Abstract
Background
Rheumatoid arthritis (RA) is a chronic and systemic inflammatory disorder that mainly affects the small joints of the hands and feet. Erythropoiesis‐stimulating agents have been used to treat anemia, one of the extra‐articular manifestations of RA. Although anemia is less of a problem now because of the reduction in inflammation due to disease‐modifying antirheumatic drugs (DMARDs), it could still be an issue in countries where DMARDs are not yet accessible.
Objectives
We assessed the clinical benefits and harms of erythropoiesis‐stimulating agents for anemia in rheumatoid arthritis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (issue 7 2012), Ovid MEDLINE and Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations (1948 to 7 August 2012), OVID EMBASE (1980 to 7 August 2012), LILACS (1982 to 7 August 2012), the Clinical Trials Search Portal of the World Health Organization, reference lists of the retrieved publications and review articles. We did not apply any language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) in patients aged 16 years or over, with a diagnosis of rheumatoid arthritis affected by anemia. We considered health‐related quality of life, fatigue and safety as the primary outcomes.
Data collection and analysis
Two authors independently performed trial selection, risk of bias assessment, and data extraction. We estimated difference in means with 95% confidence intervals (CIs) for continuous outcomes. We estimated risk ratios with 95% CIs for binary outcomes.
Main results
We included three RCTs with a total of 133 participants. All trials compared human recombinant erythropoietin (EPO), for different durations (8, 12 and 52 weeks), versus placebo. All RCTs assessed health‐related quality of life. All trials had high or unclear risk of bias for most domains, and were sponsored by the pharmaceutical industry. Two trials administered EPO by a subcutaneous route while the other used an intravenous route.
We decided not to pool results from trials, due to inconsistencies in the reporting of results.
Health‐related quality of life: subcutaneous EPO – one trial with 70 patients at 52 weeks showed a statistically significant difference in improvement of patient global assessment (median and interquartile range 3.5 (1.0 to 6.0) compared with placebo 4.5 (2.0 to 7.5) (P = 0.027) on a VAS scale (0 to 10)). The other shorter term trials (12 weeks with subcutaneous EPO and eight weeks with intravenous administration) did not find statistically significant differences between treatment and control groups in health‐related quality of life outcomes.
Change in hemoglobin: both trials of subcutaneous EPO showed a statistically significant difference in increasing hemoglobin levels; (i) at 52 weeks (one trial, 70 patients), intervention hemoglobin level (median 134, interquartile range 110 to 158 g/litre) compared with the placebo group level (median 112, interquartile range; 86 to 128 g/litre) (P = 0.0001); (ii) at 12 weeks (one trial, 24 patients) compared with placebo (difference in means 8.00, 95% CI 7.43 to 8.57). Intravenous EPO at eight weeks showed no statistically significant difference in increasing hematocrit level for EPO versus placebo (difference in means 4.69, 95% CI ‐0.17 to 9.55; P = 0.06).
Information on withdrawals due to adverse events was not reported in two trials, and one trial found no serious adverse events leading to withdrawals. None of the trials reported withdrawals due to high blood pressure, or to lack of efficacy or to fatigue.
Authors' conclusions
We found conflicting evidence for erythropoiesis‐stimulating agents to increase quality of life and hemoglobin level by treating anemia in patients with rheumatoid arthritis. However, this conclusion is based on randomized controlled trials with a high risk of bias, and relies on trials assessing human recombinant erythropoietin (EPO). The safety profile of EPO is unclear. Future trials assessing erythropoiesis‐stimulating agents for anemia in rheumatoid arthritis should be conducted by independent researchers and reported according to the CONSORT statements. Trials should be based on Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) and The Patient‐Centered Outcomes Research Institute (PCORI) approaches for combining both clinician and patient perspectives.
Keywords: Humans; Anemia; Anemia/blood; Anemia/drug therapy; Anemia/etiology; Arthritis, Rheumatoid; Arthritis, Rheumatoid/blood; Arthritis, Rheumatoid/complications; Epoetin Alfa; Erythropoietin; Erythropoietin/adverse effects; Erythropoietin/therapeutic use; Hematinics; Hematinics/adverse effects; Hematinics/therapeutic use; Hemoglobin A; Hemoglobin A/metabolism; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/adverse effects; Recombinant Proteins/therapeutic use
Plain language summary
Erythropoiesis‐stimulating agents for anemia in rheumatoid arthritis
Researchers in the Cochrane Collaboration conducted a review of the effect of erythropoiesis‐stimulating agents for anemia in patients with rheumatoid arthritis. After searching for all relevant studies, they found three studies covering 133 people. Their findings are summarised below:
The review shows that in people with anemia and rheumatoid arthritis:
‐ it is uncertain whether erythropoiesis‐stimulating agents improve quality of life or hemoglobin levels. ‐ it is unknown whether erythropoiesis‐stimulating agents improve fatigue, as this was not measured by the studies.
We do not have precise information about side effects and complications. This is particularly true for rare but serious side effects, which may include thromboembolic complications.
What is anemia in rheumatoid arthritis and what are erythropoiesis‐stimulating agents?
When you have rheumatoid arthritis, your immune system, which normally fights infection, attacks the lining of your joints. This makes them swollen, stiff and painful. The small joints of the hands and feet are usually affected first. As the disease progresses, other complications may appear, including anemia (low hemoglobin level). Hemoglobin is a protein in red blood cells that carries oxygen. Anemia is a condition in which the body does not have enough healthy red blood cells. Erythropoietin is a hormone produced in the kidney, which increases the production of red blood cells. Erythropoiesis‐stimulating agents work to increase red blood cell production.
Summary of findings
Summary of findings for the main comparison. Erythropoietin (subcutaneous or intravenous at varying dosages) compared to placebo for anemia in patients with rheumatoid arthritis.
| Erythropoietin (subcutaneous or intravenous at varying dosages) compared to placebo for anemia in patients with rheumatoid arthritis | ||||||
| Patient or population: anemia in patients with rheumatoid arthritis Settings: outpatient Intervention: erythropoietin (subcutaneous or intravenous at varying dosages) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Erythropoietin (subcutaneous or intravenous at varying dosages) | |||||
| Health‐related quality of life Follow‐up: 8 to 52 weeks | See comment | See comment | 136 (3 studies1) | ⊕⊝⊝⊝ very low2,3 |
Peeters 1996, with 70 participants followed up during 52 weeks reporting patients' global assessment (VAS score 0 to 10), found statistically significant differences comparing EPO versus placebo (median and interquartile range at 52 weeks: 3.5 (1.0 to 6.0) and 4.5 (2.0 to 7.5) P = 0.027, respectively. Pincus 1990, with 17 participants followed up during 8 weeks, reported no significant differences between groups of patient satisfaction in activities of daily living using the mHAQ5 but data were not provided. Nordström 1997, with 46 participants followed up at 12 weeks, reported no significant difference between groups in Stanford Health Assessment Questionnaire scores, Nottingham Health Profile scores, classification of functional class or joint score index scores. |
|
| Hemoglobin (Hb) level at the end of the study Follow‐up: 12 to 52 weeks4 | See comment | 116 (2 studies4) | ⊕⊕⊝⊝ low2 |
Nordström 1997 (46 participants) reported a statistically significant increase in hemoglobin level at 12 weeks in the EPO group compared with placebo group (MD 8.00, 95% CI 7.43 to 8.57 g/l; P = 0.00001) Peeters 1996 (70 participants) reported a statistically significant increase in hemoglobin level at 52 weeks in the EPO group (median 134; interquartile range 110 to 158 g/L) compared with placebo group (median 112; interquartile range 86 to 128 g/L) (P = 0.001). |
||
|
Fatigue Not reported |
See comment | See comment | Not estimable | ‐ | See comment | This outcome was not assessed by any of the trials. |
|
Safety (Adverse events including adverse drug reaction) Follow‐up: 8 to 52 weeks |
See comment | See comment | Not estimable | (3 studies1) | ⊕⊝⊝⊝ very low2,3 |
Nordström 1997 found no significant alterations in pulse, blood pressure, platelet counts, albumin, creatinine, alanine aminotransferase, or aspartate aminotransferase values .
Peeters 1996 assessed adverse events and found no significant rise in blood pressure and thromboembolic complications. Pincus 1990 found no adverse reactions. |
| Withdrawals due to adverse events | See comment | See comment | Not estimable | ‐ | See comment | Nordström 1997 reported no serious adverse events causing premature discontinuation. This outcome was not reported by the other two trials (Peeters 1996; Pincus 1990). |
| Withdrawals due to lack of efficacy | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not assessed by any of the trials. |
| Withdrawals due to high blood pressure | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not assessed by any of the trials. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Nordström 1997, Peeters 1996, Pincus 1990 2. Almost all domains had a high or unclear risk of bias. 3. Small sample size 4. Nordström 1997, Peeters 1996 5. mHAQ: modified Stanford Health Assessment Questionnaire
Background
Description of the condition
The burden of anemia in rheumatoid arthritis
Rheumatoid arthritis is a chronic and systemic inflammatory disorder that mainly affects the small joints of the hands and feet (Lee 2001). The criteria for the classification of rheumatoid arthritis are described in Arnett 1988 (Appendix 1). Recently, the immunopathogenesis of rheumatoid arthritis has been described (Imboden 2009; McInnes 2011). Rheumatoid arthritis has variable extra‐articular manifestations which are known as comorbidities (Grassi 1998; Hochberg 2008; Michaud 2007; Turesson 2003; Young 2007). Anemia is one of those comorbidities (Appendix 2), and this has a prevalence ranging from 33% to 60% (Al‐Ghamdi 2009; Furst 2009; Wilson 2004). According to the World Health Organization, hemoglobin thresholds used to define anemia are 120 g/L for non‐pregnant women (≥15 years old) and 130 g/L for men (≥15 years old) (WHO 2008). However, anemia is less of a problem now because of the reduction in inflammation due to disease‐modifying antirheumatic drugs (DMARDs) (Blumenauer 2002; Blumenauer 2003; Maxwell 2009; Mertens 2009; Navarro‐Sarabia 2005; Ruiz Garcia 2011; Singh 2009; Singh 2010a; Singh 2010b; Singh 2011), but it can still be an issue in countries where the drugs are not yet accessible.
Why do patients with rheumatoid arthritis have anemia?
Rheumatoid arthritis is a chronic inflammatory state characterized by high circulating levels of interleukin‐6 (IL‐6) (Cronstein 2007; Dayer 2010; Fonseca 2009; Kishimoto 2006; Nicolaisen 2008). IL‐6 stimulates the hepatic production of hepcidin (Dayer 2010; Ganz 2009; Raj 2009; Zhang 2009), which is the main iron regulatory hormone (Andrews 2007; Andrews 2008; Lee 2009; Muñoz 2009; Nemeth 2003; Sasu 2010). This hormone causes inhibition of iron release from macrophages which generates iron sequestration (Ganz 2009; Nemeth 2004). It reduces the iron supply to erythropoiesis‐generating anemia called 'anemia of inflammation', which shows the interplay between iron and immune function (Demirag 2009; Ganz 2009; Jayaranee 2009; Roy 2005; Weiss 2009). This anemia is also known as 'anemia of chronic disease' (Agarwal 2009; Cartwright 1966; Means 1995; Theurl 2009; Masson 2011). The term 'anemia of inflammation' reflects its pathophysiology (Ganz 2009).
Other factors for explaining the pathogenesis of anemia in rheumatoid arthritis
Anemia in rheumatoid arthritis due to serum immunoreactive erythropoietin has been described (Hochberg 1988), and lower levels of serum erythropoietin in these patients may contribute to the pathogenesis of anemia (Baer 1997). Furthermore, tumour necrosis factor alpha, interleukin‐1 (IL‐1), and interferon‐gamma (IFN‐gamma) are factors mediating impaired erythropoiesis in anemia of chronic disease in active rheumatoid arthritis (Capocasale 2008; Moreland 2009; Papadaki 2002; Smith 1992; Vreugdenhil 1992a; Zhu 2000). Patients with rheumatoid arthritis and suffering with anemia have a decreased sensitivity of bone marrow erythroblasts to interleukin‐3, which has hematopoietic growth‐promoting activity (Jaworski 2008; Wu 2003). In summary, the pathophysiology of anemia in rheumatoid arthritis involves three mechanisms: disturbances of iron homeostasis, inhibition of erythroid progenitor of proliferation and differentiation, and blunted erythropoietin response (Weiss 2002).
Description of the intervention
Erythropoietin is a glycoprotein hormone that is produced in the kidney and acts through specific receptors on hematopoietic precursor cells to increase the production of red blood cells (Glaspy 2009).
Erythropoiesis‐Stimulating Agents (ESAs)
There are two types of erythropoiesis‐stimulating agents: recombinant human erythropoietin (alpha and beta) and darbepoetin alpha. Recombinant human erythropoietin is a hemopoietic growth factor that acts as a primary regulator of erythropoiesis. It is used for the treatment of chronic anemia associated with rheumatoid arthritis (Coussons 2005). Darbepoetin alpha is an analogue of recombinant human erythropoietin. Both agents share the same mechanism of action, but darbepoetin alpha has a three‐fold longer terminal half‐life (25.3 hours), after intravenous administration, than recombinant human erythropoietin (8.5 hours) (Cases 2003; Ibbotson 2001).
Why it is important to do this review
There are randomized controlled trials (Murphy 1994; Nordström 1997; Peeters 1996; Pincus 1990), clinical trials (Arndt 2005; Fantini 1992; Gudbjörnsson 1992; Kaltwasser 2001; Kato 1994; Pettersson 1993; Pettersson 1994; Salvarani 1991; Swaak 1994; Takashina 1990; Tauchi 1990; Vreugdenhil 1992b) and case reports (Krantz 1990; Means 1989) using human recombinant erythropoietin for treating anemia in rheumatoid arthritis. However, this drug was not approved for this indication. This systematic review is important for the following issues. 1. Anemia is a comorbidity with negative impact on quality of life in patients with rheumatoid arthritis (Han 2007; Michaud 2007). Low hemoglobin levels may be associated with rheumatoid arthritis disease severity and the presence of certain comorbidities (Furst 2009). 2. Anemia is associated with the severity of rheumatoid arthritis, and its successful treatment leads to a significant improvement in the quality of life scores in patients with rheumatoid arthritis (Wilson 2004). 3. The clinical effectiveness of erythropoiesis‐stimulating agents could be affected (hyporesponsiveness to erythropoietic therapy) by chronic inflammation (Macdougall 2005; van der Putten 2008). 4. Erythropoiesis‐stimulating agents have been associated with an increased risk of mortality and cardiovascular events in cancer patients (Bennet 2008; Hershman 2009), and in patients with chronic kidney disease (Unger 2010). Both erythropoiesis‐stimulating agents have been labelled with a warning by the Food and Drug Administration due to the following adverse events: increased mortality, serious cardiovascular and thromboembolic events, and increased risk of tumor progression or recurrence (FDA 2008a; FDA 2008b).
A systematic review, with rigorous assessment of the risk of bias, of the most up‐to‐date evidence will help clinicians make informed decisions about the use of erythropoiesis‐stimulating agents for treating anemia in patients with rheumatoid arthritis.
Objectives
To assess the clinical benefits and harms of erythropoiesis‐stimulating agents for anemia in rheumatoid arthritis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials irrespective of their publication status, language and country of origin. We included trials irrespective of their follow‐up duration.
Types of participants
We included patients aged 16 years and older, with a diagnosis of rheumatoid arthritis and affected by anemia.
Diagnosis of rheumatoid arthritis was based on the American College of Rheumatology criteria (ACR) (Arnett 1988). See Appendix 1
Diagnosis of anemia was based on the World Health Organization (WHO) criteria. See Appendix 2.
Types of interventions
Intervention
Erythropoiesis‐stimulating agents (epoetin (alpha or beta) or darbepoetin alpha) alone or in combination with oral or parenteral iron supplementation.
Control
Epoetin alpha compared with epoetin beta.
Darbepoetin alpha versus epoetin (alpha or beta).
Epoetin alpha or beta versus placebo or no medication.
Darbepoetin alpha versus placebo or no medication.
Any of the erythropoiesis‐stimulating agents versus standard treatment of anemia (Hematinics ‐ iron, folic acid, packed red cell blood transfusion, or both).
Any of the erythropoiesis‐stimulating agents plus iron, folic acid, or both, versus placebo or versus standard treatment of anemia.
We accepted as potentially eligible any trials that assessed any erythropoiesis‐stimulating agents regimen, in terms of route of administration (intravenous or subcutaneous), dosage, or treatment duration. We included trials that used hematinics as co‐interventions, administered by any route, dosage or treatment duration.
Types of outcome measures
Primary outcomes
Health‐related quality of life: “the degree to which persons perceive themselves able to function physically, emotionally, mentally, and socially” (Porta 2008). To be included in the review, trials had to use standardized scales (e.g. European League Against Rheumatism (EULAR), Disease Activity Score (DAS28), Health Assessment Questionnaire Disability Index (HAQ‐DI), Routine Assessment of Patient Index Data 3 (RAPID3), Clinical Disease Activity Index (CDAI)).
Fatigue (Kirwan 2007).
Safety:
Adverse events: "any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment" (Nebeker 2004);
Adverse drug reaction: "a response to a drug which is noxious and uninitiated and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions" (Nebeker 2004). (e.g. high blood pressure).
Secondary outcomes
Hemoglobin (Hb) level at the end of the study.
Withdrawals due to adverse events.
Withdrawals due to lack of efficacy.
Withdrawals due to high blood pressure.
Withdrawals due to high disease activity.
Withdrawals due to poor compliance.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (issue 7, 2012), Ovid MEDLINE and Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations (1948 to 7 August 2012), OVID EMBASE (1980 to 17 August 2012), and LILACS (1982 to 7 August 2012). See Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8 for full search strategies.
Searching other resources
We searched the Clinical Trials Search Portal of the World Health Organization (http://apps.who.int/trialsearch/) to identify ongoing and unpublished trials.
We also searched the reference lists of the retrieved publications and review articles. We did not apply any language restrictions.
Date of the most recent search: 7 August 2012.
Data collection and analysis
Selection of studies
AMC and DS screened the search results for potentially relevant trials, and assessed them independently. They resolved disagreements through discussion with LAP and IS to reach a consensus.
Data extraction and management
AMC and DS extracted the data using the agreed form, and resolved discrepancies through discussion. IS checked the data entered into Review Manager 5 (RevMan 2011) for accuracy.
Assessment of risk of bias in included studies
All review authors independently assessed the risk of bias for each included trial, using the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All review authors checked the assessments, discussed discrepancies and achieved consensus.
The definition of each domain classification is given below:
Generation of allocation sequence (checking for possible selection bias)
Low risk of bias, if the allocation sequence was generated by a computer or random number table, drawing of lots, tossing of a coin, shuffling of cards, or throwing dice.
Unclear risk of bias, if the trial was described as randomized but the method used for the allocation sequence generation was not described.
High risk of bias, if a system involving dates, names, or admittance numbers was used for the allocation of patients. These studies are known as quasi‐randomized and were excluded from the present review when assessing beneficial effects.
Allocation concealment (checking for possible selection bias)
Low risk of bias, if the allocation of patients involved a central independent unit, on‐site locked computer, identical‐appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear risk of bias, if the trial was described as randomized but the method used to conceal the allocation was not described.
High risk of bias, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomized. The latter was excluded from the present review when assessing beneficial effects.
Blinding or masking (checking for possible performance or detection bias)
We assessed the adequacy of blinding separately for participants, carers/personnel and outcome assessors.
Low risk of bias: participants, carers/personnel and/or outcome assessors blinded from knowledge of which intervention the participant received, or the lack of blinding could not have affected the results;
High risk of bias: participants, carers/personnel and/or outcome assessors were not blinded from the knowledge of which intervention the participant received and this could have affected the results;
Unclear risk of bias: the blinding of participants, carers/personnel and outcome assessors was not reported.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Low risk of bias (any one of the following): No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods.
High risk of bias (any one of the following): reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomizations; potentially inappropriate application of simple imputation.
Unclear risk of bias (any one of the following): insufficient reporting of attrition/exclusions to permit judgement of ‘low risk’ or ‘high risk’ (e.g. number randomized not stated, no reasons for missing data provided); the study did not address this outcome.
Selective reporting bias (Outcome reporting bias)
Low risk of bias (any one of the following): the study protocol is available and all the pre‐specified (primary and secondary) outcomes were reported in the final report, or the study protocol was not available but it was clear that the published reports included all expected outcomes.
High risk of bias (any one of the following): not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk of bias insufficient information available to permit judgement of ‘Low risk’ or ‘High risk’.
Other bias
Low risk of bias, the trial appeared to be free of other components that could put it at risk of bias.
Unclear risk of bias, the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias, there were other factors in the trial that could put it at risk of bias.
AMC entered the data into RevMan 5, and DS and IS checked them.
Measures of treatment effect
If we had conducted meta‐analyses, we would have used the following procedures (and will apply these for future updates, if possible):
Both health‐related quality of life and fatigue data would be analysed as continuous variables. We would present results as summary standardized mean differences (SMDs) with 95% confidence intervals (CIs).
For dichotomous data (safety and withdrawals), we would present results as summary risk ratios (RRs) with 95% CIs.
For hemoglobin levels, we have presented results as difference of means (MD) with 95% CIs.
Dealing with missing data
We would have used the following procedures (and will apply these for future updates, if possible):
We would have noted levels of attrition and explored the impact of high levels of missing data in the overall assessment of treatment effect by using sensitivity analyses.
For all outcomes we would have carried out analysis, as far as possible, on an intention‐to‐treat basis (i.e. we would have attempted to include all participants randomized to each group in the analyses). If intention‐to‐treat analysis had not been carried out, then we would have attempted a per‐protocol or complete case analysis. The denominator for each outcome in each trial would have been the number randomized minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We would have used the following procedures (and will apply these for future updates, if possible). We would have used the I2 statistic to measure statistical heterogeneity between the trials in each analysis. This describes the percentage of total variation across trials that is due to heterogeneity rather than to sampling error (Higgins 2003). We would have considered there to be substantial statistical heterogeneity if the I2 was greater than 50% (Higgins 2011), and would have explored this by prespecified subgroup analysis.
Assessment of reporting biases
We would have used the following procedures (and will apply these for future updates, if possible). When we suspected reporting bias (see ‘Selective reporting bias’ above), we would have attempted to contact study authors to obtain missing outcome data. When this was not possible, and if the missing data were thought to introduce serious bias, we would have used sensitivity analyses to explore the impact of including such studies in the overall assessment of results.
We would also have attempted to assess whether the review is subject to publication bias, by using a funnel plot to graphically illustrate variability between trials. If asymmetry were detected, we would have explored causes other than publication bias (e.g. selective outcome reporting, poor methodological quality in smaller studies, true heterogeneity) (Higgins 2011). In future updates we will construct a funnel plot, provided we have 10 or more randomized controlled trials (Sterne 2011).
Data synthesis
We would have used the procedures previously described (and will apply these for future updates, if possible). We would have carried out statistical analysis using the RevMan 5 software (RevMan 2011) and summarized the findings using a random‐effects model, as differences would have been anticipated in terms of interventions and patients. When I2 is greater than 50%, suggesting substantial heterogeneity, we may decide not to conduct meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We would have used the following procedures (and will apply these for future updates, if possible). We anticipated clinical heterogeneity for the following participant and intervention characteristics, and therefore we would have carried out the following subgroup analyses:
type of intervention (EPO versus darbepoetin)
duration of administration
gender
erythropoiesis‐stimulating agents with and without hematinics
year of publication.
We would have restricted subgroup analysis to primary outcomes only (Higgins 2011).
Sensitivity analysis
We would have used the following procedures (and will apply these for future updates, if possible). If sufficient trials are identified, we plan to conduct a sensitivity analysis comparing the results using all the included trials. We would have compared trials with high methodological quality (studies classified as having a 'low risk of bias') versus those of lower methodological quality (classified as having an 'unclear' or 'high risk of bias') (Higgins 2011).
We would also have evaluated the risk of attrition bias, as estimated by the percentage of participants lost to follow‐up. We would have excluded from the meta‐analysis trials with a total attrition of more than 20% or with between‐group differences in attrition exceeding 10%, but would still have included them in the review.
Summary of findings tables
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the body of evidence (Guyatt 2011b). The GRADE approach classifies the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the outcome being assessed (Balshem 2011; Guyatt 2011a; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h).
The Table 1, created with GRADE software (GRADEPro 2008), includes health‐related quality of life, fatigue, hemoglobin at the end of the study, adverse events, withdrawals due to adverse events, withdrawals due to lack of efficacy, and withdrawals due to high blood pressure.
Results
Description of studies
Results of the search
We identified 337 references using our search strategy (Figure 1). Three trials with a total of 133 participants met our inclusion criteria (Nordström 1997; Peeters 1996; Pincus 1990).
1.
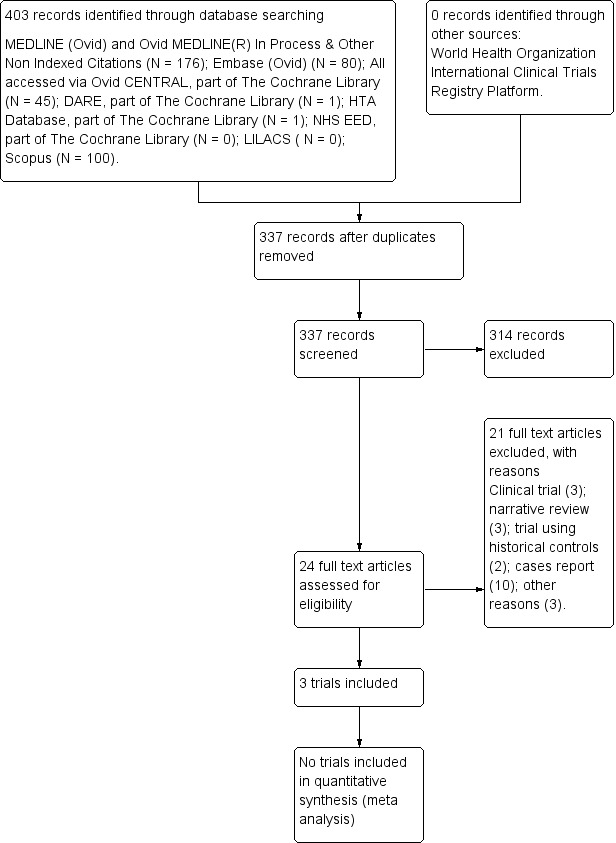
Study flow diagram (7 August 2012).
Included studies
Interventions and populations assessed in the trials
The three trials reported on EPO compared with placebo (Nordström 1997; Peeters 1996; Pincus 1990). Two trials used a subcutaneous route of administration (Nordström 1997; Peeters 1996), and one trial administered EPO by an intravenous route (Pincus 1990). The subcutaneous EPO regimen varied from 150 IU/kg‐body (Nordström 1997) to 240 IU/kg‐body (Peeters 1996). The intravenous EPO trial administered the intervention at 50 U/kg, 100 U/kg, and 150 U/kg doses (Pincus 1990). The mean percentage of female participants was 88.17 (± 5.09), with a mean age of 56.43 (± 3.38) years.
Location and timing of trials
The trials were published between 1990 and 1997. They were conducted in Sweden (Nordström 1997), the Netherlands (Peeters 1996), and the USA (Pincus 1990).
Trial methods
All three trials were conducted using a parallel study design. The trials were small with sample sizes ranging from 17 to 70, with a median sample size of 46 and a mean of 44.33 (± SD 26.54), and were conducted without a priori sample size estimation. The follow‐ups ranged from eight to 52 weeks. . Two trials were multicenter (Nordström 1997; Pincus 1990), and the setting unclear for Peeters 1996.
The Characteristics of included studies table shows a detailed description of the trials (Nordström 1997; Peeters 1996; Pincus 1990).
Excluded studies
Twenty‐one studies were excluded (Arndt 2005; Birgegard 1991; Dyjas 2005; Fantini 1992; Goodnough 1997; Kaltwasser 2001; Kato 1994; Krantz 1995; Matsuda 2001; Means 1989; Mercuriali 1996; Mercuriali 1997; Murphy 1994; Pettersson 1993a; Saikawa 1994; Salvarani 1991; Swaak 1994; Takashina 1990; Tauchi 1990; Vreugdenhil 1990; Vreugdenhil 1992). See Characteristics of excluded studies table for reasons for exclusion.
Risk of bias in included studies
The risks of bias in the included trials are summarised in Figure 2 and Figure 3, and are detailed in the Characteristics of included studies table.
2.
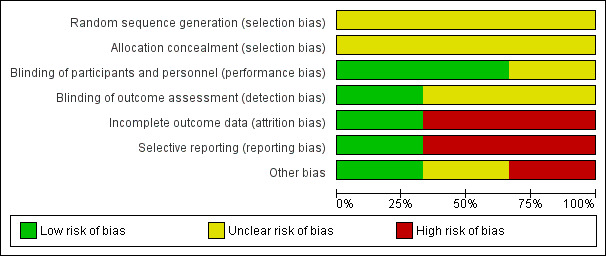
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
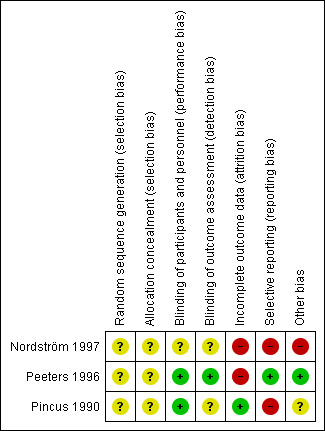
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random Sequence Generation The risk of bias arising from the method of generation of the allocation sequence was unclear in all trials (Nordström 1997; Peeters 1996; Pincus 1990). Allocation Concealment The risk of bias arising from the method of allocation concealment was unclear in all trials (Nordström 1997; Peeters 1996; Pincus 1990).
Blinding
The risk of bias due to blinding of participants and personnel was low in two trials because the drug preparations were covered to make them indistinguishable (Peeters 1996; Pincus 1990). The risk of bias was unclear in the remaining trial (Nordström 1997).
The risk of bias arising from lack of blinding of outcome assessment was low in one trial (Peeters 1996). The risk of bias was unclear in the remaining trials (Pincus 1990; Nordström 1997).
Incomplete outcome data
The risk of attrition bias was low in one trial (Pincus 1990). The risk of attrition bias was high in the remaining trials (Nordström 1997; Peeters 1996).
Selective reporting
The risk of reporting bias was low in one trial (Peeters 1996). The risk of reporting bias was high in the remaining trials (Nordström 1997; Pincus 1990).
Other potential sources of bias
One study (Nordström 1997) had high risk of bias for baseline imbalance between comparison groups for gender, duration of disease (years) and medication for treating ARA clinical variables. Pincus 1990 did not report baseline characteristics of the patients assigned to the placebo group, and there was no baseline imbalance in Peeters 1996. All included trials were suspected to have sponsorship bias, as they were funded by the pharmaceutical company, but we do not have enough information to permit judgment of 'low risk' or 'high risk' for inappropriate influence.
Effects of interventions
See: Table 1
Results are based on three randomized controlled trials (Nordström 1997; Peeters 1996; Pincus 1990). Table 1 shows evidence on outcomes reported by the trials.
Health‐related quality of life
Nordström 1997 reported a significant elevation of energy at the end of the study after week 12 (P = 0.004). Otherwise, Stanford Health Assessment Questionnaire scores, Nottingham Health Profile scores, classification of functional class or joint score index scores did not differ significantly between the comparison groups.
Peeters 1996, reporting patients' global assessment (VAS score 0 to 10), found statistically significant differences comparing the EPO group (median 3.5 and interquartile range 1.0 to 6.0) to placebo (median 4.5 and interquartile range 2.0 to 7.5) at 52 weeks (P = 0.027).
Pincus 1990 assessed patient satisfaction in activities of daily living, using the modified Stanford Health Assessment Questionnaire, and found no significant differences between comparison groups during the trial duration; however, it did not report supporting data.
Fatigue
This outcome was not assessed by any of the trials included in this review.
Safety (Adverse events including adverse drug reaction)
Nordström 1997 found no significant alterations in pulse, blood pressure, platelet counts, albumin, creatinine, alanine aminotransferase, or aspartate aminotransferase values. Peeters 1996 found no significant rise in blood pressure and thromboembolic complications. Pincus 1990 found no adverse events associated with EPO.
Hemoglobin level at the end of the study
Nordström 1997 (46 participants) reported a statistically significant increase in hemoglobin level at 12 weeks in the EPO group compared with the placebo group (mean difference (MD) 8.00, 95% confidence interval (CI) 7.43 to 8.57; P = 0.00001) .
Peeters 1996 (70 participants) reported a statistically significant increase in hemoglobin level at 52 weeks in the EPO group (median 134; interquartile range 110 to 158 g/litre) compared with the placebo group (median 112; interquartile range 86 to 128 g/litre; P = 0.001).
Pincus 1990 (17 participants) reported no significant difference between the EPO and placebo groups (MD 4.69, 95% CI ‐0.17 to 9.55; P = 0.06).
Withdrawals due to adverse events
Nordström 1997 reported no serious adverse effects causing premature discontinuation. This outcome was not reported by the other two trials (Peeters 1996; Pincus 1990).
Withdrawals due to lack of efficacy
This outcome was not assessed by the trials.
Withdrawals due to high blood pressure
This outcome was not assessed by the trials.
Withdrawals due to high disease activity
Peeters 1996, with eight events in 70 participants, reported no significant difference between the EPO and placebo groups (risk ratio (RR) 0.64, 95% CI 0.16 to 2.46, P = 0.51). The other two trials did not report this outcome (Nordström 1997; Pincus 1990).
Withdrawals due to poor compliance
Peeters 1996, with four events in 70 participants, reported no significant difference between the EPO and placebo groups (RR 1.06, 95% CI 0.16 to 7.10, P = 0.95). The other two trials did not report this outcome (Nordström 1997; Pincus 1990).
Discussion
Summary of main results
This review of erythropoiesis‐stimulating agents for treating anemia in patients with rheumatoid arthritis identified three randomized controlled trials incorporating 133 participants (Nordström 1997; Peeters 1996; Pincus 1990). Trials assessed EPO with different follow‐ups (eight weeks (Pincus 1990), 12 weeks (Nordström 1997) and 52 weeks (Peeters 1996)) compared with placebo. Two trials administered EPO by a subcutaneous route (Nordström 1997; Peeters 1996), and one used an intravenous route (Pincus 1990). The trials were conducted in developed countries (Sweden, the Netherlands, and the USA). Two trials found benefits for health‐related quality of life at 12 and 52 weeks (Nordström 1997; Peeters 1996). Two trials found benefits in increasing hemoglobin levels at 12 and 52 weeks (Nordström 1997; Peeters 1996). All trials had high risks of bias, were underpowered, and were sponsored by the pharmaceutical company. The safety profile of human recombinant erythropoietin is unclear over the course of the trials. See Table 1 for details.
Overall completeness and applicability of evidence
This Cochrane review provides inconclusive evidence on the assessed intervention, due both to heterogeneity between trials, and to inadequate information provided by trial reports (Hopewell 2010). During this review, we have identified the following issues, which we feel are particularly relevant to consider as further work is planned. Overall, heterogeneity between trials prevented the pooling of results, with the main areas of variation between trials being differences in outcome definition, and inconsistency of reported outcomes i.e., one trial reported hemoglobin level (Nordström 1997) and another trial reported hematocrit (Pincus 1990). It was not advisable to pool data on the health‐related quality of life and hemoglobin level at the end of the study, due to inconsistency in reporting the results of these outcomes. It has been suggested that trials should adopt an agreed set of core outcomes for each medical condition (Clarke 2007). This approach may reduce the impact of outcome reporting bias (Kirkham 2010). There is an international network initiated in 1992 aimed at improving outcome measurement in rheumatology, named 'Outcome Measures in Rheumatoid Arthritis Clinical Trials' (OMERACT) (Tugwell 2007). A standardized definition of rheumatoid arthritis flare is needed for research and clinical care that combines both clinician and patient perspectives (Alten 2011; Bartlett 2012; Bingham 2011).
Another approach to reduce the impact of outcome reporting bias may be to adopt the recommendations of the Patient‐Centered Outcomes Research Institute (PCORI) (PCORI 2012). It was established by the United States Congress as an independent, non‐profit organization, created to conduct research to provide information about the best available evidence to help patients and their healthcare providers make more informed decisions. PCORI’s research is intended to give patients a better understanding of the prevention, treatment and care options available, and the science that supports those options (Basch 2012; Gabriel 2012; PCORI 2012).
Quality of the evidence
The three randomized controlled trials included in this review all had a high risk of bias. The main source of bias was the lack of detail in describing the generation of randomization sequences or the allocation concealment. The trials also lacked detail about their blinding processes, but two of them described 'similar placebos' (Peeters 1996; Pincus 1990). Peeters 1996 was masked to outcome assessment. In this clinical setting, the potential harms of EPO are unknown, due to the lack of detail in presenting safety data. All the trials were underpowered, and were sponsored by the pharmaceutical industry, which reduces confidence in the results. Table 1 shows the quality of the evidence supplied by the included trials.
Potential biases in the review process
In the process of conducting a systematic review, there is a group of biases called significance‐chasing biases (Ioannidis 2010). These includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials. However, we believe that this Cochrane review has a low risk of publication bias, due to the thorough trial search process. Selective outcome reporting bias operates through suppression of information on specific outcomes and has similarities to study publication bias, in that ‘negative’ results remain unpublished (Ioannidis 2010). This Cochrane review found that two trials had high risk of selective outcome reporting (Nordström 1997; Pincus 1990).
Authors' conclusions
Implications for practice.
We found eligible randomized controlled trials only assessing human recombinant erythropoietin. They provide evidence of benefit in improving health‐related quality of life and in increasing hemoglobin levels. The results are based on trials with a high risk of bias, which were sponsored by the pharmaceutical company. The safety profile of human recombinant erythropoietin is unclear in this population. The trial results should therefore be treated with great caution.
Implications for research.
This review highlights a need for well‐designed, high‐quality randomized trials, with a priori calculations on sample sizes, to assess the benefits and harms of erythropoiesis‐stimulating agents for anemia in rheumatoid arthritis. The trials should include outcomes such as quality of life measures, fatigue, adverse events, and hemoglobin level at the end of the study. The trials should be conducted according to 'Outcome Measures in Rheumatoid Arthritis Clinical Trials' (OMERACT) recommendations for rheumatoid arthritis (Alten 2011; Bartlett 2012; Bingham 2011; Tugwell 2007) and the Patient‐Centered Outcomes Research Institute (PCORI) recommendations (Basch 2012; Gabriel 2012; PCORI 2012).
Rheumatoid arthritis is a chronic inflammatory state characterized by high circulating levels of interleukin‐6, which is the main factor causing anemia in this population; patients suffering from this disorder should therefore be treated with disease‐modifying antirheumatic drugs (DMARDs), but EPO could still be an issue in countries where DMARDs are not yet accessible. Potential trials for assessing benefits and harms of erythropoiesis‐stimulating agents to treat anemia in rheumatoid arthritis should be reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement for improving the quality of reporting of efficacy and of harms in clinical research (Ioannidis 2004; Moher 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 26 January 2010 | Amended | Change of authorship and new contact person. Criteria for inclusion and methods updated. |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 26 January 2010 | Amended | The protocol has been changed from all sections. |
| 10 September 2008 | Amended | CMSG ID: C122‐P |
| 10 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We want to express our gratitude to Elizabeth Ghogomu, Jordi Pardo, the peer‐reviewers and Editor for their comments and suggestions which improved the quality of this Cochrane review.
Appendices
Appendix 1. American Rheumatology criteria of rheumatoid arthritis
Source: Arnett 1988
Morning stiffness in and around joints lasting at least 1 hour before maximal improvement;
Soft tissue swelling (arthritis) of 3 or more joint areas observed by a physician;
Swelling (arthritis) of the proximal interphalangeal, metacarpophalangeal, or wrist joints;
Symmetric swelling (arthritis);
Rheumatoid nodules;
The presence of rheumatoid factor;
Radiographic erosions and/or periarticular osteopenia in hand and/or wrist joints.
Criteria 1 through 4 must have been present for at least 6 weeks. Rheumatoid arthritis is defined by the presence of 4 or more criteria, and no further qualifications (classic, definite, or probable) or list of exclusions are required.
Appendix 2. World Health Organization
Source: WHO 2008
2.1.‐ Hemoglobin: <120 g/l (12 g/dl) for non‐pregnant women (≥15 years).
2.2.‐ Hemoglobin: <130 g/l (13 g/dl) for men(≥15 years).
Appendix 3. Ovid Medline search strategy
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. bechterew$ disease.tw.
9. or/1‐8
10. exp Hematinics/
11. hematinic$.tw.
12. hematopoietic$.tw.
13. erythropoie$.tw.
14. (esa or esas).tw.
15. rhuepo.tw.
16. (epoetin adj (alpha or alfa or beta)).tw.
17. Abseamed.tw.
18. Binocrit.tw.
19. Culat.tw.
20. Dynepo.tw.
21. EPIAO.tw.
22. Epog?en.tw.
23. Epokine.tw.
24. Epomax.tw.
25. Epopen.tw.
26. Eporon.tw.
27. Eposino.tw.
28. Epotin.tw.
29. Epotrex.tw.
30. Epovitan.tw.
31. Epoxitin.tw.
32. Eprex.tw.
33. Erantin.tw.
34. Eritina.tw.
35. Eritrogen.tw.
36. Eritromax.tw.
37. Erypo.tw.
38. Exetin‐A.tw.
39. Globuren.tw.
40. Hemapo.tw.
41. Hemax‐Eritron.tw.
42. Hemax.tw.
43. Hemoprex.tw.
44. Hepta.tw.
45. Hypercrit.tw.
46. Mepotin.tw.
47. Mircera.tw.
48. Negortire.tw.
49. NeoRecormon.tw.
50. Procrit.tw.
51. Recormon.tw.
52. Renogen.tw.
53. Repotin.tw.
54. Retacrit.tw.
55. Silapo.tw.
56. Tinax.tw.
57. Wepox.tw.
58. Yepotin.tw.
59. or/10‐58
60. randomized controlled trial.pt.
61. controlled clinical trial.pt.
62. randomized.ab.
63. placebo.ab.
64. drug therapy.fs.
65. randomly.ab.
66. trial.ab.
67. groups.ab.
68. or/60‐67
69. (animals not (humans and animals)).sh.
70. 68 not 69
71. and/9,59,70
Appendix 4. Ovid EMBASE
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. or/1‐7
9. exp antianemic agent/
10. hematinic$.tw.
11. hematopoietic$.tw.
12. erythropoie$.tw.
13. (esa or esas).tw.
14. rhuepo.tw.
15. (epoetin adj (alpha or alfa or beta)).tw.
16. Abseamed.tw.
17. Binocrit.tw.
18. Culat.tw.
19. Dynepo.tw.
20. EPIAO.tw.
21. Epog?en.tw.
22. Epokine.tw.
23. Epomax.tw.
24. Epopen.tw.
25. Eporon.tw.
26. Eposino.tw.
27. Epotin.tw.
28. Epotrex.tw.
29. Epovitan.tw.
30. Epoxitin.tw.
31. Eprex.tw.
32. Erantin.tw.
33. Eritina.tw.
34. Eritrogen.tw.
35. Eritromax.tw.
36. Erypo.tw.
37. Exetin‐A.tw.
38. Globuren.tw.
39. Hemapo.tw.
40. Hemax‐Eritron.tw.
41. Hemax.tw.
42. Hemoprex.tw.
43. Hepta.tw.
44. Hypercrit.tw.
45. Mepotin.tw.
46. Mircera.tw.
47. Negortire.tw.
48. NeoRecormon.tw.
49. Procrit.tw.
50. Recormon.tw.
51. Renogen.tw.
52. Repotin.tw.
53. Retacrit.tw.
54. Silapo.tw.
55. Tinax.tw.
56. Wepox.tw.
57. Yepotin.tw.
58. or/9‐57
59. 8 and 58
60. (random$ or placebo$).ti,ab.
61. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
62. controlled clinical trial$.ti,ab.
63. RETRACTED ARTICLE/
64. or/60‐63
65. (animal$ not human$).sh,hw.
66. 64 not 65
67. 59 and 66
Appendix 5. LILACS search strategy
rheumatoid OR rheumatoid OR revmatoid OR rheumatic OR rheumatic OR revmatic OR reumat* OR revmarthrit* in Words
AND
hematinic* hematopoietic* or erythropoie* or rhuepo or epoetin in Words
Appendix 6. Scopus search strategy
#1 rheumatoid OR rheumatoid OR revmatoid OR rheumatic OR rheumatic OR revmatic OR reumat* OR revmarthrit*in TITLE‐ABS‐KEY
#2 hematinic* OR hematopoietic*OR erythropoie* OR rhuepo OR epoetin in TITLE‐ABS‐KEY
#3 #1 AND #2
#4 #3 limited to Conference Paper
Appendix 7. World Health Organization International Clinical Trials Registry Platform
#1 rheumatoid OR rheumatoid OR revmatoid OR rheumatic OR rheumatic OR revmatic OR reumat* OR revmarthrit*in Condition
#2 hematinic* OR hematopoietic*OR erythropoie* OR rhuepo OR epoetin in Intervention
Appendix 8. Ovid EBM Reviews
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. bechterew$ disease.tw.
9. or/1‐8
10. exp Hematinics/
11. hematinic$.tw.
12. hematopoietic$.tw.
13. erythropoie$.tw.
14. (esa or esas).tw.
15. rhuepo.tw.
16. (epoetin adj (alpha or alfa or beta)).tw.
17. Abseamed.tw.
18. Binocrit.tw.
19. Culat.tw.
20. Dynepo.tw.
21. EPIAO.tw.
22. Epog?en.tw.
23. Epokine.tw.
24. Epomax.tw.
25. Epopen.tw.
26. Eporon.tw.
27. Eposino.tw.
28. Epotin.tw.
29. Epotrex.tw.
30. Epovitan.tw.
31. Epoxitin.tw.
32. Eprex.tw.
33. Erantin.tw.
34. Eritina.tw.
35. Eritrogen.tw.
36. Eritromax.tw.
37. Erypo.tw.
38. Exetin‐A.tw.
39. Globuren.tw.
40. Hemapo.tw.
41. Hemax‐Eritron.tw.
42. Hemax.tw.
43. Hemoprex.tw.
44. Hepta.tw.
45. Hypercrit.tw.
46. Mepotin.tw.
47. Mircera.tw.
48. Negortire.tw.
49. NeoRecormon.tw.
50. Procrit.tw.
51. Recormon.tw.
52. Renogen.tw.
53. Repotin.tw.
54. Retacrit.tw.
55. Silapo.tw.
56. Tinax.tw.
57. Wepox.tw.
58. Yepotin.tw.
59. or/10‐58
71. 9 and 59
Appendix 9. Modified Paulus index
From Paulus 1990.
Ritchie index;
Number of swollen joints;
Duration of morning stiffness;
Patients' assessment of disease activity;
Observers' assessment of disease activity;
Erythrocyte sedimentation rate.
If responses in 4 of 6 selected measures were required for improvement (greater than or equal to 20% of above criteria), and by greater than or equal to 2 grades on a 5‐grade scale, or from grade 2 to grade 1 for patient's and physician's overall assessments of current disease severity).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Nordström 1997.
| Methods | 1. Design: parallel‐design (2 arms) 2. Country: Sweden (7 sites) 3. Intention‐to‐treat analysis: no 4. Follow‐up period: 12 weeks 5. Unit of randomization: patient 6. Unit of analysis: patient |
|
| Participants | 1. Randomized: 46 (Quote "three active, one placebo") (page 68)
Erythropoietin (EPO) group: 36
Placebo group: 10 2. Patients receiving drug: 46 EPO group: 36 (78.2%) Placebo group: 10 (21.7%) 3. Analyzed patients: EPO group: 26 (72.2%) Placebo group: 9 (90%) Imbalance between groups: 17.8% 4. Age (years; mean±SD; range) EPO group: 56.1±12.7; 25 to 76 Placebo group: 56.9±12.7; 29 to 79 5. Gender (female): Overall: 85% (39/46) EPO group: 80.5% (29/36) Placebo group: 100% (10/10) 6. Inclusion criteria:
7. Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not defined explicitly as primary or secondary:
|
|
| Notes | 1. Sample size calculation a priori: not reported.
2. Sponsor: Cilag AB Sweden.
3. Roll of sponsor: not specified. 4. Conflict of interest: not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was carried out in blocks of four patients (three active, one placebo) and allocation within each block was in random order” (page 68). Insufficient information to consider 'low risk' or 'high risk'. Comment: there is an imbalance in gender and duration of disease variables (page 69, table 1). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. This trial was reported as double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. This trial was reported as double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1. Lost postrandomization: 24% (11/46).
EPO group: 38.4% (10/26).
Placebo group: 11.1% (1/9).
2. Imbalance between comparison groups: 27.3% Quote: "(5 patients excluded due to entering the extension study, 5 drop‐outs) patients receiving rHuEPO over 9 patients receiving placebo (one drop out)" (page 70) 3. Comment: potentially inappropriate application of simple imputation. 4. Comment: For continuous outcome data, plausible effects size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size and potentially inappropriate application of simple imputation. |
| Selective reporting (reporting bias) | High risk | Quote: "A significant rise in Hb was noted in 26 (5 patients excluded due to entering the extension study, 5 drop‐outs) patients receiving rHuEPO over 9 patients receiving placebo (one drop‐out) (Hb elevation from 95 g/L to 107 g/L vs 93 g/L to 97 g/L, P < 0.05), from week 10 onwards that lasted throughout the study. A significant elevation of red blood cells was noted already from 4 weeks onward (P = 0.02) and Hct levels at week 12 (P = 0.009)" (page 70) Quote: "only 14.6% (N = 6) of the patients were considered responders according to the preset criteria of Hb level equaling or exceeding 120 g/l" (page 70). Quote "A significant elevation of energy (NHP variable) was noted at the end of the study after week 12 (P = 0.004). Otherwise, HAQ scores, NHP scores, classification of functional class or joint score index scores did not differ significantly between the two groups". (page 70). Comment: one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. |
| Other bias | High risk | Comments: 1. There is imbalance between comparison groups regarding gender, duration of disease (years) and medication for treating ARA variables (page 69). 2. There was a potential source of bias related to the specific study design used, with extreme baseline imbalance. |
Peeters 1996.
| Methods | 1. Design: parallel design (2 arms)
2. Country: Netherlands 3. Multicenter study: unclear. 4. Intention‐to‐treat analysis: yes 5. Per protocol analysis: yes 6. Follow‐up period: 52 weeks 7. Unit of randomization: patient 8.Unit of analysis: patient |
|
| Participants | 1. Randomized: 70
Erythropoietin (EPO) group: 34
Placebo group: 36 2. Age: years; median 5th to 95th centile EPO group: 56; 23 to 79 Placebo group: 58; 39 to 75 3. Gender (female): EPO group: 79% (27/34) Placebo group: 86% (31/36) 4. Inclusion criteria:
5. Exclusion criteria:
|
|
| Interventions | 1. Intervention group: recombinant human erythropoietin (rHuEPO ), 240 U per kg subcutaneous (sc) initially three times a week. Duration: 52 weeks. 2. Placebo: visually similar. Composition of placebo was not reported. 3. Co‐intervention: oral iron supplementation. Quote "Adjusment of the dose of disease modifying antirheumatic drugs or prednisone, as well as local measures, particularly intra‐articular corticosteroids injections, was not allowed during the study (page 740) |
|
| Outcomes | 1. Primary:
2. Secondary:
|
|
| Notes | 1. Sample size calculation a priori: not reported. 2. Sponsor: Dutch League against Rheumatism (Het Nationaal Rheuma Fonds). 3. Sponsor: Boehringer Mannheim provided rHuEPO. 4. Conflict of interest: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " randomisation of the patients...was done by a second independent observer" (page 740). Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Quote: 'Randomisation of the patients ... was done by a second independent observer.' Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo group received a visually similar placebo. After randomisation, patients in the placebo group were matched with patients in the treatment group and followed their treatment regime with respect to frequency of administration". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Disease activity measures were assessed by the first observer, who remained blinded for treatment schedules and laboratory results" (page 740). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1. Overall: 82.8% (58/70)
2. EPO group: 85% (29/34)
3. Placebo group: 80% (29/36) 4. Imbalance between groups: 5% Comment: Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. However, this was a small trial. |
| Selective reporting (reporting bias) | Low risk | This trial reported outcomes of clinical interest. |
| Other bias | Low risk | Quote: "At baseline, groups were compared using Student's t test, the Mann‐Whitney test, and the x' test where appropriate." There was no significant baseline imbalance. |
Pincus 1990.
| Methods | 1. Design: parallel design (2 arms).
2. Multicenter study: USA (5 sites).
3. Intention‐to‐treat analysis: yes.
4. Follow‐up period: 8 weeks.
5. Follow‐up period: 24 weeks open label. 6.‐ Unit of randomization: patients 7.‐ Unit of analysis: patient. |
|
| Participants | 1. Randomized: 17
Erythropoietin (EPO) group: 13
Placebo group: 4
2. Receiving EPO and placebo: 17
EPO group:13
Placebo group: 4 3. Age: years; mean range Both groups: 52; 25 to 73 By comparison group: not reported 4. Gender (female): Both group: 94.1% (16/17) By comparison group: not reported 5. Inclusion criteria: These were not reported explicitly. Quote: " All met the 1958 American Rheumatism Association criteria for rheumatoid arthritis... were being treated only with nonsteroidal anti‐inflammatory drugs and no patient was taking gold, methotrexate, azathioprine, penicillamine, or prednisone, or had taken any second‐line agents for 6 months prior to, or during, erythropoietin administration" (page 162). 6. Exclusion criteria:
|
|
| Interventions | 1. Intervention group: recombinant human erythropoietin rHuEPO (50, 100, 150 U per kg three times a week, intravenous bolus given over a 5 minute period for up to 8 weeks). 2. Placebo: schedule identical to intervention. Placebo composition was not reported. 3. Co‐intervention: oral ferrous sulphate (325 mg three times daily). |
|
| Outcomes | This trial did not explicitly address this domain. 1. Change in hematocrit, and leucocyte and platelet counts (page 163) 2. Safety (page 163) 3. Rheumatologic clinical status changes (page 165) |
|
| Notes | 1. Sample size calculation a priori: not reported.
2. Sponsor: Ortho Biotech Corporation, National Institutes of Health (grants DK‐1555, RR‐95, and T32‐07186), Jack C Massey Foundation, and Veterans Administration Medical Research Funds.
3. Role of sponsor: Ortho Biotech Corporation provided the intervention. 4.‐ This study had two phases: First phase: 8 weeks randomized double blind study. Second Phase: 24 week‐open‐label‐study "involved an open‐label protocol in which all patients received erythropoietin three times weekly" (page 163). Quote: "Following the 8 week randomized study, the 17 patients were offered the possibility of participations in a 24 week open‐label extension study" (page 162). 5. Conflict of interest: not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to consider 'low risk' or 'high risk'. Comment: there is imbalance between comparison groups regarding treated number of patients (13 treated with EPO and 4 with placebo). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to consider 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "identically appearing placebo" (page 162) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to consider 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely, so that they cannot be entered in a meta‐analysis. Comment: this trial reported only activities of daily living score and visual analog pain scale score data for intervention group. (page162). |
| Other bias | Unclear risk | Comment: this trial did not report baseline characteristics of the patients assigned to placebo group. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arndt 2005 | Clinical trial but not an RCT |
| Birgegard 1991 | Case report |
| Dyjas 2005 | Controlled clinical trial but not an RCT |
| Fantini 1992 | Case report |
| Goodnough 1997 | Narrative review of the responses to erythropoietin in patients with rheumatoid arthritis |
| Kaltwasser 2001 | Clinical trial but not an RCT |
| Kato 1994 | Trial was conducted using historical controls |
| Krantz 1995 | Narrative review of erythropoietin and the anemia of chronic disease |
| Matsuda 2001 | Trial included patients without anemia |
| Means 1989 | Case report |
| Mercuriali 1996 | Narrative review of erythropoietin alpha for autologous blood donations in patients with rheumatoid arthritis and concomitant anemia |
| Mercuriali 1997 | Trial was conducted using historical controls |
| Murphy 1994 | This study is shown as RCT involving 20 participants (10 assigned to EPO versus 10 randomized to placebo). Quote "Intervention group: recombinant human erythropoietin (rHuEPO) "starting at 40 IU per kg (patients 1‐10) and 100 U/Kg (patients 11‐20)" (page 1337), subcutaneous (sc), twice a week by 20 weeks". Therefore, it must be considered as non‐randomized. |
| Pettersson 1993a | Case report |
| Saikawa 1994 | Study including anemic and non‐anemic with rheumatoid arthritis. All participants received erythropoietin. |
| Salvarani 1991 | Case report |
| Swaak 1994 | Case report |
| Takashina 1990 | Case report |
| Tauchi 1990 | Case report |
| Vreugdenhil 1990 | Case report |
| Vreugdenhil 1992 | Case report |
Differences between protocol and review
1. Assessment of risk of bias of the included RCTs was adapted to the new Cochrane methodology recommendations. 2. We amended the terminology for number of withdrawals due to lack of efficacy, number of withdrawals due to high blood pressure, and number of withdrawals due to adverse events by withdrawals due to lack of efficacy, withdrawals due to high blood pressure, and withdrawals due to high disease activity. This was because these outcomes are binary. 3. We included withdrawals due to high disease activity and withdrawals due to poor compliance.
Contributions of authors
Conceiving the review (guarantor): Arturo Martí‐Carvajal (AMC)
Screening search results: AMC, Luís Agreda‐Pérez (LAP), Daniel Simancas (DS)
Screening retrieved papers against inclusion criteria: AMC
Appraising quality of papers: AMC, LAP, DS
Abstracting data from papers: AMC, LAP, Ivan Solà (IS).
Data management for the review: AMC, LAP
Entering data into RevMan 5.1: AMC, IS
Double entry of data: AMC, DS, IS.
Interpretation of data: AMC, IS, LAP, DS.
Statistical analysis: AMC, IS
Writing the review: AMC
Comment and editing of review drafts: AMC, IS, LAP, DS.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Iberoamerican Cochrane Centre, Spain.
Academic.
-
Cochrane Musculoskeletal Group, Canada.
Academic.
Declarations of interest
In 2004 Arturo Martí‐Carvajal was employed by Eli Lilly to run a four‐hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007 Arturo Martí‐Carvajal was employed by Merck to run a four‐hour workshop 'How to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Ivan Solà, Luís Agreda‐Pérez and Daniel Simancas: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Nordström 1997 {published data only}
- Nordström D, Lindroth Y, Marsal L, Hafstrom I, Henrich C, Rantapaa‐Dahlqvist S, et al. Availability of iron and degree of inflammation modifies the response to recombinant human erythropoietin when treating anemia of chronic disease in patients with rheumatoid arthritis. Rheumatology International 1997;17(2):67‐73. [PUBMED: 9266623] [DOI] [PubMed] [Google Scholar]
Peeters 1996 {published data only}
- Peeters HR, Jongen‐Lavrencic M, Bakker CH, Vreugdenhil G, Breedveld FC, Swaak AJ. Recombinant human erythropoietin improves health‐related quality of life in patients with rheumatoid arthritis and anaemia of chronic disease; utility measures correlate strongly with disease activity measures. Rheumatology International 1999;18(5‐6):201‐6. [PUBMED: 10399796] [DOI] [PubMed] [Google Scholar]
- Peeters HR, Jongen‐Lavrencic M, Vreugdenhil G, Swaak AJ. Effect of recombinant human erythropoietin on anaemia and disease activity in patients with rheumatoid arthritis and anaemia of chronic disease: a randomized placebo controlled double blind 52 weeks clinical trial. Annals of the Rheumatic Diseases 1996;55(10):739‐44. [PUBMED: 8984939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 1990 {published data only}
- Pincus T, Olsen NJ, Russell IJ, Wolfe F, Harris ER, Schnitzer TJ, et al. Multicenter study of recombinant human erythropoietin in correction of anemia in rheumatoid arthritis. The American Journal of Medicine 1990;89(2):161‐8. [PUBMED: 2200263] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arndt 2005 {published data only}
- Arndt U, Kaltwasser JP, Gottschalk R, Hoelzer D, Moller B. Correction of iron‐deficient erythropoiesis in the treatment of anemia of chronic disease with recombinant human erythropoietin. Annals of Hematology 2005;84(3):159‐66. [PUBMED: 15565327] [DOI] [PubMed] [Google Scholar]
Birgegard 1991 {published data only}
- Birgegard G, Gudbjronsson B, Hallgren R, Wide L. Anemia of chronic inflammatory arthritides: treatment with recombinant human erythropoietin. Contributions to Nephrology 1991;88:295‐303; discussion 304‐5. [PUBMED: 2040192] [PubMed] [Google Scholar]
Dyjas 2005 {published data only}
- Dyjas R, Bulanowski M, Ficek R, Witkowicz J, Chudek J, Wiecek A. [Influence of recombinant human erythropoietin (rHuEPO) on plasma levels of selected hormones in females with rheumatoid arthritis] [Wplyw leczenia niedokrwistosci za pomoca ludzkiej erytropoetyny uzyskanej metodt rekombinacji genetycznej (rHuEPO) na stezenie w osoczu wybranych hormonow u kobiet chorych na reumatoidalne zapalenie stawow.]. Polskie Archiwum Medycyny Wewnetrznej 2005;114(2):731‐7. [PUBMED: 16808310] [PubMed] [Google Scholar]
Fantini 1992 {published data only}
Goodnough 1997 {published data only}
- Goodnough LT, Marcus RE. The erythropoietic response to erythropoietin in patients with rheumatoid arthritis. The Journal of Laboratory and Clinical Medicine 1997;130(4):381‐6. [PUBMED: 9358076] [DOI] [PubMed] [Google Scholar]
Kaltwasser 2001 {published data only}
- Kaltwasser JP, Kessler U, Gottschalk R, Stucki G, Moller B. Effect of recombinant human erythropoietin and intravenous iron on anemia and disease activity in rheumatoid arthritis. The Journal of Rheumatology 2001;28(11):2430‐6. [PUBMED: 11708414] [PubMed] [Google Scholar]
Kato 1994 {published data only}
- Kato Y, Takagi C, Tanaka J, Masaki Y, Furuya H. Effect of daily subcutaneous administration of recombinant erythropoietin on chronic anemia in rheumatoid arthritis. Internal Medicine (Tokyo, Japan) 1994;33(4):193‐7. [PUBMED: 8069012] [DOI] [PubMed] [Google Scholar]
Krantz 1995 {published data only}
- Krantz SB. Erythropoietin and the anaemia of chronic disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association ‐ European Renal Association 1995;10 Suppl 2:10‐7. [PUBMED: 7644100] [DOI] [PubMed] [Google Scholar]
Matsuda 2001 {published data only}
- Matsuda S, Kondo M, Mashima T, Hoshino S, Shinohara N, Sumida S. Recombinant human erythropoietin therapy for autologous blood donation in rheumatoid arthritis patients undergoing total hip or knee arthroplasty. Orthopedics 2001;24(1):41‐4. [PUBMED: 11199350] [DOI] [PubMed] [Google Scholar]
Means 1989 {published data only}
- Means RT Jr, Olsen NJ, Krantz SB, Dessypris EN, Graber SE, Stone WJ, et al. Treatment of the anemia of rheumatoid arthritis with recombinant human erythropoietin: clinical and in vitro studies. Arthritis and Rheumatism 1989;32(5):638‐42. [PUBMED: 2719734] [DOI] [PubMed] [Google Scholar]
Mercuriali 1996 {published data only}
- Mercuriali F. Epoetin alfa for autologous blood donation in patients with rheumatoid arthritis and concomitant anemia. Seminars in Hematology 1996;33(2 Suppl 2):18‐20; discussion 21. [PUBMED: 8723576] [PubMed] [Google Scholar]
Mercuriali 1997 {published data only}
- Mercuriali F, Inghilleri G, Biffi E, Colotti MT, Vinci A, Sinigaglia L, et al. Comparison between intravenous and subcutaneous recombinant human erythropoietin (Epoetin alfa) administration in presurgical autologous blood donation in anemic rheumatoid arthritis patients undergoing major orthopedic surgery. Vox Sanguinis 1997;72(2):93‐100. [PUBMED: 9088076] [DOI] [PubMed] [Google Scholar]
- Mercuriali F, Gualtieri G, Biffi E, Inghilleri G, Colotti M, Vinci A, et al. Intravenous vs subcutaneous erythropoietin for autologous blood donation in anemic rheumatoid arthritis patients. Transfusion Supplement. 1994; Vol. 34. [DOI] [PubMed]
- Mercuriali F, Gualtieri G, Inghilleri G, Biffi E, Vinci A, Colotti M, et al. Erythropoietin and autologous blood donation in surgical rheumatoid arthritis patients with anemia. Transfusion Abstract. Florida, 1993; Vol. 33, issue 28.
Murphy 1994 {published data only}
- Murphy EA, Bell AL, Wojtulewski J, Brzeski M, Madhok R, Capell HA. Study of erythropoietin in treatment of anaemia in patients with rheumatoid arthritis. BMJ 1994;309(6965):1337‐8. [PUBMED: 7866082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pettersson 1993a {published data only}
- Pettersson T, Rosenlof K, Friman C, Mickos A, Teppo A M, Fyhrquist F. Successful treatment of the anemia of rheumatoid arthritis with subcutaneously administered recombinant human erythropoietin. Slower response in patients with more severe inflammation. Scandinavian Journal of Rheumatology 1993;22(4):188‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saikawa 1994 {published data only}
- Saikawa I, Hotokebuchi T, Arita C, Inaba S, Shuto T, Tsutsui H, et al. Autologous blood transfusion with recombinant erythropoietin treatment. 22 arthroplasties for rheumatoid arthritis. Acta Orthopaedica Scandinavica 1994;65(1):15‐9. [PUBMED: 8154276] [DOI] [PubMed] [Google Scholar]
Salvarani 1991 {published data only}
- Salvarani C, Lasagni D, Casali B, Macchioni P, Boiardi L, Rossi F, et al. Recombinant human erythropoietin therapy in patients with rheumatoid arthritis with the anemia of chronic disease. The Journal of Rheumatology 1991;18(8):1168‐71. [PUBMED: 1941817] [PubMed] [Google Scholar]
Swaak 1994 {published data only}
- Swaak AJ, Nieuwenhuizen C, Vreugdenhil G. Recombinant human erythropoietin (r‐hu‐EPO) treatment in patients with rheumatoid arthritis and anaemia of chronic disease (ACD). Clinical and Experimental Rheumatology 1994; Vol. 12, issue 5:577. [PUBMED: 7842542] [PubMed]
Takashina 1990 {published data only}
- Takashina N, Kondo H, Kashiwazaki S. Suppressed serum erythropoietin response to anemia and the efficacy of recombinant erythropoietin in the anemia of rheumatoid arthritis. The Journal of Rheumatology 1990;17(7):885‐7. [PUBMED: 2213753] [PubMed] [Google Scholar]
Tauchi 1990 {published data only}
Vreugdenhil 1990 {published data only}
- Vreugdenhil G, Swaak AJ. The role of erythropoietin in the anaemia of chronic disease in rheumatoid arthritis. Clinical Rheumatology 1990;9(1):22‐7. [PUBMED: 2185909] [DOI] [PubMed] [Google Scholar]
Vreugdenhil 1992 {published data only}
- Vreugdenhil G, Manger B, Nieuwenhuizen C, Feelders RA, Eijk HG, Swaak AJ. Iron stores and serum transferrin receptor levels during recombinant human erythropoietin treatment of anemia in rheumatoid arthritis. Annals of Hematology 1992;65(6):265‐8. [PUBMED: 1457588] [DOI] [PubMed] [Google Scholar]
Additional references
Agarwal 2009
- Agarwal N, Prchal JT. Anemia of chronic disease (anemia of inflammation). Acta Haematologica 2009;122:103‐8. [PUBMED: 19907147] [DOI] [PubMed] [Google Scholar]
Al‐Ghamdi 2009
- Al‐Ghamdi A, Attar SM. Extra‐articular manifestations of rheumatoid arthritis: a hospital‐based study. Annals of Saudí Medicine 2009;29:189‐93. [PUBMED: 19448378] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alten 2011
- Alten R, Pohl C, Choy EH, Christensen R, Furst DE, Hewlett SE, et al. Developing a construct to evaluate flares in rheumatoid arthritis: a conceptual report of the OMERACT RA Flare Definition Working Group. The Journal of Rheumatology 2011; Vol. 38, issue 8:1745‐50. [PUBMED: 21807796] [DOI] [PubMed]
Andrews 2007
- Andrews NC, Schmidt PJ. Iron homeostasis. Annual Review of Physiology 2007;69:69‐85. [PUBMED: 17014365] [DOI] [PubMed] [Google Scholar]
Andrews 2008
- Andrews NC. Forging a field: the golden age of iron biology. Blood 2008;112:219‐30. [PUBMED: 18606887] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31:315‐24. [PUBMED: 3358796] [DOI] [PubMed] [Google Scholar]
Baer 1997
- Baer AN, Dessypris EN, Goldwasser E, Krantz SB. Blunted erythropoietin response to anaemia in rheumatoid arthritis. British Journal of Haematology 1987;66:599‐64. [PUBMED: 3663512] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bartlett 2012
- Bartlett SJ, Hewlett S, Bingham CO 3rd, Woodworth TG, Alten R, Pohl C, et al. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Annals of the Rheumatic Diseases Jul 6. [Epub ahead of print]. [PUBMED: 22772326] [DOI] [PubMed]
Basch 2012
- Basch E, Aronson N, Berg A, Flum D, Gabriel S, Goodman SN, et al. Methodological standards and patient‐centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307(15):1636‐40. [PUBMED: 22511692] [DOI] [PubMed] [Google Scholar]
Bennet 2008
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia. JAMA 2008;299:914‐24. [PUBMED: 18314434 ] [DOI] [PubMed] [Google Scholar]
Bingham 2011
Blumenauer 2002
- Blumenauer BBTB, Judd M, Wells GA, Burls A, Cranney A, Hochberg MC, et al. Infliximab for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenauer 2003
- Blumenauer BBTB, Cranney A, Burls A, Coyle D, Hochberg MC, Tugwell P, Wells GA. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004525] [DOI] [PubMed] [Google Scholar]
Capocasale 2008
- Capocasale RJ, Makropoulos DA, Achuthanandam R, Stowell N, Quinn J, Rafferty PA, et al. Myelodysplasia and anemia of chronic disease in human tumor necrosis factor‐alpha transgenic mice. Cytometry A 2008;73:148‐59. [PUBMED: 18205195] [DOI] [PubMed] [Google Scholar]
Cartwright 1966
- Cartwright GE. The anemia of chronic disorders. Seminars in Hematology 1966;3:351‐75. [PUBMED: 5341723] [PubMed] [Google Scholar]
Cases 2003
- Cases A. Darbepoetin alfa: a novel erythropoiesis‐stimulating protein. Drugs of Today (Barc) 2003;39:477‐95. [PUBMED: 12973399] [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8:39. [PUBMED: 18039365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coussons 2005
- Coussons PJ, Baig S, Fanutti C, Grant R. Novel tissue remodelling roles for human recombinant erythropoietin. Biochemical Society Transactions 2005;33 (Pt 5):1129‐30. [PUBMED: 16246063] [DOI] [PubMed] [Google Scholar]
Cronstein 2007
- Cronstein BN. Interleukin‐6‐‐a key mediator of systemic and local symptoms in rheumatoid arthritis. Bulletin of the NYU Hospital for Joint Diseases 2007;65 (Suppl 1):11‐5. [PUBMED: 17708739 ] [PubMed] [Google Scholar]
Dayer 2010
- Dayer JM, Choy E. Therapeutic targets in rheumatoid arthritis: the interleukin‐6 receptor. Rheumatology (Oxford) 2010;49:15‐24. [PUBMED: 19854855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demirag 2009
- Demirag MD, Haznedaroglu S, Sancak B, Konca C, Gulbahar O, Ozturk MA, et al. Circulating hepcidin in the crossroads of anemia and inflammation associated with rheumatoid arthritis. Internal Medicine 2009;48:421‐6. [PUBMED: 19293540] [DOI] [PubMed] [Google Scholar]
FDA 2008a
- Food, Drug Administration. EPOGEN (epoetin alfa) solution. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=9169 [Accessed January 29, 2010].
FDA 2008b
- Food, Drug Administration. Erythropoiesis Stimulating Agents: Aranesp (darbepoetin alfa), Epogen (epoetin alfa), and Procrit (epoetin alfa). http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110251.htm March 07, 2008 (Accessed Jan 29, 2010).
Fonseca 2009
- Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin‐6 as a key player in systemic inflammation and joint destruction. Autoimmunity Reviews 2009;8:538‐42. [PUBMED: 19189867] [DOI] [PubMed] [Google Scholar]
Furst 2009
- Furst DE, Chang H, Greenberg JD, Ranganath VK, Reed G, Ozturk ZE, et al. Prevalence of low hemoglobin levels and associations with other disease parameters in rheumatoid arthritis patients: evidence from the CORRONA registry. Clinical and Experimental Rheumatology 2009;27:560‐6. [PUBMED: 19772785] [PubMed] [Google Scholar]
Gabriel 2012
Ganz 2009
- Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Seminars in Hematology 2009;46:387‐93. [PUBMED: 19786207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glaspy 2009
- Glaspy 2009. Erythropoietin in cancer patients. Annual Review of Medicine 2009;60:181‐92. [PUBMED: 18980468] [DOI] [PubMed] [Google Scholar]
GRADEPro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows 2008.
Grassi 1998
- Grassi W, Angelis R, Lamanna G, Cervini C. The clinical features of rheumatoid arthritis. European Journal of Radiology 1998;27 (Suppl 1):18‐24. [PUBMED: 9652497] [DOI] [PubMed] [Google Scholar]
Gudbjörnsson 1992
- Gudbjörnsson B, Hällgren R, Wide L, Birgegård G. Response of anaemia in rheumatoid arthritis to treatment with subcutaneous recombinant human erythropoietin. Annals of Rheumatic Diseases 1992;51:747‐52. [PUBMED: 1616357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Han 2007
- Han C, Rahman MU, Doyle MK, Bathon JM, Smolen J, Kavanaugh A, et al. Association of anemia and physical disability among patients with rheumatoid arthritis. Journal of Rheumatology 2007;34:2177‐82. [PUBMED: 17937474] [PubMed] [Google Scholar]
Hershman 2009
- Hershman DL, Buono DL, Malin J, McBride R, Tsai WY, Neugut AI. Patterns of use and risks associated with erythropoiesis‐stimulating agents among Medicare patients with cancer. Journal of the National Cancer Institute 2009;101:1633‐41. [PUBMED: 19903808 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.5 [updated September 29th, 2009]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hochberg 1988
- Hochberg MC, Arnold CM, Hogans BB, Spivak JL. Serum immunoreactive erythropoietin in rheumatoid arthritis: impaired response to anemia. Arthritis and Rheumatism 1988;31:1318‐21. [PUBMED: 3178910] [DOI] [PubMed] [Google Scholar]
Hochberg 2008
- Hochberg MC, Johnston SS, John AK. The incidence and prevalence of extra‐articular and systemic manifestations in a cohort of newly‐diagnosed patients with rheumatoid arthritis between 1999 and 2006. Current Medical Research and Opinion 2008;24:469‐80. [PUBMED: 18179735 ] [DOI] [PubMed] [Google Scholar]
Hopewell 2010
- Hopewell S, Clarke M, Higgins JPT (editors). Core reporting of outcomes in effectiveness trials. Cochrane Methods. Cochrane Database of Systematic Reviews 2010; Vol. Suppl 1:1‐29. [ISSN 2044‐4702]
Ibbotson 2001
- Ibbotson T, Goa KL. Darbepoetin alfa. Drugs 2001;61:2097‐104. [PUBMED: 11735636] [DOI] [PubMed] [Google Scholar]
Imboden 2009
- Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annual Review of Pathology 2009;4:417–34. [PUBMED: 18954286] [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Ioannidis 2010
- Ioannidis JP. The art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. [DOI] [PubMed] [Google Scholar]
Jaworski 2008
- Jaworski J, Maslinski W, Pazdur J, Sliwinska‐Stanczyk P, Kaminska‐Tchorzewska E, Jung L, et al. Decreased expression of integrins by hematopoietic cells in patients with rheumatoid arthritis and anemia: relationship with bone marrow cytokine levels. Journal of Investigational Allergology & Clinical Immunology 2008;18:17‐21. [PUBMED: 18361097] [PubMed] [Google Scholar]
Jayaranee 2009
- Jayaranee S, Sthaneshwar P, Sokkalingam S. Serum prohepcidin concentrations in rheumatoid arthritis. Pathology 2009;41:178‐82. [PUBMED: 18972320] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomized controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [PUBMED: 20156912] [DOI] [PubMed] [Google Scholar]
Kirwan 2007
- Kirwan JR, Minnock P, Adebajo A, Bresnihan B, Choy E, Wit M, et al. Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. Journal of Rheumatology 2007;34:1174‐7. [PUBMED: 17477482] [PubMed] [Google Scholar]
Kishimoto 2006
- Kishimoto T. Interleukin‐6: from basic science to medicine‐‐40 years in immunology. Annual Review of Immunology 2005;23:1‐21. [PUBMED: 15771564 ] [DOI] [PubMed] [Google Scholar]
Krantz 1990
- Krantz SB, Sawyer ST, Sawada K, Wolfe F, Bocagno J. Erythropoietin: receptors and clinical use in rheumatoid arthritis. International Journal of Cell Cloning 1990;8 (Suppl 1):181‐95. [DOI] [PubMed] [Google Scholar]
Lee 2001
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358:903‐11. [PUBMED: 11567728] [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee PL, Beutler E. Regulation of hepcidin and iron‐overload disease. Annual Review of Pathology 2009;4:489‐515. [PUBMED: 19400694] [DOI] [PubMed] [Google Scholar]
Macdougall 2005
- Macdougall IC, Cooper AC. Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. European Journal of Clinical Investigation 2005;35 (Suppl 3):32‐5. [PUBMED: 16281956] [DOI] [PubMed] [Google Scholar]
Masson 2011
- Masson C. Rheumatoid anemia. Joint, Bone, Spine 2011;78(2):131‐7. [PUBMED: 20851655] [DOI] [PubMed] [Google Scholar]
Maxwell 2009
- Maxwell L, Singh JA. Abatacept for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2011
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England Journal of Medicine 2011;365(23):2205‐19. [PUBMED: 22150039] [DOI] [PubMed] [Google Scholar]
Means 1995
- Means RT. Pathogenesis of the anemia of chronic disease: a cytokine‐mediated anemia. Stem Cells 1995;13:32‐7. [PUBMED: 7719246] [DOI] [PubMed] [Google Scholar]
Mertens 2009
- Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005121.pub3] [DOI] [PubMed] [Google Scholar]
Michaud 2007
- Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Practice & Research. Clinical Rheumatology 2007;21:885‐906. [PUBMED: 17870034] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. BMJ 2010;340:c869. [PUBMED: 20332511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreland 2009
- Moreland LW, Curtis JR. Systemic nonarticular manifestations of rheumatoid arthritis: focus on inflammatory mechanisms. Seminars in Arthritis and Rheumatism 2009;39:132‐43. [PUBMED: 19022481] [DOI] [PubMed] [Google Scholar]
Muñoz 2009
- Muñoz M, Villar I, García‐Erce JA. An update on iron physiology. World Journal of Gastroenterology 2009;15:4617‐26. [PUBMED: 19787824 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Navarro‐Sarabia 2005
- Navarro‐Sarabia F, Ariza‐Ariza R, Hernandez‐Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005113.pub2] [DOI] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140:795‐801. [PUBMED: 15148066] [DOI] [PubMed] [Google Scholar]
Nemeth 2003
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematologica 2003;122:78‐86. [PUBMED: 19907144 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nemeth 2004
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL‐6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. Journal of Clinical Investigation 2004;113:1271‐6. [PUBMED: 15124018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolaisen 2008
- Nikolaisen C, Figenschau Y, Nossent JC. Anemia in early rheumatoid arthritis is associated with interleukin 6‐mediated bone marrow suppression, but has no effect on disease course or mortality. Journal of Rheumatology 2008;35:380‐6. [PUBMED: 18260177] [PubMed] [Google Scholar]
Papadaki 2002
- Papadaki HA, Kritikos HD, Gemetzi C, Koutala H, Marsh JC, Boumpas DT, et al. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha‐mediated effect. Blood 2002;99:1610‐9. [PUBMED: 11861275] [DOI] [PubMed] [Google Scholar]
Paulus 1990
- Paulus HE, Egger MJ, Ward JR, Williams HJ. Analysis of improvement in individual rheumatoid arthritis patients treated with disease‐modifying antirheumatic drugs, based on the findings in patients treated with placebo. The Cooperative Systematic Studies of Rheumatic Diseases Group. Arthritis and rheumatism 1990;33(4):477‐84. [PUBMED: 2109613] [DOI] [PubMed] [Google Scholar]
PCORI 2012
- Patient‐Centered Outcomes Research Institute (PCORI). Preliminary draft methodology report: “Our questions, our decisions: Standards for patient‐centered outcomes research”. http://www.pcori.org/assets/Preliminary‐Draft‐Methodology‐Report.pdf (accessed 27 July 2012):1‐61.
Pettersson 1993
- Pettersson T, Rosenlöf K, Friman C, Mickos A, Teppo AM, Fyhrquist F. Successful treatment of the anemia of rheumatoid arthritis with subcutaneously administered recombinant human erythropoietin. Slower response in patients with more severe inflammation. Scandinavian Journal of Rheumatology 193;22:188‐93. [PUBMED: 8356412] [DOI] [PubMed] [Google Scholar]
Pettersson 1994
- Pettersson T, Rosenlöf K, Laitinen E, Tenhunen R. Effect of exogenous erythropoietin on haem synthesis in anaemic patients with rheumatoid arthritis. British Journal of Rheumatology 1994;33:526‐9. [PUBMED: 8205399] [DOI] [PubMed] [Google Scholar]
Porta 2008
- Porta M. A Dictionary of Epidemiology. New York: Oxford University Press, 2008. [ ISBN 978‐0‐19‐531449‐6] [Google Scholar]
Raj 2009
- Raj DS. Role of interleukin‐6 in the anemia of chronic disease. Seminars in Arthritis and Rheumatism 2009;38:382‐8. [PUBMED: 18336871] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Roy 2005
- Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Current Opinion in Hematology 2005;12:105‐11. [PUBMED: 15725899] [DOI] [PubMed] [Google Scholar]
Ruiz Garcia 2011
- Ruiz Garcia V, Jobanputra P, Burls A, Cabello JB, Gálvez Muñoz JG, Saiz Cuenca ESC, et al. Certolizumab pegol (CDP870) for rheumatoid arthritis in adults. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD007649.pub2] [DOI] [PubMed] [Google Scholar]
Sasu 2010
- Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, et al. Anti‐hepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation‐induced anemia. Blood 2010;115(17):3616‐24. [PUBMED: 20053755] [DOI] [PubMed] [Google Scholar]
Singh 2009
- Singh JA, Christensen R, Wells GA, Suarez‐Almazor ME, Buchbinder R, Lopez‐Olivo MA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2010a
- Singh JA, Noorbaloochi S, Singh G. Golimumab for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008341] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2010b
- Singh JA, Beg S, Lopez‐Olivo MA. Tocilizumab for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD008331.pub2] [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, MacDonald JK, et al. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008794.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1992
- Smith MA, Knight SM, Maddison PJ, Smith JG. Anaemia of chronic disease in rheumatoid arthritis: effect of the blunted response to erythropoietin and of interleukin 1 production by marrow macrophages. Annals of the Rheumatic Diseases 1992;51:753‐7. [PUBMED: 1616358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomized controlled trials. BMJ 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Theurl 2009
- Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 2009;113:5277‐86. [PUBMED: 19293425 ] [DOI] [PubMed] [Google Scholar]
Tugwell 2007
- Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials 2007;8:38. [PUBMED: 18039364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turesson 2003
- Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra‐articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Annals of the Rheumatic Diseases 2003;62:722‐7. [PUBMED: 12860726] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unger 2010
- Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis‐stimulating agents‐‐time for a reevaluation. New England Journal of Medicine 2010;362:189‐92. [PUBMED: 20054037] [DOI] [PubMed] [Google Scholar]
van der Putten 2008
- Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nature Clinical Practice. Nephrology 2008;4(1):47‐57. [PUBMED: 18094727] [DOI] [PubMed] [Google Scholar]
Vreugdenhil 1992a
- Vreugdenhil G, Löwenberg B, Eijk HG, Swaak AJ. Tumor necrosis factor alpha is associated with disease activity and the degree of anemia in patients with rheumatoid arthritis. European Journal of Clinical Investigation 1992;22:488‐93. [PUBMED: 1516597] [DOI] [PubMed] [Google Scholar]
Vreugdenhil 1992b
- Vreugdenhil G, Manger B, Nieuwenhuizen C, Feelders RA, Eijk HG, Swaak AJ. Iron stores and serum transferrin receptor levels during recombinant human erythropoietin treatment of anemia in rheumatoid arthritis. Annals of Hematology 1992;65:265‐8. [PUBMED: 1457588] [DOI] [PubMed] [Google Scholar]
Weiss 2002
- Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Review 2002;16:87‐96. [PUBMED: 12127952] [DOI] [PubMed] [Google Scholar]
Weiss 2009
- Weiss G. Iron metabolism in the anemia of chronic disease. Biochimica et Biophysica Acta 2009;1790:682‐93. [PUBMED: 18786614] [DOI] [PubMed] [Google Scholar]
WHO 2008
- Benoist B, McLean E, Egli I, Cogswell M, editors. Worldwide prevalence of anaemia 1993–2005 : WHO global database on anaemia. Geneva: World Health Organization, 2008. [ISBN 978 92 4 159665 7] [Google Scholar]
Wilson 2004
- Wilson A, Yu HT, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. American Journal of Medicine 2004;116(Suppl 7A):50‐7. [PUBMED: 15050886 ] [DOI] [PubMed] [Google Scholar]
Wu 2003
- Wu HH, Talpaz M, Champlin RE, Pilat SR, Kurzrock R. Sequential interleukin 3 and granulocyte‐macrophage‐colony stimulating factor therapy in patients with bone marrow failure with long‐term follow‐up of responses. Cancer 2003;98:2410‐9. [PUBMED: 14635076] [DOI] [PubMed] [Google Scholar]
Young 2007
- Young A, Koduri G. Extra‐articular manifestations and complications of rheumatoid arthritis. Best Practice & Reserach. Clinical Rheumatology 2007;21:907‐27. [PUBMED: 17870035] [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang AS, Enns CA. Molecular mechanisms of normal iron homeostasis. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2009:207‐14. [PUBMED: 20008200] [DOI] [PMC free article] [PubMed]
Zhu 2000
- Zhu Y, Ye D, Huang Z. The correlation of cytokines TNF alpha, IFN‐gamma, Epo with anemia in rheumatoid arthritis. Zhonghua Xueyexue Zazhi 2000;21:587‐90. [PUBMED: 11225250 ] [PubMed] [Google Scholar]


