Abstract
Background
Although the use of enemas during labour usually reflects the preference of the attending healthcare provider, enemas may cause discomfort for women.
Objectives
To assess the effects of enemas applied during the first stage of labour on maternal and neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2013), the Cochrane Central Register of Controlled Trials and Database of Abstracts of Reviews of Effectiveness (The Cochrane Library 2013, Issue 5), PubMed (1966 to 31 May 2013), LILACS (31 May 2013), the Search Portal of the International Clinical Trials Registry Platform (ICTRP) (31 May 2013), Health Technology Assessment Program, UK (31 May 2013), Medical Research Council, UK (31 May 2013), The Wellcome Trust, UK (31 May 2013) and reference lists of retrieved articles.
Selection criteria
Randomised controlled trials (RCTs) in which an enema was administered during the first stage of labour and which included assessment of possible neonatal or puerperal morbidity or mortality.
Data collection and analysis
Two review authors independently assessed studies for inclusion.
Main results
Four RCTs (1917 women) met the inclusion criteria. One study was judged as having a low risk of bias. In the meta‐analysis we conducted of two trials, we found no significant difference in infection rates for puerperal women (two RCTs; 594 women; risk ratio (RR) 0.66, 95% confidence (CI) 0.42 to 1.04). No significant differences were found in neonatal umbilical infection rates (two RCTs; 592 women; RR 3.16, 95% CI 0.50 to 19.82; I² 0%. In addition, meta‐analysis of two studies found that there were no significant differences in the degree of perineal tear between groups. Finally, meta‐analysis of two trials found no significant differences in the mean duration of labour.
Authors' conclusions
The evidence provided by the four included RCTs shows that enemas do not have a significant beneficial effect on infection rates such as perineal wound infection or other neonatal infections and women's satisfaction. These findings speak against the routine use of enemas during labour, therefore, such practice should be discouraged.
Keywords: Female; Humans; Pregnancy; Labor Stage, First; Umbilicus; Bacterial Infections; Bacterial Infections/epidemiology; Bacterial Infections/prevention & control; Defecation; Enema; Enema/adverse effects; Perineum; Perineum/injuries; Puerperal Infection; Puerperal Infection/epidemiology; Puerperal Infection/prevention & control; Randomized Controlled Trials as Topic; Risk
Plain language summary
Enemas during labour
Scientific research evidence does not support the routine use of enemas during the first stage of labour.
Giving women enemas during labour has been routine practice in delivery wards of many countries and settings. Occasionally women leak from their back passage whilst giving birth and it was thought an enema in early labour would reduce this soiling and the consequent embarrassment for women. It was also thought that emptying the back passage would give more room for the baby to be born, would reduce the length of labour and would reduce the chance of infection for both the mother and the baby. It was also suggested it would reduce bowel movements after birth which often cause women concern. The disadvantages suggested were that it is a very unpleasant procedure and causes increased pain for women during labour and because enemas could produce a watery faecal soiling whilst giving birth, they could potentially increase the risk of infections. The review identified four studies involving 1917 women. These studies found no significant differences in any of the outcomes assessed either for the woman or the baby. However, none of the trials assessed pain for the woman during labour and there were insufficient data to assess rare adverse outcomes. Thus the evidence speaks against the routine use of enemas during labour.
Background
Enemas are frequently used in obstetric settings depending on the preference of, and the resources available to, the person attending the delivery (Cuervo 2006; Drayton 1984). Several consumer web pages on the Internet widely recommend their use in labour (Curtis 2007; PregnancyWeekly.com). Since this intervention may generate discomfort to women, increase the cost of delivery, and the workload on wards, an evaluation of the effectiveness of enemas during labour is important. A survey that evaluated the use of routine interventions and practices in labour and birth as reported by women in the Maternity Experiences Survey of the Canadian Perinatal Surveillance System found that 5.4% (95% confidence interval 4.7 to 6.0) of women reported having an enema. The authors also found that there were regional variations in rates ranging from 1.9% to 13.0% and that younger (15 to 19 years) and older (aged 40 years and older) women having their first baby, or with lower levels of education and family income were more likely to report having had an enema (Chalmers 2009). Other studies conducted in South East Asia countries (SEA‐ORCHID Study Group 2008), Colombia (Conde‐Agudelo 2008), Brazil (Sodré 2007) and Jordan (Sweidan 2008) found high rates of unnecessary practices of enema in labour. Another study reported that 37% of hospitals (193/521) had a "no enema/suppository" policy on admission in 2003 while 88% (282/322) did have in 2007 (Levitt 2011).
Some researchers (Lopes 2001; Romney 1981) have proposed that enemas should be used because: (i) they will lessen the degree of contamination of broken skin with faeces, thus reducing puerperal infections; (ii) women may regard cleaning their bowels as something good; (iii) they hope that they may diminish neonatal and puerperal infection rates by reducing contamination with faeces; (iv) for women who have not opened their bowels in the previous 24 hours and have an obviously loaded rectum on initial pelvic examination, bowel movement soon after delivery may cause discomfort with an episiotomy.
Others (Cuervo 2006) have opposed the use of enemas on the basis of: (i) unproven effectiveness; (ii) watery faeces may increase contamination, potentially increasing maternal and neonatal infections; (iii) it is widely accepted that this intervention generates discomfort to women and increases the costs of care.
The effectiveness of enemas has been measured using different maternal and neonatal outcomes such as puerperal (approximate six‐week period lasting from childbirth to the return of normal uterine size) infections and/or neonatal (relating to the first 28 days of life) infections. The duration of follow‐up depends on the measured maternal or neonatal outcome; in one study (Cuervo 2006) the primary outcomes were the diagnosis of infections in newborns or women during the month following delivery.
Enemas may also add to the workload of delivery wards and increase the cost of delivery. Enemas are known to cause discomfort and it is unclear if their potential benefit exceeds their inconvenience, cost and potential harms (Yeat 2011). Rare serious adverse events, such as perforations of the rectosigmoid colon or septic shock induced by cleansing enemas have been reported, usually in older women (Gayer 2002; Paran 1999). Considering that there are opposing views and theories, and uncertainties about the use of this intervention, scientific methods are needed to assess the effects of enemas to allow people to make a well‐informed choice. We considered it was therefore important to assess the effects of enemas used during labour on women and newborn.
Objectives
To assess the effects of enemas applied during the first stage of labour on maternal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing the use of enema versus no enema, or comparing different types of enemas.
Types of participants
Women during the first stage of labour.
Types of interventions
Enemas (of high or low volume, soapsuds, saline solutions, medicated or tap water).
Types of outcome measures
We planned to measure the effectiveness of enemas at four to six weeks of birth. However, we also collected data on different follow‐up periods depending on the trialists report.
Primary outcomes
Maternal or neonatal mortality
Puerperal outcomes
Complications of episiotomy and perineal tears such as episiotomy dehiscence (splitting of a surgical wound) or infection (i.e. purulent effusion from perineal tears).
Endometritis (i.e. fever and purulent vaginal discharge).
Neonatal outcomes
Neonatal infections such as: umbilical infection (foul smell with periumbilical erythema (redness of the skin)); respiratory tract infections (any infection of the respiratory tract, i.e. lower and/or upper tract); meningitis (serious inflammation of the meninges); or sepsis (as defined by trialists).
Secondary outcomes
Perineal wound repair.
Perineal tear/episiotomy wound (not a pre‐specified outcome).
Need for any analgesia during labour.
Economic outcomes.
Duration of labour and its different stages.
One‐minute Apgar of the newborn.
Five‐minute Apgar of the newborn.
Faecal soiling during first stage of labour.
Faecal soiling during delivery.
Duration of labour and labour stages.
Levels of satisfaction of parturients and\or medical staff (not a pre‐specified outcome).
Overall pain during labour (not a pre‐specified outcome).
Other pelvic Infections (infections involving the tissues or organs in the pelvis i.e. vulvovaginitis, myometritis etc.).
Urinary tract infection.
Other puerperal infections.
Need for systemic antibiotics (maternal and neonatal).
Other neonatal infections (not a pre‐specified outcome).
Ophthalmic infection (i.e. purulent drainage in the eye after the sixth day of delivery) or dacryocystitis (inflammation of the lacrimal sac).
Skin infections (i.e. cellulitis or impetigo).
Intestinal infections.
Rare serious adverse effects (i.e. perforations of the rectosigmoid colon or septic shock. Not a pre‐specified outcome).
Delivery type (caesarean section) (not a pre‐specified outcome).
Birthweight (not a pre‐specified outcome).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (31 May 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL and the Database of Abstracts of Reviews of Effectiveness (DARE) (The Cochrane Library 2013, Issue 5), PubMed (1966 to 31 May 2013), LILACS (1982 to 31 May 2013) using the search strategy listed in Appendix 1.
In order to further identify ongoing RCTs and unpublished studies, we searched the following databases (31 May 2013).
Search Portal of the International Clinical Trials Registry Platform (ICTRP)
Health Technology Assessment Program (HTA) [United Kingdom]
Medical Research Council [United Kingdom]
The Wellcome Trust [United Kingdom]
See:Appendix 1 for search strategy used.
Searching other resources
We also searched the reviews previously published in the Cochrane Pregnancy and Childbirth database on enemas (Hay‐Smith 1995a; Hay‐Smith 1995b; Hay‐Smith 1995c; Hay‐Smith 1995d).
We did not apply any language restrictions.
Data collection and analysis
For the methods to be used to assess trials identified in the future updates, seeAppendix 2.
1. Study selection
One review author, Ludovic Reveiz (LR), checked the titles and abstracts identified from the searches. If it was clear that the study was not a randomised controlled trial (RCT) evaluating enemas in pregnant women during labour, we excluded it. If we were unclear about the above, then two review authors assessed the full report of the study independently (LR and Hernando Gaitan (HG)) to determine if it fulfilled the inclusion criteria. The two authors resolved disagreements by consensus. Otherwise, we would have consulted the third review author (Luis Gabriel Cuervo) but this situation did not happen. The table Characteristics of excluded studies lists the reasons behind the exclusion of trials.
2. Risk of bias assessment
Two review authors (LR, HG) independently assessed risk of bias for each study as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve disagreements through dialogue leading to consensus and if we could not reach the latter, to invite the third review author to weigh in to decide by majority. We considered the possible sources of bias described below and assessed these in the 'Risk of bias' table for each included study. We classified each one of these sources as of low, high or unclear risk of bias by answering 'yes', 'no' or 'unclear' to each one of the items in the 'Risk of bias' table. Whenever possible, we gave more information about these answers using the 'Description' boxes with a clarifying comment or a quoted sentence taken directly from the original article.
(1) Sequence generation
We described, for each included study, the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups.
(2) Allocation concealment
We described, for each included study, the methods used to conceal the allocation sequence in sufficient detail, and therefore, determined whether the intervention allocation could have been foreseen before, or during recruitment, or changed after assignment.
(3) Blinding of participants, healthcare professionals and outcome assessors
We described, for each included study, the methods used to blind study participants, healthcare professionals and outcome appraisers to the intervention the participant received. We also provided information on whether the intended blinding was effective. If blinding was unclear, we assessed whether the information provided was likely to have introduced bias.
(4) Incomplete outcome data
We described, for each included study the completeness of data. We assessed whether incomplete data were adequately addressed.
(5) Selective reporting
We described, for each included study, the possibility that outcomes were selectively reported.
(6) Other potential sources of bias
We described, for each included study, any important concerns about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high, moderate or low risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we estimated the magnitude and direction of the bias and whether we considered it was likely to have impacted on the findings.
3. Data extraction
Two review authors (LR and HG) carried out data extraction using a pre‐designed data extraction form. We resolved disagreements among all three review authors. We actively sought missing data from authors using their registered contact details, plus complementary Internet searches when needed. We included these data if retrieved.
4. Analysis
To estimate statistical differences between treatments, we pooled the results of RCTs that assessed the effects of similar interventions, and calculated a weighted‐treatment effect across RCTs using the fixed‐effect model. We expressed the results as follows:
for dichotomous outcomes: as risk ratio (RR) (RCTs, participants; RR, 95% confidence intervals (CI));
for continuous outcomes: as mean difference (MD) (RCTs; participants; MD and 95% CI).
We expressed results as number needed to treat where appropriate. We summarised available information in accordance with the review protocol. We identified and listed quasi‐randomised and non‐randomised controlled studies for reference, but we did not discuss them further. To provide a better balance of information, we also included qualitative descriptions of available information on adverse effects, when possible. We reported results as rate ratios. We imputed conservative standard deviations where necessary using the P value from an independent two‐sample T‐test (Higgins 2011). For the pooled analysis, we calculated the I² statistic, which describes the percentage of total variation across studies caused by heterogeneity; less than 25% was considered as low level heterogeneity; 25% to 50% as moderate level, and higher than 50% as high level heterogeneity (Higgins 2011).
Results
Description of studies
Results of the search
The search identified 102 references. An initial trawl through this list, undertaken by two review authors, excluded 90 references that did not comply with the inclusion criteria. We screened 12 trials: we excluded eight and included four RCTs that recruited a total of 1917 women. For this update we did not identify any additional trials for inclusion. The search of clinical trial registers (International Clinical Trials Registry Platform) identified 78 trials but found no ongoing RCTs complying with the inclusion criteria.
Included studies
Drayton 1984: the RCT was conducted at the University Hospital of Wales, Cardiff (UK). The characteristics of participating women are poorly described. Inclusion criteria were fulfilled by 370 women invited to participate, of whom 222 (60%) agreed to randomisation. The intervention group received a low‐volume disposable phosphate enema during the first stage of labour. Caesarean section rates were not described in this study. Randomisation was stratified between primigravidae (43%) and multigravidae (57%).
Kovavisarach 2005: this RCT was conducted in Thailand; 1100 term‐pregnant women admitted for delivery were randomly allocated to receive either enema or no enema (539 participants to enema and 561 participants to no enema) from 1 February 2002 to 15 June 2002. Stratification by parity was not done. Seventy‐three women, who delivered by caesarean section due to obstetric indications after an unsuccessful trial of labour were excluded (39 with enema versus 34 with no enema). Newborns were followed up for four days through clinical observation during hospitalisation to assess neonatal infections. We were unable to reach the authors by e‐mail when we sought additional information. The authors measured satisfaction level in pregnant women and medical staff with Likert scales with scores ranked as: excellent, good, average, fair or poor.
Cuervo 2006: this RCT was conducted in a tertiary care referral hospital in Bogota, Colombia. Of the women invited to participate, 16 were not eligible and one declined to participate. In total, 443 women were randomised, achieving well‐balanced groups (221 allocated to enema versus 222 to control) from February 1997 to February 1998. Twelve per cent of the participants had a caesarean section. Stratification by parity was done. The RCT did not evaluate women's preferences or known adverse effects of enemas, such as pain, discomfort, embarrassment or diarrhoea.
Clarke 2007: this RCT was conducted in the United States; 152 pregnant women admitted for delivery were randomly allocated to receive either standardised enema soap solution within 30 minutes of enrolment or no enema (75 women to enema and 77 to no enema). The characteristics of participating women are poorly described. The primary outcome was the time interval from enrolment to delivery. Secondary outcomes included intrapartum infection rate, faecal soiling at delivery, mode of delivery and patient satisfaction.
Excluded studies
Eight studies were excluded from the review for different reasons including non‐randomised studies, inadequate randomisation, different type of participants or interventions (see Excluded studies).
Risk of bias in included studies
Allocation
The allocation strategy and the method of allocation concealment were considered adequate for one trial (Cuervo 2006) and unclear for the other three (Clarke 2007; Drayton 1984; Kovavisarach 2005).
Blinding
Blinding was not used in any of the four trials.
Incomplete outcome data
Two studies were considered to have low risk of bias (Cuervo 2006; Kovavisarach 2005) while the other two had an unclear risk of bias (Clarke 2007; Drayton 1984).
Selective reporting
The study protocol was not available for any of the studies.
Other potential sources of bias
One study had a high risk of bias (Drayton 1984), while one was judged as having unclear (Clarke 2007) and two low risk of bias (Cuervo 2006; Kovavisarach 2005).
Overall, only one study was judged as having low risk of bias (Cuervo 2006). A brief description is provided for each study (see Figure 1; Figure 2). For more details, seeRisk of bias in included studies.
1.
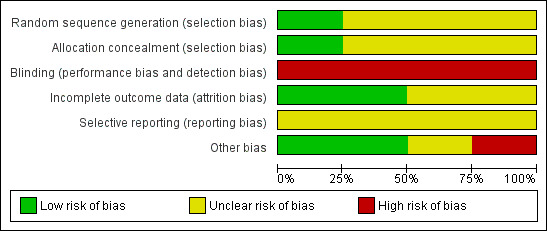
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
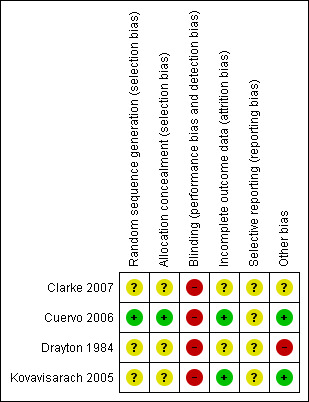
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Enema versus no enema
Four trials, involving 1917 women, fulfilled the inclusion criteria for the review: Drayton 1984, (222 women); Kovavisarach 2005, (1100 women); Cuervo 2006, (443 women) and Clarke 2007 (152 women). Findings of individual trials are found in Table 1.
1. Findings of individual studies.
| Study ID | Comments |
| Drayton 1984 | The RCT from Wales investigated the incidence of maternal and neonatal infections. None of the women had a perineal wound infection. Regarding neonatal infections, no significant differences were found between the enema and the no‐enema groups (one RCT; 222 women; risk ratio (RR) 0.89, 95% confidence interval (CI) 0.31 to 2.56; Analysis 1.2). The RCT also evaluated women's views on enemas. In the no‐enema group, 14.1% of women willingly accepted to receive a future enema compared to 39.6% in the enema group (P < 0.01). |
| Kovavisarach 2005 | In the trial from Thailand, the duration of labour was shorter in the enema group (1027 women; 409.4 minutes versus 459.8 minutes; mean difference (MD) ‐50.40, 95% CI ‐75.68 to ‐25.12; P < 0.001; Analysis 1.21) but no adjustment was done by parity. No significant differences were found in the route of delivery, degree of perineal tear and perineal wound infection rates. No neonatal infections occurred during the four‐day follow‐up, which seems a short time to identify infections comprehensively. No significant differences were found with regard to satisfaction between women receiving an enema versus those not receiving an enema, as assessed using a five‐point Likert scale (1027 women; 3.58 versus 3.58; MD 0.00, CI 95% ‐0.10 to 0.10; P = 0.922; Analysis 1.23). Satisfaction levels of labour attendants and healthcare providers were significantly higher in the enema group (P < 0.01) than in the control group (measured using the Likert scale). |
| Cuervo 2006 | The trial from Colombia investigated the effect of enemas on labour duration adjusted by parity. It found no statistically significant differences between groups for delivery types, episiotomy rates, or prescription of antibiotics. No significant differences were found in lower and upper respiratory tract infections rates. Similarly, no significant differences were found for ophthalmic infection rates, skin infections, or intestinal infections. The authors reported no significant differences in the distribution between groups for newborns' "Ballard" score, birthweight, diagnosis of neonatal apnoea, or the administration of ocular and umbilical prophylaxis. Twelve per cent of women had caesarean sections with no significant differences in rates between groups. In addition, no significant differences were found for the duration of labour (for all women for first stage of labour: median 515 minutes with enemas versus 585 minutes without enemas, P = 0.24; for second stage of labour: mean 43.2 minutes with enemas and 38 minutes without; MD 5.20, 95% CI ‐2.56 to 12.96; P=0.19; Analysis 1.22). These results could not be aggregated with the RCT from Thailand (Kovavisarach 2005) as times did not follow a normal distribution and, therefore, trialists considered non‐parametric measures (differences between medians). Finally, there were no significant difference in the degree of perineal tear between groups. The Colombian RCT found no significant differences between groups in the rate of neonatal infection after one month of follow‐up (370 newborns; RR 1.12, 95% CI 0.76 to 1.67; Analysis 1.4) |
| Clarke 2007 | In the trial from the United States, the mean times to delivery were 504.7 minutes and 392.7 minutes for enema and no enema respectively (152 women; MD 112, 95% CI 48.13 to 175.87; Analysis 1.21); we estimated the standard deviations because these were not provided by the researchers. Intrapartum infection rates were significantly higher in the enema group (RR 4.62, 95% CI 1.03 to 20.68; Analysis 1.33). However, when controlling for duration of membrane rupture, enema use fell below the level of significance for infection (no data was provided by trialists). Women who received enemas had significantly less faecal soiling at delivery (RR 0.36, 95% CI 0.17 to 0.75; Analysis 1.20). There was no significant difference in the mode of delivery between the two groups. No neonatal outcomes were reported. |
RCT: randomised controlled trial
Primary outcomes
No significant differences were found in neonatal umbilical infection rates (two RCTs ; 592 women; risk ratio (RR) 3.16, 95% confidence interval (CI) 0.50 to 19.82; I² 0%; Analysis 1.5). However, these results should be interpreted with caution as one of the studies (Drayton 1984) did not report time of follow‐up of participants and did not describe the means to assess infections.
1.5. Analysis.
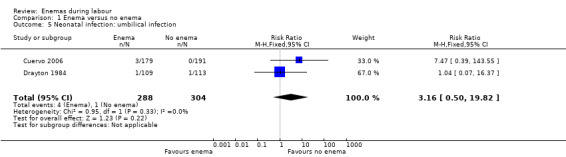
Comparison 1 Enema versus no enema, Outcome 5 Neonatal infection: umbilical infection.
No significant differences were found for any other primary outcomes.
Secondary outcomes
In meta‐analyses of two studies there were no significant differences in the degree of perineal tear between groups (Cuervo 2006; Kovavisarach 2005) (Analysis 1.10; Analysis 1.12). In the meta‐analysis we conducted of two trials (Cuervo 2006; Drayton 1984), we found no significant difference in infection rates for puerperal women (two RCTs; 594 women; RR 0.66, 95% CI 0.42 to 1.04; Analysis 1.32). Finally, meta‐analysis of two trials (Clarke 2007; Kovavisarach 2005) found no significant differences in the mean duration of labour (Analysis 1.21); however heterogeneity was very high (I² 95%).
1.10. Analysis.
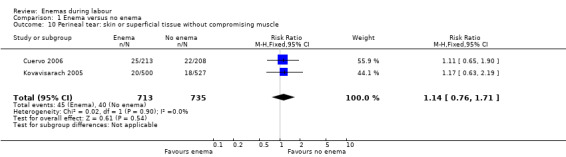
Comparison 1 Enema versus no enema, Outcome 10 Perineal tear: skin or superficial tissue without compromising muscle.
1.12. Analysis.
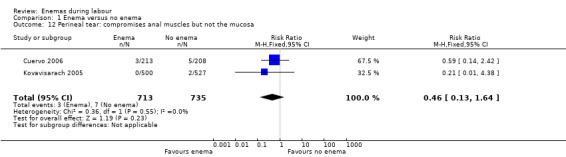
Comparison 1 Enema versus no enema, Outcome 12 Perineal tear: compromises anal muscles but not the mucosa.
1.32. Analysis.
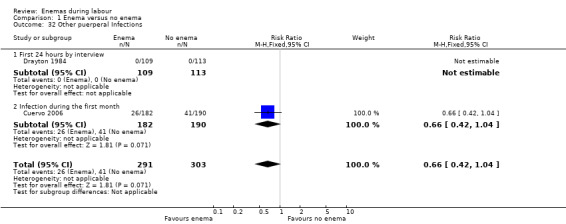
Comparison 1 Enema versus no enema, Outcome 32 Other puerperal Infections.
1.21. Analysis.
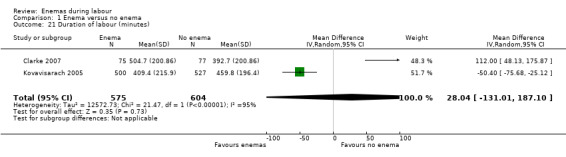
Comparison 1 Enema versus no enema, Outcome 21 Duration of labour (minutes).
There was less faecal soiling (one RCT; 152 women; RR 0.36, 95% CI 0.17 to 0.75; Analysis 1.20), higher satisfaction levels of labour attendants, accoucheurs and perineorrhaphy operators (one RCT; 1027 women; mean difference (MD) 0.17, 95% 0.08 to 0.26; MD 0.26, 95% 015 to 0.37; MD 0.11, 95% 0.02 to 0.20; Analysis 1.24; Analysis 1.25; Analysis 1.26) and more intrapartum infection rates (one RCT; 152 women; RR 4.62, 95% CI 1.03 to 20.68; Analysis 1.33) in the group of women randomised to receive enemas compared to those that did not.
1.20. Analysis.
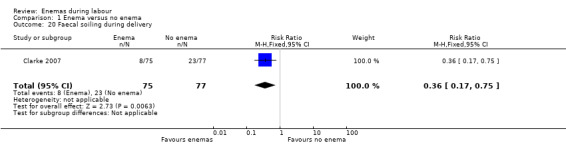
Comparison 1 Enema versus no enema, Outcome 20 Faecal soiling during delivery.
1.24. Analysis.
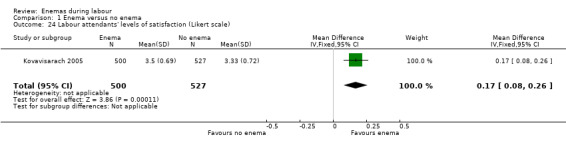
Comparison 1 Enema versus no enema, Outcome 24 Labour attendants' levels of satisfaction (Likert scale).
1.25. Analysis.
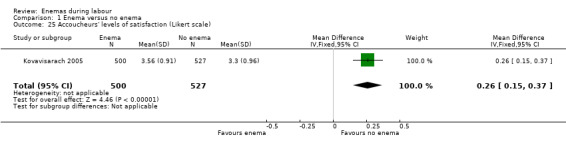
Comparison 1 Enema versus no enema, Outcome 25 Accoucheurs' levels of satisfaction (Likert scale).
1.26. Analysis.
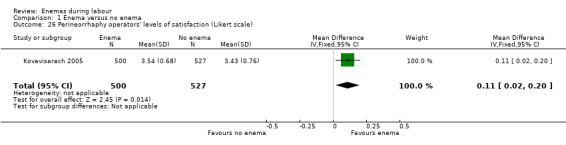
Comparison 1 Enema versus no enema, Outcome 26 Perineorrhaphy operators' levels of satisfaction (Likert scale).
1.33. Analysis.
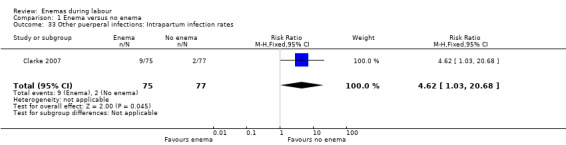
Comparison 1 Enema versus no enema, Outcome 33 Other puerperal infections: Intrapartum infection rates.
No significant differences were found for any other secondary outcomes.
The four RCTs did not report any serious adverse event.
Enema versus different type of enema
No trials were found.
Discussion
Summary of main results
The objective of this review was to address the effects of enemas during the first stage of labour. The use of enema does not result in improved maternal or neonatal outcomes: there was no difference in the rate of infection, such as perineal wound infection and neonatal infection. Pooled results of two trials (Cuervo 2006; Drayton 1984) found no significant difference in infection rates for puerperal women, in the rate of neonatal umbilical infection, differences in the degree of perineal tear between groups (Cuervo 2006; Kovavisarach 2005) or in the mean duration of labour (Cuervo 2006; Kovavisarach 2005).
Quality of the evidence
The quality of the evidence available for comparisons was high to moderate, mainly because there were only one or two studies for each treatment comparison and main results were imprecise in some cases, as reflected in wide confidence intervals.
Even though blinding of outcome assessors was not done, it is unlikely that this had any influence on the assessment of objective outcome measures most relevant to patients, specifically, infection outcomes. However, other subjective outcomes such as levels of satisfaction, could be open to biased assessments. Similarly, the selection of patients by the recruiter may have been influenced by the lack of allocation concealment in three of the four studies.
Potential biases in the review process
It is unlikely that publication bias is an issue, given that all included studies presented results not favourable for the more active interventions. Also, the search strategy was very comprehensive, including a search of the most important clinical trials registers. Contact with the authors of the included studies was made, and data were analysed and extracted independently by at least two review authors.
All relevant data for the objectives of this review were available from the publications of included studies. However, given that there was not an increased risk of specific types of infection with the interventions, it might be more relevant to use outcomes that reflect severity and timing of infection, rather than specific infections (i.e. whether before or after discharge from hospital after birth, need for antibiotics, or admission to special care). We will address this issue in the next update.
Agreements and disagreements with other studies or reviews
Our review provides evidence derived from four trials using different outcomes. The findings provide grounds against the routine administration of enemas during labour. These are in line with the findings from quasi‐experimental studies (Lopes 2001; Tzeng 2005). The results suggest that it is unlikely that enemas have any beneficial effects on clinical outcomes large enough to outweigh the inconvenience, costs or potential adverse effects.
Authors' conclusions
Implications for practice.
This review found that enemas did not improve puerperal or neonatal infection rates, episiotomy dehiscence rates or maternal satisfaction. Therefore, their use is unlikely to benefit women or newborn children, and there is no reliable scientific basis to recommend their routine use. Routine enemas should only be offered or advocated if there is clear evidence of benefit. In the absence of such evidence, these findings should discourage the routine use of enemas during labour.
Implications for research.
No additional research may be necessary to assess the overall effects of enemas in maternal or neonatal outcomes unless there is a need to assess their effects on a specific outcome. Routine enemas are not a priority topic for future research. The existing studies may have been underpowered to assess specific outcomes but, considering that these outcomes may be uncommon, the studies would require huge sample sizes making it unlikely that any findings would swing the balance as far as making the benefits outweigh potential harms, costs and inconvenience. If additional studies were to be carried out, these should perhaps focus on other factors that may be influencing the use of enemas, such as women's perceptions, pain, inconvenience, costs or any other non‐clinical motivations.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2013 | New citation required but conclusions have not changed | Review updated. |
| 29 June 2013 | New search has been performed | Search updated in May 2013. No new trials identified. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 1 November 2012 | New citation required but conclusions have not changed | Review updated but no new trials included. |
| 17 May 2012 | New search has been performed | Search updated in May 2012. One new trial identified and excluded (Lurie 2012). |
| 18 January 2012 | Amended | Contact details updated. |
| 28 February 2010 | New search has been performed | Search updated, which identified one new RCT (Clarke 2007). There are now a total of four trials included in the review. The inclusion of the new included study did not change the overall conclusions. In addition, a 'Risk of bias' assessment was performed for all RCTs. |
| 12 May 2009 | Amended | Contact details updated. |
| 2 September 2008 | Amended | Converted to new review format. |
| 30 July 2007 | New citation required and conclusions have changed | New authors. |
| 1 April 2007 | New search has been performed | This update is based on a search run in March 2007, which identified one new included study (Kovavisarach 2005) and two new excluded studies (Lopes 2001; Tzeng 2005). |
Acknowledgements
We would like to thank Lynn Hampson for her assistance with the search for trials and Sonja Henderson, Denise Atherton and Rebecca Smyth from the Cochrane Pregnancy and Childbirth Group.
This review is published as a Cochrane Review in the Cochrane Database of Systematic Reviews 2013, Issue 7. Cochrane Reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the Review.
The Pan American Health Organization and Hernando G Gaitán retain copyright and all other rights in their respective contributions to the manuscript of this Review as submitted for publication.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
Review authors searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews of Effectiveness (DARE) (The Cochrane Library 2013, Issue 5), PubMed (1966 to 31 May 2013) and LILACS (1982 to 31 May 2013) using the search strategy:
enema* AND (labor OR labour OR intrapartum OR delivery OR pregnan*).
Review authors also searched the following on 31 May 2013 using the word 'enema':
Search Portal of the International Clinical Trials Registry Platform (ICTRP)
Health Technology Assessment Program (HTA) [United Kingdom]
Medical Research Council [United Kingdom]
The Wellcome Trust [United Kingdom]
Appendix 2. Methods to be used in future updates
Data extraction and management
We will design a form to extract data. For eligible studies, at least two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult the third author. We will enter data into Review Manager software (RevMan 2012) and check for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third author.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator),
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) or,
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This study design is not eligible for inclusion in this review.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We will regard heterogeneity as substantial if I2 is greater than 30% and either T2 is greater than zero, or there is a low P‐value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2012). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful and, if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
1. Parity (primiparous versus non‐primiparous).
2. Gestational age.
The following primary outcomes will be used in subgroup analyses:
Complications of episiotomy and perineal tears.
Endometritis.
Neonatal infections.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We will report the results of subgroup analyses quoting the χ2 statistic and p‐value, and the interaction test I² value.
Sensitivity analysis
In the event of significant heterogeneity, we will perform sensitivity analysis excluding trials with greater risk of bias to determine the effect on the results. Studies with high or unclear risk of bias for selection and/or attrition bias will be considered at high risk of bias and excluded in sensitivity analyses.
Data and analyses
Comparison 1. Enema versus no enema.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Episiotomy dehiscence | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.14] |
| 2 Neonatal infection (all infections, including umbilical) | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.31, 2.56] |
| 3 Neonatal infection (not specified) at 4 days | 1 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Neonatal Infection (any infectious outcome, during the first month of life) | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.76, 1.67] |
| 5 Neonatal infection: umbilical infection | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.50, 19.82] |
| 6 Neonatal infection: respiratory tract infection (high ‐ during first month) | 1 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.73, 4.52] |
| 7 Neonatal infection: respiratory tract infection (low ‐ during first month) | 1 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.73] |
| 8 Neonatal infection: meningitis | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neontal infection: sepsis | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Perineal tear: skin or superficial tissue without compromising muscle | 2 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.76, 1.71] |
| 11 Perineal tear: perineal muscle without anal muscles | 1 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.40] |
| 12 Perineal tear: compromises anal muscles but not the mucosa | 2 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.13, 1.64] |
| 13 Perineal tear: complete tear that compromises anal mucosa | 1 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.51] |
| 14 No episiotomy wound ‐ no further tear | 1 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.43, 1.27] |
| 15 No episiotomy wound ‐ further tear: 1st degree tear | 1 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.19] |
| 16 Episiotomy wound ‐ no further tear | 1 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.05] |
| 17 Episiotomy wound ‐ further tear: 3rd degree tear | 1 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.38] |
| 18 One‐minute Apgar < 7 | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.57, 3.06] |
| 19 Five‐minute Apgar < 7 | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.57, 3.06] |
| 20 Faecal soiling during delivery | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| 21 Duration of labour (minutes) | 2 | 1179 | Mean Difference (IV, Random, 95% CI) | 28.04 [‐131.01, 187.10] |
| 22 Duration of labour (second stage) | 1 | 347 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [‐2.56, 12.96] |
| 23 Parturients' levels of satisfaction (Likert scale) | 1 | 1027 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 24 Labour attendants' levels of satisfaction (Likert scale) | 1 | 1027 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.08, 0.26] |
| 25 Accoucheurs' levels of satisfaction (Likert scale) | 1 | 1027 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.15, 0.37] |
| 26 Perineorrhaphy operators' levels of satisfaction (Likert scale) | 1 | 1027 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.02, 0.20] |
| 27 Pelvic infection: infected episiotomy | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.18, 2.00] |
| 28 Pelvic infection: vulvovaginitis | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.87] |
| 29 Pelvic infection: endometritis | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.31] |
| 30 Pelvic infection: myometritis | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 76.37] |
| 31 Urinary tract infection | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.18, 2.00] |
| 32 Other puerperal Infections | 2 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.04] |
| 32.1 First 24 hours by interview | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Infection during the first month | 1 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.04] |
| 33 Other puerperal infections: Intrapartum infection rates | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [1.03, 20.68] |
| 34 Need for systemic antibiotics (postpartum) | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.73, 1.84] |
| 35 Need for systemic antibiotics (neonatal ‐ after hospital discharge during the first month) | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.80] |
| 36 Opthalmic infection (dacriocistitis or conjunctivitis in first month) | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.62, 1.71] |
| 37 Skin infection (first month) | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.27, 9.47] |
| 38 Intestinal infection | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.94] |
1.1. Analysis.
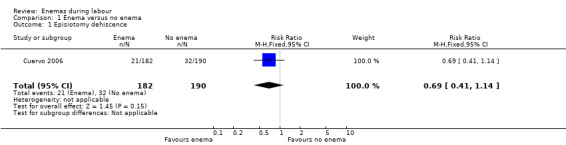
Comparison 1 Enema versus no enema, Outcome 1 Episiotomy dehiscence.
1.2. Analysis.

Comparison 1 Enema versus no enema, Outcome 2 Neonatal infection (all infections, including umbilical).
1.3. Analysis.
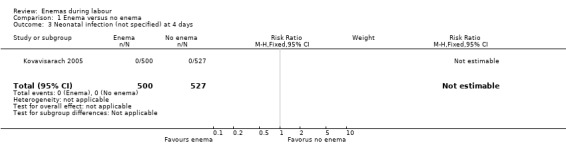
Comparison 1 Enema versus no enema, Outcome 3 Neonatal infection (not specified) at 4 days.
1.4. Analysis.
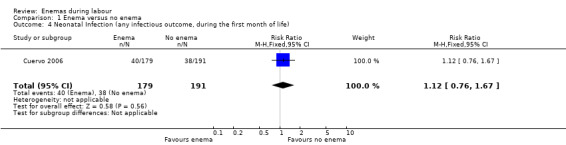
Comparison 1 Enema versus no enema, Outcome 4 Neonatal Infection (any infectious outcome, during the first month of life).
1.6. Analysis.
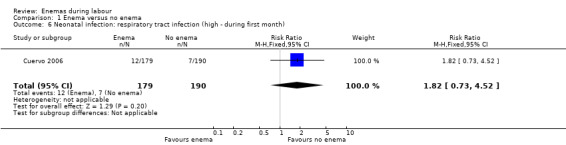
Comparison 1 Enema versus no enema, Outcome 6 Neonatal infection: respiratory tract infection (high ‐ during first month).
1.7. Analysis.
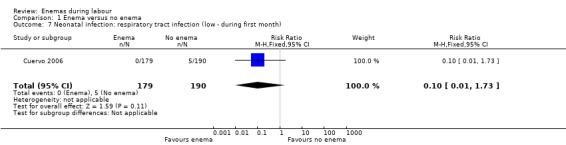
Comparison 1 Enema versus no enema, Outcome 7 Neonatal infection: respiratory tract infection (low ‐ during first month).
1.8. Analysis.
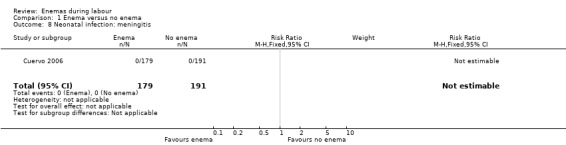
Comparison 1 Enema versus no enema, Outcome 8 Neonatal infection: meningitis.
1.9. Analysis.
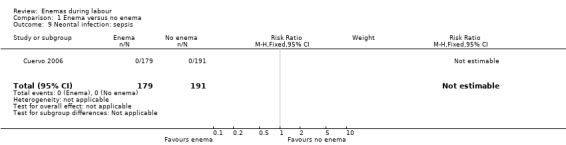
Comparison 1 Enema versus no enema, Outcome 9 Neontal infection: sepsis.
1.11. Analysis.
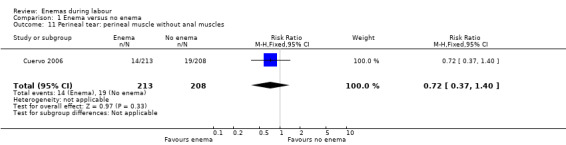
Comparison 1 Enema versus no enema, Outcome 11 Perineal tear: perineal muscle without anal muscles.
1.13. Analysis.
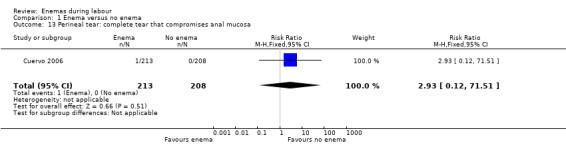
Comparison 1 Enema versus no enema, Outcome 13 Perineal tear: complete tear that compromises anal mucosa.
1.14. Analysis.
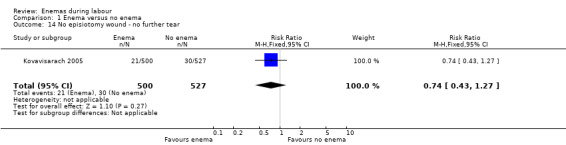
Comparison 1 Enema versus no enema, Outcome 14 No episiotomy wound ‐ no further tear.
1.15. Analysis.
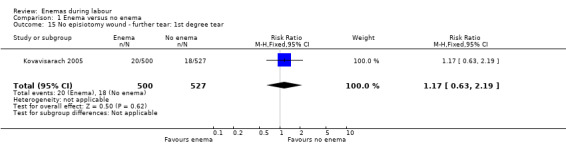
Comparison 1 Enema versus no enema, Outcome 15 No episiotomy wound ‐ further tear: 1st degree tear.
1.16. Analysis.
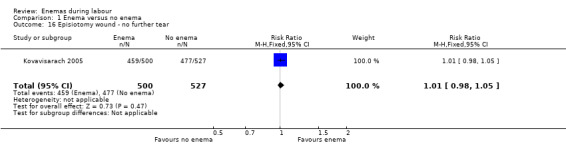
Comparison 1 Enema versus no enema, Outcome 16 Episiotomy wound ‐ no further tear.
1.17. Analysis.
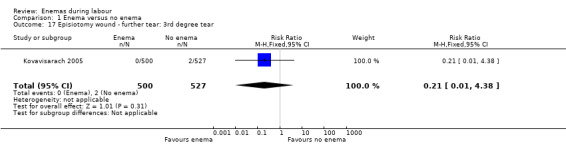
Comparison 1 Enema versus no enema, Outcome 17 Episiotomy wound ‐ further tear: 3rd degree tear.
1.18. Analysis.
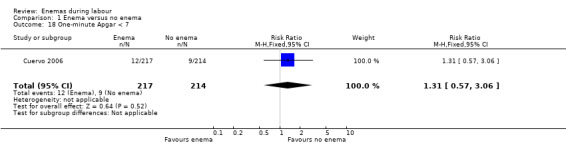
Comparison 1 Enema versus no enema, Outcome 18 One‐minute Apgar < 7.
1.19. Analysis.
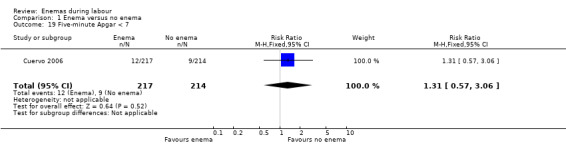
Comparison 1 Enema versus no enema, Outcome 19 Five‐minute Apgar < 7.
1.22. Analysis.
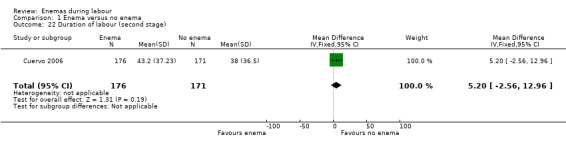
Comparison 1 Enema versus no enema, Outcome 22 Duration of labour (second stage).
1.23. Analysis.
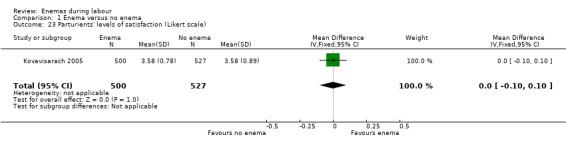
Comparison 1 Enema versus no enema, Outcome 23 Parturients' levels of satisfaction (Likert scale).
1.27. Analysis.
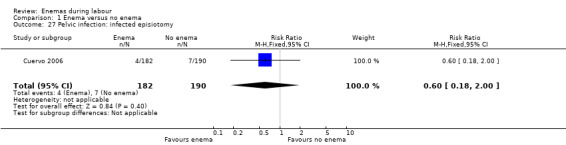
Comparison 1 Enema versus no enema, Outcome 27 Pelvic infection: infected episiotomy.
1.28. Analysis.
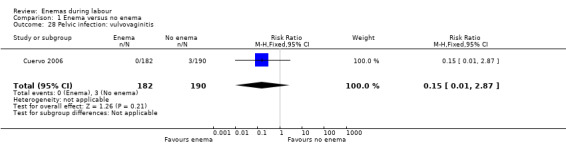
Comparison 1 Enema versus no enema, Outcome 28 Pelvic infection: vulvovaginitis.
1.29. Analysis.
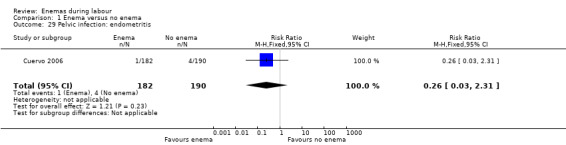
Comparison 1 Enema versus no enema, Outcome 29 Pelvic infection: endometritis.
1.30. Analysis.
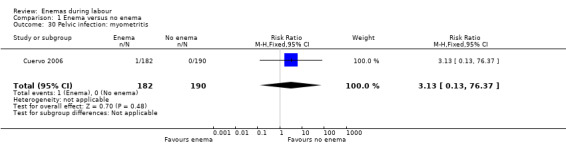
Comparison 1 Enema versus no enema, Outcome 30 Pelvic infection: myometritis.
1.31. Analysis.
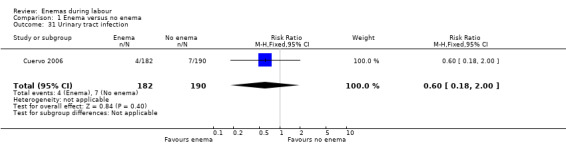
Comparison 1 Enema versus no enema, Outcome 31 Urinary tract infection.
1.34. Analysis.
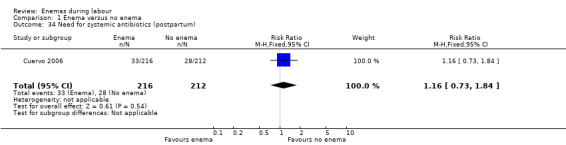
Comparison 1 Enema versus no enema, Outcome 34 Need for systemic antibiotics (postpartum).
1.35. Analysis.
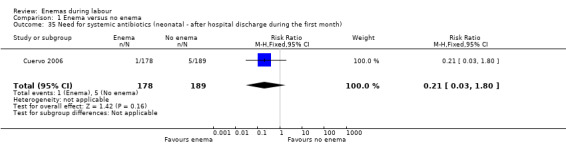
Comparison 1 Enema versus no enema, Outcome 35 Need for systemic antibiotics (neonatal ‐ after hospital discharge during the first month).
1.36. Analysis.
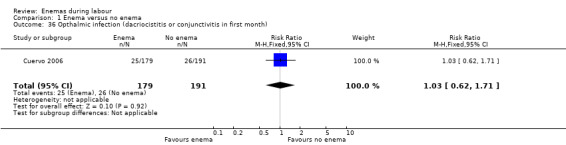
Comparison 1 Enema versus no enema, Outcome 36 Opthalmic infection (dacriocistitis or conjunctivitis in first month).
1.37. Analysis.
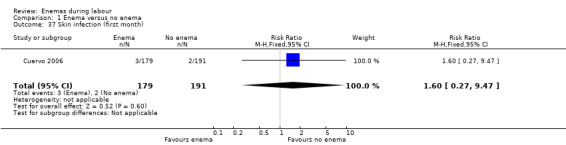
Comparison 1 Enema versus no enema, Outcome 37 Skin infection (first month).
1.38. Analysis.
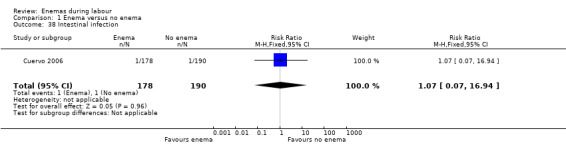
Comparison 1 Enema versus no enema, Outcome 38 Intestinal infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clarke 2007.
| Methods | Randomised clinical trial. Randomisation method was not explained. | |
| Participants | 152 pregnant women admitted for delivery. The characteristics of participating women are not described. | |
| Interventions | Women were randomly allocated to receive either standardised enema soap solution within 30 minutes of enrolment or no enema (75 women to enema and 77 to no enema). | |
| Outcomes | The primary outcome was time interval from enrolment to delivery. Secondary outcomes included intrapartum infection rate, faecal soiling at delivery, mode of delivery and patient satisfaction. | |
| Notes | Abstract publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are some missing data in outcomes' results. Women's satisfaction and neonatal outcomes were not reported. No standard deviation was provided |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study is not available. |
| Other bias | Unclear risk | Declarations of interest are not declared. No information was provided concerning baseline demographics |
Cuervo 2006.
| Methods | Randomised clinical trial. Block randomisations in blocks of 2 (20%), 4 (60%) and 6 (20%) using sealed envelopes when participants visited the obstetrics admission ward and filled inclusion criteria. | |
| Participants | Women attending a tertiary care hospital in Bogota, Colombia for delivery. Inclusion criteria included: living and staying in Bogota the month following delivery; gestational age of 36 or more weeks; willingness to participate. Exclusion criteria: medical emergency; use of antibiotics the week prior to admission; rupture of amniotic membranes; cervical dilatation over 7 cm. | |
| Interventions | High volume (1000 mL) saline solution enema or no enema. | |
| Outcomes | Participants and newborns were followed for 1 month after delivery. Visits were carried out at the puerperium and paediatrics ward and neonatal intensive care unit. Participants were evaluated through telephone interviews and/or physical examination carried out 1 and 4 weeks after delivery. 24‐hour pager service was offered to inform of any health problems. Telephone follow‐up was performed when patients failed to attend programmed visits. Infections were diagnosed on clinical grounds. Neonatal infections included: ocular, umbilical or skin infection; lower or upper respiratory tract infection; intestinal infection; meningitis or sepsis. Puerperal infections included: dehiscence of the episiorraphy suture; purulent effusion from episiorraphy; urinary tract infection; pelvic inflammatory disease or vulvovaginitis. | |
| Notes | Blinding was not possible although an effort was made to keep the hypothesis unknown to staff and participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in blocks of 2 (20%), 4 (60%) and 6 (20%) using Ralloc® allocation software. |
| Allocation concealment (selection bias) | Low risk | Opaque envelope with sequential numbering and instructions was opened. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 12 protocol violations; women who did not fulfil the inclusion criteria were identified and excluded from the final analysis (4 allocated to enema, 8 to no enema). The remaining women were analysed by intention‐to‐treat; losses to follow‐up were 35/217 (16%) in the enema group and 24/214 (11%) in the control group (P = 0.14). Losses to follow‐up among newborns were 18% in the enema group and 11% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study is not available. |
| Other bias | Low risk | Information was provided concerning baseline demographics. Declarations of interest are declared. |
Drayton 1984.
| Methods | Randomised clinical trial, with stratified allocation (primigravid vs multigravid). Randomisation method was not explained. | |
| Participants | Women entering labour ward during the first stage of labour, for vaginal delivery, single pregnancy and 37 or more weeks of gestation. Exclusion criteria included diabetes, cardiac disease or pregnancy complicated by antepartum haemorrhage or severe pre‐eclampsia. 370 women were eligible and 222 (60%) agreed to participate. | |
| Interventions | Low‐volume disposable phosphate enema in the study group vs no enema. Inclusion bias could have occurred when the clinic staff avoided the inclusion of women who had faecal deposition prior to admission. | |
| Outcomes | Faecal contamination was evaluated with an arbitrary scale, not validated, previously described by Romney and Gordon (Romney 1981). Infection evaluation is not clear. Follow‐up time is not clearly discussed, but there are no data suggesting that women were followed up after leaving the hospital. Infections were confirmed bacteriologically and association between the organism and soiling was established by a microbiologist and a research sister. Duration of labour: it was stratified between primigravidae and multigravidae. Data were collected in 6 ordinal and arbitrary categories. No clear time of follow‐up. | |
| Notes | Stratification would be better done by parity instead of gravidity. Randomisation method was not discussed in the article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are some missing data in outcomes' results. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study is not available. In the Drayton 1984 study the means to assess infection as well as the time of follow‐up was not reported by the authors. Infection rates were remarkably low in newborns and puerperal women as compared to the study from Colombia (Cuervo 2006). |
| Other bias | High risk | Inclusion bias seemed to happen when clinic staff excluded women if they had faecal soiling prior to their evaluation (time span unspecified). The authors provide no demographic data that would be relevant to know the external validity of the study. |
Kovavisarach 2005.
| Methods | Randomised clinical trial. Randomisation method not explained. | |
| Participants | 1027 pregnant women with labour pain were randomised to receive enema vs no enema. Inclusion criteria: all were in the gestational age range of 37‐42 weeks and met the inclusion criteria consisting of living singleton pregnancy, vertex presentation, having normal bowel function, and with true labour. Exclusion criteria: the pregnancies with medical or obstetric complications such as history of premature rupture of membranes, unexplained vaginal bleeding, previous uterine scar or previous antibiotics usage within 7 days before admission were excluded from the present study. | |
| Interventions | Experiment group: the enema in the present study (Uni‐ma enema) consisted of sodium biphosphate and sodium phosphate 118 mL. Control group: no enema. | |
| Outcomes | Faecal contamination rate during the second stage of labour with an arbitrary scale; neonatal infection; duration of labour; route of delivery; degree of perineal tear; and satisfactory level of parturients and medical staff using the Likert scale. Women and babies were followed up for 4 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no missing data in outcomes' results. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study is not available. |
| Other bias | Low risk | Information was provided concerning baseline demographics. Declarations of interest are not declared. |
cm: centimetre mL: millilitre vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lopes 2001 | Inadequate randomisation by hospital file number. 90 pregnant women (43 normal births, 27 forceps and 20 caesarean sections) were included in the study. Enema group did not provide faster labour and did not reduce faecal contamination. |
| Lurie 2012 | Enemas given before cesarean section. |
| Mathie 1959 | This is a trial that used for controls women receiving other interventions such as oral administration of castor oil and hot baths. It evaluated outcomes through physiologic measurements with a tocodynamometer. The studies were performed before labour. Neonatal or maternal morbidity or mortality was not evaluated. Labour duration was not evaluated either. It does not comply with inclusion criteria for this review. |
| Romney 1981 | This was not a randomised controlled trial. A pilot study was done with a population of 84 consecutively admitted women who had no enema and compared them with 111 women admitted for induction of labour who received an enema. Later, they recruited 50 women with a haphazard allocation of enema vs no enema. The authors grouped the populations studied in the pilot and the main study together. No information regarding sample size selection is described. There is no description of the methodology of statistical analysis. |
| Rosenfield 1958 | This is not a randomised controlled trial. There was no information about the characteristics of the included women. |
| Rutgers 1993 | This was a case‐control study as described in the abstract. Sample size is not adjusted and its calculation is not based on risk analysis for case‐control studies. |
| Tzeng 2005 | This is not a randomised controlled trial. |
| Whitley 1980 | Observational study. Contamination was the main outcome and was measured according to the opinion of researchers. Labour duration, morbidity and mortality were not assessed. |
vs: versus
Differences between protocol and review
Levels of satisfaction of parturients and medical staff, delivery types (caesarean section), other neonatal infections, perineal tear/episiotomy wound, serious adverse effects, respiratory infection, overall pain during labour and birthweight were not pre‐specified outcomes but were included in the review as secondary outcomes.
Contributions of authors
All authors approved the final version of the 2013 update.
Ludovic Reveiz assessed the identified papers, drafted the conclusions and contributed to the writing of the review. He ensured the appropriate use of the statistical tests used and extracted the data. Ludovic Reveiz is now the guarantor of the review.
Hernando Gaitan assessed the identified papers, drafted the conclusions and contributed to the writing of the review.
Luis Gabriel Cuervo led the development of the first version and the first update of the review and commented on drafts of this update.
Declarations of interest
Dr Luis Gabriel Cuervo conducted a randomised controlled trial included in this review. His study fulfilled the inclusion criteria for this review. For this review, he was not involved in the selection of studies or in the appraisal of his study. Information from this study was independently collected and appraised by the other review authors.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Clarke 2007 {published data only}
- Clarke NT, Jenkins TR. Randomized prospective trial of the effects of an enema during labor [abstract]. Obstetrics & Gynecology 2007;109(4 Suppl):7S. [Google Scholar]
Cuervo 2006 {published data only}
- Cuervo LG, Bernal MP, Mendoza N. Effects of high volume saline enemas vs no enema during labour – the N‐Ma randomised controlled trial. BMC Pregnancy and Childbirth 2006;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drayton 1984 {published data only}
- Drayton S, Rees C. They know what they're doing. Do nurses know why they give pregnant women enemas?. Nursing Mirror 1984;159(5):4‐8. [PubMed] [Google Scholar]
Kovavisarach 2005 {published data only}
- Kovavisarach E, Sringamvong W. Enema versus no‐enema in pregnant women on admission in labor: a randomized controlled trial. Journal of the Medical Association of Thailand 2005;88(12):1763‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Lopes 2001 {published data only}
- Lopes MHB, Silva MAS, Christoforo FFM, Andrade DCJ, Bellini NR, Cervi RC. The use of intestinal cleansers to prepare for labor: analysis of advantages and disadvantages [O uso do enteroclisma no preparo para o parto: análise de suas antagens e desvantagens]. Revista Latino‐Americana de Enfermagem 2001;9(6):49‐55. [DOI] [PubMed] [Google Scholar]
Lurie 2012 {published data only}
- Lurie S, Baider C, Glickman H, Golan A, Sadan O. Are enemas given before cesarean section useful? A prospective randomized controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;163(11):27‐9. [DOI] [PubMed] [Google Scholar]
Mathie 1959 {published data only}
- Mathie JG, Dawson BH. Effect of castor oil, soap enema and hot bath on the pregnant human uterus near term. A tocographic study. Britiish Medical Journal 1959;1(5130):1162‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romney 1981 {published data only}
- Romney ML, Gordon H. Is your enema really necessary?. British Medical Journal 1981;282(6272):1269‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenfield 1958 {published data only}
- Rosenfield HH, Burke L, Rubin H. Disposable enema unit in obstetrics. Obstetrics & Gynecology 1958;11(2):222‐5. [PubMed] [Google Scholar]
Rutgers 1993 {published data only}
- Rutgers S. Hot, high and horrible. Should routine enemas still be given to women in labour?. Central African Journal of Medicine 1993;39(6):117‐20. [PubMed] [Google Scholar]
Tzeng 2005 {published data only}
- Tzeng YL, Shih YJ, Teng YK, Chiu CY, Huang MY. Enema prior to labor: a controversial routine in Taiwan. Journal of Nursing Research 2005;13(4):263‐70. [PubMed] [Google Scholar]
Whitley 1980 {published data only}
- Whitley N, Mack E. Are enemas justified for women in labor?. American Journal of Nursing 1980;80(7):1339. [DOI] [PubMed] [Google Scholar]
Additional references
Chalmers 2009
- Chalmers B, Kaczorowski J, Levitt C, Dzakpasu S, O'Brien B, Lee L, et al. Use of routine interventions in vaginal labor and birth: findings from the Maternity Experiences Survey. Birth 2009;36(1):13‐25. [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2008
- Conde‐Agudelo A, Rosas‐Bermudez A, Gülmezoglu AM. Evidence‐based intrapartum care in Cali, Colombia: a quantitative and qualitative study. BJOG: an international journal of obstetrics and gynaecology 2008;115(12):1547‐56. [DOI] [PubMed] [Google Scholar]
Curtis 2007
- Curtis GB. Your pregnancy week by week. http://www.mdadvice.com/library/urpreg/wbw37.htm (accessed March 12 2007).
Gayer 2002
- Gayer G, Zissin R, Apter S, Oscadchy A, Hertz M. Perforations of the rectosigmoid colon induced by cleansing enema: CT findings in 14 patients. Abdominal Imaging 2002;27(4):453‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levitt 2011
- Levitt C, Hanvey L, Bartholomew S, Kaczorowski J, Chalmers B, Heaman M, et al. Use of routine interventions in labour and birth in Canadian hospitals: comparing results of the 1993 and 2007 Canadian hospital maternity policies and practices surveys. Journal of Obstetrics and Gynaecology Canada 2011;33(12):1208‐17. [DOI] [PubMed] [Google Scholar]
Paran 1999
- Paran H, Butnaru G, Neufeld D, Magen A, Freund U. Enema‐induced perforation of the rectum in chronically constipated patients. Diseases of the Colon and Rectum 1999;42(12):1609‐12. [DOI] [PubMed] [Google Scholar]
PregnancyWeekly.com
- PregnancyWeekly.com. Enema. http://www.pregnancyweekly.com/pregnancy_information/enema.htm (accessed March 12 2007).
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
SEA‐ORCHID Study Group 2008
- SEA‐ORCHID Study Group, Laopaiboon M, Lumbiganon P, McDonald SJ, Henderson‐Smart DJ, Green S, Crowther CA. Use of evidence‐based practices in pregnancy and childbirth: South East Asia Optimising Reproductive and Child Health in Developing Countries project. PLoS One 2008;3(7):e2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sodré 2007
- Sodré TM, Lacerda RA. The working process in labor care in Londrina‐PR. Revista da Escola de Enfermagem da USP 2007;41(1):82‐9. [DOI] [PubMed] [Google Scholar]
Sweidan 2008
- Sweidan M, Mahfoud Z, DeJong J. Hospital policies and practices concerning normal childbirth in Jordan. Studies in Family Planning 2008;39(1):59‐68. [DOI] [PubMed] [Google Scholar]
Yeat 2011
- Yeat SK, Chen SC, Lee HH. Enema resulting in rectal prolapse and colostomy in a term pregnant woman. Taiwanese Journal of Obstetrics and Gynecology 2011;50(3):370‐1. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cuervo 1999
- Cuervo LG, Rodríguez MN, Delgado MB. Enemas during labour. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000330] [DOI] [PubMed] [Google Scholar]
Hay‐Smith 1995a
- Hay‐Smith J. Routine enema on admission in labour. [revised 27 January 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hay‐Smith 1995b
- Hay‐Smith J. Soapsuds vs tapwater enema on admission in labour. [revised 26 January 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hay‐Smith 1995c
- Hay‐Smith J. `Medicated' vs soapsuds enema on admission in labour. [revised 26 January 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hay‐Smith 1995d
- Hay‐Smith J. `Medicated' vs tapwater enema on admission in labour. [revised 27 January 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Reveiz 2007
- Reveiz L, Gaitán HG, Cuervo LG. Enemas during labour. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000330.pub2] [DOI] [PubMed] [Google Scholar]


