Abstract
Background
Chronic lung disease (CLD) is a major cause of mortality and morbidity in very low birth weight infants despite increased use of antenatal steroids and surfactant therapy. Ventilator injury and oxygen toxicity are thought to be important factors in the pathogenesis of chronic pulmonary disease. Evidence from animal studies and from adult human studies indicates that high‐frequency jet ventilation may reduce the severity of lung injury associated with mechanical ventilation.
Objectives
To compare use of high‐frequency jet ventilation (HFJV) versus conventional ventilation (CV) in preterm infants with severe pulmonary dysfunction.
Subgroup analyses include the following.
• Trials with and without surfactant replacement therapy.
• Trials with and without strategies to maintain lung volume.
• Trials with infants of different gestational ages and birth weights (specific subgroups to include < 28 weeks' gestation and < 1000 grams).
• Trials with and without adequate humidification of inspired gases.
Search methods
The original search included MEDLINE (1966 to August 2005), the Cochrane Central Register of Controlled Trials (CENTRAL; 2005, Issue 3) and EMBASE (1988 to August 2005). We also obtained information from experts in the field and checked cross‐references. We updated the electronic search in June 2013 and again in June 2015.
Selection criteria
We included in this systematic review randomised and quasi‐randomised controlled trials of rescue high‐frequency jet ventilation versus conventional ventilation in preterm infants born at less than 35 weeks' gestation or with birth weight less than 2000 grams in respiratory distress.
Data collection and analysis
We used standard methods of the Cochrane Neonatal Review Group, including independent trial assessment and data extraction. We analysed data using risk ratios (RRs) and risk differences (RDs).
Main results
We included only one trial in the review. Keszler 1991 randomly assigned 166 preterm infants; reported data on 144 infants; and permitted cross‐over to the alternate treatment if initial treatment failed. Investigators found no statistically significant differences in overall mortality (including survival after cross‐over) between the two groups (RR 1.07, 95% confidence interval (CI) 0.67 to 1.72). In a secondary analysis of infants up to the time of cross‐over, rescue treatment with HFJV was associated with lower mortality (RR 0.66, 95% CI 0.45 to 0.97). Researchers reported no significant differences in the incidence of CLD among survivors at 28 days of age, nor in the incidence of intraventricular haemorrhage, new air leaks, airway obstruction and necrotising tracheobronchitis.
Authors' conclusions
Study authors reported no significant differences in overall mortality between rescue high‐frequency jet ventilation and conventional ventilation and presented highly imprecise results for important adverse effects such as intraventricular haemorrhage, new air leaks, airway obstruction and necrotising tracheobronchitis.
The overall quality of evidence is affected by limitations in trial design and by imprecision due to the small number of infants in the included study. Existing evidence does not support the use of high‐frequency jet ventilation as rescue therapy in preterm infants.
Studies that target populations at greatest risk and that have sufficient power to assess important outcomes are needed. These trials should incorporate long‐term pulmonary and neurodevelopmental outcomes.
Plain language summary
Rescue high‐frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants
Background: In very low birth weight infants who require support on breathing machines (ventilators), ventilator‐associated lung injury and toxic effects of oxygen may be important factors in creating a chronic disturbance in lung function. Compared with routine conventional ventilators, high‐frequency jet ventilators (breathing machines that introduce short‐duration pulses of gas under pressure into the airway at a very fast rate) may reduce the severity of lung injury associated with mechanical ventilation.
Question: In preterm infants with severe respiratory dysfunction despite attempts at conventional ventilator support, does use of rescue high‐frequency jet ventilation compared with ongoing conventional ventilation decrease the risk of lung injury (chronic lung disease) or death?
Study characteristics: One study randomly assigned 166 preterm infants and reported data on 144 infants. The included study was completed before the introduction of surfactant and widespread use of antenatal steroids.
Key Results: This trial demonstrated no differences in outcomes among infants who received high‐frequency jet ventilation. In this trial, cross‐over to the alternate treatment was permitted if initial treatment failed. Investigators found no statistically significant differences in overall mortality (including survival after cross‐over) between the two groups. In a secondary analysis, researchers showed that rescue treatment with HFJV, up to the time of cross‐over, was associated with lower mortality. Researchers reported no differences in the incidence of chronic lung disease among survivors at 28 days of age, and they found no differences in intraventricular haemorrhage, new air leaks, airway obstruction and necrotising tracheobronchitis.
Conclusions: Existing evidence does not support the use of rescue high‐frequency jet ventilation compared with conventional mechanical ventilation for treatment of preterm infants with severe pulmonary problems. Additional research is needed.
Summary of findings
Summary of findings for the main comparison. Rescue high‐frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants.
| Rescue high‐frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants | ||||||
| Patient or population: preterm infants with severe pulmonary dysfunction Setting: neonatal intensive care unit in the United States Intervention: high‐frequency jet ventilation Comparison: conventional ventilation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with conventional ventilation | Risk with high‐frequency jet ventilation | |||||
| Overall mortality | 314 per 1000 | 324 per 1000 (201 to 522) | RR 1.03 (0.64 to 1.66) | 144 (1 RCT) | ⊕⊕◯◯ LOWa | Downgraded 2 levels because of: • serious risk of bias (limitations in trial design due to high cross‐over rates between alternative therapies). • serious imprecision (95% confidence interval includes both no effect and appreciable harm. Total number of events does not meet optimal information size) |
| Mortality before cross‐over | 529 per 1000 | 349 per 1000 (238 to 513) |
RR 0.66 (0.45 to 0.97) | 144 (1 RCT) | ⊕⊕⊕◯ MODERATEa | Downgraded 1 level because of: • serious imprecision (total number of participants does not meet the optimal information size; OIS is > 260 participants) • reasons other than limitations in study design, as mortality before cross‐over is not affected by design limitations (see above) |
| Chronic lung disease at 28 days of birth (CLD 28 days) | 667 per 1000 | 513 per 1000 (360 to 713) | RR 0.77 (0.54 to 1.07) | 97 (1 RCT) | ⊕⊕◯◯ LOWa,b | Downgraded 2 levels because of: • serious risk of bias (limitations in trial design (lack of blinding of outcome assessment) • serious imprecision (95% confidence interval includes both no effect and appreciable harm. Total number of events does not meet optimal information size) |
| Pulmonary air leak (new) | 357 per 1000 | 271 per 1000 (164 to 439) | RR 0.76 (0.46 to 1.23) | 144 (1 RCT) | ⊕⊕◯◯ LOWa,b | Downgraded 2 levels because of: • serious risk of bias (limitations in trial design (lack of blinding of participants, providers and outcome assessor) • serious imprecision (95% confidence interval includes both no effect and appreciable harm. Total number of events does not meet optimal information size) |
| Severe intraventricular haemorrhage (grades 3 and 4) | 345 per 1000 | 256 per 1000 (145 to 442) | RR 0.74 (0.42 to 1.28) | 118 (1 RCT) | ⊕⊕◯◯ LOWa,b | Downgraded 2 levels because of: • serious risk of bias (limitations in trial design (lack of blinding of participants, providers and outcome assessor) • serious imprecision (95% CI includes both no effect and appreciable harm. Total number of events does not meet OIS) |
| Chronic lung disease at 36 weeks postmenstrual age (CLD 36 wPMA) | See comments | See comments | Not reported | |||
| Long‐term neurodevelopmental outcomes | See comments | See comments | Not reported | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but it may be substantially different. Low quality: Our confidence in the effect of the estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of the effect. | ||||||
a95% confidence interval includes both no effect and appreciable harm. Total number of events does not meet optimal information size.
bDowngraded because of lack of blinding of participants, providers and outcome assessors.
Background
Description of the condition
Pulmonary disease continues to be a major cause of mortality and morbidity in very low birth weight infants despite increased use of antenatal steroids and surfactant therapy. In addition to immaturity, ventilator injury and oxygen toxicity are thought to be important factors in the pathogenesis of chronic pulmonary disease (Jobe 2000). Animal studies (Barringer 1982; Carlon 1983; Hoff 1981) and adult human studies (Carlon 1981; Turnbull 1981) have provided evidence that high‐frequency jet ventilation may reduce the severity of lung injury associated with mechanical ventilation.
Description of the intervention
High‐frequency jet ventilators (HFJVs) deliver high‐flow, short‐duration pulses of pressurised gas directly into the upper airway through a specially designed endotracheal lumen. Pulses are delivered to the upper airway and are superimposed on a background gas flow from a conventional ventilator, which provides positive end‐expiratory pressure (PEEP). Conventional breaths may be delivered in conjunction with jet ventilation. Systems operate at a rate of 150 to 600 breaths per minute. Exhalation is passive.
How the intervention might work
High‐frequency jet ventilation (HFJV) is substantially different from high‐frequency oscillatory ventilation (HFOV), which uses an electromagnetically driven diaphragm to generate a sinusoidal pattern of pressure within the ventilatory circuit. Oscillating movement of the diaphragm causes active inspiratory and expiratory phases that drive the mixing of gases between circuits and alveoli. The amplitude of pressure generated by the diaphragm and mean airway pressure can be adjusted independently (Avery 2005). The major difference between HFJV and HFOV is seen in the inspiratory/expiratory ratios that they generate. Piston‐driven HFOV devices have a mandatory 1:1 ratio. The SensorMedics HFOV is capable of a range of ratios but usually is used at a ratio of 1:2. The Bunnel HFJV is usually used at a 1:6 ratio. This extremely short I:E ratio makes it effective in treating patients with interstitial emphysema or a bronchopleural fistula.
Potential advantages of HFJV include use of small tidal volumes, effective management of ventilation and oxygenation and safer use of mean airway pressure, thereby reducing ventilator‐related lung injury, improving gas exchange and decreasing oxygen requirements (Lampland 2007). Some interventions will influence any ventilator strategy used in preterm infants. Surfactant, by its surface tension‐reducing property, renders alveoli stable and prevents their collapse while increasing alveolar recruitment. Thus, use of surfactant has been reported to have a beneficial effect among infants with hyaline membrane disease (Soll 1998). High lung volume strategies have proved effective in reducing mortality and chronic lung disease. The adverse effect of necrotising tracheobronchitis, thought to be due to inadequate humidification of inspired gases (Elzouki 2011), has been reported in some animal studies. Therefore, we made the decision to conduct subgroup analyses based on use of surfactant; use of ventilation strategies to maintain optimal lung volumes, gestational age and weight; and use or no use of adequate humidification a priori.
Why it is important to do this review
This review updates the previous review of "Rescue high frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants" (Joshi 2006). Included in this systematic review were trials in which participants were randomly assigned after they failed to adequately ventilate on conventional ventilation (CV), or when complications of CV developed or were likely to develop. Elective use of HFJV was assessed in another review (Bhuta 2002).
Objectives
To compare use of high‐frequency jet ventilation (HFJV) versus conventional ventilation (CV) in preterm infants with severe pulmonary dysfunction.
Subgroup analyses include the following.
Trials with and without surfactant replacement therapy.
Trials with and without strategies to maintain lung volume.
Trials with infants of different gestational ages and birth weights (specific subgroups to include < 28 weeks' gestation and < 1000 grams).
Trials with and without adequate humidification of inspired gases.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised controlled trials.
Types of participants
Preterm infants at less than 35 weeks' gestational age at birth or with birth weight less than 2000 grams with severe pulmonary dysfunction, including pulmonary interstitial emphysema, and an unsatisfactory response to conventional ventilation.
Types of interventions
High‐frequency jet ventilation (HFJV) used as rescue intervention in infants failing adequate conventional ventilation (CV), or presenting with complications such as pulmonary interstitial emphysema (PIE), and in preterm infants with severe pulmonary dysfunction (i.e. failing with adequate CV, or presenting complications such as pulmonary interstitial emphysema) for whom CV was used as continued therapy.
Trials assessing any of the following modes of ventilation were included.
HFJV.
High flow.
Short‐duration pulses of pressurised gas directly into the upper airway.
Respiratory rate of 150 to 600 breaths per minute.
CV.
Time‐cycled.
Pressure‐limited ventilation through an endotracheal lumen.
Respiratory rate of approximately 30 to 80 breaths per minute.
Elective use of HFJV is not included here and is assessed in another review (Bhuta 2002).
Types of outcome measures
Primary outcomes
Mortality at 28 to 30 days and at hospital discharge.
Chronic lung disease at 28 days (defined as oxygen supplementation at 28 days).
Chronic lung disease at 36 weeks' postmenstrual age (defined as supplemental oxygen at 36 weeks' gestation).
Chronic lung disease or death at 36 weeks' postmenstrual age.*
Secondary outcomes
Pneumothorax.*
Pulmonary air leak syndromes (pneumothorax, pneumomediastinum, pulmonary interstitial emphysema).
Intraventricular haemorrhage (all grades with severe IVH defined as grades 3 and 4) (Papile 1978).
Periventricular echodensities.
Necrotising tracheobronchitis.
Sepsis.*
Necrotising enterocolitis (Bell 1978).*
Retinopathy of prematurity (ICCROP 2005).*
Long‐term pulmonary outcome (including rehospitalisation for respiratory causes, asthma and/or pulmonary function testing).*
Long‐term neurodevelopmental outcome (measured at approximately two years' corrected age; acceptable range 18 months to 28 months), including cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity) and hearing deficit (aided or < 60 dB on audiometric testing); impairment defined as including any of the aforementioned deficits.
Adverse events: airway obstruction (added post hoc).
*Added as an outcome in the 2015 update.
Search methods for identification of studies
Electronic searches
The original search was conducted as described in Appendix 1.
In March 2015, we updated the search for published manuscripts through the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL), EBSCOhost, EMBASE, MEDLINE via PubMed, The Cochrane Library from 2005 to 2015, using the following search terms: (oscillatory ventilation OR mechanical ventilation OR jet ventilation OR volume‐targeted ventilation OR pressure‐limited ventilation) AND (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial).
We searched for ongoing or registered clinical trials on clinicaltrials.gov and controlled‐trials.com using the same search terms.
Searching other resources
For the original review, information was obtained from experts in the field and from cross‐references in published articles.
We conducted a search of the database of abstracts of the Pediatric Academic Societies from 2000 to 2015 using the term 'high‐frequency jet ventilation'.
Data collection and analysis
We used the standard review methods of the Neonatal Review Group, as documented in The Cochrane Library.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. Two review authors (MX and PO) reviewed results of the updated search and selected studies for inclusion.
Data extraction and management
Each review author extracted data separately, then compared and resolved differences.
We requested additional data from one study author regarding 22 babies who were excluded post randomisation, but we could not obtain outcomes for these babies.
Assessment of risk of bias in included studies
We assessed the methodological quality of studies using the following key criteria: sequence generation,allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow‐up and blinding of outcome measurement/assessment. For each criterion, assessment was yes, no or cannot determine. We included this information in the table Characteristics of included studies. In addition, review authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the methodological quality of studies by using the following criteria.
Sequence generation (evaluating possible selection bias): For each included study, we described the method used to generate the allocation sequence as adequate (any truly random process, e.g. random number table, computer random number generator); inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or unclear.
Allocation concealment (evaluating possible selection bias): For each included study, we described the method used to conceal the allocation sequence as adequate (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes); inadequate (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or unclear.
Blinding (evaluating possible performance bias): For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods as adequate, inadequate or unclear for participants; adequate, inadequate or unclear for study personnel; and adequate, inadequate or unclear for outcome assessors.
Incomplete outcome data (evaluating possible attrition bias through withdrawals, drop‐outs, protocol deviations): For each included study and for each outcome, we described completeness of data, including attrition and exclusions from analysis. We stated whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomly assigned participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. We assessed methods as adequate (< 20% missing data); inadequate (≥ 20% missing data); or unclear.
Selective reporting bias: For each included study for which the protocol is available (through trial registers), we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed methods as adequate (when it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported); inadequate (when not all of the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or the study failed to include results of a key outcome that would have been expected to have been reported); or unclear.
Other sources of bias. We noted other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias.
We made explicit judgements regarding whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the criteria above, we assessed the likely magnitude and direction of bias and the likelihood that it would impact our findings. If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis below).
Quality of evidence
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a). This methodological approach considers randomised controlled trials (RCTs) as providing high‐quality evidence that may be "down‐rated" by limitations in any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias (Guyatt 2011a). The GRADE approach can be used to assess the quality of a body of evidence by one of the following four grades. High: We are very confident that the true effect lies close to that of the estimate of effect. Moderate: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect but may be substantially different. Low: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of effect. Very low: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect (Schünemann 2013).
Review authors independently assessed the quality of evidence found for outcomes identified as critical or important for clinical decision making: mortality at 28 to 30 days and at hospital discharge, chronic lung disease at 28 days (defined as oxygen supplementation at 28 days), chronic lung disease at 36 weeks' postmenstrual age (defined as supplemental oxygen at 36 weeks' gestation), chronic lung disease or death at 36 weeks' postmenstrual age, pulmonary air leak syndromes (pneumothorax, pneumomediastinum, pulmonary interstitial emphysema), intraventricular haemorrhage (all grades and grades 3 and 4) and long‐term neurodevelopmental outcomes (including cerebral palsy, delayed neurodevelopment, legal blindness, hearing deficit and impairment defined as including any one of the aforementioned deficits).
When study authors did not take measures to ensure concealment of allocation, randomised assignment, completed follow‐up or blinded outcome assessment, we downgraded the quality of the evidence because of design limitations (Guyatt 2011b). We evaluated consistency using similarity of point estimates, extent of overlap of confidence intervals and statistical criteria including tests for heterogeneity (I2). We downgraded the quality of the evidence when inconsistency across study results was present in a large and unexplained way (i.e. some studies suggest important benefit, and others no effect or harm without a clinical explanation) (Guyatt 2011d). We assessed precision with the 95% confidence interval around the pooled estimation (Guyatt 2011c). When trials were conducted in populations other than the target population, we downgraded the quality of the evidence because of indirectness (Guyatt 2011e).
We entered data (i.e. pooled estimates of effects and corresponding 95% confidence Intervals) and explicit judgements for each of the assessed aspects described above into the Guideline Development Tool 2015 ‐ the software used to create 'Summary of findings' (SoF) tables. In footnotes or comments in the SoF table, we explained all judgements involved in assessment of study characteristics as described above (Table 1).
Measures of treatment effect
We used the standard methods of the Neonatal Review Group, including those for categorical data, and we used risk ratios (RRs) and risk differences (RDs). From 1/RD, we calculated the number needed to treat for an additional benefit (NNTB) or the number needed to treat for an additional harm (NNTH) to identify adverse effects, and we calculated their 95% confidence intervals (CIs). For continuous data, we used standardised mean differences (SMDs) with 95% CIs.
Unit of analysis issues
For clinical outcomes such as episodes of sepsis, we analysed the data as proportions of neonates having one or more episodes.
Dealing with missing data
For included studies, we noted levels of attrition. We used sensitivity analysis to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
We performed all outcomes analyses on an intention‐to‐treat basis (i.e. we included in these analyses all participants randomly assigned to each group). The denominator for each outcome in each trial was the number randomly assigned minus the number of participants whose outcomes were known to be missing.
We requested additional data from one study author regarding 22 babies who were excluded post randomisation. We could not obtain outcomes for these babies from the study author.
Assessment of heterogeneity
We estimated treatment effects of individual trials and examined heterogeneity among trials by inspecting forest plots and quantifying the impact of heterogeneity using the I2 statistic. We graded the degree of heterogeneity as < 25% no heterogeneity; 25% to 49% low heterogeneity; 50% to 75% moderate heterogeneity; and > 75% substantial heterogeneity. If we noted statistical heterogeneity (I2 > 50%), we explored possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments).
Assessment of reporting biases
We planned to assess possible publication bias and other biases using symmetry/asymmetry of funnel plots.
For included trials that were recently performed (and therefore prospectively registered), we explored possible selective reporting of study outcomes by comparing primary and secondary outcomes provided in the reports versus primary and secondary outcomes proposed at trial registration, using the websites www.clinicaltrials.gov and www.controlled‐trials.com. If we found such discrepancies, we planned to contact the primary investigators to obtain missing data for outcomes prespecified at trial registration.
Data synthesis
When meta‐analysis was judged to be appropriate, we performed the analysis using Review Manager software (RevMan 2011), supplied by The Cochrane Collaboration. We used the Mantel‐Haenszel method to determine estimates of typical risk ratios and risk differences. We included no continuous outcomes in this review.
We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses included the following.
Trials with and without surfactant replacement therapy.
Trials with and without strategies to maintain lung volume.
Trials with infants of different gestational ages and birth weights (specific subgroups to include < 28 weeks' gestation and < 1000 g).
Trials with and without adequate humidification of inspired gases.
Sensitivity analysis
We planned sensitivity analyses for situations that might affect interpretation of significant results (e.g. when risk of bias was associated with the quality of some of the included trials, when outcome data were missing). We determined that no such analyses were necessary for this review.
Results
Description of studies
Results of the search
In the original review, a total of six potentially relevant trials were identified; five were randomised controlled trials (RCTs) (Keszler 1991; Carlo 1990; Engle 1997; Keszler 1997; Wiswell 1996), and one was a non‐randomised study (Davis 1992). Of the five RCTs, only one met our inclusion criteria; three of the remaining trials (Carlo 1990; Keszler 1997; Wiswell 1996) used HFJV as an elective intervention ‐ not as "rescue" ‐ and one (Engle 1997) was conducted in near term or term infants and did not match the population specified in the inclusion criteria.
From the updated search, we obtained 489 records; we screened 457 records after removing duplicates (Figure 1). We excluded a total of 455 records on the basis of review of titles and abstracts. We selected two as potentially eligible studies (Kushnir 2014; Plavka 2006). Assessment of full text revealed that none of the remaining records matched the inclusion criteria for this review (Characteristics of excluded studies).
1.

Study flow diagram.
Included studies
Keszler 1991 is a multi‐centre RCT conducted from January 1987 to March 1989. Keszler 1991 compared HFJV versus CV when given to infants younger than 7 days of age and weighing 750 grams or more at birth, in whom pulmonary interstitial emphysema (PIE) developed during CV. The primary outcome was improved PIE, clinical or radiographic, with reduced mean airway pressure (Paw). We included 144 infants in the analysis. Infants who did not respond to the initial mode of ventilation and met the specific criteria for treatment failure were permitted to cross over to other ventilation therapy.
Criteria for treatment failure included the following.
Worsening PIE, as demonstrated by significant radiographic worsening or by development of intractable air leaks, accompanied by deteriorating gas exchange requiring increasing ventilator support to maintain target blood gas values.
Lack of improvement, defined as no improvement in PIE after 96 hours, accompanied by deteriorating gas exchange.
Inadequate gas exchange during maximal support, including arterial oxygen tension < 40 mmHg or arterial carbon dioxide tension > 65 mmHg on mean airway pressure > 15 cmH2O and fraction of inspired oxygen equal to one.
Acute deterioration, demonstrated by sudden worsening of participant status, so that continued participation in the study would be contrary to his or her best interest. In all, 39% of babies from the HFJV group were crossed over to CV, and 63% of babies from the CV group were crossed over to HFJV, when criteria for treatment failure were met. In this review, we analysed infants in the groups to which they were originally randomly assigned. None of the infants in the study received surfactant. See Characteristics of included studies for details.
Excluded studies
In the original review, five studies were excluded (Carlo 1990; Davis 1992; Engle 1997; Keszler 1997; Wiswell 1996) (see Characteristics of excluded studies).
Engle 1997 was not included because the study population was restricted to near term and term infants. Davis 1992 was not included as it was neither randomised nor quasi‐randomised.
In addition to the studies of Engle 1997 and Davis 1992, three studies evaluating the effects of elective use of HFJV were excluded (Carlo 1990; Keszler 1997; Wiswell 1996). These studies are included in the systematic review, "Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants" (Bhuta 2002).
For this updated review, we excluded two studies (Kushnir 2014; Plavka 2006).
Kushnir 2014 was an observational trial of infants being transitioned from HFOV to HFJV.
Plavka 2006 was a single‐centre (Prague), non‐randomised pilot study in which 10 preterm infants with refractory respiratory failure (RRF) were assigned to rescue HFJV. Primary outcomes included reducing the PaCO2 and the oxygenation index (OI). The main reason for exclusion was that the study population did not match the inclusion criteria for this review. Plavka 2006 explored the effects of HFJV on oxygenation, ventilation and ease of extubation in preterm infants with evolving chronic lung disease (CLD) and refractory respiratory failure (RRF) (see Characteristics of excluded studies).
Risk of bias in included studies
Details on the methodological quality of the included study are provided in the table Characteristics of included studies. Randomisation was adequate and allocation was concealed, but treatment was not blinded. Of 166 patients who were entered into the study, 22 (13%) were excluded from further consideration because of the presence of unrecognised exclusion criteria (nine patients), significant deviation from the protocol (eight), presence of a conflicting research protocol (three) or other reasons. We were not able to obtain from the study author data regarding babies excluded from each randomly assigned group.
Effects of interventions
See: Table 1
The single included trial (Keszler 1991) reported on 144 infants (166 were randomly assigned). Results for critical and important outcomes follow here.
Primary outcomes
Mortality
Overall mortality
No statistically significant differences in overall mortality were noted between HFJV and CV groups (RR 1.03, 95% CI 0.64 to 1.66; RD 0.01, 95% CI ‐0.14 to 0.16). The quality of this evidence is low because of limitations in trial designed and imprecision of estimates.
Mortality before cross‐over
Mortality in the HFJV group was significantly lower than in the CV group (RR 0.66, 95% CI 0.45 to 0.97; RD ‐0.18, 95% CI ‐0.34 to ‐0.02). The quality of evidence for this outcome is moderate.
Mortality at 28 to 30 days
Not reported.
Mortality at hospital discharge
Not reported.
Chronic lung disease
Chronic lung disease at 28 days
No statistically significant differences were found in the incidence of CLD among survivors (RR 0.77, 95% CI 0.54 to 1.07; RD ‐0.16, 95% CI ‐0.35 to 0.04). The quality of this evidence is low because of limitations in trial design and imprecision of estimates.
Chronic lung disease at 36 weeks' postmenstrual age
Not reported.
Chronic lung disease or death at 36 weeks' postmenstrual age
Not reported.
Secondary outcomes
Pulmonary air leak syndromes (pneumothorax, pneumomediastinum, pulmonary interstitial emphysema)
New air leak
No statistically significant differences were noted in the incidence of new air leaks (RR 0.76, 95% CI 0.46 to1.23; RD ‐0.09, 95% CI ‐0.24 to 0.06). The quality of this evidence is low because of limitations in trial design and imprecision of estimates.
Pneumothorax
Not reported.
Intraventricular haemorrhage
Intraventricular haemorrhage all grades
Not reported.
Intraventricular haemorrhage grades 3 and 4
No statistically significant differences were noted in the incidence of severe IVH (grades 3 and 4) (RR 0.74, 95% CI 0.42 to1.28; RD ‐ 0.09, 95% CI ‐0.26 to 0.07). The incidence of new IVH in infants assessed was lower in the rescue HFJV group, but this finding was not statistically significant (RR 0.49, 95% CI 0.19 to1.24). The quality of this evidence is low because of limitations in trial design and imprecision of estimates.
Periventricular leukomalacia (cystic or haemorrhage) and periventricular echodensities
Keszler 1991 did not report on these outcomes.
Necrotising tracheobronchitis
No statistically significant differences were noted in the incidence of necrotising tracheobronchitis at autopsy in 17 infants (RR 1.33, 95% CI 0.29 to 6.06; RD 0.08, 95% CI ‐0.35 to 0.51).
Airway obstruction
No statistically significant differences were noted between the two groups (RR 3.78, 95% CI 0.43 to 33.03; RD 0.04, 95% CI ‐0.02 to 0.10).
Sepsis
Not reported.
Necrotising enterocolitis
Not reported.
Retinopathy of prematurity
Not reported.
Long‐term pulmonary outcomes (including rehospitalisation for respiratory causes, asthma and/or pulmonary function testing)
Not reported.
Long‐term neurodevelopmental outcomes
Not reported.
Discussion
Only one trial satisfied the eligibility criteria and was included in this review. It was conducted in the late 1980s, and none of the infants in the study received exogenous surfactant. The study was designed to investigate short‐term pulmonary outcomes such as resolution of pulmonary interstitial emphysema (PIE) associated with radiographic improvement. The cross‐over that was permitted hampered the ability of investigators to demonstrate an advantage of one ventilator over the other in reducing important complications.
Regarding outcomes identified as critical or important for clinical decision making, the included study showed no differences in overall mortality (including survival after cross‐over) among infants treated with high‐frequency jet ventilation (HFJV) versus conventional ventilation (CV). Survival by original assignment did not differ between groups; however, because of the cross‐over design of the trial and the high cross‐over rate, the meaning of data on this important outcome is unclear. Mortality up until the point of cross‐over was lower in the HFJV group than in the group given CV.
Although reduction in the incidence of chronic lung disease (CLD) at 28 days was a potential benefit, this finding was not statistically significant. A possible explanation for this is that the intervention in infants with severe respiratory failure and established barotrauma may have come too late to have an impact on the incidence of CLD.
The trial reported on new pulmonary air leak syndrome but failed to show statistically significant differences in risk of pneumothorax, pneumomediastinum or PIE between HFJV and CV.
Among desirable short‐term and long‐term neurological outcomes, the included trial reported only on rates of severe intraventricular haemorrhage (IVH; grades 3 and 4), but lack of precision in estimates permits no conclusions on the effects of HFJV on this outcome. Researchers did not assess periventricular leukomalacia (PVL) and long‐term neurodevelopmental outcomes.
The overall quality of evidence among these critical outcomes ranges from low to moderate because of imprecision of estimates and lack of blinding of participants and of outcome assessors. For CLD, pulmonary air leak syndrome and IVH, the quality of evidence is low, which means that our confidence in the effect estimate is limited, and it may be substantially different from the true effect.
The included trial reported on other important pulmonary outcomes such as incidence of airway obstruction and necrotising tracheobronchitis, but estimates of the effects of assessed interventions on these outcomes are very imprecise, leaving it unclear whether HFJV leads to significantly more or less airway injury.
No evidence showed the effect that HFJV may have on other important neonatal outcomes, including sepsis, necrotising enterocolitis, retinopathy of prematurity or long‐term pulmonary outcomes such as rehospitalisation for respiratory causes, asthma or pulmonary function.
Evidence on effects of HFJV as rescue intervention for severe pulmonary dysfunction was derived from just from one trial involving 144 infants. Limitations in trial design such as lack of blinding of outcome assessment; reports of imprecise results for most assessed outcomes; and failure to report important long‐term neurodevelopmental outcomes do not support use of this intervention in neonates.
Authors' conclusions
Implications for practice.
This study showed no significant differences in overall mortality between rescue high‐frequency jet ventilation and conventional ventilation; investigators reported highly imprecise results for adverse effects such as intraventricular haemorrhage, new air leaks, airway obstruction and necrotising tracheobronchitis, which do not allow conclusions regarding effects of high‐frequency jet ventilation as rescue intervention on these important safety outcomes.
The overall quality of evidence is related to the fact that the study was done before surfactant therapy was introduced and before antenatal steroids (which have shown significant impact in lung disease and survival of preterm infants) were used routinely, as well as to limitations in trial design and imprecision resulting from the small number of infants included in the study.
Thus, existing evidence is of low quality and does not support the use of high‐frequency jet ventilation as rescue therapy in preterm infants.
Implications for research.
New studies assessing high‐frequency jet ventilation as rescue therapy for preterm infants under current practices in neonatal care units could be useful for determining the real effects of this intervention. These new studies should have enough power to define the effects of the intervention on different subgroups of infants according to use of surfactant and prenatal steroids, and should undertake comparisons of conventional ventilation versus more gentle ventilation modes currently available, such as trigger modes and volume guarantee. These trials should incorporate long‐term neurodevelopmental outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2015 | New search has been performed | GRADE assessment and 'Summary of findings' table were added |
| 30 June 2015 | New citation required but conclusions have not changed | Search was updated in June 2015. No new trials were identified for inclusion |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 22 July 2013 | New citation required but conclusions have not changed | Updated June 30, 2013 |
| 20 July 2013 | New search has been performed | Electronic search updated in June 2013. No new studies identified for inclusion. Conclusions unchanged |
| 16 October 2008 | Amended | Converted to new review format |
Acknowledgements
From the original review: We thank Martin Keszler for answering our queries about the study, and David Henderson‐Smart, who participated in protocol development.
We acknowledge Prof. David J. Henderson‐Smart (deceased), Dr. Tushar Bhuta and Dr. Vinay H. Joshi for their previous work on this review.
The review authors thank Colleen Ovelman, Information Specialist from the Cochrane Neonatal Group, for running new searches for this review update.
The Faculty of Medicine of the Pontificia Universidad Javeriana, Bogotá, Colombia, supported the time dedicated by María Ximena Rojas to update this review as part of an agreement with The Cochrane Collaboration. Paola Orrego is a medical student participant from the Systematic Reviews Seed Research Program of the Cochrane Colaborating Centre "Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine of the Pontificia Universidad Javeriana, Bogotá, Colombia".
Appendices
Appendix 1. Original search strategies
Search was made of MEDLINE by means of the MeSH and text words, 'high frequency ventilation', 'high frequency jet ventilation', 'jet ventilation' from the years 1966 to August 2005; EMBASE (1988 to August 2005); and trials register held by the Neonatal Review Group of The Cochrane Collaboration (2005, Issue 3; The Cochrane Library).
In June 2013 and February 2015, we updated the search as follows: MEDLINE (search via PubMed), CINAHL, EMBASE and CENTRAL (The Cochrane Library) were searched from 2005 to 2013, and subsequently from 2013 to 2015. Search string: (high frequency ventilation OR high frequency jet ventilation OR jet ventilation) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])).
In June 2013, we also searched clinicaltrials.gov and controlled‐trials.com for relevant studies.
Data and analyses
Comparison 1. Rescue HFJV vs CV in preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality | 1 | 144 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.14, 0.16] |
| 2 Mortality before cross‐over | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.97] |
| 3 CLD at 28 to 30 days in survivors | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.07] |
| 4 Pulmonary air leak (new) | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.23] |
| 5 Severe IVH (grades 3 and 4) | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.28] |
| 6 Necrotising tracheobronchitis at autopsy | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.29, 6.06] |
| 7 Airway obstruction | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.43, 33.03] |
1.1. Analysis.

Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 1 Overall mortality.
1.2. Analysis.

Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 2 Mortality before cross‐over.
1.3. Analysis.
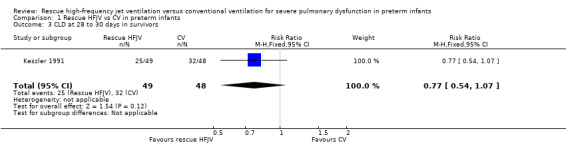
Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 3 CLD at 28 to 30 days in survivors.
1.4. Analysis.
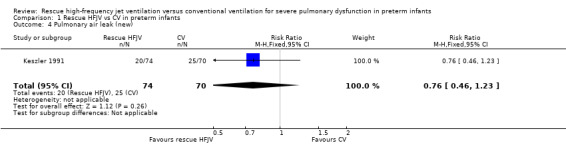
Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 4 Pulmonary air leak (new).
1.5. Analysis.
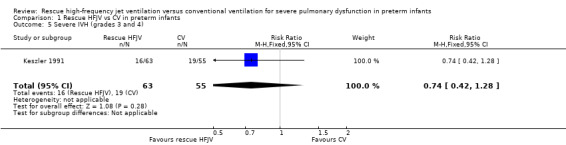
Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 5 Severe IVH (grades 3 and 4).
1.6. Analysis.

Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 6 Necrotising tracheobronchitis at autopsy.
1.7. Analysis.

Comparison 1 Rescue HFJV vs CV in preterm infants, Outcome 7 Airway obstruction.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Keszler 1991.
| Methods | Multi‐centre trial, enrolment between January 1987 and March 1989 Concealment at randomisation ‐ yes. Randomisation was performed centrally by calling a 24‐hour hotline Blinding of intervention ‐ no Complete follow‐up ‐ 22 of 166 infants were excluded after initial randomisation Blinding of outcome assessment ‐ no | |
| Participants | 166 preterm infants younger than 7 days of age and weighing ≤ 750 grams at birth, with pulmonary interstitial emphysema. Eligible infants were stratified by birth weight and by severity of illness. Among 144 infants analysed, mean birth weight was 1340 grams and mean gestational age at study entry was 29.3 weeks | |
| Interventions | High‐frequency jet ventilation (HFJV) with 400 to 450 cycles/min (treatment group), conventional ventilation (CV) with rates of 60 to 100 breaths/min, short inspiratory time (control group). 70 infants were assigned to CV, and 74 infants to HFJV. Cross‐over to alternate therapy was allowed if infants failed allocated ventilator therapy (Treatment failure was defined as worsening pulmonary interstitial emphysema (PIE), lack of improvement, inadequate gas exchange during maximal support or acute deterioration) Effective gas heating and humidification system | |
| Outcomes | Mortality at 28 to 30 days, success in the original assignment, chronic lung disease (CLD) at 28 to 30 days, intraventricular haemorrhage (IVH) (grades 3 and 4), new air leak, necrotising tracheobronchitis, airway obstruction, CLD in survivors | |
| Notes | Prenatal steroids were not reported. None of the babies received exogenous surfactant. Study was supported by Bunnell Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Concealment at randomisation ‐ yes. Randomisation performed centrally by calling 24‐hour hotline |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 of 166 infants were excluded after initial randomisation |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carlo 1990 | Elective high‐frequency jet ventilation (HFJV) Carlo 1990 randomly selected 42 infants with clinical and radiographic evidence of severe respiratory distress syndrome to receive HFJV or conventional ventilation (CV) RESULTS: Mortality rates (19% among infants receiving HFJV vs 24% among infants receiving CV), incidence of air leaks (48% vs 52%), bronchopulmonary dysplasia (39% vs 41%), intraventricular haemorrhage (33% vs 43%) and assignment cross‐overs (14% vs 24%) did not differ significantly between treatment groups. We conclude that early use of HFJV does not prevent or substantially reduce mortality or morbidity rates associated with assisted ventilation |
| Davis 1992 | Non‐randomised trial Twenty‐eight newborn infants (birth weight 2.4 ± 1.1 kg; gestational age 34.6 ± 6.1 weeks) with respiratory distress syndrome (RDS), meconium aspiration syndrome or pneumonia, who deteriorated in spite of optimal conventional mechanical ventilation (CMV) and exogenous surfactant therapy, were treated with high‐frequency jet ventilation (HFJV) and continued surfactant therapy Infants had to have a limited response to surfactant therapy and conventional ventilation, and had to meet clinical criteria that confirmed clinical deterioration and severity of illness Participants initially responded to HFJV alone with significant improvement in several respiratory variables, but their condition deteriorated subsequently and they received additional doses of exogenous surfactant on HFJV. Exogenous surfactant and HFJV resulted in significant and sustained improvement in several respiratory variables |
| Engle 1997 | The study population was restricted to term and near term neonates |
| Keszler 1997 | Multi‐centre, randomised, controlled clinical trial of high‐frequency jet ventilation (HFJV) and conventional ventilation (CV). Participants were to remain on assigned therapy for 14 days or until extubation, whichever came first. Cross‐over from CV to HFJV was allowed if bilateral pulmonary interstitial emphysema or bronchopleural fistula developed. Participants could cross over to the other ventilatory mode if failure criteria were met. The optimal lung volume strategy was mandated for HFJV by protocol to provide alveolar recruitment and optimise lung volume and ventilation/perfusion matching, while minimising pressure amplitude and O2 requirements. CV management was not controlled by protocol SETTING: 8 tertiary neonatal intensive care units PARTICIPANTS: Preterm infants with birth weights between 700 and 1500 grams and gestational age < 36 weeks who required mechanical ventilation with fraction of inspired oxygen (FIO2) > 0.30 at 2 to 12 hours after surfactant administration, received surfactant by 8 hours of age, were < 20 hours old and had been ventilated for < 12 hours OUTCOME MEASURES: Primary outcome variables were bronchopulmonary dysplasia (BPD) at 28 days' and 36 weeks' postconceptional age. Secondary outcome variables were survival, gas exchange, airway pressures, air leak, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL) and other non‐pulmonary complications RESULTS: A total of 130 participants were included in the final analysis; 65 were randomly assigned to HFJV and 65 to CV. Groups were of comparable birth weight, gestational age, severity of illness, postnatal age and other demographics. The incidence of BPD at 36 weeks' postconceptional age was significantly lower in babies randomly assigned to HFJV compared with CV (20.0% vs 40.4%). The need for home oxygen was significantly lower in infants receiving HFJV compared with CV (5.5% vs 23.1%). Survival, incidence of BPD at 28 days, retinopathy of prematurity, air leak, pulmonary haemorrhage, grade 1 to 2 IVH and other complications were similar |
| Kushnir 2014 | OBJECTIVE: to study the response of infants with hypoxaemic respiratory failure whose respiratory support was switched from high‐frequency oscillatory ventilation (HFOV) to high‐frequency jet ventilation (HFJV) Retrospective data analysis of 15 infants from 1/2011 to 9/2013 was conducted for infants with hypoxaemic refractory respiratory failure (HRRF) who were started on HFJV. Data were collected while patients were being switched from HFOV to HFJV and for 24 hours after the switch PARTICIPANTS: patients being switched from HFOV to HFJV OUTCOME MEASURES: Ventilator settings, oxygen saturations and oxygenation index (OI) were collected every 2 hours for 8 hours, then every 4 hours for 16 hours. Respiratory failure was defined as OI > 20, with mean airway pressure (MAP) providing optimum lung expansion on HFOV. Oxygenation settings on HFOV were compared with settings on HFJV initially and during the first 24 hours Over 20 months, 15 neonates with mean gestational age of 25.1 weeks and birth weight of 723 grams qualified for the study. A significant decrease in the fraction of inspired oxygen (FiO2) was noted at 30 minutes (0.93 to 0.78, P value = 0.01), within the first 12 hours (0.64, P value < 0.001) and within 24 hours (0.57, P value < 0.001) following the change to HFJV. A decrease in the OI was observed within the first 12 hours (37 to 19.6, P value = 0.006) and within 24 hours (17.8, P value = 0.005) post change. MAP decreased within the first 24 hours (14.4 to 12.8, P value = 0.06). Median postnatal age at change to HFJV was 15 days, and patients remained on the jet ventilator for an average of 12 days E‐PAS2014:2937.522 |
| Plavka 2006 | Evaluated the effects of high‐frequency jet ventilation (HFJV) on oxygenation, ventilation and ease of extubation in preterm infants with evolving chronic lung disease (CLD) and refractory respiratory failure (RRF) in a non‐randomised pilot trial |
| Wiswell 1996 | 73 premature infants who met the inclusion criteria (gestational age < 33 weeks, birth weight > 500 grams, age < 24 hours, need for assisted ventilation with peak inspiratory pressure > 16 and fraction of inspired oxygen (FIO2) > 0.30 and roentgenographic evidence of respiratory distress syndrome) were randomly assigned to conventional (n = 36) or to high‐frequency jet (n = 37) ventilation (HFJV). Attempts were made to maintain infants on the assigned ventilator for ≥ 7 days unless they could be extubated or could meet cross‐over criteria RESULTS: The 2 groups of infants were similar in all obstetrical, perinatal and neonatal demographic characteristics. Mean birth weight and gestational age in the conventional group were 930 g and 26.6 weeks, and in the HFJV group, 961 g and 26.9 weeks. Infants were randomly assigned at similar ages (7.1 and 7.3 hours of life, respectively). Their prerandomisation ventilator settings and arterial blood gases were nearly identical. No differences were noted in pulmonary outcomes (occurrence of air leaks, need for oxygen or ventilation at 36 weeks' postmenstrual age), and no differences were reported in the mean number of days oxygen was required, the number of days ventilated or the length of hospital stay. Infants ventilated with HFJV were significantly more likely to develop cystic periventricular leukomalacia (10 vs 2, P value = .022) or to have a poor outcome (grade 4 haemorrhage, cystic periventricular leukomalacia or death) (17 vs 7, P value = .016). Logistic regression analysis revealed HFJV to be a significant independent predictor of both cystic periventricular leukomalacia and a poor outcome |
Differences between protocol and review
New outcomes were added to the 2015 update (chronic lung disease or death at 36 weeks' postmenstrual age, pneumothorax, sepsis, necrotising enterocolitis (Bell 1978), retinopathy of prematurity (ICCROP 2005), long‐term pulmonary outcomes (including rehospitalisation for respiratory causes, asthma and/or pulmonary function testing), long‐term neurodevelopmental outcomes (measured at approximately two years' corrected age; acceptable range 18 months to 28 months) including cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity) and hearing deficit (aided or < 60 dB on audiometric testing), or impairment defined as having any one of the aforementioned deficits, and adverse events including airway obstruction.
GRADE assessment and a 'Summary of findings' table were added to the 2015 update.
Contributions of authors
Vinay Joshi and Tushar Bhuta wrote the first version of this review.
María Ximena Rojas‐Reyes and Paola Orrego‐Rojas drafted the update protocol and conducted the updating process.
Sources of support
Internal sources
Royal North Shore Hospital, Sydney, Australia.
Sydney Children's Hospital, Sydney, Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None of the authors declared that they had a conflict of interest over the past three years.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Keszler 1991 {published data only}
- Keszler M, Donn SM, Bucciarelli RL, Alverson DC, Hart M, Lunyong V, et al. Multicenter controlled trial comparing high‐frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema. Journal of Pediatrics 1990;119(1 Pt 1):85‐93. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carlo 1990 {published data only}
- Carlo WA, Siner B, Chatburn RL, Robertson S, Martin RJ. Early randomized intervention with high‐frequency jet ventilation in respiratory distress syndrome. Journal of Pediatrics 1990;117(5):765‐70. [DOI] [PubMed] [Google Scholar]
Davis 1992 {published data only}
- Davis JM, Richter SE, Kending JW, Notter RH. High‐frequency jet ventilation and surfactant treatment of newborns with severe respiratory failure. Pediatric Pulmonology 1992;13(2):108‐12. [DOI] [PubMed] [Google Scholar]
Engle 1997 {published data only}
- Engle WA, Yoder MC, Andreoli SP, Darragh RK, Langefeld CD, Hui SL. Controlled prospective randomized comparison of high‐frequency jet ventilation and conventional ventilation in neonates with respiratory failure and persistent pulmonary hypertension. Journal of Perinatology 1997;17(1):3‐9. [PubMed] [Google Scholar]
Keszler 1997 {published data only}
- Keszler M, Modanlou HD, Brudno DS, Clark FI, Cohen RS, Ryan RM, et al. Multicenter controlled clinical trial of high‐frequency jet ventilation in preterm infants with uncomplicated respiratory distress syndrome. Pediatrics 1997;100(4):593‐9. [DOI] [PubMed] [Google Scholar]
Kushnir 2014 {published data only}
- Kushnir A, Kemble N, Saslow J. Oxygenation in neonates with hypoxemic refractory respiratory failure (HRRF) after switching from high‐frequency oscillatory ventilator (HFOV) to high‐frequency jet ventilator (HFJV) [abstract]. Pediatric Academic Societies 2014.
Plavka 2006 {published data only}
- Plavka R, Dokoupilová M, Pazderová L, Kopecký P, Sebron V, Zapadlo M, et al. High‐frequency jet ventilation improves gas exchange in extremely immature infants with evolving chronic lung disease. American Journal of Perinatology 2006;23(8):467‐72. [DOI] [PubMed] [Google Scholar]
Wiswell 1996 {published data only}
- Wiswell TE, Graziani LJ, Kornhauser MS, Cullen J, Merton DA, McKee L, et al. High‐frequency jet ventilation in the early management of respiratory distress syndrome is associated with a greater risk for adverse outcomes. Pediatrics 1996;98(6 Pt 1):1035‐43. [PubMed] [Google Scholar]
Additional references
Avery 2005
- Avery GB, MacDonald MG, Seshia MMK, Mullett MD. Avery’s Neonatology: Pathophysiology & Management of the Newborn. 6th Edition. Philadelphia: Lippincott Williams & Wilkins, 2005. [Google Scholar]
Barringer 1982
- Barringer M, Meredith J, Prough D, Gibson R, Blinkhorn R. Effectiveness of high‐frequency jet ventilation in management of experimental bronchopleural fistula. American Surgeon 1982;48(12):610‐3. [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhuta 2002
- Bhuta T, Henderson‐Smart DJ. Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlon 1981
- Carlon GC, Kahn RC, Howland WS, Ray C Jr, Turnbull AD. Clinical experience with high frequency jet ventilation. Critical Care Medicine 1981;9(1):1‐6. [DOI] [PubMed] [Google Scholar]
Carlon 1983
- Carlon GC, Griffin J, Ray C Jr, Groeger JS, Patrick K. High frequency jet ventilation in experimental airway disruption. Critical Care Medicine 1983;11(5):353‐5. [DOI] [PubMed] [Google Scholar]
Elzouki 2011
- Kaam AH, Keszler M. Mechanical ventilation: HFV. In: Elzouki AY, Harfi HA, Nazer HM, Stapleton FB, Oh W, Whitley RJ editor(s). Textbook of Clinical Pediatrics [Internet]. Berlin, Heidelberg: Springer, 2012:245‐50. [Google Scholar]
GradePro 2014 [Computer program]
- McMaster University. GradePro [Version 3.2 for Windows]. Hamilton, Ontario, Canada: McMaster University, 2014.
Guideline Development Tool 2015 [Computer program]
- McMaster University and Evidence Prime Inc.. Guideline Development Tool. Hamilton, Ontario, Canada: McMaster University and Evidence Prime Inc., 2015. [http://www.guidelinedevelopment.org/]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hoff 1981
- Smith RB, Cutaia F, Hoff BH, Babinski M, Gelineau J. Long‐term transtracheal high frequency ventilation in dogs. Critical Care Medicine 1981;9(4):311‐4. [DOI] [PubMed] [Google Scholar]
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI] [PubMed] [Google Scholar]
Jobe 2000
- Jobe AH, Ikegami M. Lung development and function in preterm infants in the surfactant era. Annual Review of Physiology 2000;62:825‐46. [DOI] [PubMed] [Google Scholar]
Lampland 2007
- Lampland AL, Mammel MC. The role of high‐frequency ventilation in neonates: evidence‐based recommendations. Clinical Perinatology 2007;Mar 34(1):129–44, viii. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. RADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated October 2013.
Soll 1998
- Soll R. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD001149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnbull 1981
- Turnbull AD, Carlon G, Howland WS, Beattie EJ Jr. High‐frequency jet ventilation in major airway or pulmonary disruption. Annals of Thoracic Surgery 1981;32(5):468‐74. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Joshi 2006
- Joshi VH, Bhuta T. Rescue high frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000437.pub2] [DOI] [PubMed] [Google Scholar]


