Abstract
Background:
Elevated production of the pro-inflammatory cytokine interleukin-6 (IL-6) and dysfunction of IL-6 signaling promotes tumorigenesis and are associated with poor survival outcomes in multiple cancer types. Recent studies showed that the IL-6/GP130/STAT3 signaling pathway plays a pivotal role in pancreatic cancer development and maintenance.
Objective:
We aim to develop effective treatments through inhibition of IL-6/GP130 signaling in pancreatic cancer.
Method:
The effects on cell viability and cell proliferation were measured by MTT and BrdU assays, respectively. The effects on glycolysis was determined by cell-based assays to measure lactate levels. Protein expression changes were evaluated by western blotting and immunoprecipitation. siRNA transfection was used to knock down estrogen receptor α gene expression. Colony forming ability was determined by colony forming cell assay.
Results:
We demonstrated that IL-6 can induce pancreatic cancer cell viability/proliferation and glycolysis. We also showed that a repurposing FDA-approved drug bazedoxifene could inhibit the IL-6/IL-6R/GP130 complexes. Bazedoxifene also inhibited JAK1 binding to IL-6/IL-6R/GP130 complexes and STAT3 phosphorylation. In addition, bazedoxifene impeded IL-6 mediated cell viability/proliferation and glycolysis in pancreatic cancer cells. Consistently, other IL-6/GP130 inhibitors SC144 and evista showed similar inhibition of IL-6 stimulated cell viability, cell proliferation and glycolysis. Furthermore, all three IL-6/GP130 inhibitors reduced the colony forming ability in pancreatic cancer cells.
Conclusion:
Our findings demonstrated that IL-6 stimulates pancreatic cancer cell proliferation, survival and glycolysis, and supported persistent IL-6 signaling is a viable therapeutic target for pancreatic cancer using IL-6/GP130 inhibitors.
Keywords: IL-6/GP130 signaling, bazedoxifene, pancreatic cancer, cell viability, cell proliferation, glycolysis, colony forming
1. INTRODUCTION
Pancreatic cancer is a lethal malignancy with poor prognosis and little treatment options, and is expected to surpass colon cancer, becoming the second leading cause of cancerrelated deaths by the year 2030 [1]. About 53,670 people are expected to be diagnosed with pancreatic cancer and estimated about 43,090 people will die of pancreatic cancer in the United States in 2017. Currently, there are no validated treatment approaches for pancreatic cancer, and surgery remains the only option for therapy, despite only a minority of patients deemed eligible [2]. The standard therapy for advanced pancreatic cancer is the combination of gemcitabine and nab-paclitaxel (mGNabP) or aggressive chemotherapy, such as FOLFIRINOX (combination of 5-FU, leu-covorin, oxapliplatin and irinotecan) in the curative stage [3–6]. Although effective, these regimens are linked to noticeable cumulative hematological toxicities and cost. Searching for novel strategies with potential for long-term clinical relevance and cures for pancreatic cancer remains an urgent task.
Interleukin-6 (IL-6) is the archetypal member of the GP130-related cytokine family, and is known as a regulator by the involvement in inflammation response, immune defense as well as in modulation of development and differentiation in many human malignancies [7–9]. IL-6 also controls homeostatic functions, including regulation of glucose metabolism [10]. IL-6 fulfills its pleiotropic effects by binding a non-signaling α-receptor (IL-6R) to recruit GP130 homodimer and form IL-6/IL-6R/GP130 hexamer, initiating intra-cellular signaling cascades consisting of the JAK/STAT (Janus kinase/signal transducer and activator of transcription), Ras/Raf/MEK/MAPK (mitogen-activated protein kinase), and PI3K/AKT (phosphatidylinositol 3-kinase, a serine/threonine kinase) pathways [11,12]. JAK/STAT signaling is considered as the most prominent and accumulating evidence indicates that IL-6/JAK/STAT3 signaling pathway is activated in a variety of common solid tumors contributing to cancer progression [13–17]. In light of this, inhibition of IL-6/JAK/STAT3 signaling might be a new cancer therapeutic option, and IL-6 and IL-6R blocking antibodies, JAK inhibitors as well as STAT3 inhibitors have been explored [16,18–22]. Many IL-6 ligand binding antibodies and IL-6R blocking antibodies have been developed and are recently in clinical trials. However, dramatically increases in systemic IL-6 and risk of infections were observed [23].
Numerous studies unveiled that excess production of IL-6 and dysfunction of IL-6 signaling pathway, particularly the IL-6/JAK/STAT3 signaling pathway was strongly associated with the human pancreatic cancer development and progression [24–26]. A recent study conducted on genetically engineered mouse models demonstrated the significance of impediment of IL-6/JAK/STAT3 pathway to prevent pancreatic cancer progression [27]. New functions of classical small molecular inhibitors play critical roles in drug discovery. In our previous studies, FDA-approved drug bazedoxifene has been re-purposed as a novel small molecular inhibitor of trimetric IL-6/IL-6R/GP130 complex, resulting in apoptosis and suppressing tumor growth in human cancer cell lines and a xenograft model [28, 29]. The objective of our study is to evaluate the efficacy of bazedoxifene on IL-6/IL-6R/GP130 and functions in pancreatic cancer cells. As a result, bazedoxifene not only selectively inhibited IL-6 induced cell viability, cell proliferation and glycolysis, but also blocked colony formation, supporting that bazedoxifene could be a potent inhibitor against the IL-6/GP130 signaling in pancreatic cancer cells.
2. METHOD
2.1. Cell Culture and Reagents
Human pancreatic cancer cell lines (PANC-1, HPAF-II, Capan-1, BxPC-3, and MIA PaCa-2) were purchased from the ATCC. Short-term cultured primary murine pancreatic cancer cell lines (8–365APR and 22–614APR) from a clinically relevant p16lox/lox; LSL-KrasGI2D; Pdx1-Cre genetically engineered mouse model were provided by Dr. Gloria H. Su at Columbia University Medical Center. Cells were cultured in 1 × Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech, #10013 CV) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, #S11150) and 1% Penicillin/Streptomycin (P/S) (Sigma, #P0781) in incubators with 5% CO2 at 37 °C.
All reagents in the study are as follows: recombinant human IL-6 (Cell Signaling Technology, #8904SF), recombinant mouse IL-6 (Cell Signaling Technology, #5216SF), bazedoxifene (Sigma, #PZ0018), SC144 (Sigma, #SML 0763), evista (Sigma, #R1402), dimethyl sulfoxide (DMSO) (Sigma, #D2650), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma, #M5655), N, N-dimethylformamide (DMF) (Fisher, #D119–4) and crystal violet (Sigma, #C6158). The stock solution of drugs was prepared by transferring 10 mg to the DMSO at a concentration of 20 mM. IL-6 powder was dissolved in sterile PBS to make a 100 ng/μL stock solution. Aliquots of the stock solutions were stored at −20 °C. All other chemicals used were analytical grade without purification.
2.2. MTT Assay
Cells were seeded in 96-well plates at a density of 3,000 cells per well in triplicate and allowed to adhere overnight. Cells were treated with IL-6 and/or other inhibitors with different concentrations in the presence of 0% FBS medium for 48 hours at 37 °C. MTT (20 μL, 5 mg/mL) was added to each well. The plates were incubated at 37 °C for 4 hours followed by the addition with 150 μL of DMF solubilization solution at gentle shaking overnight. Absorbance was measured at 595 nm.
2.3. BrdU (Bromodeoxyuridine) Cell Proliferation Assay
Cell proliferation was measured using BrdU Cell Proliferation Assay Kit (Cell Signaling Technology, # 6813S). Cells were seeded in 96-well plates at a density of 8,000 cells per well in triplicate and incubated overnight in DMEM, starving overnight with serum free medium before being exposed to serial dilutions of IL-6 and/or inhibitors for 24 hours at 37 °C to induce proliferation and incorporation of BrdU during S-phase. The rest of procedure was performed following the manufacturer’s instructions. The BrdU incorporation was detected at 450 nm.
2.4. Western Blotting Assay
Cells were washed with cold PBS and harvested with a rubber scraper after the desired treatment. Cell pellets were kept on ice and lysed for 20 minutes in cell lysis buffer (Cell Signaling Technology, #9803) contained Tris-HCl (20 mM, pH 7.5), NaCl (150 mM), Na2EDTA (1 mM), EGTA (1 mM), Triton (1%), sodium pyrophosphate (2.5 mM), β-glycerophosphate (1 mM), Na3VO4 (1 mM) and leupeptin (1 μg/mL) with protease and phosphatase inhibitors. The lysates were cleared by centrifugation, and the supernatant fractions were collected. Subsequently, cell lysates were separated by 10% SDS-PAGE and subjected to western blotting analysis with 1:1,000 dilutions of primary antibodies and 1:10,000 horseradish peroxidase-conjugated secondary antibodies. Rabbit primary antibodies against phosphorylated STAT3 (Y705), phosphorylated AKT (Ser473), phosphorylated p44/42 MAPK (ERK1/2) (Thr202/Tyr204), STAT3, phosphor-S6 ribosomal protein (Ser235/236), cyclin D1, cleaved caspase-3 and β-Actin, as well as the anti-rabbit IgG, HRP-linked secondary antibody were used for western blotting. All of them were provided from Cell Signaling Technology. β-Actin served as the loading control in all experiments. Membranes (GE Healthcare, #10600023) were analyzed using SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo, #34096).
2.5. Glycolysis
Extracellular L-lactate in cultured pancreatic cancer cells was measured using Glycolysis Cell-Based Assay Kit (Cayman, Ann Arbor, MI). Assays were conducted following the manufacturer’s instructions. Cells were cultured and seeded at a density of 1 × 104 cells per well in 96-well plates in triplicate and given adhere overnight, followed by treatment with IL-6 and/or other inhibitors in serum free medium. After 48 hours incubation, supernatant (10 μL) from each well was transferred to the corresponding wells on the new plates. Reaction solution (100 μL) composed of assay buffer (phosphate buffered saline), glycolysis assay substrate (2-(4-lodophenyl)-3(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride), glycolysis assay cofactor (NAD+), and glycolysis assay enzyme mixture (diaphorase and L-lactic dehydrogenase) was prepared and added to each well with gentle shaking for 30 minutes at room temperature, then the optical density was detected at 490 nm.
2.6. Transfection
HPAF-II cells were seeded in 10-cm plate and allowed to adhere overnight. The cells were transfected with negative control siRNA (Thermo Fisher, Waltham, MA) or estrogen receptor α siRNA (Santa Cruz Biotechnology, Inc, Dallas, TX) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. After 48 hours, the cells were collected and lysed for western blotting assay, and reseeded in 96-well plates at a density of 3,000 cells per well and incubated for 48 hours. Cell viability was determined by MTT assay as described above.
2.7. Caspase-3/7 Activity Assay
Cells cultured in 96-well plates were left untreated or treated with bazedoxifene (0, 5, 10 and 15 μM). The caspase-3/7 activity was detected using Caspase-3/7 Fluorescence Assay Kit (Cayman, Ann Arbor, MI) according to the manufacture’s instruction.
2.8. Colony Forming Cell Assay
Cells were treated with bazedoxifene, SC144 and evista for 16 hours. After that, cells were harvested and reseeded on 6-cm plates with drug-free medium for approximately 2 weeks incubation. Colonies were fixed with methanol and stained with crystal violet dye (0.1% w/v).
2.9. Immunoprecipitation
Cells were pretreated with bazedoxifene (20 μM) in serum free medium for 4 hours followed by adding IL-6 (50 ng/mL). After additional incubation for 30 minutes and 6 hours respectively, cells were harvested and lysed. Cell lysates were precleared using protein A/G agarose (Pierce Biotechnology, Rockford, IL), incubating with gentle mixing at 4 °C for 2 hours. GP130 was immunoprecipitated by incubating cell lysates with anti-GP130 antibody (EMD Millipore Corporation, Temecula, CA) overnight at 4 °C. Protein agarose slurry was added and further incubated for 2 hours at 4 °C. At the end of incubation, protein A/G agarose was washed three times with IP buffer (Thermo, #28379) and proteins bound to GP130 were collected by boiling the samples in SDS loading buffer. Supernatant was then separated by 10% SDS-PAGE and subjected to western blotting analysis. Monoclonal human IL-6Rα mouse antibody, phosphorylated STAT3 (Y705) rabbit antibody, STAT3 rabbit antibody, GP130 rabbit antibody and the anti-mouse IgG, HRP-linked secondary antibody were purchased from Cell Signaling Technology. JAK1 mouse primary antibody was obtained from R&D System (#MAB42601).
2.10. Statistical Analysis
All results are presented as the means ± standard error of the mean (SEM). The difference between two groups was analyzed using the Student’s t-test (n=3). All statistical analyses were performed using the GraphPad Prism 5.0 software. (*P < 0.05, **P < 0.01, ***P < 0.001)
3. RESULTS
3.1. IL-6 Stimulates Cell Viability in Human and Murine Pancreatic Cancer Cells
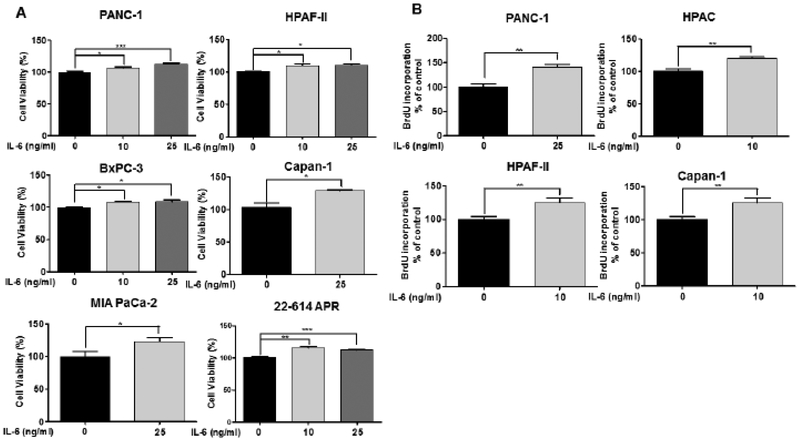
Accumulating evidence suggested that elevated production of IL-6 in serum is strongly associated with aggression of pancreatic cancer [30,31]. To further investigate the role of IL-6, five human pancreatic cancer cells (PANC-1, HPAF-II, Capan-1, BxPC-3 and MIA PaCa-2) and one primary murine pancreatic cancer cells (22–614APR) were treated with different concentrations of recombinant IL-6 (0 −25 ng/mL). Cell viability was detected using MTT assay. Our results showed that cell viability were induced in all six pancreatic cells in response to IL-6. 10 ng/mL and 25 ng/mL of IL-6 significantly induced cell viability in PANC-1, HPAF-II, BxPC-3 cells and 22–614APR cells, while only 25 ng/mL of IL-6 showed statistically significant induction of cell viability in Capan-1 and MIA PaCa-2 cells (Fig. 1A).
Fig. (1).
IL-6 stimulates cell viability and proliferation in pancreatic cancer cells. (A) Human pancreatic cancer cell lines (PANC-1, HPAF-II, BxPC-3, Capan-1 and MIA PaCa-2) and murine primary pancreatic cancer cells (22–614APR) were exposed with recombinant IL-6 (0, 10 and 25 ng/mL) for 48 hours. Cell viability was measured using MTT assay in triplicate. (B) PANC-1, HPAC, HPAF-II and Capan-1 cells were treated with recombinant human IL-6 (0, 10 and 25 ng/mL) for 24 hours. Cell proliferation was detected by BrdU assay.
3.2. IL-6 Promotes Cell Proliferation and Activates STAT3 Phosphorylation in Human Pancreatic Cancer Cells
Due to the stimulation of cell viability in pancreatic cancer cells, we further tested the effect of exogenous human IL-6 on the cell proliferation of pancreatic cancer cells using BrdU incorporation assay, in which cell proliferative changes over time was detected through the incorporation of BrdU into newly synthesized DNA in cells. Four pancreatic cancer cell lines, PANC-1, HPAC, HPAF-II and Capan-1 were treated with different concentrations of recombinant human IL-6 (0 – 25 ng/mL), and BrdU incorporation into DNA was remarkably increased in all four pancreatic cell lines by IL-6 stimulation. Specifically, 10 ng/mL of IL-6 exhibited significant stimulation of cell proliferation in HPAC, HPAF-II, and Capan-1 cells, and the cell proliferation was increased statistically in PANC-1 cells by 25 ng/mL of IL-6 (Fig. 1B).
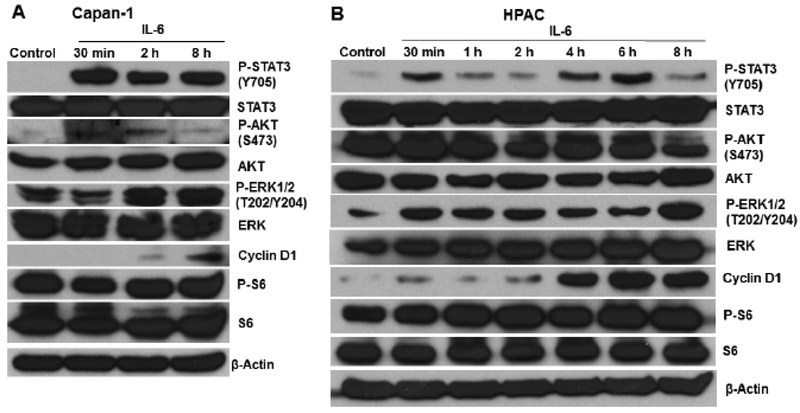
To examine the influence of exogenous IL-6 on the protein expression of phosphorylated STAT3, the principal IL-6 activated signaling molecule, we treated Capan-1 and HPAC cells with human IL-6 (50 ng/mL) and observed the changes in STAT3 phosphorylation at different interval time (0 – 8 hours). Western blotting analysis revealed that IL-6 remarkably increased the phosphorylated STAT3 protein levels in Capan-1 and HPAC cells at different time points. In addition, IL-6 time-dependently induced phosphorylated p44/42 MAPK (ERK1/2) (Thr202/Tyr204) and cyclin D1 in Capan-1 and HPAC cell lines (Fig. 2).
Fig. (2).
IL-6 stimulates the phosphorylation of STAT3 in human pancreatic cancer cells. Western blotting analysis of P-STAT3 (Y705), STAT3, P-AKT (S473), AKT, P-ERK (T202/Y204), ERK, P-S6, S6 and cyclin D1 were performed after addition of IL-6 (50 ng/mL) in (A) Capan-1 and (B) HPAC cells.
3.3. Bazedoxifene Inhibits IL-6 mediated Cell Viability in Pancreatic Cancer Cells
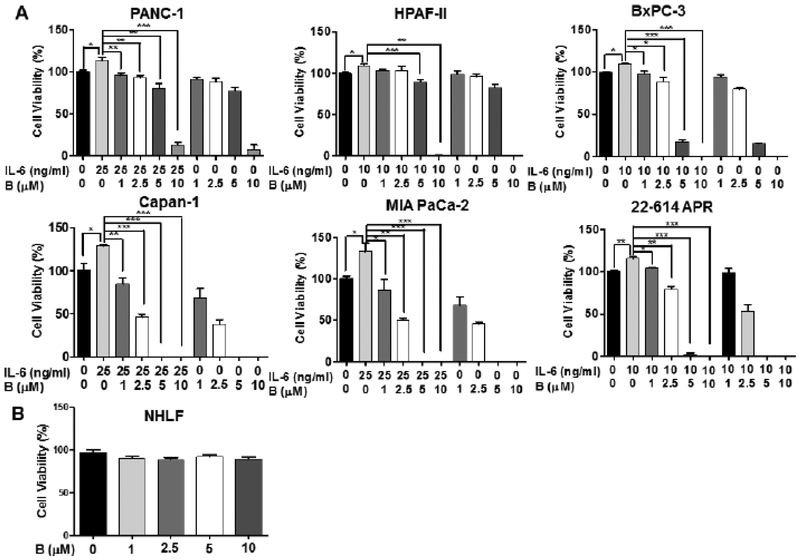
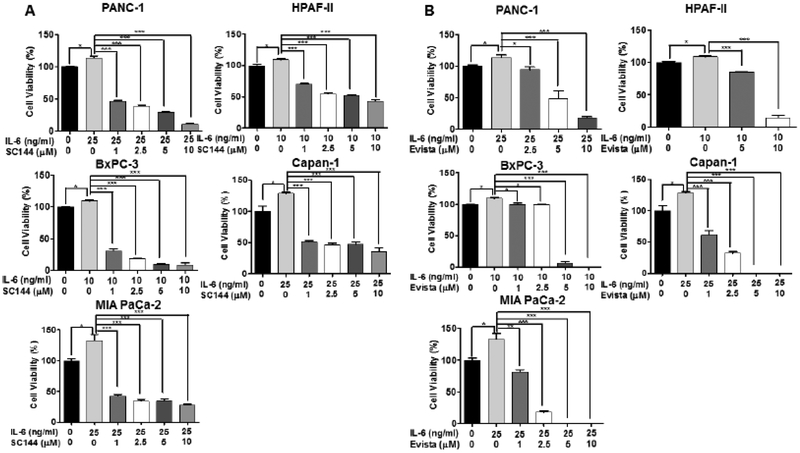
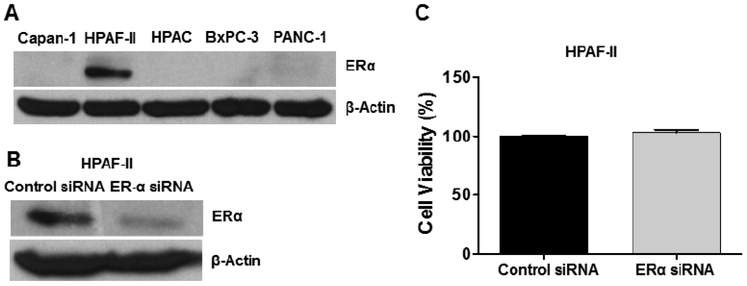
Bazedoxifene is known as a selective estrogen receptor modulator (SERM) and increasing studies implicated that bazedoxifene was able to mediate anti-tumor activity [28, 29, 32]. To investigate whether bazedoxifene suppresses cell viability mediated by IL-6, we pretreated five human pancreatic cancer cell lines (PANC-1, HPAF-II, Capan-1, BxPC-3 and MIA PaCa-2), and one primary murine pancreatic cancer cell line (22–614APR) with different concentrations of bazedoxifene (0, 1, 2.5, 5 and 10 μM) for 4 hours, then added exogenous IL-6. After 48 hours of treatments, a dose-dependent suppression of IL-6-induced cell viability was observed (Fig. 3A). Furthermore, we also tested previously reported GP130 inhibitor, SC144 and IL-6/GP130 inhibitor, evista (raloxifene). Consistently, SC144 and evista also inhibited cell viability of pancreatic cancer cells induced by IL-6 (Fig. 4). To test the effect of bazedoxifene on the secretion of IL-6, we examined the secretion level of IL-6 in human pancreatic cells after the bazedoxifene treatment and found that bazedoxifene could reduce IL-6 secretion in pancreatic cancer cells (data not shown). To examine the potential toxicity of bazedoxifene in normal human cells, we treated Normal Human Lung Fibroblasts (NHLF) with the same doses of bazedoxifene used in cancer cells. The results showed that bazedoxifene did not exhibit significant inhibition of cell viability in NHLF cells (Fig. 3B). Additionally, to exclude the possibility that the effect of bazedoxifene may depend upon the inhibition of estrogen receptor α (ERα) signaling, we measured the protein expression of ERα in Capan-1, HPAF-II, HPAC, BxPC-3 and PANC-1 cells, and found that only HPAF-II cells expressed detectable ERα protein (Fig. 5A). These results suggest that the ability of bazedoxifene to inhibit cell viability on Capan-1, HPAC, BxPC-3 and PANC-1 cells is independent of inhibiting ERα signaling since they do not express detectable ERα.
Fig. (3).
Bazedoxifene inhibits IL-6 induced cell viability in pancreatic cancer cells and has little effects in human normal cells. (A) Pancreatic cancer cells. (B) Human normal cells (NHLF). PANC-1, HPAF-II, BxPC-3, Capan-1, MIA PaCa-2 and 22–614APR cells were pretreated with bazedoxifene (0, 1, 2.5, 5 and 10 μM) for 4 hours, followed by addition of IL-6 (10 ng/mL for HPAF-II, BxPC-3 and 22–614APR cells; 25 ng/mL for PANC-1, Capan-1 and MIA PaCa-2 cells). After 48 hours treatment, cell viability was measured using MTT assay in triplicate. NHLF cells was treated with bazedoxifene (0, 1, 2.5, 5 and 10 μM) for 48 hours. B: bazedoxifene.
Fig. (4).
SC144 and evista inhibit IL-6 mediated cell viability in pancreatic cancer cells. (A) SC144; (B) evista. Cells were pretreated with SC144 (0, 1, 2.5, 5 and 10 μM) or evista (0, 1, 2.5, 5 and 10 μM) for 4 hours, followed by addition of IL-6 (10 ng/mL for HPAF-II and BxPC-3; 25 ng/mL for PANC-1, Capan-1 and MIA PaCa-2 cells).
Fig. (5).
(A) Western blotting analysis of ERα protein in Capan-1, HPAF-II, HPAC, BxPC-3 and PANC-1 cells. (B) ERα gene is knocked down in HPAF-II cells using ERα siRNA. Cells were transfected with negative control siRNA or ERα siRNA using Lipofectamine 2000. After 48 hours, the cells were collected and lysed for western blotting assay. (C) ERα has no impact on cell viability in HPAF-II cells. Cells were seeded and incubated for 48 hours. Cell viability was determined by MTT assay in triplicate.
To determine whether ERα signaling is relevant to cell viability in HPAF-II cells, we knocked down the ERα gene using ERα siRNA (Fig. 5B) and found that the cell viability was not significantly reduced (Fig. 5C), suggesting that ERα may not play a key role on the cell viability of HPAF-II cells. Even if it is possible that bazedoxifene may target ERα besides IL-6/GP130 in HPAF-II cells, we believe that blocking of IL-6/GP130 signaling is still the major mechanism of cell viability inhibition.
3.4. Bazedoxifene Suppresses IL-6 Induced Cell Proliferation in Human Pancreatic Cancer Cells
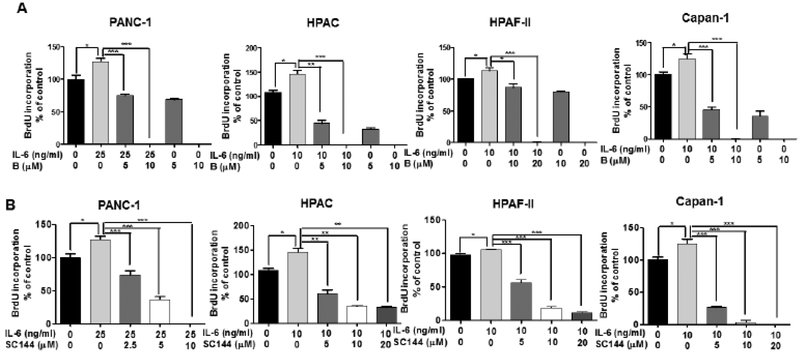
Next, we investigated the suppressive activity of bazedoxifene on IL-6 induced cell proliferation of human pancreatic cancer cells. We pretreated HAPC, PANC-1, Capan-1 and HPAF-II cell lines with different doses of bazedoxifene (0, 5, 10 and 20 μM) for 4 hours followed by addition of exogenous IL-6 (10 ng/mL), and performed the BrdU incorporation test after 24 hours. The results were presented in Fig. (6A). Bazedoxifene showed dose-dependent inhibition of IL-6 stimulated cell proliferation. Compared with bazedoxifene, SC144 treatment also decreased IL-6 induced cell proliferation, while bazedoxifene exhibited more potential than SC144 at 10 μM on HPAC and Capan-1 cells, and 20 μM on HPAF-II cells (Fig. 6B).
Fig. (6).
Bazedoxifene and GP130 inhibitor SC144 moderate IL-6 mediated cell proliferation in human pancreatic cancer cells. (A) Bazedoxifene; (B) SC144. PANC-1, HPAC, Capan-1 and HPAF-II cells were pretreated with bazedoxifene (0, 5, 10 and 20 μM), or SC144 (0, 5, 10 and 20 μM) for 4 hours, followed by treatment of IL-6 (10 ng/mL for HPAC, Capan-1 and HPAF-II; 25 ng/mL for PANC-1). After 24 hours, cell proliferation was detected using BrdU assay in triplicate. B: bazedoxifene.
3.5. Bazedoxifene Inhibits Glycolysis Stimulated by IL-6 in Human Pancreatic Cancer Cells
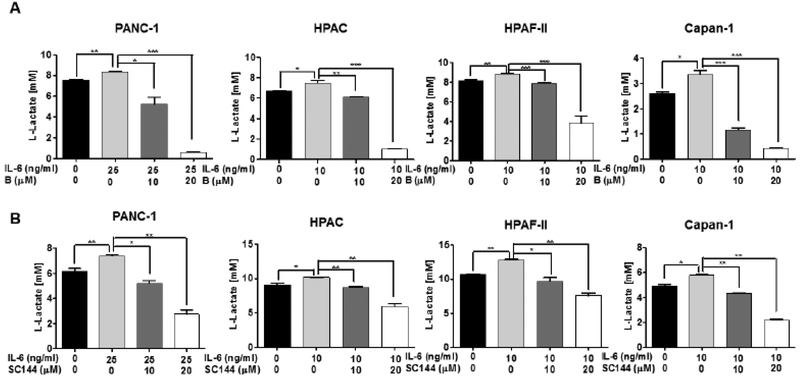
Otto Warburg and coworkers reported that the elevation of the flux of glucose to lactate is associated with tumor aggression [33,34]. To evaluate the effect of IL-6 signaling on glucose metabolism in human pancreatic cancer cells, we treated the HAPC, PANC-1, Capan-1 and HPAF-II cells with recombinant human IL-6 (10 ng/mL for HPAC, HPAF-II and Capan-1 and 25 ng/mL for PANC-1) and observed an increase in the corresponding metabolite lactate, suggesting that IL-6 promoted the uptake of glucose (Fig. 7). Importantly, bazedoxifene treatment resulted in a dose-dependent decrease of lactate induced by IL-6, supporting that reducing of glycolysis by bazedoxifene was associated with inhibition of IL-6 signaling pathway in human pancreatic cancer cells (Fig. 7A). Consistently, SC144 also displayed inhibition of IL-6 mediated cell glycolysis in those four same cell lines (Fig. 7B). In addition, we examined the effect of bazedoxifene on the protein expression of hexokinase, phosphofructokinase, and pyruvate kinase in HPAC cells and observed that bazedoxifene could slightly inhibit the expression of phosphofructokinase but not hexokinase and pyruvate kinase (data not shown).
Fig. (7).
IL-6 stimulates glycolysis in human pancreatic cancer cells and is inhibited by bazedoxifene and SC144. (A) Bazedoxifene; (B) SC144. PANC-1, HPAC, Capan-1 and HPAF-II cells were pretreated with bazedoxifene (0, 5, 10 and 20 μM), or SC144 (0, 5, 10 and 20 μM) for 4 hours, followed by addition of recombinant IL-6 (10 ng/mL for HPAC, Capan-1 and HPAF-II; 25 ng/mL for PANC-1). After 48 hours, the production of lactate were measured using glycolysis assay. B: bazedoxifene.
3.6. Bazedoxifene Induces Apoptosis in Human and Murine Pancreatic Cancer Cells
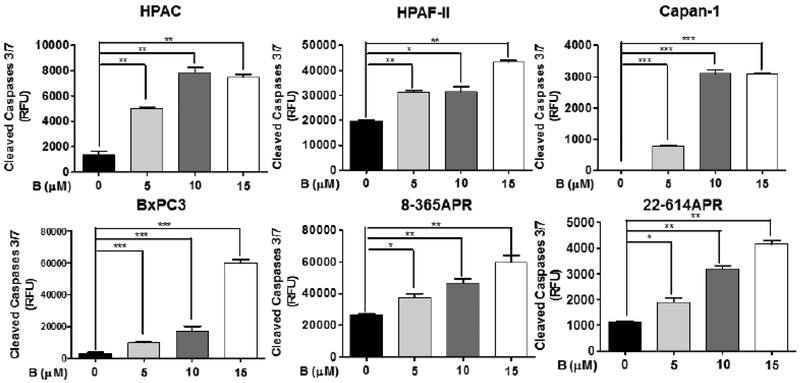
Although apoptosis can be triggered by different pathways, each of them merges to a common execution cascade of apoptosis that needs proteolytic activation of caspase-3/7 [35, 36]. Therefore, the activation of caspase-3/7 is considered as a marker of apoptosis. To investigate the ability of bazedoxifene in the induction of apoptosis in pancreatic cancer cells, we detected the active caspase-3/7 in four human pancreatic cancer cells (HPAC, HPAF-II, Capan-1 and BxPC-3) and two primary murine pancreatic cancer cells (8–365APR and 22–614APR) treated with bazedoxifene using the caspase-3/7 fluorescence assay. This assay uses a specific substrate, N-Ac-DEVD-N’-MC-R110, which can be cleaved by active caspase-3/7, and produces a highly fluorescent compound that can be detected by excitation and emission wavelengths (485 and 535 nm). As shown in Fig. (8), the amount of active caspase-3/7 in all pancreatic cell lines significantly increased after bazedoxifene treatment. Furthermore, we treated HPAC cells with bazedoxifene for 12 hours and performed western blotting analysis. The result suggested that bazedoxifene could induce cleaved caspase-3 protein in HPAC cells (data not shown). Taken together, our results indicated that bazedoxifene induces apoptosis in human pancreatic cancer cells and murine primary cancer cells that express IL-6.
Fig. (8).
Bazedoxifene induces apoptosis in human pancreatic cancer cells and murine primary cancer cells. Active caspase-3/7 treated with bazedoxifene (0, 5, 10 and 15 μM) for 5 hours. B: bazedoxifene.
3.7. Bazedoxifene Inhibits Colony Forming Ability of Human Pancreatic Cancer Cells
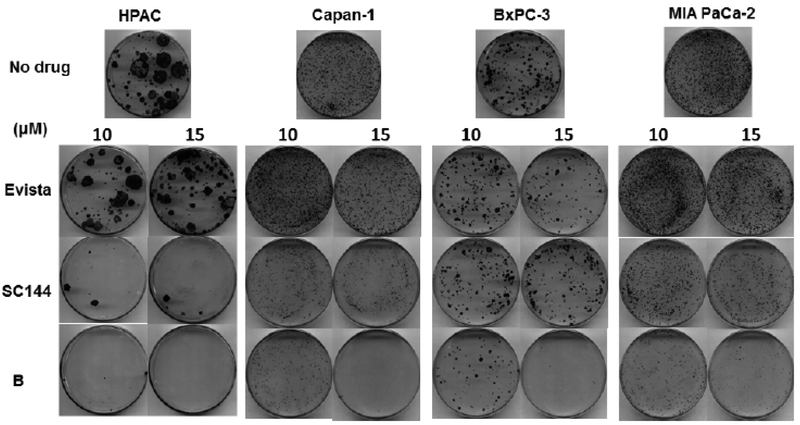
To evaluate the therapeutic potential of bazedoxifene in pancreatic cancer cells, we treated HPAC, Capan-1, BxPC-3 and MIA PaCa-2 cells with bazedoxifene (0, 10 and 15 μM), SC144 (0, 10 and 15 μM), or evista (0, 10 and 15 μM) and observed that bazedoxifene dose-dependently inhibited the capacity of HPAC, Capan-1, BxPC-3 and MIA PaCa-2 cells to grow and form colonies after treatment (Fig. 9). Notably, bazedoxifene is more potent than SC144 and evista, supporting that bazedoxifene is a promising IL-6/GP130/ STAT3 inhibitor in the treatment of pancreatic cancer.
Fig. (9).
Bazedoxifene, SC144 and evista inhibit the colony forming ability in human pancreatic cancer cells. HPAC, Capan-1, BxPC-3 and MIA PaCa-2 cells were treated with bazedoxifene (0, 10 and 15 μM), SC144 (0, 10 and 15 μM) or evista (0, 10 and 15 μM) for 16 hours, then cells were harvested and reseeded on 6-cm plates with a drug-free medium for an additional incubation of 2 weeks. Colonies were fixed with methanol and stained with crystal violet dye (0.1% w/v). B: bazedoxifene.
3.8. Bazedoxifene Blocks IL-6/GP130/IL-6R/JAK1 Complex Formation
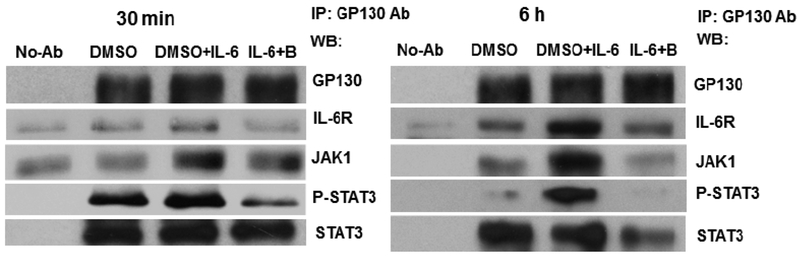
To further explore whether bazedoxifene down-regulates IL-6 signaling by blocking IL-6/IL-6R/GP130 complex formation in human pancreatic cancer cells, we performed immunoprecipitation in HPAC cells, and found that bazedoxifene treatment noticeably blocked IL-6R binding to GP130 (Fig. 10). Bazedoxifene also significantly reduced JAK1 and phosphorylated STAT3 binding to GP130 after 6 hours treatment. Our results indicated that bazedoxifene could block GP130 to interact with IL-6R, JAK1 and to phosphorylated STAT3, and thus inhibit IL-6/IL-6R/GP130 signaling to JAK1/STAT3.
Fig. (10).
Bazedoxifene blocks GP130 to interact with IL-6R and JAK1/STAT3 in HPAC cells. Cells were pretreated with bazedoxifene (20 μM) for 4 hours, followed by the addition of recombinant human IL-6 (50 ng/mL). After 30 minutes or 6 hours stimulation, whole cell lysates were prepared and GP130 was immunoprecipitated. GP130 binding proteins included IL-6R, JAK1, P-STAT3 (Y705) and STAT3 were analyzed by Western blotting. No-Ab: without GP130 immunoprecipitation. B: bazedoxifene. IP: immunoprecipitation.
4. DISCUSSIONS
Pancreatic cancer remains one of the most aggressive malignancies with a dismal 7% overall 5-year survival rate and limited treatment options [37]. Gemcitabine-based regimens have been the only treatment for decades. However, many patients appear gemcitabine resistance. Although its combination with novel agents has resulted in modest improvement in the overall survival, the regimens are found to be associated with significant toxicities [38]. Thus, searching for alternative therapy options remains an urgent matter. It has been reported that inflammatory environment is a vital component in numerous types of human cancer, yet our mechanistic understanding of the effect inflammation during cancer progression is incomplete. One well-known pro-tumorigenic inflammatory factor is the pleiotropic cytokine IL-6. Increasing evidence suggests that enhanced production of IL-6 and dysfunction of IL-6 signaling is strongly associated with pancreatic cancer progression [24, 30, 31]. Given the important role IL-6 plays during the initiation of pancreatic cancer, we investigated whether pancreatic cancer cell viability/proliferation is impacted by IL-6. Interestingly, our findings revealed that IL-6 significantly stimulated cell viability and cell proliferation of pancreatic cancer cells, supporting that IL-6 is indispensable in the growth of pancreatic cancer cells. IL-6 transduces signals through binding to membrane receptor complexes containing the ubiquitously-expressed GP130 co-receptor, in turn activating a complicated series of signaling downstream included Ras/Raf/MEK/MAPK, PI3K/AKT, and JAK/STAT pathways [39]. Herein, we found that IL-6 mediated JAK/STAT3 pathway in human pancreatic cancer cells.
IL-6 targeting regimens have been considered as new therapeutic options in pancreatic cancer but with restricted success. This is potentially due to blockade of IL-6 result in dramatic elevated in systemic IL-6 [40]. Another possibility would be humanized monoclonal antibody treatment targeting IL-6R, tocilizumab, whereas its potential therapeutic benefit on pancreatic cancer has not yet been established [23]. Selective molecule sgp130Fc (a combination of a human IgG1-Fc part with two GP130 extracellular domains) exhibits potential clinical significance, similar to sgp130 but with much higher efficacy, nevertheless, an optimized sgp130Fc variant is still undergoing clinical trials [41]. Recent data from literature reported that inhibition of IL-6 signaling remarkably suppressed tumor growth in animal models of pancreatic cancer [27]. In our previous studies, we demonstrated that both FDA-approved drugs bazedoxifene and evista as IL-6/GP130 inhibitors inhibited IL-6 induced phosphorylated STAT3 activation in pancreatic cancer cells [28,29]. Bazedoxifene is a third generation selective estrogen receptor modulator, and is applied in the prevention of the osteoporosis in postmenopausal women with improved selectivity and safety over tamoxifen [42, 43]. Furthermore, in phase III clinical studies, bazedoxifene showed a favorable reproductive safety profile in postmenopausal women over three and seven years respectively [44, 45]. Therefore, bazedoxifene is a potential drug candidate as a novel IL-6/GP130 signaling antagonist. In this study, we demonstrated that inhibition of IL-6 signaling by bazedoxifene resulted in a remarkably reduction in IL-6 mediated cell viability and cell proliferation in pancreatic cancer cells. Consistently, GP130 inhibitor, SC144, and IL-6/GP130 inhibitor, evista, also effectively inhibited cell viability and cell proliferation stimulated by IL-6 in pancreatic cancer cells. These results suggested that targeting of IL-6 signaling pathway might be an effective approach for pancreatic cancer therapy.
Otto Warburg and co-workers demonstrated that increased aerobic glycolysis is one of the prominent phenotypes of tumor, not only promote cell proliferation but also offer energy to cancer cells [34, 46]. Several studies have shown that IL-6 signaling could enhance glucose transporter type 4 (GLUT4) translocation [47, 48]. Importantly, it has recently emerged that IL-6 mediated promotion of glycolysis relies on the JAK/STAT3 signaling pathway via the increased hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKRB-3) expression [49, 50], nonetheless, it is unclear whether IL-6 can enhance glucose metabolism through IL-6/GP130/STAT3 axis to provide biomass intermediates and energy in pancreatic cancer progression. Additionally, lactate is known as the end-product in glycolysis. Elevated lactate level is an indicator of the glycolytic adaptation in cancer cells [51]. Therefore, we examined the cell glycolysis using lactate production experiments, and discovered that IL-6 significantly promotes lactate production in pancreatic cancer cells, demonstrating that IL-6 may play a role in promoting pancreatic cancer glycolysis and/or metabolism. In light of this, targeting IL-6/GP130/STAT3 pathway to inhibit glucose metabolism might be a potential therapeutic strategy for pancreatic cancer. Herein, down-regulating of IL-6/GP130/STAT3 signaling by bazedoxifene treatment remarkably reduced IL-6-mediated glycolysis in human pancreatic cancer cells. SC144 also decreased IL-6-stimulated glycolysis in human pancreatic cancer cells, implicating that IL-6/gP130/STAT3 axis is a potential target for pancreatic cancer treatment.
Our results also showed that bazedoxifene induced apoptosis of pancreatic cancer cells. Furthermore, the effects of bazedoxifene by inhibiting colony formation of pancreatic cancer cells exhibited more potent than other reported inhibitors, evista and SC144, supporting that bazedoxifene could be a potent inhibitor against the IL-6/GP130/STAT3 signaling pathway. Additionally, we also explored the molecular regulation of bazedoxifene inhibition in pancreatic cancer cells, and demonstrated that bazedoxifene treatment blocked IL-6R binding to GP130, and also reduced STAT3 and JAK1 binding to GP130. Consistently, these results supported that bazedoxifene could block GP130 to interact with IL-6R, JAK1, and STAT3, and thus inhibit IL-6/IL-6R/GP130 signaling to JAK1/STAT3.
CONCLUSION
In summary, we provided information to demonstrate IL-6 signaling regulates cell viability, cell proliferation and glycolysis in pancreatic cancer cells, implicating that IL-6 signaling is a potential molecular target for pancreatic cancer treatment. In addition, we investigated the effects of a novel small molecular inhibitor, bazedoxifene, on pancreatic cancer cells in vitro, and demonstrated that it could not only inhibit IL-6 induced cell viability, cell proliferation, and glycolysis, but also reduce the clonogenic capacity in pancreatic cancer cells. We further confirmed that bazedoxifene down-regulated IL-6/GP130/STAT3 signaling pathway via blocking GP130 to interact with IL-6R and JAK1/STAT3. Our findings strongly supported the use of IL-6/GP130 signaling inhibitors as a novel therapeutic approach for pancreatic cancer. Since Bazedoxifene has already been approved by FDA for treating other human disease with established safety profile, the potential to this drug into evaluation for pancreatic cancer patients may be clinically feasible in a short term.
ACKNOWLEDGEMENTS
This research was supported by the University of Maryland School of Medicine and Comprehensive Cancer Center start up fund. Authors thanks to Dr. David Weber for providing the fluorescent 96-well plate reader.
LIST OF ABBREVIATIONS
- 5-FU
Fluorouracil
- GP130
Glycoprotein 130
- IL-6
Interleukin-6
- IL-6R
Interleukin-6 α-receptor
- JAK
Janus kinase
- STAT3
Signal transducer and activator of transcription 3
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
REFERENCES
- [1].Rahib L; Smith BD; Aizenberg R; Rosenzweig AB; Flesh-man JM; Matrisian LM Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res,2014, 74 (11), 2913–2921. [DOI] [PubMed] [Google Scholar]
- [2].Winter JM; Brennan MF; Tang LH; D’Angelica MI; De-Matteo RP; Fong Y; Klimstra DS; Jarnagin WR; Allen PJ Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann. Surg. Oncol,2012, 19 (1), 169–175. [DOI] [PubMed] [Google Scholar]
- [3].Al-Hajeili M; Azmi AS; Choi M Nab-paclitaxel: potential for the treatment of advanced pancreatic cancer. Onco Targets Ther,2014, 7, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moorcraft SY; Khan K; Peckitt C; Watkins D; Rao S; Cun-ningham D; Chau I FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin. Colorectal Canc,2014, 13 (4), 232–238. [DOI] [PubMed] [Google Scholar]
- [5].Von Hoff DD; Ervin T; Arena FP; Chiorean EG; Infante J; Moore M; Seay T; Tjulandin SA; Ma WW; Saleh MN; Harris M; Reni M; Dowden S; Laheru D; Bahary N; Rama-nathan RK; Tabernero J; Hidalgo M; Goldstein D; Van Cut-sem E; Wei X; Iglesias J; Renschler MF Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl. J. Med, 2013, 369 (18), 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Conroy T; Desseigne F; Ychou M; Bouche O; Guimbaud R; Becouarn Y; Adenis A; Raoul JL; Gourgou-Bourgade S; de la Fouchardiere C; Bennouna J; Bachet JB; Khemissa-Akouz F; Pere-Verge D; Delbaldo C; Assenat E; Chauffert B; Michel P; Montoto-Grillot C; Ducreux M; Groupe Tumeurs Digestives of, U.; Intergroup P FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl. J. Med,2011, 364 (19), 1817–1825. [DOI] [PubMed] [Google Scholar]
- [7].Hunter CA; Jones SA IL-6 as a keystone cytokine in health and disease. Nat. Immunol,2015, 16 (5), 448–457. [DOI] [PubMed] [Google Scholar]
- [8].Hong DS; Angelo LS; Kurzrock R Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer,2007, 110 (9), 1911–1928. [DOI] [PubMed] [Google Scholar]
- [9].Scheller J; Garbers C; Rose-John S Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol,2014, 26 (1), 2–12. [DOI] [PubMed] [Google Scholar]
- [10].Wunderlich FT; Strohle P; Konner AC; Gruber S; Tovar S; Bronneke HS; Juntti-Berggren L; Li LS; van Rooijen N; Libert C; Berggren PO; Bruning JC Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. CellMetab,2010,12 (3), 237–249. [DOI] [PubMed] [Google Scholar]
- [11].Yamasaki K; Taga T; Hirata Y; Yawata H; Kawanishi Y; Seed B; Taniguchi T; Hirano T; Kishimoto T Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science,1988, 241 (4867), 825–828. [DOI] [PubMed] [Google Scholar]
- [12].Lokau J; Garbers C Signal transduction of Interleukin-11 and Interleukin-6 α -Receptors. Receptors Clin. Investig,2016, 3 (2), e1190. [Google Scholar]
- [13].Jones SA; Scheller J; Rose-John S Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest,2011, 121 (9), 3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Garbers C; Aparicio-Siegmund S; Rose-John S The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr. Opin. Immunol,2015, 34, 75–82. [DOI] [PubMed] [Google Scholar]
- [15].Roxburgh CS; McMillan DC Therapeutics targeting innate immune/inflammatory responses through the interleukin-6/JAK/STAT signal transduction pathway in patients with cancer. Transl. Res,2016, 167 (1), 61–66. [DOI] [PubMed] [Google Scholar]
- [16].Yao X; Huang J; Zhong H; Shen N; Faggioni R; Fung M; Yao Y Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther,2014, 141 (2), 125–139. [DOI] [PubMed] [Google Scholar]
- [17].Hu B; Zhang K; Li S; Li H; Yan Z; Huang L; Wu J; Han X; Jiang W; Mulatibieke T HIC1 attenuates invasion and metas-tasis by inhibiting the IL-6/STAT3 signalling pathway in human pancreatic cancer. Cancer Lett,2016, 376 (2), 387–398. [DOI] [PubMed] [Google Scholar]
- [18].Xu S; Neamati N gp130: a promising drug target for cancer therapy. Expert. Opin. Ther. Tar,2013, 17 (11), 1303–1328. [DOI] [PubMed] [Google Scholar]
- [19].Bournazou E; Bromberg J Targeting the tumor microenvironment: JAK-STAT3 signaling. JATSTAT,2013, 2 (2), e23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heo TH; Wahler J; Suh N Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget,2016, 7 (13), 15460–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bharti R; Dey G; Banerjee I; Dey KK; Parida S; Kumar BP; Das CK; Pal I; Mukherjee M; Misra M Somatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapy. Cancer Lett,2017, 388, 292–302. [DOI] [PubMed] [Google Scholar]
- [22].Lee B-R; Kwon B-E; Hong E-H; Shim A; Song J-H; Kim H-M; Chang S-Y; Kim Y-J; Kweon M-N; Youn J-I Interleukin-10 attenuates tumour growth by inhibiting interleukin-6/signal transducer and activator of transcription 3 signalling in myeloid-derived suppressor cells. Cancer Lett,2016, 381 (1), 156–164. [DOI] [PubMed] [Google Scholar]
- [23].Tanaka T; Narazaki M; Kishimoto T Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol,2012, 52, 199–219. [DOI] [PubMed] [Google Scholar]
- [24].Holmer R; Goumas FA; Waetzig GH; Rose-John S; Kalthoff H Interleukin-6: a villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat. Dis. Int,2014, 13 (4), 371–380. [DOI] [PubMed] [Google Scholar]
- [25].Denley SM; Jamieson NB; McCall P; Oien KA; Morton JP; Carter CR; Edwards J; McKay CJ Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. J. Gastrointest. Surg,2013, 17 (5), 887–898. [DOI] [PubMed] [Google Scholar]
- [26].Lesina M; Kurkowski MU; Ludes K; Rose-John S; Treiber M; Kloppel G; Yoshimura A; Reindl W; Sipos B; Akira S Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell,2011, 19 (4), 456–469. [DOI] [PubMed] [Google Scholar]
- [27].Goumas FA; Holmer R; Egberts JH; Gontarewicz A; Heneweer C; Geisen U; Hauser C; Mende MM; Legler K; Röcken C Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer,2015, 137 (5), 1035–1046. [DOI] [PubMed] [Google Scholar]
- [28].Li H; Xiao H; Lin L; Jou D; Kumari V; Lin J; Li C Drug design targeting protein-protein interactions (PPIs) using multiple ligand simultaneous docking (MLSD) and drug repositioning: discovery of raloxifene and bazedoxifene as novel inhibitors of IL-6/GP130 interface. J. Med. Chem,2014, 57 (3), 632–641. [DOI] [PubMed] [Google Scholar]
- [29].Wu X; Cao Y; Xiao H; Li C; Lin J Bazedoxifene as a Novel GP130 Inhibitor for Pancreatic Cancer Therapy. Mol. Cancer Ther,2016,15 (11), 2609–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pop V-V; Seicean A; Lupan I; Samasca G; Burz C-C IL-6 roles-Molecular pathway and clinical implication in pancreatic cancer-A systemic review. Immunol Lett,2016, 181, 45–50. [DOI] [PubMed] [Google Scholar]
- [31].Miura T; Mitsunaga S; Ikeda M; Shimizu S; Ohno I; Takahashi H; Furuse J; Inagaki M; Higashi S; Kato H Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas,2015, 44 (5), 756–763. [DOI] [PubMed] [Google Scholar]
- [32].Yadav A; Kumar B; Teknos TN; Kumar P Bazedoxifene enhances the anti-tumor effects of cisplatin and radiation treatment by blocking IL-6 signaling in head and neck cancer. Oncotarget,2016, 8 (40), 66912–66924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Racker E Bioenergetics and the problem of tumor growth: an understanding of the mechanism of the generation and control of biological energy may shed light on the problem of tumor growth. Am. Sci,1972, 60 (1), 56–63. [PubMed] [Google Scholar]
- [34].Koppenol WH; Bounds PL; Dang CV Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer,2011, 11 (5), 325–337. [DOI] [PubMed] [Google Scholar]
- [35].Ghavami S; Hashemi M; Ande SR; Yeganeh B; Xiao W; Eshraghi M; Bus CJ; Kadkhoda K; Wiechec E; Halayko AJ Apoptosis and cancer: mutations within caspase genes. J. Med. Genet,2009, 46 (8), 497–510. [DOI] [PubMed] [Google Scholar]
- [36].McIlwain DR; Berger T; Mak TW Caspase functions in cell death and disease. Cold Spring Harbor perspectives in biology,2013, 5 (4), a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Siegel R; Naishadham D; Jemal A Cancer statistics, 2013. CA Cancer J. Clin,2013, 63 (1), 11–30. [DOI] [PubMed] [Google Scholar]
- [38].Moore MJ; Goldstein D; Hamm J; Figer A; Hecht JR; Gall-inger S; Au HJ; Murawa P; Walde D; Wolff RA Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol,2007, 25 (15), 1960–1966. [DOI] [PubMed] [Google Scholar]
- [39].Scheller J; Chalaris A; Schmidt-Arras D; Rose-John S The pro-and anti-inflammatory properties of the cytokine interleukin-6. BBA-Mol. Cell. Res,2011, 1813 (5), 878–888. [DOI] [PubMed] [Google Scholar]
- [40].Calabrese LH; Rose-John S IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol,2014, 10 (12), 720–727. [DOI] [PubMed] [Google Scholar]
- [41].Tenhumberg S; Waetzig GH; Chalaris A; Rabe B; Seegert D; Scheller J; Rose-John S; Grotzinger J Structure-guided optimization of the interleukin-6 trans-signaling antagonist sgp130. J. Biol. Chem,2008, 283 (40), 27200–27207. [DOI] [PubMed] [Google Scholar]
- [42].Chines AA; Komm BS Bazedoxifene acetate: a novel selective estrogen receptor modulator for the prevention and treatment of postmenopausal osteoporosis. Drugs Today (Barc).2009, 45 (7), 507–520. [DOI] [PubMed] [Google Scholar]
- [43].Komm BS; Kharode YP; Bodine PV; Harris HA; Miller CP; Lyttle CR Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology,2005, 146 (9), 3999–4008. [DOI] [PubMed] [Google Scholar]
- [44].Archer DF; Pinkerton JV; Utian WH; Menegoci JC; de Villiers TJ; Yuen CK; Levine AB; Chines AA; Constantine GD Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Meno-pause,2009, 16 (6), 1109–1115. [DOI] [PubMed] [Google Scholar]
- [45].Palacios S; de Villiers TJ; Nardone FDC; Levine AB; Williams R; Hines T; Mirkin S; Chines AA; Group BS Assessment of the safety of long-term bazedoxifene treatment on the reproductive tract in postmenopausal women with osteoporosis: results of a 7-year, randomized, placebo-controlled, phase 3 study. Maturitas,2013, 76 (1), 81–87. [DOI] [PubMed] [Google Scholar]
- [46].Vander Heiden MG; Cantley LC; Thompson CB Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science,2009, 324 (5930), 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cron L; Allen T; Febbraio MA The role of gp130 receptor cytokines in the regulation of metabolic homeostasis. J. Exp. Biol,2016, 219 (2), 259–265. [DOI] [PubMed] [Google Scholar]
- [48].Carey AL; Steinberg GR; Macaulay SL; Thomas WG; Holmes AG; Ramm G; Prelovsek O; Hohnen-Behrens C; Watt MJ; James DE Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes,2006, 55 (10), 2688–2697. [DOI] [PubMed] [Google Scholar]
- [49].Ando M; Uehara I; Kogure K; Asano Y; Nakajima W; Abe Y; Kawauchi K; Tanaka N Interleukin 6 enhances glycolysis through expression of the glycolytic enzymes hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3. J. Nippon. Med. Sch,2010, 77 (2), 97–105. [DOI] [PubMed] [Google Scholar]
- [50].Han J; Meng Q; Xi Q; Zhang Y; Zhuang Q; Han Y; Jiang Y; Ding Q; Wu G Interleukin-6 stimulates aerobic glycolysis by regulating PFKFB3 at early stage of colorectal cancer. Int. J. Oncol,2016, 48 (1), 215–224. [DOI] [PubMed] [Google Scholar]
- [51].Hirschhaeuser F; Sattler UG; Mueller-Klieser W Lactate: a metabolic key player in cancer. Cancer Res,2011, 71 (22), 6921–6925. [DOI] [PubMed] [Google Scholar]