel to the alignment of MWCNT-S and the lowest modulus in the perpendicular direction. The anisotropy is discussed in supporting information Figure S2–Figure S6. After the first layer is fabricated, the adjacent layer is gradually rotated about its normal axis, thereby creating Bouligand-type MWCNT-S layers. The cross-section microscopy image shows the same structure with Bouligand type biological organisms (Figure 1d).
Figure 1.
Biomimetic architectures with Bouligand-type MWCNT-S can be recreated by electrically assisted 3D printing. a) The diagram of Homarus americanus and the microstructure of claws made with Bouligand-type Chitin-protein fibers 16; b) Schematic diagram of different alignment of carbon nanotubes by the rotation of the electrodes; c) Surface optical microscopy images and SEM images of fracture surface for different alignment of MWCNT-S corresponding to b; d) Schematic diagram of layer by layer bioinspired Bouligand-type MWCNT-S fabricated by the electrically assisted nanocomposite 3D printing.
Electrically assisted nanocomposite 3D printing set-up
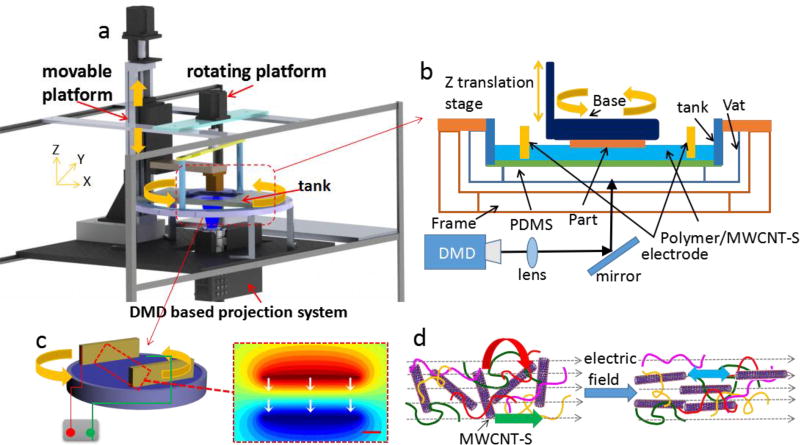
Figure 2a shows the set up process of electrically assisted 3D printing platform, Polymer A (PA purchased from Makerjuice)/MWCNT-S nanocomposites were deposited in a transparent glass tank. The photo curable resin (PA) is cured after mask images are projected upwards onto the bottom of the substrate by the Digital Micro-mirror Device (DMD) based projection system 41–44. A mask-image-projection-based stereolithography (MIP-SL) process is used due to its high quality surface finish, dimensional accuracy, high fabrication speed and low machine cost. Different from a laser-based SLA, a digital micro-mirror device (DMD) is used in the MIP-SL process to dynamically define mask images that are projected on a photo curable resin surface (Figure 2b). Compared with other methods (mechanical forces, shear flows and magnetic field)45, 46 DC voltage is preferred for its easy processibility and high efficiency in the alignment of carbon nanotubes 47. Two parallel plate electrodes were used with DC voltages to get the parallel alignment (Figure 2c, gaps 3cm, 900V). The alignment relaxation time is determined by τ−1 = (F(D)/3η) G, G = ε0εE2/2 Where ε0 is the electric permittivity of vacuum, η is the matrix viscosity, G is rotational torque, ε is anisotropic dielectric constant, F(D) is the shape factor including aspect ratio D. The relaxation time is in proportional relation with the matrix viscosity. In order to reduce the time for alignment, a photo curable resin with low viscosity (90cp, 20 °C) is chosen. The alignment of carbon nanotubes in PA/MWCNT-S with 1.5wt% filler loading takes 60s. The three key forces that dominate the rotation of carbon nanotubes are torque, Coulombic and electrophoresis forces that act on each nanotube due to the electric field (Figure 2d). The polarization of CNT generated by the electric field leads to a torque force (red arrow). Coulombic attraction is generated among oppositely charged ends of different CNTs (blue arrow). The electrophoresis force is induced by the presence of charged surface (green arrow) 48.
Figure 2.
Schematic diagram of the electrically assisted 3D printing platform for the creation of reinforcement anisotropic composites. a) Diagram of electrically assisted 3D printing device, the rotation of electrodes is controlled by the platform; b) A bottom-up projection process; c) Two parallel electrodes with applied DC electric field and the electrical potential simulated by Comsol Multiphysics; d) Schematic diagram shows rotation of CNT in polymer resin under the application of electric field.
The simulation by Comsol Multiphysics shows the direction of electric field that controls the alignment of MWCNT-S (Figure 2e). After the first layer is cured, the container with nanocomposites and electrodes is rotated by a stepper motor and the base is moved up for a certain distance (Figure 2b). Hence, when the base is moved down to the container, the alignment of MWCNT-S has been changed due to the controlled rotation of nanocomposites relative to the base to achieve a Bouligand-type structure. An accuracy of 0.5 degree control of the rotation angle by the motor is designed to study its effects on the impact resistance. Besides, different alignment of carbon nanotubes is successfully achieved by controlling the types of electric field. Figure S11b and S11c (in supporting information) shows the simulation of the electric field by using two needle electrodes and needle-arc electrodes (300V/cm), respectively. This two kinds of electrodes will result in circumferential and radial (Figure 5e) alignments of carbon nanotubes.
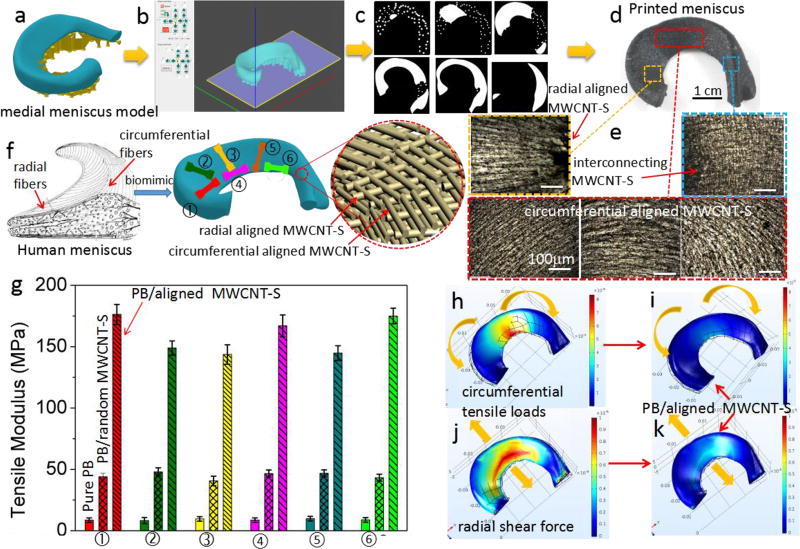
Figure 5.
The schematic diagram of artificial meniscus fabricated by electrically assisted 3D printing. a) model of medial meniscus by Solidworks; b) Model was sliced in the DMD-based SL software to generate different types of patterns for different layers (c); d) Printed medial meniscus; e) Optical microscopy images show radial and circumferential alignment MWCNT-S for one layer and interconnecting for adjacent two layers; f) Schematic diagram of aligned fibers in Human meniscus and the bio-mimic, dog-bone bars for tensile test cut from different parts in the medial meniscus, g) Comparison of tensile modulus in different parts of printed meniscus made by using the pure resin B, PB/random MWCNT-S and PB/aligned MWCNT-S, Simulation by Comsol Multiphysics to show tears in human meniscus, vertical tear (h) and radial tear (j) of human meniscus under circumferential tensile loads and radial shear forces, the relative strain of the printed meniscus with the reinforcement of aligned MWCNT-S under the same forces (i and k).
Functional electrically assisted 3D printed architectures
A Menger structure is designed to demonstrate the electrically assisted 3D printing with Bouligand-type MWCNT-S to enable a new class of strong and lightweight composites. Figure 3 shows the 3D printing process of the Menger sponge model by using PA/MWCNT-S composites (see Methods). The electric field was employed to enable the direction of MWCNT-S during printing. The microscopy images in Figure 3g show that the model (5mm×5mm) was successfully built and the layer thickness is 25µm. The lengths of squares in the model are 2mm, 750µm and 250µm, respectively (different colors of triangles are labels that show the microscopy in different portions of the model). The cross section demonstrates the layer by layer fabrication process. And the uniformity of the layer thickness shows the control of the electrically assisted 3d printing in building reinforced architectures. SEM images show that there is no defect between layers, which suggests the interlayer bonding is strong.
Figure 3.
Schematic diagram of the printing process of functional models by electrically assisted 3D printing. a) the Menger sponge model by solid works, b) sliced in our digital micro-mirror device based Stereolithography (DMD-based SL) software to generate different patterns for projection as shown in c) and f); d) The diagram of the electrically assisted 3D printer; e) Photo of the fabricated Menger model; g) The optical microscopy images of different portions in e) on the model different colors of triangles are marked with magnified SEM images to show the details.
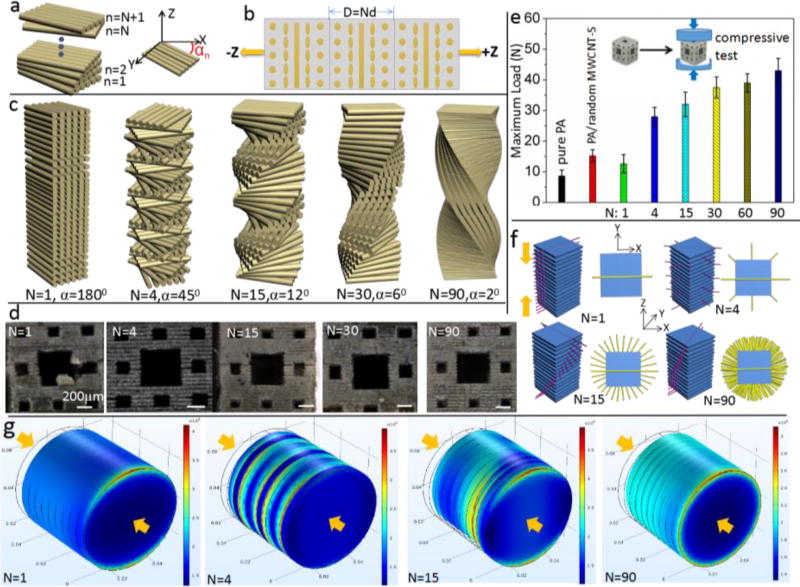
The Bouligand structure of fibers is shown to enhance the impact resistance under static loading conditions49. Here a static compression force was act on the printed Menger structure (a smaller Menger model, 2.4 mm×2.4 mm, with layer thickness of 50 µm) by Instron-5942 to test the ability of impact resistance with different angles of Bouligand-type MWCNT-S (Figure S7, Supporting information). Schematic diagram (Figure 4a) shows that the layer starts from n=1 to n=N+1. Each nth layer forms an angle αn = (n-1)α with the global x-axis, where n is the layer number and α is the rotating angle formed between adjacent two layers. N layers complete a 180° rotation (αN=180°) through a pitch distance D = Nd (d is the layer thickness). Figure 4b shows three cycles of unit cell repetition along the z-axis for N=4, which shows that the aligned MWCNT-S layer starts from the x-axis with different rotations for following layers and return to the x-axis at N=5. Figure 4c shows schematic diagram of layered structures for different values of N. For undirectional N=1, the rotation angle is 180°. For N=4, the aligned CNT rotates α=45° to form the second layer, 90° to form the third layer until it reaches the 5th layer to complete a 180°rotation. For N=15, the rotation angle is α=12°, α=6° for N=30 and α=2° for N=90. The accurate control of the alignment of MWCNT-S bundles is discussed in Figure S10 (supporting information).
Figure 4.
Impact resistance test for Menger models with different rotation angles. a) Diagram of the rotation for different layers; b) unit cell repetition along z-axis for N=4; c) Schematic diagram of different types of layered pitch; d) micrograph of the fraction of electrically assisted 3D printed models by the same compression load (30N); e) Comparison of load of fracture for the models printed by pure resin, random MWCNT-S and aligned MWCNT-S with different N values; (f) Schematic diagram showing the direction of crack propagation (red lines) and crack arrest (yellow lines) for different rotation angles in the printed structure; g) Simulations by Comsol Multiphysics show the stress distribution for different values of N under the same compression (200 k Pa)-arrows show the direction of the force.
The comparison of impact resistance under the same load for different N is shown in Figure 4d. The results show an increased maximum load (the load for initial fracture) with the increment of N (Figure 4e; Figure S7, supporting information). The sample will deform in the xy plane under the compression in the z direction. The schematic diagram in Figure 4f shows the crack lines in each layer under compression. The cracks will follow the path of least resistance (red lines parallel to the alignment of CNT), propagating between aligned CNT rather than severing them. For example, for N=1, if a crack in the matrix occurs in the 0° direction on the bottom layer, the stress concentration will affect the directionality of the damage zone in the second and subsequent layers (see Figure 4g, the stress is concentrated on the red area with the maximum stress higher than 400kPa). The crack propagation will easily follow the red lines without the need to sever MWCNT-S all through the sample, which leads to catastrophic failure in the compression direction (Figure 4d). Thus the alignment of N=1 leads to a decrease of maximum load even compared with the random distributed MWCNT-S composites. A quasi-isotropic layup (N=4, α=45°) was chosen as it is an aerospace industry standard design and a robust baseline architecture50. Figure 4f shows the rotation of crack propagation direction while the yellow line is perpendicular to the alignment of CNT and represents the direction of crack arrest. With the increment of N, the crack is redirected and twisted through the thickness of the sample, leading to a more tortuous crack path thus greater energy dissipation. The vertical views from the z direction show that the crack is arrested throughout the xy plane for N=90 compared with only one direction for N=1 (Figure 4f). Through in-plane spreading of cracks and crack redirection, catastrophic propagation of damage through the thickness of the sample is prevented 51. The studies of compressive strength show that it increases with the increment of N (Figure S8, Supporting information). This observation is consistent with previous studies that show a wide in-plane spread of damage and an increase in stiffness for a smaller fiber rotation angle 50. No delamination between interlayers is observed after fracture, which indicates the interface area is strong during compression (Figure S8, Supporting information). A larger pitch length indicates a smaller rotation angle and a larger number of aligned MWCNT-S layers, which result in increased energy dissipation (Figure 4g). Compared with the concentrated stress in N=1, the stress is distributed in various layers (N=4 and N=15) until all through the sample (N=90, Figure 4g). Under a compression of 200 k Pa, the maximum stress is 400 k Pa for N=1 and decrease to 300 k Pa for N=90. Additionally, as elastic properties are a function of fiber angle, the graded design with a larger value of N results in a smooth change in the in-plane stiffness and will reduce the interlaminar shear stresses (a key source of delamination) 52, 53. Thus a smaller rotation angle will enhance the ability to withstand greater deflections. Meanwhile, the strain is increased for a large N. This results in a rotating crack front, which yields a large surface area per unit crack length in the direction of crack propagation38.
Bioinspired Meniscus with circumferential and radial aligned MWCNT-S by electrically assisted 3D printing
The meniscus plays a crucial role in load bearing, shock absorption, as well as lubrication and nutrition of articular cartilage. The multiple roles leave the menisci susceptible to permanent post traumatic and degenerative lesions54. In recent decades, extensive scientific investigations have been established to study the structure, tear mechanism and repair solution of meniscus55. The mechanical function of human meniscus is dependent on its unique fiber-aligned (circumferential and radial) collagen architecture (Figure 5f). Vertical (Fig. 5h) and radial (Fig. 5j) tears caused by circumferential tensile and radial shear forces under compression and radial deformation55, 56. This architecture is interrupted after the meniscus tear and the ability of transmitting load is compromised. Therefore, both the circumferential and radial modulus should be enhanced for artificial meniscus in order to prevent the failure.
The nature of oriented collagen fibers in native menisci inspires us to solve the problem by mimicking the alignment to achieve enhanced anisotropic mechanical property (Figure 5f) 56, 58. Here an artificial meniscus with highly oriented carbon nanotubes (radial and circumferential) was fabricated by electrically assisted 3D printing. Polymer resin B was chosen due to its soft and photo curable properties. Compared with the collagen fiber, carbon nanotubes make greater contributions to the biomechanical properties due to their excellent mechanical property along the direction of the load. Figure 5a–d show the electrically assisted 3D printing process of medial meniscus with a pair of needle electrodes and needle-arc electrodes (Figure S11, supporting information). Circumferential, radial alignments as well as the interconnecting of MWCNT-S between adjacent layers were observed (Figure 5e). Figure 5f shows the schematic diagram of printed medial meniscus, different colors of dog-bone samples show the position on the meniscus for the tensile test. The results show that both the radial and circumferential tensile moduli were enhanced (Figure 5g). The tensile modulus of pure resin B is about 8.7MPa. With the incorporation of random distributed carbon nanotubes (1.5 wt%), this value is increased to 43 MPa. The results show enhanced circumferential moduli (②,③,⑤) of 176 MPa (higher than the circumferential moduli of human menisci (120MPa)) and radial moduli (①,④,⑥) of 143MPa (3 times of the radial moduli of human menisci (48MPa) 57, 58. The enhancement of tensile modulus is attributed to the alignment of carbon nanotubes, which will make a great contribution to prevent the meniscus from failure. Specifically, the circumferential aligned MWCNT-S will prevent the radial tear (Figure 5h and i) and the radial alignment will prevent the circumferential tears (Figure 5j and k). The replacement used for meniscus tissue engineering must endure multiple mechanical stresses to provide adequate mechanical support. The stress-strain curves and compressive properties of printed meniscus were studied (Fig. S12, Supporting information). The results show that the printed meniscus has a little higher compressive modulus, higher tensile strength and strain at rupture than human menisci 59. The compressive modulus (0.79MPa) is comparable to the human meniscus (0.69 MPa). This result suggestions that the printed meniscus can function as a shock absorber and enhance the tear resistance to prolong the life time of usage. The ability for circumventing the crack propagation is important for the biomimetic printed meniscus. Two groups of samples (no pre-cut and pre-cut) prepared with pure PB, PB/random MWCNT-S and PB/aligned MWCNT-S (with circumferential and radial alignments) were tested. The results (discussed in Figure S13, supporting information) show enhanced fracture energy as the interfacial friction between aligned MWCNT-S bundles and polymer matrix and crack deflection. The enhanced modulus and fracture energy demonstrated that the electrically assisted 3D printed meniscus holds promise as a replica for tissue constructs to circumvent meniscus tear. Besides, 3D printing offers a new way to make customized replacement using computer-aided design (CAD) models that are individualized to a specific patient and his/her meniscus defect.
3. Conclusion
Here we propose a way to build different bio-inspired structure by controlling the alignment of MWCNT-S using electric field during 3D printing. The new printing process leads us an effective approach to the generation of reinforced architectures. Bouligand MWCNT-S reinforced composites provide insight into toughening mechanism and reveal guidelines for the design of new impact resistant materials. With the precision orientation control of MWCNT-S over the reinforcing architecture, more complex and functional materials created via additive manufacturing will find application in a wide range of engineering disciplines. The potential usage of electrically assisted 3D printed meniscus lies in the combination with Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) scans to print custom-made individual implants60. A photocurable bioink containing cells might also be processed with the proposed approach. The ultimate goal is to mimic the native structure and mechanical properties of the target tissue to develop the printed structures into a functional tissue.
In summary, the electrically assisted 3D printing shows a great promise as a new manufacturing tool to design the reinforced structure for maximized properties, especially for bioinspired architectures. The Menger structures were successfully fabricated with Bouligand-type MWCNT-S to mimic the Bouligand collagen or chitin fibers in nature. The maximum load is profoundly enhanced attributed to the fracture resistance of the Bouligand pattern as shown in natural armour. The results show that a smaller rotation angle leads to a greater energy dissipation and impact resistance. The study will enable the design of improved engineered biomimetic structural materials. Besides, this work provides a feasible method for printing artificial meniscus with enhanced mechanical performance to prevent the tear disease. The printed menisci will find wide application in the repair of meniscal defects and other fibrous tissues. This electrically assisted 3D printing technology enables us to design and evolve reinforced architectures with arbitrary 3D geometries and MWCNT-S orientations, which offers tremendous possibilities for applications in aerospace, mechanical and tissue engineering.
4. Experimental section
Surface modification of MWCNT
0.5 gram of MWCNT-OH (Bucky USA. Inc) was first chemically treated with 30ml of 10N sulfuric acid in the presence of 1g of potassium dichromate for 1h at 80°C. It was then filtered and washed with hot and cold water several times to remove the chromic acid and dried in oven at 90 °C. Then 0.5ml 3-aminopropyltriethoxysilane dissolved in acetone was added to the carbon nanotube dispersed in water and the stirring was continued for 1h at 80 °C. The resultant was filtered and washed with acetone (Supporting information, Fig. S1a).
Preparation of Polymer/MINT-S composite resin
Polymer resin A (PA) from Makerjuice (MakerJuice Labs, Kansas, US) is selected in our study due to its excellent photo-sensitivity and mechanical property. It contains high tensile epoxy diacrylate (~9000 psi) and the glycol diacrylate as well as the photoinitiator. This particular resin has a very high tensile strength (63MPa). MWCNT-S were mixed with polymer resin for 2 hours under a magnetic stirring and then ultrasonic bath for 30min. The composite was degassed in the vacuum before fabrication. PB/MWCNT-S nanocomposite was prepared with the same method.
Electrically assisted printing of polymer A/MWCNT-S architectures
PA/MWCNT-S nanocomposite was deposited in a transparent glass tank (Figure 2a), the photo curable resin is cured after mask images are projected upwards onto the bottom of the substrate by the projector. Two parallel plate electrodes (1cm×3cm) were used with DC voltages to get the parallel alignment. Uniform thin layers are then re-coated by moving the platform to form a desired gap between the previously cured layers and the glass substrate. The container is rotated by a motor after the base is moved up to the highest level. In this case, when the base is moved down to the container, the alignment is changed. Through controlling rotation angles of the container at different layers, Bouligand-type anisotropy can be introduced in the final MWCNT-S reinforced architecture. The resolution of the DMD chip (Texas Instrument, Dallas, TX) is 1024×768, the output light intensity of the projector is 3.16 mW/cm2.
Electrically assisted printing of Menger model
The Menger sponge model was first created using Solid works (Figure 3a) and then sliced by an in-house developed ‘DMD-based SL’ software (Figure 3b) to get different patterns (Figure 3c and f). Those patterns were then projected to build different layers. For example, the first layer was built by projecting the 1st pattern until we get the 100th layer (Figure 3f). For the fabrication process, different patterns will be projected on the surface of PA/MWCNT-S nanocomposite. The composites will become cured attributed to the photo curable property of the resin. After the first layer was cured on the base, the base will move up, then move down for the second layer to be projected and so on. Rotating nanocomposites with electric field was employed to control the orientation of MWCNT-S related to the base. During the movement, the layer thickness is controlled to be less than the cure depth to make sure that the following layer is stick onto the previous layers. In our study, the movement in the z direction is controlled to be 25–100µm and the cure depth is about 180µm for 1s. Different rotation angles (from 2°–180°) were used to study its effect on the ability of impact resistance. The curing time of each layer is 10s and the movement of the base takes about 5s. The alignment of MWCNT-S for 60s is done in the beginning of the building process and will keep the same orientation afterwards. The platform with the electrodes is rotated during the building process to control the MWCNT-S alignment relative to the base. The whole fabrication process takes about 25 minutes for 100 layers. Besides, different models with complicated shapes were successfully fabricated by electrically assisted 3D printing technology (Figure S9, Supporting information).
Electrically assisted Meniscus printing
Human medial and lateral menisci are wedge shaped and semi lunar with distinctly different dimensions. Polymer resin B was purchased from 3D Systems. Inc. The best performance with 1.5 wt% loading of MWCNT-S was chosen for the fabrication process. Fig.5a-d shows the electrically assisted 3D printing process of medial meniscus. Different kinds of electrodes are used for the promotion of circumferential (a pair of needle electrodes) and radial (needle-arc electrodes) alignment of MWCNT-S (Figure S11, Supporting information). The models by Solid works was firstly sliced in the DMD-based SL software (Fig. 5b) to generate different types of patterns for different layers (Fig. 5c). Medial meniscus was printed with pure resin B and PB/aligned MWCNT-S nanocomposites (Figure 5d). The circumferential alignment is controlled by a pair of needle electrodes to get the first layer, after that, the voltage was shut down, then the radial alignment on second layer is achieved by applying a DC voltage on the two needle electrodes. In this case, two types of alignment of MWCNT-S were got by alternatively applying the DC voltages. The artificial menisci with both types of aligned MWCNT-S were successfully fabricated by the electrically assisted 3D printing process.
Test of mechanical properties
A smaller Menger model was fabricated with dimension 2.4mm×2.4mm in order not to exceed the maximum load of the testing machine. The fabrication process was the same as the Menger model with dimension of 6mm×6mm. A universal testing machine (Instron 5492 Dual Column Testing Systems, Instron, Massachusetts, USA) was used. Before the test, the sample was put in the middle of the test plate with the compression force vertical to the alignment of MWCNT-S. The static compression was chosen and the velocity of compression is 1mm/min with a maximum compression of 2mm. The pictures from the side view was instantly taken after the test by an optical microscopy. Slices of the printed meniscus were cut into tensile samples using a 3D printed dumb bell shaped punch (central width 3mm and central length 10mm.) Six different meniscal specimens were tested to represent different meniscal regions (Figure 5f). In the tensile tests, the velocity of extension is 2mm/min with a maximum extension of 3mm at room temperature. In all of tests, 5 samples with the same alignment were tested to reduce the experimental error.
DC voltages were applied by using Model 210-02R high voltage power supply. FTIR spectra were collected by using a FT/IR 420 Fourier Transform Infrared Spectrometer. The optical images were obtained on a Micro Vu Sol 161 Microscope. SEM images of fracture cross section of printed Menger model were taken by using JSM-6610LV Scanning Electron Microscope.
Simulation by Comsol Multiphysics
No sliding was observed between the sample and the clamp plates during the compression test. Compression was modeled by clamps with fixed boundary conditions on the bottom and a compression of 200 k Pa on the top. The model was designed by Solid works and then imported to Comsol Multiphysics. 10 layers were fabricated and the material was chosen orthotropic for each layer and the modulus was defined from our test results. Different rotating coordinates were applied on each layer, as such, the rotating angles (0°, 45°, 12°, 2°) can be chosen according to our design. For example, in Figure 4g, the rotation angle is 180° for N=1. For N=4, the rotation angle is 45°, thus, the third layer rotates 90° until the 5th layer to 180°. The relative strain of medial meniscus was simulated by importing the model from Solid works into Comsol Multiphysics. Circumferential tensile loads and radial shear forces (200N) are applied on the model to study the deformation, respectively. The modulus of human menisci was used from references (55, 56) to get the representation of meniscus tear (Figure 5h and j). The moduli used for the simulation of relative strain of printed meniscus (Figure 5i and k) were based on the data from our study.
Supplementary Material
Acknowledgments
We thank the support from National Science Foundation (NSF) CMMI-1335476, NSF-CMMI 1151191 and National Institutes of Health (NIH) Grant P41-EB002182, and NIH 1R01HL118650 and China Scholarship Council. We also acknowledge Prof. Qiming Wang and Zheming Gao for their help on the mechanical tests and Center for Electron Microscopy and Microanalysis at USC for the SEM images measurement.
Footnotes
Supporting information accompanies this paper
References
- 1.Ferrand HL, Bouville F, Niebel TP, Studart AR. Magnetically assisted slip casting of bioinspired heterogeneous composites. Nature Mater. 2015;14:1172–1179. doi: 10.1038/nmat4419. [DOI] [PubMed] [Google Scholar]
- 2.Bouville F, Maire E, Meille S, Moortele BV, Stevenson AH, Deville S. Strong, tough and stiff bioinspired ceramics from brittle constituents. Nature Mater. 2014;13:508–514. doi: 10.1038/nmat3915. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Cheng Q, Tang Z. Layered nanocomposites inspired by the structure and mechanical properites of nacre. Chem. Soc. Rev. 2012;41:945–1404. doi: 10.1039/c1cs15106a. [DOI] [PubMed] [Google Scholar]
- 4.Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nature Mater. 2015;14:23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 5.Yaraghi NA, et al. A Sinusoidally Architected Helicoidal Biocomposite. Adv. Mater. 2016;28:6835–6844. doi: 10.1002/adma.201600786. [DOI] [PubMed] [Google Scholar]
- 6.Martin JJ, Fiore BE, Erb RM. Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nature Commun. 2015;6:8641. doi: 10.1038/ncomms9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinosa HD, et al. Tablet-level origin of toughening in abalone shells and translation to synthetic composite materials. Nature Commun. 2011;2:173. doi: 10.1038/ncomms1172. [DOI] [PubMed] [Google Scholar]
- 8.Nawroth JC, et al. A tissue-engineered jellyfish with biomimetic propulsion. Nature Biotech. 2012;30:792–800. doi: 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver JC, et al. The stomatopod dactyl club: a formidable damage-tolerant biological hammer. Science. 2012;336:1275–1280. doi: 10.1126/science.1218764. [DOI] [PubMed] [Google Scholar]
- 10.Grunenfelder LK, et al. Bio-inspired impact-resistant composites. Acta Biomater. 2014;10:3997–4008. doi: 10.1016/j.actbio.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Finnemore A, et al. Biomimetic layer-by-layer assembly of artificial nacre. Nature Commun. 2012;3:966. doi: 10.1038/ncomms1970. [DOI] [PubMed] [Google Scholar]
- 12.Espinosa HD, et al. Tablet level origin of toughening in abalone shells and translation to synthetic composite materials. Nature Commun. 2012;2:173. doi: 10.1038/ncomms1172. [DOI] [PubMed] [Google Scholar]
- 13.Kokkinis D, Schaffner M, Studart AR. Multimaterial magnetically assisted 3D printing of composite materials. Nature Commun. 2015;6:8643. doi: 10.1038/ncomms9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patek SN, Korff WL, Caldwell RL. Biomechanics: Deadly strike mechanism of a mantis shrimp. Nature. 2004;428:819–820. doi: 10.1038/428819a. [DOI] [PubMed] [Google Scholar]
- 15.Raabe D, Sachs C, Romano P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005;53:4281–4292. [Google Scholar]
- 16.Romano P, Fabritius H, Raabe D. The exoskeleton of the lobster Homarus americanus as an example of a smart anisotropic biological material. Acta Biomater. 2007;3:301–309. doi: 10.1016/j.actbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann EA. Mechanical adaptability of the Bouligand-type structure in natural dermal armour. Nature Commun. 2013;4:2634. doi: 10.1038/ncomms3634. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Wang L, Karlsson AM. Mechanics-based analysis of selected features of the exoskeletal microstructure of Popillia japonica. J. Mater. Res. 2009;24:3253–3267. [Google Scholar]
- 19.Daly IM. Dynamic polarization vision in mantis shrimps. Nature Commun. 2016;7:12140. doi: 10.1038/ncomms12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawalmih AA. Microtexture and Chitin/Calcite Orientation Relationship in the Mineralized Exoskeleton of the American Lobster. Adv. Fun. Mater. 2008;18:3307–3314. [Google Scholar]
- 21.Fabritius HO, Sachs C, Triguero PR, Raabe D. Influence of Structural Principles on the Mechanics of a Biological Fiber-Based Composite Material with Hierarchical Organization: The Exoskeleton of the Lobster Homarus americanus. Adv. Mater. 2009;21:391–400. [Google Scholar]
- 22.Holmes LR, JR, Riddick JC. Research summary of an Additive Manufacturing technology for the fabrication of 3D composites with tailored internal structure. The journal of the Minerals, Metals & Materials Society. 2014;66:270–274. [Google Scholar]
- 23.Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nature Mater. 2015;14:23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 24.Dimas LS, Bratzel GH, Eylon I, Buehler MJ. Tough Composites inspired by mineralized natural materials: computation, 3D printing, and testing. Adv. Funct. Mater. 2013;23:4629–4638. [Google Scholar]
- 25.Compton BG, Lewis JA. 3D-printing of lightweight cellular composites. Adv. Mater. 2014;26:5930–5935. doi: 10.1002/adma.201401804. [DOI] [PubMed] [Google Scholar]
- 26.Erb RM, Libanori R, Rothfuchs N, Studart AR. Composites Reinforced in Three Dimensions by Using Low Magnetic Fields. Science. 2012;335:199–204. doi: 10.1126/science.1210822. [DOI] [PubMed] [Google Scholar]
- 27.Schadler LS, Giannaris SC, Ajayan PM. Load transfer in carbon nanotube epoxy composites. Appl. Phys. Lett. 1998;73:3842–3845. [Google Scholar]
- 28.Huang S, Liu P, Mokasdar A, Hou L. Additive manufacturing and its societal impact: a literature review. Int. J. Adv. Manuf. Technol. 2013;67:1191–1203. [Google Scholar]
- 29.Gao W, et al. The Status, Challenges, and Future of Additive Manufacturing in Engineering. Comput. Aided Design. 2015;69:65–89. [Google Scholar]
- 30.Hutchinson ID, Moran CJ, Potter HG, Warren RF, Rodeo SA. Restoration of the meniscus: form and function. Am J. Sports Med. 2014;42:987–998. doi: 10.1177/0363546513498503. [DOI] [PubMed] [Google Scholar]
- 31.Wei J, et al. 3D printing of an extremely tough hydrogel. RSC Adv. 2015;5:81324–81329. [Google Scholar]
- 32.Multilayered silk scaffolds for meniscus tissue engineering. Mandal Biman B, Park Sang-Hyug, Gil Eun S, Kaplan David L. Biomaterials. 2011;32:639–651. doi: 10.1016/j.biomaterials.2010.08.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi M, Chang YS, Oka M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials. 2005;26:3243–3248. doi: 10.1016/j.biomaterials.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Baker BM, Mauck RL. The Effect of Nanofiber Alignment on the Maturation of Engineered Meniscus Constructs. Biomaterials. 2007;28:1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nature Mater. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 36.Gonçalves EM, et al. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J Biomed Mater Res B Appl Biomater. 2016;104:1210–1219. doi: 10.1002/jbm.b.33432. [DOI] [PubMed] [Google Scholar]
- 37.Nikolov S, et al. Revealing the Design Principles of High-Performance Biological Composites Using Ab initio and Multiscale Simulations: The Example of Lobster Cuticle. Adv. Mater. 2010;22:519–526. doi: 10.1002/adma.200902019. [DOI] [PubMed] [Google Scholar]
- 38.Weaver JC, et al. The Stomatopod Dactyl Club:A Formidable Damage-Tolerant Biological Hammer. Science. 2012;336:1275–1280. doi: 10.1126/science.1218764. [DOI] [PubMed] [Google Scholar]
- 39.Bruet BJF, Song J, Boyce MC, Ortiz C. Materials design principles of ancient fish armour. Nature Mater. 2008;7:748–756. doi: 10.1038/nmat2231. [DOI] [PubMed] [Google Scholar]
- 40.Sachs C, Fabritius H, Raabe D. Influence of microstructure on deformation anisotropy of mineralized cuticle from the lobster Homarus americanus. J. Struct. Biol. 2008;161:120–132. doi: 10.1016/j.jsb.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, et al. Three dimensional printing of high dielectric capacitor using projection based stereolithography method. Nano Energy. 2016;22:414–421. [Google Scholar]
- 42.Chen Z, et al. 3D printing of piezoelectric element for energy focusing and ultrasonic sensing. Nano Energy. 2016;27:78–86. [Google Scholar]
- 43.Pan Y, Zhao X, Zhou C, Chen Y. Smooth surface fabrication in mask projection based stereolithography. J. Manuf. Process. 2012;14:460–470. [Google Scholar]
- 44.Song Xuan, Chen Yong, Lee TW, Wu SH, Cheng LX. Ceramic fabrication using mask image projection based stereolithography integrated with tape casting. Journal of Manufacturing Processes. 2015;20:456–464. [Google Scholar]
- 45.Zhao H, et al. A facile method to align carbon nanotubes on polymeric membrane substrate. Scientific Reports. 2013;3:3480. doi: 10.1038/srep03480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinert BW, Dean DR. Magnetic field alignment and electrical properties of solution cast PET-carbon nanotube composite films. Polymer. 2009;50:898–904. [Google Scholar]
- 47.Park C, et al. Aligned Single-Wall Carbon Nanotube Polymer Composites Using an Electric Field. J. Polym. Sci. Pol. Phys. 2006;44:1751–1762. [Google Scholar]
- 48.Takahashi T, Murayama T, Higuchi A, Awano H, Yonetake K. Aligning vapor grown carbon fibers in polydimethysioxane using DC electric of magnetic field. Carbon. 2006;44:1180–1187. [Google Scholar]
- 49.Zimmermann EA, et al. Mechanical adaptability of the Bouligand-type structure in natural dermal armour. Nature Commun. 2013;4:2634. doi: 10.1038/ncomms3634. [DOI] [PubMed] [Google Scholar]
- 50.Grunenfelder LK, et al. Bio-inspired impact-resistant composites. Acta Biomater. 2014;10:3997–4008. doi: 10.1016/j.actbio.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Meyers MA, McKittrick J, Chen PY. Structural biological materials: critical mehcanics-materials connections. Science. 2013;339:773–779. doi: 10.1126/science.1220854. [DOI] [PubMed] [Google Scholar]
- 52.Suresh S. Graded materials for resistance to contact deformation and damage. Science. 2001;292:2447–2451. doi: 10.1126/science.1059716. [DOI] [PubMed] [Google Scholar]
- 53.Apichattrabrut T, Ravi CK. Helicoidal composites. Mech. Adv. Mater. Struct. 2006;13:61–76. [Google Scholar]
- 54.Tissakht M, Ahmed AM, Chan KC. Calculated stress-shielding in the distal femur after total knee replacement corresponds to the reported location of bone loss. J. Orthop. Res. 1996;14:778–785. doi: 10.1002/jor.1100140515. [DOI] [PubMed] [Google Scholar]
- 55.Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health. 2012;4:340–351. doi: 10.1177/1941738111429419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sweigart MA, Athanasiou KA. Toward tissue engineering of the knee meniscus. Tissue. Eng. 2001;7:111–129. doi: 10.1089/107632701300062697. [DOI] [PubMed] [Google Scholar]
- 57.Tissakht M, Ahmed AM. Tensile stress strain characteristics of the human meniscal material. J. Biomech. 1995;28:411–422. doi: 10.1016/0021-9290(94)00081-e. [DOI] [PubMed] [Google Scholar]
- 58.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: Structure function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chia HN, Hull ML. Compressive Moduli of the Human Medial Meniscus in the Axial and Radial Directions at Equilibrium and at a Physiological Strain Rate. J. Orthop. Res. 2008;26:951–956. doi: 10.1002/jor.20573. [DOI] [PubMed] [Google Scholar]
- 60.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.