Abstract
Background
Risperidone is one of the 'new generation' antipsychotics. As well as its reputed tendency to cause fewer movement disorders than the older drugs such as chlorpromazine and haloperidol, it is claimed that risperidone may improve negative symptoms.
Objectives
To evaluate the effects of risperidone for schizophrenia in comparison to 'conventional' neuroleptic drugs.
Search methods
The original electronic searches of Biological Abstracts (1980‐1997), Cochrane Schizophrenia Group's Register (1997), The Cochrane Library (1997, Issue 1), EMBASE (1980‐1997), MEDLINE (1966‐1997), PsycLIT (1974‐1997), and SCISEARCH (1997) were updated with a new electronic search of the same databases in 2002. The search term used in the update was identical to that used in 1997. Any new studies or relevant references were added to the review. In addition, references of all identified studies were searched for further trial citations. Pharmaceutical companies and authors of trials were also contacted.
Selection criteria
All randomised trials comparing risperidone to any 'conventional' neuroleptic treatment for people with schizophrenia or other similar serious mental illnesses.
Data collection and analysis
Citations and, where possible, abstracts were independently inspected by reviewers, papers ordered, re‐inspected and quality assessed. Data were also independently extracted. Where possible, sensitivity analyses on dose of risperidone, haloperidol and duration of illness were undertaken for the primary outcomes of clinical improvement, side effects (movement disorders) and acceptability of treatment. For homogeneous dichotomous data the Relative Risk (RR), 95% confidence interval (CI) and, where appropriate, the number needed to treat/harm (NNT/H) were calculated on an intention‐to‐treat basis.
Main results
In the short‐term, risperidone was more likely to produce an improvement in the Positive and Negative Syndrome Scale (PANSS) when compared with haloperidol (n=2368, 9 RCTs, RR not 20% improved 0.72 CI 0.59 to 0.88 NNT 8). A similar, favourable outcome for risperidone was found in long‐term studies (n=859, 2RCTs RR not 20% improved 0.51 CI 0.38 to 0.67 NNT 4;n=675 1RCT, RR not improved 40% 0.75 CI 0.66 to 0.84 NNT 5; n=675, 1 RCT, RR not 60% improved 0.90 CI 0.84 to 0.96, NNT 11). Risperidone was also more likely to reduce relapse at one year follow up, compared with haloperidol (n=367, 1 RCT, RR 0.64 CI 0.41 to 0.99, NNT 7). Less people allocated risperidone left studies before completion, both for short‐term (n=3066, 16 RCTs, RR 0.76 CI 0.63 to 0.92, NNT 6) and long‐term trials (n=1270, 4RCTs, RR 0.55 CI 0.42 to 0.73 NNT 4). For general movement disorders results favoured risperidone. People given risperidone had significantly fewer general movement disorders (including extrapyramidal side effects) than those receiving older typical antipsychotics (n=2702, 10 RCTs, RR 0.63 CI 0.56 to 0.71, NNT 3). Significantly fewer people given risperidone used antiparkinsonian drugs (n=2524, 11 RCTs, RR 0.66 CI 0.58 to 0.74, NNT 4). As regards body weight, however, four studies (n=1708) found people were more likely to gain weight if allocated risperidone compared to typical antipsychotics (RR 1.55 CI 1.25 to 1.93, NNH 3). Risperidone was no more or less likely than haloperidol to cause sexual problems such as erectile dysfunction (n=106, 2 RCTs, RR 1.55 CI 0.58 to 4.20). Finally, some results found risperidone was more likely to cause rhinitis than conventional antipsychotics (n=656, 3 RCTs, RR1.99 CI 1.24 to 3.19, NNH 3).
Authors' conclusions
Risperidone may be more acceptable to those with schizophrenia than older antipsychotics and have marginal benefits in terms of limited clinical improvement. Its adverse effect profile may be better than haloperidol. With the addition of more studies to this review, the publication bias evident in previous versions is no longer a significant issue. Any marginal benefits this drug may have have to be balanced against its greater cost and increased tendency to cause side effects such as weight gain.
Recent important longer term data favouring risperidone's effect on relapse needs to be replicated by researchers independently of the manufacturers of the drug.
Plain language summary
Risperidone versus typical antipsychotic medication for schizophrenia
Risperidone is one of the most widely used new generation of antipsychotic drugs. This review summarises its effects compared with the older antipsychotics. In essence, risperidone may be equally clinically effective to relatively high doses of haloperidol, for an outcome that is difficult to interpret as clinically meaningful. Risperidone causes less adverse effects than the side‐effect prone haloperidol.
Background
The 'conventional' neuroleptic drugs such as haloperidol and chlorpromazine are frequently used as the first line treatment for people with schizophrenia (Kane 1990, Kane 1993). However, about 5‐25% of these people show poor response to these treatments (Davis 1977, Christison 1991, Meltzer 1992). In addition, adverse effects such as movement disorders (i.e. distressing restlessness, stiffness, potentially disfiguring repetitive movements of the mouth and face) and sedation often makes compliance with the 'older generation' of drug treatment problematic (Kane 1990). The effects of these medications with respect to positive symptoms, such as fixed, false beliefs (delusions) and perceptions with no cause (hallucinations) is well described (Joy 2000, Thornley 2003). However, little evidence exists that 'conventional' antipsychotic treatment has any effect on the 'negative' symptoms of schizophrenia, that is symptoms such as poverty of speech, lack of motivation and apathy, and impoverished ability to express emotion (Crow 1980, Andreasen 1985).
Risperidone is one of the 'new generation' neuroleptic compounds. These drugs have also been termed 'atypical' antipsychotics, as they have a decreased tendency to cause movement disorders. Risperidone has a well‐described mechanism of action in the brain (5HT1a, 2a, 2c, H1, M1 and D1‐4 receptor blockade) and was developed following the observation that a selective serotonin receptor blocker (ritanserin) produced a beneficial effect when combined with conventional neuroleptics (Gupta 1994, Curtis 1995). Risperidone was first made available for the care of those with schizophrenia in 1986 and since then clinical trials have been conducted to evaluate its efficacy and safety (He 1995, Chouinard 1993b).
As well as its reputed tendency to cause fewer movement disorders, it is claimed that risperidone may improve negative symptoms (Livingston 1994, Edwards 1994). Unlike many neuroleptic drugs, both 'conventional' and 'atypical', risperidone does not bind to the sites in the brain (cholinergic/muscarinic receptors) that cause the dry mouth, blurred vision, constipation and urinary retention commonly seen with the 'conventional' class of drugs (Edwards 1994, He 1995, Keltner 1995). It is also claimed that risperidone may reduce the decline in memory, attention and concentrations associated with schizophrenia which 'conventional' neuroleptics fail to reverse or prevent (Meltzer 1994, Stip 1996).
Adverse effects commonly associated with risperidone include anxiety, agitation, insomnia, headache and weight gain (Remington 1993, Janssen‐Cilag 1996). Concern has been raised that the emphasis on primarily controlling the positive symptoms of schizophrenia may deflect attention from such apparently minor side effects and that this is often illustrated by the paucity of information regarding such effects in drug trials (Kane 1990).
Risperidone is a relatively expensive neuroleptic. Treatment for one year (6mg daily) costs £1,423 (pounds sterling) as compared to £127 with 10 mg daily haloperidol (figures provided by MIMS & Scottish Drug Tariff 2003). However calculating cost‐effectiveness in mental health is complex and it can be argued for example that improved treatment efficacy may reduce costs incurred as a result of hospitalisation (Addington 1993, Guest 1996).
Individuals with schizophrenia previously resistant to treatment may be helped by treatment with risperidone and it therefore may act as an alternative to clozapine (Edwards 1994, Meltzer 1994). Its use for those experiencing their first episode of schizophrenia is also being explored (Kopala 1996).
Objectives
To review the effects of risperidone compared with placebo and conventional neuroleptic drugs for people with schizophrenia.
It was also proposed to see if: 1. People whose illnesses were described as 'treatment resistant' differed in their treatment response from those whose illnesses were not designated as such; and if 2. Those experiencing their first episode of schizophrenia differed in their response from people who had had the illness longer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
People with schizophrenia and other types of schizophrenia‐like psychoses (schizophreniform and schizoaffective disorders) were included. There is no evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
1. Risperidone: any dose 2. Placebo 3. 'Typical' neuroleptic drugs: such as haloperidol, chlorpromazine, any dose.
Types of outcome measures
Outcomes were grouped into those measured in the short term (up to 12 weeks), medium term (13 to 26 weeks) and long term (over 26 weeks).
Primary outcomes
Four outcomes were considered of principal interest:
1. Global outcome: Relapse as defined in individual studies
2. Mental state: No clinically important change in general mental state (as defined in individual studies) with particular reference to the positive and negative symptoms of schizophrenia
3. Adverse effects: particularly movement disorders (extrapyramidal side effects) and those side effects considered to occur commonly with risperidone (anxiety, agitation, insomnia and headache) (Janssen‐Cilag 1996)
4. Acceptability of treatment: both assessed directly by questioning trial participants and indirectly by analysing the numbers of people who dropped out of the studies
Secondary outcomes
1. Death ‐ suicide and natural causes
2. Global state 2.1 Time to relapse 2.2 No clinically important change in global state 2.3 Not any change in global state 2.4 Average endpoint global state score 2.5 Average change in global state scores
3. Service outcomes 3.1 Hospitalisation 3.2 Time to hospitalisation
4. Mental state 4.1 Not any change in general mental state 4.2 Average endpoint general mental state score 4.3 Average change in general mental state scores 4.4 No clinically important change in specific symptoms 4.5 Not any change in specific symptoms 4.6 Average endpoint specific symptom score 4.7 Average change in specific symptom scores
5. General functioning 5.1 No clinically important change in general functioning 5.2 Not any change in general functioning 5.3 Average endpoint general functioning score 5.4 Average change in general functioning scores 5.5 No clinically important change in specific aspects of functioning, such as social or life skills 5.6 Not any change in specific aspects of functioning, such as social or life skills 5.7 Average endpoint specific aspects of functioning, such as social or life skills 5.8 Average change in specific aspects of functioning, such as social or life skills
6. Behaviour 6.1 No clinically important change in general behaviour 6.2 Not any change in general behaviour 6.3 Average endpoint general behaviour score 6.4 Average change in general behaviour scores 6.5 No clinically important change in specific aspects of behaviour 6.6 Not any change in specific aspects of behaviour 6.7 Average endpoint specific aspects of behaviour 6.8 Average change in specific aspects of behaviour
7. Adverse effects 7.1 No clinically important general adverse effects 7.2 Not any general adverse effects 7.3 Average endpoint general adverse effect score 7.4 Average change in general adverse effect scores 7.5 No clinically important change in specific adverse effects 7.6 Not any change in specific adverse effects 7.7 Average endpoint specific adverse effects 7.8 Average change in specific adverse effects
8. Engagement with services 8.1 No clinically important engagement 8.2 Not any engagement 8.3 Average endpoint engagement score 8.4 Average change in engagement scores
9. Satisfaction with treatment 9.1 Recipient of care not satisfied with treatment 9.2 Recipient of care average satisfaction score 9.3 Recipient of care average change in satisfaction scores 9.4 Carer not satisfied with treatment 9.5 Carer average satisfaction score 9.6 Carer average change in satisfaction scores
10. Quality of life 10.1 No clinically important change in quality of life 10.2 Not any change in quality of life 10.3 Average endpoint quality of life score 10.4 Average change in quality of life scores 10.5 No clinically important change in specific aspects of quality of life 10.6 Not any change in specific aspects of quality of life 10.7 Average endpoint specific aspects of quality of life 10.8 Average change in specific aspects of quality of life
11. Economic outcomes 11.1 Direct costs 11.2 Indirect costs
Search methods for identification of studies
Electronic searches
1. The Cochrane Schizophrenia Group's Register of Randomised Controlled Trials (July 2001) was searched using the phrase:
[risperidone or risperdal or 9‐OH‐risperidone or #42 = 142]
(#42 is the field in this Register that contains a code for each intervention.)
2. Searches of commercial databases Biological Abstracts (1980‐2001), The Cochrane Library (Issue 1, Feburary 2001), EMBASE (1980‐2001), PsycLIT (1974‐2001) and MEDLINE (1966‐2001) were searched using the Cochrane Schizophrenia Group's phrase for randomised controlled trials and schizophrenia (see Group search strategy) combined with the phrase:
[and (risperidone or risperdal or 9‐OH‐risperidone)]
Searching other resources
1. Reference lists. All references of articles selected were searched for further relevant trials.
2. Pharmaceutical Companies. Those companies performing trials (Janssen‐Cilag) with risperidone were contacted directly to obtain data on unpublished trials.
Data collection and analysis
1. Study selection CJ inspected all reports of studies identified as above. Randomly selected samples of 10% of all reports were re‐inspected by RH in order to allow selection to be reliable. Where disagreement occurred this was resolved by discussion, and where there was still doubt the full article was acquired for further inspection. Once the full articles were obtained EK and FS independently decided whether the studies met the review criteria. EK was blinded to the names of the authors, institutions and journal of publication. Where disagreement occurred this was resolved by discussion and when this was not possible further information was sought. These trials were added to the list of those awaiting assessment pending acquisition of further information. For the 2001 update, the reviewer (CJ) inspected all reports identified in the new search. Randomly selected samples of 10% of all new reports were re‐inspected by RH. Again once full reports were obtained, CJ and RH resolved disputes over whether studies meet inclusion criteria by discussion.
2. Quality assessment Trials were allocated to three quality categories, as described in the Cochrane Collaboration Handbook (Clarke 2000) by each reviewer. When disputes arose as to which category a trial was allocated to, resolution was attempted by discussion. When this was not possible and further information was necessary, data were not entered and the trial was allocated to the list of those awaiting assessment. Only trials in Category A or B were included in the review.
3. Assessing the presence of publication bias Data from all included trials were entered into a funnel graph (trial effect versus trial size or 'precision') in an attempt to investigate the likelihood of overt publication bias. Where only 3‐4 studies reported an outcome or there was little variety in sample size (or precision estimate) between studies ‐ funnel plot analysis was not appropriate. There is currently no consensus about the validity of formal statistical tests to investigate funnel plot asymmetry, one test, proposed by Egger 1997 has been subject to criticism (Irwig 1998). Further versions of this review will include such tests when their validity has been proven. 4. Data extraction Data from selected trials were independently extracted by EK, FS, RH and SG. When disputes arose resolution was attempted by discussion. When this was not possible and further information was necessary to resolve the dilemma, data were not entered and this trial was added to the list of those awaiting assessment. For the 2001 update, CJ extracted data, a random sample of extracted data were independently checked by SG.
5. Data synthesis Data types: Outcomes were assessed using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such a 'little change', 'moderate change' or 'much change') or dichotomous measures (for example, either 'no important changes' or 'important changes' in a persons behaviour). Currently RevMan does not support categorical data so they were presented only in the text of the review.
5.1 Dichotomous data Where possible, efforts were made to convert outcome measures to dichotomous data; this may be done by identifying cut off points on rating scales and dividing subjects accordingly into 'clinically improved' or 'not clinically improved'. If the authors of a study had used a designated cut off point for determining clinical effectiveness this was used by the reviewers where appropriate. For dichotomous outcomes, a random effects Relative Risk (RR) with the 95% confidence interval (CI) around this was estimated. As a summary measure of effectiveness, the number needed to treat statistic (NNT) was also calculated.
5.2 Continuous data 5.2.1 Scale derived data A wide range of rating scales is available to measure outcomes in mental health trials. These scales vary in quality and many are questionably validated, or even ad hoc. It is generally accepted that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure). Before publication of an instrument, most scientific journals insist that reliability and validity be demonstrated to the satisfaction of referees. It was therefore decided, as a minimum standard, not to include any data from a rating scale in this review unless its properties had been published in a peer‐reviewed journal. In addition, the following minimum standards for rating scales were set: the rating scale should either be a self‐report, or completed by an independent rater or relative. More stringent standards for instruments may be set in future editions of this review.
Whenever possible we took the opportunity to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. Where continuous data was presented from different scales rating the same effect, both sets of data were presented and the general direction of effect inspected.
5.2.2 Normal data Data on outcomes in neuroleptic trials are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data the following standards were applied to data derived from continuous measures of end point 'state'. The criteria were used before inclusion: i. Standard deviations and means were reported in the paper or were obtainable from the authors; ii. The standard deviation (SD), when multiplied by 2 was less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution) (Altman 1996). Data that did not meet the first or second standard were not entered into RevMan software for analysis, but were reported in the text of the 'Results' section.
For continuous mean change data (endpoint minus baseline) the situation is even more problematic. In the absence of individual patient data it is impossible to know if change data is skewed. The RevMan meta‐analyses of continuous data are based on the assumption that the data are, at least to a reasonable degree, normally distributed. It is quite feasible that change data is skewed but, after consulting the ALLSTAT 1988 electronic statistics mailing list (http://www.stats.gla.ac.uk/allstat/index.html), it was entered into RevMan in order to summarise the available information. In doing this it is assumed that either data were not skewed or that the analyses within RevMan could cope with the unknown degree of skew. Without individual trial data it is not possible to formally check this assumption.
5.2.3 Summary statistic We estimated a weighted mean difference, random effects model (WMD) for continuous outcomes.
5.3 Intention to treat analysis We hoped we would produce a review of risperidone that was pragmatic and answered the question as to whether, on average, those who take this new drug do better or worse than if they had taken none, an old drug or a similar new medication. The purpose of this review was not to ask the question "does risperidone work with people who are very compliant with medication and complete the course?" as this greatly limits the generalisability (external validity) of the findings. The reviewers therefore undertook an intention to treat analysis assuming that those who dropped out ‐ from whatever group ‐ had an unfavourable outcome. The reviewers investigated the sensitivity of the principle dichotomous outcomes to this assumption. For continuous, summary data it is not possible to include such an assumption so non‐intention to treat data were presented. Such data, however, were excluded if studies had more than 50% of the people lost to follow‐up.
5.4 Cluster trials Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems: Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) ‐ whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated ‐ causing type I errors (Bland 1997, Gulliford 1999). Secondly, RevMan does not currently support meta‐analytic pooling of clustered dichotomous data, even when these are correctly analysed by the authors of primary studies, since the 'design effect' (a statistical correction for clustering) cannot be incorporated.
Where clustering was not accounted for in primary studies, we presented the data in a table, with an (*) symbol ‐ to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to seek intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). If clustering had been incorporated into the analysis of primary studies, we also presented these data in a table. No further secondary analysis (including meta‐analytic pooling) will be attempted until there is consensus on the best methods of doing so, and until RevMan, or any other software, allows this. A Cochrane Statistical Methods Workgroup is currently addressing this issue. In the interim, individual studies were very crudely classified as positive or negative, according to whether a statistically significant result (p<0.05) was obtained for the outcome in question, using an analytic method which allowed for clustering.
5.5 Test for heterogeneity A Chi‐square test was used, as well as visual inspection of graphs, to investigate the possibility of heterogeneity. A significance level less than 0.10 was interpreted as evidence of heterogeneity. If heterogeneity was found, the studies responsible for heterogeneity were not added to the main body of homogeneous trials, but summated and presented separately and reasons for heterogeneity investigated.
6. Addressing publication bias Data from all included studies were entered into a funnel graph (trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias (Egger 1997).
7. Sensitivity analyses The effect of including studies with high attrition rates was analysed in a sensitivity analysis.
8. General Where possible, reviewers entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for risperidone.
Results
Description of studies
1. Excluded A total of 30 studies have now been excluded from this review. Details are presented in the 'Excluded studies' table. All trials were excluded only after acquisition and inspection of a hard copy. Half of the 30 studies did not meet the inclusion criteria because they were not randomised. Six of these were reviews and did not report randomised studies. Meltzer 1996 was excluded because it was a withdrawal study, rather than investigating the instigation of treatment. Six more studies were excluded because they compared risperidone to an atypical antipsychotic. A further eleven were trials that, at the moment, are only available to the authors as conference proceedings. Extraction of information from short abstracts is difficult and all data presented in these studies were unusable.
2. Awaiting assessment There are currently no trials awaiting assessment.
3. Ongoing As far as the authors are aware, there are six ongoing randomised trials that may be relevant to this review. It is not clear how many people are to be included in these trials.
4. Included Twenty three studies have now been identified for inclusion in this review. Details of these can be found in the 'Included studies' table.
4.1 Length of trials Eighteen of the included studies fell into the 'short term' (up to 12 weeks) category, with a duration of only eight weeks being most common (Ceskova 1993, Chouinard 1993a, Hoyberg 1993, Marder 1994, Mesotten 1991, Min 1993, Peuskens 1995, Wirshing 1999). Blin 1996 and Potkin 1997 were only four weeks in duration. Only Malyarov 1999 reported outcomes for the 'medium term' (13‐26 weeks) category. The remaining four trials were of longer duration. Mahmoud 1998 and Purdon 2000 followed people for about one year, while Bouchard 1998 and Csernansky 1999 reported outcomes at two years and beyond. 4.2 Participants A total of 2595 people were randomised to receive risperidone compared with a total of 1850 people receiving either a typical antipsychotic or placebo. The uneven numbers in some studies are due to the fact that trialists randomised to several different doses of risperidone versus one comparator group. Nearly all of the studies included people diagnosed with schizophrenia according to a diagnostic manual. Most (fourteen) used the Diagnostic and Statistical Manual of Mental Disorders, third edition Revised (DSM‐III‐R). Purdon 2000 and See 1999 used the 4th edition (DSM ‐IV) and three diagnosed people according to ICD‐9 or ICD ‐10 (the International Classification of Diseases). Four trials did not explicitly report how they diagnosed people with schizophrenia (Bouchard 1998, Csernansky 1999, Mahmoud 1998, Purdon 2000). Further information is being sought.
All studies included both men and women, although participants were predominantly male (about 70%). The mean age was about 33 years. The age range for most trials was 18‐65 years except for Kaleda 2000, a study that focused on young people (age range 16‐20 years). A few studies did not describe age and sex of the participants and further information is being sought. Most people in the trials had previously been admitted to hospital and were chronically ill. Only a few trials included people who were not chronically ill and/or in hospital. Two of these, Bouchard 1998 and Csernansky 1999, involved people who were able to be outpatients, with Csernansky 1999 specifically requiring participants to be in a stable condition. Emsley 1995 included only those experiencing their first episode of schizophrenia, and Blin 1996, Huttunen 1995 and Malyarov 1999 included people who were in an acute phase of their illness. Finally, Wirshing 1999 focused on people whose illness was designated to be treatment resistant, and See 1999 on those whose illness had been only partially responsive to neuroleptics.
4.3 Settings Trials took place in a mixture of outpatient and inpatient settings although the majority of trials were conducted in a hospital. Twelve of the 30 included trials were multi‐centre and overall, participants were recruited from a diverse range of cultural backgrounds including Australia, Canada, China, Czechoslovakia, France, Korea, North America, Scandinavia, South Africa and the UK.
4.4 Study size A total of 4445 people participated in the included studies. The largest trial was a 15 nation multi‐centre study (Peuskens 1995) that involved 1362 participants. Next in size was Mahmoud 1998 with 675 people randomised. Chouinard 1993a and Marder 1994, which were in effect one study, sometimes referred to as the North American Study, collectively randomised 523 people. All other studies were comparatively small ranging from 183 (Emsley 1995) to only 20 (See 1999). 4.5 Interventions Risperidone doses varied and were either fixed or flexible. Seven trials used fixed doses, ranging from a minimum of 1 mg/day (Peuskens 1995), to a maximum of 16mg/day (Peuskens 1995, Marder 1994, Chouinard 1993a). The remaining studies allowed the dose to be individually titrated according to response (range 2‐24mg/day). Wirshing 1999 used both fixed and varied doses of risperidone. This trial used a fixed dose of 6mg/day for the first four weeks and then switched for the remaining four weeks to a dose that could vary according to need (range 3‐15mg/day).
The control interventions varied. Twelve of the trials used haloperidol as the control intervention. Five of these used a fixed dose ranging from a minimum of 5mg/day (Min 1993) to a maximum of 20mg/day (Chouinard 1993a, Marder 1994). All other haloperidol trials used flexible dose regimes, individually titrating the dose according to response. The dose range for these studies was 2mg/day (Borison 1991) to 30mg/day (Wirshing 1999). Only two small studies compared risperidone with a neuroleptic other than haloperidol. Hoyberg 1993 used perphenazine (16‐48mg/day) and Huttunen 1995 zuclopenthixol (10‐100mg/day). Mahmoud 1998 compared risperidone with 'conventional treatment strategy'. Six trials involved three comparison groups (Blin 1996, Borison 1991, Chouinard 1993a, Malyarov 1999, Marder 1994, Purdon 2000). All of these studies had haloperidol as one of the control interventions with the other groups being randomised to methotrimeprazine (Blin 1996), olanzapine (Purdon 2000, Malyarov 1999) or placebo. Data relating to methotrimeprazine is not used in this review, as this compound, despite being a phenothiazine, is not one that is in common usage. The inclusion of this would introduce a potential source of heterogeneity whilst gaining little extra external validity. Olanzapine data is also not used, as this is an atypical compound.
4.6 Outcomes 4.6.1 Missing Outcomes None of the studies mentioned death, suicide or self‐harm. Other outcomes notable by their absence were quality of life, social functioning, employment status and cost effectiveness.
4.6.2 Scales Twenty four different instruments were used to collect scale data. Only six, however, provided useful data. Seven studies reported usable scale data for clinical improvement but employed three different scales (BPRS, CGI, PANSS, for details see below). Six studies used scales to help rate adverse effects. Again, however, they employed three different scales (ESRS, CGI, UKU). Reasons for exclusion of data from other instruments are given under 'Outcomes' in the 'Included studies' section. Overall scale data were poorly presented; frequently means were reported with no variance and/or not reported at all.
4.6.2.1 Details of scales that reported usable data a. Global state ‐ Clinical Global Impression Scale ‐ CGI Scale (Guy 1976) This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven‐point scoring system is usually used with low scores showing decreased severity and/or overall improvement.
b. Mental state i. Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) This is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a seven‐point scale varying from 'not present' to 'extremely severe', scoring from 0‐6 or 1‐7. Scores can range from 0‐126, with high scores indicating more severe symptoms.
ii. Positive and Negative Syndrome Scale ‐ PANSS (Kay 1987) This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from 1 ‐ absent to 7 ‐ extreme. This scale can be divided into three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity.
c. Adverse effects i. Extrapyramidal Symptom Rating Scale ‐ ESRS (Chouinard 1980) This consists of a questionnaire relating to parkinsonian symptoms (nine items), a physician's examination for parkinsonism and dyskinetic movements (eight items), and a clinical global impression of tardive dyskinesia. High scores indicate severe levels of movement disorder.
ii. Simpson Angus Scale ‐ SAS (Simpson 1970) This ten‐item scale, with a scoring system of 0‐4 for each item, measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of parkinsonism.
iii. UKU Side Effects Rating Scale ‐ UKU‐SERS (Lingjaerde 1987). The UKU rates four major topics: psychological side effects (10 items), neurological side effects (eight items), autonomic side effects (11 items) and other side effects (19 items). Each item is defined by means of a four‐point scale where zero means not or doubtfully present. Scoring range is 0‐144.
Risk of bias in included studies
1. Randomisation All included studies were reported as randomised but only two, Peuskens 1995 and Wirshing 1999, described how this was achieved. In both cases computer generated numbers were used to allocate participants to groups. Min 1993 stated allocation was concealed by use of sealed envelopes, and a few studies mentioned the use of 'randomised blind schedules'.
2. Blindness All but three studies were reported to be double blind. Bouchard 1998 was an open label trial and therefore involved no blinding. Mahmoud 1998 did not state if blinding occurred and Malyarov 1999 said that only raters were blind to medication. The other 20 studies all stated they were double blind with half describing how this was achieved (by identical capsules or oral solutions) and the other half giving no further explanation. No trial tested whether their attempts at blinding had been successful.
3. Leaving the study early All but two trials, Kaleda 2000 and See 1999, reported the number of people leaving the study before completion and most trials attempted to ascribe reasons for this. It was assumed that the two trials which did not describe withdrawals, had no loss.
4. Jadad quality scores According to the Cochrane criteria described above, all studies were at moderate risk of bias. Jadad scores were 0‐2 for randomisation, 0‐2 for blindness and 0‐1 for description of withdrawals (high scores indicate better quality and less risk of inclusion of bias). Apart from Peuskens 1998 and Wirshing 1999, all studies received one point for their descriptions of randomisation (Peuskens 1995 and Wirshing 1999 received two). Eight trials scored two for blindness (Blin 1996, Borison 1991, Ceskova 1993, Chouinard 1993a, Claus 1991, Hoyberg 1993, Mesotten 1991, Min 1993), three (Bouchard 1998, Kaleda 2000, Mahmoud 1998) scored none, and all others received one. Finally, all were scored as 'one' for description of withdrawals except for the two studies that were rated at zero (Kaleda 2000, See 1999). These ratings resulted in a mean score of 2.5.
5. Data reporting Reporting of data was poor. Overall, it was only possible to use relatively small amounts of the data presented in the 23 included trials. Continuous data were particularly problematic. The most common reason for exclusion of scale data was lack of standard deviations and/or failure to give any information about outcomes at all. Many studies also presented findings in graphs, in percentiles or by inexact p‐values. 'P'‐values are commonly used as a measure of association between intervention and outcomes instead of showing the strength of the association.
Effects of interventions
1. The search Sixty citations were identified by the initial search. Of these 46 were selected for further inspection, yielding 29 reports of the 14 included trials. 349 new citations were found in the 2001 update, 156 of these were selected for further inspection and 120 of these were either further references to trials already included in the review or not relevant. The remaining papers yielded nine new trials that could be included and 23 that eventually were excluded. Clive Rogers of Janssen‐Cilag Ltd kindly ran a search through their databases. Although a substantial list of citations was obtained, no additional studies were identified.
2. COMPARISON: RISPERIDONE versus TYPICAL NEUROLEPTIC MEDICATION
2.1 Global state: not improved to a clinically important degree 'Clinical improvement' was measured in several different ways. Eleven studies prestated that 'clinical improvement' was a 20% reduction in total PANSS score from baseline. Emsley 1995 defined it as a 50% reduction while Mahmoud 1998 considered 20%, 40% and 60% reductions in the same scale. Two studies used a 20% reduction in total Brief Psychiatric Rating Scale (BPRS) score from baseline as a cut off for 'clinical improvement' (Borison 1991, Wirshing 1999) and eight employed the Clinical Global Impression (CGI). Finally, Wirshing 1999 used personal ratings by the participants.
Results derived from the BPRS and CGI scales are equivocal. Two studies using the BPRS appear to show a favourable result for risperidone in the short‐term but the difference is not statistically significant (n=173, 2 RCTs, RR 0.80 CI 0.6 to 1.02). Again with the CGI, it appears as though risperidone has a favourable effect on global state in the short‐term . Eight studies found a slight difference but it was not statistically significant (n=1126 8 RCTs, RR 0.78 CI 0.61 to 1.0). Results from the PANSS scale mainly favoured risperidone. Eleven studies used a 20% reduction in PANSS score as a cut off for clinical improvement. Risperidone was more likely to produce this degree of improvement, both in the short and long‐term, when compared with haloperidol (short‐term n=2368, 9 RCTs RR 0.85 CI 0.77 to 0.93 NNT 8; long term n=859, 2 RCTs RR 0.73 CI 0.65 to 0.83 NNT 4). Emsley 1995 found risperidone was no more likely to produce a 40% reduction PANSS scores than haloperidol in the short‐term (n=183, 1 RCT, RR 0.85 CI 0.6 to 1.2) but Mahmoud 1998 found a significant difference, favouring risperidone in the long‐term (n=675, 1 RCT, RR 0.75 CI 0.67 to 0.84 NNT 5). Mahmoud 1998 also obtained similar results for a 60% reduction (n=675, RR 0.90 CI 0.84 to 0.96, NNT 11). Only Wirshing 1999 used participant rating as a measurement of clinical improvement. This small, short‐term study (n=67) found no difference between groups (RR 0.72 CI 0.47 to 1.09).
Csernansky 1999 reported the global outcome of 'relapse'. The limited data found risperidone was more likely to reduce relapse by one year, compared with haloperidol (n=367, 1 RCT, RR 0.64 CI 0.41 to 0.99, NNT 7). The same study reported on 'time to relapse'. Results did favour people allocated to risperidone (data are too skewed to be presented graphically). Ceskova 1993 found no difference between risperidone and haloperidol for discharge from hospital by 8 weeks (n=62, 1 RCT, RR 1.07 CI 0.65 to 1.76).
A few studies used a continuous measure of global change (change in CGI score). They provided short‐term data and no difference was found between risperidone and haloperidol groups (n=503, 4 RCTs, WMD ‐0.11 CI ‐0.3 to 0.1).
2.2 Mental state Five studies measured improvement in mental state by change in total PANSS scores. Results are equivocal. For this outcome risperidone, in the short‐term, is not clearly different to haloperidol (n=1352, 5RCTs, WMD ‐1.52 CI ‐4.01 to 0.97). Again no significant differences were found by 12 weeks between people allocated risperidone compared with those given haloperidol for either positive PANSS score changes (n=1352, 5RCTs, WMD ‐0.24 CI ‐1.09 to 0.60) or negative PANSS score changes (n=1352, 5RCTs, WMD ‐0.77 CI ‐1.62 to 0.07).
See 1999 reported limited data for endpoint PANSS scores. Here there was a significant difference, favouring risperidone, in endpoint scores by 6 weeks (n=20, 1RCT, WMD ‐2.75 CI ‐3.94 to ‐1.56).
Results from trials using the BPRS to measure mental state are more favourable for risperidone. Four trials found risperidone more likely to produce a statistically significantly greater mean change in total BPRS score compared to haloperidol by 12 weeks (n=1352, 4RCTs, WMD ‐1.70 CI ‐3.22 to ‐0.1).
Levels of the specific symptom of anxiety/tension in the short‐term were reported in five studies. Results do not favour either risperidone or haloperidol (n=2087, 5RCTs, RR1.05 CI 0.8 to 1.4). Ceskova 1993 and Hoyberg 1993 reported whether people were depressed. Again, there were no differences for people receiving risperidone compared with those on haloperidol (n=169, 2RCTs, RR 0.72 CI 0.4 to 1.3).
2.3 Behaviour Data are limited. Four studies (n=724) report short‐term data for agitation and found no significant differences between groups (RR 0.88 CI 0.6 to 1.2) and similar non‐significant results were found for the need for sedative medication (n=198, 3 RCTs, RR 1.04 CI 0.8 to 1.4).
2.4 Cognitive function For outcomes of poor concentration, risperidone is not different to haloperidol (n=1548, 4 RCTs, RR 0.90 CI 0.8 to 1.1), and the outcome for poor memory is similar (n=1469, 2 RCTs, RR 0.84 CI 0.7 to 1.0).
2.5 Acceptability of treatment Acceptability of treatment was measured in two ways; numbers of people leaving the study before completion and by direct questioning. For the outcome, 'leaving the study early' there were three subcategories: leaving for any reason, leaving due to adverse effects and leaving due to insufficient response. In the short‐term, numbers of people leaving a study early for any reason was significantly lower for people in the risperidone groups (n=3066, 16 RCTs, RR 0.76 CI 0.6 to 0.9, NNT 6) and a similar result was found for those leaving after a 26 week period (n=1270, 4 RCTs, RR 0.55 CI 0.4 to 0.73 NNT 4). Only one study provided medium‐term (12‐26 weeks) data and here no difference between groups was found (n=28, 1RCT, RR 1.24 CI 0.2 to 9.0). The numbers of people leaving a study for particular reasons appeared to be lower for risperidone but the differences for both adverse effect and insufficient response were not statistically significant (n=2243, 11 RCTs, RR 0.82 CI 0.61 to 1.09; n=2498, 11 RCTs, RR 0.84 CI 0.67 to 1.06 respectively).
Only Mesotten 1991(n=60) measured acceptability of treatment by direct questioning. Here no significant difference was found between groups (RR 0.70 CI 0.4 to 1.2).
2.6 Adverse events For overall difference in number of adverse events between groups, risperidone was no more likely to cause adverse effects than other typical antipsychotics (n=1278, 9 RCTs, RR 0.98 CI 0.9 to 1.0).
Results for specific adverse events, and in particular, general movement disorders were more favourable for risperidone. People given risperidone had significantly fewer symptoms of general movement disorders (including extrapyramidal side effects) (n=2702, 10 RCTs, RR 0.63 CI 0.56 to 0.71, NNT 3). Significantly fewer people given risperidone used antiparkinsonian drugs compared with, in the main, haloperidol (n=2524, 11 RCTs, RR 0.66 CI 0.58 to 0.74, NNT 4). Only Heck 2000 (n=88) reported the numbers of people able to stop antiparkinsonian drugs during the trial and found no difference between groups (RR 1.07 CI 0.39 to 2.29).
For specific movement disorders, Ceskova 1993 (n=62) reported data for tardive dyskinesia and found no significant difference between groups (RR 1.00 CI 0.07 to 15.28). For akathisia, however, results significantly favoured risperidone (n=731, 4 RCTs, RR 0.68 CI 0.52 to 0.89, NNT 5). No significant differences were found for dystonia (n=169, 2 RCTs, RR 0.72 CI 0.26 to 1.99), parkinsonism (n=62, 1 RCT, RR 0.89 CI 0.70 to 1.12), or tremor (n=101, 2 RCTs, RR 0.97 CI0.51 to 1.83).
Data from three studies found the change of severity of dyskinesia CGI scores were not significantly different for people on risperidone compared with those taking haloperidol (n=1778, 3 RCTs, WMD ‐0.20 CI ‐0.34 to ‐0.06), but were significantly different for severity of parkinsonism with the result favouring risperidone (n=468, 2 RCTs, WMD ‐0.78 CI ‐1.18 to ‐0.39). For change in Extrapyrimidal Symptom Rating Scale (ESRS) scores, people given risperidone had favourable results for severity of parkinsonism scores with a significantly lower mean change (n=2507, 3 RCTs, WMD ‐2.07 CI ‐2.91 to ‐1.23) and people on risperidone also had a significantly better total score (n=1708, 3 RCTs, WMD ‐0.68 CI ‐1.22 to ‐0.15). Peuskens 1995 provided data for change in UKU Side Effects Rating Scale score. The result favoured the risperidone group (n=1350, WMD ‐0.49 CI ‐0.51 to ‐0.47). In one small study (See 1999, n=20) the Simpson and Angus Scale (SAS) endpoint score was just significantly in favour of the risperidone group (n=20 WMD ‐0.35 CI ‐0.55 to ‐0.15).
Anticholinergic effects were reported in a few studies. Dry mouth (n=1616, 5 RCTs, RR 0.80 CI 0.59 to 1.09), blurred vision (n=1635, 5 RCTs, RR 0.83 CI 0.62 to 1.10), constipation (n=2165, 7 RCTs, RR 1.04 CI 0.80 to 1.37) and difficulty in passing urine (n=210, 3 RCTs, RR 0.24 CI 0.03 to 2.12) is no more common if receiving risperidone, compared largely with haloperidol.
Risperidone was also no more likely to cause sleep problems than the control medications. No significant differences between groups were found for insomnia (n=2899, 7 RCTs, RR 0.96 CI 0.82 to 1.14), decreased quality of duration (n=1510, 3 RCTs, RR 0.89 CI 0.69 to 1.14), increased duration (n=1469, 2 RCTs, RR 0.95 CI 0.76 to 1.18) or somnolence (n=1711, 12 RCTs, RR 0.89 CI 0.79 to 1.01).
As regards body weight, four studies (n=1708) found people were more likely to gain weight if allocated risperidone compared to typical antipsychotics (4 RCTs, RR 1.55 CI 1.25 to 1.93, NNH 3). No significant difference between groups was found for weight loss (n=1469, 2 RCTs, RR 1.23 CI 0.98 to 1.56).
Results for gastrointestinal problems found no significant differences between groups. The outcomes with usable data were dyspepsia (n=322, 1 RCT, RR 1.55 CI 0.47 to 5.09), nausea (n=366, 2 RCTs, RR 1.18 CI 0.57 to 2.44) and vomiting (n=689, 2 RCTs, RR 0.74 CI 0.43 to 1.26)
Risperidone is no more likely to cause dizziness than haloperidol (n=2426, 7 RCTs, RR 1.15 CI 0.92 to1.43), nor is it more prone to cause headaches (n=2891, 10 RCTs, RR 1.06 CI 0.88 to 1.27).
Again no significant differences between groups were found for cardiovascular problems (n=419, 3 RCTs, RR tachycardia 0.85 CI 0.39 to 1.82; n=85, 2RCTs, RR palpitations 0.85 CI 0.32 to 2.26; n=103, 2 RCTs, RR hypotension 0.96 CI 0.38 to 2.43; n=367, 1 RCT, RR hypertension 0.67 CI 0.35 to 1.27).
Risperidone was no more likely to cause sexual problems such as erectile dysfunction (n=106, 2 RCTs, RR 1.55 CI 0.58 to 4.20) ejaculatory dysfunction (n=29, 1 RCT, RR 4.12 CI 0.21 to 78.9) orgastic function (n=107, 1 RCT, RR 1.89 CI 0.36 to 9.9) or sexual desire (n=1469, 2 RCTs, RR 0.96 CI 0.66 to 1.41).
Women allocated risperidone had the same frequency of menstrual problems as those on typical antipsychotic drugs (n=45, 2 RCTs, RR amenorrhoea 1.86 CI 0.4 to 8.2; n=30, 1 RCT, RR menorrhoea 3.0 CI 0.13 to 68).
Finally, some results found risperidone was more likely to cause rhinitis (n=656, 3 RCTs, RR 1.24 to 3.19, NNH 3), but no more likely to cause sweating (n=1506, 5 RCTs, RR 0.75 CI 0.55 to 1.02) or hypersalivation (n=183, 1 RCT, RR 0.42 CI 0.08 to 2.26), psychosis (n=367 RR0.70 CI 0.47 to 1.04) or injury (n=367 RR 1.23 CI 0.68 to 2.22).
3. Funnel plots When funnel plots of effect size versus sample size were applied to outcomes where there were more than five studies reporting useable data, there was no evidence of obvious asymmetry. Statistical tests for funnel plot asymmetry were non significant in all cases.
4. Investigator misconduct It has come to the attention of the reviewers that Dr Richard Borison and Dr Bruce Diamond have been convicted of theft, making false statements and violations of state racketeering law in the USA. At this point, it seems that crimes were to do with criminal diversion of funds, rather than falsifying study data (http://www.the‐scientist.com/yr1998/oct/notebook_981026.html). Nevertheless studies involving either of these authors were temporarily removed from the analyses to see if this made a substantive difference to the findings. In all cases where data from these trials had been added to those of others, removal of the studies resulted in no substantive changes in the findings.
Discussion
1. Publication bias In a previous version of this review the possibility of publication bias, as evidenced by funnel plot asymmetry was raised. The conduct of further searches and addition of more studies has greatly reduced this potential source of bias, and the results of this review can be interpreted with a greater level of confidence.
2. Applicability of findings 2.1 Diagnoses Despite new data from nine studies being included in the review, the overall results remain remarkably similar to those of the previous version. Methodology across all trials was virtually identical. As for the studies in the first version of this review, the majority of the new studies used the DSM‐III‐R as a diagnostic tool, were short in duration, yet lost about 30% of their participants before study completion. They also used haloperidol as the comparator.
Studies included in this review involved large numbers of participants from a wide range of cultural settings. All but nine studies involved people with a strict operational DSM‐III‐R diagnosis. As such, the homogeneity of the patient group can be assumed. However, this means of diagnosis excludes many people seen in routine practice who also receive anti‐psychotic medication for schizophrenia and related disorders. For example, many people will receive anti‐psychotic medication for presumed schizophrenia‐like disorders in the absence of 'DSM‐III‐R' psychotic symptoms or before exhibiting continuous disturbance for six months (a major DSM‐III‐R criterion). Similarly, many will have co‐existing substance abuse disorders or other co‐morbid mental disorders, such as depression, or substance misuse. The results of the review may be internally valid and applicable to those with DSM‐III‐R schizophrenia, but can only be assumed to be externally valid and applicable to the large numbers of people in routine clinical practice who fall outside of the rigid DSM‐III‐R classificatory system, yet require anti‐psychotic medication.
2.2 Adherence to trial protocol About 30% of people left the studies, even before 6‐8 weeks. This figure is perhaps higher than that seen in routine practice, particularly considering that the majority of participants were recruited and managed in an inpatient setting. This presumably reflects the rigidity of study protocols, which, whilst improving internal validity, potentially reduces the external applicability of results. The high default rates that are observed when a rigid trial design is imposed on routine care for schizophrenia (or any disorder) suggest that the randomised study design should more closely replicate routine care. There is no information on the subsequent care of people leaving the studies.
2.3 History Although most trials included people who had long histories of schizophrenia, only Emsley 1995 exclusively focused on those experiencing their first episode. These people do not appear to differ substantially in their response, or lack of it, to risperidone.
2.4 Interventions This is essentially a review of the effectiveness of risperidone compared to haloperidol as 18 of the 23 included trials used haloperidol as at least one of its comparators. It is therefore problematic to generalise the findings to other 'conventional' neuroleptic drugs. Many of the studies included in this review used relatively high doses of haloperidol. A companion review has examined the relationship of dose of haloperidol to clinical outcome (Waraich 2002).
2.5 Duration Again most of included trials (18 of the 23) were 'short term' (less than twelve weeks duration) so little can be concluded from this review regarding the long‐term effects of risperidone. Only Csernansky 1999 provided some data on relapse by one year.
3. COMPARISON: RISPERIDONE versus TYPICAL NEUROLEPTIC MEDICATION
3.1 Global clinical improvement Risperidone is slightly more effective than control (largely haloperidol) in achieving 'global clinical improvement'. Most studies defined this as a 20% reduction in the BPRS or PANSS scale. The clinical meaning of this is unclear. The PANSS and BPRS are rating scales that are not commonly used in routine clinical practice. They both include a restricted range of items relating to various aspects of psychopathology (including positive and negative symptoms). Each of these individual items attracts equal weight. The validity of using a 20% reduction in this scale must be viewed with a degree of caution. It is far from clear whether a 20% reduction in scores represents an externally valid and clinically important improvement in mental state, which people with schizophrenia and clinicians would generally regard as a successful outcome that was worth achieving. It is quite possible to record improvement amongst a small number of the items to achieve a 20% reduction, whilst still retaining the most disabling and distressing features of a schizophrenic episode. Clinicians will need to consider the significance of having to treat 10 people with risperidone instead of haloperidol in order to achieve one clinical outcome equivalent to a 20% score reduction. In the original review we stated 'that more studies are appearing that record greater degrees of clinical improvement, such as 40% and 60% is encouraging, but these findings, as reported from Emsley 1995 and Mahmoud 1998, should be replicated'. No new studies in the update provided usable data for the greater degrees of clinical improvement with eleven studies still using a 20% reduction as a cut off point.
The Clinical Global Impression (CGI) scale is different from the BPRS and PANSS. It is less focused on specific symptoms and would take into account behavioural and social aspects of a person's day‐to‐day functioning. This scale failed to show any difference between risperidone and, largely, haloperidol. It could be argued that by focussing less on specific aspects of psychopathology, it represents a more valid measure of the overall impact of schizophrenia and its treatment on wider aspects of functioning and quality of life. The absence of any difference in this outcome further throws into doubt the external validity and clinical meaning of the observed changes in BPRS and PANSS scores. Whilst being a crude and imprecise measure of clinical outcome, it is perhaps a more externally valid global measure than the BPRS and PANSS.
Risperidone's effect on relapse was investigated in one study and findings look promising for risperidone. Csernansky 1999 found a significant reduction in relapse if allocated risperidone. These important findings, however, should be replicated.
3.2 Mental state Results for mental state are mixed according to the scale which was used to measure improvement, making it difficult to draw firm conclusions regarding the effect of risperidone. No marked differences in mental state between groups were found from the data provided by five studies using the mean change in PANSS scores. However, four trials used the BPRS and did find risperidone more likely to produce a greater mean change in score. One trial provided limited favourable data for the endpoint score on the PANSS. Overall it seems as though risperidone does not differ greatly from the older drugs in terms of mental state outcomes.
It is not possible to make any confident statements regarding the effect of risperidone on negative symptoms, due to limitations of the data. In the absence of individual patient data it was not possible to check for the presence of skew. The validity therefore of parametric tests for these change data is not known. Caution should subsequently be exercised when interpreting the results. Four trials (n=1820) were pooled in the analysis. Overall, there appeared to be no significant differences in mean change of negative symptoms between risperidone and control group.
The difficulty in differentiating negative symptoms from movement disorders such as slowness and poverty of movement (van Putten 1987) may favour risperidone over haloperidol, especially when haloperidol is used in relatively high dose. It is noteworthy that less difference is observed between risperidone and haloperidol in terms of negative symptoms in the European study where 10mg/day of haloperidol is used (Peuskens 1995) as opposed to the North American studies where the dose of haloperidol was 20mg/day (Chouinard 1993a, Marder 1994).
The same problems arise for the understanding of ratings on 'positive' symptoms. In ten studies positive symptoms were assessed using the positive subscale of the PANSS scale. Mean change data and standard deviations were presented in four trials. As with negative symptoms, there was no reliable means of detecting skew and so caution should be exercised in interpretation. Risperidone appears to be no more effective than control in treating 'positive' symptoms as in those studies that did report standard deviations (n=1820) no statistically significant differences between risperidone and control were observed.
3.3 Behaviour Again, no significant differences in behaviour were found between groups. The data are again limited and so no firm conclusion about this result can be made until more data is available.
3.4 Cognitive function Although more recent studies specifically investigated cognitive functioning, data presentation was poor and insufficient information was available to assess the impact of risperidone on cognitive function. Limited information, available from side effect scales, reveals no significant differences between risperidone and control for causing memory or concentration difficulties.
3.5 Acceptability of treatment When the acceptability of treatment is assessed indirectly by the numbers of people leaving the studies early, risperidone does appear to be more acceptable to those with schizophrenia than older typical antipsychotics.
3.6 Adverse events The main objective of this review was to compare the effects of risperidone with that of 'conventional' neuroleptic drugs. Essentially, however, this review is a comparison of risperidone with haloperidol. Findings comparing side effect profiles with risperidone should be interpreted with this in mind. Data derived from the new included studies were similar and no new changes in the adverse events results were found for this update. The extra data, however, made some results more robust.
3.6.1 Movement disorders People treated with risperidone experienced less movement disorders (extrapyramidal symptoms) than did those in control groups. This finding was robust and consistent across all studies. Excluding studies that used higher doses of haloperidol (>10 mg/day) did not materially change the results and confirms that haloperidol has a high propensity to cause extrapyramidal symptoms, even at lower doses (Joy 2000). The availability of a greater number of randomised studies directly comparing risperidone with other conventional neuroleptics with a lower potential to cause extrapyramidal symptoms would allow more meaningful and externally valid comparisons to be made.
The use of antiparkinsonian medication, used to alleviate these extrapyramidal symptoms, unsurprisingly reflects the results relating directly to movement disorders. Far fewer people treated with risperidone required anti‐parkinsonian medication. Again, this finding reflects comparisons between risperidone and largely haloperidol, a conventional antipsychotic with an unusually high propensity to cause parkinsonian side effects. In clinical practice, many patients will be commenced on an antipsychotic with less potent parkinsonian side effects or will be adequately managed with occasional antiparkinsonian medications. The appropriateness of these routine therapeutic options cannot be established from the studies reviewed.
3.6.2 Anxiety, agitation, insomnia and headache No significant differences were observed between risperidone and control for those side effects that are considered 'common' with risperidone (anxiety, agitation, insomnia and headache). Although insomnia is no more likely to occur with risperidone than with control people treated with risperidone appear marginally less likely to experience somnolence.
3.6.3 Dry mouth, blurred vision, and constipation No difference between risperidone and control was revealed in terms of causing anticholinergic side effects (dry mouth, blurred vision, and constipation). However, like risperidone, haloperidol (the main control treatment), is reputed to have a limited propensity to cause these and therefore greater differences may have been observed had other 'conventional' neuroleptics such as chlorpromazine been used as the control treatment.
3.6.4 Other outcomes After the update and inclusion of more studies with usable data, we found that people treated with risperidone are more likely to gain weight than are those treated with the control drugs. Risperidone also appears more likely to cause rhinitis (nasal inflammation). This finding, similar to that found in the original review, has been replicated by further studies in the update. No differences were observed between risperidone and control for other miscellaneous side effects such as gastrointestinal, cardiovascular, sexual or menstrual problems. 3.7 Missing outcomes Very limited data or no information at all was provided regarding quality of life, social functioning, employment status or cost effectiveness.
4. Heterogeneity Only one result was statistically significantly heterogeneous (blurring of vision). This result must be considered alongside the other outcomes and the probability of a heterogeneous result arising by chance (1 in 20). The principal outcomes under consideration were homogeneous between studies and the source of heterogeneity for blurring of vision was not apparent and was not investigated further.
5. Sensitivity analyses As no trial focused exclusively on people with treatment resistant schizophrenia it was impossible to undertake sensitivity analyses on this important subgroup of people.
Only Emsley 1995 (n=183) included patients experiencing their first episode of schizophrenia. The results of this study appear consistent with other studies and excluding this trial did not materially change any of the main outcomes of interest. However, because this was not a large study, it is difficult to draw any firm conclusions.
Three studies (n=1885) compared multiple fixed doses of risperidone with one fixed dose of haloperidol (Chouinard 1993a, Marder 1994, Peuskens 1995). These multiple doses of risperidone were pooled together for the purposes of comparison. This meant pooling doses of risperidone of 1mg/day or 2mg/day that are arguably sub‐therapeutic. Excluding these lower doses, however, did not materially change the results for the principal outcomes of interest.
In most studies the mean daily dose of haloperidol at endpoint was 10mg/day or less. If studies where the dose of haloperidol at endpoint was greater than this are excluded, the beneficial effect of risperidone in causing clinical improvement is no longer statistically significant. However, the magnitude of this change is very small and is most probably attributable to loss of power as a result of excluding trials. Similarly, excluding data from those given higher doses of haloperidol does marginally weaken the results relating to leaving studies early, making it no longer statistically significant. Again, this may well be due to loss of power rather than a substantive change in effect. Excluding the higher doses of haloperidol did not materially change the results in terms of extrapyramidal side effects and the strong beneficial effect of risperidone over control was retained.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia Risperidone appears to have a marginally greater effect than typical neuroleptics in improving a limited number of the symptoms associated with schizophrenia. Many of these symptoms are used in the diagnosis of schizophrenia and may or may not be the ones which people find most troublesome. One extra person out of every 10 treated will achieve this gain in the short term. It appears however to have little or no additional effect on the positive and negative symptoms of schizophrenia, although, because of limited data, it is not possible to be confident about this. Risperidone has an advantage over haloperidol in that it does have reduced tendency to cause movement disorders, although haloperidol is particularly prone to cause this, and, had the people designing the studies chosen another typical antipsychotic risperidone may not have appeared to be so advantageous. However, by treating 4 people with risperidone one less patient will experience movement disorders or require medication to alleviate this, when compared with haloperidol. Risperidone seems to be more acceptable to people than, largely, haloperidol, with one less person out of every 6 treated dropping out before the study ends. Risperidone, however, makes weight gain more likely, with one additional person out of every three treated gaining weight.
People with schizophrenia may want to consider carefully whether they want to enter studies involving risperidone. Consumer advocates may wish to lobby for the release of data for which informed consent was given.
2. For clinicians The question of whether risperidone is more effective than other 'conventional' neuroleptics needs to be answered. It does appear to have some advantages over haloperidol in terms of limited alleviation of symptoms and side effect profile. It may be more acceptable to those with schizophrenia, perhaps due to decreased sedation, despite a tendency to increase weight more than drugs such as haloperidol. There is little known from trials about long term effects.
3. For managers or policy makers Surprisingly limited data exist on the clinical implications of using risperidone and there are almost no data relevant to service utilisation, hospitalisation or functioning in the community. The potential benefits of risperidone, clinically and in reducing extrapyramidal side effects, over and above, largely, haloperidol, need to be balanced against the greater costs of this drug. Whether risperidone is more effective than other low cost 'conventional' neuroleptic drugs, such as chlorpromazine, needs to be proven.
Implications for research.
1. Publication bias Those undertaking reviews are unaware of the extent to which research goes unpublished and will never be sure that they are in possession of all of the available research evidence. The current review is less prone to publication bias than the previous version. However, statutory legislation requiring prospective registration of research studies is the only solution to the problem of publication bias (Dickersin 1992). Further, there should be a requirement on the part of those who undertake research (published or unpublished) to make all data available to those undertaking systematic reviews (Naylor 1997).
2. Methodology Clearly more trials of risperidone are needed. The short duration of the studies included in this review make it impossible to inform people with schizophrenia about the effects of risperidone in the medium and long term. Long, well‐designed, conducted and reported trials are needed.
Protocols for trials are now encouraged as publications quite separate from the final report (Horton 1997). Appropriate power calculations, the proposed selection of participants, randomisation process and its concealment and recording of outcomes could be presented and clearly described. Final reports should present a table of baseline characteristics of those in each group to reassure readers that the groups were similar and should describe reasons for every post‐randomisation loss to follow‐up. Clinically useful and understandable outcomes (binary and, if required, continuous) should be presented and an intention‐to‐treat analysis undertaken.
2.1 Participants Pragmatic entry criteria to trials would allow greater applicability of results.
2.2 Interventions Clearly there is a need to evaluate the effectiveness of risperidone against 'conventional' neuroleptic drugs other than haloperidol. It is possible that comparisons with, for example, chlorpromazine, or sulpiride may show risperidone to have fewer advantages for minimising movement disorders. The significantly greater cost of risperidone makes it all the more important to establish its efficacy over these cheaper alternatives.
2.3 Outcome Measures Most trials used rating scales. These provided information relating to symptoms and adverse effects that was difficult to translate into anything clinically meaningful. In future studies it would be helpful to target key symptoms of interest and present data in a dichotomous form. For example, it was difficult to get a clear idea about the impact of risperidone on anxiety or depression. These symptoms could have been targeted and the numbers of people experiencing a clinically important degree of such symptoms at baseline and endpoint reported.
Important outcomes such as cognitive function, social functioning, quality of life, employment status, discharge from hospital and relapse rates were insufficiently reported.
Feedback
Methods
Summary
The test for skewness is acceptable for testing continuous endpoint data but not for continuous change data. The normal distribution of change data may cross the zero line, which increases the likelihood that 2SDs is greater than the mean. Thus if the intervention is ineffective, the outcome change data are closer to zero, so more likely to be excluded. This produces a selection bias of the included studies, in that the smaller the treatment effect the less likely the study will be included in the meta‐analysis. This could profoundly alter your results.
Reply
The limitation of the doubling of the standard deviation for testing for skewness is now highlighted in the Methods section of the review. In addition the following paragraph has been added: "For continuous mean change data (endpoint minus baseline) the situation is even more problematic. In the absence of individual patient data it is impossible to know if change data is skewed. The RevMan meta‐analyses of continuous data are based on the assumption that the data are, at least to a reasonable degree, normally distributed. It is quite feasible that change data is skewed but, after consulting the ALLSTAT 1988 electronic statistics mailing list (http://www.stats.gla.ac.uk/allstat/index.html), it was entered into RevMan in order to summarise the available information. In doing this it is assumed that either data were not skewed or that the analyses within RevMan could cope with the unknown degree of skewness. Without individual trial data it is not possible to formally check this assumption." As a result summated change data is presented in graphical form.
Contributors
Comment received from Kristian Wahlbeck, Helsinki, Finland, November 1997 Reply from Alan Clark, Glasgow, August 1998
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 15 February 2010 | Amended | Contact details updated. |
| 31 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Clive Adams, Leanne Roberts and Gill Rizzello at the Cochrane Schizophrenia Group Editorial Base, first in Oxford, and then in Leeds, for their help and support. Richard German and Jackie Cahoon at Gartnavel Royal Hospital Library are gratefully acknowledged for their efforts in retrieving papers for the first version of this review. We also thank Clive Rogers and his colleagues from Janssen‐Cilag Ltd. for providing information regarding published and unpublished trials.
Data and analyses
Comparison 1. RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Not improved to a clinically important degree (using BPRS) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 short‐term (up to 12 weeks) | 2 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.02] |
| 2 Global state: 2. Not improved to a clinically important degree (using CGI) | 8 | 1126 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.61, 1.00] |
| 2.1 short‐term (up to 12 weeks) | 8 | 1126 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.61, 1.00] |
| 3 Global state: 3. Not improved to a clinically important degree (no greater than 20% change in PANSS score) | 11 | 3227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.74, 0.87] |
| 3.1 short‐term (up to 12 weeks) | 9 | 2368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 3.2 long‐term (over 26 weeks) | 2 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.83] |
| 4 Global state: 4. Not improved to a clinically important degree (no greater than 40% change in PANSS score) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 short‐term (up to 12 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 4.2 long‐term (over 26 weeks) | 1 | 675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] |
| 5 Global state: 5. Not improved to a clinically important degree (no greater than 60% chnage in PANSS score) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.3 long‐term (over 26 weeks) | 1 | 675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.96] |
| 6 Global state: 6. Not improved to a clinically important degree (as rated by participants) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.09] |
| 6.1 short‐term (up to 12 weeks) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.09] |
| 7 Global state: 7a. Relapse ‐ by 1 year | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.99] |
| 8 Global state: 7b. Relapse ‐ average number of days to relapse (skewed data) | Other data | No numeric data | ||
| 9 Global state: 6. Unable to be discharged from hospital by 8 weeks | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.76] |
| 10 Global state: 6. Change in CGI (continuous, high score = poor)* | 4 | 503 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.33, 0.11] |
| 10.1 short‐term (up to 12 weeks) | 4 | 503 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.33, 0.11] |
| 11 Mental state: 1. Change in PANSS by 12 weeks ‐ total (continuous, high score = poor)* | 5 | 1815 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐4.01, 0.97] |
| 12 Mental state: 2. Change in PANSS by 12 weeks ‐ negative (continuous, high score = poor)* | 4 | 1820 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.62, 0.07] |
| 13 Mental state: 3. Change in PANSS by 12 weeks ‐ positive (continuous, high score = poor)* | 4 | 1820 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.09, 0.60] |
| 14 Mental state: 4. Change in BPRS by 12 weeks (continuous, high score = poor)* | 4 | 1820 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.22, ‐0.17] |
| 15 Mental state: 5. PANSS endpoint score by 6 weeks (continuous, high score = poor) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.75 [‐3.94, ‐1.56] |
| 16 Mental state: 6. Anxiety/tension: short‐term (up to 12 weeks) | 5 | 2087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.38] |
| 17 Mental state: 7. Depression: short‐term (up to 12 weeks) | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.25] |
| 18 Behaviour: 1. Agitation: short‐term (up to 12 weeks) | 4 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| 19 Behaviour: 2. Use of medication for sedation: short‐term (up to 12 weeks) | 3 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.79, 1.37] |
| 20 Cognitive function: short‐term (up to 12 weeks) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 concentration difficulties | 4 | 1548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| 20.2 memory difficulties | 2 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.69, 1.03] |
| 21 Acceptability of treatment: 1a. Leaving study early ‐ adverse effect | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 short‐term (up to 12 weeks) | 11 | 2243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.09] |
| 22 Acceptability of treatment: 1b. Leaving study early ‐ insufficient response | 11 | 2498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.60, 1.08] |
| 22.1 short‐term (up to 12 weeks) | 11 | 2498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.60, 1.08] |
| 23 Acceptability of treatment: 1c. Leaving the study early ‐ any reason | 23 | 4364 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.59, 0.80] |
| 23.1 short‐term (up to 12 weeks) | 18 | 3066 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.63, 0.92] |
| 23.2 medium term (12‐26 weeks) | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.17, 9.04] |
| 23.3 long‐term (over 26 weeks) | 4 | 1270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.42, 0.73] |
| 24 Acceptability of treatment: 2. Not acceptable ‐ as measured by direct questioning | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.40, 1.21] |
| 24.1 short‐term (up to 12 weeks) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.40, 1.21] |
| 25 Adverse effects: 1. Experiencing at least one adverse effect | 9 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.02] |
| 26 Adverse effects: 2. Movement disorders ‐ general | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 general movement disorders (extrapyramidal side effects) | 10 | 2702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.56, 0.71] |
| 26.2 use of medication for movement disorders (antiparkinsonian drugs) | 11 | 2524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.58, 0.74] |
| 26.3 number of people whose need for antiparkinson medication ceased during trial | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.39, 2.92] |
| 27 Adverse effects: 3. Movement disorders ‐ specific | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 abnormal involuntary movements (tardive dyskinesia) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.28] |
| 27.2 distressing restlessness (akathesia) | 4 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.52, 0.89] |
| 27.3 postural disturbance (dystonia) | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 1.99] |
| 27.4 slow impovershed movement, stiffness (parkinsonism) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 27.5 tremor | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.83] |
| 28 Adverse effects: 4. Movement disorders ‐ change in CGI (high score = poor) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 28.1 severity of dyskinesia | 3 | 1778 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.34, ‐0.06] |
| 28.2 severity of parkinsonism | 3 | 468 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.18, ‐0.39] |
| 29 Adverse effects: 5. Movement disorders ‐ change in ESRS (high score = poor) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 29.1 severity of parkinsonism | 3 | 1507 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐2.91, ‐1.23] |
| 29.2 total | 3 | 1708 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐1.22, ‐0.15] |
| 30 Adverse effects: 6. Movement disorders ‐ change in UKU (high score = poor) | 1 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.51, ‐0.47] |
| 31 Adverse effects: 7. SAS endpoint score by 6 weeks ( high score = poor) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.55, ‐0.15] |
| 32 Adverse effects: 8. Anticholinergic effects (dry mouth, blurred vision, constipation, urinary difficulties) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 dry mouth | 5 | 1616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.09] |
| 32.2 blurred vision | 5 | 1635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.10] |
| 32.3 constipation | 7 | 2165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.80, 1.37] |
| 32.4 difficulties passing urine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.12] |
| 33 Adverse effects: 9. Sleep problems | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 reduction ‐ insomnia | 7 | 2699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 33.2 reduction ‐ decreased duration or quality | 3 | 1510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 33.3 increase ‐ duration of sleep | 2 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 33.4 increase ‐ day time sleepiness (somnolence) | 12 | 2711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.01] |
| 34 Adverse effects: 10. Weight change | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 34.1 loss | 2 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.98, 1.56] |
| 34.2 gain | 4 | 1708 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.25, 1.93] |
| 35 Adverse effects: 11. Gastrointestinal problems | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 dyspepsia | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.47, 5.09] |
| 35.2 nausea | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.57, 2.44] |
| 35.3 vomiting | 2 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.43, 1.26] |
| 36 Adverse effects: 12. Central nervous system | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 dizziness | 7 | 2426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.43] |
| 36.2 headache | 10 | 2891 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.88, 1.27] |
| 37 Adverse effects: 13. Cardiovascular | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 increased heart rate (tachycardia) | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.39, 1.82] |
| 37.2 palpitations | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.32, 2.26] |
| 37.3 low blood pressure (hypotension) | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.38, 2.43] |
| 37.4 high blood pressure (hypertension) | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.27] |
| 38 Adverse effects: 14. Sexual problems | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 erectile dysfunction | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.58, 4.20] |
| 38.2 ejaculatory dysfunction | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [0.21, 78.89] |
| 38.3 orgastic dysfunction | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.36, 9.89] |
| 38.4 diminished sexual desire | 2 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 39 Adverse effects: 15. Menstrual problems | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 39.1 amenorrhea | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.42, 8.17] |
| 39.2 menorrhagia | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 40 Adverse effects: 16. Other | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 40.1 nasal inflammation (rhinitis) | 3 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.24, 3.19] |
| 40.2 sweating | 5 | 1606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| 40.3 too much saliva | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.08, 2.26] |
| 40.4 psychosis | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.04] |
| 40.5 injury | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.68, 2.22] |
1.1. Analysis.
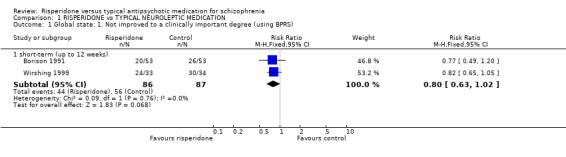
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 1 Global state: 1. Not improved to a clinically important degree (using BPRS).
1.2. Analysis.
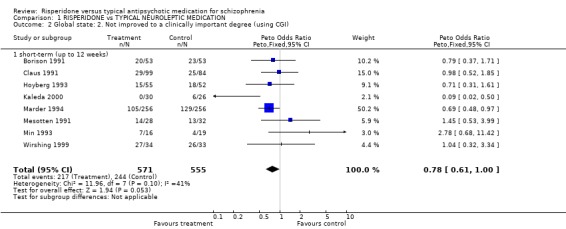
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 2 Global state: 2. Not improved to a clinically important degree (using CGI).
1.3. Analysis.
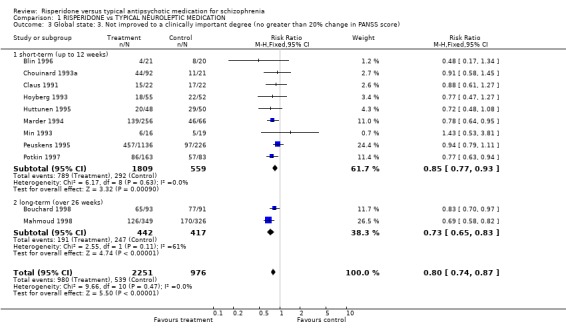
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 3 Global state: 3. Not improved to a clinically important degree (no greater than 20% change in PANSS score).
1.4. Analysis.
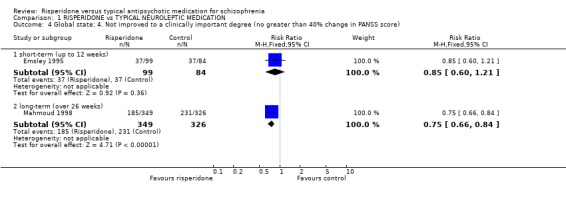
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 4 Global state: 4. Not improved to a clinically important degree (no greater than 40% change in PANSS score).
1.5. Analysis.
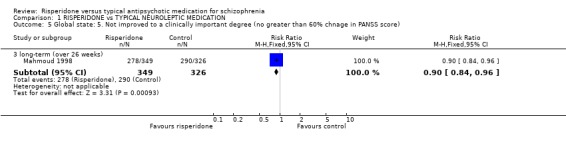
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 5 Global state: 5. Not improved to a clinically important degree (no greater than 60% chnage in PANSS score).
1.6. Analysis.
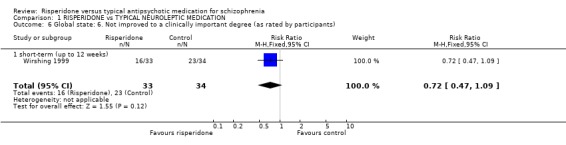
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 6 Global state: 6. Not improved to a clinically important degree (as rated by participants).
1.7. Analysis.
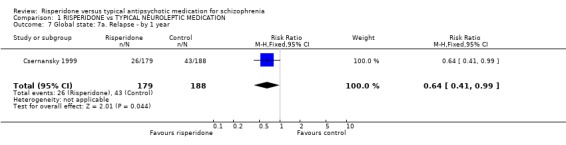
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 7 Global state: 7a. Relapse ‐ by 1 year.
1.8. Analysis.
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 8 Global state: 7b. Relapse ‐ average number of days to relapse (skewed data).
| Global state: 7b. Relapse ‐ average number of days to relapse (skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| Csernansky 1999 | Risperidone | 452.23 | 236.5 | 179 |
| Csernansky 1999 | Haloperidol | 391.33 | 299 | 188 |
1.9. Analysis.
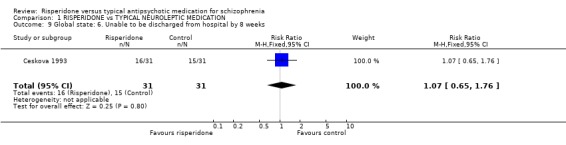
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 9 Global state: 6. Unable to be discharged from hospital by 8 weeks.
1.10. Analysis.
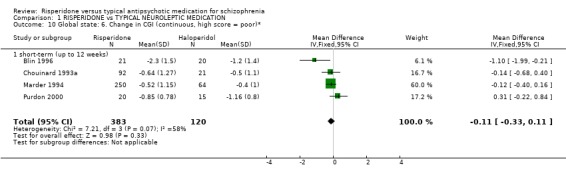
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 10 Global state: 6. Change in CGI (continuous, high score = poor)*.
1.11. Analysis.
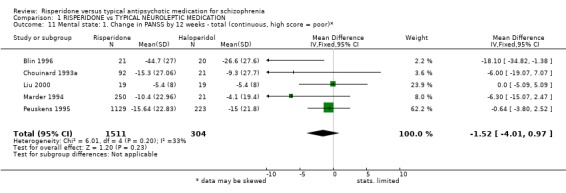
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 11 Mental state: 1. Change in PANSS by 12 weeks ‐ total (continuous, high score = poor)*.
1.12. Analysis.
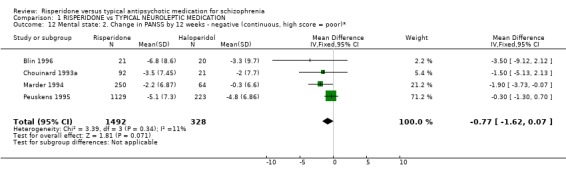
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 12 Mental state: 2. Change in PANSS by 12 weeks ‐ negative (continuous, high score = poor)*.
1.13. Analysis.
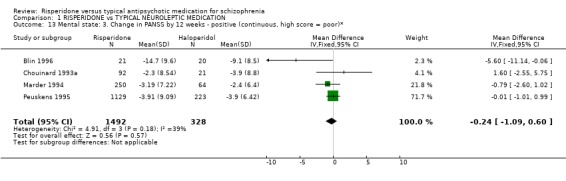
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 13 Mental state: 3. Change in PANSS by 12 weeks ‐ positive (continuous, high score = poor)*.
1.14. Analysis.
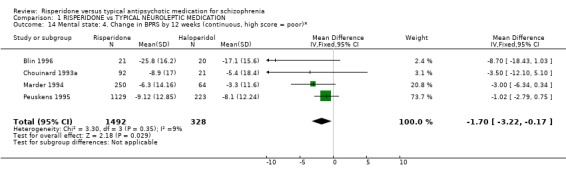
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 14 Mental state: 4. Change in BPRS by 12 weeks (continuous, high score = poor)*.
1.15. Analysis.
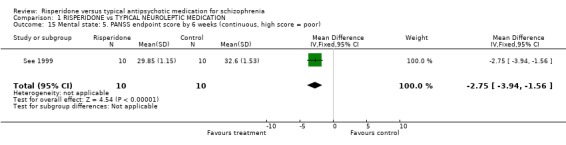
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 15 Mental state: 5. PANSS endpoint score by 6 weeks (continuous, high score = poor).
1.16. Analysis.
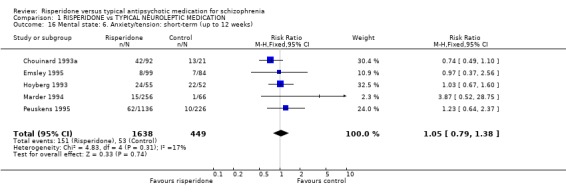
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 16 Mental state: 6. Anxiety/tension: short‐term (up to 12 weeks).
1.17. Analysis.
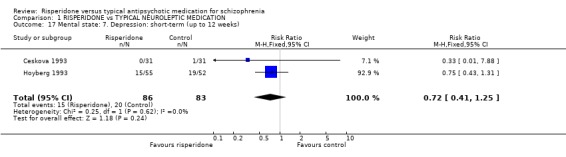
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 17 Mental state: 7. Depression: short‐term (up to 12 weeks).
1.18. Analysis.
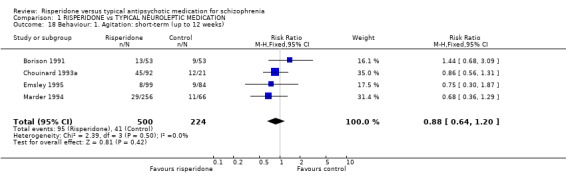
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 18 Behaviour: 1. Agitation: short‐term (up to 12 weeks).
1.19. Analysis.
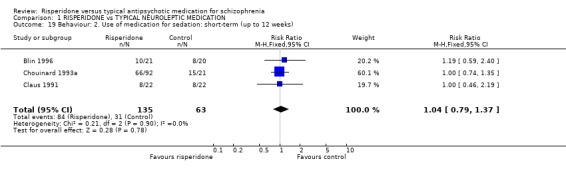
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 19 Behaviour: 2. Use of medication for sedation: short‐term (up to 12 weeks).
1.20. Analysis.
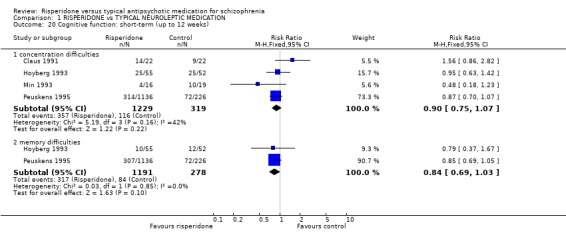
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 20 Cognitive function: short‐term (up to 12 weeks).
1.21. Analysis.
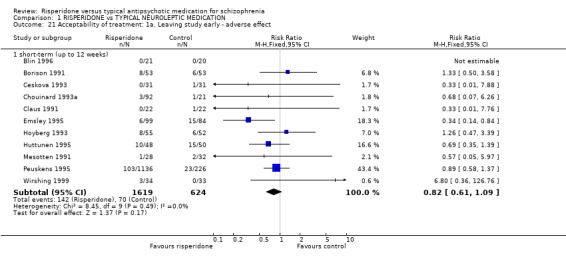
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 21 Acceptability of treatment: 1a. Leaving study early ‐ adverse effect.
1.22. Analysis.
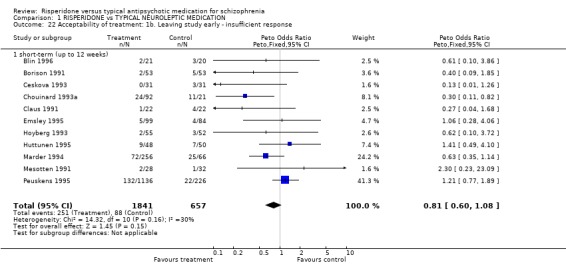
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 22 Acceptability of treatment: 1b. Leaving study early ‐ insufficient response.
1.23. Analysis.
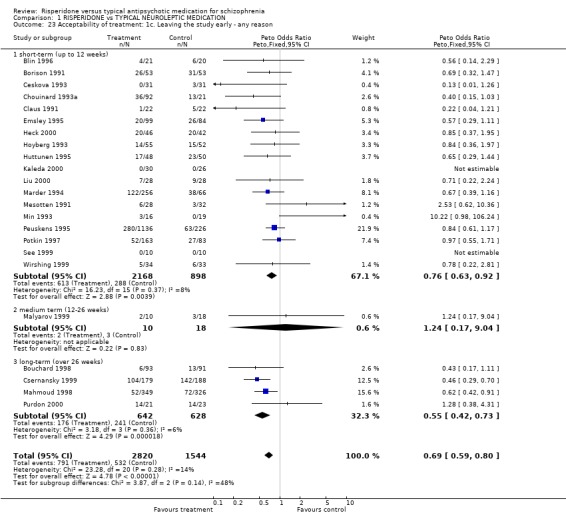
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 23 Acceptability of treatment: 1c. Leaving the study early ‐ any reason.
1.24. Analysis.
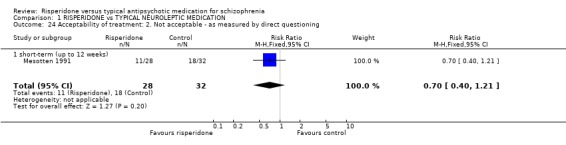
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 24 Acceptability of treatment: 2. Not acceptable ‐ as measured by direct questioning.
1.25. Analysis.
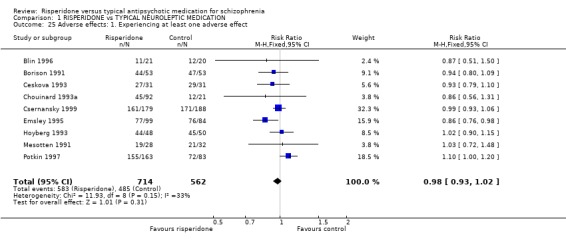
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 25 Adverse effects: 1. Experiencing at least one adverse effect.
1.26. Analysis.
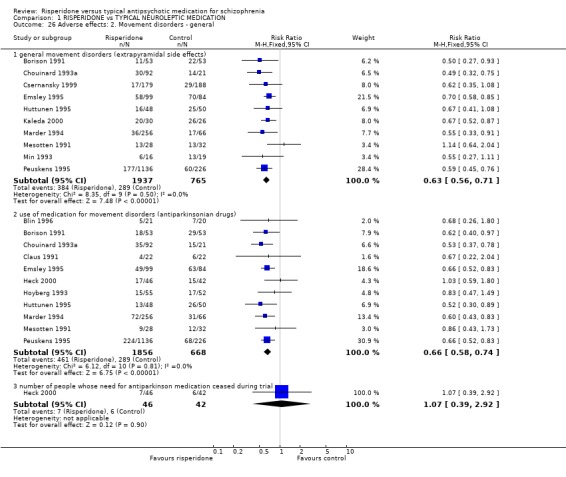
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 26 Adverse effects: 2. Movement disorders ‐ general.
1.27. Analysis.
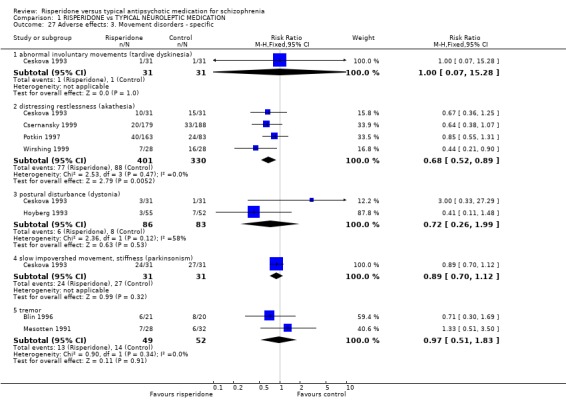
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 27 Adverse effects: 3. Movement disorders ‐ specific.
1.28. Analysis.
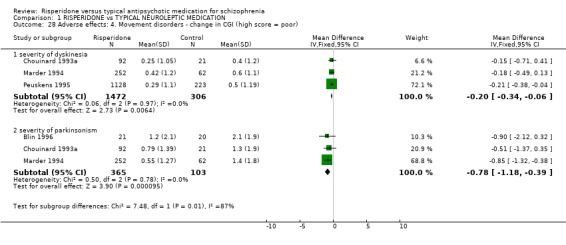
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 28 Adverse effects: 4. Movement disorders ‐ change in CGI (high score = poor).
1.29. Analysis.
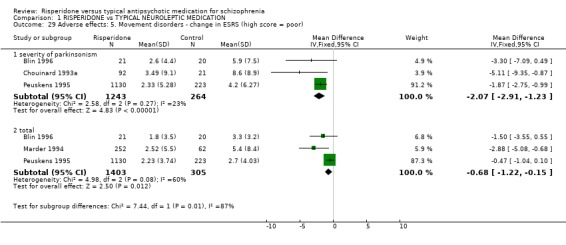
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 29 Adverse effects: 5. Movement disorders ‐ change in ESRS (high score = poor).
1.30. Analysis.
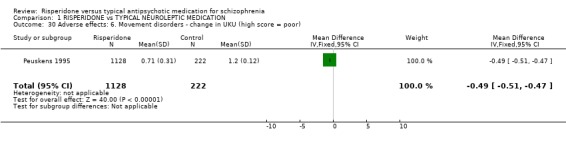
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 30 Adverse effects: 6. Movement disorders ‐ change in UKU (high score = poor).
1.31. Analysis.
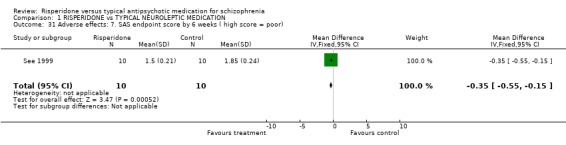
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 31 Adverse effects: 7. SAS endpoint score by 6 weeks ( high score = poor).
1.32. Analysis.
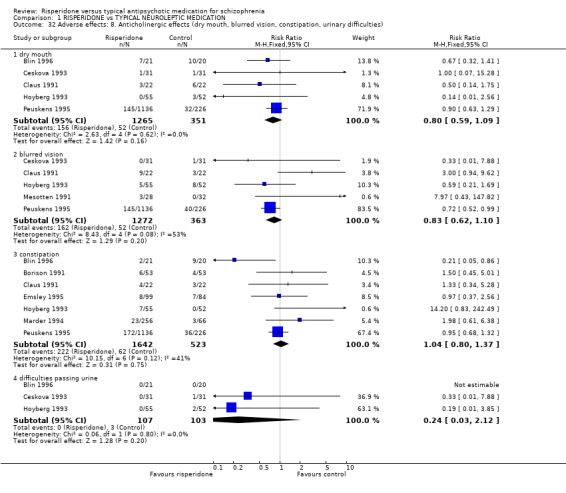
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 32 Adverse effects: 8. Anticholinergic effects (dry mouth, blurred vision, constipation, urinary difficulties).
1.33. Analysis.
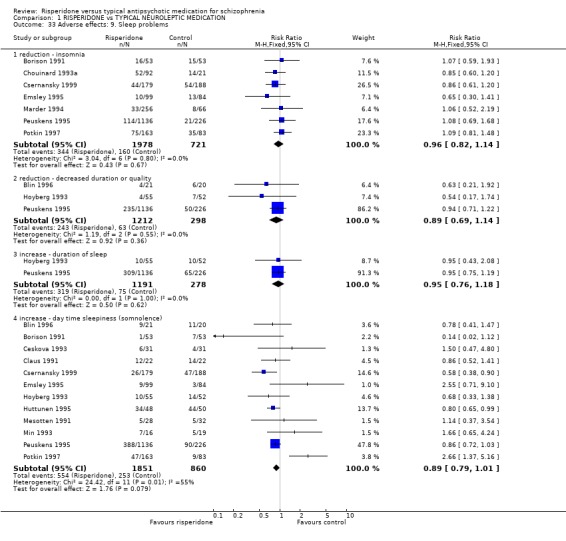
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 33 Adverse effects: 9. Sleep problems.
1.34. Analysis.
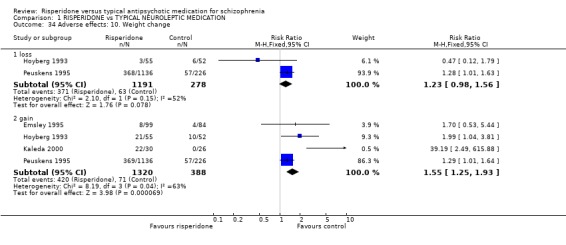
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 34 Adverse effects: 10. Weight change.
1.35. Analysis.
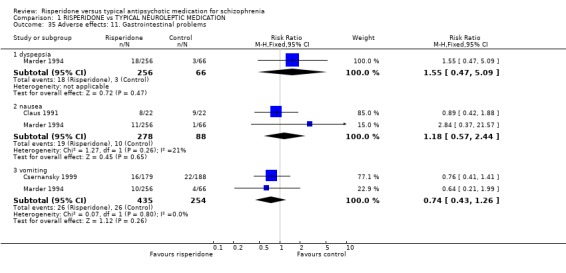
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 35 Adverse effects: 11. Gastrointestinal problems.
1.36. Analysis.
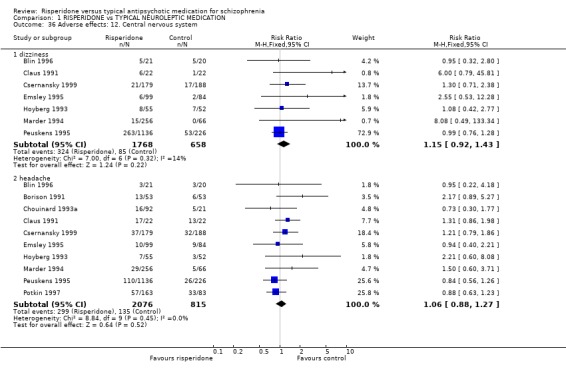
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 36 Adverse effects: 12. Central nervous system.
1.37. Analysis.
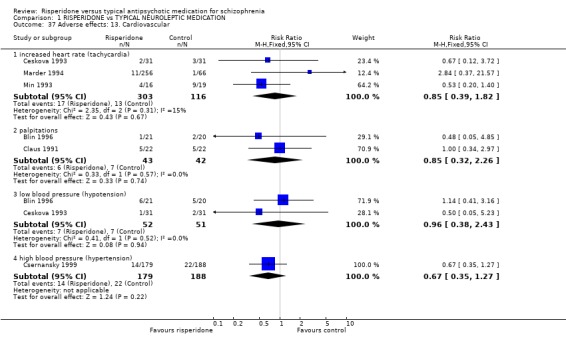
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 37 Adverse effects: 13. Cardiovascular.
1.38. Analysis.
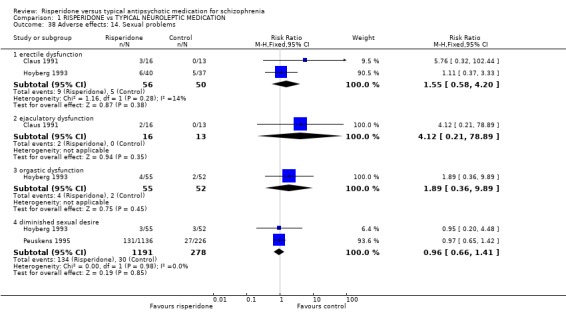
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 38 Adverse effects: 14. Sexual problems.
1.39. Analysis.
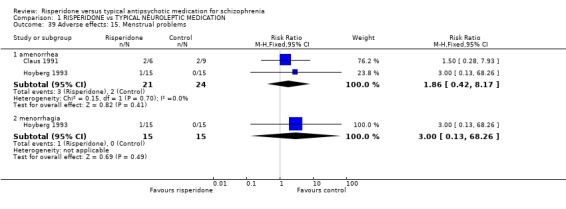
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 39 Adverse effects: 15. Menstrual problems.
1.40. Analysis.
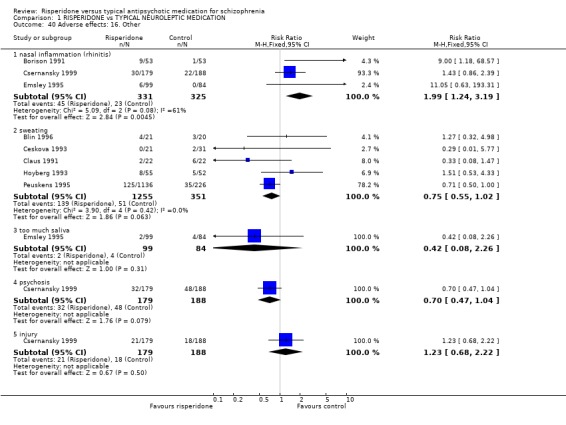
Comparison 1 RISPERIDONE vs TYPICAL NEUROLEPTIC MEDICATION, Outcome 40 Adverse effects: 16. Other.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blin 1996.
| Methods | Allocation: randomised (no further description). Blindness: double (medication in identical capsules). Duration: 4 weeks. Consent: written. Setting: multicentre, hospital. | |
| Participants | Diagnosis: schizophenia (DSM‐III‐R). N=62. Sex: 38M, 24F. Age: 16‐63 years, mean ˜ 34 years. History: hospitalised, currently experiencing acute exacerbation. Exclusions: schizo‐affective or severe somatic disorders, pregnant or nursing women, substance abuse, abnormal lab results, long‐acting antipsychotic within 4 weeks of trial or short‐acting antipsychotics within 48 hours of trial. | |
| Interventions | 1. Risperidone: 4‐12mg/day, initial dose of 4mg/day adjusted as required every 2 days. N=21.
2. Haloperidol: 4‐12mg/day, initial dose of 4 mg/day adjusted as required every 2 days. N=20.
3. Methotrimeprazine: 50‐150mg/day, initial dose of 50mg/day adjusted as required every 2 days. N=21*. loprazolam, diazepam, heptaminol hydrochloride as required. |
|
| Outcomes | Clinical improvement: 20% reduction in PANSS score.
Global effect: CGI improved/not improved, CGI score.
Mental state: PANSS, PANSS‐derived BPRS, PAS.
Behaviour: use of sedative medication.
Side effects: Asberg Scale, ESRS.
Leaving the study early. Unable to use ‐ Physiological monitoring: ECG, lab tests (insufficient data). |
|
| Notes | * Methotimeprazine data not used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Borison 1991.
| Methods | Allocation: randomised (no further description). Blindness: double (medication identical). Duration: 6 weeks (preceeded by one week placebo washout). Consent: not stated. Setting: hospital. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=160. Sex: 154M, 6F. Age: 21‐63 years, mean ˜ 40 years. History: chronic, mean duration of illness ˜ 15 years. Exclusions: other significant physical or psychological illness, abnormal lab results, depot antipsychotics within 2 weeks of trial, other psychotropic medication within 3 days of trial, recent substance abuse, non response to classic neuroleptics, child bearing potential. | |
| Interventions | 1. Risperidone: 1‐10mg/day, initial dose of 1mg/day adjusted as required days 1‐14, FD thereafter. N=53.
2. Haloperidol: 2‐20mg/day, initial dose of 2mg/day adjusted as required days 1‐14, FD thereafter. N=53.
3. Placebo: tablet number adjusted as required days 1‐14, FD thereafter. N=54. lorazepam,sodium amytal, chloral hydrate, benztropine or trihexyphenidyl as required. |
|
| Outcomes | Global effect: BPRS, CGI improved/not improved.
Side effects: various observed effects, use of antiparkinsonian medication.
Leaving the study early. Unable to use ‐ Global effect: CGI score (no SD). Mental state: BPRS, SANS (no SD). Physiological monitoring: ECG, lab tests (insufficient data) Side effects: ESRS, AIMS (no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bouchard 1998.
| Methods | Allocation: randomised (no further description). Blindness: none, open label. Duration: 2 years. Consent: not stated. Setting: need trial for update. | |
| Participants | Diagnosis: schizophrenia (PANSS score 60‐120). N=184. Age: not stated. Sex: not stated. History: 81% outpatients. | |
| Interventions | 1. Risperidone: mean dose 5.5 mg/day. N=93. 2. Classical neuroleptics: mean dose 1mg/day chlorpromazine equivalents. N=91. | |
| Outcomes | Clinical improvement: 20% reduction in PANSS.
Leaving the study early. Unable to use ‐ Global effect: CGI score (no data). Subjective tolerance: ESRS Part 1 (insufficient data). Movement disorders: ESRS Part 2 (insufficient data). |
|
| Notes | Ongoing study ‐ 12 month data only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ceskova 1993.
| Methods | Allocation: randomised (no further description). Blindness: double (administered as monotherapy in oral solution). Duration: 8 weeks. Consent: not stated. Setting: hospital. | |
| Participants | Diagnosis: schizophrenia & schizoaffective disorder (ICD‐9). N=62. Sex: 45M, 17F. Age: mean ˜ 36 years. History: hospitalised, chronic with mean duration of illness ˜ 10 years. Exclusions: not stated. | |
| Interventions | 1. Risperidone: 2‐20mg/day. N=31.
2. Haloperidol: 2‐20mg/day. N=31. Antiparkinsonian medication, minor tranquillizers or promethazine, dihydroergotamine as required. |
|
| Outcomes | Adverse effects: various observed effects.
Leaving the study early.
Discharge from hospital. Unable to use ‐ Global effect: Serejskij's modified scale (no SD). Mental state: BPRS (no SD). Side effects: DVP (no SD), use of antiparkison medication (individual group data not given). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chouinard 1993a.
| Methods | Allocation: randomised (no further description). Blindness: double (identical tablets). Duration: 8 weeks (preceeded by one week washout). Consent: written. Setting: multicentre. | |
| Participants | Diagnosis:schizophrenia (DSM‐III‐R). N=135. Sex: 96M, 39F. Age: 19‐67 years, mean ˜ 37 years. History: chronic, hospitalised with mean duration of current hospitalisation ˜ 2 years. Exclusions: other significant physical or psychological illness, pregnant or nursing females, females without adequate contraception, substance abuse. | |
| Interventions | 1. Risperidone: 2mg/day FD. N=24.
2. Risperidone: 6mg/day FD. N=22.
3. Risperidone: 10 mg/day FD. N=22.
4. Risperidone: 16mg/day FD. N=24.
5. Haloperidol: 20mg/day FD. N=21.
6. Placebo. N=22. chloral hydrate, benzodiazepine, procyclidine or biperidin as required. |
|
| Outcomes | Clinical improvement: 20% reduction of total PANSS score.
Global effect: CGI score.
Mental state: BPRS, PANSS.
Adverse effects: ESRS, observed events on the UKU*, use of antiparkinsonian and/or sedative medication.
Leaving the study early. Unable to use ‐ Physiological monitoring: ECG, lab tests (no data) |
|
| Notes | Only haloperidol data used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Claus 1991.
| Methods | Allocation: randomised (no further description). Blindness: double (described). Duration: 12 weeks (preceeded by one week placebo washout). Consent: written. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=44. Sex: 28M, 14F. Age: 20‐66 years, mean ˜ 38 years. History: chronic, hospitalised. Exclusions: other significant physical or psychological illness, pregnant or nursing females. | |
| Interventions | 1. Risperidone: 2‐20mg/day, an initial dose of 2mg/day was adjusted as required days 1‐42, FD thereafter. N=21.
2. Haloperidol: 2‐20mg/day, an initial dose of 2mg/day was adjusted as required days 1‐42, FD thereafter. N=21. Diazepam, dexetimide, tybezatropine IM as required. |
|
| Outcomes | Clinical improvement: 20% reduction of total PANSS.
Global effect: CGI improved/not improved
Adverse effects: various observed effects.
Leaving the study early. Unable to use ‐ Global effect: CGI (no SD). Mental state: PANSS, SADS‐C (no SD). Behaviour: NOISE‐30, Individual target symptom (visual analogue scale), sleep quality (visual analogue scale) (no SD). Physiological monitoring: ECG, lab tests (no data) Side effects: ESRS (no SD). |
|
| Notes | Intention to treat analysis for adverse effects, two patients excluded from efficacy analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Csernansky 1999.
| Methods | Allocation: randomised (no further description). Blindness: double (no further description). Duration: ˜ 1 year (trial continued until last patient had completed 1 year. Consent: not stated. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia. N=397. Sex: 255M, 110F*. Age: mean ˜ 40 years. History: stable outpatients. Exclusions: not stated. | |
| Interventions | 1. Risperidone: 4 mg/day FD days 1‐3 followed by 4 wks 2‐8 mg/day, most suitable dose thereafter. N=179 2. Haloperidol: 10mg/day FD days 1‐3 followed by 4 wks VD 5‐20 mg/day, most suitable dose thereafter. N=188. | |
| Outcomes | Leaving the study early.
Adverse effects: various observed effects.
Relapse: defined by either hospitalisation, significant increase in PANSS or CGI, deliberate self injury or violent behaviour, time to relapse. Unable to use ‐ Mental state: PANSS (mean presented in graph, no SD). Side effects: ESRS (>50% loss). |
|
| Notes | If patients relapsed twice they were discontinued from the trial. Data available for 367 participants only as data from one centre due to the centre's failure to achieve the quality of reporting set out in the trial protocol. This exclusion did not affect any of the results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Emsley 1995.
| Methods | Allocation: randomised (no further description). Blindness: double (no further description). Duration: 6 weeks. Consent: not stated. Setting: multicentre, multinational. | |
| Participants | Diagnosis:schizophrenia or schizophreniform disorder (DSM‐III‐R). N=183. Sex: 122M, 61F. Age: 15‐50 years. History: first‐episode. Exclusions: not stated | |
| Interventions | 1. Risperidone: 2‐16 mg/day, an initial dose of 4mg/day was adjusted as required throughout the trial. N=99. 2. Haloperidol: 1‐16 mg/day, an initial dose of 4mg/day was adjusted as required throughout the trial. N=84. | |
| Outcomes | Clinical improvement: >50% in PANSS.
Adverse effects: various observed effects.
Leaving the study early. Unable to use ‐ Global effect: CGI score (no SD). Mental state BPRS ‐ PANSS derived, PANSS (no SD). Physiological monitoring: ECG, lab tests (no data) Adverse effects: ESRS (no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Heck 2000.
| Methods | Allocation: randomised (no further description). Blindness: double. Duration: 7 weeks (preceeded by one week baseline period). Consent: given. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=77. Age: 23‐68 years. Sex: 38M, 39F. History: chronic, neuroleptic induced EPS. Exclusions: standard exclusion criteria (Declaration of Helsinki). | |
| Interventions | 1. Risperidone: Day 1. 2mg/day FD, Day 2. 4mg/day FD, Days 3‐7. 6‐14mg/day as required, most appropriate dose thereafter with a maximum of 16mg/day allowed. N=40.
2. Haloperidol: Day 1. 3mg/day FD, Day 2. 6 mg/day, Days 3‐7. 9‐21mg/day as required, most appropriate dose thereafter with a maximum of 24mg/day allowed. N=37. benzodiazepine as required. |
|
| Outcomes | Adverse effects: use of anitparkinson medication.
Leaving the study early. Unable to use ‐ Global effect: CGI score (no SD). Mental state: BPRS (no SD). Adverse effects: ESRS (no SD), observed effects (data not given for both groups). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hoyberg 1993.
| Methods | Allocation: randomised (no further description). Blindness: double (identicle appearance of medication). Duration: 8 weeks. Consent: given. Setting: multicentre, multinational. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=107. Sex: 77M, 30F. Age: 20‐67 years, mean ˜ 36 years. History: chronic. Exclusions: other significant physical or psychological illness, abnormal lab results, substance abuse, oral neuroleptics within 3 days of trial, depot neuroleptics within 3 weeks of trial, pregnant or nursing females, females of child bearing potential without adequate contraception. | |
| Interventions | 1. Risperidone: initially 5mg/day adjusted according to clincial judgement, 5‐15mg/day 1‐28, FD (if possible) thereafter. N=55. 2. Perphenazine: 16‐48mg/day, an initial dose of 16mg/day was adjusted as required days 1‐28, FD (if possible) thereafter. N=52. | |
| Outcomes | Clinical improvement: >20% reduction in total PANSS or BPRS score,
Global effect: CGI improved/not improved.
Adverse effects: use of antiparkinsonian medication.
Leaving the study early. Unable to use ‐ Severity of illness: CGI severity (no SD). Mental state: PANSS, BPRS‐PANSS derived (no SD). Physiological monitoring: ECG, lab tests (insufficient data). Adverse effects: ESRS, UKU (no SD). |
|
| Notes | Intention‐to‐treat analysis for adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Huttunen 1995.
| Methods | Allocation: randomised (no further description). Blindness: double (no further description). Duration: 6 weeks. Consent: given. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=98. Sex: 47M, 51F. Age: 11‐43 years, mean ˜ 36 years. History: chronic, currently acutely ill. | |
| Interventions | 1. Risperidone: 2‐20 mg/day, dose decided by clincial judgement. N=48. 2. Zuclopenthixol: 10‐100mg/day, dose decided by clincial judgement. N=50. | |
| Outcomes | Clinical improvement: 20% reduction in PANSS.
Comparison with previous medication: Categorical scale.
Adverse effects: use of antiparkinson medication, other various observed effects.
Leaving the study early. Unable to use ‐ Global effect: CGI (no SD). Mental state: PANSS, BPRS ‐ PANSS derived (no SD). Physiological monitoring: ECG, lab tests (no data). Adverse effects: ESRS, UKU (no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kaleda 2000.
| Methods | Allocation: randomised (no further description). Blindness: not stated. Duration: 3 months. Consent: not stated. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (ICD‐10). N=56. Sex: not stated. Age: 16‐20 years, mean ˜ 18 years. History: not stated. Exclusions: not stated. | |
| Interventions | 1. Risperidone: 8‐22mg/day. N=30. 2. Haloperidol: 9‐40 mg/day. N=26. | |
| Outcomes | Global effect: CGI improved/not improved.
Adverse effects: ESRS, various other observed effects.
Leaving the study early.* Unable to use ‐ Mental state: PANSS (no SD). Adverse effects: ESRS (no data). |
|
| Notes | Conference abstract only. * Not stated, assumed to be zero. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Liu 2000.
| Methods | Allocation: randomised (block procedure, block size of 4). Blindness: double. Duration: 12 weeks (preceeded by one week washout). Consent: written. Setting: hospital and outpatient clinic. | |
| Participants | Diagnosis: schizophrenia (DSM‐II‐R). N=56. Age: mean ˜ 34 years. Sex: 15M, 23F*. History: average length of illness ˜ 8 years. Exclusions: other significant physical or psychological illness, substance abuse. | |
| Interventions | 1. Risperidone: dose unknown. N=28.
2. Haloperidol: dose unknown. N=28. Trihexyhenidyl, lorazepam, propanolol as required. |
|
| Outcomes | Mental state: PANSS.
Adverse effects: ESRS.
Leaving the study early. Unable to use ‐ CPT: outcome not relevant. |
|
| Notes | * Sex of those who completed the trial only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mahmoud 1998.
| Methods | Allocation: randomised (stratified by number of prior hospitalisations). Blindness: none. Duration: 1 year. Consent: not stated. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia. N=684. Age: mean ˜ 39 years. Sex: History: diagnosed before age 35 years , ill for at least 2 years, currently not hospitialised within 60 days of trial but hospitalised at least once before. Exclusions: clozapine use. | |
| Interventions | 1. Risperidone. N=349. 2. Conventional treatment strategy. N=326. | |
| Outcomes | Clinical Improvement: 20%,40%,60% reduction in PANSS.
Leaving the study early. Unable to use ‐ Adverse effects: SARS, BAS, AIMS (no SD). Quality of life: (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Malyarov 1999.
| Methods | Allocation: randomised (no further description). Blindness: independent observers blind to treatment. Duration: 6 months. Consent: not stated. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia (ICD‐10). N=43. Age: mean ˜ 25 years. Sex: 28M, 15F. History: acute or post‐acute phase, hospitalised, duration of illness < 3 years. Exclusions: not stated. | |
| Interventions | 1. Risperidone: 3‐6mg/day. N=10. 2. Haloperidol: 5‐20mg/day. N=18. 3. Olanzapine: 5‐15mg/day. N=15. | |
| Outcomes | Leaving the study early. Unable to use ‐ Global effect: (no data). Mental State: PANSS (no SD). |
|
| Notes | Conference abstract only. Only risperidone and haloperidol data used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marder 1994.
| Methods | Allocation: randomised (in blocks of 12). Blindness: double (no further description). Duration: 8 weeks (preceeded by one week placebowashout). Consent: written. Setting: hospital | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=388. Age: 18‐65 years, mean ˜ 37 years.. Sex: 340M, 48F. History: duration of illness, mean 15.7 years; duration of current hospitalisation, mean 29 weeks; number of previous hospitalisations, mean 9.1, range 0‐61. Setting: hosptial. Exclusions: PANSS score < 60, people with schizoaffective disorder | |
| Interventions | 1. Risperidone: 2mg/day. N=63.
2. Risperidone: 6mg/day. N=64.
3. Risperidone: 10mg/day. N=65.
4. Risperidone: 16mg/day. N=64.
5. Haloperidol: 20mg/day. N=66.
6. Placebo: N=66.
Medication titrated to fixed maintainance dose during week 1. Chloral hydrate, lorazepam, EPS medication as required. |
|
| Outcomes | Clinical improvement: 20% reduction in total PANSS.
Time to clinical improvement.*
Global effect: CGI improved/not improved, CGI score.
Mental state: BPRS, PANSS.
Adverse effects: ESRS, various observed effects.
Leaving the study early. Unable to use ‐ Physiological monitoring: ECG, lab tests (no data). Adverse effects: UKU (used modified version of this scale). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mesotten 1991.
| Methods | Allocation: randomised (no further description). Blindness: double (identical medication). Duration: 8 weeks (preceeded by one week washout). Consent: not stated. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia, other serious psychotic disorders (DSM‐III). N=60. Age: 20‐65 years, mean ˜ 40 years. Sex: 37M, 23F. History: hospitalised, mean duration of hospitalisation ˜ 5 years. Exclusions: continuous neuroleptic treatment for > 5 years, other significant physical of psychological illness, depot neuroleptics within 4 weeks of trial, pregnant or nursing females or those of reproductive age without adequate contraception. | |
| Interventions | 1. Risperidone: 2‐20 mg/day, and initial dose of 2mg/day was adjusted as required days 1‐28, FD thereafter. N=28. 2. Haloperidol: 2‐20 mg/day, an initial dose of 2 mg/day was adjusted as required days 1‐28, FD thereafter. N=32. | |
| Outcomes | Global effect: CGI improved/not improved.
Adverse effects: use of EPS medication, other various observed effects.
Leaving the study early.
Acceptability of treatment. Unable to use ‐ Global effect: CGI score (no SD). Mental state: BPRS (no SD). Behaviour: NOISE (no SD). Physiological monitoring: ECG, lab tests (insufficient data). Adverse effects: ESRS (no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Min 1993.
| Methods | Allocation: randomised (sealed envelopes, no description of how code generated). Blindness: double (identical medication). Duration: 8 weeks (preceeded by 1 week placebo washout). Consent: not stated.* Setting: hospital. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=35. Age: 18‐59 years, mean ˜ 34 years. Sex: 17M, 18F. History: hospitalised, mean duration of hospitalisation ˜ 6 months, mean number of previous hospitalisations ˜3. Exclusions: other significant physical of psychological illness, substance abuse, participation in other drug trial within 4 weeks of this study, pregnant or nursing females or those of reproductive age without adequate contraception. | |
| Interventions | 1. Risperidone: 5 or 10mg/day, an initial FD of 5mg/day was given days 1‐14, if necessary this was increased to a FD of 10mg/day for the remaining 6 weeks. N=16.
2. Haloperidol: 5 or 10mg/day, an initial FD of 5 mg/day was given days 1‐14, if necessary this was increased to a FD of 10mg/day for the remaining 6 weeks. N=19 Lorazepam, oxazepam, benztropine mesylate as required. |
|
| Outcomes | Clinical improvement: 20% reduction in PANSS.
Global effect: CGI improved/not improved.
Leaving the study early. Unable to use ‐ Global effect: CGI score (no SD). Mental state: BPRS ‐ PANSS derive, PANSS (no SD). Physiological monitoring: ECG, lab tests (no data). Satisfaction with treatment: (no data) Adverse effects: ESRS (no SD), UKU (no SD, modified version used). |
|
| Notes | * trial performed in accordance with the Declaration of Helsiniki. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peuskens 1995.
| Methods | Allocation: randomised (random permuted block procedure with randomisation list transferred to sealed envelopes). Blindness: double (no further description). Duration: 8 weeks (preceded by one week washout). Consent: not stated. Setting: multi‐centre, multi‐national. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=1362. Age: mean ˜ 38 years. Sex: 894M, 467F. History: chronic, mean duration of illness ˜17 years, Exclusions: PANSS score < 60 | |
| Interventions | 1. Risperidone: 1mg/day. N=229.
2. Risperidone: 4mg/day. N=227.
3. Risperidone: 8mg/day. N=230.
4. Risperidone: 12mg/day. N=226.
5. Risperidone: 16mg/day. N=224.
6. Haloperidol: 10mg/day. N=226. Medication titrated to fixed maintainance dose during week |
|
| Outcomes | Clinical improvement: 20% reduction PANSS.
Mental state:BPRS ‐ PANSS derived, PANSS.
Adverse effects: CGI, ESRS, modified UKU, use of antiparkinsonian medication.
Leaving the study early. Unable to use ‐ Physiological monitoring: ECG, lab tests (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Potkin 1997.
| Methods | Allocation: randomised (no futher description). Blindness: double (no further description). Duration: 28 days. Consent: not stated. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia. N=246. Age: 18‐66 years.* Sex: 96M, 50F.* History: not stated. Exclusions: not stated. | |
| Interventions | 1. Risperidone: 4 mg/day FD. N=163. 2. Risperidone: 8mg/day FD. 3. Placebo. N=83. | |
| Outcomes | Mental state: PANSS.
Adverse effects: various observed effects.
Leaving the study early. Unable to use ‐ Clinical improvement: mean reduction in PANSS (no SD). Adverse effects: ESRS (no SD). |
|
| Notes | Data taken from conference poster. * Numbers given for Age ‐ only 236 people. Sex ‐ only 146 people. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Purdon 2000.
| Methods | Allocation: randomised (no further desscription). Blindness: double (no further description). Duration: 54 weeks(preceeded by 4 weeks prestudy stablisation period followed by 2‐9 day washout). Consent: given. Setting: multicentre. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=65. Age: 18‐65 years. Sex: History: under 5 years of exposure to neuroleptics. Exclusions: other significant physical or psychological illness, pregnant or nursing females, substance abuse within 30 days of trial. | |
| Interventions | 1. Risperidone: dose of 2mg/day increased by 2mg/day to maximum of 6mg/day during week 1, 4‐10 mg/day thereafter. N=21. 2. Haloperidol: 10mg/day FD during week 1, 5‐20 mg/day thereafter. N=23. 3. Olanzapine: 10 mg/day FD during week 1, 5‐20 mg/day thereafter. N=21. | |
| Outcomes | Leaving the study early.
Adverse effects:use of anticholinergic medication. Unable to use ‐ Mental state: PANSS (endpoint data only, by this point loss is >50%). Adverse effects: ESRS (endpoint data only, by this point loss is >50%). Cognitive functioning: General Cognitive Index (scale is not referenced, endpoint data only, by this point loss is >50%). |
|
| Notes | Olanzapine data not used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
See 1999.
| Methods | Allocation: randomised (no further description). Blindness: double (no further description). Duration: 5 weeks (preceeded by 3 weeks prestudy stablisation with trifluoperazine 20‐30 mg/day. This was reduced to 5‐10mg/day during week 1 and discontinued during week 2). Consent: given. Setting: hospital. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=20. Age: >18 years. Sex: 14M, 6F. History: chronic, partial responders to neuroleptics, residual sypmptoms. Exclusions: not stated. | |
| Interventions | 1. Risperidone: 4‐6 mg/day. N=10.
2. Haloperidol: 15‐30 mg/day. N=10. Benztropine mesylate as required. |
|
| Outcomes | Mental state: PANSS. Adverse effects: SAS. Leaving the study early.* | |
| Notes | * Not stated, assumed to be zero. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wirshing 1999.
| Methods | Allocation: randomised (computer generated numbers). Blindness: double (no further description). Duration: 8 weeks (preceeded by 3 weeks stablisation with 15‐30 mg/day haloperidol followed by one week placebo washout). Consent: written. Setting: hospital and medical centre. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=67. Sex: 55M, 12F. Age: 18‐60 years. History: treatment resistant. Exclusions: other significant pyschological or physical illness, recent substance abuse, risk to self or others, history of risperidone treatment failure*. | |
| Interventions | 1. Risperidone: 4 weeks FD (fixed dose) 6 mg/day followed by 4 weeks 3‐15 mg/day. N=34.
2. Haloperidol: 4weeks FD 15 mg/day followed by 4 weeks 5‐30 mg/day. N=33. Benztropine, biperiden, propanolol, lorazepam, temazepam as required. |
|
| Outcomes | Global effect: improved/not improved, 20% improvement in BPRS scores and/or <3 on CGI.
Leaving the study early.
Neurocognitive functioning**: Wisconsin Card Sorting Test, Spatial Working Memory Trials A & B, California Verbal Learning Test.
Patient satisfaction**: DAI.
Side effects**: other various observed effects, use of EPS medication. Unable to use ‐ Global effect: CGI score (no SD). Mental state: BPRS (no SD), PANSS (no mean, SD). Side effects: AIMS, BAS, SAS (no usuable data). PsychomMotor Ability: Serial Reaction Time, Pin Test, Pursuit Rotor (no mean, SD). Emotional perception: Facial and Voice Emotional Identification Tests, Videotape Affect Perception Test (no usuable data). Emergence of depressive or obsessive compulsive symptoms: Yale Brown OCS Scale (modified version used), Hamiliton Depression Scale (no usuable data). |
|
| Notes | *excluded if non responsive to or intolerant of risperidone **data for these outcomes comes from studies using subgroups of patients participating in the original trial. data used in analysis is that at the end of the trial i.e. after the 4 weeks variable dose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
General abreviations ECG ‐ Electrocardiograph. EPS ‐ Extrapyramidal side effects. FD ‐ Fixed dose IM ‐ intramuscular. lab ‐ laboratory. SD ‐ standard deviation.
Diagnostic tools DSM‐III‐R ‐ Diagnostic and Statistical Manual of Mental disorders, third edition,revised. ICD‐9 ‐ International Classificaiton of Diseases, ninth revision.
Global effect scales CGI ‐ Clinical Global Impression. GCI ‐ Global Clinical Impression.
Behaviour scale NOISE ‐ Nursing Observation Rating Scale for Inpatient Evaluation.
Mental state scales BPRS ‐ Brief Psychiatric Rating Scale. PANSS ‐ Positive and Negative Symptom Scale. PAS ‐ Psychotic Anxiety Scale. SADS‐C ‐ Schedule for Affective Disorders and Schizophrenia. SANS‐Scale for Assessment of Negative Symptoms.
Side effect scales AIMS‐ Abnormal Involuntary Movement Scale. BAS‐ Barnes Akathisia Scale. CGI ‐ Clinical Global Impression, side effects. DVP ‐ scale for drug tolerance. ESRS ‐ Extrapyramidal Symptom Rating Scale. SARS‐ Simpson‐Angus Rating Scale for Extrapyramidal Side Effects. UKU ‐ UKUSERS Scale.
Other scales CPT ‐ Continuous Performance Test DAI ‐ Drug Attitude Inventory. Yale Brown OCS ‐ Yale Brown Obessive Compulsive Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1994 | Allocation: not randomised ‐ review. |
| Axelson 1996 | Allocation: not randomised. Interventions: risperidone vs risperidone + other antipsychotic drugs. |
| Barak 2000 | Allocation: randomised. Participants: DSM‐IV schizophrenia. Interventions: risperidone vs typical neuroleptics. Outcomes: information taken from conference abstract, all data unusable, further information being sought. |
| Berman 1995 | Allocation: randomised. Participants: DSM‐III‐R schizophrenia. Interventions: risperidone vs haloperidol. Outcomes: information taken from conference abstract, all data unusable as numbers randomised to each group not given, further information being sought. |
| Bilder 2001 | Allocation: randomised. Participants: treatment resistant DSM‐IV schizophrenia or schizoaffective disorder. Interventions: risperidone vs haloperidol, olanzapine or clozapine. Outcomes: information taken from conference poster, all data unusable as numbers randomised to each group not given, further information being sought. |
| Bondolfi 1996 | Allocation: randomised. Participants: treatment resistant schizophrenia. Interventions: risperidone vs clozapine, atypical comparison. |
| Brecher 1998 | Allocation: randomised. Blindness: double. Participants: schizophrenia, schizoaffective disorder. Interventions: risperidone vs olanzapine, atypcial comparison. |
| Carman 1995 | Allocation: not randomised ‐ review. |
| Ceskova 1994 | Allocation: randomised. Participants: ICD‐10 schizophrenia or schizoaffective psychoses. Interventions: risperidone vs perphenazine. Outcomes: all data unusable as no individual group data given. |
| Cetin 1999 | Allocation: randomised. Participants: schizophrenia. Interventions: risperidone vs haloperidol. Outcomes: no usable data, data taken from conference abstract, further information being sought. |
| Czober 1993 | Allocation: not randomised. |
| Daniel 1996 | Allocation: randomised. Participants: schizophrenia/schizoaffective disorder. Interventions: risperidone vs clozapine, atypical comparison. |
| Deshmukh 1998 | Allocation: unclear if randomised, seeking further information. |
| Estrella 1996 | Allocation: randomised. Participants: treatment resistant schizophrenia. Interventions: risperidone vs clozapine, atypical comaprison. |
| Gallhofer 1995 | Allocation: randomised. Participants: schizophrenia. Interventions: risperidone vs conventional neruoleptics. Outcomes: no usable data, information available in conference abstract only, further information being sought. |
| Gelders 1989 | Allocation: not randomised, review. |
| Gureje 1998 | Allocation: randomised. Blindness: double. Participants: schizophrenia, schizophreniform disorder, schizoaffective disorder. Interventions: risperidone vs olanzapine, atypical comparison. |
| Heinrich 1994 | Allocation: randomised. Participants: schizophrenia. Interventions: risperidone vs clozapine, atypical comparison. |
| Heylen 1992 | Allocation: not randomised, review. |
| Kleiser 1995 | Allocation: randomised. Participants: chronic schizophrenia. Interventions: risperidone vs clozapine, atypical comparison. |
| Klieser 1996 | Allocation: not randomised, review. |
| Kopala 1996 | Allocation: not randomised, review. |
| Meltzer 1996 | Allocation: randomised. Participants: schizophrenia. Interventions: clozapine withdrawl vs typical neuroleptic. |
| Muller 1998 | Allocation: randomised. Participants: schizophrenia, schizoaffective, major depression with psychotic features. Interventions: risperidone vs haloperidol. Outcomes: unable to use data from those with schizophrenia or schizoaffective disorder alone, further information being sought. |
| Murasaki 1995 | Allocation: unclear if randomised, seeking further information. |
| Nagao 1998 | Allocation: unclear if randomised. Participants: people with DSM‐IV schizophrenia. Interventions: risperidone vs haloperidol, cross‐over. Outcomes: no relevant or usuable outcomes, further information being sought. |
| Pathiraja 1995 | Allocation: randomised. Participants: DSM‐II‐R schizophrenia. Interventions: risperidone vs haloperidol, MAR 327 or placebo. Outcomes: unusable data, information taken from conference abstract, further information being sought. |
| Sikich 2001 | Allocation: randomised. Participants: DSM‐VI psychotic disorder. Interventions: risperidone vs haloperidol or olanzapine. Outcomes: all data unusable, information taken from conference abstract, further information being sought. |
| Zhou 1998 | Allocation: randomised. Participants: chronic schizophrnenia. Interventions: risperidone vs haloperidol. Outcomes: all data unusable, information taken from english abstract, further information and translation being sought. |
| Zinner 1992 | Allocation: unclear if randomised. Participants: people with chronic DSM‐III‐R schizophrenia. Interventions: risperidone vs haloperidol. Outcomes: all data unusable, information taken from conference abstract, further information being sought. |
DSM ‐ Diagnostic Statistical Manual (versions II to IV, R=revised)
Characteristics of ongoing studies [ordered by study ID]
Armenteros 2001.
| Trial name or title | Risperidone treatment of adolescents with schizophrenia. |
| Methods | |
| Participants | Adolescents with DSM IV schizophrenia and in need of hospitalisation. |
| Interventions | 1. Risperidone. 2. Placebo. |
| Outcomes | Mental state: PANSS. Adverse effects. Lab results. |
| Starting date | March 1998 |
| Contact information | JL Armenteros University of Miami Box 016159 Miami, FL 33101 |
| Notes | Allocation: random
Blindness: double
Duration: 4 weeks Finish date: Feb 2003 |
Lacro 2001.
| Trial name or title | Antipsychotic treatment in late life schizophrenia. |
| Methods | |
| Participants | Older people with schizophrenia. |
| Interventions | 1. Risperidone. 2. Haloperidol. |
| Outcomes | Mental state. Cognitive effects. Social improvement: health related quality of life. Adverse effects. Lab results. |
| Starting date | July 1997 |
| Contact information | JP Lacro Veterans Medical Research Foundation San Diego CA 921 61 |
| Notes | Allocation: random.
Blindness: double.
Duration: 12 weeks. Due to Finish Dec 2002 |
Lieberman 2001.
| Trial name or title | Risperidone and clozapine in chronic schizophrenia. |
| Methods | |
| Participants | People severely ill with treatment resistant schizophrenia. |
| Interventions | 1. Risperidone: 6 mg/day. 2. Risperidone: 16 mg/day. 3. Haloperidol. 4. Clozapine. |
| Outcomes | Global state: CGI. Mental state: PANSS. Behaviour: NOSIE, Overt Aggression Scale. Social improvement: Quality of Life Interview. Cognitive effects. Adverse effects: ESRS. |
| Starting date | |
| Contact information | Sites of study: Bronx Psychiatric Centre (J Lieberman. Rockland Psychiatric Center, Manhattan Psychiatric Center (J Volavka) |
| Notes | Allocation: random. Blindness: double. Duration: 12 weeks. |
Reveley 2000.
| Trial name or title | Ris‐int‐35 a double blind evaluation of risperidone versus haloperidol on the long‐term morbidity of early psychotic patients |
| Methods | |
| Participants | People with early psychotic symptoms. |
| Interventions | 1. Risperidone. 2. Haloperidol. |
| Outcomes | Relapse. Morbidity. |
| Starting date | Nov 1996 |
| Contact information | J Reveley: University of Leicester. Leicestershire& Rutland Healthcare |
| Notes | Allocation: random.
Blindness: double.
Duration: 'long‐term' Due to finish Oct 1999 ‐ as yet not published. |
Tonmoy 2000.
| Trial name or title | A double‐blind evaluation of risperidone versus haloperidol on the long‐term morbidity of early psychotic patients. |
| Methods | |
| Participants | People with first episode psychotic disorders. |
| Interventions | 1. Risperidone. 2. Haloperidol. |
| Outcomes | Morbidity after 2‐4 years. |
| Starting date | Trial now complete but not yet published |
| Contact information | Dr Tonmoy Sharma Institute of Psychiatry De Crespigny Park Denmark Hill London |
| Notes | National Research Register 2002. |
Woods 2000.
| Trial name or title | Atypical antipsychotic medications ‐ outcomes and costs. |
| Methods | |
| Participants | People with schizophrenia. |
| Interventions | 1. Atypical antipsychotics. 2. Typical antipsychotics. |
| Outcomes | Clinical effects. Cost of treatment. |
| Starting date | Not given. |
| Contact information | National Research Register 2000 |
| Notes | Allocation: random. Blindness: double. Duration: 2‐4 years. |
DSM IV ‐ DSM‐III‐R ‐ Diagnostic and Statistical Manual of Mental disorders, fourth edition. CGI ‐ Clinical Global Impression ESRS ‐ ESRS ‐ Extrapyramidal Symptom Rating Scale NOSIE ‐ Nursing Observation Rating Scale for Inpatient Evaluation PANSS ‐ Positive and Negative Symptom Scale.
Contributions of authors
Eilis Kennedy ‐ protocol formulation, searching, trial selection, data extraction and assimilation, report writing.
Fujian Song ‐ initial protocol formulation, searching, trial selection, data extraction and assimilation, report writing.
Robert Hunter ‐ protocol formulation, searching, update, trial selection, data extraction and assimilation, report writing, help with update.
Alan Clarke ‐ data extraction and assimilation, report writing.
Simon Gilbody ‐ protocol formulation, searching, trial selection, data extraction and assimilation, report writing, help with update.
Claire Joy ‐ update searching, update trial selection, update data exctaction and assimilation, update report writing
Sources of support
Internal sources
Research and Development Directorate, Glasgow, UK.
NHS Centre for Reviews and Dissemination, University of York, UK.
External sources
Greater Glasgow Primary Care NHS Trust, UK.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Blin 1996 {published and unpublished data}
- Blin O, Azorin J M, Bouhours P. Antipsychotic and anxiolytic properties of risperidone, haloperidol and methotrimeprazine in schizophrenic patients. Journal of Clinical Psychopharmacology 1996;16:38‐44. [DOI] [PubMed] [Google Scholar]
- Tatossian A. Comparitive double‐blind trial of the efficacy of risperidone, haloperidol and levomepromazine (methotrimeprazine) in patients with an acute exacerbation of schizophhrenia presenting psychotic anxiety symptoms. Clinical research report (RIS‐FRA‐9003) 1991(unpublished data from Janssen).
Borison 1991 {published data only}
- Borison R. Risperidone versus haloperidol versus placebo in the treatment of schizophrenia. Clinical research report (RIS‐USA‐9001) 1991(unpublished data from Janssen).
- Borison R, Pathiraja A, Diamond B, Meibach R. Risperidone: clinical safety and efficacy in schizophrenia. Psychopharmacology Bulletin 1992;28:213‐8. [PubMed] [Google Scholar]
Bouchard 1998 {published data only}
- Bouchard RH, Demers MF, Merette C, Pourcher E. Classical neuroleptics (CNLP) vs risperdone (RIS) interim 12 months efficacy of a long‐term, naturalistic study in schizophrenics. 11th CINP Congress, Glasgow, Scotland. July 12‐16, 1998.
Ceskova 1993 {published data only}
- Ceskova E, Svestka J. Double‐blind comparison of risperidone and haloperidol in schizophrenic and schizoaffective psychoses. Pharmacopsychiatry 1993;26:121‐4. [PubMed] [Google Scholar]
Chouinard 1993a {published data only}
- Chouinard G. Effects of risperidone in tardive dyskinesia: an analysis of the Canadian multicenter risperidone study. Journal of Clinical Psychopharmacology 1995;15(Suppl 1):36‐44. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan G, et al. A Canadian multicenter placebo controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. Journal of Clinical Psychopharmacology 1993;13:25‐40. [PubMed] [Google Scholar]
- Moller H J, Moller H, Borison R, Schooler N, Chouinard G. A path‐analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients: a re‐evaluation of the North American risperidone study. European Achives of Pychiatry and Clinical Neurosciences 1995;245:45‐9. [DOI] [PubMed] [Google Scholar]
- Schooler N. Negative symptoms in schizophrenia: assesment of the effect of risperidone. Journal of Clinical Psychiatry 1994;55(Suppl 5):22‐8. [PubMed] [Google Scholar]
Claus 1991 {published data only}
- Claus A, Bollen J, Cuyper H, Eneman M, Malfroid M, Peuskens J, et al. Risperidone versus haloperidol in the treatment of chronic schizophrenic inpatients: a multicentre double‐blind comparitive study. Acta Psychiatrica Scandinavica 1992;85:295‐305. [DOI] [PubMed] [Google Scholar]
- Wilms G, Ongeval C, Baert AL, Claus A, Bollen J, Cuyper H, Eneman M, Malfroid M, Peuskens J, Heylen S. Ventricular enlargement, clinical correlates and treatment outcome in chronic schizophrenic inpatients. Acta Psychiatrica Scandinavica 1992;85:306‐12. [DOI] [PubMed] [Google Scholar]
Csernansky 1999 {published data only}
- Csernansky J, Okamoto A. [Risperidone vs Haloperidol for prevention of relapse in schizophrenia and schizoaffective disorders: A long‐term double‐blind comparison]. 11th World Congress of Psychiatry; 1999 August; Hamburg. 1999.
- Csernansky JG, Mahmoud R, Brenner R, Risperidone‐USA‐Study Group. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. The New England Journal of Medicine 2002;346(1):16‐22. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Okamoto A, Brecher M. Risperidone vs haloperidol: prevention of relapse in schizophrenia. Journal of the European College of Neuropsychopharmacology (Abstracts of the 12th ECNP Congress; 1999 September 21‐25th; London,UK. 1999.
- Harvey P, Meltzer H, Green M. Long‐term cognitive effects of risperidone treatment in schizophrenia. Abstracts of the XXIInd CINP Congress; 2000 July 9‐13th; Brussels, Belgium. 2000.
- Harvey PD. Long‐term cognitive effects of risperidone treatment in schizophrenia. 52nd Institute on Psychiatric Services; 2000 October 25‐29th; Phildelphia PA, USA. 2000.
- Harvey PD, Lyons BE, Mahmoud R. Long term cognitive effects of risperidone. Schizophrenia Research 2000;41(1):200‐1. [Google Scholar]
- Myers J, Mahmoud R, Keith SJ, Csernansky JG. [The long‐term benefit of risperidone versus haloperidol for affective symptoms in schizophrenia and schizoaffective disorder]. Abstracts of the VIII International Congress on Schizophrenia Research; 2001 April 28th ‐May 2nd; British Columbia, Canada. 2001.
Emsley 1995 {unpublished data only}
- Emsley R, McCreadie R, Livingston M, Smedt G, Lemmens P. Risperidone in the treatment of first‐episode schizophrenic patients with schizophreniform disorder: a double blind study. Poster at the Congress of the International Academy for Biomedical and Drug Research on Critical Issues in the Treatment of Schizophrenia 1995:N 112027/1.
Heck 2000 {published data only}
- Heck AH, Haffmans PMJ, Groot IW, Hoencamp E. Risperidone versus haloperidol in psychotic patients with disturbing neuroleptic‐induced extrapyramidal symptoms: a double‐blind, multi‐centre trial. Schizophrenia Research 2000;46:97‐105. [DOI] [PubMed] [Google Scholar]
Hoyberg 1993 {published data only}
- Hoyberg O, Fensbo C, Remvig J, LingJaerde O, Sloth‐Neilsen M, Salvesen I. Risperidone versus perphenazine in the treatment of chronic schizophrenic patients with acute exacerbations. Acta Psychiatrica Scandinavica 1993;88:395‐402. [DOI] [PubMed] [Google Scholar]
Huttunen 1995 {published data only}
- Huttunen M, Piepponen T, Rantanen H, Larmo I, Nyholm R, Raitasuo V. Risperidone versus zuclopenthixol in the treatment of acute schizophrenic episodes: a double‐blind parallel group trial. Acta Psychiatrica Scandinavica 1995;91:271‐7. [DOI] [PubMed] [Google Scholar]
Kaleda 2000 {published data only}
- Kaleda VG, Oleichik IV, Artiokh VV, Naddour SA. [Risperidone vs haloperidol in the therapy of adolescent schizophrenia and schizoaffective disorders: an open comparative medium‐term efficacy and tolerability study]. Abstracts of the XXIInd CINP Congress; 2000 July 9‐13th; Brussels, Belgium. 2000.
Liu 2000 {published data only}
- Liu SK, Chen WJ, Chang CJ, Lin HN. Effects of atypical neuroleptics on sustained attention deficits in schizophrenia: A trial of risperidone versus haloperidol. Neuropsychopharmacology 2000;22(3):311‐9. [DOI] [PubMed] [Google Scholar]
Mahmoud 1998 {published data only}
- Mahmoud RA, Engelhart LM, Risperidone Outcome Study of Effectiveness (ROSE) Group Janssen Research Foundation. Risperidone versus conventional antipsychotics in usual care: a prospective randomised effectiveness trial of outcomes for patients with schizophrenia and schizoaffective disorder. 11th CINP Congress; Glasgow, Scotland,. July 12‐16 1998.
- Merideth C, Mahmoud RA, Englehart LM, Ramirez L, the ROSE Group. Clinical and quality of life superiority of risperidone over conventional antipsychotics under usual care conditions: a prospective randomised trial in schizophrenia and schizoaffective disorder. 151st Annual Meeting of the American Psychiatric Association. May 30‐June 4 1998.
Malyarov 1999 {published data only}
- Malyarov S, Dzub G. Comparative assessment of the positive and negative symptom dynamics in schizophrenic patients treated with atypical antipsychotics or haloperidol. Abstracts of the 12th ENCP Congress;1999 September 21‐25th; London, UK. 1999.
Marder 1994 {published data only}
- Blaeser‐Kiel G. Schizophrenes negativsyndrom risperidone erfolgreich. Neurologie Psychiatrie 1994;11:614‐5. [Google Scholar]
- Czobor P, Volavka J, Meibach R. Effect of risperidone on hostility in schizophrenia. Journal of Clinical Psychopharmacology 1995;15:243‐9. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Grochowski S, Hyman R. Five factor model of schizophrenia: replication across samples. Schizophrenia Research 1995;14:229‐34. [DOI] [PubMed] [Google Scholar]
- Marder S, Meibach R. Risperidone in the treatment of schizophrenia. American Journal of Psychiatry 1994;151:825‐35. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. Efficacy of risperidone on positive features of schizophrenia. Journal of Clinical Psychiatry 1994;55(suppl 5):18‐21. [PubMed] [Google Scholar]
- Schooler N. Negative symptoms in schizophrenia: assesment of the effect of risperidone. Journal of Clinical Psychiatry 1994;55(suppl 5):22‐8. [PubMed] [Google Scholar]
Mesotten 1991 {unpublished data only}
- Messotten F. Risperidone versus haloperidol in the teatment of chronic psychotic patients: a multicentre double‐blind study. Clinical research report (RIS‐INT‐2, N 85163) 1991(unpublished data from Janssen).
Min 1993 {published data only}
- Min S, Rhee C, Kim C, Kang D. Risperidone versus haloperidol in the treatment of chronic schizophrenic patients: a parallel group double‐blind comparative trial. Yansei Medical Journal 1993;34:179‐90. [DOI] [PubMed] [Google Scholar]
Peuskens 1995 {published and unpublished data}
- Lindstrom E, Knorring L. Changes in single symptoms and separate factors of the schizophrenic syndrome after treatment with risperidone or haloperidol. Pharmacopsychiatry 1994;7:108‐13. [DOI] [PubMed] [Google Scholar]
- Muller‐Spahn. Risperidone in the treatment of chronic schizophrenic patients: an inernational double‐blind parallel group study versus haloperidol. Clinical Neuropharmacology 1992;15(suppl 1):90A‐1A. [DOI] [PubMed] [Google Scholar]
- Peuskens J. Risperidone in the treatment of chronic schizophrenic patients: an international double blind parallel group comparative study versus haloperidol. Clinical Research Report (RIS‐INT‐2) 1992 (unpublished data from Janssen). [DOI] [PubMed]
- Peuskens J, The Risperidone Study Group. Risperidone in the treatment of chronic schizophrenic patients: an international multicentre double‐blind comparative study versus haloperidol. British Journal of Psychiatry 1995;166:97‐9. [Google Scholar]
Potkin 1997 {published data only}
- Gutierrez R, Lemmens P, Potkin S. [Safety and efficacy of once‐daily risperidone in the treatment of schizophrenia]. Sixth World Congress of Biological Psychiatry; 1997 June 22‐27th; Nice, France. 1997.
- Potkin SG, Gutierrez R. 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22nd; San Diego, California, USA. 1997.
Purdon 2000 {published data only}
- Canadian Cognition and Outcome Study Group. Neuropsychological change in early phase schizophrenia over twelve months of treatment with olanzapine, risperidone or haloperidol. Schizophrenia Research 1998;29(1‐2):152. [Google Scholar]
- Jones B. Olanzapine versus risperidone and haloperidol in the treatment of schizophrenia. American Psychiatric Association Annual Meeting; 1998 May 30 ‐ June 4th; Toronto, Ontario, Canada. 1998.
- Purdon SE, Jones B, Labelle A, Addington D, Tollefson G, Canadian Cognition and Outcome Study Group. A multicentre comparison of olanzapine, risperidone, and haloperidol on working memory, new learning, and delayed recall of verbal and nonverbal materials in early‐phase schizophrenia over a 12‐month prospective double‐blind clinical trial. Abstracts of the VIIth International Congress on Schizophrenia Research; 1999 April 17‐21st; Santa Fe, New Mexico, USA. 1999.
- Purdon SE, Jones BDW, Stip E, Labelle A, Addingtion D, David SR, Breier A, Tollefson GD. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone or haloperidol. Archives of General Psychiatry 2000;57:249‐58. [DOI] [PubMed] [Google Scholar]
See 1999 {published data only}
- See RE, Fido AA, Maurice M, Ibrahim MM, Salama GMS. Risperidone‐induced increase of plasma norepinephrine is not correlated with symptom improvement in chronic schizophrenia. Society of Biological Psychiatry 1999;45:1653‐6. [DOI] [PubMed] [Google Scholar]
Wirshing 1999 {published data only}
- Ames D, Wirshing WC, Marder SR, Hwang SS, German CA, Mintz J, Goldstein D. Risperidone vs Haloperidol: Relative liabilities for OCD and depression. Schizophrenia Research 1996;18(2‐3):129. [Google Scholar]
- Ames D, Wirshing WC, Marder SR, Hwang SS, German CA, Strough AB. Subjective response to risperidone and haloperidol: Preliminary results. Schizophrenia Research 1996;18:129. [Google Scholar]
- Ames D, Wirshing WC, Marshall BD, Green MF, McGurk SR, Mintz J, Marder SR. [Treatment‐resistant schizophreina: efficacy of risperidone versus haloperidol]. American Psychiatric Association 150th Annual Meeting; 1997; San Diego, USA. 1997.
- Green MF, Marshall BD, Wirshing WC, Ames D, Marder SR, McGurk S, Kern RS, Mintz J. Does risperidone improve verbal working memory in treatment‐resistant schizophrenia?. American Journal of Psychiatry 1997;154(6):799‐804. [DOI] [PubMed] [Google Scholar]
- Kee KS, Kern RS, Marshall BD, Green MF. [Risperidone versus haloperidol for perception of emotion in treatment‐resistant schizophrenia ‐ preliminary findings]. American Psychiatric Association Annual Meeting; 1998 May 30 ‐ June 4th; Toronto, Ontario, Canada. 1998. [DOI] [PubMed]
- Kee KS, Kern RS, Marshall BD, Green MF. Risperidone versus haloperidol for perception of emotion in treatment‐resistant schizophrenia: preliminary findings. Schizphrenia Research 1998;31:159‐65. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Marshall BD, Wirshing WC, Ames D, Marder SR, McGurk S, Mintz J. Risperidone vs haloperidol on reaction time and fine motor speed. 6th International Congress on schizophrenia research;1997; Colorado Springs, Colorado, USA. 1997.
- Kern RS, Green MF, Marshall BD, Wirshing WC, Wirshing D, McGurk S, Marder S, Mintz J. Risperidone vs. haloperidol on reaction time, manual dexterity, and motor learning in treatment‐resistant schizophrenic patients. Society of Biological Psychiatry 1998;44:726‐32. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Marshall BD, Wirshing WC, Wirshing D, McGurk SR, Marder SR, Mintz J. Risperidone versus haloperidol on secondary memory: Can newer medications aid learning?. Schizophrenia Bulletin 1999;25(2):223‐32. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Green MF, Wirshing WC, Donna A, Marder BD, Marder SR, Koehn H. The effects of risperidone versus haloperidol on frontal lobe functioning in treatment‐resistant schizophrenia. American Psychiatric Association 150th Annual Meeting;1997; San Diego, USA. 1997.
- Wirshing DA, Marshall BD, Green MF, Mintz J, Marder SR, Wirshing WC. Risperidone in treatment‐refractory schizophrenia. American Journal of Psychiatry 1999;156(9):1374‐9. [DOI] [PubMed] [Google Scholar]
- Wirshing WC, Ames D, Bray MP, Marshall BD, Green MF, Marder SR. Risperidone versus haloperidol in treatment refractory schizophrenia: preliminary results. 148th Annual Meeting of the American Psychiatric Association; 1995 May 20‐25th; Miami, Florida, USA. 1995.
- Wirshing WC, Ames D, Green MF, Marshall BD, Marder SR. Risperidone versus Haloperidol in treatment‐refractory schizophrenia: preliminary results. Psychopharmacology Bulletin 1995;31(3):633. [Google Scholar]
- Wirshing WC, Ames D, Marder SR, Marshall BD, Green MF, McGurk S. Risperidone in treatment resistant schizophrenia. 8th Biennial Winter Workshop on Schizophrenia, 1996 February ; Crans Montana, Switzerland. 1996.
- Wirshing WC, Ames D, Marder SR, Marshall BD, Green MF, McGurk S. Risperidone versus haloperidol in treatment resistant schizophrenia: preliminary results. Schizophrenia Research 1996:130. [DOI] [PubMed] [Google Scholar]
- Wirshing WC, Green MF, Ames D, Marshall BD, McGurk SR, Mintz J, Marder SR. [Risperidone vs haloperidol in treament‐resistant schizophrenia]. Sixth World Congress of Biological Psychiatry; 1997 June 22‐27th; Nice, France. 1997.
References to studies excluded from this review
Anonymous 1994 {published data only}
- Anonymous. New antipsychotic agent, risperidone, introduced. American Journal of Hospital Pharmacy 1994;51:1145. [PubMed] [Google Scholar]
Axelson 1996 {published data only}
- Axelson R, Lindstrom E. Dyskinesia in psychotic patients. Xth World Congress of Psychiatry; 1996 August 23rd ‐ 28th; Madrid, Spain. 1996.
Barak 2000 {published data only}
- Barak Y, Shamir E, Weizman R. [Risperidone compared with typical neuroleptic treatment in elderly schizophrenic patients]. Abstracts of XXIInd CINP Congress; 2000 July 9‐13th; Brussels, Belgium. 2000.
Berman 1995 {published data only}
- Berman I, Allan ER, Pappas D, Sison CE, Merson A. The cognitive effect of risperidone in elderly schizophrenic patients. A pilot double‐blind comparison study with haloperidol. American Psychiatric Association 150th Annual Meeting; 1997; San Diego, USA. 1997.
- Berman I, Merson A, Allan E, Alexis C, Losonczy M. Effect of risperidone on cognitive performance in elderly schizophrenic patients: a double‐blind comparison study with haloperidol. Psychological Bulletin 1995;31(3):552. [Google Scholar]
Bilder 2001 {published data only}
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment‐resistant patients with schizophrenia and schizoaffective disorder. International Congress on Schizophrenia Research; 2001 April 28 ‐ May 2nd; Whistler, British Columbia, USA. 2001.
Bondolfi 1996 {published data only}
- Bondolfi G, Baumann P, Dufour H. Treatment‐resistant schizophrenia ‐ clinical experience with new antipsychotics. European Neuropsychopharmacology 1996;6(Suppl 2):21‐5. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Baumann P, Patris M, May J, Billeter U, Dufour H. A randomized double‐bind trial of risperidone versus clozapine for treatment‐resistant chronic schizophrenia. 8th European College of Neuropsychopharmacology Congress; 1995 September 30th ‐ October 4th; Venice, Italy. 1995.
Brecher 1998 {published data only}
- Brecher M, The Risperidone/Olanzapine Study Group. Risperidone versus olanzepine in the treatment of patients with schizophrenia or schizoaffective disorder. 11th CINP Congress; 1998 July 12‐16th; Glasgow, Scotland. 1998.
Carman 1995 {published data only}
- Carman J, Peuskens J, Vangeneugden A. Risperidone in the treatment of negative symptoms of schizophrenia: a meta‐analysis. International Journal of Clinical Psychopharmacology 1995;10:207‐13. [DOI] [PubMed] [Google Scholar]
Ceskova 1994 {published data only}
- Ceskova E, Svestka J. [Efficacy and tolerability of risperidone in different dose levels]. XIXth Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1994 June 27 ‐ July 1; Washington, D.C., USA. 1994.
- Ceskova E, Svestka J. Risperidone vs perphenazine ‐ a double blind comparison and prolactin plasma levels. Psychiatrica Danubina 1994;6(3‐4):151‐5. [Google Scholar]
Cetin 1999 {published data only}
- Cetin M, Ebrinc S, Agargun MY, Basoglu C, Yigit S, Yilmaz V, Secil M, Cobanoglu N, Can S. Determination of the optimal dose of risperidone in patients with schizophrenia. 12th Congress of the European College of Neuropsychopharmacology; 1999 September 21‐25th; London, UK. 1999.
Czober 1993 {published data only}
- Czober P, Volavka J. Quantative electroencephalogram examination of effects of risperidone in schizophrenic patients. Journal of Clinical Psychopharmacology 1993;13:322‐42. [PubMed] [Google Scholar]
Daniel 1996 {published data only}
- Daniel DG, Goldberg TE, Weinberger DR, Kleinman JE, Pickar D, Lubick LJ, Williams TS. Different side effect profiles of risperidone and clozapine in 20 outpatients with schizophrenia or schizoaffective disorder: a pilot study. American Journal of Psychiatry 1996;153:417‐9. [DOI] [PubMed] [Google Scholar]
Deshmukh 1998 {published data only}
- Deshmukh DK, Patil B, Save D, Matcheswalla YA, Vaya L. A side effect profile of risperidone vs haloperidol in a double blind randomized comparative group. Psychopharmacology 1998;40:S26. [Google Scholar]
Estrella 1996 {published data only}
- Estrella MH, Soria FL, Gonzalez CJ, Butron MA, Torres JA, Escareco RR. Cost‐effectiveness of Clozapine versus risperidone for treatment‐resistant schizophrenic patients. Xth World Congress of Psychiatry; 1996 August 23rd‐28th; Madrid, Spain. 1996.
Gallhofer 1995 {published data only}
Gelders 1989 {published data only}
- Gelders YG. Thymosthenic agents, a novel approach in the treatment of schizophrenia. British Journal of Psychiatry 1989;155(Suppl 5):33‐6S. [PubMed] [Google Scholar]
Gureje 1998 {published data only}
- Gureje O, Catts S, Fraser H, Hustig N, Keks Y, Lambert J, McGrath W, Miles A, Thomas D, Grainger S, Andersen G, Tollefson, Australasian Olanzepine Study Group. Olanzapine versus risperidone in the treatment of schizophrenia and related psychotic disorders. 11th CINP Congress; 1998 July 12‐16th; Glasgow, Scotland. 1998.
Heinrich 1994 {published data only}
- Heinrich K, Klieser E, Lehmann E, Kinzler E, Hruschka H. Risperidone versus clozapine in the treatment of schizophrenic patients with acute symptoms: a double blind, randomized trial. Progress in Neuropsychopharmacology and Biological Psychiatry 1994;18:129‐37. [DOI] [PubMed] [Google Scholar]
Heylen 1992 {published data only}
- Heylen SL, Gelders YG. Risperidone, a new antipsychotic with serotonin 5‐HT2 and dopamine D2 antagonistic properties. Clinical Neuropharmacology 1992;15(Suppl 1):180a‐1a. [DOI] [PubMed] [Google Scholar]
Kleiser 1995 {published data only}
- Klieser E, Lehmann E, Kinzler E, Wurthmann C, Heinrich K. Randomized, double‐blind, controlled trial of risperidone versus clozapine in patients with chronic schizophrenia. Journal of Clinical Psychopharmacology 1995;15:45S‐51S. [DOI] [PubMed] [Google Scholar]
Klieser 1996 {published data only}
- Klieser E, Lehman E, Heinrich K. Risperidone in comparison with various treatments of schizophrenia. In: JM Kane, HJ Moller, F Awouters editor(s). Serotonin in Antipsychotic Treatment. Mechanisms and Clinical Practice. New York, Basel, Hong Kong: Marcel Dekker Inc, 1996:331‐42. [Google Scholar]
Kopala 1996 {published data only}
- Kopala LC, Frederikson D, Good KP, Honer WG. Symptoms in neuroleptic‐naive, first‐episode schizophrenia ‐ response to risperidone. Biological Psychiatry 1996;39:296‐8. [DOI] [PubMed] [Google Scholar]
Meltzer 1996 {published data only}
- Meltzer HY, Lee MA, Ranjan R, Mason EA, Cola PA. Relapse following clozapine withdrawal ‐ effect of neuroleptic drugs and cyproheptadine. Psychopharmacology 1996;124:176‐87. [DOI] [PubMed] [Google Scholar]
Muller 1998 {published data only}
- Muller MJ, Wetzel H, Benkert O. Depressive symptoms in acute psychotic disorders: evaluation methods and treatment strategies. 11th CINP Congress; 1998 July 12‐16th; Glasgow, Scotland. 1998.
Murasaki 1995 {published data only}
- Murasaki M, Miura S. [Risperidone in the treatment of schizophrenia: A double‐blind comparison with haloperidol]. 8th ECNP ( European College of Neuropyschopharmacology) Congress; 1995 September 30th ‐ October 4th; Venice, Italy. 1995.
- Murasaki M, Yamashita I, Machiyama Y, Yamauchi T, Okuma T, Toru M, Ushijima S, Kamijima K, Yagi G, Miura S. Efficacy of a new antipsychotic, risperidone on schizophrenia. Clinical Evaluation 1993;21:221‐59. [Google Scholar]
Nagao 1998 {published data only}
- Nagao M, Mori K, Kobayakawa M, Hirayama S, Harada Y, Kashiwagi K, Irisawa T, Nagao K. Quantitative electoencephalography in schizophrenia: therapeutic effects of haloperidol and risperidone. 11th Congress of the European College of Neuropsychopharmacology; 1998 October 31‐ November 4; Paris, France. 1998.
Pathiraja 1995 {published data only}
- Pathiraja AP, Diamond BI, Borison RL, Meibech RC, Anand R. Relationship between creatine phosphokinase, psychotic symptoms and novel antipsychotic drugs. Schizophrenia Research 1995;15(1‐2):160‐1. [Google Scholar]
Sikich 2001 {published data only}
- Sikich L, Hooper SR, Malekpour AH, Sheitman BB, Lieberman JA. A double blind comparison of typical versus atypical antipsychotic agents on selected neurocognitive functions in children and adolescents with psychotic disorders. Abstracts of the VIII International Congress on Schizophrenia Research; 2001 April 28‐May 2; British Columbia, Canada. 2001.
Zhou 1998 {published data only}
- Zhang XY, Zhou DF, Zhang PY, Shen YC. Effects of risperidone and haloperidol on paroxetine‐stimulated neuroendocrine responses and blood SOD in schizophrenia. 11th CINP Congress; 1998 July 12‐16th; Glasgow, Scotland. 1998.
- Zhou D, Zhang X, Shen Y, et al. Effects of risperidone and haloperidol on paroxetine‐evoked neuroendocrine responses in schizophrenia. Chinese Journal of Psychiatry 1999;32(2):84‐7. [Google Scholar]
Zinner 1992 {published data only}
- Zinner HJ, Fuger J, Kasper S, Middendorf E, Moller H. Changes of positive and negative syndromes ‐ a double‐blind clinical trial with risperidone versus haloperidol in chronic schizophrenia. Pharmacopsychiatry 1992;25:118. [Google Scholar]
References to ongoing studies
Armenteros 2001 {published data only}
- Risperidone treatment of adolescents with schizophrenia.. Ongoing study March 1998.
Lacro 2001 {published data only}
- Antipsychotic treatment in late life schizophrenia.. Ongoing study July 1997.
Lieberman 2001 {published data only}
- Risperidone and clozapine in chronic schizophrenia.. Ongoing study Starting date of trial not provided. Contact author for more information.
Reveley 2000 {published data only}
- Ris‐int‐35 a double blind evaluation of risperidone versus haloperidol on the long‐term morbidity of early psychotic patients. Ongoing study Nov 1996.
Tonmoy 2000 {published data only}
- A double‐blind evaluation of risperidone versus haloperidol on the long‐term morbidity of early psychotic patients.. Ongoing study Trial now complete but not yet published.
Woods 2000 {published data only}
- Atypical antipsychotic medications ‐ outcomes and costs.. Ongoing study Not given..
Additional references
Addington 1993
- Addington DE, Jones B, Bloom D. Reduction of hospital days in chronic schizophrenic patients treated with risperidone: a retrospective study. Clinical Therapeutics 1993;15:917‐26. [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecing skewness from summary information. BMJ 1996;313:1200. [RSPT020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1985
- Andreasen NC. Negative syndrome in schizophrenia: strategies for long‐term management. Advances in Biochemical Psychopharmacology 1985;40:1‐7. [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Chouinard 1980
- Chouinard G, Ross‐Chouinard A, Annable L, Jones BD. Extrapyramidal Rating Scale. Canadian Journal of Neurological Science 1980;7:233. [Google Scholar]
Chouinard 1993b
- Chouinard G, Arnott W. Clinical review of risperidone. Canadian Journal of Psychiatry 1993;38(suppl 3):89‐95. [PubMed] [Google Scholar]
Christison 1991
- Christison GW, Kirch DG, Wyatt RJ. When symptoms persist: choosing among alternative somatic symptoms for schizophrenia. Schizophrenia Bulletin 1991;17:217‐45. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD. Cochrane Collaboration Handbook. The Cochrane Library [database on disk & CD ROM] The Cochrane Collaboration 2000, Issue 1. [Google Scholar]
Crow 1980
- Crow TJ. Positve and negative schizophrenic symptoms and the role of dopamine. British Journal of Psychiatry 1980;137:383‐6. [PubMed] [Google Scholar]
Curtis 1995
- Curtis V, Kerwin R. A risk benefit assessment of risperidone in schizophrenia. Drug Safety 1995;12:139‐45. [DOI] [PubMed] [Google Scholar]
Davis 1977
- Davis JM, Casper R. Antipsycotic drugs: clinical pharmacology and therapeutic use. Drugs 1977;14:260‐82. [DOI] [PubMed] [Google Scholar]
Dickersin 1992
- Dickersin K. Why register clinical trials?. Controlled Clinical Trials 1992;13:170‐7. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Edwards 1994
- Edwards JG. Risperidone for schizophrenia. BMJ 1994;308:1311‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guest 1996
- Guest JF, Hart WM, Cookson RF, Lindstrom E. Pharmocoeconomic evaluation of long‐term treatment with risperidone for patients with chronic schizophrenia. British Journal of Medical Economics 1996;10:59‐67. [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Gupta 1994
- Gupta S, Black D, Smith D. Risperidone: review of its pharmacology and therapeutic use in schizophrenia. Annals of Clinical Psychiatry 1994;6:173‐80. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. Washington DC: National Institute of Mental Health, 1976. [Google Scholar]
He 1995
- He H, Richardson JS. A pharmacological, pharmacokinetic and clinical overview of risperidone, a new antipsychotic that blocks serotonin 5‐HT2 and dopamine D2 receptors. International Clinical Psychopharmacology 1995;10:19‐30. [DOI] [PubMed] [Google Scholar]
Horton 1997
- Horton R. Pardonable revisions and protocol reviews. Lancet 1997;349:6. [DOI] [PubMed] [Google Scholar]
Irwig 1998
- Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta‐analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ 1998;316:470. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Janssen‐Cilag 1996
- Janssen‐Cilag Ltd. Risperidal (risperidone) summary of product characteristics. UK: Janssen‐Cilag Ltd, 1996. [Google Scholar]
Joy 2000
- Joy CB, Adams CE, Lawrie SM. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2000, Issue 2. [Google Scholar]
Kane 1990
- Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica 1990;358 (suppl):151‐7. [DOI] [PubMed] [Google Scholar]
Kane 1993
- Kane JM. Newer antipsychotic drugs. A review of their pharmacology and therapeutic potential. Drugs 1993;46:585‐93. [DOI] [PubMed] [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐7. [DOI] [PubMed] [Google Scholar]
Keltner 1995
- Keltner Nl. Risperidone: the search for a better antipsychotic. Perspectives in Psychiatric Care 1995;31:30‐3. [DOI] [PubMed] [Google Scholar]
Lingjaerde 1987
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatrica Scandinavicia Supplementum 1987;334:1‐100. [DOI] [PubMed] [Google Scholar]
Livingston 1994
- Livingston MG. Risperidone. Lancet 1994;343:457‐60. [DOI] [PubMed] [Google Scholar]
Meltzer 1992
- Meltzer HY. Treatment of the neuroleptic‐nonresponsive schizophrenic patient. Schizophrenia Bulletin 992;18:515‐42. [DOI] [PubMed] [Google Scholar]
Meltzer 1994
- Meltzer HY, Lee MA, Ranjan R. Recent advances in the pharmacotherapy of schizophrenia. Acta Psychiatrica Scandinavica 1994;90:s95‐101. [DOI] [PubMed] [Google Scholar]
Naylor 1997
- Naylor CD. Meta‐analysis and the meta‐epidemiology of clinical research. BMJ 1997;315:617‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Remington 1993
- Remington GJ. Clinical considerations in the use of risperidone. Canadian Journal of Psychiatry 1993;38:96‐100. [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JWS. A rating scale for extra‐pyramidal side‐effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. [DOI] [PubMed] [Google Scholar]
Stip 1996
- Stip E, Lussier I. The effect of risperidone on cognition in patients with schizophrenia. Canadian Journal of Psychiatry 1996;41:35‐40. [DOI] [PubMed] [Google Scholar]
Thornley 2003
- Thornley B, Rathbone J, Adams CE, Awad G. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI] [PubMed] [Google Scholar]
van Putten 1987
- Putten T, Marder SR. Behavioural toxicity of antipsychotic drugs. Journal of Clinical Psychiatry 1987;48(Suppl 9):13‐19. [PubMed] [Google Scholar]
Waraich 2002
- Waraich PS, Adams CE, Roque M, Hamill KM, Marti J. Haloperidol dose for the acute phase of schizophrenia. The Cochrane Library. Oxford: Update Software, 2002. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Song 1997
- Song F. Risperidone in the treatment of schizophrenia: a metanalysis of randomized controlled trials. Journal of Psychopharmacology 1997;11:65‐71. [DOI] [PubMed] [Google Scholar]


